Abstract
Rationale:
Antibacterials are largely prescribed to the intensive care unit (ICU) patients due to high prevalence of infections. However, appropriate use of antibacterials is imperative; since the misuse of antibacterials increases antibacterial resistance and ultimately, it has negative impact on health care and economic system. Hence, continuous antibacterials prescription assessments are very important to judge and improve prescription patterns. The present work was carried out at public and private hospitals to assess the differences in antibacterial prescribing pattern.
Methods:
The present study was conducted at three public and two private hospitals over the period of 14 months. Demographic and drug use details were captured daily from patients admitted to medical ICUs to assess the World Health Organization indicators.
Results:
A total of 700 patients were enrolled across the five centers (140 per center), among them 424 were male and 276 were female. Average number of drugs and antibacterials prescribed at public hospitals are significantly higher than the private hospital. However, percentage of antibacterial agents prescribed at public hospitals was significantly lower than the private hospitals (P = 0.0381). Private hospitals had significantly lower percentage of antibacterial agents prescribed by generic name (P < 0.0001). Differences in change of antibacterial agents required were not statistically significantly different (P = 0.1888); however, significant difference was observed in percentage of patients who received antibacterial treatment as per sensitivity pattern (P = 0.0385) between public and private hospitals. Significantly higher mortality was observed in public hospitals compared to private hospitals (<0.0001).
Conclusions:
More generic prescriptions and more number of prescriptions as per the sensitivity pattern are required at each public and private hospital.
Keywords: Antibacterial resistance, antibacterials, indicators, medical intensive care unit, public and private hospitals
INTRODUCTION
Patients admitted to the medical intensive care unit (MICU) of tertiary referral centers are critically ill and incidences of nosocomial infections are also very high.[1,2] Antibacterial agents are the most prescribed drugs for rapid control of serious infections to reduce the mortality and morbidity.[2,3,4] The burden of bacterial disease in India is among highest in the world and the inappropriate use of antibacterial agents leads to increase the development of antibacterial resistance.[1,5,6] Antibiotic resistance has been a low-priority area in most developing and many developed countries. To overcome the burden of infectious disease and rising antibiotic resistance prevalence in India, the Global Antibiotic Resistance Partnership-India Working Group have recommended important interventions which include surveillance of antibiotic use, distributing standard treatment guidelines, increasing the use of diagnostics tests, infection control interventions, educational approaches, and improving antibiotic supply chain and quality.[5]
A drug utilization study is a potential tool which not only assesses the current prescribing pattern of drugs but also assess the disease burden as well resistance pattern of microorganisms and recommends necessary interventions to be used to achieve rational prescribing practice.[7]
Resistance to important antibacterials such as vancomycin and colistin are increasing globally.[8,9] Majority of antibacterial prescription data comes from single-center studies. In a developing country like India, there might be differences in prescription patterns between public and private hospitals due to differences in referral patterns, hospital resources, patients load, and availability of workforce. Hence, this prospective multicentric study was conducted at five centers across India to compare the prescription pattern of antibacterials and cost pattern of antibacterial treatment between three public and two private tertiary referral hospitals with special focus on vancomycin and colistin.
METHODS
Ethics
The study was initiated after obtaining approval from the Institutional Ethics Committee (IEC) of Public Hospital 1. Individual IEC approval of each four participating center was also obtained and the study was registered with the Clinical Trial Registry of India. Written informed consent was obtained from patient or legally acceptable representative of the patient only at Public Hospital 1 and waiver of consent were sought at other four participating centers and which was granted by each of IEC.
Study design and duration
This multicentric, prospective, observation antibacterial utilization study was carried out from May 20, 2014 to July 1, 2015.
Study sites
There were five centers across India. These included:
Public hospitals
Public Hospital 1 is a 2200-bedded hospital with capacity of 22 MICU beds
Public Hospital 2 is a 1623-bedded hospital with capacity of 23 MICU beds
Public Hospital 3 is a 600-bedded specialist cancer treatment and research center having a 14-bedded mixed medical–surgical ICU.
Private hospitals
Private Hospital 1 is a 725-bedded hospital having 18-bedded MICU
Private Hospital 2 is a 1044-bedded private hospital having 18-bedded MICU.
Study population
Consecutive patients admitted to the MICU regardless of previous admission history were enrolled in the study with day 1 of admission into MICU being considered as the 1st day of the study. Each admission was considered as a patient encounter[10] and all patients were followed up until death, discharge or transfer to the general ward.
Study procedure
Patient's medical records were scrutinized daily. Demographic details, clinical diagnosis, details of antibacterial use (name, dose, frequency, route and length of treatment, prescribed as generic or brand, change in antibacterial agent, fixed-dose combination [FDC]), total number of drugs prescribed, culture and sensitivity data, length of stay, and cost of antibacterial treatment was recorded in an online Google spreadsheet.
Cost calculation
The costs of antibacterials were obtained from hospital pharmacy price list (for those available on hospital schedule) and from the Monthly Index of Medical Specialties (MIMS_http://www.mims.com/assessed during the study period).
Definitions
For the purpose of the study, following definitions were considered:
Antibacterial: An agent that interferes with the growth and reproduction of bacteria. For the purpose of this study, antimalarials, antifungals, antifilarials, antischistosomals, antileprosy drugs, antituberculosis drugs, anti-amoebic, antigiardiasis drugs, antileishmaniasis drugs, and antitrypanosomal drugs were not considered as an antibacterial
-
Antibacterial use was classified into empiric use or definitive use:[11]
- Empiric use of antibacterial agent: It was defined as administration of an antibacterial agent within 72 h of admission in MICU, while microbiologic cultures results are pending or use of antibacterial agents in situations after 72 h of admission when microbiologic cultures do not yield a pathogen
- Definitive (therapeutic) use of antibacterial agent: It was defined as the use of any antibacterial agent at a time when microbiologic culture results and susceptibility data are available. This was at the time of initiation of therapy or after empiric antimicrobial use has been initiated once microbiological culture results are available.
FDC drug: The FDC of two or more active antibacterial agents was counted as separate antibacterial agents. If one of the agents in the FDC was inactive, that is, did not have direct antibacterial activity, then it was not counted as an antibacterial agent.[12]
World Health Organization indicators:[13,14] Below-mentioned indicators related to antibacterial use are divided into three main sections
Prescribing indicators: Average number of drugs prescribed per patient, percentage of antibacterial agents prescribed, percentage of patients who received an antibacterial agent, average number of antibacterial agents prescribed per patient, percentage of antibacterial agents prescribed by generic name, percentage of antibacterial agents prescribed by intravenous route, percentage of antibacterial agents available in hospital pharmacy, percentage of patients in whom a change of antibacterial agent was made, percentage of antibacterial agents prescribed as FDC, average length of antibacterial treatment, average length of empirical use of antibacterials, and average antibacterial treatment cost per patient
Patient care indicators: Average length of MICU stay and percentage of patients died during MICU stay
Supplemental indicators: Percentage of patients who received antibacterial treatment as per the sensitivity pattern.
Sample size calculation
Sample size was calculated using nMaster 1.0, Department of Biostatistics, CMC, Vellore, Tamilnadu, India. Vancomycin use in Indian population varies between 3% and 10%. No data were available on the prevalence of use of colistin. Hence, keeping the prevalence of vancomycin use as 10%, with alpha error and precision as 5%, we have enrolled 140 patients per center; collectively 700 patients were enrolled from five centers.
Statistical analysis
Descriptive statistics was used and data were expressed using measures of central tendency. Numerical data were tested for normality using the Kolmogorov–Smirnov test. Unpaired t-test, Fisher exact test, and Chi-square test (nonparametric) were used to compare the data between the centers and P ≤ 0.05 was considered statistically significant. All analyses were done with Statistical Package for the Social Sciences (SPSS) 20, IBM, Armonk, NY, United States of America and GraphPad version 3.06, (GraphPad Software, California, USA).
RESULTS
Demographics
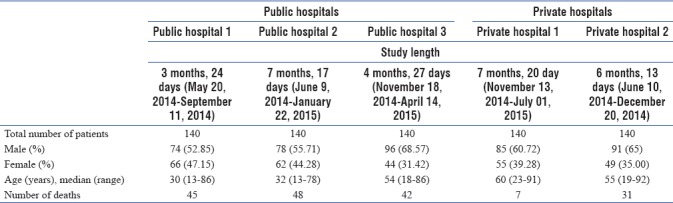
A total of 700 patients were enrolled across the five centers (140 per center). The median age of the patients across the centers was 48 (13, 92), of which 424 (60.57%) were male and 276 (39.43%) were female. Demographic details and study length of individual center are depicted in Table 1.
Table 1.
Study length and demographic details
More number of patients with respiratory disease, central nervous system disease, heart disease, and fever were admitted to Public Hospital 1 and 2. At Public Hospital 3, patients with respiratory disease, postoperative infections, heart disease, and central nervous system disease contributed most of the ICU admissions. Where at Private Hospital 1 and 2, more number of patients with heart disease, respiratory disease, central nervous system disease, and trauma were admitted to ICU.
Indicators
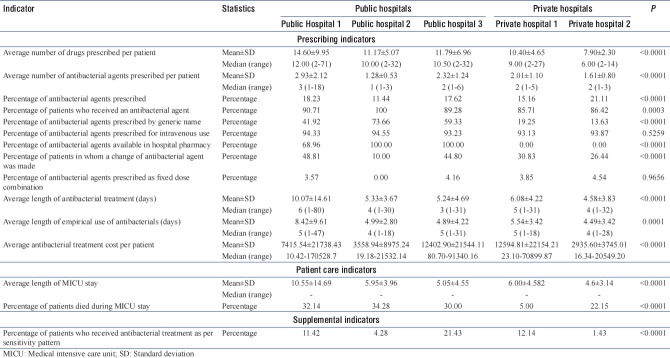
In public hospitals, the average number of drugs and average number of antibacterials prescribed were significantly higher compared to private hospitals; the values are depicted in Table 2.
Table 2.
World Health Organization indicators (public vs. private hospitals)
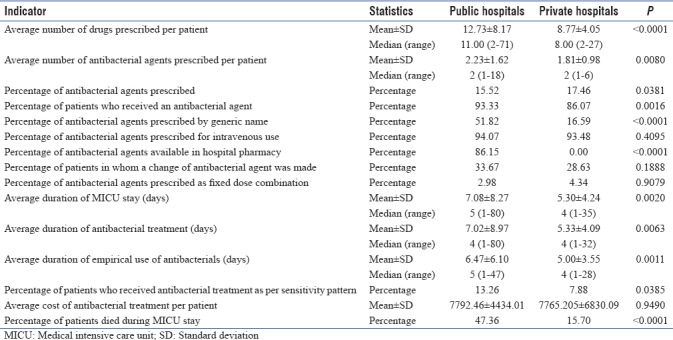
Percentage of antibacterial agents prescribed at public hospitals was significantly lower than the private hospitals (P = 0.0381). The individual values of each participating center are depicted in Table 3. Percentage of patients received antibacterials was significantly different between public and private hospitals (P = 0.0016) and between the individual centers (P = 0.0003). Private hospitals had significantly lower percentage of antibacterial agents prescribed by generic name (P < 0.0001). There was no significant difference in intravenous use (P = 0.4095) of antibacterials and the FDCs (P = 1.0000). At private hospitals, none of the patients received antibacterial from the hospital pharmacy.
Table 3.
World Health Organization indicators (individual)
Differences in change of antibacterial agents required were not statistically significantly different (P = 0.1888); however, significant difference was observed in percentage of patients who received antibacterial treatment as per sensitivity pattern (P = 0.0385) between public and private hospitals. Public hospitals had a longer average length of antibacterial treatment and empirical use of antibacterials as well as longer duration of MICU stay as compared to private hospitals. Antibacterial treatment cost was not significantly different between public and private hospitals; significantly higher mortality was observed in public hospitals compared to private hospitals (<0.0001). Details of the prescribed antibacterials are presented in Figure 1.
Figure 1.
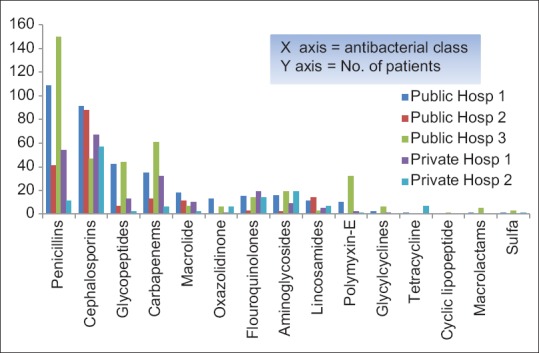
Class-wise distribution of antibacterials
Pooled data
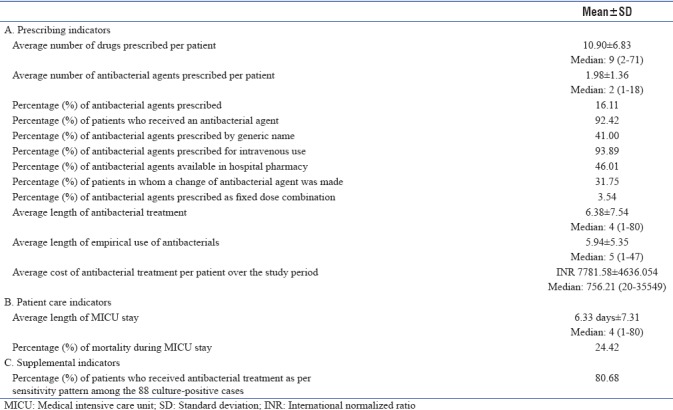
Patients in MICU received as many as 10.90 (±6.83) drugs, of which only 1.98 ± 1.36 (16.11%) were antibacterials. Over the study period, 92.42% patients received antibacterials of which 41% antibacterials were prescribed by generic name. A total of 93.89% antibacterials were prescribed intravenously. Only 46.01% antibacterials were prescribed from the hospital pharmacy. Change of antibacterial agent was made in 31.75% patients and only 3.54% antibacterials were prescribed as FDCs. The average length of MICU stay was 6.33 days (±7.31), average length of antibacterial treatment was 6.38 days (±7.54), and average duration of empirical treatment was 5.94 days (±5.35). Across the centers, average antibacterial cost per patient was INR 7781.58 (±4636.054). A total of 88 culture tests were positive among 212 patients, from which 71 (80.68%) patients received antibacterials as per sensitivity pattern. Pooled data indicators have been presented below in Table 4.
Table 4.
Pooled data indicators
DISCUSSION
The World Health Organization defines drug utilization as “the marketing, distribution, prescription, and use of drugs in a society with special emphasis on the resulting medical, social, and economic consequences.[10] To the best of our knowledge, this is the first study which has assessed and compared the prescribing pattern of antibacterials between public and private tertiary care hospitals of India. At all five centers, the majority of patients were male was also seen in most reported literature.[15,16,17,18] Respiratory disorders are a common problem faced in the ICUs, and at our centers also a large number of patients with respiratory disease were admitted to MICU.[19,20,21,22,23] The mean number of drugs received by patients at public hospitals (12.73 ± 8.17) was comparatively higher than the private hospitals and study conducted at various regions of India.[16,18,24] Extensive polypharmacy (100%), that is, more than five drugs were prescribed at all the centers; since ICU patients require more drugs due to multiple comorbidities and prophylaxis needs. However, it is also essential to keep a balance between the number of drugs and effective pharmacotherapy. Our findings are closely similar with the study conducted by Sireesha et al. (100%), Hussain et al. (97.27%), and Badar and Navale (83.00%) but lower than with the study conducted by Pandiamunian and Somasundaram (57%).[15,16,18,23] Patients admitted to tertiary referral centers are critically ill and are needs to be treated with antibacterials for prophylactic, suspected or proven bacterial infections; majority of patients of public as well private hospitals had received antibacterials during their ICU stay. Cephalosporin antibacterials were largely prescribed at each center and these findings are similar to some reported studies.[24,25,26,27,28] Very low proportion of generic prescription at private centers was observed and among our participating centers, therefore, there is need to increase the prescription of antibacterials by generics at every center to reduce the antibacterial treatment cost. Some of the antibacterial cost was taken from MIMS and this is one of the limitations of the present study. The intravenous use of antibacterial was very high among the centers, and at each center, more than 90% of the antibacterials were administered intravenously possibly to reduce the mortality and morbidity in an emergent situation since these all are tertiary referral centers and most of the patients come with a fairly advanced disease requiring emergent action.
An antibacterials need to be prescribed as per the sensitivity pattern; since inappropriate use of antibacterials increases the risk of bacterial resistance.
CONCLUSIONS
The number of antibacterials and drugs prescribed at public hospitals are significantly higher than the private hospitals. Generic prescriptions are very low at private hospitals; however, more generic prescriptions are suggested at each public and private hospital. In addition, more number of prescriptions as per the sensitivity pattern is required to reduce prolonged empirical use of antibacterials.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Dr. Avinash Supe, director (Medical Education & Major Hospitals) and dean of Seth G.S. Medical College & KEM Hospital for granting permission to publish this manuscript.
REFERENCES
- 1.Venkataraman R, Divatia JV, Ramakrishnan N, Chawla R, Amin P, Gopal P, et al. Multicenter observational study to evaluate epidemiology and resistance patterns of common Intensive Care Unit-infections. Indian J Crit Care Med. 2018;22:20–6. doi: 10.4103/ijccm.IJCCM_394_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravi KP, Durairajan S, Parivar S, Venkataraman R, Ramasubramanian V, Ramakrishnan N. Epidemiology of Intensive Care Unit infections and impact of infectious disease consultants in managing resistant infections. Am J Infect Dis. 2013;9:30–3. [Google Scholar]
- 3.McKenzie C. Antibiotic dosing in critical illness. J Antimicrob Chemother. 2011;66(Suppl 2):ii25–31. doi: 10.1093/jac/dkq516. [DOI] [PubMed] [Google Scholar]
- 4.Gangwar A, Kumar N, Kothiyal P. Antibiotic prescription and cost patterns in an Intensive Care Unit: A review of literature. Pharm Innovation. 2012;1:68–72. [Google Scholar]
- 5.Ganguly NK, Arora NK, Chandy SJ, Fairoze MN, Gill JP, Gupta U, et al. Rationalizing antibiotic use to limit antibiotic resistance in India. Indian J Med Res. 2011;134:281–94. [PMC free article] [PubMed] [Google Scholar]
- 6.Divatia JV, Amin PR, Ramakrishnan N, Kapadia FN, Todi S, Sahu S, et al. Intensive care in India: The Indian intensive care case mix and practice patterns study. Indian J Crit Care Med. 2016;20:216–25. doi: 10.4103/0972-5229.180042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srishyla MV, Mahesh K, Nagarani M, Mary CS, Andrade C, Venkataraman B. Prescription audit in an Indian hospital setting using the DDD (Defined Daily Dose) concept. Indian J Pharm. 2016;26:23–8. [Google Scholar]
- 8.Orsi GB, Ciorba V. Vancomycin resistant enterococci healthcare associated infections. Ann Ig. 2013;25:485–92. doi: 10.7416/ai.2013.1948. [DOI] [PubMed] [Google Scholar]
- 9.Ah Y-M, Kim A-J, Lee J-Y. Colistin resistance in Klebsiella pneumoniae. Int J Antimicrob Agents. 2014;44:8–15. doi: 10.1016/j.ijantimicag.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Introduction to Drug Utilization Research. World Health Organization. 2003. [Last accessed on 2017 Dec 27]. Available from: http://www.apps.who.int/medicinedocs/pdf/s4876e/s4876e.pdf .
- 11.Camins BC, King MD, Wells JB, Googe HL, Patel M, Kourbatova EV, et al. Impact of an antimicrobial utilization program on antimicrobial use at a large teaching hospital: A randomized controlled trial. Infect Control Hosp Epidemiol. 2009;30:931–8. doi: 10.1086/605924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gautam CS, Saha L. Fixed dose drug combinations (FDCs): Rational or irrational: A view point. Br J Clin Pharmacol. 2008;65:795–6. doi: 10.1111/j.1365-2125.2007.03089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. How to Investigate Drug use in Health Facilities: Selected Drug Use Indicators. World Health Organization. 1993. [Last accessed on 2018 Jan 08]. Available from: http://www.apps.who.int/medicinedocs/en/d/Js2289e/
- 14.World Health Organization. How to Investigate Antimicrobial Use in Hospitals: Selected Indicators. World Health Organization. 2012. [Last accessed on 2018 Jan 08]. Available from: http://www.apps.who.int/medicinedocs/documents/s21031en/s21031en.pdf .
- 15.Hussain M, Syed N, Shobha JC. Prescription patterns of antibiotics in acute medical care units of a tertiary care hospital in India. Int J Curr Microbiol App Sci. 2014;5:673–679. [Google Scholar]
- 16.Pandiamunian J, Somasundaram G. A study on prescribing pattern of anti microbial agents in the medical Intensive Care Unit of a tertiary care teaching hospital in Puducherry union. Int J Pharm Pharm Sci. 2014;6:25. [Google Scholar]
- 17.Ansari AA, Khan MA, Khan JA, Hariz ML. Antibiotic prescription patterns in an Intensive Care Unit in the Kingdom of Bahrain: An observational prospective study. Int J Sci Res. 2013;2:371–4. [Google Scholar]
- 18.Badar VA, Navale SB. Study of prescribing pattern of antimicrobial agents in medicine Intensive Care Unit of a teaching hospital in central India. J Assoc Phys India. 2012;60:20–3. [PubMed] [Google Scholar]
- 19.Hug BL, Rossi M. A year's review of bacterial pneumonia at the central hospital of Lucerne, Switzerland. Swiss Med Wkly. 2001;131:687–92. doi: 10.4414/smw.2001.09806. [DOI] [PubMed] [Google Scholar]
- 20.Curcio D. Latin American Antibiotic Use in Intensive Care Unit Group†. Antibiotic prescriptions in critically-ill patients: A Latin American experience. Ann Med Health Sci Res. 2013;3:220–8. doi: 10.4103/2141-9248.113666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanssens Y, Ismaeil BB, Kamha AA, Elshafie SS, Adheir FS, Saleh TM, et al. Antibiotic prescribing pattern in a medical Intensive Care Unit in Qatar. Saudi Med J. 2005;26:1269–76. [PubMed] [Google Scholar]
- 22.Paruk F, Richards G, Scribante J, Bhagwanjee S, Mer M, Perrie H. Antibiotic prescription practices and their relationship to outcome in South African Intensive Care Units: Findings of the prevalence of infection in South African Intensive Care Units (PISA) study. S Afr Med J. 2012;102:613–6. doi: 10.7196/samj.5833. [DOI] [PubMed] [Google Scholar]
- 23.Sireesha GB, Tiwari P, Gombar S, D'Cruz S, Sachdev A. Antimicrobial utilization in multidisciplinary Intensive Care Units of a public tertiary care hospital. J Pharm Pharm. 2014;5:252–4. doi: 10.4103/0976-500X.142441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.John LJ, Devi P, Guido S. Drug utilization study of antimicrobial agents in medical Intensive Care Unit of a tertiary care hospital. Asian J Pharam Clin Res. 2011;4:81–4. [Google Scholar]
- 25.Mahajan B, Kaushal S, Chopra SC. A drug utilization study of antimicrobial agents (AMAs) in the Intensive Care Units (ICUs) at medical college hospital of North India. JK Sci. 2013;15:129–32. [Google Scholar]
- 26.Luciana T, Andrajati R, Rianti A, Khan AH. Rational antimicrobial use in an Intensive Care Unit in Jakarta, Indonesia: A hospital-based, cross-sectional study. Trop J Pharm Res. 2015;14:707–14. [Google Scholar]
- 27.Intensive Care Society. Cuthbertson BH, Thompson M, Sherry A, Wright MM, Bellingan GJ, et al. Antibiotic-treated infections in intensive care patients in the UK. Anaesthesia. 2004;59:885–90. doi: 10.1111/j.1365-2044.2004.03742.x. [DOI] [PubMed] [Google Scholar]
- 28.Shrikala B, Kranthi K, Nafisa A Prospective study on evaluation of antibiotic prescription practices in an Intensive Care Unit of a tertiary care hospital. J Clin Diagn Res. 2010;4:3387–91. [Google Scholar]