Abstract
Aim of the Study:
Metabolic acidosis is associated with increased mortality in critically ill patients. We hypothesized that early correction of acidosis of presumed metabolic origin results in improved outcomes.
Patients and Methods:
We conducted a prospective, observational study from February 2015 to June 2016 in a 12 bed mixed intensive care unit (ICU) of a 1000 bed tertiary care hospital in the north of India. ICU patients aged above 18 years with an admission pH ≥7.0 to <7.35 of presumed metabolic origin were included. Arterial blood gas parameters including pH, PaO2, PaCO2, HCO3−, Na+, K+, Cl−, anion gap (AG), base excess, and lactate at 0, 6, and 24 h along with other standard laboratory investigations were recorded. The primary outcome was to assess the impact of early pH changes on mortality at day 28 of ICU.
Results:
A total of 104 patients with 60.6% males and 91.3% medical patients were included in the study. Sepsis of lung origin (60.6%) was the predominant etiology. By day 28, 68 (65.4%) patients had died. Median age was 49.5 years, weight 61.7 kg, Sequential Organ Failure Assessment, and Acute Physiologic and Chronic Health Evaluation II scores were 16 and 12, respectively. Nonsurvivors had a higher vasopressor index (P < 0.01), lactate and central venous oxygen saturation (P < 0.05), and lower pH (P < 0.05). A pH correction/change of ≥1.16% during the first 24 h had the best receiver operating characteristic for predicting survival at day 28, with area under the curve (95% confidence interval, 0.72 [0.62–0.82], P < 0.05) compared to HCO3-, BE, lactate, and AG.
Conclusions:
Metabolic acidosis is associated with higher mortality in ICU. The rate of change in pH may better predict ICU mortality than other metabolic indices.
Keywords: Metabolic acidosis, mortality, pH change
INTRODUCTION
Metabolic acidosis is a challenging acid–base disorder, especially in the critically ill.[1,2] It can result from an increase in the concentration of hydrogen (H+) ions or a decrease in bicarbonate (HCO3-) ions, respectively. Various etiologies of metabolic acidosis include sepsis, cardiogenic shock, severe hypoxemia, hepatic failure, and intoxication. Metabolic acidosis can be classified as high or normal anion gap (AG) acidosis.[3,4] Lactic acidosis is a common cause of high AG metabolic acidosis.[5,6,7,8] It is further classified as Type-A (anaerobic) and Type-B (aerobic). Type-A lactic acidosis is associated with tissue hypoperfusion with inadequate oxygen delivery although with intact mitochondrial function. In Type-B lactic acidosis, there is an adequate cellular oxygen supply, no tissue hypoxia, and acidosis occurs due to abnormal carbohydrate metabolism, as in hepatic failure, diabetes, or phenformin intoxication.[9,10] The development of lactic acidosis depends on the magnitude of lactate elevation, the buffering capacity of the body, and coexistence of other conditions producing tachypnea and alkalosis (e.g., liver disease, sepsis). Thus, hyperlactatemia may be associated with a normal pH, acidemia, or alkalemia.
Metabolic acidosis is often caused by hyperlactatemia in critically ill patients.[3,11,12] Severe academia in sepsis contributes to hemodynamic instability owing to reduced myocardial contractility, arterial vasodilation, and impaired catecholamine responsiveness. In severe acidemia (blood pH <7.1), these effects can result in organ dysfunction and contribute to increased morbidity and mortality. Irrespective of the etiology, metabolic acidosis is associated with increased mortality in critically ill patients.[1,11,13,14] Survivors of severe sepsis and septic shock had better acidosis resolution than nonsurvivors.[1]
Enough supportive evidence exists to suggest that early lactate clearance is associated with reduced mortality and morbidity.[3,15,16,17] However, the same, though believed, cannot yet be claimed about early pH correction. We hypothesized that early hour clearance of metabolic acidosis, of either septic or nonseptic origin, is associated with improved outcomes. We proposed to do this prospective observational study to assess the effect of early hour pH changes on mortality in critically ill patients.
PATIENTS AND METHODS
Ethics and consent
After prior approval from the institute's ethics committee and written informed consent from patients or their first of kin, we conducted the present prospective observational study. The study period was from February 2015 to June 2016. A 12 bed mixed intensive care unit (ICU) of a tertiary care referral hospital and academic institute in the north of India was utilized for this purpose.
Inclusion criteria
Critically ill ICU patients aged 18 years or above, with an admission pH ≥7.0 but <7.35, and presumed to be of metabolic origin were included in the study.
Exclusion criteria
Patient with a pH <7.0, acidosis presumed to be respiratory origin, age below 18 years, and refusal of consent were excluded from the study.
Definitions
Acidosis
Acidosis was defined as an arterial pH of <7.35. It was arbitrarily considered corrected if the arterial pH was >7.35. Acidosis was further categorized as metabolic, respiratory, or mixed. Metabolic acidosis was defined as a state of decreased systemic pH, resulting from either a primary increase in H+ or a decrease in HCO3− blood concentration.[18]
Hemodynamics
Hemodynamic status during the first 24 h was defined by the vasopressor index.[19,20,21] We modified the vasopressor index to include vasopressin in place of phenylephrine as vasopressin is the most commonly used vasopressor after noradrenaline in septic shock. This was calculated as per the formula ((Dopamine dose × 1) + (Dobutamine dose × 1) + (Adrenaline dose × 100) + (Noradrenaline dose × 100) + (Vasopressin dose × 10)].
All doses were in μg/kg/min, except vasopressin which was in units/hour.
Study protocol
Following admission to our ICU, consecutive patients satisfying the inclusion criteria were included in the study. Our observations and analysis were disclosed to the ICU treating team. However, any alteration in clinical management based on our analysis was entirely at their discretion. Arterial blood gas (ABG) analysis with lactate was measured at 0, 6, and 24 h on the day of admission. All patients were followed until day 28 of ICU stay or discharge or death whichever occurred earlier for measurement of outcomes.
Measurements
Arterial blood samples were obtained from the existing invasive hemodynamic intra-arterial catheter (either radial or femoral artery) or through direct arterial puncture in case the intra-arterial catheter was yet not inserted. The ABG samples were used for determination of pH, PaO2, PaCO2, HCO3−, Na+, K+, Cl−, AG, base excess (BE), and lactate at 0, 6, and 24 h by automated blood gas analyzer. Also determined simultaneously were serum lactate from the same ABG sample and central venous oxygen saturation (ScVO2) from venous blood from the central vein catheter. Additional ABG samples, biochemistry, and other investigations were obtained whenever required as per the judgment of the attending ICU physician.
Arterial blood gas machine
ABG analysis was performed on Cobas b 221 system (Roche Diagnostics GmbH, Mannheim, Germany) with the integrated AutoQC drawer option and electrodes for pH, PO2, PCO2, reference electrode, and an ion selective electrode measuring sodium, potassium, and chloride. Specimens were analyzed using our standard operating procedure which included preanalytical protocols (such as safety, temperature, and transportation time) by trained authorized laboratory technicians, with automated internal quality control. External quality controls were performed at least 4 times a year with samples. Two milliliter nonheparinized syringes were used for collection of the arterial samples. These samples were then immediately transported at room temperature to the ABG machine housed within a minutes walking distance from our ICU.
Data collection
Demographic (age, gender, type of patient/admission, category of illness, etc.), clinicophysiologic characteristics (coexisting illness, organ failure, hemodynamic, and respiratory status), biochemical characteristics (Hb%, complete blood count, serum electrolytes, renal and liver function test, random blood glucose, and procalcitonin [PCT], etc.), ICU severity scores (Acute Physiologic and Chronic Health Evaluation-II [APACHE-II] and Sequential Organ Failure Assessment [SOFA]) were all recorded. ABG indices, lactate, and ScVO2 were also determined as previously mentioned. Organ supportive interventions such as fluid therapy, blood and blood product transfusions, sodium bicarbonate administration, vasoactive agents, vasopressor index, mechanical ventilation (MV), and renal replacement therapy (RRT) were also documented. Outcomes such as mortality, length of MV, and length of ICU/Hospital stay were recorded until 28 days of ICU stay or discharge or death whichever occurred earlier.
Sample size
Sample size was calculated assuming a proportion of pH change (≥0.10) equal to 0.50 in survivors and 0.20 in nonsurvivors at a minimum of 80% power of study and 95% confidence interval. Minimum sample size of 65 in survivors and 34 in nonsurvivors was needed to detect a difference in group proportions of 0.30. Sample size was calculated using software power analysis and sample size (NCSS(2008) PASS, Kaysville, UT).
Statistical analysis
Normality of continuous data was tested using Shapiro–Wilk test. Nonnormal, continuous data were expressed as median (interquartile range), while categorical data were expressed as frequency and percentage. Mann–Whitney U-test was used to compare the medians between survivors and nonsurvivors. Receiver operating characteristic (ROC) curve was drawn to predict survival at day 28 of ICU stay. Chi-square test was used to compare the proportions/test the association between groups. For repeated observations over time, Friedman analysis of variance (ANOVA) was used to estimate the significance level among the time points. If in Friedman ANOVA, the P value was observed to be significant then the difference in medians between individual groups was further assessed using the Wilcoxon Signed Rank test. A two-tailed P < 0.05 was considered statistically significant. IBM, SPSS version 23 (SPSS Inc., Chicago IL, USA) was used for statistical analysis.
RESULTS
Baseline characteristics
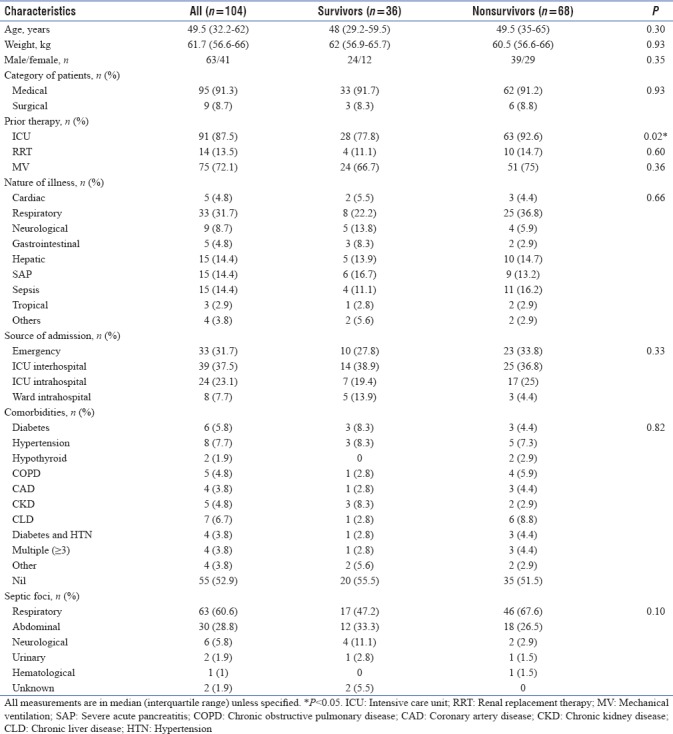
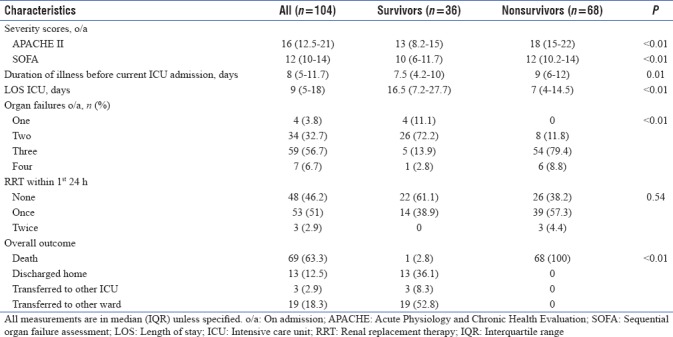
A total of 104 patients, 63 males (60.6%) and 95 (91.3%) medical patients were included in the study. The nature of illness for which these patients got admitted to our ICU was mainly respiratory (33/104, 31.7%). Sepsis of lung origin (63/104, 60.6%) was the predominant etiology. Most of the patients studied had already received prior ICU care (91/104, 87.5%), MV (75/104, 72.1%), and RRT (14/104, 13.5%) before this admission. Half of those who had previously undergone RRT also received it within the first 24 h of our ICU admission. A little over half (55/104, 52.9%) of the patients in the study did not have any coexisting illness. By day 28 of ICU stay, 68 (65.4%) patients succumbed to their illness. Thirteen of the 36 (12.5%) who survived were discharged home directly from the ICU. Nonsurvivors did not differ significantly from survivors, except for prior ICU care (P = 0.02). Baseline characteristics of patients and their comparison between survivors and nonsurvivors were as depicted in Table 1.
Table 1.
Baseline characteristics of the study population
Physiochemical characteristics
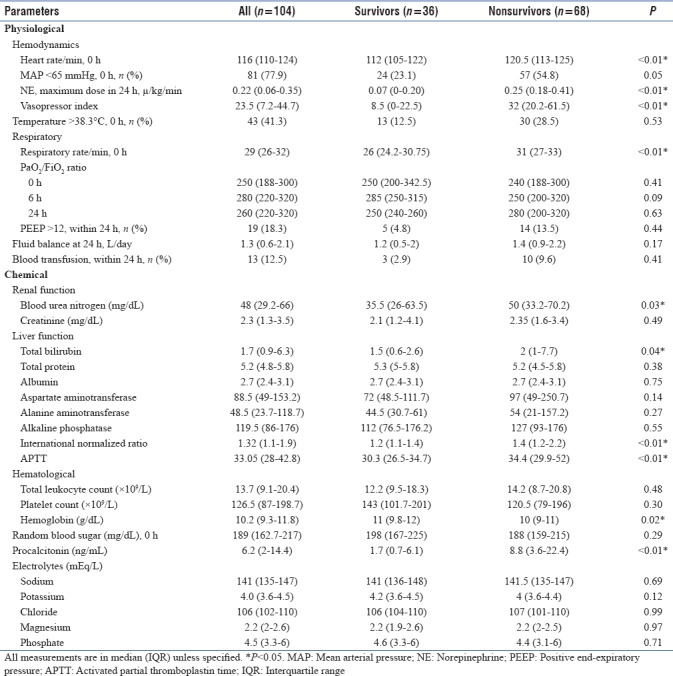
Nonsurvivors had significantly greater tachycardia (120.5 [113–125] vs. 112 [105–122] in survivors; P < 0.001), tachypnea (31 [27–33] vs. 26 [(24.2–30.7] in survivors; P < 0.001), and vasopressor index (32 [20.2–61.5] vs. 8.5[(0–22.5] in survivors; P < 0.001). Oxygenation (PaO2/FiO2 ratio) and positive end-expiratory pressure at 0, 6, and 24 h were comparable. No significant differences in fluid balance or need for blood transfusions were observed at or within 24 h period of admission, between groups. Among biochemical parameters, coagulation profile (International normalized ratio and activated partial thromboplastin time), blood urea nitrogen (BUN), and PCT were significantly higher in nonsurvivors (P < 0.05), while hemoglobin was significantly lower (P = 0.02). Electrolyte levels done during the first 24 h of admission were also comparable. The physiochemical characteristics of the study population were as depicted in Table 2.
Table 2.
Physiochemical characteristics of the study population
Acid–Base characteristics
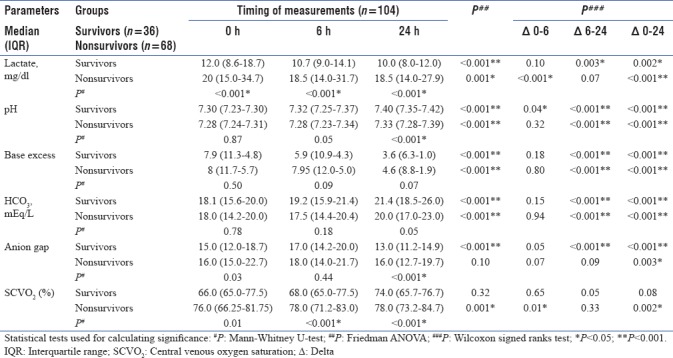
Nonsurvivors had significantly higher lactate and ScVO2 at 0, 6, and 24 h (P < 0.001). AG was also significantly higher at 0 and 24 h (P < 0.05); pH was significantly lower at 24 h in nonsurvivors. Trends over time were significantly unidirectional for most parameters, except for AG. Significant change in metabolic profile was observed for all parameters at Δ0–24 h, except ScVO2 in survivors. However, the changes were insignificantly minimal at Δ0–6 h, except for lactate (nonsurvivors; P < 0.001), pH (survivors; P = 0.04), and ScVO2(nonsurvivors; P = 0.01). In the middle period of Δ6–24 h, the majority of the parameters differed significantly [Table 3].
Table 3.
Metabolic trends over time of the study population
Hyperlactatemia
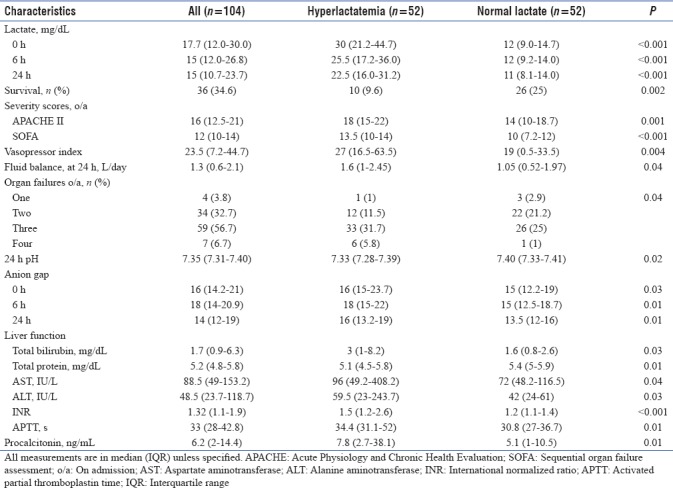
Hyperlactatemia (≥18 mg/dL) was observed in half (n = 52) of the patients. The majority of these patients (42/52, 80.8%) did not survive, as compared to those with normal levels (26/52, 50%; P = 0.002). Survivors among patients with normal lactate had lower lactate levels compared to nonsurvivors at 0, 6, and 24 h. However, lactate profiles were comparable between groups in patients with hyperlactatemia. A larger proportion of patients had ≥3 organ failures in the group with hyperlactatemia (P = 0.036). They also had significantly higher APACHE-II, SOFA, vasopressor index, AG, PCT, and 24 h fluid balance. Furthermore, these patients had lower 24 h pH and poorer liver function and coagulation profile [Table 4].
Table 4.
Hyperlactatemia (≥18 mg/dL)-based characteristics of the study population
Outcomes
Nonsurvivors had significantly higher APACHE-II and SOFA scores (P < 0.001). They also had a significantly longer median time interval between the onset of illness and admission to our ICU (9 [6–12] vs. 8 [5–11.7] in survivors; P = 0.01). They, however, had a shorter ICU stay (7 [4–14.5] vs. 16.5 [7.2–27.7] in survivors; P < 0.01). Sixty of the 68 (88%) nonsurvivors had three or more organ failures at ICU admission (P < 0.001). Two (34/104, 32.7%) and three (59/104, 56.7%) organ failures occurred at admission. Three or more organ failures (60/68, 88%) occurred more commonly in nonsurvivors (P < 0.001). A higher proportion of nonsurvivors (61.8%) required one or more RRT sessions within the first 24 h of ICU admission, although this was not statistically significant. The outcome-related characteristics of the study population were as depicted in Table 5.
Table 5.
Outcome-based characteristics of the study population
Survival prediction
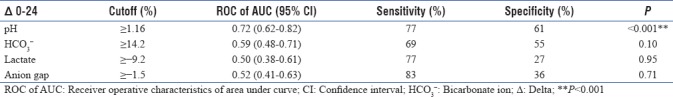
Among all ABG parameters, pH was significantly higher in survivors. pH correction/delta change ≥1.16% during the first 24 h had the best ROC, predicting survival at day 28, with area under the curve (AUC) (95% [confidence interval], 0.72 [0.62–0.82]; P < 0.001) with sensitivity and specificity of 77% and 61%, respectively. Delta change of other ABG parameters such as HCO3-, BE, lactate, and AG over first 24 h had lower AUC of ROC for predicting survival at day 28 [Table 6].
Table 6.
Delta changes of arterial blood gas parameters over 24 h predicting survival at day 28
DISCUSSION
The diagnosis and management of major acid–base disturbances are standard care aspects in critically ill patients. Metabolic acidosis is commonly encountered in ICU in severe sepsis and septic shock, irrespective of renal function and is often associated with poor outcomes.[1,22,23] Although hyperchloremic acidosis is associated with poor outcomes, studies in ICU patients have failed to detect a significant attributable effect on mortality.[11] The main findings of our prospective observational study were as follows: (a) significant physiochemical and acid–base derangements [Tables 2 and 3] contributed to the higher morbidity and mortality in nonsurvivors [Table 5], (b) patients with hyperlactatemia had worse outcomes [Table 4], and (c) changes in pH had better predictability for ICU survival than other ABG parameters.
Metabolic acidosis and prognosis
Noritomi et al.[1] in a study of 60 patients with severe sepsis or septic shock, concluded that nonsurvivors exhibited a complex metabolic acidosis at ICU admission. Severity of metabolic acidosis predicts ICU and hospital mortality,[1,11,14,24,26,27] length of stay, hospital morbidity,[28,29] and transfusion requirements[30] in medical, surgical, or trauma ICU patients. While AG predicts short-term mortality, strong ion gap predicts both short- and long-term mortality, in AKI patients with metabolic acidosis.[31] However, apart from lactate, there is no consensus about the clinical relevance of individual components of metabolic acidosis.
In the present study, we observed that patients with earlier acidosis resolution had lower mortality. The pH, relative to other ABG parameters, was significantly higher in survivors. On the other hand, nonsurvivors had higher ICU severity scores (APACHE II and SOFA), higher BUN, PCT, and more deranged coagulation profile and organ failures.
Lactic acidosis and mortality
Lactic acidosis is a common cause of metabolic acidosis in ICU. Mortality in patients with lactic acidosis, persisting beyond 24 h, approaches 70%.[32] In a prospective observational study of 126 patients admitted emergently with severe sepsis or septic shock, Lee et al.[13] observed that lactic acidosis and not hyperlactatemia, accurately predicted mortality in severe sepsis and septic shock. Noritomi et al.[1] in a study of 60 patients with severe sepsis or septic shock, concluded that in survivors, the resolution of acidosis was attributable to the decrease in strong ion gap and lactate. Furthermore, in another prospective observational study on 111 patients from both emergency and ICU, Nguyen et al.[3] reported that patients with lactate clearance ≥10% had a greater reduction in APACHE II score at 72 h and also a lower 60-day mortality (P = 0.007), relative to patients with a lactate clearance <10%. Contrary to this, Marik and Bellomo[16] in his review on lactate suggested that lactate clearance should not be used as the end-point of resuscitation in patients with sepsis. Significant majority of our patients with hyperlactatemia (42/52, 80.8%) did not survive, as compared to those with normal lactate levels (26/52, 50%; P = 0.002). Furthermore, survivors with normal lactate had lower levels at 0, 6, and 24 h.
Metabolic acidosis correction
Early recognition and prompt correction of metabolic acidosis are essential to survival. Treatment of metabolic acidosis is multimodal, involving identification and treatment of the underlying cause with simultaneous appropriate resuscitative and organ supportive measures. Although RRT seems attractive, particularly in patients with renal dysfunction, randomized controlled studies are needed to prove benefits of this strategy in treatment of lactic acidosis. Acidosis resolution in survivors was attributable to a decrease in strong ion gap and lactate.[1] A prospective randomized, double-blind, controlled clinical trial by Chen et al.[33] in 2013, conducted on 65 critically ill septic shock patients with lactic acidosis, concluded that bicarbonate therapy significantly reduced the incidence of multiple organ dysfunction, duration of MV, ICU and hospital length of stay, and mortality. Kim et al.[34] in 2013, in a retrospective analysis, reported that septic patients with metabolic acidosis, especially those with high AG, had higher mortality with sodium bicarbonate administration. However, although evidence is minimal, it is usually recommended to treat serum pH <7.1, empirically with sodium bicarbonate, 1 mEq/Kg, unless the underlying acidosis is believed to be immediately responsive to therapy.[35] In a study of chronic hemodialysis patients, correcting metabolic acidosis resulted in improved growth hormone insensitivity, increased and normalized plasma free T3 concentration, and improved plasma albumin.[36] A recently published multicenter, open-label, randomized controlled, phase 3 trial, in 26 French ICUs, concluded that in patients with severe metabolic acidemia, sodium bicarbonate infusion did not affect 28-day mortality and presence of at least one organ failure at day 7. However, 28-day mortality and need for RRT was decreased in a subset of AKI patients.[37] In our study, we used sodium bicarbonate infusion in three patients when the pH was <7.10; only one patient among these survived at day 28. Though our study is an observational study not designed to address the impact of sodium bicarbonate infusion in severe metabolic acidosis, inference from the French ICU study,[37] despite their limitations, is quite meaningful. In real-world scenario, a substantial proportion of AKI patients with metabolic acidosis would receive sodium bicarbonate to either defer or delay a dialysis depending on the acuity of illness. We also did not find any significant difference in fluid balance at 24 h and need of blood transfusion within 24-h period between survivors and nonsurvivors. Interestingly, RRT support was almost equally distributed in either group. Furthermore, we observed that pH correction/delta change of ≥1.16% during first 24 h had the best ROC for predicting survival at day 28 with an AUC of 0.72 (0.62–0.82) and P < 0.05, compared to other parameters such as HCO3−, BE, lactate, and AG.
Limitations
The several limitations that existed in our study were as follows: (a) single-center study with a small sample size; (b) variability in time of onset of illness and ICU admission, resuscitation, and organ supportive therapy before our admission and the quality of care provided during inter-/intra-hospital transportation may have all influenced the acid–base milieu; (c) presence of mixed acid–base disorders may have affected the pH; (d) multiple factors during our ICU admission such as MV, RRT, fluids, organ dysfunction/failure, antibiotics and source control, and the timing and intensity of life supportive interventions make it difficult to ascertain the dominating influence; (e) ABG errors during sample withdrawal, collection, transportation, and analysis were not accounted for in the study. Limitations b, c, and d would be difficult to remove and shall continue to influence any pH-based study. Despite the above limitations, ours is a sincere attempt to study the impact of early hour pH changes on mortality and morbidity in critically ill ICU patients.
CONCLUSIONS
Metabolic acidosis in ICU patients is frequently associated with varied etiologies and poor outcomes. Survivors have better acidosis resolution than nonsurvivors. As compared to lactate and other metabolic indices, early hour pH correction is a better predictor of ICU survival. However, due to the abovementioned limitations, generalization of our results to other ICU patients' needs to be done cautiously. We further suggest future trials with specific interventions to rectify metabolic acidosis early, before the true impact of pH change can be used for prognostication in critically ill.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors wish to thank the Department of Biostatistics and Health Informatics of the institute for the support provided in statistical analysis of the results.
REFERENCES
- 1.Noritomi DT, Soriano FG, Kellum JA, Cappi SB, Biselli PJ, Libório AB, et al. Metabolic acidosis in patients with severe sepsis and septic shock: A longitudinal quantitative study. Crit Care Med. 2009;37:2733–9. doi: 10.1097/ccm.0b013e3181a59165. [DOI] [PubMed] [Google Scholar]
- 2.Gauthier PM, Szerlip HM. Metabolic acidosis in the Intensive Care Unit. Crit Care Clin. 2002;18:289–308, vi. doi: 10.1016/s0749-0704(01)00012-4. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32:1637–42. doi: 10.1097/01.ccm.0000132904.35713.a7. [DOI] [PubMed] [Google Scholar]
- 4.Oh MS, Carroll HJ. The anion gap. N Engl J Med. 1977;297:814–7. doi: 10.1056/NEJM197710132971507. [DOI] [PubMed] [Google Scholar]
- 5.Kraut JA, Kurtz I. Use of base in the treatment of acute severe organic acidosis by nephrologists and critical care physicians: Results of an online survey. Clin Exp Nephrol. 2006;10:111–7. doi: 10.1007/s10157-006-0408-9. [DOI] [PubMed] [Google Scholar]
- 6.Kraut JA, Madias NE. Lactic acidosis. N Engl J Med. 2014;371:2309–19. doi: 10.1056/NEJMra1309483. [DOI] [PubMed] [Google Scholar]
- 7.Madias NE. Lactic acidosis. Kidney Int. 1986;29:752–74. doi: 10.1038/ki.1986.62. [DOI] [PubMed] [Google Scholar]
- 8.Stacpoole PW. Lactic acidosis. Endocrinol Metab Clin North Am. 1993;22:221–45. [PubMed] [Google Scholar]
- 9.Arieff AI. Indications for use of bicarbonate in patients with metabolic acidosis. Br J Anaesth. 1991;67:165–77. doi: 10.1093/bja/67.2.165. [DOI] [PubMed] [Google Scholar]
- 10.Hindman BJ. Sodium bicarbonate in the treatment of subtypes of acute lactic acidosis: Physiologic considerations. Anesthesiology. 1990;72:1064–76. [PubMed] [Google Scholar]
- 11.Gunnerson KJ, Saul M, He S, Kellum JA. Lactate versus non-lactate metabolic acidosis: A retrospective outcome evaluation of critically ill patients. Crit Care. 2006;10:R22. doi: 10.1186/cc3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khosravani H, Shahpori R, Stelfox HT, Kirkpatrick AW, Laupland KB. Occurrence and adverse effect on outcome of hyperlactatemia in the critically ill. Crit Care. 2009;13:R90. doi: 10.1186/cc7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SW, Hong YS, Park DW, Choi SH, Moon SW, Park JS, et al. Lactic acidosis not hyperlactatemia as a predictor of in hospital mortality in septic emergency patients. Emerg Med J. 2008;25:659–65. doi: 10.1136/emj.2007.055558. [DOI] [PubMed] [Google Scholar]
- 14.Allyn J, Vandroux D, Jabot J, Brulliard C, Galliot R, Tabatchnik X, et al. Prognosis of patients presenting extreme acidosis (pH<7) on admission to Intensive Care Unit. J Crit Care. 2016;31:243–8. doi: 10.1016/j.jcrc.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Jansen TC, van Bommel J, Mulder PG, Lima AP, van der Hoven B, Rommes JH, et al. Prognostic value of blood lactate levels: Does the clinical diagnosis at admission matter? J Trauma. 2009;66:377–85. doi: 10.1097/TA.0b013e3181648e2f. [DOI] [PubMed] [Google Scholar]
- 16.Marik PE, Bellomo R. Lactate clearance as a target of therapy in sepsis: A flawed paradigm. OA Crit Care. 2013;1:3. [Google Scholar]
- 17.Lee YK, Hwang SY, Shin TG, Jo IJ, Suh GY, Jeon K, et al. Prognostic value of lactate and central venous oxygen saturation after early resuscitation in sepsis patients. PLoS One. 2016;11:e0153305. doi: 10.1371/journal.pone.0153305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seifter JL. Acid-base disorders. In: Goldman L, Schafer AI, editors. Goldman-Cecil Medicine. 25th ed. Elsevier: Philadelphia; 2016. pp. 762–74. [Google Scholar]
- 19.Wernovsky G, Wypij D, Jonas RA, Mayer JE, Jr, Hanley FL, Hickey PR, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92:2226–35. doi: 10.1161/01.cir.92.8.2226. [DOI] [PubMed] [Google Scholar]
- 20.Zuppa AF, Nadkarni V, Davis L, Adamson PC, Helfaer MA, Elliott MR, et al. The effect of a thyroid hormone infusion on vasopressor support in critically ill children with cessation of neurologic function. Crit Care Med. 2004;32:2318–22. doi: 10.1097/01.ccm.0000146133.52982.17. [DOI] [PubMed] [Google Scholar]
- 21.Cruz DN, Antonelli M, Fumagalli R, Foltran F, Brienza N, Donati A, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: The EUPHAS randomized controlled trial. JAMA. 2009;301:2445–52. doi: 10.1001/jama.2009.856. [DOI] [PubMed] [Google Scholar]
- 22.Mecher C, Rackow EC, Astiz ME, Weil MH. Unaccounted for anion in metabolic acidosis during severe sepsis in humans. Crit Care Med. 1991;19:705–11. doi: 10.1097/00003246-199105000-00018. [DOI] [PubMed] [Google Scholar]
- 23.O'Dell E, Tibby SM, Durward A, Murdoch IA. Hyperchloremia is the dominant cause of metabolic acidosis in the postresuscitation phase of pediatric meningococcal sepsis. Crit Care Med. 2007;35:2390–4. doi: 10.1097/01.CCM.0000284588.17760.99. [DOI] [PubMed] [Google Scholar]
- 24.Kincaid EH, Miller PR, Meredith JW, Rahman N, Chang MC. Elevated arterial base deficit in trauma patients: A marker of impaired oxygen utilization. J Am Coll Surg. 1998;187:384–92. doi: 10.1016/s1072-7515(98)00202-6. [DOI] [PubMed] [Google Scholar]
- 25.Rocktaeschel J, Morimatsu H, Uchino S, Bellomo R. Unmeasured anions in critically ill patients: Can they predict mortality? Crit Care Med. 2003;31:2131–6. doi: 10.1097/01.CCM.0000079819.27515.8E. [DOI] [PubMed] [Google Scholar]
- 26.Husain FA, Martin MJ, Mullenix PS, Steele SR, Elliott DC. Serum lactate and base deficit as predictors of mortality and morbidity. Am J Surg. 2003;185:485–91. doi: 10.1016/s0002-9610(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 27.Randolph LC, Takacs M, Davis KA. Resuscitation in the pediatric trauma population: Admission base deficit remains an important prognostic indicator. J Trauma. 2002;53:838–42. doi: 10.1097/00005373-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez-Lozada R, Chapa-Azuela O, Gutiérrez-Vega R, Fernández-Hidalgo E. Use of base deficit as a prognosis factor for acute pancreatitis. Gac Med Mex. 2003;139:108–11. [PubMed] [Google Scholar]
- 29.Piper G, Patel NA, Chandela S, Benckart DH, Young JC, Collela JJ, et al. Short-term predictors and long-term outcome after ruptured abdominal aortic aneurysm repair. Am Surg. 2003;69:703–9. [PubMed] [Google Scholar]
- 30.Rutherford EJ, Morris JA, Jr, Reed GW, Hall KS. Base deficit stratifies mortality and determines therapy. J Trauma. 1992;33:417–23. doi: 10.1097/00005373-199209000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Zheng CM, Liu WC, Zheng JQ, Liao MT, Ma WY, Hung KC, et al. Metabolic acidosis and strong ion gap in critically ill patients with acute kidney injury. Biomed Res Int 2014. 2014:819528. doi: 10.1155/2014/819528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forrest DM, Russell JA. Metabolic acidosis. In: Webb A, Shapiro M, Singer M, Suter P, editors. Oxford Textbook of Critical Care. 1st ed. USA: Oxford University Press; 1999. pp. 573–7. [Google Scholar]
- 33.Chen XF, Ye JL, Zhu ZY. The use of sodium bicarbonate in stages in treating hypoperfusion induced lactic acidemia in septic shock. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2013;25:24–7. doi: 10.3760/cma.j.issn.2095-4352.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Kim HJ, Son YK, An WS. Effect of sodium bicarbonate administration on mortality in patients with lactic acidosis: A retrospective analysis. PLoS One. 2013;8:e65283. doi: 10.1371/journal.pone.0065283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kraut JA, Madias NE. Metabolic acidosis: Pathophysiology, diagnosis and management. Nat Rev Nephrol. 2010;6:274–85. doi: 10.1038/nrneph.2010.33. [DOI] [PubMed] [Google Scholar]
- 36.Wiederkehr MR, Kalogiros J, Krapf R. Correction of metabolic acidosis improves thyroid and growth hormone axes in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1190–7. doi: 10.1093/ndt/gfh096. [DOI] [PubMed] [Google Scholar]
- 37.Jaber S, Paugam C, Futier E, Lefrant JY, Lasocki S, Lescot T, et al. Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the Intensive Care Unit (BICAR-ICU): A multicentre, open-label, randomised controlled, phase 3 trial. Lancet. 2018;392:31–40. doi: 10.1016/S0140-6736(18)31080-8. [DOI] [PubMed] [Google Scholar]


