Abstract
Background
Insulin resistance and beta cell dysfunction were reported to be responsible for gestational diabetes mellitus (GDM). However, little is known about the heterogeneity of these factors and its influences on perinatal outcomes. We investigated whether subtypes of insulin resistance and beta cell dysfunction in gestational diabetes mellitus have different impacts on perinatal outcomes.
Methods
In this prospective cohort study, we followed 554 pregnant women and glucose challenge test was performed at 24–28th weeks of their gestation. Women with plasma glucose ≥ 7.8 mmol/L would be included and advised to undergo the diagnostic 75-g, 3-h oral glucose tolerance test. According to indices of measuring insulin resistance or beta cell function were below the 25th percentile of women with normal glucose tolerance (NGT), women with GDM were defined as three subtypes: GDM with the beta cell dysfunction, GDM with the insulin resistance defect or GDM with both traits mentioned above (GDM-mixed). Perinatal outcomes were documented.
Results
The levels of prepregnancy and maternal BMI in the GDM-mix group were higher compared to women in the NGT group (23.2 ± 4.0 vs 20.8 ± 3.7 kg/m2, P < 0.001; 24.5 ± 4.3 vs 21.8 ± 3.4 kg/m2, P < 0.001, respectively). Furthermore, women in GDM-mix group more likely to be subjected to LGA (P = 0.008) adverse perinatal outcomes (P = 0.005), although these differences were normalized after adjusting age, prepregnancy and maternal BMI (GDM-mix vs. NGT: P = 0.141 for LGA and P = 0.186 for adverse outcomes). On the other hand, all perinatal outcomes were similar between other two GDM subgroups and NGT group.
Conclusions
Women with GDM display respective characteristics on metabolism disorders and confer discriminating risks of adverse perinatal outcomes because of this heterogeneity.
Electronic supplementary material
The online version of this article (10.1186/s12967-018-1666-5) contains supplementary material, which is available to authorized users.
Keywords: Gestational diabetes mellitus, Insulin resistance, Beta cell dysfunction, Perinatal outcome
Background
Gestational diabetes mellitus (GDM), a glucose intolerance with onset or first diagnosed during pregnancy [1], makes a tremendous contribution to adverse perinatal outcomes to both the mother and developing fetus [2]: Common maternal outcomes of GDM include the incremental risk of caesarean section, preeclampsia or proteinuria and likelihood of developing type 2 diabetes (T2D) in the future; for infants, fetal complications, connected with GDM, comprise neonatal hypoglycemia, stillbirth and macrosomia or large for gestational age (LGA), sometimes birth trauma, shoulder dystocia especially, as well [3–5].
Although exact mechanisms responsible for the development of hyperglycemia during pregnancy are not fully understood, insulin resistance has been identified as the catalyst of GDM with advancing gestation. Chronic inflammation factors, such as tumor necrosis factor α, and placental-derived hormones, such as placental lactogen and growth hormone, have been verified to be contributors to this increasing level of insulin resistance [6, 7] and when women fail to adapt to these physiological changes, they develop the glucose intolerance during pregnancy. In addition, the majority of women who develop GDM also experience a significant impairment in beta cell function which leads to initial postprandial and later fasting hyperglycemia [8, 9], and an elevated ratio of proinsulin to insulin was also observed in women with GDM to indicate beta cell dysfunction [10].
It has been suggested that GDM, the same as T2D, is a heterogeneous disease [11] and most studies focused on diverse subtypes of maternal body mass index (BMI), weight gain or level of glucose [12–16]. However, few studies characterized GDM based on insulin resistance and beta cell dysfunction, which are linked up with the BMI and circulating glucose concentrations [17], important predictors of perinatal outcomes. Therefore, we carried out a prospective cohort study to clarify the heterogeneous impact of insulin resistance and beta cell dysfunction in GDM on perinatal outcomes.
Methods
Study population and design
A prospective cohort study was conducted and all subjects were recruited in a prospective cohort of pregnant women from January 2015 to June 2016 at the Department of Obstetrics and Gynecology of Shanghai Jiao-Tong University Affiliated Sixth People’s Hospital. Their clinical and biochemical profiles longitudinally were collected from initial visit to delivery. In order to include as many as pregnant women with GDM, 50-g 1-h oral glucose tolerance was firstly performed among them at 24–28th weeks of gestation. Women with plasma glucose ≥ 7.8 mmol/L were included and advised to undergo the diagnostic 75-g, 3-h oral glucose tolerance test (OGTT), and GDM was diagnosed according to the International Association of Diabetes and Pregnancy Study Group (IADPSG) [18]. Women with the history of diabetes and multiple gestations were excluded. Five hundred and fifty-five women in total were enrolled at the baseline. We excluded 1 woman with the history of diabetes, 16 women with preeclampsia, 12 women because of multiple gestations, 70 women because of missing data on covariates. Finally, 456 subjects were eligible and their clinical data were available in the study. This study was approved and supervised by the Ethics Committee of the Shanghai Jiao-Tong University Affiliated Sixth People’s Hospital. All the participants signed the informed consents. The study was administered according to the Declaration of Helsinki.
To outline the heterogeneity of GDM, we reckoned the distributions of insulin sensitivity and secretion in women with normal glucose tolerance (NGT) as the reference. We considered women with GDM to have an insulin resistance or beta cell dysfunction if indices of measuring insulin resistance or beta cell function were below the 25th percentile, respectively. Women with GDM were classified into four subtypes based on the characteristic present: GDM with a predominant beta cell dysfunction (GDM-dysfunction), GDM with a predominant insulin resistance defect (GDM-resistance), or GDM with both traits mentioned above (GDM-mixed). If one participant with GDM had indices of both insulin sensitivity and beta cell function above the 25th percentile, she was not included from subgroup analyses (Table 1 and Additional file 1: Table S1 listed clinical information and perinatal outcomes of women in this group—GDM-normal).
Table 1.
Baseline characteristics of pregnant women in different GDM subgroups
| GDM-resistance | P value | GDM-dysfunction | P value | GDM-mix | P value | GDM-normal | P value | All GDM | P value | NGT | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (n) | 35 (17.0% of GDM) | 43 (20.9% of GDM) | 79 (38.3% of GDM) | 49 (23.8% of GDM) | 206 | 250 | |||||
| Age (years) | 31.0 (7.5) | > 0.999 | 31.0 (6.0) | 0.021 | 32.0 (6.0) | < 0.001 | 31.0 (4.0) | 0.824 | 32.0 (5.0) | < 0.001 | 29.0 (5.0) |
| Prepregnancy BMI (kg/m2) | 21.5 (3.2) | 0.231 | 21.6 (3.2) | 0.405 | 23.2 (4.0) | < 0.001 | 20.7 (3.3) | > 0.999 | 22.0 (3.7) | < 0.001 | 20.8 (3.7) |
| Maternal BMI (kg/m2) | 23.1 (4.0) | 0.071 | 22.2 (2.9) | 0.45 | 24.5 (4.3) | < 0.001 | 21.3 (3.3) | > 0.999 | 23.2 (3.7) | < 0.001 | 21.8 (3.4) |
| Gestational age at weight measuring (weeks) | 13.3 (3.5) | – | 13.7 (3.3) | – | 13.9 (2.9) | – | 13.9 (2.4) | – | 13.7 (2.8) | 0.073 | 14.0 (3.6) |
| Maternal BMI change (kg/m2) | 0.8 (0.9) | – | 1.1 (1.1) | – | 0.8 (1.3) | – | 0.8 (0.8) | – | 0.8 (1.0) | 0.969 | 0.8 (1.1) |
| ALT (U/L) | 13 (7.5) | – | 14.5 (13.0) | – | 13 (10.0) | – | 12 (8.5) | – | 13 (10.8) | 0.753 | 13 (11.0) |
| History of hypertension (n) | 0 | – | 0 | – | 4 (5.1) | – | 2 (4.1) | – | 6 (2.9) | 0.007 | 0 |
| Smoking before or during pregnancy (n) | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 |
| Nulliparous (n) | 25 (71.4) | – | 27 (62.8) | – | 53 (67.1) | – | 32 (65.3) | – | 137 (66.5) | 0.173 | 181 (72.4) |
Data are median (IQR) for continuous variables and n (%) for categorical variables
Differences between GDM subgroups and women with NGT were evaluated with the Kruskal–Wallis test for continuous variables and χ2 or Fisher exact test for categorical variables
When the P value was significant (< 0.05), Dunn’s multiple comparisons test or χ2 or Fisher exact test was performed to evaluate the difference between each GDM subtype and NGT group. P values for pairwise comparisons were adjusted by Bonferroni correction
Data collection and laboratory measurements
All the participants completed a questionnaire with the following data: general information about the present and previous illness, reproductive history, medication, alcohol consumption and smoking status. Height and weight were measured by the same physician during the health check-up. BMI was calculated as body weight (in kg)/height (in m2). The level of serum alanine aminotransferase (ALT) was measured in 13th–16th gestation week and determined by an automatic analyzer (7600-020 biochemistry automatic analyzer, Hitachi, Tokyo, Japan) and the normal range was 0–65 U/L. Plasma glucose and insulin values at 0, 30, 60, 120, and 180 min were obtained from the diagnostic 75-g, 3-h OGTT and levels of plasma glucose were determined by glucose oxidase method, the linearity range was 0–35 mmol/L. HbA1c and glycated serum albumin (GA) were measured at 24–28th weeks of gestation and determined by high-pressure liquid chromatography and by the liquid enzymatic assay, respectively. Insulin concentrations were determined with a 2-site chemiluminescent enzyme immunometric assay for the immulite automated analyzer (Diagnostic Products, Los Angeles, CA), the linearity range was 0.02–1000 μU/mL. HOMA2-IR at http://www.dtu.ox.ac.uk (accessed on 11 January 2016). Insulinogenic indices (a measure of insulin release) were calculated as the ratio between Δ (0–30 min) for insulin and glucose during OGTT [19]. Insulin sensitivity was assessed using the Matsuda index [20]. Beta cell function was measured by disposition index (insulinogenic index/HOMA2-IR).
Perinatal outcomes
Gestational week at delivery, mode of delivery, birth weights, gender of offspring and Apgar score were obtained from the medical record. Fetal growth was assessed in 1 week before the delivery by a trained technician, and measurements were obtained using a ultrasonography (Philips iE33 ultrasound system and a 5-MHz probe). Biparietal diameter (BPD), femur length (FL) and amniotic fluid index (AFI) were used to evaluate the fetal growth. Adverse outcomes are including: LGA, neonatal hypoglycemia and cesarean delivery. LGA was defined as birth weight ≥ 90th percentile for completed week of gestational age based on the sex-specific Ref. [21] and birth weight z value was calculated also by this reference. Neonatal hypoglycemia was diagnosed according to the criterion that the blood glucose concentration of is less than 2.6 mmol/L that McKinlay et al. [22] claimed.
Statistical methods
Data were expressed as median ± interquartile range (IQR) for continuous variables and percentages (%) for categorical variables. Differences between GDM subgroups and women with NGT were evaluated with the Kruskal–Wallis test for continuous variables and χ2 or Fisher exact test for categorical variables. When the P value was significant (< 0.05), Dunn’s multiple comparisons test or χ2 or Fisher exact test was performed to evaluate the difference between each GDM subtype and NGT group. P values for pairwise comparisons were adjusted by Bonferroni correction. In order to eliminate impacts of confounds on difference among groups, we conducted linear or logistic regression models to adjust covariates. All the statistical analysis was performed by SPSS 22.0 (SPSS Inc., Chicago, IL). A two-sided P < 0.05 was considered statistically significant.
Results
Baseline characteristics
In these 456 pregnant women, two hundred and six women finally developed GDM. Further, 35 women were classified into GDM-resistance group. 43 women were eligible for the GDM-dysfunction group and 79 women, commodity with insulin resistance and beta cell dysfunction, were in the GDM-mix group. Baseline characteristics of the NGT (n = 250), GDM-resistance (n = 35), GDM-dysfunction (n = 43), GDM-mix (n = 79) and all GDM groups (n = 206) are presented in Table 1. Women in the GDM group were older than women with NGT (32 ± 5.0 vs 29 ± 5.0 years, P < 0.001) and prepregnancy and maternal BMI in the GDM group were higher than those in the NGT group (22.0 ± 3.7 vs 20.8 ± 3.7 kg/m2, P < 0.001; 23.2 ± 3.7 vs 21.8 ± 3.4 kg/m2, P < 0.001, respectively). Among different subtypes in GDM, women in the GDM-mix group were also older than women with NGT (32 ± 6.0 vs 29 ± 5.0 years, P < 0.001) and the levels of prepregnancy and maternal BMI were much higher compared to women in the NGT group (23.2 ± 4.0 vs 20.8 ± 3.7 kg/m2, P < 0.001; 24.5 ± 4.3 vs 21.8 ± 3.4 kg/m2, P < 0.001, respectively). Interestingly, women with GDM-resistance and women with NGT were similar in terms of age, prepregnancy and maternal BMI. Still, we observed similar levels of BMI change and the percentage of nulliparous between three subtype groups and NGT group.
Glucose, insulin and indices of beta cell function and insulin resistance
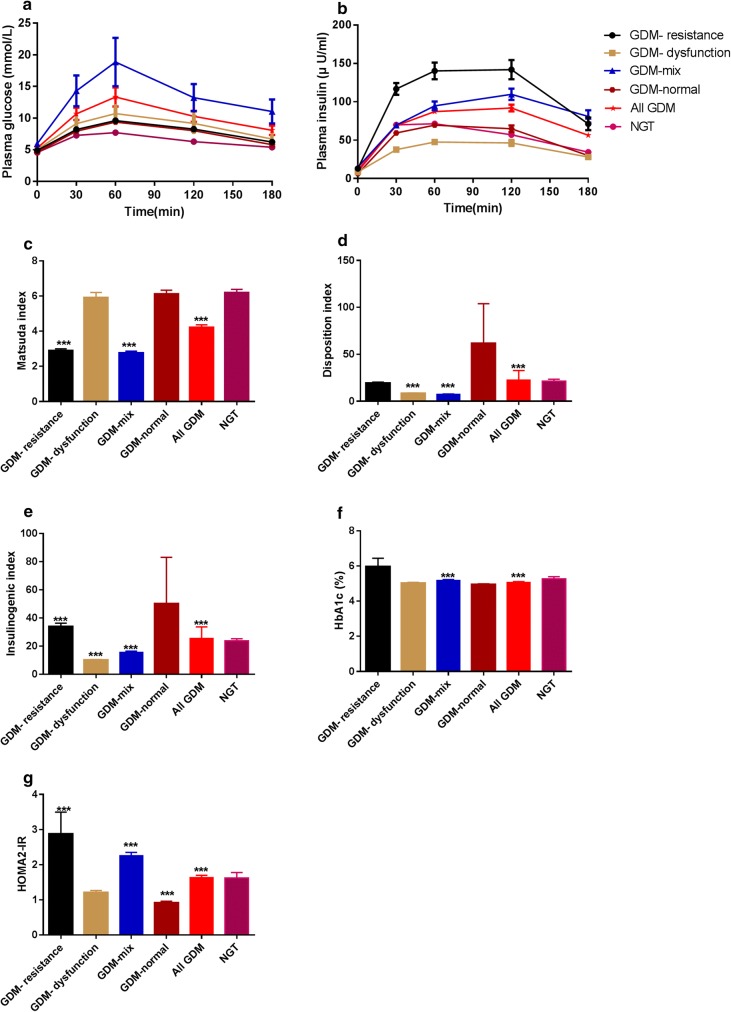
Parameters of 75 g 3 h OGTT, measurements of beta cell function and insulin resistance and status of glucose metabolism (GA and HbA1c) were listed in Table 2 and the dynamic responses of glucose and insulin are portrayed in Fig. 1. Noticeably, levels of glucose on all the time points in the GDM-mix group were significantly higher than those in the NGT group (All P < 0.001), whereas the other two groups exhibited elevated levels of glucose on all the time points except for glucose 180 min compared to the NGT group. Moreover, three subgroups showed respective traits in insulin secretion patterns: levels of insulin in all the time points in the GDM-resistance group showed significant increment than those in the NGT group; women in GDM-dysfunction showed a lower beta cell responsiveness in early phase (30 min and 60 min) of insulin secretion according to the reference of women in the NGT group and levels of insulin on other time points showed no difference between these two groups; in addition, except of insulin 30 min, levels of insulin on all the other time points in the GDM-mix group were significantly higher than those in the NGT group.
Table 2.
Comparison of glucose levels during 3 h 75 g OGTT and insulin secreting parameters among GDM subgroups and women with NGT
| GDM-resistance | P value | GDM-dysfunction | P value | GDM-mix | P value | All GDM | P value | NGT | |
|---|---|---|---|---|---|---|---|---|---|
| Glucose 0 min (mmol/L) | 4.8 (0.6) | 0.017 | 5.1 (0.7) | < 0.001 | 5.2 (1.0) | < 0.001 | 5.0 (0.8) | < 0.001 | 4.6 (0.4) |
| Glucose 30 min (mmol/L) | 8.3 (1.7) | 0.008 | 8.2 (1.4) | < 0.001 | 9.0 (1.6) | < 0.001 | 8.5 (1.6) | < 0.001 | 7.3 (1.5) |
| Glucose 60 min (mmol/L) | 9.8 (2.3) | < 0.001 | 10.1 (1.9) | < 0.001 | 10.6 (1.9) | < 0.001 | 10.2 (2.0) | < 0.001 | 10.2 (2.0) |
| Glucose 120 min (mmol/L) | 8.6 (2.1) | < 0.001 | 8.0 (2.8) | < 0.001 | 9.2 (2.7) | < 0.001 | 8.6 (2.3) | < 0.001 | 7.7 (1.9) |
| Glucose 180 min (mmol/L) | 6.1 (2.3) | 0.069 | 6.2 (2.5) | > 0.999 | 7.5 (2.9) | < 0.001 | 6.5 (3.0) | < 0.001 | 6.4 (1.8) |
| Insulin 0 min (μU/mL) | 11.9 (4.9) | < 0.001 | 7.6 (4.2) | 0.628 | 13.6 (5.9) | < 0.001 | 10.4 (7.1) | < 0.001 | 8.3 (5.3) |
| Insulin 30 min (μU/mL) | 106.9 (40.2) | < 0.001 | 37.0 (27.3) | < 0.001 | 64.8 (39.9) | > 0.999 | 59.4 (43.2) | 0.356 | 62.9 (48.9) |
| Insulin 60 min (μU/mL) | 122.0 (61.6) | < 0.001 | 46.0 (30.2) | 0.001 | 86.2 (48.1) | < 0.001 | 76.8 (54.9) | 0.006 | 65.0 (46.0) |
| Insulin 120 min (μU/mL) | 126.7 (86.2) | < 0.001 | 47.9 (38.8) | > 0.999 | 95.9 (68.0) | < 0.001 | 77.2 (69.6) | < 0.001 | 49.3 (53.2) |
| Insulin 180 min (μU/mL) | 63.0 (61.2) | 0.003 | 22.3 (35.5) | > 0.999 | 67.0 (72.0) | < 0.001 | 41.5 (59.2) | < 0.001 | 27.3 (38.2) |
| HOMA2-IR | 1.7 (0.7) | < 0.001 | 1.2 (0.9) | > 0.999 | 2.1 (1.1) | < 0.001 | 1.5 (1.1) | < 0.001 | 1.2 (0.8) |
| Matsuda index | 2.9 (0.9) | < 0.001 | 5.4 (1.6) | > 0.999 | 2.8 (1.2) | < 0.001 | 3.7 (2.7) | < 0.001 | 5.6 (3.4) |
| Insulinogenic index | 29.21 (18.5) | < 0.001 | 9.1 (5.5) | < 0.001 | 14.4 (9.5) | < 0.001 | 14.7 (11.5) | < 0.001 | 21.1 (14.5) |
| Disposition index | 16.0 (9.1) | > 0.999 | 8.3 (3.4) | < 0.001 | 7.0 (4.6) | < 0.001 | 10.3 (8.4) | < 0.001 | 17.8 (12.1) |
| GA (%) | 11.0 (1.7) | 0.017 | 11.7 (1.9) | > 0.999 | 11.5 (2.0) | > 0.999 | 11.6 (2.0) | 0.975 | 11.6 (2.1) |
| HbA1c (%) | 5.2 (0.6) | 0.125 | 5.0 (0.5) | > 0.999 | 5.2 (0.4) | < 0.001 | 5.1 (0.4) | < 0.001 | 5.0 (0.4) |
Data are median (IQR) for continuous variables
Differences between GDM subgroups and women with NGT were evaluated with the Kruskal–Wallis test for continuous variables
When the P value was significant (< 0.05), Dunn’s multiple comparisons test was performed to evaluate the difference between each GDM subtype and NGT group. P values for pairwise comparisons were adjusted by Bonferroni correction
Fig. 1.
Dynamic responses of 3 h 75 g OGTT and glucose metabolism parameters among different GDM subgroups. Data are mean ± SEM. ***P < 0.001
Reduced insulin sensitivity assessed by HOMA2-IR and Matsuda index was observed in the GDM-resistance group and the GDM-mix group compared to these in the NGT group. Furthermore, beta cell function adjusted for insulin resistance (HOMA2-IR)-disposition index showed a significant reduction in the GDM-dysfunction group and the GDM-mix group in comparison to the NGT group. Additionally, higher levels of GA and HbA1c were observed in the GDM-resistance group and the GDM-mix group, respectively (Table 2).
Heterogeneity of perinatal outcomes in subtypes groups
Perinatal outcomes in subtypes groups were shown in Table 3. Compared with perinatal outcomes of women with NGT, women in GDM-mix group more likely to be subjected to LGA (P = 0.008) and adverse perinatal outcomes (P = 0.005). Nevertheless, these differences were normalized after adjusting age, prepregnancy and maternal BMI (GDM-mix vs. NGT: P = 0.186 for LGA and P = 0.141 for adverse outcomes) perinatal outcomes of women in the GDM-resistance and GDM-dysfunction group exhibited no difference with these of women with NGT. Interestingly, All the women diagnosed with GDM were more vulnerable to undergo cesarean delivery and one of adverse outcomes compared to women with NGT, whereas possibilities of other individual GDM-associated adverse outcomes exhibited no difference between them.
Table 3.
Perinatal outcomes among groups with different glucose tolerance and insulin secreting status
| GDM-resistance | P value | GDM-dysfunction | P value | GDM-mix | P value | All GDM | P value | NGT | |
|---|---|---|---|---|---|---|---|---|---|
| Gestational age (week) | 39.7 (1.8) | – | 39.4 (1.2) | – | 39.3 (2.0) | – | 39.4 (1.6) | 0.964 | 39.5 (1.6) |
| Infant birth weight (g) | 3260.0 (547.5) | – | 3380.00 (575.0) | – | 3380.0 (720.0) | – | 3365.0 (527.5) | 0.546 | 3340.0 (520.0) |
| Infant birth weight (z score) | 0.20 (1.1) | – | 0.36 (1.4) | – | 0.41 (1.5) | – | 0.31 (1.3) | 0.186 | 0.14 (1.2) |
| BPD (cm) | 93 (5.3) | – | 93 (4.3) | – | 93 (7.0) | – | 93 (6.0) | 0.149 | 94 (5.0) |
| FL (cm) | 68 (3.5) | – | 69 (4.3) | – | 69 (4.0) | – | 69 (4.0) | 0.836 | 69 (4.0) |
| AFI (mm) | 125 (49.8) | – | 115 (32.0) | – | 116 (41.0) | – | 118 (43.75) | 0.92 | 119 (38.5) |
| Apgar score | 10 (0) | – | 10 (0) | – | 10 (0) | – | 10 (0) | 0.364 | 10 (0) |
| Infant male gender [n, (%)] | 17 (48.6) | – | 20 (46.5) | – | 40 (50.6) | – | 104 (50.5) | 0.456 | 135 (54.0) |
| LGA [n, (%)] | 2 (5.7) | 0.4 | 6 (14.0) | 0.808 | 20 (25.3) | 0.008 | 34 (16.5) | 0.263 | 32 (12.8) |
| Cesarean delivery [n, (%)] | 17 (48.6) | – | 19 (44.2) | – | 40 (50.6) | – | 95 (46.1) | 0.029 | 90 (36.0) |
| Neonate hypoglycemia [n, (%)] | 4 (11.4) | – | 4 (9.3) | – | 11 (13.9) | – | 21 (10.2) | 0.510 | 21 (8.4) |
| Any adverse outcome [n, (%)] | 19 (54.3) | 0.313 | 22 (51.2) | 0.469 | 50 (63.3) | 0.005 | 114 (55.3) | 0.031 | 113 (45.2) |
Data are median (IQR) for continuous variables and n (%) for categorical variables
Differences between GDM subgroups and women with NGT were evaluated with the Kruskal–Wallis test for continuous variables and χ2 or Fisher exact test for categorical variables
When the P value was significant (< 0.05), Dunn’s multiple comparisons test or χ2 or Fisher exact test was performed to evaluate the difference between each GDM subtype and NGT group. P values for pairwise comparisons were adjusted by Bonferroni correction
Discussion
Here, we conducted a prospective cohort study in a relatively large number of women with gestational diabetes and analyzed perinatal outcomes according to the heterogeneity of insulin resistance and beta cell dysfunction-a novel perspective about GDM. Three phenotypic subgroups were defined: GDM-resistance group, GDM-dysfunction group and GDM-mix group. Then women in the GDM-resistance and GDM-dysfunction was further characterized by a predominant insulin resistance (Matsuda index, OGTT) or a decreased beta cell function in response to oral glucose (disposition index, OGTT) and women in the GDM-mix group hold the combination of features about these two groups, so the most divergent metabolic disorder was observed in the GDM-mix group compared with women in the NGT group, but other two groups. Not surprisingly, the incremental risk of GDM-related complications and LGA were observed in the GDM-mix group compared to women in the NGT group, although this difference was attenuated for the adjustment of confounding factors including age, prepregnancy and maternal BMI.
One strength of our study is that we performed a clinical investigation with detailed responses of glucose and insulin to OGTT and the association between pathophysiology of GDM and perinatal outcomes in a prospective cohort of pregnant women without prior diabetes history. Five individual time points (0 min, 30 min, 60 min, 120 min and 180 min) measurements of glucose and insulin outlined a detailed and complete picture of glucose and insulin fluctuations during OGTT, which provided clear and exact information about insulin resistance and beta cell function on different phenotypes about GDM. Moreover, the level of prepregnancy BMI was documented and it played, to some extent, an index in estimating the insulin sensitivity before pregnancy.
Given similar genetic background and pathophysiological mechanism [23, 24], GDM should be considered as a heterogeneous disease, like T2D. We used indices evaluating insulin resistance and beta cell function to investigate whether their heterogeneity contributes to GDM-related complications, though many researches applied other parameters, such as BMI or glucose [5, 12–15], as the reference. Recently, Powe et al. [3] also investigated the relationship between insulin resistance and beta cell function and perinatal outcomes in women with GDM. Different from our discoveries, they found that women with GDM undergoing impaired insulin sensitivity were more likely to confer GDM-related complications, independent from maternal BMI. Factors that may have contributed to this difference include: firstly, they took the advantage of the Stumvoll first-phase estimate to exhibit beta cell function, not DI, a more widely used index and adjusted for insulin resistance [17]; second, we conducted a complete Chinese women cohort, therefore, the divergent ethnological genetic and environmental background induced a disparate metabolic pattern on GDM: as we can see, levels of fasting insulin were much higher in the GDM-resistance and GDM-mix group than these in the NGT group, however, this observation was completely opposite from Powe’s investigation. In that, we are inclined to draw this explanation that ethnological differences prompted separate a metabolic pattern and disorder in our cohorts and it may leaded to differences on perinatal outcomes between these two researches. Indeed, the number of subjects in our cohorts was much smaller than Powe’s investigation and it may undermine our conclusion and confounded our observation. However, considering the collective role of insulin resistance and beta cell dysfunction in promoting the occurrence of GDM [23, 24], it is reasonable to draw the conclusion that women in the GDM-mix group hold most severe metabolic disorder and further greatest risk of GDM-related complications among three subtypes.
A limitation of our investigation is unavailable of assessing levels of glucose and insulin before, after and during the pregnancy, and these longitude transformations of insulin resistance and beta cell function facilitate us to portrait a more clear picture about impacts of heterogeneous insulin resistance and beta cell dysfunction on prenatal outcomes. Moreover, the limited sample size and monotonous ethnological subjects may partly diminish our conclusion. Furthermore, the lack of data about levels of insulin in infants hampers further analyses and evaluations on impacts of different subtypes on the neonate hyperinsulinemia, or the association between hyperinsulinemia and birthweight or neonate hypoglycemia. Finally, our study did not include genetic information and adipokines associated with insulin resistance and beta cell function.
Conclusion
In general, our study revealed that women with GDM display respective characteristics on metabolism disorders and confer discriminating risk of adverse perinatal outcomes in accordance with various phenotypes of insulin resistance and beta cell function. Among three subtypes of GDM, women with the combination of predominant insulin resistance and beta cell dysfunction conferred the greatest risk of adverse perinatal outcomes. Currently, few studies concentrated on the heterogeneity of GDM and its influences on perinatal outcomes, especially on the base of pathogenesis of GDM. Future researches, including large number of subjects and diverse races, may help to explain the underlying causal and effect link between subtypes in pathogenesis of GDM and perinatal outcomes.
Additional file
Additional file 1: Table S1. Perinatal Outcomes of Women with GDM Who were not taken into subgroup analyses.
Authors’ contributions
The study was designed by FL and WLH. YJH and MFT planned and conducted all the obstetric and surgical clinical practice. JMP and JLT were responsible for evaluating clinical characteristics and measuring biochemistry parameters. XYM and WLH collected biochemistry measurement data. YFL and FL were responsible for writing the article, analyzing data and critical revisions. All authors read and approved the final manuscript.
Acknowledgements
None
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data in this manuscript cannot be revealed, due to the consent that every participant signed and security considerations.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved and supervised by the Ethics Committee of the Shanghai Jiao-Tong University Affiliated Sixth People’s Hospital. All the participants signed the informed consents. The study was administered according to the Declaration of Helsinki.
Funding
We thank support from the grants of National Key R & D Program of China (2017YFC1309601 for Fang Liu), National Natural Science Foundation of China (81770802 for Fang Liu), Shanghai Science & Technology Pillar Program in the Field of Medicine and Agriculture (15411953100 to Fang Liu) and Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (20152232).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- GDM
gestational diabetes mellitus
- T2D
type 2 diabetes
- LGA
large for gestational age
- OGTT
oral glucose tolerance test
- IADPSG
International Association of Diabetes and Pregnancy Study Group
- BMI
body mass index
- NGT
normal glucose tolerance
- GDM-dysfunction
GDM with a predominant beta cell dysfunction
- GDM-resistance
GDM with a predominant insulin resistance defect
- GDM-mixed
GDM with both traits mentioned above
- GA
glycated serum albumin
- GDM-normal
GDM without insulin resistance defect and beta cell dsyfunction
Contributor Information
Yingfeng Liu, Email: wertianjy@163.com.
Wolin Hou, Email: hwlin25628@sina.com.
Xiyan Meng, Email: 876118013@qq.com.
Weijing Zhao, Email: z811wj@163.com.
Jiemin Pan, Email: lilypjm@hotmail.com.
Junling Tang, Email: tjunling@hotmail.com.
Yajuan Huang, Email: huang_yajuan@hotmail.com.
Minfang Tao, Email: taomf@126.com.
Fang Liu, Phone: +86 21 64369181, Email: f-liu@sjtu.edu.cn.
References
- 1.American Diabetes A 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40:S11–S24. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 2.Reece EA, Leguizamon G, Wiznitzer A. Gestational diabetes: the need for a common ground. Lancet. 2009;373:1789–1797. doi: 10.1016/S0140-6736(09)60515-8. [DOI] [PubMed] [Google Scholar]
- 3.Powe CE, Allard C, Battista MC, Doyon M, Bouchard L, Ecker JL, et al. Heterogeneous contribution of insulin sensitivity and secretion defects to gestational diabetes mellitus. Diabetes Care. 2016;39:1052–1055. doi: 10.2337/dc15-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, Hsu-Hage B, Zhang H, Zhang C, Zhang Y, Zhang C. Women with impaired glucose tolerance during pregnancy have significantly poor pregnancy outcomes. Diabetes Care. 2002;25:1619–1624. doi: 10.2337/diacare.25.9.1619. [DOI] [PubMed] [Google Scholar]
- 5.Wendland EM, Torloni MR, Falavigna M, Trujillo J, Dode MA, Campos MA, et al. Gestational diabetes and pregnancy outcomes—a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth. 2012;12:23. doi: 10.1186/1471-2393-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guillemette L, Lacroix M, Battista MC, Doyon M, Moreau J, Menard J, et al. TNFalpha dynamics during the oral glucose tolerance test vary according to the level of insulin resistance in pregnant women. J Clin Endocrinol Metab. 2014;99:1862–1869. doi: 10.1210/jc.2013-4016. [DOI] [PubMed] [Google Scholar]
- 7.Catalano PM. Obesity, insulin resistance, and pregnancy outcome. Reproduction. 2010;140:365–371. doi: 10.1530/REP-10-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Homko C, Sivan E, Chen X, Reece EA, Boden G. Insulin secretion during and after pregnancy in patients with gestational diabetes mellitus. J Clin Endocrinol Metab. 2001;86:568–573. doi: 10.1210/jcem.86.2.7137. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan TA. Pancreatic B-cell defects in gestational diabetes: implications for the pathogenesis and prevention of type 2 diabetes. J Clin Endocrinol Metab. 2001;86:989–993. doi: 10.1210/jcem.86.3.7339. [DOI] [PubMed] [Google Scholar]
- 10.Swinn RA, Wareham NJ, Gregory R, Curling V, Clark PM, Dalton KJ, et al. Excessive secretion of insulin precursors characterizes and predicts gestational diabetes. Diabetes. 1995;44:911–915. doi: 10.2337/diab.44.8.911. [DOI] [PubMed] [Google Scholar]
- 11.Cundy T, Holt RI. Gestational diabetes: paradigm lost? Diabet Med. 2017;34:8–13. doi: 10.1111/dme.13200. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. 2017;317:2207–2225. doi: 10.1001/jama.2017.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Disse E, Graeppi-Dulac J, Joncour-Mills G, Dupuis O, Thivolet C. Heterogeneity of pregnancy outcomes and risk of LGA neonates in Caucasian females according to IADPSG criteria for gestational diabetes mellitus. Diabetes Metab. 2013;39:132–138. doi: 10.1016/j.diabet.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Black MH, Sacks DA, Xiang AH, Lawrence JM. The relative contribution of prepregnancy overweight and obesity, gestational weight gain, and IADPSG-defined gestational diabetes mellitus to fetal overgrowth. Diabetes Care. 2013;36:56–62. doi: 10.2337/dc12-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos MJ, Fernandes V, Marques O, Pereira ML. Effect of maternal body mass index and weight gain in women with gestational diabetes on the incidence of large-for-gestational-age infants. Diabetes Metab. 2016;42:471–474. doi: 10.1016/j.diabet.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Group HSCR. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 17.Chandler-Laney PC, Shepard DN, Schneider CR, Flagg LA, Granger WM, Mancuso MS, et al. Relatively low beta-cell responsiveness contributes to the association of BMI with circulating glucose concentrations measured under free-living conditions among pregnant African American women. J Nutr. 2016;146:994–1000. doi: 10.3945/jn.115.222877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes A. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 21.Dai L, Deng C, Li Y, Zhu J, Mu Y, Deng Y, et al. Birth weight reference percentiles for Chinese. PLoS ONE. 2014;9:e104779. doi: 10.1371/journal.pone.0104779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKinlay CJ, Alsweiler JM, Ansell JM, Anstice NS, Chase JG, Gamble GD, et al. Neonatal glycemia and neurodevelopmental outcomes at 2 years. N Engl J Med. 2015;373:1507–1518. doi: 10.1056/NEJMoa1504909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bozkurt L, Gobl CS, Pfligl L, Leitner K, Bancher-Todesca D, Luger A, et al. Pathophysiological characteristics and effects of obesity in women with early and late manifestation of gestational diabetes diagnosed by the International Association of Diabetes and Pregnancy Study Groups criteria. J Clin Endocrinol Metab. 2015;100:1113–1120. doi: 10.1210/jc.2014-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agha-Jaffar R, Oliver N, Johnston D, Robinson S. Gestational diabetes mellitus: does an effective prevention strategy exist? Nat Rev Endocrinol. 2016;12:533–546. doi: 10.1038/nrendo.2016.88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Perinatal Outcomes of Women with GDM Who were not taken into subgroup analyses.
Data Availability Statement
The data in this manuscript cannot be revealed, due to the consent that every participant signed and security considerations.