Abstract
Adverse prognosis of internal tandem duplication in the FMS-like tyrosine kinase 3 gene(s) (FLT3-ITD) among AML patients may depend on allelic burden.
We compared post-remission therapies of chemotherapy and hematopoietic stem cell transplantation (HSCT) in 169 FLT3-ITDmut intermediate cytogenetic risk AML patients with allelic ratio evaluable at diagnosis who achieved first complete remission (CR1) with induction therapy. To minimize selection bias, the analysis was limited to patients who remained in CR1 for at least 4 months (median time to HSCT) after achieving CR1 and propensity score matching was implemented. Sensitivity analysis including patients who remained in CR1 for at least 3 months was also applied.
HSCT in CR1 was associated with longer relapse free and overall survival (RFS and OS) with 3-year estimates of 10% vs. 38% (p<0.001) and 18% and 43% (p<0.001) for chemotherapy and HSCT patients respectively. Multivariate regression models showed that HSCT remained statistically significant with improved RFS and OS independent of FLT3-ITD allelic ratio and NPM1 status. Irrespective of post-remission therapy, relapse remains the main reason of treatment failure with 3-year incidence of 68% vs. 41% in chemotherapy and HSCT patients respectively.
Allogeneic HSCT improved disease outcomes compared with chemotherapy after propensity score matching was applied. The improvement observed for RFS (HR=0.55, p=0.09) and OS (HR=0.58, p=0.1) with HSCT as post-remission therapy in patients who remained in CR1 for at least 4 months did not reach statistical significance. However, the sensitivity analyses including patients who remained in CR1 for at least 3 months showed significant improvement for both RFS (HR=0.31, p=0.002) and OS (HR=0.27, p=0.02) after propensity score matching.
Our results indicate that HSCT in CR1 AML FLT3-ITDmut patients is associated with a longer RFS and OS. Innovative transplantation approaches to improve relapse incidence are urgently needed.
Keywords: AML, FLT3-ITD mutation, allogeneic stem cell transplant, post-remission therapy
INTRODUCTION
Acute myeloid leukemia (AML) is a heterogeneous disease with varying prognosis. Overall, FMS-like tyrosine kinase 3 (gen)s (FLT3-ITD) mutations occur in 23–25% of patients with AML at diagnosis (1, 2). FLT3-ITDmut AML represents a distinct entity in patients with intermediate-risk karyotype conferring poor prognosis (1–3). The probability of reaching first complete remission (CR1) is similar in FLT3-ITDmut AML and in other ‘intermediate-risk’ AML, both in young patients and in the elderly(3–5). However, the high risk of relapse, frequently in the first months, is the cause of the shorter disease-free survival (DFS) and overall survival (OS) in this group. Recent studies have shown that the risk conferred by FLT3-ITDmut has been related to specific characteristics, such as the allelic burden, length of the mutation, or site of insertion (4, 6). Moreover, the coexistence of other poor-risk molecular markers in FLT3-ITDmut AML can further worsen the prognosis, as demonstrated for WT1 and DMNT3A mutations(7). In contrast, whether certain additional genetic mutations in FLT3-ITDmut AML makes the outcome relatively favorable, as is the case with NPM1 mutations, is a matter of debate. Mutations of the NPM1 gene are found in 50% of cytogenetically normal AML (CN-AML)(8) and lead to favorable survival. Of note, in 20% of patients with NPM1 mutation, FLT3-ITD is also identified and presence of FLT3-ITD may negate the favorable impact of mutated NPM1 (9–11).
Hematopoietic cell transplantation (HSCT) is usually indicated for patients with FLT3-ITDmut AML due to their short relapse free survival (RFS) even after achieving CR1 and resistance to salvage chemotherapies. Despite this common practice, some authors still argue that the benefit of this high-risk procedure in CR1 of FLT3-ITDmutAML has yet to be proven (12, 13). Several studies showed improved survival with allogeneic HSCT compared with chemotherapy when performed in CR1 but those were criticized for comparing outcomes with historical controls or not including matched unrelated donors as the donor source(11, 14–16). More recently, the importance of taking into account not only the mutational status of FLT3-ITD at diagnosis but also the allele ratio has been addressed and improved outcomes after HSCT even in patients with high allelic ratio HSCT have been reported (17).
In this study, we compared the post-remission therapies of chemotherapy and allogeneic HSCT in intermediate risk AML patients with FLT3-ITDmut who achieved CR1 after induction chemotherapy. We particularly aimed to investigate the impact of FLT3-ITD allelic ratio and presence of NPM1 at diagnosis on the outcomes so that a subgroup of patients who might not need to proceed with HSCT in CR1 would be identified.
MATERIALS AND METHODS
The study population included 227 adult AML patients (age 18 or older), with FLT3-ITDmut, who were diagnosed and treated at MD Anderson Cancer Center from July 2000 through November 2013. Patients who received anti-leukemia therapy at an outside institution before referral to MDACC were excluded. Patients were eligible for analysis if they achieved CR1 with induction chemotherapy and had not had prior HSCT for other hematological malignancy (Figure 1). Patients with diagnostic cytogenetic abnormalities including poor risk features like del5q/-5 and/or del7q/-7 were excluded and only patients with intermediate risk cytogenetics were included. Patients who received HSCT as consolidation in CR1 using mismatched donors including cord blood units were excluded from this analysis. To minimize time to transplant selection bias, the analysis was limited to patients who remained in CR1 for at least 4 months (median time to HSCT) after achieving CR1. Thus, 169 patients were included in this analysis.
Figure 1:
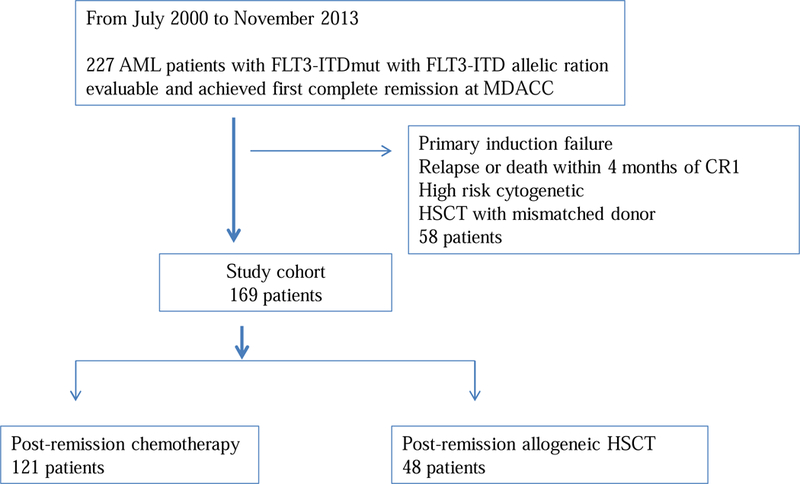
Flow chart for patient selection strategy: Among all AML patients diagnosed and treated at MDACC between July 2000 through November 2013, only ones with FLT-ITDmut and FLT3 allelic ratio available were included if they achieved first complete remission with anti-leukemia treatment. Final study cohort included 169 patients.
Analysis of FLT3-ITD, FLT3-ITDwild type allelic burden and NPM1
All samples were obtained at diagnosis and detection of NPM1 and FLT3-ITD mutations were performed on genomic DNA from bone marrow aspirates. FLT3-ITD and codon 835/836 tyrosine-kinase domain mutational status were determined using DNA from unsorted bone marrow aspirate samples obtained at initial presentation using a semiquantitative DNA-based PCR-capillary electrophoresis assay, as described previously(6) with an analytical sensitivity of 1% to 2% mutation-bearing cell. FLT3-ITDwild allelic burden at diagnosis was calculated as the ratio of the area under the curve of mutant and wild-type alleles (FLT3-ITD/FLT3wild)(18). In cases with >1 mutation, all FLT3-ITD was summed. NPM1 exon12 MUTs were determined by PCR amplifications as described previously (19).
Treatments
Induction therapies were according to age appropriate frontline regimens and were all given at MDACC during the study period. At our institution, induction chemotherapy for AML has been preferred to be combination chemotherapy including high dose cytarabine for patients aged 65 and younger if they are medically fit. Older patients are treated with combination chemotherapies based on low dose cytarabine or hypomethylating agents. Overall, induction chemotherapy predominantly comprised of high dose cytarabine based regimens in 119 (70%), hypomethylating agents in 28 (17%), clofarabine in 9 (5%) or cladrabine in 6(4%) patients. Most patients aged ≤65 were treated with high dose cytarabine based regimens (90%). Patients who did not proceed with HSCT as post-remission therapy received consolidation chemotherapy similar to their induction therapy. Of 169 patients, 37(22%) received FLT3 inhibitors as a part of their induction/consolidation chemotherapy. In our study cohort, patients were enrolled into clinical trials (including high dose cytarabine based, hypomethylating agent based or low dose cytarabine based front line regimens) based on patient age and organ function; majority of the patients (n=134, 79%) were treated on clinical trials.
In general, all high risk patients including cytogenetics and molecular findings (including FLT3-ITD mutated patients) were referred for stem cell consultation. The decision to proceed with stem cell transplant in CR1 was finalized based on the donor availability, patients’ preference and treating physicians’ preference. Post-remission therapy was consolidation chemotherapy (chemo-group) in 121 (71.6%) and allogeneic HSCT in 48 (28.4%) patients. Among 121 patients in chemo-group, 5 patients received HSCT following disease relapse and 2 patients received HSCT without relapse but later on during their disease course and they were analyzed in “chemo-group”.
Of 48 patients who received HSCT as the post-remission consolidation therapy, 45 had HSCT at our institution. The source of hematopoietic stem cells was peripheral blood (PB) in 30 patients (66.6%) and bone marrow (BM) in 15 patients (33.3%). Serologic or low-resolution molecular techniques were used for class I antigens and high-resolution molecular typing using polymerase chain reaction for class II alleles until July 2005. After July 2005, all donors had high-resolution molecular typing of class I and II antigens and matched at -A, -B, -C, and -DRB1 with the recipients. Donors were matched unrelated (n=26, 57.8%), as described by Weisdorf et al.(20) and the rest had matched related donor (n=19, 42.2%).
Conditioning regimen for HSCT was myeloablative (MAC) in 33 of 45 (73.3%) patients and consisted of Fludarabine given as 40 mg/m2 for 4 days with intravenous Busulfan either at a fixed dose of 130 mg/m2 or targeted dose of area under the curve (AUC) of 5000–6000 for 4 days. Reduced intensity conditioning (RIC) given to 12 patients consisted of Fludarabine given as 25–40 mg/m2 for 3–4 days with either 1) Melphalan 140 mg/m2 or 100 mg/m2 or 2) Busulfan either at a fixed dose of 100 mg/m2 or targeted dose of AUC of 4000 for 4 days. Tacrolimus (0.015 mg/kg) and methotrexate 5 mg/m2 intravenously on days 1, 3, 6, and 11 after transplantation were used for graft-versus-host disease (GVHD) prophylaxis in the majority of the patients (93.3%). Patients with an unrelated donor received rabbit-ATG (Thymoglobulin® , Genzyme Inc., Cambridge, MA), 0.5 mg/kg on day −3, 1.5 mg/kg on day −2, and 2.0 mg/kg on day −1).
Approval was obtained from The University of Texas M. D. Anderson Cancer Center’s institutional review board for these studies. Informed consent was provided in accordance with the Declaration of Helsinki.
Statistical Methods
Patient characteristics were tabulated by status of stem cell transplantation. Differences between categorical covariates were tested using Fisher’s exact tests, and differences between continuous covariates were compared by the Wilcoxon rank-sum tests. OS was defined as the time interval between diagnosis date and death date, and was censored at the last follow-up date for patients who were alive. RFS was defined as the time interval between remission dates and relapse date or death date, whichever came first, and was censored at last follow-up date for patients who were alive without relapse. Survival curves were estimated using the Kaplan-Meier methods(21). Univariate and multivariate Cox proportional hazards regression models(22) were used to assess the association between patient characteristics and OS or RFS. Patient characteristics that were significant in the univariate models at the 0.10 level were included in the multivariate model. Backward elimination was implemented until all remaining predictors had a p-value less than 0.05. Predictive variables were transformed as appropriate.
We implemented propensity score matching to reduce the possibility of selection bias since the decision for post-remission therapy in our study was not randomized (23, 24). The Greedy 8→1 digit match algorithm was applied in propensity score matching. Age at diagnosis, FLT3-ITD allelic ratio, presence of NPM1 mutation, receiving intermediate/high dose cytarabine as part of induction and/or consolidation chemotherapy and year of diagnosis (before vs. after 2008) were the criteria used to estimate the propensity scores. The year of 2008 was used as a cutoff for matching as FLT3 inhibitors in frontline and salvage setting has been used most since that year. HSCT patients were matched to chemotherapy patients with a ratio of 1:1. For the matched data, differences in patient characteristics were evaluated using McNemar’s tests for categorical covariates with 2 levels, generalized estimating equation (GEE) methods for categorical covariates with 3 levels, and Wilcoxon signed rank tests for continuous covariates. Stratified log-rank test was applied to assess the difference in OS and RFS between the two matched groups (i.e., HSCT vs. chemotherapy). Univariate and multivariate Cox proportional hazards regression models stratifying on the matched pairs were used to assess the association between patient characteristics and OS or RFS. Statistical analyses were performed using SAS 9.3 (SAS Institute, Cary NC) and graphics were created using Stata (StataCorp LP, College Station, TX)
RESULTS
Table 1 presents the disease and patient characteristics by post-remission therapy patients received in CR1. Treatment groups were comparable except that patients in the HSCT group were younger (median age 55 vs. 62, p=0.002) and more often received high dose cytarabine as a part of their induction/consolidation chemotherapy (87.5% vs. 58.7%, p<0.001) compared with those in the chemotherapy group. ECOG performance status (PS)(25) at diagnosis was available in 155 of 169 patients and more than three quarters of patients in both treatment groups had ECOG PS of 0–1 (83.6 % vs. 93.3% in chemo and HSCT groups respectively).
1:
Patient characteristics before and after propensity score matching.
| Study cohort without propensity score matching | Patients matched by propensity score | ||||||
|---|---|---|---|---|---|---|---|
| All patients, n=169 | Chemo-group, n=121 |
HSCT group, n=48 (%) |
p | Chemo-group, n=41 |
HSCT group, n=41 |
p | |
| Median age diagnosis (median, IQR) | 59 (50–68) | 62 (52–70) | 55 (47–62) | 0.002 | 56 (49–65) | 56 (52–62) | 0.48 |
| WBC at diagnosis (median, IQR) | 11.6 (3.8–29.7) | 12 (4–28.2) | 9 (3.1–36.75) | 0.99 | 9.8 (4.9–19.4) | 11.3 (4.2–51.4) | 0.24 |
| Platelet count at diagnosis (median, IQR) | 45 (25–74) | 45 (25–71) | 45 (22.5–83) | 0.99 | 43 (30–79) | 52 (31–91) | 0.73 |
| BM blast at diagnosis (median, IQR) | 43 (12–74) | 46 (13–75) | 32.5 (6.5–63.5) | 0.18 | 58 (28–82) | 32 (7–64) | 0.07 |
| Cytogenetics at diagnosis | 164/169 | 119/121 | 45/48 | ||||
| Diploid | 128 (78%) | 95 (79.8%) | 33 (73.3%) | 34(85%) | 29(74.4%) | ||
| Other | 36 (22%) | 24 (20.2%) | 12 (26.7%) | 0.40 | 6(15%) | 10(25.6%) | 0.13 |
| No of FLT3-ITD mutations at diagnosis | 163/169 | 116/121 | 47/48 | ||||
| 1 | 130 (79.8%) | 95 (81.9%) | 35 (74.5%) | 33(80.5%) | 31(75.6%) | ||
| >1 | 33 (20.2%) | 21 (18.1%) | 12 (25.5%) | 0.29 | 8(19.5%) | 10(24.4%) | 0.56 |
| FLT3-ITD allelic ratio at diagnosis(median, IQR) | 0.34 (0.11–0.48) | 0.35 (0.12–0.49) | 0.3 (0.04–0.48) | 0.29 | 0.18 (0.04–0.45) | 0.32 (0.05–0.48) | 0.46 |
| FLT3-ITD allelic ration ≥0.3 at diagnosis | 163/169 | 116/121 | 47/48 | ||||
| Yes | 88 (54%) | 65 (56%) | 23 (48.9%) | 19(46.3%) | 22(53.7%) | ||
| No | 75 (46%) | 51 (44%) | 24 (51.1%) | 0.49 | 22(53.7%) | 19(46.3%) | 0.44 |
| FLT3-ITD allelic ration ≥0.5 at diagnosis | 163/169 | 116/121 | 47/48 | ||||
| Yes | 37 (22.7%) | 29 (25%) | 8 (17%) | 9(22%) | 7(17.1%) | ||
| No | 126 (77.3%) | 87 (75%) | 39 (83%) | 0.31 | 32(78%) | 34(82.9%) | 0.62 |
| Presence of NPM1 mutation | |||||||
| Yes | 56 (33.1%) | 37 (30.6%) | 19 (39.6%) | 18(43.9%) | 17(41.5%) | ||
| No | 57 (33.7%) | 42 (34.7%) | 15 (31.3%) | 12(29.3%) | 14(34.1%) | ||
| Unknown | 56 (33.1%) | 42 (34.7%) | 14 (29.2%) | 0.55 | 11(26.8%) | 10(24.4%) | 0.85 |
| Use of FLT3 inhibitors as induction/consolidation | |||||||
| Yes | 37 (21.9%) | 27 (22.3%) | 10 (20.8%) | 10(24.4%) | 8(19.5%) | ||
| No | 132 (78.2%) | 94 (77.7%) | 38 (79.2%) | 1.0 | 31(75.6%) | 33(80.5%) | 0.53 |
| Year of diagnosis after 2008 | |||||||
| Yes | 106 (62.7%) | 68 (56.2%) | 38 (79.2%) | 31(75.6%) | 31(75.6%) | ||
| No | 63 (37.3%) | 53 (43.8%) | 10 (20.8%) | 0.01 | 10(24.4%) | 10(24.4%) | 1.00 |
| Use of high dose cytarabine regimen before HCT | |||||||
| Yes | 113(66.9%) | 71(58.7%) | 42(87.5%) | 34(82.9%) | 35(85.4%) | ||
| No | 56(33.1%) | 50(41.3%) | 6(12.5%) | <0.001 | 7(17.1%) | 6(14.6%) | 0.65 |
| ECOG performance status at diagnosis | 155/169 | 110/121 | 45/48 | 36/41 | 38/41 | ||
| 0–1 | 134 (86.5%) | 92 (83.6%) | 42 (93.3%) | 31(86.1%) | 36(94.7%) | ||
| 2–3 | 55 (13.5%) | 18 (16.4%) | 3 (6.7%) | 0.1 | 5 (13.9%) | 2 (5.3%) | 0.2 |
Abbreviations: HSCT, hematopoietic stem cell transplantation; IQR, interquartile range; BM, bone marrow.
The median follow-up from the date of achieving CR1 was 29.4 months for the HSCT and 32.8 months for the chemo-group (p=0.71). The median time from diagnosis to transplantation among the 48 HSCT patients was 4.1 months (range, 2.5 to 8.9 months).
Relapse Free Survival
Of 169 patients, 121 (72%) had disease relapse or died and 48 (28%) were alive without disease relapse at last follow-up. The median RFS was 8.5 months (95% confidence interval (CI), 7.0–10.9 months) and the 3-year RFS rate was 26% (95% CI, 20%−34%). Figure 2a shows the Kaplan-Meier estimates for RFS by post-remission therapy. Among the 48 HSCT patients, 25 patients relapsed or died. The median RFS was 20 months (95% CI, 11.8 months – not estimable) and the 3-year RFS rate was 46% (95% CI, 33%−64%). Among the 121 chemotherapy patients, 96 patients relapsed or died. The median RFS was 6.4 months (95% CI, 5.6–8.8 months) and the 3-year RFS rate was 18% (95% CI, 12%−26%). There was a significant difference in RFS between HSCT and chemotherapy patients (log-rank test p-value<0.001).
Figure 2:
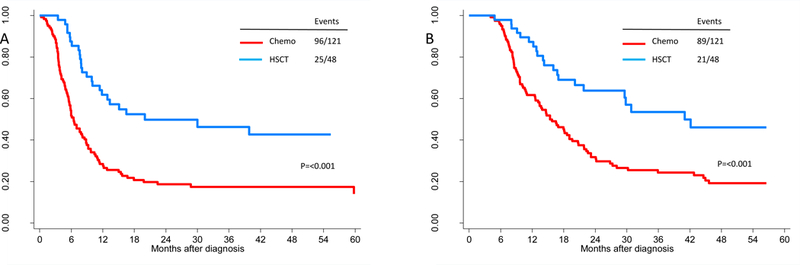
Relapse-free (A) and overall survival (B) by post-remission therapy in first complete remission. Relapse free survival at 3-years was 18% with chemotherapy and 46% with HSCT as post-remission therapy in CR1. Overall survival was also different between treatment groups; 24% vs. 54% with chemotherapy and HSCT.
Table 2 presents the results of univariate Cox proportional hazards model for RFS. While log(WBC at diagnosis) (Hazard ratio (HR)=1.16; 95%CI, 1.01–1.33, p=0.03) and log(FLT3-ITD allelic ratio at diagnosis) (HR=1.16; 95%CI, 1.0–1.35, p=0.05) were poor prognostic factors, receiving high dose cytarabine as part of induction/consolidation chemotherapy (HR=0.61, 95%CI, 0.42–0.89, p=0.001), allogeneic HSCT as post-remission therapy (HR=0.39; 95%CI, 0.25–0.61, p<0.001) and presence of NPM1 mutation (HR=0.56; 95%CI, 0.36–0.88, p=0.01) were associated with improved RFS. When these prognostic variables were fitted into a multivariate regression model, allogeneic HSCT as post-remission therapy remained statistically significant with improved RFS (HR=0.42, 95% CI, 0.27–0.66, p<0.001) and log(WBC at diagnosis) (HR=1.18; 95% CI, 1.03–1.35, p=0.02) was a poor prognostic factor for RFS (Table 3).
Table 2:
Univarite analysis for relapse free and overall survival with all patients in the study cohort.
| RFS | OS | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95%CI | p | HR | 95%CI | p |
| Age | 1.01 | 0.99–1.02 | 0.32 | 1.01 | 1–1.03 | 0.05 |
| Log (WBC) at diagnosis | 1.16 | 1.01–1.33 | 0.03 | 1.19 | 1.03–1.37 | 0.02 |
| Log (Platelet) count at diagnosis | 1.13 | 0.91–1.4 | 0.26 | 1.12 | 0.90–1.4 | 0.3 |
| BM blast at diagnosis | 1 | 1–1.01 | 0.63 | 1 | 0.99–1.01 | 0.79 |
| Cytogenetics at diagnosis (Diploid vs. other) | 1.04 | 0.67–1.61 | 0.87 | 1.45 | 0.89–2.36 | 0.13 |
| No of FLT3-ITD mutations at diagnosis (>1 vs. 1) | 0.79 | 0.50–1.26 | 0.32 | 0.78 | 0.48–1.26 | 0.31 |
| Log (FLT3-ITD allelic ratio) at diagnosis | 1.16 | 1–1.35 | 0.05 | 1.18 | 1–1.38 | 0.05 |
| FLT3-ITD allelic ratio ≥0.3 at diagnosis (Yes vs. no) | 1.3 | 0.9–1.87 | 0.16 | 1.29 | 0.87–1.9 | 0.2 |
| FLT3-ITD allelic ratio ≥0.5 at diagnosis (Yes vs. no) | 0.94 | 0.6–1.46 | 0.77 | 1.07 | 0.68–1.68 | 0.77 |
| Presence of NPM1 mutation | ||||||
| Yes vs. no | 0.56 | 0.36–0.88 | 0.01 | 0.64 | 0.4–1.04 | 0.07 |
| Unknown vs. no | 0.8 | 0.53–1.22 | 0.3 | 0.91 | 0.58–1.42 | 0.68 |
| Use of FLT3 inhibitors as induction/consolidation (Yes vs. no) | 1.1 | 0.71–1.71 | 0.67 | 1.18 | 0.75–1.84 | 0.47 |
| Year of diagnosis after 2008 (Yes vs. no) | 0.82 | 0.57–1.17 | 0.27 | 0.82 | 0.56–1.2 | 0.3 |
| Allogeneic HSCT (Yes vs. no) | 0.39 | 0.25–0.61 | <0.001 | 0.43 | 0.27–0.69 | <0.001 |
| Use of high dose cytarabine regimen before HCT (Yes vs. no) | 0.61 | 0.42–0.89 | 0.01 | 0.68 | 0.46–1.01 | 0.06 |
Abbreviations: RFS, relapse-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; HSCT, hematopoietic stem cell transplantation; BM, bone marrow.
Table 3:
Multivariate regression for relapse free and overall survival with all patients in the study cohort*
| RFS | OS | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95%CI | p | HR | 95%CI | p |
| Log (WBC) at diagnosis | 1.18 | 1.03–1.35 | 0.02 | 1.20 | 1.04–1.39 | 0.01 |
| Allogeneic HSCT (Yes vs. no) | 0.42 | 0.27–0.66 | <0.001 | 0.47 | 0.29–0.76 | 0.002 |
The regression model included log (FLT3-ITD allelic ratio at diagnosis, presence of NPM1 mutation and the use of intermediate/high dose of cytarabine as induction/consolidation chemotherapy.
Abbreviations: RFS, relapse-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; HSCT, hematopoietic stem cell transplantation; BM, bone marrow.
Overall Survival
Of 169 patients, 110 (65%) died and 59 (35%) were alive at last follow-up. The median OS among the 59 survivors was 33.6 months (range, 5.0 to 159.4 months). Figure 2b shows the Kaplan-Meier estimates for OS by post-remission therapy. Among the 48 HSCT patients, 21 died, the median OS was 41 months (95% CI, 29.6 months – not estimable) and the 3-year OS rate was 54% (95% CI, 40%−72%). For the 121 chemotherapy patients, 89 died, the median OS was 15.4 months (95% CI, 13.1–19.6 months) and the 3-year OS rate was 24% (95% CI, 17%−34%). OS was significantly better in HSCT patients compared to chemotherapy patients (p-value<0.001).
Table 2 presents the results of univariate Cox proportional hazards models for OS. While older age (HR=1.01; 95%CI, 1–1.03, p=0.05), log(WBC at diagnosis) (HR=1.19; 95%CI, 1.03–1.37, p=0.02) and log(FLT3-ITD allelic ratio at diagnosis) (HR=1.18; 95%CI, 1–1.38, p=0.05) were poor risk factors for OS, receiving high dose cytarabine as part of induction/consolidation chemotherapy (HR=0.68, 95%CI, 0.46–1.01, p=0.06) and HSCT as post-remission therapy improved the OS (HR=0.43, 95%CI, 0.27–0.69, p<0.001). Multivariate analysis showed that HSCT was associated with improved OS (HR=0.47; 95% CI, 0.29–0.76; P=0.002) and log(WBC at diagnosis) (HR=1.2; 95% CI, 1.04–1.39, p=0.01) was a poor prognostic factor for OS (Table 3).
Interaction between HSCT and FLT3-ITD allelic ratio at diagnosis, NPM1 or cytarabine regimens
The impact of HSCT and FLT3-ITD allelic ratio at diagnosis on RFS and OS was further investigated by adding the interaction term between HSCT and FLT3-ITD allelic ratio (≥0.3 vs. <0.3) in the Cox proportional regression model. The effects of the interaction on RFS and OS were not significant (p=0.91 and p=0.18 respectively), which suggests that the effect of HSCT on RFS and OS were not influenced by FLT3-ITD allelic ratio at diagnosis. Similar interaction effects were examined for the presence of NPM1 and the effect of HSCT. The effects of the interaction between the presence of NPM1 and HSCT was not significant for RFS (p=0.42) and OS (p=0.43). Similarly the use of high dose cytarabine as part of induction/consolidation chemotherapy did not influence the effect of HSCT on RFS and OS with non-significant interaction between the use of high dose cytarabine and HSCT (p=0.11 and p=0.24 respectively).
Relapse and Non-Relapse Mortality Incidence
The 3-year cumulative incidence rates of relapse among HSCT and chemotherapy patients were 41% (95%CI, 26%−55%) and 68% (95%CI, 58%−76%), respectively when non-relapse mortality was the competing event (Figure 3a). The 3-year cumulative incidence rates of non-relapse mortality among HSCT and chemotherapy patients were 13% (95%CI, 5%−25%) and 15% (95%CI, 9%−22%) (Figure 3b), respectively.
Figure 3:

Cumulative incidence of relapse with non-relapse mortality as the competing event (A) and non-relapse mortality with relapse as the competing event (B) by the type of post-remission therapy in first complete remission. The 3-year cumulative incidence of relapse was 68% and 41% with chemotherapy and HSCT respectively. The 3-year cumulative incidence of non-relapse mortality was 15% and 13% with chemotherapy and HSCT respectively.
Calculation of Propensity Scores and Propensity Score Matching
We used a propensity score-based approach for the comparison of outcomes between patients in the chemo and HSCT groups as described above in “Statistical Methods” because post-remission therapy with chemotherapy or allogeneic HSCT was not allocated through randomization. From 169 patients, we selected 82 propensity score-matched chemo and allogeneic HSCT patients for comparison.
Table 1 shows the comparison of patient characteristics by post-remission therapy before and after propensity score matching. In the original population, patients between groups were significantly different for age at diagnosis, year of diagnosis and the use of high dose cytarabine as a part of induction or consolidation chemotherapy. After propensity score matching, all patient and disease characteristics were similar between groups (table 1). Median age at diagnosis was 56 in both groups (p=0.48); high dose cytarabine was used in 34 of 41 chemo patients and 35 of 41 allogeneic HSCT patients (p=0.65).
Relapse free and overall survival
Of 82 patients, 47 (57%) had disease relapse or died and 35 (43%) were alive without disease relapse at last follow-up. The median follow-up of survivors from the date of achieving CR1 in chemo and HSCT groups were 44.6 and 30.7 months, respectively. Figure 4a shows the Kaplan-Meier estimates for RFS by post-remission therapy status. Among the 41 HSCT patients, the median RFS was 30 months (95% CI, 11.4 months-not estimable) and among the 41 chemotherapy patients, the median RFS was 8.0 months (95% CI, 5.9 months-not estimable). This difference observed in RFS between HSCT and chemotherapy patients (stratified log-rank test p-value=0.09) did not reach statistical significance. RFS at 3-year was 47% (95% CI, 30%−62%) vs. 34% (95% CI, 20%−49%) for HSCT and chemo groups respectively.
Figure 4:

The estimates of relapse-free survival (A), overall survival (B), cumulative incidence of relapse (C) and non-relapse mortality (D) by post-remission therapy in 82 patients with first complete remission for at least 4 months after propensity score matching. Relapse free survival at 3-years was 34% with chemotherapy and 47% with HSCT as post-remission therapy in CR1. Overall survival was also different between treatment groups; 39% vs. 54% with chemotherapy and HSCT. The 3-year cumulative incidence of relapse was 45% vs. 40% with chemotherapy and HSCT while the non-relapse mortality was 21% vs. 13%.
Figure 4b shows the Kaplan-Meier estimates for OS by HSCT. Among HSCT and chemo patients, the median OS was 42.2 months (95% CI, 21.8 months – not estimable) vs. 16.5 months (95% CI, 12.6 months-not estimable). Similar to RFS, OS between HSCT and chemotherapy patients (stratified log-rank test p-value=0.14) did not reach statistical significance. The OS at 3-years was 54% (95% CI, 36%−69%) vs. 39% (95% CI, 24%−55%) for HSCT and chemo patients respectively.
Table 4 presents the results of stratified univariate Cox proportional hazards models for RFS and OS. Among the variables tested, allogeneic HSCT as post-remission therapy improved RFS (HR=0.55, 95%CI, 0.27–1.1, p=0.09) and OS (HR=0.58, 95% CI, 0.28–1.22, p=0.1) but did not reach statistical significance. No other variable was found to be a significant prognostic factor for RFS and OS.
Table 4:
Univariate analyses for relapse free and overall survival after propensity score matching*.
| RFS | OS | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95%CI | p | HR | 95%CI | p |
| Age | 0.99 | 0.96–1.02 | 0.54 | 1.01 | 0.97 , 1.05 | 0.66 |
| Log (WBC) at diagnosis | 0.97 | 0.65–1.47 | 0.90 | 1.05 | 0.69–1.60 | 0.80 |
| Log (Platelet) count at diagnosis | 1.17 | 0.62–2.19 | 0.63 | 1.00 | 0.52–1.92 | 0.99 |
| BM blast at diagnosis | 1.00 | 0.98–1.02 | 0.97 | 1.00 | 0.98–1.02 | 0.96 |
| Cytogenetics at diagnosis (Diploid vs. other) | 4.00 | 0.85–18.84 | 0.08 | 4.00 | 0.85–18.84 | 0.08 |
| Log (FLT3-ITD allelic ratio) at diagnosis | 0.93 | 0.62–1.37 | 0.70 | 0.92 | 0.61–1.38 | 0.68 |
| FLT3-ITD allelic ration ≥0.3 at diagnosis (Yes vs. no) | 1.40 | 0.44–4.41 | 0.57 | 1.40 | 0.44–4.41 | 0.57 |
| Presence of NPM1 mutation | ||||||
| Yes vs. no | 0.77 | 0.26–2.31 | 0.64 | 0.79 | 0.24–2.64 | 0.70 |
| Unknown vs. no | 0.67 | 0.15–3.05 | 0.61 | 0.52 | 0.10–2.63 | 0.43 |
| Year of diagnosis after 2008 (Yes vs. no) | 1.50 | 0.25–8.98 | 0.66 | 4.00 | 0.45–35.79 | 0.22 |
| Allogeneic HSCT (Yes vs. no) | 0.55 | 0.27–1.10 | 0.09 | 0.58 | 0.28–1.22 | 0.15 |
| Use of high dose cytarabine (Yes vs. no) | 0.33 | 0.03–3.20 | 0.34 | 0.33 | 0.03–3.20 | 0.34 |
Number of FLT3-ITD mutation and the use of FLT3 inhibitors were not analyzed for their impact on RFS and OS due to sample size<10 in groups.
Abbreviations: RFS, relapse-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; HSCT, hematopoietic stem cell transplantation; BM, bone marrow.
The cumulative incidence of relapse at 3-years was 40% (95%CI, 24%−56%) and 45% (95%CI, 28%−60%) for patients with HSCT and chemo (Figure 4c). There was no difference in non-relapse mortality between HSCT and chemo (Figure 4d).
Sensitivity Analyses for Relapse free and overall Survival
We repeated our analyses with another “minimum time” to be in CR1 and alive to address lead time bias and confirm our results with a sensitivity analysis. In the repeat analyses, we included patients who remained in CR1 for at least 3 months. This increased the study cohort to 184 patients; 136 with chemotherapy and 48 with HSCT as post-remission therapy. Similar to cohort with patients who remained in CR1 for at least 4 months, patients with HSCT were younger (median age of 55 vs. 61.5, p=0.003), more often diagnosed after 2008 (79.2% vs. 55.9%, p=0.01) and received high dose cytarabine as a part of their induction/consolidation more often (87.5% vs. 61%, p=0.001) (Supplementary Table 1). Univariate and multivariate regression analyses were very similar (supplementary Table 2 and sTable 3) compared with the results of study cohort with lead time bias of 4 months. Multivariate regressions showed that HSCT as post-remission therapy improved RFS (HR=0.39, 95%CI=0.25–0.6, p<0.001) and OS (HR=0.43, 95%CI=0.26–0.68, p<0.001) while log (WBC) was a poor prognostic factor for both outcomes.
When propensity score matching applied to this cohort that remained in CR1 for at least 3 months, we had a group of 44 patients with HSCT and 44 with chemotherapy as post-remission therapy. Age at diagnosis, log (FLT3-ITD AR), diagnosis after 2008, presence of NPM1 mutation, and the use of high dose cytarabine as a part of induction/consolidation were the criteria used to estimate the propensity scores.
RFS and OS at 3 years were significantly improved with HSCT as post-remission therapy as shown in Figure 5a-b. RFS at 3-years was 45.5% (95% CI, 29%−60.7%) vs. 21.8% (95%CI, 10.3%−38.4%) for HSCT and chemo groups respectively. Univariate regression in this group showed that the use of HSCT as post-remission therapy vs. chemotherapy was the only prognostic factor for RFS (HR=0.31, p=0.002) and OS (HR=0.27, p=0.02) (Supplementary Table 4).
Figure 5:
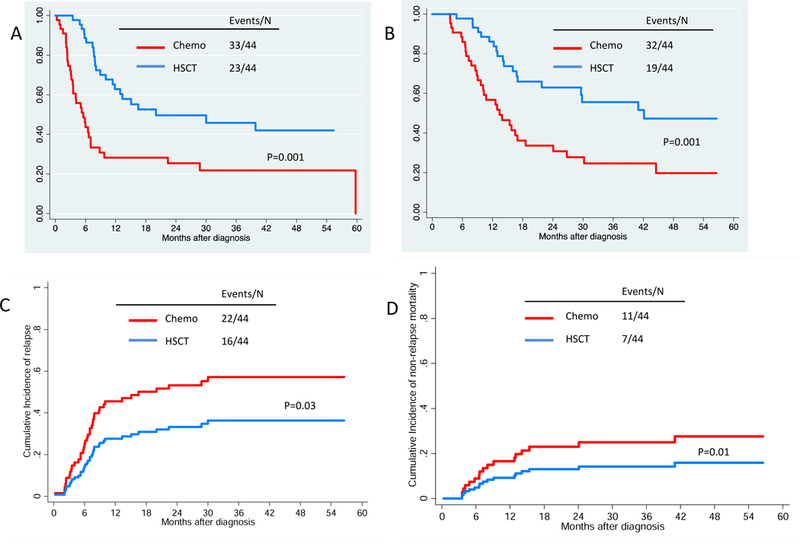
The estimates of relapse-free survival (A), overall survival (B), cumulative incidence of relapse (C) and non-relapse mortality (D) by post-remission therapy in 88 patients with first complete remission for at least 3 months after propensity score matching. RFS and OS at 3-years was 46% and 55.5% vs. 22% and 24.6% for HSCT and chemo groups respectively. The cumulative incidence of relapse was 40% in patients who proceeded HSCT as compared with 55% with chemo as post-remission therapy.
The cumulative incidence of relapse was also improved in patients with HSCT as shown in Figure 5c. The incidence of relapse at 3-years was 40% (95% CI, 24%−55%) vs. 55% (95% CI, 37%−69%) for HSCT and chemo groups respectively. Non-relapse mortality between treatment groups was similar as shown in Figure 5d.
DISCUSSION
As use of molecular data in assigning prognosis in AML has become mainstream, it is important to define role of HSCT in molecularly defined prognostic groups. Our study evaluated the impact of allogeneic HSCT among 169 patients with AML and FLT3-ITD mutation in first CR compared with chemotherapy as post-remission therapy on clinical outcomes after taking FLT3-ITD allelic ratio and NPM1 mutation into consideration. Our results indicate that allogeneic HSCT in CR1 is associated with prolonged RFS and OS independent of the FLT3-ITD allelic ratio and NPM1 mutation status in FLT3-ITDmut patients. As slightly less than a quarter of patients received FLT3 inhibitors as part of induction, our study is not adequately powered to analyze its impact in outcome.
Since Gale et al. published their experience of AML FLT3-ITDmut patients in CR1, where patients were grouped according to the availability of a matched-related donor as donor versus no donor group, and showed a lower but non-significant relapse incidence without impact on overall survival in the donor group, the role of allogeneic HSCT has been a source of debate in FLT3-ITDmut AML(12). More recently reported data indicated a clinical benefit in FLT3-ITDmut AML after allogeneic HSCT, with both RFS and OS rates being significantly improved(11, 16, 26). Our results also support the notion that allogeneic HSCT provides improvement in RFS and OS in AML FLT3-ITDmut patients if performed in CR1 with a matched donor.
Given the retrospective nature of our study, we used propensity score adjustment to accurately identify the impact of chemotherapy and allogeneic HSCT as post-remission therapy on patients’ outcome by balancing the covariates in the 2 groups and reducing selection bias when treatment assignment was not random(24). However, this might lead to a large reduction in sample size while counting for the selection bias associated with observed confounding variables but not observed latent confounding variable. We also included only patients who did not die or relapse within 4 months after achieving CR1, which was the median time to HSCT in AML patients at our institution, to reduce the lead-time bias and make the study population as homogeneous as possible. We selected another “minimum time” to be alive and in CR1 and repeated our analyses to confirm our findings with a sensitivity analysis. Mismatched donor recipients were also excluded to increase the homogeneity of the study population. Despite all these efforts, the limitations of a non-prospective and non-randomized study remain as is true for other available data so far. As patients did not receive FLT3 inhibitors uniformly post relapse, our study cannot address the issue of any potential favorable impact of FLT3 inhibitors as salvage therapy on OS. Therefore our results should be interpreted cautiously until well-designed prospective clinical trials confirm the findings.
AML is a polyclonal disease and the allelic ratio is to some degree a reflection of the clonal burden of the FLT3-ITDmut cells within the leukemia cell population. Despite several studies showing that higher mutant to wild-type allelic ratio is predictive of worse outcomes, the importance of taking into account not only the mutational status of FLT3-ITD at diagnosis but also the allelic ratio for post-remission therapy was not addressed until recently. Studies by German-Austrian AML Study Group(27) (AMLSG) and the Spanish cooperative group CETLAM(28) showed that the benefit of allogeneic HSCT performed in CR1 may be restricted to patients with an allelic ratio of ≥0.51 and allogeneic HSCT did not improve outcome of patients with a low allelic ratio, suggesting that, in these patients, the risk associated with allogeneic HSCT was not outweighed by its benefit. Our results however show improvement in RFS and OS after allogeneic HSCT in CR1 independent of FLT3 allelic ratio. Our study was different from the previously published series that the FLT3-ITD allelic ratio at diagnosis was lower with a median of 0.3. After propensity score matching, the limited study group had even a lower allelic ratio at diagnosis with a median of 0.18. The number of patients with allelic ratio of 0.5 or higher, which is the usually accepted cut-off for high allelic ratio, was limited in our study cohort. Despite the lower allelic ratios at diagnosis compared with previously published studies, relapse incidence was the major reason for failure in both chemo and HSCT groups and the relapse incidences were similar with reports of higher allelic ratios. These results suggest that chemoresistance can be observed with any subclone of FLT3-ITD mutated cells during chemotherapy and be the primary reason of treatment failure.
Similar to clinical impact of FLT3-ITD allelic ratio, the impact of NPM1 mutation in FLT3-ITDmut patients is unclear (9, 10, 29, 30). The interaction of NPM1 status and FLT3-ITD mutant level is important particularly for post-remission therapy decision. In our study, two thirds of the patients had NPM1 status evaluable and one third of study population had NPM1mutation. Although the presence of NPM1 was associated with favorable RFS and OS, this effect lost its significance with multivariate regression suggesting that FLT3-ITDmut trumps the favorable prognosis of NPM1 mutations and those patients should be considered in the high risk category. We believe that FLT3-ITDmut and NPM1mut patients would benefit from aggressive consolidative therapy with HSCT. The reported discrepancies in the literature may arise because of the small size of the minor subgroups in some of the studies, the use of different thresholds for FLT3-ITD levels, and because FLT3-ITD levels might be underestimated in samples with low leukemic cell purity. However, a larger cohort of intermediate risk patients treated through Medical Research Council showed similar outcomes in NPM1mut patients when adjusted by high or low FLT3-ITD allelic ratio suggesting that NPM1mut patients with low allelic ratio of FLT3-ITD should not yet be considered different from patients with higher allelic ratios(30).
Despite improved outcomes compared with post-remission chemotherapy, relapse remains to be the major reason of failure after allogeneic HSCT in AML. A recent EBMT analyses showed that FLT3-ITDmut patients had 2-year cumulative incidence of relapse in the range of 30% after allogeneic HSCT, which was twice the incidence observed in FLT3-ITDwild group(15). A recent study investigating the transplant outcomes in poor risk patients by cytogenetic and the presence of FLT3-ITDmut showed 3-year relapse incidence of 28%−36% in normal karyotype AML patients with FLT3-ITDmut (26). These results argue for innovative strategies to reduce relapse incidence and improve LFS in FLT3-ITDmut AML (31, 32).
FLT3 kinase inhibitors with promising evidence of clinical efficacy have been investigated alone or with combination with chemotherapy not only in front-line and salvage AML therapy (33–37) but also in the post-transplant setting to prevent relapse as well. Most recently Stone et al.(38), reported improved survival with addition of FLT3 kinase inhibitor to standard chemotherapy compared with standard chemotherapy in a multi-center phase III trial. Similarly, safety and efficacy of using FLT3 kinase inhibitors in the post-transplant setting as maintenance therapy have been reported. Chen et al. (39) showed 2-year progression-free survival (PFS) of 86% if sorafenib was given as maintenance therapy in FLT3-ITDmut patients transplanted in CR1 or CR2. It is also plausible that the addition of FLT3 inhibitors to induction and/or consolidation therapy prior to stem cell transplantation will yield a potential benefit of reducing early relapses and increasing the likelihood of proceeding with HSCT. Recent studies with improved CR rates and prolonged CR duration when FLT3 inhibitors are used in combination with hypomethylating agents(35) or chemotherapy(40–42) is promising that more patients can be brought to allogeneic HSCT without early relapse while logistics of allogeneic HSCT like donor identification process is ongoing. We believe that it is worth investigating the feasibility of using new generation FLT3 inhibitors incorporated into leukemia treatment before HSCT, to conditioning regimen as a part of HSCT and then in post-transplant maintenance after HSCT, in FLT3-ITDmut patients. It is plausible that using such an integrated approach throughout different stages of leukemia treatment may lead prolonged LFS with low relapse rates and change the prognosis of FLT-ITDmut patients.
In summary, our analyses showed that allogeneic HSCT with a matched related or unrelated donor provides favorable outcomes compared with consolidation chemotherapy in FLT3-ITDmut CR1 patients independent of their allelic ratio and NMP1mutation status. With the introduction of kinase inhibitors at different stages of disease treatment, transplant outcomes may further improve. Well-designed prospective studies are needed to define what these promising drugs can offer when integrated to current treatment approaches.
Supplementary Material
Highlights.
HSCT in AML with FLT3-ITDmut as post-remission therapy in CR1 is associated with longer relapse free and overall survival compared with consolidation chemotherapy.
Irrespective of post-remission therapy, relapse remains the main reason of treatment failure
Innovative transplantation approaches to improve relapse incidence are urgently needed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Dr. Cortes received research support from Ambit, Arog, Novartis, Kyowa, and Astellas. Dr. Champlin received research support from Otsuka, and Sanofi Corporations. Dr. Andersson received research support from Otsuka Corporation.
The other authors have no conflict of interest to disclose.
REFERENCES
- 1.Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood 2001;98(6):1752–9. [DOI] [PubMed] [Google Scholar]
- 2.Frohling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood 2002;100(13):4372–80. [DOI] [PubMed] [Google Scholar]
- 3.Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 2002;99(12):4326–35. [DOI] [PubMed] [Google Scholar]
- 4.Gale RE, Green C, Allen C, Mead AJ, Burnett AK, Hills RK, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood 2008;111(5):2776–84. [DOI] [PubMed] [Google Scholar]
- 5.Whitman SP, Maharry K, Radmacher MD, Becker H, Mrozek K, Margeson D, et al. FLT3 internal tandem duplication associates with adverse outcome and gene-and microRNA-expression signatures in patients 60 years of age or older with primary cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood 2010;116(18):3622–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santos FPS, Jones D, Qiao W, Cortes JE, Ravandi F, Estey EE, et al. Prognostic Value of FLT3 Mutations Among Different Cytogenetic Subgroups in Acute Myeloid Leukemia. Cancer 2011;117(10):2145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med 2012;366(12):1079–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thiede C, Koch S, Creutzig E, Steudel C, Illmer T, Schaich M, et al. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML). Blood 2006;107(10):4011–20. [DOI] [PubMed] [Google Scholar]
- 9.Pratcorona M, Brunet S, Nomdedeu J, Ribera JM, Tormo M, Duarte R, et al. Favorable outcome of patients with acute myeloid leukemia harboring a low-allelic burden FLT3-ITD mutation and concomitant NPM1 mutation: relevance to post-remission therapy. Blood 2013;121(14):2734–8. [DOI] [PubMed] [Google Scholar]
- 10.Schnittger S, Bacher U, Kern W, Alpermann T, Haferlach C, Haferlach T. Prognostic impact of FLT3-ITD load in NPM1 mutated acute myeloid leukemia. Leukemia 2011;25(8):1297–304. [DOI] [PubMed] [Google Scholar]
- 11.Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med 2008;358(18):1909–18. [DOI] [PubMed] [Google Scholar]
- 12.Gale RE, Hills R, Kottaridis PD, Srirangan S, Wheatley K, Burnett AK, et al. No evidence that FLT3 status should be considered as an indicator for transplantation in acute myeloid leukemia (AML): an analysis of 1135 patients, excluding acute promyelocytic leukemia, from the UK MRC AML 10 and 12 trials. Blood 2005;106(10):3658–65. [DOI] [PubMed] [Google Scholar]
- 13.Sengsayadeth SM, Jagasia M, Engelhardt BG, Kassim A, Strickland SA, Goodman S, et al. Allo-SCT for high-risk AML-CR1 in the molecular era: impact of FLT3/ITD outweighs the conventional markers. Bone Marrow Transplant 2012;47(12):1535–7. [DOI] [PubMed] [Google Scholar]
- 14.Bornhauser M, Illmer T, Schaich M, Soucek S, Ehninger G, Thiede C. Improved outcome after stem-cell transplantation in FLT3/ITD-positive AML. Blood 2007;109(5):2264–5; author reply 5. [DOI] [PubMed] [Google Scholar]
- 15.Brunet S, Labopin M, Esteve J, Cornelissen J, Socie G, Iori AP, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. J Clin Oncol 2012;30(7):735–41. [DOI] [PubMed] [Google Scholar]
- 16.DeZern AE, Sung A, Kim S, Smith BD, Karp JE, Gore SD, et al. Role of allogeneic transplantation for FLT3/ITD acute myeloid leukemia: outcomes from 133 consecutive newly diagnosed patients from a single institution. Biol Blood Marrow Transplant 2011;17(9):1404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehninger G, Bornhauser M, Kramer M, Rollig C, Wandt H, Hanel M, et al. A Strong Immune Effect by Allogeneic Stem Cell Transplantation May Improve Survival in AML Patients with a High Ratio of the FLT3-ITD Mutation to the Wt-FLT3 Allele: Results from an Analysis of 257 Patients Treated in the SAL AML-2003 Trial. Blood 2011;118(21):232–. [Google Scholar]
- 18.Chen WN, Jones D, Medeiros LJ, Luthra R, Lin P. Acute myeloid leukaemia with FLT3 gene mutations of both internal tandem duplication and point mutation type. British journal of haematology 2005;130(5):726–8. [DOI] [PubMed] [Google Scholar]
- 19.Jain P, Kantarjian H, Patel K, Faderl S, Garcia-Manero G, Benjamini O, et al. Mutated NPM1 in patients with acute myeloid leukemia in remission and relapse. Leukemia & lymphoma 2014;55(6):1337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weisdorf D, Spellman S, Haagenson M, Horowitz M, Lee S, Anasetti C, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant 2008;14(7):748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. J Am Stat Assoc 1958;53(282):457–81. [Google Scholar]
- 22.Cox DR. Regression Models and Life-Tables. J R Stat Soc B 1972;34(2):187–+. [Google Scholar]
- 23.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17(19):2265–81. [DOI] [PubMed] [Google Scholar]
- 25.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5(6):649–55. [PubMed] [Google Scholar]
- 26.Oran B, Jimenez AM, De Lima M, Popat UR, Bassett R, Andersson B, et al. Age and Modified European LeukemiaNet Classification to Predict Transplant Outcomes: An Integrated Approach for Acute Myelogenous Leukemia Patients Undergoing Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant 2015. [DOI] [PMC free article] [PubMed]
- 27.Schlenk RF, Kayser S, Bullinger L, Kobbe G, Casper J, Ringhoffer M, et al. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood 2014;124(23):3441–9. [DOI] [PubMed] [Google Scholar]
- 28.Pratcorona M, Brunet S, Nomdedeu J, Ribera JM, Tormo M, Duarte R, et al. Favorable outcome of patients with acute myeloid leukemia harboring a low-allelic burden FLT3-ITD mutation and concomitant NPM1 mutation: relevance to post-remission therapy. Blood 2013;121(14):2734–8. [DOI] [PubMed] [Google Scholar]
- 29.Schneider F, Hoster E, Unterhalt M, Schneider S, Dufour A, Benthaus T, et al. The FLT3ITD mRNA level has a high prognostic impact in NPM1 mutated, but not in NPM1 unmutated, AML with a normal karyotype. Blood 2012;119(19):4383–6. [DOI] [PubMed] [Google Scholar]
- 30.Linch DC, Hills RK, Burnett AK, Khwaja A, Gale RE. Impact of FLT3(ITD) mutant allele level on relapse risk in intermediate-risk acute myeloid leukemia. Blood 2014;124(2):273–6. [DOI] [PubMed] [Google Scholar]
- 31.Oran B, de Lima M. Prevention and treatment of acute myeloid leukemia relapse after allogeneic stem cell transplantation. Curr Opin Hematol 2011;18(6):388–94. [DOI] [PubMed] [Google Scholar]
- 32.de Lima M, Porter DL, Battiwalla M, Bishop MR, Giralt SA, Hardy NM, et al. Proceedings from the National Cancer Institute’s Second International Workshop on the Biology, Prevention, and Treatment of Relapse After Hematopoietic Stem Cell Transplantation: part III. Prevention and treatment of relapse after allogeneic transplantation. Biol Blood Marrow Transplant 2014;20(1):4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kindler T, Lipka DB, Fischer T. FLT3 as a therapeutic target in AML: still challenging after all these years. Blood 2010;116(24):5089–102. [DOI] [PubMed] [Google Scholar]
- 34.Small D. FLT3 mutations: biology and treatment. Hematology Am Soc Hematol Educ Program 2006:178–84. [DOI] [PubMed]
- 35.Ravandi F, Alattar ML, Grunwald MR, Rudek MA, Rajkhowa T, Richie MA, et al. Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood 2013;121(23):4655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravandi F, Cortes JE, Jones D, Faderl S, Garcia-Manero G, Konopleva MY, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol 2010;28(11):1856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ravandi F, Arana Yi C, Cortes JE, Levis M, Faderl S, Garcia-Manero G, et al. Final report of phase II study of sorafenib, cytarabine and idarubicin for initial therapy in younger patients with acute myeloid leukemia. Leukemia 2014;28(7):1543–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stone RM; Mandrekar S; Sanford BL; Geyer S; Bloomfield CD; Dohner K; Thiede C; Marcucci G; Lo-CoCo F; Klisovic RB; et al. The multi-kinase inhibitor midostaurin (M) prolongs survival compared with placebo (P) in combination with daunorubicin (D)/cytarabine (C) induction (ind), high-dose c consolidation (consol), and as maintenance (maint) therapy in newly diagnosed acute myeloid leukemia (AML) patients (pts) age 18–60 with FLT3mutations (muts): An international prospective randomized (rand) p-controlled double-blind trial (CALGB 10603/RATIFY [Alliance]). Proceedings of the American Society of Hematology Annual Meeting, Orlando, FL, USA, 5–8 December 2015. [Google Scholar]
- 39.Chen YB, Li S, Lane AA, Connolly C, Del Rio C, Valles B, et al. Phase I trial of maintenance sorafenib after allogeneic hematopoietic stem cell transplantation for fms-like tyrosine kinase 3 internal tandem duplication acute myeloid leukemia. Biol Blood Marrow Transplant 2014;20(12):2042–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altman JK, Foran JM, Pratz KW, Trone D, Gammon G, Cortes JE, et al. Results Of a Phase 1 Study Of Quizartinib (AC220, ASP2689) In Combination With Induction and Consolidation Chemotherapy In Younger Patients With Newly Diagnosed Acute Myeloid Leukemia. Blood 2013;122(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serve H, Krug U, Wagner R, Sauerland MC, Heinecke A, Brunnberg U, et al. Sorafenib in combination with intensive chemotherapy in elderly patients with acute myeloid leukemia: results from a randomized, placebo-controlled trial. J Clin Oncol 2013;31(25):3110–8. [DOI] [PubMed] [Google Scholar]
- 42.Inaba H, Rubnitz JE, Coustan-Smith E, Li L, Furmanski BD, Mascara GP, et al. Phase I pharmacokinetic and pharmacodynamic study of the multikinase inhibitor sorafenib in combination with clofarabine and cytarabine in pediatric relapsed/refractory leukemia. J Clin Oncol 2011;29(24):3293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


