Abstract
A water-soluble tetra-S-glycosylated porphyrin (P-Glu4) is absorbed by MDA-MB-231 human breast cancer cells whereupon irradiation with visible light causes necrosis or apoptosis depending on the concentration of the porphyrin and the power of the light. With the same amount of light irradiation power (9.4 W m−2), at 10–20 μM concentrations necrosis is predominantly observed, while at <10 μM concentrations, apoptosis is the principal cause of cell death. Of the various possible pathways for the induction of apoptosis, experiments demonstrate that calcium is released from the endoplasmic reticulum, cytochrome c is liberated from the mitochondria to the cytosol, pro-caspase-3 is activated, poly-(ADP-ribose) polymerase is cleaved, and the chromatin is condensed subsequent to photodynamic treatment of these cells. Confocal microscopy indicates a substantial portion of the P-Glu4 is located in the endoplasmic reticulum at <10 μM. These data indicate that the photodynamic treatment of MDA-MB-231 cells using low concentrations of the P-Glu4 porphyrin and low light induces apoptosis mostly initiated from stress produced to the endoplasmic reticulum.
Introduction
Apoptosis, programmed physiological cell death, is an essential and well-regulated cell function that allows for the ordered removal of superfluous, aged, or damaged cells.1,2 Several million cells in the human body undergo apoptosis every second. Insufficient apoptosis may prompt oncogenesis by allowing cell accumulation, while excessive apoptosis may be the basis of degenerative diseases such as Huntington’s and Alzheimer’s.2 Apoptosis is manifested by both biochemical and morphological changes including: cell shrinkage, chromatin condensation, DNA fragmentation, plasma membrane blebbing and vesiculating, and phosphatidylserine lipid redistribution to the cell surface. In contrast, the pathology of necrosis is characterized by significant degradation of membrane integrity and leakage of cell contents.
Photodynamic therapy (PDT) is an approved treatment for a variety of cancers that can be exposed to a high flux of light— either white or a band centered at a particular wavelength. Since PDT has been extensively reviewed,3–5 it is described only in broad terms here. The concept is that the patient is dosed with a photosensitizing dye and the specificity arises largely from the selective irradiation of target tissue with light in the visible region of the electromagnetic spectrum. Upon irradiation the dye becomes reactive and/or toxic, or it photosensitizes the formation of reactive and/or toxic species in vivo. The chromophores used in current technologies, and those in the immediate pipeline, are generally not selective for cancer tissue beyond what would be expected from the greater metabolism. These agents are generally believed to photosensitize the formation of singlet oxygen. Singlet oxygen then reacts with a variety of cellular components including, aromatic amino acids, double bonds in lipids, a variety of redox enzymes and cofactors, both the bases and the phosphate backbones of DNA and RNA. The mechanism(s) of action of PDT agents arise from both the photophysical properties of the chromophore and the specific localization of the porphyrin in the cell or tissue. The uptake and localization of the photodynamic agent in the cell depends exquisitely on the exact chemical structure of the dye and any covalently bound auxiliary motifs, and this topic has been reviewed.4,6,7
We report herein studies on the initiation of apoptosis using low concentrations of a non-hydrolyzable tetraglycosylated porphyrin (P-Glu4, Scheme 1) with low light irradiation. Phototoxicity studies reveals that as the concentration decreases from 20 μM to 10 μM of P-Glu4 the percentage of immediately necrotic cells decrease and the percentage of cells that exhibit a delayed response increase (Fig. 1). A variety of assays indicate the delayed response is mostly due to apoptosis (see below). Studies at 5 μM show less necrosis and the percentage of apoptotic cells is less, and at 1 μM neither necrosis nor apoptosis are indicated but the cells are qualitatively less aggressive by a cell migration assay.8 As illustrated below, the specific concentration and localization of the photodynamic chromophore, as well as the light intensity, dictates the mode of cell death.
Scheme 1.
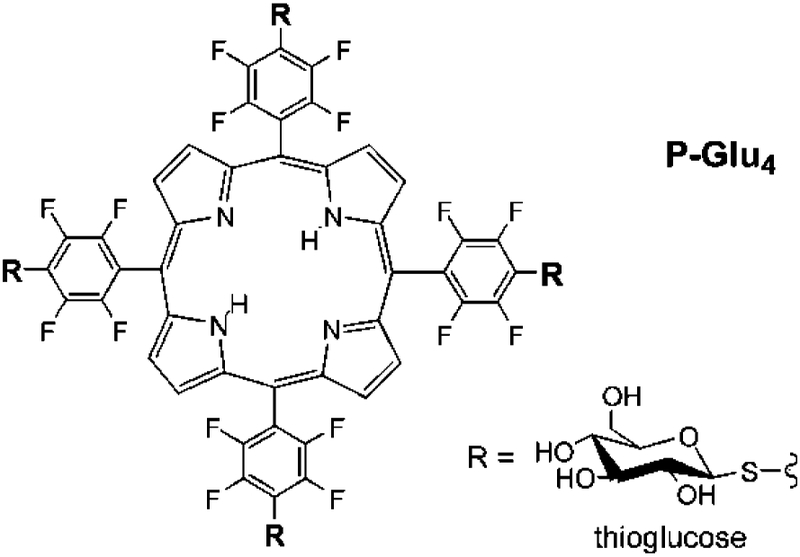
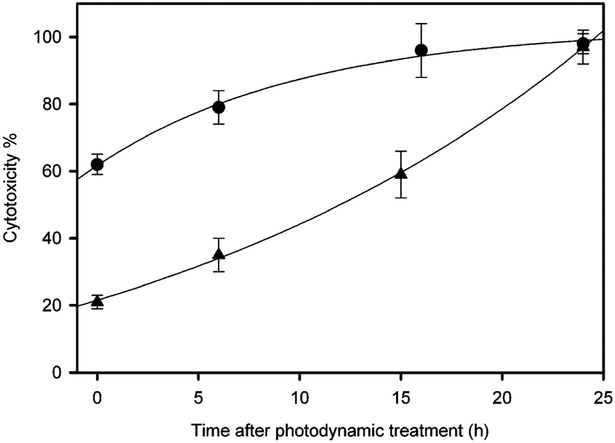
Fig. 1.
Photocytotoxic effects on human breast cancer MDA-MB-231 cells. Cells were treated with (▲) 10 μM P-Glu4 and (●) 20 μM P-Glu4, for 24 h, rinsed by exchanging the growth medium, and irradiated under a white 13 W fluorescent light (0.94 mW cm−2 for 20 min; 11.28 kJ m−2). The non-viable cells were counted with hemacytometer after staining with 0.4% w/v trypan blue at various lengths of time after photodynamic treatment.
Of the vast number of reports on apoptosis,2 there are a few on the induction of apoptosis by photodynamic treatment.6–19 Cellular responses to photodynamic treatment depend on the cell type, the specific photosensitizer, the dosage of both photosensitizer and light, and other factors. The specific subcellular localizations of the photosensitizer dictates the sites of primary photo damage, thus the potential apoptosis initiation point(s). To date, the photosensitizers used in PDT are found to localize mostly in the mitochondria, lysosomes, endoplasmic reticulum (ER), and cell membranes.19 Concerning apoptosis, after its activation, the mitochondrial potential is lost, which is followed by the release of cytochrome c to the cytosol. Cytochrome c, upon binding to apoptotic protease activating factor-1 (Apaf-1) and pro-caspase-9 (cystein aspartate-specific protease), activates the caspases. The activation of caspase-9 then triggers a cascade of proteases. The induction of the caspases also triggers a variety of other responses in the cell via signaling pathways, such as chromatin condensation, DNA cleavage, and the cleavage of repair enzymes such as poly-(ADP ribose) polymerase (PARP). The detection of these activities is generally considered as biochemical markers of apoptosis. Release of cytochrome c from between the inner and outer membranes of the mitochondria has been shown to accompany apoptosis in every circumstance with every cell line studied to date,20 though the mechanism of the release of this enzyme remains a topic of interest.
The entry and partition of photosensitizers in cells is a complex issue that depends on nonspecific properties such as hydrophobicity and the specific substituents. It may be that many compounds enter into cells via more than one pathway and partition into several cellular components with a time-dependence. Some photosensitizers preferentially bind the plasma membrane, where a number of signaling pathways including apoptosis may be induced, though the mechanisms remain controversial.21 Apoptosis is rapidly induced at the plasma membrane level via activation of “death-inducing signaling complexes” (DISCs) that involve cell surface receptors such as Fas and tumor necrosis factor acceptor (TNFR).21
Research on porphyrins appended with sugar moieties has been of great interest in the last decade.22,23 Glycosylated porphyrins can have greater water solubility than most naturally occurring and synthetic porphyrins. Amphipathic solubility can not only increase the efficacy of drug delivery but also assist the drug elimination from the organism after treatment. The proper amphipathicity of neutral saccharide conjugated porphyrins enables them to permeate better into both lipophilic and hydrophilic biological structures. Furthermore, they can have specific interactions with saccharide transporter and other proteins on cell membranes, and thus exhibit specific targeting of cancer cells.23
Results
Since our previous report, we have found that concentrations of P-Glu4 over ca. 10 μM form nanoscaled aggregates so may enter and partition into the cells differently than the non-aggregated compound.8 The entry and partition of this compound depends both on concentration and incubation time and will be the subject of a future report.
Our investigations on the mechanism of apoptosis induction by light and P-Glu4 in MDA-MB-231 breast cancer cells is evaluated by several assays. Confocal microscopy indicates that when treated with <10 μM, the porphyrin binds predominantly to the endoplasmic reticulum. Although the mechanism is partially unclear, it is known that stresses to the ER can activate apoptosis and that the release of calcium from the ER to the cytoplasm is one of the steps involved.24 Several observations are consistent with our hypothesis that under low light and low concentrations, P-Glu4 induces apoptosis primarily by ER-stress in MDA-MB-231 breast cancer cells. This evidence shows that calcium is released from the endoplasmic reticulum, which subsequently is followed by cytochrome c release from the mitochondria. After the liberation of cytochrome c from the mitochondria membrane, pro-caspase-3 is activated, PARP cleavage is observed, and then the chromatin condenses.
Photocytotoxicity
P-Glu4 with light is a potent mediator of cell death for MDA-MB-231 cells in culture.8 Since cell death can be caused either directly, necrosis, or indirectly by initiating apoptosis,1 several assays were performed to delineate the extent of each. Cells were incubated for 24 h with 10 μM P-Glu4, the growth media was then exchanged to remove unbound porphyrin, and the culture irradiated with 0.94 mW cm−2 white light for 20 min (11.28 kJ m−2). About 20% of the cells were non-viable immediately after photodynamic treatment (within the few minutes it takes to place them under a light contrast microscope, (Fig. 1). Both cell morphology and trypan blue staining were used as an assay with the caveat that there may be some unstained cells that are non-viable or vice versa. Yet, these assays also reveal that the percentage of non-viable cells continues to increase with time until nearly 100% are non-viable 24 h post irradiation. The continued demise of these cells implies that under these conditions a secondary process such as apoptosis is causing cell death. When the porphyrin concentration was doubled to 20 μM, ~60% of the cells observed to be non-viable just after irradiation with the same energy; and similarly, the percentage of cytotoxic cells reached 100% within 24 h of photodynamic treatment. Control experiments show there is no significant effect without both light and the P-Glu4. With 10 μM P-Glu4 our data suggests that around 20% percent of the cell are non-viable just after radiation. The process of apoptosis takes longer time than necrosis, and the mechanisms of apoptosis induction is the focus of the present research.
Confocal fluorescence microscopy
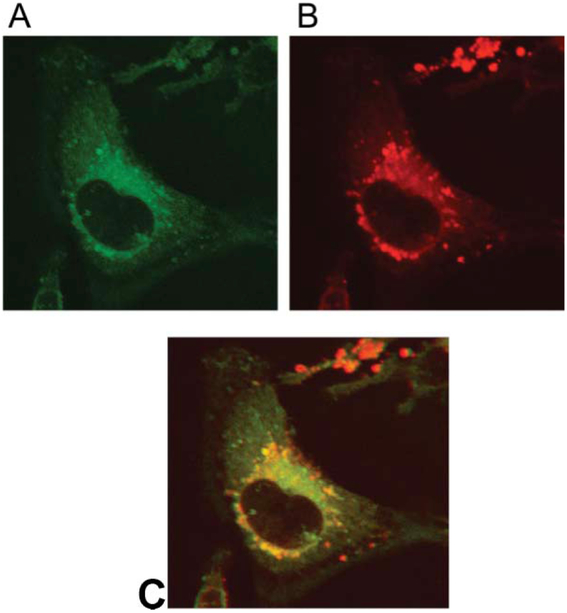
ER-Tracker Green® (Molecular Probes) is a dye specific to endoplasmic reticulum and luminesces green when exited with blue light, and the tetraarylporphyrin core of P-Glu4 fluoresces in the red region. The combination of the two dyes allows an evaluation of the location of the glycosylated porphyrin in MDA-MB-231 cells. Confocal fluorescence images of cells treated with both 10 μM P-Glu4 and with ER-tracker Green exhibit both the porphyrin fluorescence (red) and the ER Green fluorescence (Fig. 2). These experiments indicate that after exposure at this concentration ca. 90% of P-Glu4 in the cell is localized at the ER. This supports our hypothesis that photodynamic treatment of cells with P-Glu4 can induce apoptosis by stress to the ER. Similar experiments using Mito-Tracker®R (Molecular Probes) reveal only a small correlation with the fluorescence of P-Glu4 in the mitochondria.
Fig. 2.
P-Glu4 is mainly localized in endoplasmic reticulum in MDA-MB-231 cells. Cells were incubated with 10 μM P-Glu4 for 24 h (red), rinsed, treated with ER Tracker Green, rinsed and fixed with 4% paraformaldehyde solution. Fluorescence of A: ER-Tracker Green, and B: P-Glu4. C: the overlapped image of A and B. Confocal fluorescence images were taken under identical conditions, magnification is 60×.
Release of calcium from the endoplasmic reticulum
To confirm the localization of the P-Glu4 and that free calcium is released from the ER upon photodynamic treatment, the calcium binding fluorophore Fluor-4 was used as an assay for monitoring free calcium. The fluorescence of Fluor-4 significantly increases when bound to free calcium. Two different experiments were performed to measure the release of calcium from the ER to the cytoplasm. First, the MDA-MB-231 cells were treated with 10 μM of P-Glu4 for 24 h, rinsed, incubated with Fluor-4 as described in the method section, and irradiated for 5 min or 10 min with white light from a 13 W fluorescent bulb. Controls examined show no calcium release without both P-Glu4 and light (see electronic supporting information, ESI†). In the second experiment, MDA-MB-231 cells were treated with 10 μM of the P-Glu4 conjugate for 24 h, incubated for 30 min with 1 μM of Fluor 4, rinsed three times, and incubated again for another 30 min with normal incubation conditions. Fluorescence images were then taken every minute while irradiating with low intensity white light to observe calcium release in the cells in real time; e.g. before and after the cells were irradiated with white light for 5 min (Fig. 3). Significant free Ca2+ is observed after photo irradiation of the cells in the presence of P-Glu4.
Fig. 3.
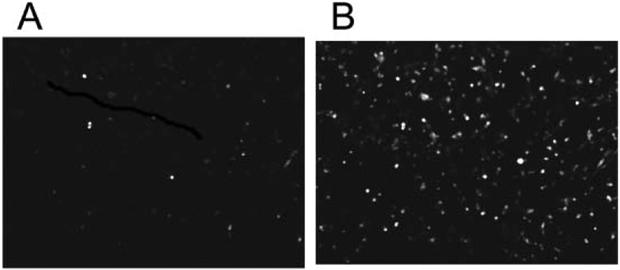
Release of calcium from the endoplasmic reticulum to the cytosol can be monitored in real time. Cells were incubated with 10 μM P-Glu4 and the Ca2+ sensor Fluor-4, rinsed, placed under the microscope and images obtained every minute without moving the plate from the microscope, while the cells were irradiated with 0.84 mW cm−2 (2.52 kJ m−2) white light. A: before irradiation, and B: after 10 min irradiation. Images are 20× and taken under identical conditions.
Release of cytochrome c from the mitochondria
If apoptosis proceeds through a mitochondria pathway, cytochrome c can be released to the cytosol, which in turn triggers caspase cascades and ultimately results in apoptosis. Though the mechanism of release remains under investigation, it has been demonstrated that two cytosolic proteins collaborate with cytochrome c to induce proteolytic processing and activation of caspase-3 in vitro.11,25,26 To assay the mitochondrial involvement in apoptosis, the MDA-MB-231 cells were treated with 4 μM of the glucose-porphyrin conjugate for 24 h, rinsed, and irradiated for 30 min or 60 min durations with white light from a 13 W fluorescent bulb. Five hours later a mitochondria/cytosol fractionation kit (BioVision) was used to separate the cytosol from the mitochondria. Western blots of the cytosolic and mitochondrial fractions were then used to detect cytochrome c. These results show that mild photodynamic conditions with P-Glu4 cause cytochrome c release from the mitochondria to the cytosol (Fig. 4).
Fig. 4.
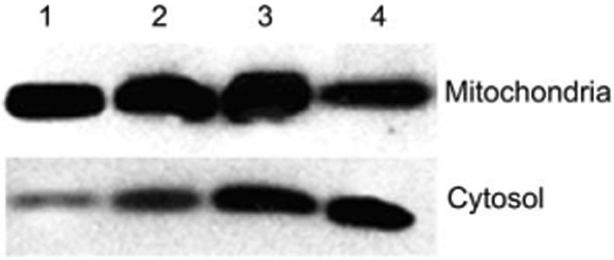
Cytochrome c release from mitochondria to cytosol. 0 or 4 μM P-Glu4 was incubated with human breast cancer MDA-MB-231 cells for 24 h and after rinsing, irradiated with 13 W fluorescent white bulb for 30 or 60 min at 0.96 mW cm−2 (17.28 or 34.56 kJ m−2). Five hours later a mitochondria/cytosol fractionation kit was used to separate mitochondria and cytosol. The fractions were subjected to western blot to detect cytochrome c. Lane 1: control (no porphyrin, no light). Lane 2: control (4 μM porphyrin, no light). Lane 3: 4 μM porphyrin, 30 min irradiation. Lane 4: 4 μM porphyrin, 60 min irradiation.
Pro-caspase-3 cleavage/activation
Caspase-3 is activated during most apoptotic processes and is believed to be the main executioner caspase.25 Caspase-3 activation is essential for DNA fragmentation as well as chromatin condensation and plasma membrane blebbing.26 Caspase-3 activation can be stimulated by cytochrome c release from the mitochondria via caspase-9/Apaf-1 or by other pathways. For these experiments the cells were treated with 0, 4, or 10 μM porphyrin conjugate, rinsed by exchanging the media, and irradiated for 20 or 40 min with white light. This experiment shows that pro-caspase-3 is indeed cleaved to yield the active caspase after mild photodynamic treatment of MDA-MB-231 cells in the presence of P-Glu4 (Fig. 5).
Fig. 5.
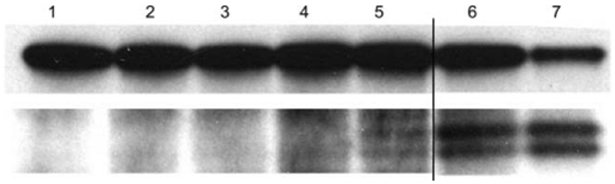
Detection of pro-caspase-3 cleavage. 0, 4 or 10 μM P-Glu4 was incubated with human breast cancer MDA-MB-231 cells for 24 h and irradiated with 13 W fluorescent white bulb for 20 or 40 min at 0.96 mW cm−22 (11.52 or 23.04 kJ m−2). Seven hours later, cells were collected and lysed. The supernatant of the lysate was applied to a western blot to detect pro-caspase-3. Lane 1: control (no porphyrin, no irradiation). Lane 2: control (no porphyrin, 20 min irradiation). Lane 3: control (4 μM porphyrin, no irradiation). Lane 4: 4 μM porphyrin, 20 min irradiation. Lane 5: 4 μM porphyrin, 40 min irradiation. Lane 6: 10 μM porphyrin, 20 min irradiation. Lane 7: 10 μM porphyrin, 40 min irradiation.
PARP Cleavage
Given the evidence of subcellular localization of P-Glu4 at the endoplasmic reticulum, cytochrome c release, pro-caspase-3 activation, and chromatin condensation (see below), it not surprising to find that later stages of apoptosis, such as poly-ADP-ribose-polymerase (PARP) cleavage, are also observed27 (ESI†). PARP is one of the best-examined targets of activated caspases and is a common indicator of the action of caspase-3 in apoptosis.28
DAPI staining
To examine the morphological changes in the MDA-MB-231 chromatin after photodynamic treatment in the presence of P-Glu4, DAPI (4′,6-diamino-2-phenylindole) staining experiments were used. DAPI binds to dA-T rich regions and is widely used as a DNA probe because of its large increase in fluorescence quantum yield upon DNA binding.29 DAPI fluorescence images of the photodynamicly treated MDA-MB-231 cells reveal that the nuclei are condensed and split compared to a parallel control experiment (ESI†). The observed condensed and split chromatin morphology is typical of apoptotic cells and further indicates that photodynamic treatment using low concentrations of the glycosylated porphyrin and low light irradiation is capable of inducing apoptosis.
Discussion
We previously reported that a tetraglucose-porphyrin conjugate, 5,10,15,20-tetrakis-(4-1 ′-thio-glucosyl-2,3,5,6-tetrafluoropheny1)- porphyrin, (P-Glu4, (Scheme 1) can be made in >90% yield in two steps8,22 from commercially available meso-tetrakis(pentafluoro-phenyl)-porphyrin (TPPF20) and a thioglucose derivative.30–32 The porphyrin-glucose bond of P-Glu4 does not hydrolyze under physiological conditions. Human breast cancer MDA-MB-231 cells preferentially absorb tetraaryl porphyrins with four glucose moieties 2–3 fold greater than the corresponding tetragalactose derivatives. We also find that P-Glu4 also is taken up by this cell line 2–3 times better than several well-studied hydrophilic porphyrin derivatives such as the tetracationic tetrakis(4-N-methylpyridinium)porphyrin. Additionally, P-Glu4 is highly selective toward 3Y1v-Src transformed cells compared to normal 3Y1 cells.
Doseametric studies reveal that these saccharide-porphyrin conjugates exhibit varying photodynamic responses depending on drug concentration and irradiation energy. (1) 20 μM conjugate and greater irradiation energy (>22.56 kJ m−2) induces cell death presumably by necrosis. (2) When 10–20 μM conjugate and less irradiation energy are used, both the initial necrosis and later apoptosis are observed. (3) Using < 10 μM and the least irradiation energy (<0.75 kJ m−2), a significant reduction in cell migration is observed, which indicates a reduction in aggressiveness of the cancer cells.8
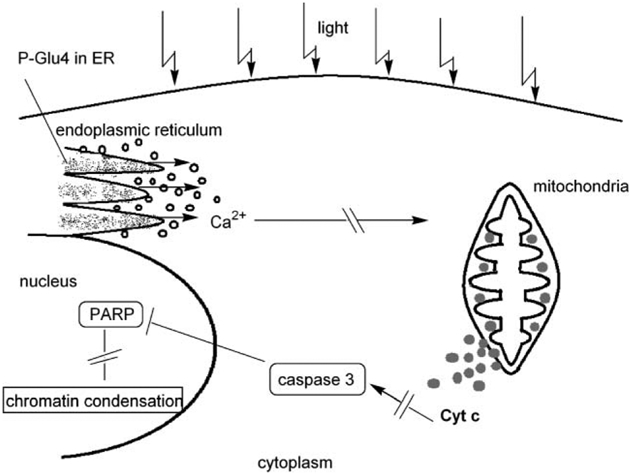
While greater concentrations of this porphyrin and greater irradiation with white light leads directly to necrosis, lower concentrations and less light lead to a delayed cell death predominantly by apoptosis. Confocal microscopy data indicate that at low P-Glu4 concentrations ca. 90% of the porphyrin absorbed by MDA-MB-231 cells is localized at the ER, and calcium is released from the ER after the cells are exposed to the light. Thus, it is reasonable to conclude that a significant fraction of the observed apoptosis is a consequence of the stress induced to the ER after photodynamic treatment. The uptake of P-Glu4 by the ER is expected because the ER is known to use sugar for glycosylation and is a large inner cell structure.33 After activation of P-Glu4 and release of calcium from the ER to the cytoplasm, cytochrome c is released from the mitochondria, caspase 3 is activated, PARP is cleaved, and chromatin condenses (Scheme 2).
Scheme 2.
There are a variety of other cellular structures and/or functions that can serve as initiation points for the cascade of events that lead to apoptosis that can be affected by the remaining ca. 10% of P-Glu4 distributed throughout the cell. These include processes originating in the nucleus and some cationic porphyrins are well known to interact and cleave with DNA under photodynamic conditions,29,34 but note that fluorescence microscopy indicates little, if any, of P-Glu4 enters the cell nucleus (ESI†). The 200 octanol/water partition coefficient for P-Glu4 between pH 7 and pH 4.75 renders the compound amphiphilic because the water soluble sugars in the 4-phenyl positions do not effectively surround the hydrophobic porphyrin core.
The enhanced activity of P-Glu4 relative to other saccharide conjugates8,23 can be attributed to several factors including the stability of the S-saccharide bonds to hydrolysis. The saccharide- porphyrin conjugates are more robust under light because of the added oxidative stability imparted by the 16 fluoro groups.35 The sugar moieties likely remain on the porphyrin even upon oxidation of the sulfur to the sulfoxide or sulfone. The reduced fluorescence intensity of P-Glu4 compared to many other meso tetraaryl porphryins is indirect evidence of a greater triplet quantum yield, which results in greater yields of singlet oxygen8 and thus of oxidative stress in treated cells. These and previous results indicate that TPPF20 may be an ideal scaffold to build a variety of porphyrin-saccharide conjugates and other biomolecular recognition motifs for diverse applications.36 Since other cancer cell types take up different sugars such as galactose,37 this approach to the formation of PDT agents is amenable to the rapid synthesis and evaluation of compounds designed to be specific to a given cancer cell type or biomolecular target.38–40
Experimental
Materials
All chemicals were purchased from Sigma-Aldrich. Dulbecco’s Modified Eagle Medium (DMEM) and antimycotic for cell culture were obtained from GibcoBRL. Bovine calf serum was obtained from HyClone. PBS (136 mM NaCl, 2.6 mM KCl, 1.4 mM KH2PO4, 4.2 mM Na2HPO4) was obtained from Invetrogen. The 13 W fluorescent bulb was from Sanco. The antibodies against PARP, cytochrome c, and pro-caspase-3 were from Cell Signaling Technology. The mitochondrial/cytosol fractionation kit was from BioVision, and ER-tracker Green and the Fluor-4 was purchased from Invitrogen. P-Glu4 was synthesized as described previously,8,22 and has a fluorescence quantum yield in cell culture medium of about 5%.
Cell culture
Cells were maintained in DMEM, 10% bovine calf serum, 1% antimycotic, at 37 °C and 5% CO2 atmosphere.8 Typically, ~2 × 105 cells mL−1 were seeded in cell culture plates and allowed to grow for 24 h. For experiments involving the porphyrin saccharide conjugate, P-Glu4 was added to the cells 24 h prior to the photodynamic experiments and biochemical assays to allow it to be taken up by the cells. The cultures were rinsed 2–3 times with fresh DMEM to remove any unbound porphyrinic compounds before proceeding to the various assays. Fluorescence microscopy indicates no unbound porphyrin remains.
Phototoxicity assays
Cell viability was quantified by trypan blue dye exclusion. After various experiments, cells were harvested with trypsin, a 0.4% w/v trypan blue solution added to the cells, and the mixture incubated at room temperature for 10 min. Cells that had taken up trypan blue were counted with a hemacytometer and considered non-viable.
Confocal microscopy
Cells were plated onto cover slips in cell culture dishes. Porphyrins dissolved in methanol were added to the cultures to a final concentration of 10 μM (methanol concentrations were <0.2%). After incubation for 24 h the cells were rinsed, treated with ER-Tracker Green (final concentration 1 μM) in growth medium, and incubated for 30 min under conditions outlined above. Cells were then washed twice with PBS and incubated with a 4% paraformaldehyde solution in growth medium for 15 min at 37 °C under cell growth conditions. Cells were then washed three times with PBS, mounted in Dako fluorescence mounting medium, and visualized using a Zeiss LSM510 laser scanning confocal microscope where images were captured. For MitoTracker green: excitation 476 nm, emission 490–510; for P-Glu4: excitation at 633 nm, emission 650–670 nm.
Inverted epifluorescence microscope
Cells were plated onto cover slips in cell culture dishes. Porphyrins dissolved in methanol were added to the cultures to a final concentration of 10 μM. After incubation for 24 h the cells were washed three times with PBS. The cells were incubated for 30 min in growth medium with Fluor-4 (final concentration 1 mM) for 30 min. Cells were then washed twice with PBS and incubated for another 30 min in normal growth medium. Imagines were taken using Nikon Eclipse TE 200 inverted epifluorescence microscope.
Western blots
Cells were treated with porphyrin for 24 h, rinsed and irradiated as described in above. After a period of time appropriate for the given experiment, cells were washed with cold PBS twice before lyses with RIPA buffer (50 mM Tris–HCl, 1% NP40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 μg mL−1 aprotinin, leupeptin, and pepstatin each, 1 mM Na3VO4, and NaF). The lysates were gently rocked at 4 °C for 25 min, centrifuged at maximum speed for 10 min, and the supernatant applied to a Western blot.8 In the assay for cytochrome c, the cytosol was further fractionated from the mitochondria with a kit designed for this purpose (purchased from Biovision) and both the cytosolic and mitochondrial fractions were examined by a Western blot. Equal amounts of protein were adjusted into gelloading buffer (50 mM Tris-HCl, pH 6.8, 100 mM dithiothreitol, 2% SDS, 0.1% bromophenol blue, 10% glycerol), and heated for 5 min at 100 °C prior to separation by SDS-polyacrylamide (8%) gel electrophoresis. After transferring to nitrocellulose membranes (Osmonics), membrane filters were blocked overnight at 4 °C with 5% non-fat dry milk in PBS. The nitrocellulose filters were washed three times for 5 min in PBS with 0.05% Tween-20 (Bio-Rad), before incubation with anti-cytochrome c, or anti-pro-caspase-3, or anti-PARP antibodies for one hour at room temperature. Antimouse IgG conjugated with horseradish peroxidase was used as a secondary antibody. The bands were visualized using an enhanced chemiluminescent detection system (Amersham).
DAPI staining
Cells were placed onto cover slips in cell culture dishes. Porphyrins dissolved in methanol were added to the cultures to a final solution of 10 μM (ca. 2 μM methanol), and 24 h later irradiated with white light from a 13 W fluorescent bulb with the energy stated in the text. The photodynamically treated cells were kept in the dark for eight hours, washed twice with PBS, and fixed with 4% paraformaldehyde solution in PBS for 20 min at room temperature.8,23 The cells were then washed with PBS 5 times, permeablized by ice-cold methanol for 2 min, and blocked by DMEM with 10% bovine calf serum for 30 min at room temperature.
Conclusions
The various causes of MDA-MB-231 cell death mediated by the glycosyl-porphyrin conjugate depend both on light energy and drug concentration; nonetheless the elimination of cancer cells, via any mechanism, is the goal. Similar studies of cell death from necrosis and apoptosis with other photosensitizers are reported.41 These results indicate that highly vasculated tissues near surfaces accessible to light irradiation that receive greater doses of both drug and light may be eliminated by necrosis, whereas areas of the tumor that absorb less drug and are further away from the light source may be eliminated by apoptosis. MDA-MB-231 cells are rendered less aggressive with yet less P-Glu4 and lower light.8 Thus there is an array of responses by this breast cancer cell line that are elicited by this saccharide-porphyrin conjugate that depend on the amount of porphyrin absorbed and the amount of light reaching the cell.
Based on our results, when low doses of PGlu4 (or under low light) are activated by light, apoptosis is initiated at the ER, and the release of Ca2+ then starts a cascade of events42 that leads to activation of caspase-3, significant cytochrome c release to the cytosol, PARP cleavage, and chromatin condensation (Scheme 2).
Supplementary Material
Acknowledgements
The authors acknowledge support from the National Institutes of Health (NIH)-SCORE program (GM60654) to C.M.D. and D.A.F.; the PSC-CUNY fund and National Science Foundation (CHE-0554703) to C.M.D.; and the National Cancer Institute (CA46677) to D.A.F. Support for infrastructure and instrumentation in the sciences at Hunter College comes from the National Science Foundation, National Institutes of Health, including the RCMI program (RR-03037), and the City University of New York.
Abbreviations
- Apaf
Apoptotic protease activating factor
- Caspase
Cysteine aspartate-specific proteases
- DAPI
4′,6-Diamidino-2-phenylindole
- DISCs
Death-inducing signaling complexes
- DMEM
Dulbecco’s modified eagle medium
- ER
Endoplasmic reticulum
- PARP
Poly(ADP-ribose)polymerase
- PBS
Phosphate buffered saline
- PDT
Photodynamic therapy
- P-Glu4
5,10,15,20-Tetrakis (4,1′-thio-glucose-2,3,5,6-tetrafluorophenyl)porphyrin
- PMSF
Phenylmethylsulfonyl fluoride
- TNRA
Tumor necrosis factor acceptor
- TPPF20
meso-Tetrakis(pentafluorophenyl)porphyrin
Footnotes
Electronic supplementary information (ESI) available: Fluorescence images of P-Glu4 in MDA-MB-231 cells, detection of pro-caspase-3 cleavage and calcium released from ER. See DOI: 10.1039/b806536e
Notes and references
- 1.Kroemer G, Dallaporta B and Resche-Rigon M, The mitochondrial death/life regulator in apoptosis and necrosis, Annu. Rev. Physiol, 1998, 60, 619–642. [DOI] [PubMed] [Google Scholar]
- 2.Lawen A, Apoptosis-an introduction, BioEssays, 2003, 25, 888–896. [DOI] [PubMed] [Google Scholar]
- 3.Sternberg ED, Bruckner C and Dolphin D, Porphyrin-based photosensitizers for use in photodynamic therapy, Tetrahedron, 1998, 54,4151–4202. [Google Scholar]
- 4.Osterloh J and Vicente MGH, Mechanisms of porphyrinoid localization in tumors, J. Porphyrins Phthalocyanines, 2002, 6, 305–324. [Google Scholar]
- 5.Bonnett R, Photosensitizers of the porphyrin the phthalocyanine series for photodynamic therapy, Chem. Soc. Rev, 1995, 24, 19–32. [Google Scholar]
- 6.Oleinick NL, Morris RL and Belichenko I, The role of apoptosis in response to photodynamic therapy: what, where, why, and how, Photochem. Photobiol. Sci, 2002, 1, 1–21. [DOI] [PubMed] [Google Scholar]
- 7.Kessel D and Luo Y, Photodynamic therapy: A mitochondrial inducer of apoptosis, Cell Death Differentiation, 1999, 6, 28–35. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Hui L, Foster DA and Drain CM, Efficient synthesis and photodynamic activity of porphyrin-saccharide conjugates: targeting and incapacitating cancer cells, Biochemistry, 2004, 43, 10918–10929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varnes ME, Chiu S-M, Xue L-Y and Oleinick NL, Photodynamic therapy-induced apoptosis in lymphoma cells: Translocation of cytochrome c causes inhibition of respiration as well as caspase activation, Biochem. Biophys. Res. Commun, 1999, 255, 673–679. [DOI] [PubMed] [Google Scholar]
- 10.Granville DJ and Hunt DWC, Porphyrin-mediated photosensitization – Taking the apoptosis fast lane, Curr. Opin. Drug Discovery Develop, 2000, 3, 232–243. [PubMed] [Google Scholar]
- 11.Yslas I, Alvarez MG, Marty C, Mori G, Durantini EN and Rivarola V, Expression of Fas antigen and apoptosis caused by 5,10,15,20-tetra(4-methoxyphenyl)porphyrin (TMP) on carcinoma cells: implication for photodynamic therapy, Toxicology, 2000, 149, 69–74. [DOI] [PubMed] [Google Scholar]
- 12.Kessel D and Luo Y, Intracellular sites of photodamage as a factor in apoptotic cell death, J. Porphyrins Phthalocyanines, 2001, 5, 181–184. [Google Scholar]
- 13.McGarrity TJ, Peiffer LP, Granville DJ, Carthy CM, Levy JG, Khandelwal M and W C D. Hunt, Apoptosis associated with esophageal adenocarcinoma: influence of photodynamic therapy, Cancer Lett, 2001, 163, 33–41. [DOI] [PubMed] [Google Scholar]
- 14.Nowis D, Makowski M, Stoklosa T, Legat M, Issat T and Golab J, Direct tumor damage mechanisms of photodynamic therapy, Acta Biochim. Polonica, 2005, 52, 339–352. [PubMed] [Google Scholar]
- 15.Lunardi CN and Tedesco AC, Synergic photosensitizers: A new trend in photodynamic therapy, Curr. Org. Chem, 2005, 9, 813–821. [Google Scholar]
- 16.Plaetzer K, Kiesslich T, Oberdanner CB and Krammer B, Apoptosis following photodynamic tumor therapy: Induction, mechanisms and detection, Curr. Pharm. Design, 2005, 11, 1151–1165. [DOI] [PubMed] [Google Scholar]
- 17.Almeida RD, Manadas BJ, Carvalho AP and Duarte CB, Intracellular signaling mechanisms in photodynamic therapy, Biochim. Biophys. Acta - Rev. Cancer, 2004, 1704, 59–86. [DOI] [PubMed] [Google Scholar]
- 18.Peng T-I, Chang C-J, Guo M-J, Wang Y-H, Yu J-S, Wu H-Y and Jou M-J, Mitochondria-targeted photosensitizer enhances the photodynamic effect-induced mitochondrial dysfunction and apoptosis, Ann. N. Y. Acad. Sci, 2005, 1042, 419–428. [DOI] [PubMed] [Google Scholar]
- 19.Buytaert E, Dewaele M and Agostinis P, Molecular effectors of multiple cell death pathways initiated by photodynamic therapy, Biochim. Biophys. Acta - Rev. Cancer, 2007, 1776, 86–107. [DOI] [PubMed] [Google Scholar]
- 20.Reed JC, Cytochrome c: can’t live with it—can’t live without it, Cell, 1997, 91, 559–562. [DOI] [PubMed] [Google Scholar]
- 21.Moor ACE, Signaling pathways in cell death and survival after photodynamic therapy, J. Photochem. Photobiol., B, 2000, 57, 1–13. [DOI] [PubMed] [Google Scholar]
- 22.Pasetto P, Chen X, Drain CM and Franck RW, Synthesis of hydrolytically stable porphyrin C- and S-glycoconjugates in high yields, Chem. Commun, 2001, 81–82. [Google Scholar]
- 23.Chen X and Drain CM, Photodynamic therapy using carbohydrate conjugated porphyrins, Drug Design Rev. Online, 2004, 1, 215–234. [Google Scholar]
- 24.Xu C, Bailly-Maitre B and Reed JC, Endoplasmic reticulum stress: cell life and death decisions, J. Clin. Invest, 2005, 115, 2656–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slee EA, Adrain C and Martin SJ, Serial killers: ordering caspase activation events in apoptosis, Cell Death Differentiation, 1999, 6,1067–1074. [DOI] [PubMed] [Google Scholar]
- 26.Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu K, McGarry TJ, Kirschner MW, Koths K, Kwiatkowski DJ and Williams LT, Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis, Science, 1997, 278, 294–298. [DOI] [PubMed] [Google Scholar]
- 27.Yu S-W, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM and Dawson VL, Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor, Science, 2002, 297, 259–263. [DOI] [PubMed] [Google Scholar]
- 28.de Murcia G and de Murcia JM, Poly(ADP-ribose) polymerase: a molecular nick-sensor, Trends Biochem. Sci, 1994, 19, 172–176. [DOI] [PubMed] [Google Scholar]
- 29.Reddy BSP, Sondhi SM and Lown JW, Synthetic DNA minor groove-binding drugs, Pharm. Therap, 1999, 84, 1–111. [DOI] [PubMed] [Google Scholar]
- 30.Drain CM and Gong X, Synthesis of meso substituted porphyrins in air without solvents or catalysts, Chem. Commun, 1997, 2117–2118. [Google Scholar]
- 31.Lindsey JS, Schreiman IC, Hsu HC, Kearney PC and Marguerettaz AM, Rothemund and Adler-Longo reactions revisited: Synthesis of tetraphenylporphyrins under equilibrium conditions, J. Org. Chem, 1987, 52, 827–836. [Google Scholar]
- 32.Adler AD, Longo FR, Finarelli JD, Goldmacher J, Assour J and Korsakoff L, A simplified synthesis for meso-tetraphenylporphine, J. Org. Chem, 1967, 32, 476–476. [Google Scholar]
- 33.Caramelo J and Parodi AJ, How sugars convey information on protein conformation in the endoplasmic reticulum, Seminars Cell Developmental Biol, 2007, 18, 732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drain CM, Gong X, Ruta V, Soll CE and Chicoineau PF, Combinatorial synthesis and modification of functional porphyrin libraries: identification of new, amphipathic motifs for biomolecule binding, J. Comb. Chem, 1999, 1, 286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grinstaff MW, Hill MG, Labinger JA and Gray HB, Mechanism of catalytic oxygenation of alkanes by halogenated iron porphyrins, Science, 264, 1311–1313. [DOI] [PubMed] [Google Scholar]
- 36.Samaroo D, Vinodu M, Chen X and Drain CM, meso-Tetra(pentafluorophenyl)porphyrin as an efficient platform for combinatorial synthesis and the selection of new photodynamic therapeutics using a cancer cell line, J. Comb. Chem, 2007, 9, 998–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujimoto K, Miyata T and Aoyama Y, Saccharide-directed cell recognition and molecular delivery using macrocyclic saccharide clusters: Masking of hydrophobicity to enhance the saccharide specificity, J. Am. Chem. Soc, 2000, 122, 3558–3559. [Google Scholar]
- 38.Lam M, Oleinick NL and Nieminen A-L, Photodynamic therapy-induced apoptosis in epidermoid carcinoma cells. Reactive oxygen species and mitochondrial inner membrane permeabilization, J. Biol. Chem, 2001, 276, 47379–47386. [DOI] [PubMed] [Google Scholar]
- 39.Pandey RK, Recent advances in photodynamic therapy, J. Porphyrins Phthalocyanines, 2000, 4, 368–373. [Google Scholar]
- 40.Srivastava M, Ahmad N, Gupta S and Mukhtar H, Involvement of Bcl-2 and Bax in photodynamic therapy-mediated apoptosis. Antisense Bcl-2 oligonucleotide sensitizes RIF 1 cells to photodynamic therapy apoptosis, J. Biol. Chem, 2001, 276, 15481–15488. [DOI] [PubMed] [Google Scholar]
- 41.Luo Y and Kessel D, Initiation of apoptosis versus necrosis by photodynamic therapy with chloroaluminum phthalocyanine, Photochem. Photobiol, 1997, 66, 479–483. [DOI] [PubMed] [Google Scholar]
- 42.Moor ACE, Signaling pathways in cell death and survival after photodynamic therapy, J. Photochem. Photobiol., B, 2000, 57, 1–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.