Abstract
Background
Adults with class II/III obesity [body mass index (in kg/m2) ≥35] may present with a phenotype characterized by low lean mass and excess fat mass, a condition known as sarcopenic obesity (SO). Little is known about the prevalence and relevance of SO in these individuals, primarily due to a lack of relevant diagnostic criteria.
Objective
Here, we explored the definition of SO based on physical function as an outcome of interest in adults with class II/III obesity and applied this definition to compare clinical characteristics between SO and non-SO patients.
Methods
In this cross-sectional analysis, patients’ demographic, anthropometric, and biochemical characteristics, as well as comorbidities and physical activity levels, were collected at an obesity specialty clinic prior to any treatment. Body composition was assessed by dual-energy X-ray absorptiometry. Physical function was assessed by self-reported difficulties with activities of daily living (ADLs) from an 11-item questionnaire. Five SO definitions were tested against reported difficulty with ADLs with the use of receiver operating characteristic (ROC) analysis.
Results
A total of 120 subjects (86% women) aged 46 ± 11 y were included. Based on ROC analysis, SO was best defined by an appendicular skeletal mass (ASM)/weight x 100 (%) <19.35% for women and <24.33% for men, resulting in a prevalence of 25% (n = 30, women 22.3%, men 41.2%). SO was significantly associated with older age, higher waist circumference, higher triglycerides, greater use of antihypertensive medications, and lower physical activity.
Conclusions
In this sample of adults with class II/III obesity, difficulties with ADLs were best associated with measures of ASM in relation to total body weight. Patients identified with SO using this criterion presented with poorer clinical outcomes such as factors of elevated cardiometabolic risk.
Keywords: activities of daily living, appendicular skeletal mass, class II/III obesity, dual-energy X-ray absorptiometry, lean soft tissue, sarcopenia
Introduction
Low lean mass, also termed sarcopenia, is a condition primarily studied in the elderly as part of the aging trajectory (1). Lower lean mass is associated with low physical activity, illness, inflammation, certain hormonal and neurologic conditions, and poorer quality of life, among other health consequences (2). Increases in fat mass (FM) are typically seen from middle age up to age 60–70 y, and are associated with a high prevalence of obesity among this age group.
In addition to aging-related changes in body composition, weight loss from obesity treatment may result in reductions in both FM and lean mass. Weight cycling (weight gain after weight loss) is common (3–5) and is associated with increased FM with lean mass remaining lower than
baseline (i.e., prior to weight loss) (6). Repeated weight loss attempts, combined with the normal aging trajectory, can put individuals at risk for sarcopenia over time (5).
Sarcopenic obesity (SO) is described as the coexistence of low lean mass with excess FM (7). Several definitions for sarcopenia exist, mostly based on sex-specific cutpoints for low muscle mass derived either from a young reference population or from a study cohort (8). Definitions based on a health outcome of interest are highly relevant and have been increasingly used (9–11). Due to the variety of definitions (for sarcopenia and obesity) and methods to assess body composition, the estimates of the prevalence of SO are highly variable (12). Studies have identified SO in older adults (≥65 y of age) and those with certain health conditions (11, 13, 14). In older adults, sarcopenia is associated with difficulties with activities of daily living (ADLs) (15, 16) and functional impairment (17, 18). Excess FM and low lean mass can compound negative effects on cardiovascular and metabolic health (19–23), impacting disability and mortality (24, 25). Although most studies to date have focused on older adults (≥60 y of age), recent data suggest younger and middle-aged individuals may also present with SO (26, 27). Nonetheless, the lack of diagnostic criteria for sarcopenia in younger individuals, especially those with class II/III obesity [BMI (in kg/m2) ≥35], has limited our understanding of the prevalence and clinical characteristics of this condition in these individuals. Therefore, the primary purpose of this study was to explore the definition of sarcopenia that best discriminated adults with class II/III obesity based on a clinical outcome of interest (data-driven approach), namely physical function. The second was to apply this definition and compare the clinical characteristics of subjects with and without SO.
Methods
Subjects
A cross-sectional analysis was conducted on patients from a multidisciplinary obesity specialty clinic in Northern Alberta, Canada (population 1.2 million) (28). Patient history and clinical assessment were completed at the initial visit (i.e., prior to any weight loss treatment). Height (centimeters), weight (kilograms), waist circumference (centimeters), and blood pressure (expressed in mm Hg) were measured using standardized procedures, and BMI calculated as weight over height squared (kg/m2), and categorized according to WHO criteria (29). Availability of dual-energy X-ray absorptiometry (DXA) scans and further details on data collection have been previously described (8). All subjects were adults (18–69 y of age) with class II/III obesity who completed the initial assessment and had a DXA body composition scan in their medical record (available from consecutive patients seen from January 2009 to June 2012). Exclusion criteria included age (≥70 y), and those who had incomplete DXA data. Ethics approval was obtained from the University of Alberta Research Ethics Board.
Body Composition
DXA scans were conducted using Hologic Discovery A/W (Hologic Inc., Bedford, MA) at a local imaging center, based on which FM, lean mass [better termed lean soft tissue (LST)], fat-free mass (FFM; composed of LST and bone) for the whole body, and segmental values [appendicular skeletal mass (ASM); composed of the LST from the arms and legs] were computed. These measures were adjusted by height in square meters to calculate the FM index and ASM index (30).
Biochemical Analysis and Comorbidities
Biochemical data was collected from medical records and included inflammatory markers, lipid panel, glucose-related measurements, kidney function, albumin, and vitamin D concentrations. Subjects with abnormal biochemical values meeting criteria for either diabetes or prediabetes were combined as a dichotomous variable.
Comorbid conditions were identified based upon review of medical history and prescription medications and clinical and laboratory values collected at the initial assessment. Metabolic syndrome was defined using the National Cholesterol Education Program Adult Treatment Panel III reference values; ≥3 out of 5 criteria being met indicated its presence (31).
As was done previously (32–34), a multimorbidity categorical score (0, 1, 2, ≥3) was used including 8 comorbidities: diabetes or prediabetes, hypertension, dyslipidemia, metabolic syndrome, mental health, chronic kidney disease, sleep apnea, and osteoarthritis.
Activity Level and Difficulties with Activities of Daily Living
Physical activity levels were categorized as either “met” or “did not meet” Canadian Physical Activity Guidelines for Adults, defined as accumulating ≥150 min of moderate-to-vigorous intensity activities in a week, in bouts of ≥10 min (35).
An occupational therapy referral screening questionnaire based on the Canadian Model of Occupational Performance (36) was used (see Supplemental Table 1). This self-administered questionnaire included 11 items that asked about subjects’ experiences with managing ADLs and the ability to perform a variety of tasks including: transferring in and out of the car, bed, bathtub, or on and off the toilet; bathing; skin care; personal hygiene; household tasks; and leisure activities. Other items asked about home accessibility, falls or balance concerns, the impact of excess skin on completing daily activities, the impact of fatigue on completing daily activities, and whether a client uses or has considered custom footwear or orthotics. Options included “yes” or “no” answers to each item, scored as 1 or 0, respectively. Subjects also had the choice to report, “I have help” for some items, in which case a score of 1 was assigned. A higher overall score represented more items reporting difficulty with ADLs. A composite score for items on the questionnaire was calculated as a continuous variable (range 0–11). Additionally, a dichotomous variable defined as “0–2” or “≥3” items was created to explore the selected definition for SO, as described next.
SO Definition
Although all subjects had obesity (BMI ≥35), the variability of percentage of FM was explored to avoid misclassification. As reported previously (and in the hereby shown results), percentage of FM fell within several accepted criteria to define excess FM (8) for all patients. Based upon this previous study (8), 5 previously published DXA-derived definitions of sarcopenia were explored (8, 21, 37-39; see Table 1). Because these definitions were not tested in regards to predictive ability, the current study identified sarcopenia based on a clinically meaningful cutpoint (data-driven approach) whereby a value was specified below which individuals would be at increased risk for a health outcome (9-11, 40, 41).
TABLE 1.
Bivariate correlations (Pearson's r, P < 0.05) for the number of items (0–11) for self-reported difficulty with activities of daily living with continuous body composition variables for the definitions of sarcopenia applied to the study sample1
| Correlations with ADL difficulties, r (P value) | ||||
|---|---|---|---|---|
| Definitions | Reference | All2 (n = 111) | Women (n = 94) | Men3 (n = 17) |
| ASM/weight x 100, % | Levine and Crimmins, 2012 (21) | –0.262 (0.005) | –0.232 (0.024) | –0.510 (0.037) |
| ASM/BMI, kg/m2 | Batsis et al., 2015 (37) | –0.187 (0.049) | –0.158 (0.127) | –0.526 (0.021) |
| ASM adjusted for height and FM | Newman et al., 2003 (38) | 0.108 (0.268) | 0.195 (0.060) | –0.177 (0.496) |
| (residuals) | Johnson Stoklossa et al., 2017 (8) | –0.084 (0.380) | –0.150 (0.148) | 0.239 (0.356) |
| FM:FFM ratio, centile | Siervo et al., 2015 (39) | 0.230 (0.015) | 0.231 (0.025) | 0.453 (0.068) |
Based on definitions identified/discussed in Johnson Stoklossa et al. (8). ADL, activity of daily living; ASM, appendicular skeletal mass; FMM, fat-free mass: FM, fat mass.
Self-reported difficulty with ADLs available for n = 111.
Spearman's r reported for men due to small sample size (n < 30).
The choice of difficulty with ADLs as the outcome of interest was due to the association with reduced physical function, increased disability, and poor quality of life (15, 24, 40, 42). ADL difficulties are notably a common health consequence of sarcopenia (43). Therefore, our sex-specific cutpoints were developed based on its discriminative and predictive ability, similar to previous studies in health and clinical conditions (18, 24, 26, 41).
Correlation of the continuous body composition variable and the continuous variable for difficulty with ADLs was evaluated for each definition (Table 1), the definition of choice being the one most significantly correlated with difficulty with ADLs. Receiver operating characteristic (ROC) curves were used to determine sex-specific cutpoints of the continuous body composition variable selected to define sarcopenia based upon the 2 categories for difficulty with ADLs (0–2 items compared to ≥3 items), with high sensitivity as the priority criteria. The AUC was then explored to identify optimal sex-specific cutpoints for the total difficulties with ADL score (continuous variable). These were used to create a dichotomous variable: SO compared to non-SO based on impaired and non-impaired ADLs, respectively. The body composition variable calculated with the new cohort-derived cutpoints was then compared, using chi-square test and kappa statistics, with the variable calculated using the original study cutpoints from the selected SO definition, to determine the level of agreement between the 2 definitions.
Statistical Analysis
Descriptive statistics were used as appropriate. Normality testing was completed with the Shapiro–Wilk test. Mean values of 2 groups were compared using independent samples t tests (Mann–Whitney U for nonparametric data). Chi-square or Fisher's exact test were used as appropriate. Pearson's or Spearman's correlations were used to test the correlation between 2 continuous variables for normally and non-normally distributed variables, respectively. Cronbach's alpha was used to determine the internal consistency of the items on the questionnaire for difficulty with ADLs. A P value of <0.05, based on 2-tailed tests, was considered statistically significant. Data analysis was completed with IBM SPSS Statistics v23 (IBM Corp., Armonk, NY).
Results
Subject Characteristics
Of 167 cases reviewed, 120 had available DXA data. Subject demographics and anthropometrics are presented in Table 2. The sample was predominately female, middle-aged, married or common law, educated, and working outside the home. In terms of body composition, women had lower LST and higher FM than did men, with similar findings between derivatives of these variables. Results for biochemical analysis (serum, fasting) are presented in Supplemental Table 2. Five subjects (4.3%) had renal impairment [estimated glomular filtration rate (eGFR) <60 mL · min−1 · 1.73 m−2]. All subjects presented with ≥1 comorbidity in addition to obesity (see Supplemental Table 3 for subjects’ clinical characteristics). The most prevalent comorbidities were dyslipidemia, followed by metabolic syndrome and hypertension. Multimorbidity was highly prevalent with 80.8% of patients having ≥3 comorbid conditions in addition to class II/III obesity. No differences were observed by sex (P = 0.695) or BMI category (P = 0.427) between those meeting or not meeting physical activity guidelines.
TABLE 2.
Demographic, anthropometric, and body composition characteristics of adults with class II/III obesity (n = 120)1
| Variables | % or mean ± SD; median (range) |
|---|---|
| Demographics | |
| Sex | |
| Female | 85.8 |
| Age, y | 46.9 ± 11.1; 49.0 (23–69) |
| Marital status | |
| Single | 23.3 |
| Married/common law | 67.5 |
| Divorced/separated/widowed | 9.2 |
| Education | |
| Some high school | 2.5 |
| Completed high school | 97.5 |
| Completed postsecondary | 53.3 |
| Employment | |
| Full-time | 53.3 |
| Part-time | 14.2 |
| Unemployed | 6.7 |
| On disability | 7.5 |
| Homemaker | 5.8 |
| Retired | 12.5 |
| Smoking | |
| Never | 45.5 |
| Former | 47.3 |
| Current | 7.3 |
| Age of obesity onset | |
| Pediatric, ≤19 y | 46.7 |
| Adult, ≥20 y | 53.3 |
| Anthropometrics | |
| Height, cm | 166.0 ± 7.64; 165.0 (148.6–187.1) |
| Weight,2 kg | 120.2 ± 19.6; 116.2 (88.9–180.7) |
| BMI,2 kg/m2 | 43.5 ± 5.7; 42.3(34.9–58.5) |
| 35.0–39.9 | 33.3 |
| 40.0–44.9 | 32.5 |
| 45.0–49.0 | 19.2 |
| ≥50.0 | 15.0 |
| Waist circumference, cm3 | 123.3 ± 13.2; 122.5 (93.5–163.0) |
| Body composition4 | |
| ASM, kg | F: 24.7 ± 3.7; 24.2 (17.4–35.4)2 |
| M: 34.2 ± 5.2; 35.3 (27.0–44.9) | |
| ASMI, kg/m2 | F: 9.2 ± 1.2; 9.1 (6.7–12.8) |
| M: 10.9 ± 1.3; 10.9 (8.7–13.6) | |
| ASM/weight x 100, % | F: 21.2 ± 2.1; 21.0 (16.2–28.3) |
| M: 24.9 ± 2.8; 24.9 (20.2–29.2) | |
| FM, % | F: 48.0 ± 4.2; 48.3 (32.3–57.4) |
| M: 41.4 ± 5.6; 39.7 (31.9–53.2) | |
| FMI, kg/m2 | F: 20.6 ± 3.8; 20.0 (13.9–30.9)2 |
| M: 18.0 ± 3.7; 16.9 (13.4–24.9) |
ASM, appendicular skeletal mass; ASMI, ASM index; FM, fat mass; FMI, fat mass index.
Variable not normally distributed for women.
Waist circumference available for 78% (n = 94).
All body composition variables were statistically different between sexes.
Of the 111 respondents (n = 9 with missing data), 11% reported no difficulties with ADLs with no sex differences noted. Subjects across all ages and BMI categories reported difficulties with ADLs, and >50% reported difficulty with ≥3 items. Prevalence of comorbidities was not different comparing subjects reporting difficulty with 0–2 items compared to those reporting ≥3 items.
SO Definition
From the 5 sarcopenia definitions explored (Table 1), 2 were significantly correlated with items of difficulty with ADLs: ASM/weight x 100 (%) (21, 22) (r = –0.262, P = 0.005) and FM:FFM ratio (39) (r = 0.230, P = 0.015). For both definitions, lower lean mass was associated with a higher number of items of difficulty with ADLs. Nonetheless, only ASM/weight x 100 (%) was significantly correlated for both sexes [women (r = –0.232, P = 0.024); men (rs = –0.510, P = 0.037)]. Therefore, this variable was selected to define sarcopenia in our cohort.
ASM/weight x 100 (%) for the entire cohort (n = 120) was 21.7% ± 2.6%; median and range: 21.5% (16.2–29.2%). Cohort-derived sex-specific cutpoints were then determined; for women the value was 19.4% (sensitivity 86%, specificity 29%) and it was 24.3% (90% sensitivity, 86% specificity) for men. Differences were not driven solely by men, as the best agreement was achieved with the sex-specific cutpoint applied to the entire data-set (data not shown). Applying these cutpoints, the prevalence of SO in the entire sample was 25% (22.3% women; 41.2% men). Mean values for ASM/weight x 100 (%) were 19.3% ± 1.8% for subjects with SO and 22.5% ± 2.3% for the group with non-SO (P < 0.0001).
Correlations for SO definition (as dichotomous variable: SO compared to non-SO) with difficulty with ADLs (0–2 compared to ≥3 items) were significant (P = 0.006). The ability for these ADL categories to discriminate sarcopenia was moderate for women (AUC = 0.60; 95% CI: 0.49, 0.71) and strong for men (AUC = 0.90; 95% CI: 0.74, 1.00). There was a high level of agreement (k = 0.915) between our cohort-specific definition and Levine and Crimmins’ (21) published cutpoints for ASM/weight x 100 (%) derived from a young reference group.
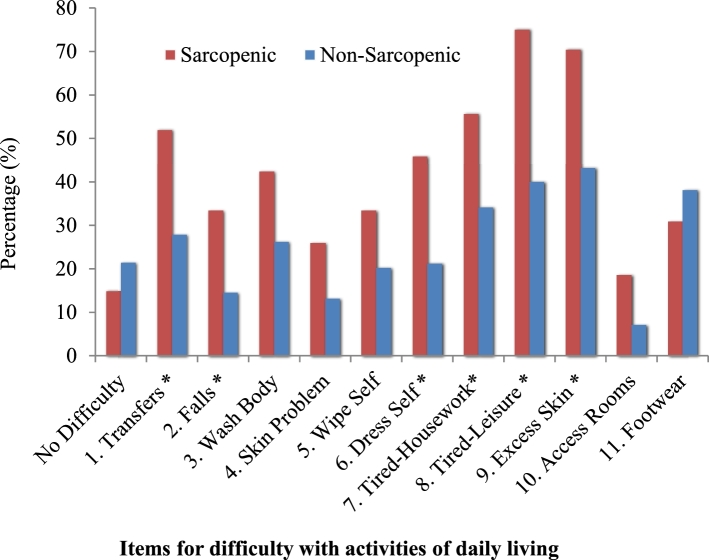
The ADL questionnaire had good internal consistency (11 items, α = 0.848). As such, nearly three-quarters of subjects with SO reported difficulty with ≥3 items compared to less than half (44%) of non-SO subjects. Values for ASM/weight x 100 (%) were lower for subjects who responded “yes” to 5 separate ADL items: transfers (P = 0.046), wiping self (P = 0.030), fatigue as a barrier to household tasks (P = 0.017) and leisure activities (P = 0.009), and access to household rooms (P = 0.020). Overall prevalence of difficulty with ADLs by SO status is presented in Figure 1, with differences identified for 6 of the 11 items: transfers (P = 0.023), falls (P = 0.030), dress self (P = 0.021), fatigue as a barrier to household (P = 0.048) and leisure activities (P = 0.004), and excess skin (P = 0.025).
FIGURE 1.
Comparison of items for self-reported difficulty with activities of daily living with body composition phenotype groups: sarcopenic obese (n = 27, left bar) compared to nonsarcopenic obese (n = 84, right bar). The occupational therapy screening questionnaire was completed by each subject at the initial clinic assessment with 11 items that asked about their experiences and ability to perform a variety of activities of daily living tasks. *Results for items that were significantly different between the groups, P < 0.05.
Clinical Characteristics of SO
No differences for most demographic variables were observed by sarcopenia status, except for age and sex (prevalence by sex as already reported). Age range was 23–69 and 24–68 y for SO compared to non-SO groups, respectively. Mean age was higher for the SO group (50.7 ± 12.7 compared to 45.7 ± 10.3 y, P = 0.033). Prevalence of SO was significantly higher among subjects aged ≥ 65 y (66.7% compared to 22.8%, P = 0.016). Smoking status (never compared to former) was not different between groups and no current smokers were identified with SO. Although all waist circumference measurements exceeded recommendations, individuals with SO presented with a higher mean waist circumference compared to their counterparts (130.2 ± 21.1 compared to 121.1 ± 11.7 cm, P = 0.004).
A comparison of clinical variables (metabolic and functional) by sarcopenia status is presented in Table 3. No differences between groups were identified for most biochemical variables, except for albumin and triglycerides. Only 2 subjects had hypoalbuminemia and both had sarcopenia. SO was associated with higher triglyceride concentrations (2.06 ± 1.00 compared to 1.62 ± 0.73 mmol/L, P = 0.040), which in turn overall exceeded the normal reference value (<1.7 mmol/L). Only a trend towards a difference was found between groups comparing abnormal to normal triglyceride concentrations (see Table 3).
TABLE 3.
Comparison of clinical variables between sarcopenic obese versus nonsarcopenic obese groups (n = 120)1
| Variables2 | All3 (n) | Sarcopenic obese4 (n = 30) | Nonsarcopenic obese4 (n = 90) | P value |
|---|---|---|---|---|
| Biochemical, % | ||||
| Fasting glucose > 6.0 mmol/L | 114 | 30.8 | 31.8 | 0.511 |
| HbA1C > 6.5 | 115 | 18.5 | 28.4 | 0.306 |
| eGFR < 60 mL · min−1 · 1.73 m−2 | 116 | 10.7 | 2.3 | 0.055 |
| TChol > 6.2 mmol/L | 115 | 11.1 | 9.1 | 0.755 |
| LDL > 3.2 mmol/L | 115 | 29.6 | 38.6 | 0.495 |
| HDL: F < 1.3, M < 1.0 mmol/L | 115 | 55.6 | 63.6 | 0.450 |
| TG > 1.7 mmol/L | 115 | 59.3 | 38.6 | 0.059 |
| CRP > 10.0 mg/L | 112 | 28.0 | 28.7 | 0.943 |
| Albumin < 35 g/L | 114 | 100 | 0.0 | 0.009 |
| 25-OH vitamin D3 <80 nmol/L | 113 | 61.5 | 65.5 | 0.710 |
| Comorbidities, % | ||||
| Abnormal glycemic control | 114 | 34.6 | 43.2 | 0.436 |
| On medication | 120 | 13.3 | 18.9 | 0.588 |
| Hypertension | 115 | 71.4 | 69.0 | 0.805 |
| On medication | 120 | 50.0 | 28.9 | 0.035 |
| Dyslipidemia | 115 | 96.4 | 90.9 | 0.342 |
| On medication | 120 | 26.7 | 15.6 | 0.173 |
| Mental health | 120 | 56.7 | 55.6 | 0.915 |
| On medication | 120 | 36.7 | 27.8 | 0.358 |
| Metabolic syndrome | 117 | 72.4 | 69.3 | 0.819 |
| Osteoarthritis | 120 | 40.0 | 23.3 | 0.077 |
| Sleep apnea | 120 | 43.3 | 34.4 | 0.382 |
| Multimorbidity score, % | 120 | |||
| ≥3 | 80.0 | 81.1 | 0.885 | |
| Activity level, % | 120 | 0.007 | ||
| Meets guidelines | 3.3 | 25.6 | ||
| Difficulty with ADLs, % | 111 | |||
| Any item | 85.2 | 78.6 | 0.584 | |
| Less vs. more items | 0.008 | |||
| 0–2 items | 25.9 | 56.0 | ||
| ≥3 items | 74.1 | 44.0 |
ADL, activity of daily living; CRP, C-reactive protein; eGFR, estimated glomular filtration rate; HbA1C, glycated hemoglobin; TChol, total cholesterol; TG, triglyceride.
Variables were compared by category: biochemical (abnormal vs. normal); comorbidities (present vs. absent); multimorbidity score (<3 vs. ≥3); difficulty with ADLs: any item (yes vs. no).
All: total number of participants with data for each variable from the entire cohort.
Percentages reported as a total within each body composition group.
SO was associated with a greater prevalence of renal impairment (eGFR <60 mL · min−1 · 1.73 m−2), yet overall the majority of the cohort had normal renal function. There were no differences for mean C-reactive protein (CRP) concentrations (SO, 8.07 ± 5.40 compared to 7.58 ± 6.51 mg/L for non-SO, P = 0.731). Presence of comorbid conditions was not different between groups. Use of medications for hypertension was higher among SO patients, although no difference was identified for the prevalence of hypertension or blood pressure measures among groups (data not shown).
Individuals with SO were less likely to meet physical activity guidelines (see Table 3). For subjects who met the guidelines, 95.8% were in the non-SO group.
Discussion
This is the first study to define SO in an adult cohort with class II/III obesity using a clinically relevant outcome of interest, ADL difficulty. Maintaining ADL functioning is essential for independence and optimal quality of life (44, 45). In our definition (ASM/weight x 100, %), reduced ADLs in an adult cohort with class II/III obesity were predicted by the amount of body weight (load) in proportion to their lean mass (capacity—load bearing) (12). In other words, this concept accounts for the magnitude of body weight in relation to lean mass that would lead to functional impairments. This concept is in accordance with other studies that reported an association between lower relative lean mass and greater difficulty with ADLs and function (18, 21, 46). We propose that the relation between body composition and physical function may provide a useful marker for the clinical diagnosis of SO in adults with class II/III obesity defined as measure of ASM in relation to total body weight. Using this definition, we reported that, although present across the age spectrum, SO was associated with older age, higher waist circumference, higher triglycerides, use of anti-hypertensive medications, and inactivity.
Defining SO is challenging (12). As explored in our previous publication (8), definitions accounting for measures of body mass or FM may better identify these individuals. Here, lower ASM/weight x 100 (%) was correlated with a higher number of items for difficulty with ADLs. Per our previous study (8), Levine and Crimmins’ (21) cutpoints identified 23.2% women and 58.5% men with SO in the present cohort. Due to the uniqueness of our cohort (non-elderly adults with class II/III obesity) and the interest to tie the definition to a clinical outcome, cohort-and sex-specific cutpoints were explored using the same body composition variable (ASM/weight x 100, %). The resulting cutpoints (women <19.35%, men <24.33%) were very similar for women and slightly lower for men, compared to the Levine and Crimmins (21) values, leading to an overall higher prevalence of SO (25% compared to 10% for the former study; sex-specific prevalence not reported).
SO was identified in more men than women (41.2% compared to 22.3%). Sex differences in the prevalence of SO are variable, with some studies reporting a higher prevalence for women (38, 46), others for men (15, 26, 47), or mixed results depending on the definition applied (37, 48). Interestingly, we showed a consistently higher prevalence in men, among 18 definitions (8). Differences in body composition between the sexes may be associated with sex hormones including estrogen and testosterone, influencing both fat and lean tissues (49-52).
There were not as many differences as expected when comparing biochemical variables between sarcopenic and nonsarcopenic obese groups. Although hypoalbuminemia was correlated with SO, our n = 2 precludes any meaningful conclusion. Elevated CRP, a biomarker for systemic inflammation and associated with obesity, sarcopenia, metabolic syndrome, and cardiovascular disease, was identified in ∼30% of the study cohort, but the prevalence was not different between groups. Our results are in agreement with those of Levine and Crimmins (21), who found no difference in CRP values between both sets of subjects in their obese groups (sarcopenic and nonsarcopenic). Although studies of older adults report an association between SO with metabolic syndrome and insulin resistance (21, 22), we were unable to observe such differences. Unfortunately, fasting insulin was not available for our cohort.
Although the prevalence of metabolic syndrome was similar between groups, a greater prevalence of reporting 2 of the 5 criteria for metabolic syndrome (high triglycerides and high waist circumference) was reported among the SO group. The hypertriglyceridemic waist phenotype has been used to identify those with increased visceral adiposity, which in turn is associated with increased cardiometabolic risk and mortality (53, 54). In addition to cardiometabolic risk, the difference in serum triglyceride concentrations between subjects with SO and those without may serve as a biomarker for other abnormalities.
Regarding physical activity, 80% of subjects did not meet activity guidelines, which is similar to nationally reported levels (78%) (55). No difference by sex or BMI categories between those who met or did not meet the guidelines was observed, but a greater proportion of individuals with SO did not meet the activity guidelines. It is possible that a proportion of SO patients were unable to perform physical activity, and hence did not meet the guidelines.
Our study has several limitations including the tool to assess ADLs. Although each item addressed a category of ADL difficulty, such as transfers, the question as stated often included >1 option (i.e., car, bed, bathtub, and toilet). It was not possible to evaluate, for example, if patients only had difficulty with transferring in and out of their car or if they experienced difficulty with transfers for all the examples provided. Additionally, reasons for the difficulties or the level of difficulty experienced cannot be ascertained. Nonetheless, this tool was able to differentiate patients by sarcopenia status, highlighting the potential use of this or similar tools in research and clinical settings. Unfortunately, measures of muscle strength, quality, or function were not available. Further assessment of factors influencing difficulty in participation and performance of ADLs, including physical function, cognition, and strength, is needed (45). Additional limitations of our study include a small sample size of a convenience sample, and the cross-sectional design. Our data-driven approach to define this abnormal body composition type should not be seen as a limitation, as similar approaches have been previously used (18, 24, 26, 41, 56). Nonetheless, the definition hereby used requires external validation in different patient cohorts.
Our study used state-of-the-art body composition data (DXA) contributing to the limited research on subjects with BMI >40 who may be excluded from studies due to equipment weight capacity limits (57). Additionally, this study highlights the impact of SO in younger and middle-aged adults, who, contrary to expectations, may also experience SO and its clinical consequences. Further research is needed to validate cutpoints for SO in relation to clinical outcomes, such as the items of self-reported difficulty with ADLs, for adults with class II/III obesity. Unfortunately, we were unable to evaluate whether the performance and discriminative capacity of SO are body size dependent—i.e., valid for those with obesity class I. Likewise, our definition of SO does not account for age (although <6% of participants were ≥65 y old), which is an important determinant of difficulties with ADLs and would probably lead to lower cutpoints for younger individuals. Notably, age was not different by sarcopenia status.
Supplementary Material
Acknowledgments
We thank the Edmonton Adult Bariatric Specialty Clinic and Alberta Health Services for their support and funding from the Campus Alberta Innovates Program. The authors’ contributions were as follows—CMP: designed research; CAJS, MF, AMS, TT, MS, and CMP: conducted research; PAH, AMS, MBS, YLMM, and VEB: provided essential materials; CAJS, SSG, MF, AMS, TT, MS, MBS, YLMM, and CMP: analyzed data or performed statistical analysis; CAJS and CMP: had primary responsibility for the final content; and all authors: wrote the manuscript, and read and approved the final manuscript.
Notes
Supported by the Campus Alberta Innovation Program.
Author disclosures: CAJS, SSG, MF, AMS, TT, MS, VEB, RSP, PAH, MBS, YLMM, and CMP, no conflicts of interest. A version of this manuscript was based upon the first author's Master's thesis.
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used:
- ADL
activity of daily living
- ASM
appendicular skeletal mass
- CRP
C-reactive protein
- DXA
dual-energy X-ray absorptiometry
- eGFR
estimated glomular filtration rate
- FFM
fat-free mass
- FM
fat mass
- LST
lean soft tissue
- ROC
receiver operating characteristic
- SO
sarcopenic obesity
References
- 1. Pasco JA, Gould H, Brennan SL, Nicholson GC, Kotowicz MA. Musculoskeletal deterioration in men accompanies increases in body fat. Obesity (Silver Spring) 2014;22(3):863–7. [DOI] [PubMed] [Google Scholar]
- 2. Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care 2008;11(6):693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Santarpia L, Contaldo F, Pasanisi F. Body composition changes after weight-loss interventions for overweight and obesity. Clin Nutr 2013;32(2):157–61. [DOI] [PubMed] [Google Scholar]
- 4. Dixon JB, Strauss BJ, Laurie C, O'Brien PE. Changes in body composition with weight loss: obese subjects randomized to surgical and medical programs. Obesity (Silver Spring) 2007;15(5):1187–98. [DOI] [PubMed] [Google Scholar]
- 5. Pownall HJ, Bray GA, Wagenknecht LE, Walkup MP, Heshka S, Hubbard VS, Hill J, Kahn SE, Nathan DM, Schwartz AV, et al. Changes in body composition over 8 years in a randomized trial of a lifestyle intervention: the Look AHEAD study. Obesity (Silver Spring) 2015;23(3):565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beavers KM, Lyles MF, Davis CC, Wang X, Beavers DP, Nicklas BJ. Is lost lean mass from intentional weight loss recovered during weight regain in postmenopausal women? Am J Clin Nutr 2011;94(3):767–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res 2004;12(6):887–8. [DOI] [PubMed] [Google Scholar]
- 8. Johnson Stoklossa CA, Sharma AM, Forhan M, Siervo M, Padwal RS, Prado CM. Prevalence of sarcopenic obesity in adults with class II/III obesity using different diagnostic criteria. J Nutr Metab 2017;11. doi: 10.1155/2017/7307618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alley DE, Shardell MD, Peters KW, McLean RR, Dam T-TL, Kenny AM, Fragala MS, Harris TB, Kiel DP, Guralnik JM, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci 2014;69(5):559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cawthon PM, Peters KW, Shardell MD, McLean RR, Dam TT, Kenny AM, Fragala MS, Harris TB, Kiel DP, Guralnik JM, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci 2014;69(5):567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008;9(7):629–35. [DOI] [PubMed] [Google Scholar]
- 12. Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr 2012;31(5):583–601. [DOI] [PubMed] [Google Scholar]
- 13. Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res 2009;15(22):6973–9. [DOI] [PubMed] [Google Scholar]
- 14. Pereira RA, Cordeiro AC, Avesani CM, Carrero JJ, Lindholm B, Amparo FC, Amodeo C, Cuppari L, Kamimura MA. Sarcopenia in chronic kidney disease on conservative therapy: prevalence and association with mortality. Nephrol Dial Transplant 2015;30(10):1718–25. [DOI] [PubMed] [Google Scholar]
- 15. Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res 2004;12(12):1995–2004. [DOI] [PubMed] [Google Scholar]
- 16. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147(8):755–63. [DOI] [PubMed] [Google Scholar]
- 17. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014;69(5):547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatri Soc 2002;50(5):889–96. [DOI] [PubMed] [Google Scholar]
- 19. Atkins JL, Whincup PH, Morris RW, Lennon LT, Papacosta O, Wannamethee SG. Sarcopenic obesity and risk of cardiovascular disease and mortality: a population-based cohort study of older men. J Am Geriatr Soc 2014;62(2):253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr 2014;68(9):1001–7. [DOI] [PubMed] [Google Scholar]
- 21. Levine M, Crimmins E. The impact of insulin resistance and inflammation on the association between sarcopenic obesity and physical functioning. Obesity (Silver Spring) 2012;20:2101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ, Kim KW, Lim JY, Park KS, Jang HC. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care 2010;33(7):1652–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poggiogalle E, Lubrano C, Sergi G, Coin A, Gnessi L, Mariani S, Lenzi A, Donini LM. Sarcopenic obesity and metabolic syndrome in adult Caucasian subjects. J Nutr Health Aging 2016;20(9):958–63. [DOI] [PubMed] [Google Scholar]
- 24. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39(4):412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Lorenzo A, Soldati L, Sarlo F, Calvani M, Di Lorenzo N, Di Renzo L. New obesity classification criteria as a tool for bariatric surgery indication. World J Gastroenterol 2016;22(2):681–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prado CM, Siervo M, Mire E, Heymsfield SB, Stephan BC, Broyles S, Smith SR, Wells JC, Katzmarzyk PT. A population-based approach to define body-composition phenotypes. Am J Clin Nutr 2014;99(6):1369–77. [DOI] [PubMed] [Google Scholar]
- 27. Cherin P, Voronska E, Fraoucene N, de Jaeger C. Prevalence of sarcopenia among healthy ambulatory subjects: the sarcopenia begins from 45 years. Aging Clin Exp Res 2014;26(2):137–46. [DOI] [PubMed] [Google Scholar]
- 28. Table 051-0056—Estimates of population by census metropolitan areas, sex and age group for July 1, based on the Standard Geographical Classification (SGC) 2011 [Internet] [cited 2016 Jan 12]. Available from: http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/demo05a-eng.htm.
- 29. World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation Geneva: World Health Organization, 2000. [PubMed] [Google Scholar]
- 30. Heymsfield SB, Gallagher D, Mayer L, Beetsch J, Pietrobelli A. Scaling of human body composition to stature: new insights into body mass index. Am J Clin Nutr 2007;86(1):82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005;112(17):2735–52. [DOI] [PubMed] [Google Scholar]
- 32. Agborsangaya CB, Majumdar SR, Sharma AM, Gregg EW, Padwal RS. Multimorbidity in a prospective cohort: prevalence and associations with weight loss and health status in severely obese patients. Obesity (Silver Spring) 2015;23(3):707–12. [DOI] [PubMed] [Google Scholar]
- 33. Roberts KC, Rao DP, Bennett TL, Loukine L, Jayaraman GC. Prevalence and patterns of chronic disease multimorbidity and associated determinants in Canada. Health Promot Chronic Dis Prev Can 2015;35(6):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Padwal RS, Majumdar SR, Klarenbach S, Birch DW, Karmali S, McCargar L, Fassbender K, Sharma AM. The Alberta population-based prospective evaluation of the quality of life outcomes and economic impact of bariatric surgery (APPLES) study: background, design and rationale. BMC Health Serv Res 2010;10:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Canadian Society for Exercise Physiology. Canada's Physical Activity Guidelines for Adults 18–64 years. Ottawa, ON: Canadian Society for Exercise Physiology; 2011. [Google Scholar]
- 36. Townsend EA, Polatajko HJ. Enabling occupation II: advancing an occupational therapy vision for health, well-being & justice through occupation. Ottawa, ON: CAOT Publications ACE; 2007. [Google Scholar]
- 37. Batsis JA, Mackenzie TA, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity, and functional impairments in older adults: National Health and Nutrition Examination Surveys 1999–2004. Nutr Res 2015;35(12):1031–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc 2003;51(11):1602–9. [DOI] [PubMed] [Google Scholar]
- 39. Siervo M, Prado CM, Mire E, Broyles S, Wells JC, Heymsfield S, Katzmarzyk PT. Body composition indices of a load-capacity model: gender- and BMI-specific reference curves. Public Health Nutr 2015;18(7):1245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol 2004;159(4):413–21. [DOI] [PubMed] [Google Scholar]
- 41. McLean RR, Shardell MD, Alley DE, Cawthon PM, Fragala MS, Harris TB, Kenny AM, Peters KW, Ferrucci L, Guralnik JM, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the Foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci 2014;69(5):576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tyrovolas S, Koyanagi A, Olaya B, Ayuso-Mateos JL, Miret M, Chatterji S, Tobiasz-Adamczyk B, Koskinen S, Leonardi M, Haro JM. The role of muscle mass and body fat on disability among older adults: a cross-national analysis. Exp Gerontol 2015;69:27–35. [DOI] [PubMed] [Google Scholar]
- 43. Cruz-Jentoft AJ, Landi F, Schneider SM, Zúñiga C, Arai H, Boirie Y, Chen LK, Fielding RA, Martin FC, Michel JP, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014;43(6):748–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang WW, Perera S, VanSwearingen J, Studenski S. Performance measures predict the onset of basic ADL difficulty in community-dwelling older adults. J Am Geriatr Soc 2010;58(5):844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Forhan M, Gill SV. Obesity, functional mobility and quality of life. Best Pract Res Clin Endocrinol Metab 2013;27(2):129–37. [DOI] [PubMed] [Google Scholar]
- 46. Oh C, Jho S, No JK, Kim HS. Body composition changes were related to nutrient intakes in elderly men but elderly women had a higher prevalence of sarcopenic obesity in a population of Korean adults. Nutr Res 2015;35(1):1–6. [DOI] [PubMed] [Google Scholar]
- 47. Bouchard D, Dionne I, Brochu M. Sarcopenic/obesity and physical capacity in older men and women: data from the Nutrition as a Determinant of Successful Aging (NuAge)—the Quebec Longitudinal Study. Obesity (Silver Spring) 2009;17:2082–8. [DOI] [PubMed] [Google Scholar]
- 48. Kim TN, Yang SJ, Yoo HJ, Lim KI, Kang HJ, Song W, Seo JA, Kim SG, Kim NH, Baik SH, et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean sarcopenic obesity study. Int J Obes (Lond) 2009;33(8):885–92. [DOI] [PubMed] [Google Scholar]
- 49. Maggio M, Lauretani F, Ceda GP. Sex hormones and sarcopenia in older persons. Curr Opin Clin Nutr 2013;16(1):3–13. [DOI] [PubMed] [Google Scholar]
- 50. Herbst KL, Bhasin S. Testosterone action on skeletal muscle. Curr Opin Clin Nutr 2004;7(3):271–7. [DOI] [PubMed] [Google Scholar]
- 51. Gallagher D, Visser M, De Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN, Harris T, Heymsfield SB. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol (1985) 1997;83(1):229–39. [DOI] [PubMed] [Google Scholar]
- 52. Gallagher D, Heymsfield SB. Muscle distribution: variations with body weight, gender, and age. Appl Radiat Isot 1998;49(5–6):733–4. [DOI] [PubMed] [Google Scholar]
- 53. Irving BA, Davis CK, Brock DW, Weltman JY, Swift D, Barrett EJ, Gaesser GA, Weltman A. The metabolic syndrome, hypertriglyceridemic waist, and cardiometabolic risk factor profile in obese women. Obes Metab 2007;3(2):50–7. [PMC free article] [PubMed] [Google Scholar]
- 54. Arsenault BJ, Lemieux I, Després J-P, Wareham NJ, Kastelein JJP, Khaw K-T, Boekholdt SM. The hypertriglyceridemic-waist phenotype and the risk of coronary artery disease: results from the EPIC-Norfolk prospective population study. Can Med Assoc J 2010;182(13):1427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Directly measured physical activity of Canadian adults 2012–2013 [Internet] [cited 2016 Jan 12]. Available from: http://www.statcan.gc.ca/pub/82-625-x/2015001/article/14135-eng.htm.
- 56. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12(5):489–95. [DOI] [PubMed] [Google Scholar]
- 57. Johnson Stoklossa CA, Forhan M, Padwal RS, Gonzalez MC, Prado CM. Practical considerations for body composition assessment of adults with class II/III obesity using bioelectrical impedance analysis or dual-energy X-ray absorptiometry. Curr Obes Rep 2016;5(4):389–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.