Abstract
Background
Hepatocellular carcinoma (HCC) remains difficult to diagnose at an early stage. Aldo-keto reductase family 1 member B10 (AKR1B10) is an oxidoreductase that is upregulated in some chronic liver diseases. The aim of this study was to use immunohistochemistry to evaluate the expression of AKR1B10 in liver tissue from patients with HCC of different stages.
Material/Methods
Forty-four patients with a tissue diagnosis of HCC (35 males and 9 females) with 37 control samples of liver tissue containing liver cirrhosis were studied using immunohistochemistry for the expression of AKR1B10. Histological examination determined the grade of HCC; the stage of HCC was determined according to the Barcelona Clinic Liver Cancer (BCLC) staging system. Serum alpha-fetoprotein (AFP) levels were measured and compared between the patients with HCC.
Results
Immunohistochemistry showed increased expression of AKR1B10 in moderately-differentiated HCC compared with well-differentiated HCC, poorly-differentiated HCC, and liver cirrhosis (P<0.05). Sensitivity and specificity of AKR1B10 expression in HCC were high at a cutoff integral optical density (IOD) value of 89.5. A significant increase in AKR1B10 expression was found in early-stage HCC (P<0.05). Serum AFP levels were increased in patients with poorly-differentiated HCC, were increased in intermediate-stage HCC, and were significantly increased in advanced-stage HCC (P<0.05).
Conclusions
Immunohistochemistry showed that the expression of AKR1B10 was increased in tumor tissue from patients with early-stage HCC. Further studies are needed to determine the role of AKR1B10 in the early detection of HCC.
MeSH Keywords: alpha-Fetoproteins; Carcinoma, Hepatocellular; Early Detection of Cancer
Background
Despite improved screening methods for the detection of hepatocellular carcinoma (HCC), most cases are still diagnosed at an advanced stage and are associated with intra-hepatic or extra-hepatic metastases. HCC has a high patient mortality rate and is currently ranked as the third most common cause of cancer-related mortality, after lung cancer and gastric cancer [1]. There are several known risk factors for HCC, including infection with hepatitis B virus (HBV) and hepatitis C virus (HCV) that are also associated with chronic hepatitis and cirrhosis, which are pathological processes thought to be the underlying causes for HCC.
In China, the majority of cases of HCC (about 80%) are associated with HBV and HCV infection [2], which continues to rise each year [3]. Surgical resection for HCC is still considered to be an effective primary form of clinical management of early-stage HCC, with a good outcome in most cases. However, because there are limited diagnostic approaches available to detect people at high risk of developing HCC, most patients with HCC present with advanced-stage disease, resulting in less effective treatment options, including surgical resection and liver transplantation and locoregional procedures, including radiofrequency ablation [4].
In the past decade, published studies have identified serum biomarkers that might have the potential for the early diagnosis of HCC [5]. In particular, serum alpha-fetoprotein (AFP) levels have been shown to be increased in patients with advanced HCC [6,7]. However, the use of serum AFP levels are unreliable for the detection of early-stage HCC, and normal serum AFP levels can be found in some patients with histologically confirmed HCC [8]. Therefore, the use of serum AFP measurement as the main choice for a screening method for the detection of early-stage HCC has limitations.
Recently, several biomarkers have been investigated as potential candidates for the early detection of HCC and have been included in clinical screening programs, including AFP-L3 and des-gamma-carboxy prothrombin (DCP) [5,9–14]. Also, miR-4417, which may function as an oncogene in HCC, has been identified as a potential screening biomarker for HCC [15]. Retinoblastoma-binding protein 2 (RBP2) has been reported to be an independent prognostic factor for disease-free survival (DFS) and overall survival (OS) in patients with HCC [16]. However, each of these potential new diagnostic biomarkers appears to be poorly sensitive for the diagnosis of early-stage HCC. Therefore, there remains a need to identify novel and reliable biomarkers for the detection of early-stage HCC.
Aldo-keto reductase family 1 member B10 (AKR1B10) is an oxidoreductase and a key member of the aldo-keto reductase superfamily that modulates cell growth and survival by regulating lipid synthesis [17,18] and eliminating carbonyl compounds [19–24]. AKR1B10 has been shown to be involved in the proliferation and development of tumors through the regulation of the retinoic acid signaling pathway [25,26]. AKR1B10 is overexpressed in several types of tumor tissues [19], including tissues containing human HCC [27,28]. AKR1B10 is mainly expressed in the cytoplasm and can be secreted through a lysosome-mediated non-classical pathway and is regulated by lysosome exocytosis signaling [29]. As a potential independent risk factor for the development of HCC, AKR1B10 can exert its regulatory role in predicting or monitoring the initiation and development of HCC, suggesting that AKR1B10 is involved in molecular signaling pathways leading to HCC, but these pathways remain poorly understood [30–32]. Increased expression of AKR1B10 in HCC patients has previously been reported to be associated with well-differentiated, or low-grade HCC in liver tissues while HCC patients with low or negative expression of AKR1B10 have previously been reported to have a poorer prognosis when resected liver tumors have been studied [27,33,34]. However, the pattern of expression of AKR1B10 in patients with early-stage HCC remains unclear.
Therefore the aim of this study was to use immunohistochemistry to evaluate the expression of AKR1B10 in liver tissue from patients with HCC of different stages, according to the Barcelona Clinic Liver Cancer (BCLC) staging system, together with serum AFP levels, liver function tests, and hepatitis virus serology.
Material and Methods
Patients
Forty-four patients with liver resection specimens containing HCC (35 males and 9 females) were included in the study, who had undergone liver surgery between October 2014 to February 2016 in the Department of Hepatobiliary Surgery, Shengjing Hospital, China Medical University. The study protocol was reviewed and approved by the Intuitional Review Board (IRB) and the Ethics Committee of Shengjing Hospital, China Medical University. Informed consent was obtained from the study participants. The identity of each participant, whose tissue samples and the corresponding medical records were obtained, were coded and later decoded after data analysis was completed.
Eligibility criteria for inclusion in the study included patients with histologically confirmed HCC, diagnosed by two experienced pathologists. The degree of differentiation of HCC (grade) was defined according to the Edmondson–Steiner grading system [35]. The stage of HCC was determined according to the BCLC staging system. The number and size of the HCC tumor nodules and the presence of portal vein invasion were identified by computed tomography (CT) or magnetic resonance imaging (MRI). Pathologic examination confirmed the presence of lymph node metastases. Liver cirrhosis tissues were used as control tissues and were obtained from surgically resected adjacent tissues from 37 patients who underwent hepatectomy for HCC in this study.
Laboratory investigations
The Clinical Laboratory of our hospital tested blood samples all patients included in the study. A range of biochemical tests, including liver function tests, were analyzed and included alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, total bilirubin (TBil), prothrombin time (PT) international normalized ratio (INR). Serum hepatitis B virus (HBV) and hepatitis C virus (HCV) viral load was detected by real-time polymerase chain reaction (PCR) using the COBAS AmpliPrep/COBAS TaqMan HBV/HCV Test (version 2.0). Hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg) and hepatitis C antibody were detected by electrochemiluminescence using the ARCHITECT PLUS i2000. Serum AFP levels were measured by electrochemiluminescence using a Roche E170 immunoassay analyzer.
Immunohistochemistry
The Pathology Department of Shengjing Hospital provided Formalin-fixed, paraffin-embedded (FFPE) tissue sections of HCC and cirrhotic liver. Tissue sections at a thickness of 4 μm were prepared onto glass slides, deparaffinized with xylene, and serial dilutions of alcohol. The tissue sections were processed by heat-induced antigen retrieval using 0.01 mmol/L citrate buffer at pH 6.0 for 20 minutes in a microwave oven. After blocking endogenous peroxidase activity with 0.3% H2O2, the sections were blocked for nonspecific antibody binding with 5% normal goat serum. The tissue sections were then incubated with the primary rabbit monoclonal antibody against AKR1B10 (ab192856) (Abcam, Cambridge, UK) at a dilution of 1: 500, overnight at 4°C. After washing with phosphate-buffered saline (PBS), the sections were incubated with a biotinylated polyclonal goat anti-rabbit immunoglobulin G (IgG) antibody for 20 minutes at room temperature, followed by incubation with peroxidase-conjugated streptavidin using the UltraSensitive S-P detection kit (KIT-9710, Maxim Biotechnologies, China). The brown chromogen, 3,3′-diaminobenzidine tetrahydrochloride was incubated on the slides for 90 seconds. Following washing, the slides were counterstained with hematoxylin and mounted with glass coverslips. Negative controls were performed by replacing the primary antibody with normal mouse immunoglobin (I8765) (Sigma-Aldrich Biochemicals, USA).
On light microscopy, positive AKR1B10 immunostaining was based on positive cytoplasmic staining. The average integral optical density (IOD) of five randomly selected fields at ×200 magnification was quantitatively assessed by using NIS-Elements BR Analysis 4.50.00 software (Nikon Corporation, Japan).
Statistical analysis
Statistical analysis was performed using the SPSS statistical software version 17.0 (IBM, New York, USA). One-way analysis of variance (ANOVA) and Fisher’s exact test were used for statistical analysis, when appropriate. If one-way ANOVA identified a significant difference, a post hoc test was used to determine the difference between the groups. A P-value of <0.05 was considered to be statistically significant.
Results
Patient clinical and laboratory characteristics
The clinical, demographic, and biochemical findings of the 44 patients with resected liver samples containing HCC who were enrolled in this study are summarized in Table 1. Of the 44 patients in the HCC group, five patients (11.36%) had decreased albumin levels (<35 g/L), three patients had elevated TBil levels (>34 μmol/L), and twenty-nine patients (65.9%) had serum AFP levels of <100 ng/ml. There were 35 patients with HCC (79.6%) who were confirmed to have chronic HBV infection, three patients (6.8%) were confirmed to have chronic HCV infection, and six patients (13.6%) were confirmed to have no infection with hepatitis virus. Three of the 35 patients with HCC and chronic HBV infection were negative for HBV DNA.
Table 1.
Characteristics of all participants.
| Characteristics | Hepatocellular carcinoma | P-value | ||
|---|---|---|---|---|
| Well differentiated (n=16) | Moderately differentiated (n=19) | Poorly differentiated (n=9) | ||
| Gender | 0.975# | |||
| Male | 13 | 15 | 7 | |
| Female | 3 | 4 | 2 | |
| Age (yaer) | 51.4±7.8 | 54.6±9.6 | 55.9±5.8 | 0.371@ |
| Aetiology | 0.581# | |||
| HBV Infection | 14 (87.5) | 15 (79.0) | 6 (66.7) | |
| HCV Infection | 0 (0.0) | 2 (10.5) | 1 (11.1) | |
| Noninfectious | 2 (12.5) | 2 (10.5) | 2 (22.2) | |
| ALT (U/L) | 75.4±97.2 | 63.5±73.0 | 43.1±22.0 | 0.603@ |
| AST (U/L) | 72.1±106.2 | 44.8±27.3 | 37.6±21.1 | 0.371@ |
| Albumin (g/L) | 41.6±5.5 | 40.3±7.7 | 42.4±4.6 | 0.701@ |
| TBil (μmol/L) | 17.8±10.2 | 18.3±12.0 | 14.0±6.3 | 0.580@ |
| PT INR | 1.2±0.1 | 1.1±0.1 | 1.1±0.1 | 0.447@ |
| HBsAg§ | 0.503# | |||
| >250 IU/ml | 10 (71.4) | 13 (86.7) | 4 (66.7) | |
| <250 IU/ml | 4 (28.6) | 2 (13.3) | 2 (33.3) | |
| HBeAg§ | 0.725# | |||
| Positive | 4 (28.6) | 6 (40.0) | 3 (50.0) | |
| Negative | 10 (71.4) | 9 (60.0) | 3 (50.0) | |
| HBV DNA§ | 0.436# | |||
| <3 log IU/ml | 6 (42.9) | 7 (46.6) | 0 (0.0) | |
| 3–6 log IU/ml | 6 (42.9) | 6 (40.0) | 4 (66.6) | |
| >6 log IU/ml | 1 (7.1) | 1 (6.7) | 1 (16.7) | |
| Unkonwn | 1 (7.1) | 1 (6.7) | 1 (16.7) | |
| HCV RNA§ | 0.333# | |||
| <3 log IU/ml | 0 | 2 (100.0) | 0 (0.0) | |
| >3 log IU/ml | 0 | 0 (0.0) | 1 (100.0) | |
| Nodule size (cm) | 4.4±2.4 | 3.8±1.7 | 7.1±3.8* | 0.009@ |
| Number of Nodules | 0.581# | |||
| 1 | 14 (87.5) | 13 (68.4) | 8 (88.9) | |
| 2 | 2 (12.5) | 3 (15.8) | 1 (11.1) | |
| 3 | 0 (0.0) | 3 (15.8) | 0 (0.0) | |
| Lymphatic metastasis | 0.075# | |||
| Positve | 3 (18.7) | 0 (0.0) | 2 (22.2) | |
| Negative | 13 (81.3) | 19 (100.0) | 7 (77.8) | |
| Portal invasion | 0.092# | |||
| Positive | 1 (6.2) | 0 (0.0) | 2 (22.2) | |
| Negative | 15 (93.8) | 19 (100.0) | 7 (77.8) | |
| BCLC stage | 0.007# | |||
| Early | 11 (68.8) | 13 (68.4) | 5 (55.6) | |
| Intermediate | 1 (6.2) | 6 (31.6) | 0 (0.0) | |
| Advanced | 4 (25.0) | 0 (0.0) | 4 (44.4) | |
Data are described as mean ± standard deviation or number (%).
One-way analysis of variance (ANOVA);
Fisher’s exact test;
data are not available for all patients.
P-values are for comparisons between patients with and without poorly-differentiated HCC tissue.
AKR1B10 – aldo-keto reductase family 1 member B10; AFP – alpha-fetoprotein; ALT – alanine aminotransferase; AST – aspartate aminotransferase; TBil – total bilirubin; PT INR – prothrombin time international normalized ratio; HBsAg – hepatitis B surface antigen; HBeAg – hepatitis e antigen; BCLC – Barcelona Clinic Liver Cancer.
Patient imaging findings
On imaging, the mean HCC tumor nodule size was significantly increased in patients with poorly-differentiated HCC (7.1±3.8 cm) (n=19) compared with patients with well-differentiated HCC (4.4±2.4 cm) (n=16), and with moderately-differentiated HCC (3.8±1.7 cm) (n=9) (P<0.05).
Grade and stage of HCC in 44 patients studied
The BCLC staging system and the Edmondson–Steiner grading system were used. Of the 44 patients with HCC, there were 16 patients with well-differentiated HCC, 16 patients with moderately-differentiated HCC, and 9 patients with poorly-differentiated HCC. There were statistically significant associations between the stage of HCC and with the tumor grade (P<0.05). There were 44.4% of patients with poorly-differentiated HCC (4/9), 25.0% of patients with moderately-differentiated HCC (4/16) and 0% of patients with well-differentiated HCC (0/16) who had advanced-stage HCC.
Immunohistochemical detection of AKR1B10 expression and serum AFP levels in patients with different grades of HCC
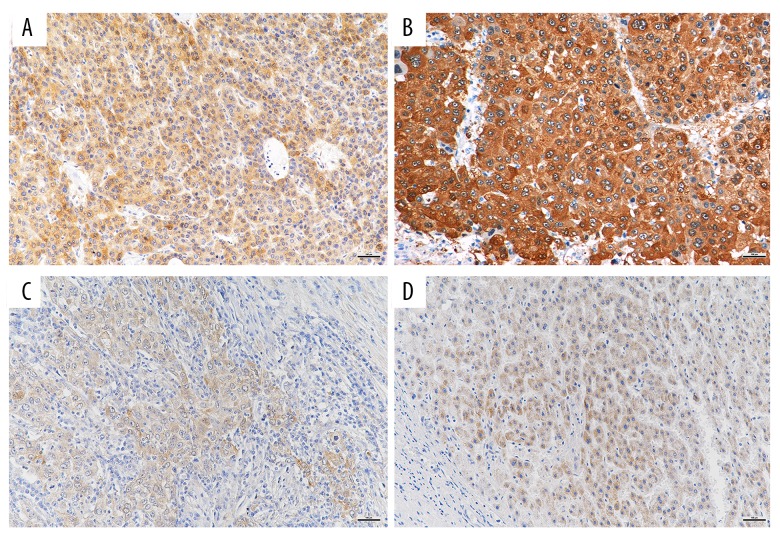
Figure 1 shows the immunostaining for AKR1B10 in tissue sections containing HCC and tissue sections containing liver cirrhosis. The AKR1B10 immunostaining was located in the cytoplasm.
Figure 1.
Photomicrographs of the immunohistochemical staining of aldo-keto reductase family 1 member B10 (AKR1B10) showing cytoplasmic immunoreactivity in hepatocellular carcinoma (HCC) tissue and liver cirrhosis tissue. (A) Well-differentiated HCC tissue stained for AKR1B10 using immunohistochemistry shows cytoplasmic immunostaining. A significant increase in AKR1B10 immunostaining of the cytoplasm is seen. Original magnification ×200. (B) Moderately-differentiated HCC tissue stained for AKR1B10 using immunohistochemistry. Original magnification ×200. (C) Poorly-differentiated HCC tissue stained for AKR1B10 using immunohistochemistry. Original magnification ×200. (D) Liver cirrhosis tissue stained for AKR1B10 using immunohistochemistry. Original magnification ×200.
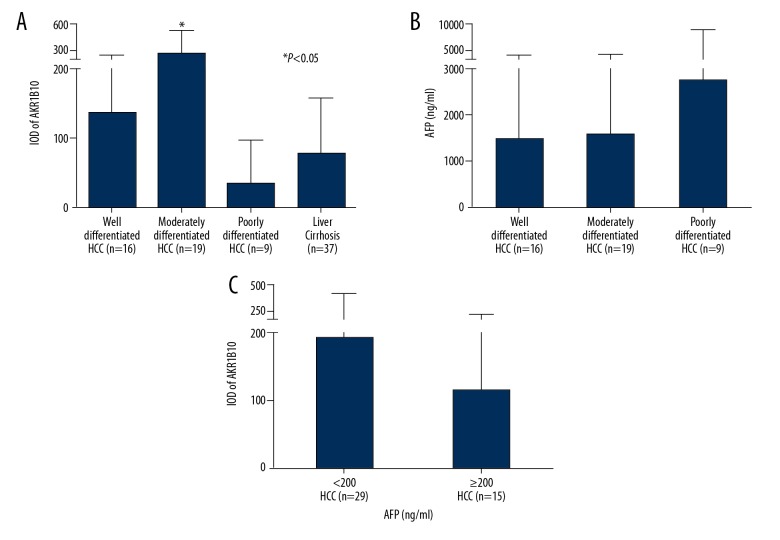
The IOD of AKR1B10 immunostaining obtained from different specimens were compared using one-way ANOVA, and the results are shown in Table 2 and Figure 2. The mean expression levels of AKR1B10 were significantly increased in liver tissue containing moderately-differentiated HCC (255.6±276.8) (n=19) compared with in liver tissue containing well-differentiated HCC (135.4±109.8) (n=16), in liver tissue containing poorly differentiated (33.8±63.4) (n=9), and in liver tissue containing cirrhosis (76.7±82.5) (n=37) (P<0.05) (Figure 2A). Although the mean expression levels of AKR1B10 were increased in liver tissue containing well-differentiated HCC, the difference between the liver tissue containing well-differentiated HCC, poorly-differentiated HCC, and liver cirrhosis was not statistically significant.
Table 2.
Tissue AKR1B10 expression of all participants.
| Characteristics | Number | IOD of AKR1B10 | AFP (ng/ml) |
|---|---|---|---|
| Well-differentiated HCC | 16 | 135.4±109.8 | 1485.2±4047.9 |
| Moderately-differentiated HCC | 19 | 255.6±276.8* | 1582.3±3921.1 |
| Poorly-differentiated HCC | 9 | 33.8±63.4 | 2745.8±6303.6 |
| Liver cirrhosis | 37 | 76.7±82.5 | – |
| P-value | <0.001@ | 0.775@ |
Data are described as mean ± standard deviation.
One-way analysis of variance (ANOVA);
P-values are for comparisons between patients with and without moderately-differentiated HCC tissue.
AKR1B10 – aldo-keto reductase family 1 member B10; HCC – hepatocellular carcinoma; IOD – integral optical density.
Figure 2.
Bar graphs of the integral optical density (IOD) of tissue aldo-keto reductase family 1 member B10 (AKR1B10) and serum alpha-fetoprotein (AFP) levels. (A) IOD of tissue AKR1B10. (B) Serum AFP levels in patients with HCC. (C) IOD of tissue AKR1B10 between groups with different serum AFP levels.
The sensitivity and specificity of tissue expression of AKR1B10 using immunohistochemistry for the diagnosis of grade and stage of HCC were determined by creating a receiver operating characteristic (ROC) curve (Figure 3). When a cutoff value of 89.5 was used, the sensitivity and specificity of tissue expression of AKR1B10 expression using immunohistochemistry were 52.3% and 73.0%, respectively.
Figure 3.
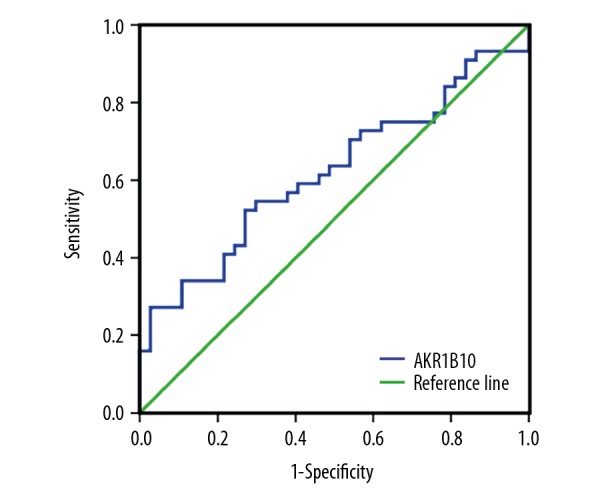
Receiver operating characteristic (ROC) curves of the sensitivity and specificity of tissue aldo-keto reductase family 1 member B10 (AKR1B10) and the association with hepatocellular carcinoma (HCC).
The mean serum AFP levels were increased in patients with poorly-differentiated HCC (2745.8±6303.6 ng/ml) (n=9) compared with patients with well-differentiated HCC (1485.2±4047.9 ng/ml) (n=16), or moderately-differentiated HCC (1582.3±3921.1 ng/ml) (n=18), but these differences did not reach statistical significance (P=0.775) (Figure 2B). Of the 44 patients with HCC, 65.9% of patients (29/44) had serum AFP levels lower than 200 ng/ml, which was set as the local diagnostic threshold for the detection of HCC in the clinic.
There was no statistically significant difference between the mean tissue expression levels of AKR1B10 between the HCC tissues and serum AFP levels when <200 ng/ml (193.3±238.6) (n=29) and when ≥200 ng/ml (114.7±136.8) (n=15) (P=0.293) (Figure 2C). Also, in patients with HCC and an IOD of tissue ARK1B10 >89.5, only 12 patients had serum AFP levels <16 ng/ml, which has been previously reported to be the relative diagnostic threshold of AFP with the highest sensitivity and specificity [36]. This additional study finding added support to the potential clinical value of measuring tissue ARK1B10 levels rather than serum AFP.
Correlation between the HCC tissue expression of AKR1B10 and serum AFP levels with the stage of HCC
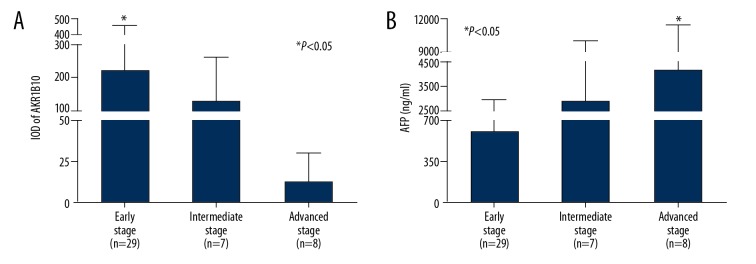
The results of the correlation between liver tissue expression of AKR1B10 in patients with HCC and serum AFP levels associated with tumor stage are summarized in Table 3. Patients with HCC that were in early, intermediate, and advanced stages were selected for further sub-group analysis. One-way ANOVA analyzed multiple comparisons of mean tissue AKR1B10 and serum AFP levels obtained from patients with HCC at different stages, and the overall difference between the groups was determined, followed by post hoc least significant difference (LSD) analysis. As shown in Figure 4, there was a statistically significant difference between tumor tissue expression of AKR1B10 and serum AFP levels in a stage-dependent manner. The highest expression levels of AKR1B10 were found in patients with early-stage HCC (219.1±232.8) (n=29), but were reduced in patients with intermediate-stage HCC (124.5±138.5) (n=7) and in patients with advanced-stage HCC (12.6±17.8) (n=8) (P<0.05) (Figure 4A). In contrast, the highest serum AFP levels were found in patients with advanced-stage HCC (4097.1±7302.7 ng/ml) (n=8), when compared within patients with early-stage HCC (605.6±2316.1 ng/ml) (n=29) and patients with intermediate-stage HCC (4028.7±5903.4 ng/ml) (n=7) (P<0.05) (Figure 4B). These findings, from the present study, showed a difference in expression of AKR1B10 and AFP in the development of HCC and an increase in AKR1B10, but not serum AFP, in early-stage HCC. The findings of this study support that AKR1B10 could be involved in the development of HCC and requires further investigation as a possible diagnostic tissue biomarker for the detection of early-stage HCC.
Table 3.
Tissue AKR1B10 expression and serum AFP levels with HCC at the different BCLC stages.
| BCLC stage | Number | IOD of AKR1B10 | AFP (ng/ml) |
|---|---|---|---|
| Early stage | 29 | 219.1±232.8* | 605.6±2316.1 |
| Intermediate stage | 7 | 124.5±138.5 | 4028.7±5903.4 |
| Advanced stage | 8 | 12.6±17.8 | 4097.1±7302.7# |
| P-value | 0.038@ | 0.046@ |
Data are described as mean ± standard deviation.
One-way analysis of variance (ANOVA);
P-values are for comparisons between HCC patients with and without early stage;
P-values are for comparisons between HCC patients with and without advanced stage.
AKR1B10 – aldo-keto reductase family 1 member B10; AFP – alpha-fetoprotein; HCC – hepatocellular carcinoma; BCLC – Barcelona Clinic Liver Cancer; IOD – integral optical density.
Figure 4.
Bar graphs of the integral optical density (IOD) of tissue aldo-keto reductase family 1 member B10 (AKR1B10) and serum alpha-fetoprotein (AFP) levels in patients with hepatocellular carcinoma (HCC) at different clinical stages, classified using the Barcelona Clinic Liver Cancer staging system. (A) Bar graph of the IOD of tissue AKR1B10 shows a significant increase in AKR1B10 in early-stage HCC. (B) Bar graph of serum AFP levels shows a significant increase in AFP was in advanced-stage HCC.
Discussion
HCC has an insidious onset but rapid progression, leading to a high mortality rate. When first diagnosed, the majority of patients with HCC are already in the advanced stage, often presenting with intrahepatic and extrahepatic metastases. Methods of detecting asymptomatic early-stage HCC are still needed to reduce mortality and improve the prognosis of patients with HCC [37].
In this study, immunohistochemistry was used to evaluate the expression of AKR1B10 in liver tissue from patients with HCC of different grades and stages, in 44 patients from a single center in China. The findings showed that patients with moderately-differentiated HCC had significantly increased expression of tissue AKR1B10 compared with the patients with poorly-differentiated HCC and liver cirrhosis. A significant increase in the expression of tissue AKR1B10 was detected in patients with early-stage HCC, while serum AFP levels were significantly increased in patients with advanced-stage HCC.
Serum AFP levels are commonly measured to detect HCC, but this type of malignancy is not always homogeneous, and some patients with HCC may have normal or only mildly elevated serum AFP levels. For high-risk populations, serum AFP levels and liver ultrasonography have recommended every six months as a screening procedure for HCC [38]. However, the effectiveness of this type of screening approach is limited by the relatively low sensitivity and specificity of AFP as a reliable biomarker for identifying early-stage HCC, as for an AFP cutoff value of 16 ng/ml, the sensitivity and specificity for diagnosing HCC have been shown to be between 60–80% and between 70–90%, respectively [36]. However, serum AFP levels have been shown to be negative in between 30–40% of HCC patients, which makes this diagnostic approach less reliable [8]. In the present study, in 61.4% of HCC patients (27/44), increased tumor tissue expression of ARK1B10 was associated with early-stage HCC, while serum AFP levels was still below its threshold for the diagnosis of HCC. Also, 65.9% of HCC patients (29/44) had mean serum AFP levels <200 ng/ml, which is higher than the optimal diagnostic value. This finding indicates that the expression of AKR1B10 correlated with the development of HCC, and might be a potential biomarker for early detection of HCC.
Previously published studies have shown that AKR1B10 expression is upregulated in liver diseases, including HCC, but its biological significance in molecular and pathological processes in HCC remains poorly understood. The findings of the present study showed that HCC tissue expression of AKR1B10 was significantly increased compared with non-tumor tissue, in this case with liver cirrhosis, and further analysis showed a strong correlation between AKR1B10 and HCC, which is a finding supported by a previously published study [27]. In the present study, a cutoff value for AKR1B10 of 89.5, as determined by the ROC curve in this study, showed a sensitivity and specificity for AKR1B10 at a diagnostic level for HCC could be achieved.
Also, in the present study, the correlation between HCC tissue expression of AKR1B10 and tumor stage using the BCLC staging system, showed a significant increase in tissue AKR1B10 of patients with early-stage and intermediate-stage HCC, but not in advanced-stage HCC. This unique pattern of tissue expression of AKR1B10 is the opposite of that found for serum AFP levels, which increase with tumor stage in patients with HCC. These findings indicate that there might be a close relationship between the expression of ARK1B10 and the early development of HCC.
However, previously published studies have shown that the expression of hepatic AKR1B10 is upregulated in patients with HBV and HCV infections, as well as in hepatic fibrosis, which are also changes that are associated with the development of HCC [30–33]. Therefore, it might be possible to hypothesize that chronic hepatitis, with upregulation of AKR1B10, could be associated with the development of early-stage or well-differentiated HCC. These findings, together with those of previous studies, indicate that the expression of ARK1B10 during early-stage HCC support its potential role as a biomarker for early-stage HCC, particularly when compared to the use of serum AFP levels.
This study had several limitations. In this study, serum levels of AKR1B10 were not measured or compared with the grade and stage of HCC in the patients studied. This study was performed in a single center in China, with a small study population. Therefore, future prospective studies are recommended that include analysis of larger study populations from multiple centers to further analyze the association between serum levels of AKR1B10 and HCC. Also, because the expression of AKR1B10 has been previously reported in several liver diseases and is not exclusive to HCC, further studies are required to characterize the specific role of AKR1B10 in HCC.
Conclusions
An immunohistochemical study showed that tissue expression of AKR1B10 in HCC tissues was increased in a stage-dependent manner and could be a potential biomarker for diagnosing early-stage HCC.
Footnotes
Source of support: The National Science and Technology Major Project (2017ZX10201201, 2017ZX10202202, 2017ZX10202203); the China National Science and Technology Key Project for Infectious Diseases Control for the Consecutive 12th Five-Year Plan Period (2012ZX10002003-003-010); the Liaoning Provincial Science and Technology Key Project for Translational Medicine (2016509); the Liaoning Provincial Science and Technology Key Project for Translational Medicine (2014225020); the Science and Technology Project of Liaoning Province (2013225021); and the Outstanding Scientific Fund of Shengjing Hospital (201102)
References
- 1.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Dicker D, Pain A, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1:505–27. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19:223–38. doi: 10.1016/j.cld.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X, Gao JY, Wang J, Cheng J. The impact of anti-HBV treatment on the occurrence and recurrence of hepatocellular carcinoma: Focus on Asian studies. Discov Med. 2015;19:89–99. [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 5.Tsuchiya N, Sawada Y, Endo I, et al. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2015;21:10573–83. doi: 10.3748/wjg.v21.i37.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aghoram R, Cai P, Dickinson JA. Alpha-foetoprotein and/or liver ultrasonography for screening of hepatocellular carcinoma in patients with chronic hepatitis B. Cochrane Database Syst Rev. 2012;(9):CD002799. doi: 10.1002/14651858.CD002799.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun S, Rhie SY, Ki CS, et al. Evaluation of alpha-fetoprotein as a screening marker for hepatocellular carcinoma in hepatitis prevalent areas. Ann Hepatol. 2015;14:882–88. doi: 10.5604/16652681.1171776. [DOI] [PubMed] [Google Scholar]
- 8.Giannini EG, Marenco S, Borgonovo G, et al. Alpha-fetoprotein has no prognostic role in small hepatocellular carcinoma identified during surveillance in compensated cirrhosis. Hepatology. 2012;56:1371–79. doi: 10.1002/hep.25814. [DOI] [PubMed] [Google Scholar]
- 9.Li D, Mallory T, Satomura S. AFP-L3: A new generation of tumor marker for hepatocellular carcinoma. Clin Chim Acta. 2001;313:15–19. doi: 10.1016/s0009-8981(01)00644-1. [DOI] [PubMed] [Google Scholar]
- 10.Marrero JA, Feng Z, Wang Y, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110–18. doi: 10.1053/j.gastro.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sterling RK, Jeffers L, Gordon F, et al. Utility of Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein and des-gamma-carboxy prothrombin, alone or in combination, as biomarkers for hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2009;7:104–13. doi: 10.1016/j.cgh.2008.08.041. [DOI] [PubMed] [Google Scholar]
- 12.Lok AS, Sterling RK, Everhart JE, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493–502. doi: 10.1053/j.gastro.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ertle JM, Heider D, Wichert M, et al. A combination of alpha-fetoprotein and des-gamma-carboxy prothrombin is superior in detection of hepatocellular carcinoma. Digestion. 2013;87:121–31. doi: 10.1159/000346080. [DOI] [PubMed] [Google Scholar]
- 14.Pote N, Cauchy F, Albuquerque M, et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62:848–54. doi: 10.1016/j.jhep.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Song L, Zhang W, Chang Z, et al. miR-4417 targets tripartite motif-containing 35 (TRIM35) and regulates pyruvate kinase muscle 2 (PKM2) phosphorylation to promote proliferation and suppress apoptosis in hepatocellular carcinoma cells. Med Sci Monit. 2017;23:1741–50. doi: 10.12659/MSM.900296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang ZY, Yang J, Liu CK, Shen SQ. High expression of retinoblastoma-binding protein 2 (RBP2) in patients with hepatocellular carcinoma and its prognostic significance. Med Sci Monit. 2017;23:2736–44. doi: 10.12659/MSM.905262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma J, Yan R, Zu X, et al. Aldo-keto reductase family 1 B10 affects fatty acid synthesis by regulating the stability of acetyl-CoA carboxylase-alpha in breast cancer cells. J Biol Chem. 2008;283:3418–23. doi: 10.1074/jbc.M707650200. [DOI] [PubMed] [Google Scholar]
- 18.Wang C, Yan R, Luo D, et al. Aldo-keto reductase family 1 member B10 promotes cell survival by regulating lipid synthesis and eliminating carbonyls. J Biol Chem. 2009;284:26742–48. doi: 10.1074/jbc.M109.022897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao D, Fan ST, Chung SS. Identification and characterization of a novel human aldose reductase-like gene. J Biol Chem. 1998;273:11429–35. doi: 10.1074/jbc.273.19.11429. [DOI] [PubMed] [Google Scholar]
- 20.Conklin D, Prough R, Bhatanagar A. Aldehyde metabolism in the cardiovascular system. Mol Biosyst. 2007;3:136–50. doi: 10.1039/b612702a. [DOI] [PubMed] [Google Scholar]
- 21.Spite M, Baba SP, Ahmed Y, et al. Substrate specificity and catalytic efficiency of aldo-keto reductases with phospholipid aldehydes. Biochem J. 2007;405:95–105. doi: 10.1042/BJ20061743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan R, Zu X, Ma J, Liu Z, et al. Aldo-keto reductase family 1 B10 gene silencing results in growth inhibition of colorectal cancer cells: Implication for cancer intervention. Int J Cancer. 2007;121:2301–6. doi: 10.1002/ijc.22933. [DOI] [PubMed] [Google Scholar]
- 23.Martin HJ, Maser E. Role of human aldo-keto-reductase AKR1B10 in the protection against toxic aldehydes. Chem Biol Interact. 2009;178:145–50. doi: 10.1016/j.cbi.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 24.Zhong L, Liu Z, Yan R, et al. Aldo-keto reductase family 1 B10 protein detoxifies dietary and lipid-derived alpha, beta-unsaturated carbonyls at physiological levels. Biochem Biophys Res Commun. 2009;387:245–50. doi: 10.1016/j.bbrc.2009.06.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crosas B, Hyndman DJ, Gallego O, et al. Human aldose reductase and human small intestine aldose reductase are efficient retinal reductases: Consequences for retinoid metabolism. Biochem J. 2003;373:973–79. doi: 10.1042/BJ20021818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallego O, Belyaeva OV, Porte S, et al. Comparative functional analysis of human medium-chain dehydrogenases, short-chain dehydrogenases/reductases and aldo-keto reductases with retinoids. Biochem J. 2006;399:101–9. doi: 10.1042/BJ20051988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matkowskyj KA, Bai H, Liao J, et al. Aldoketoreductase family 1B10 (AKR1B10) as a biomarker to distinguish hepatocellular carcinoma from benign liver lesions. Hum Pathol. 2014;45:834–43. doi: 10.1016/j.humpath.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heringlake S, Hofdmann M, Fiebeler A, et al. Identification and expression analysis of the aldo-ketoreductase1-B10 gene in primary malignant liver tumours. J Hepatol. 2010;52:220–27. doi: 10.1016/j.jhep.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Luo DX, Huang MC, Ma J, et al. Aldo-keto reductase family 1, member B10 is secreted through a lysosome-mediated non-classical pathway. Biochem J. 2011;438:71–80. doi: 10.1042/BJ20110111. [DOI] [PubMed] [Google Scholar]
- 30.Sato S, Genda T, Hirano K, et al. Up-regulated aldo-keto reductase family 1 member B10 in chronic hepatitis C: Association with serum alpha-fetoprotein and hepatocellular carcinoma. Liver Int. 2012;32:1382–90. doi: 10.1111/j.1478-3231.2012.02827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mori M, Genda T, Ichida T, et al. Aldo-keto reductase family 1 member B10 is associated with hepatitis B virus-related hepatocellular carcinoma risk. Hepatol Res. 2017;47:E85–93. doi: 10.1111/hepr.12725. [DOI] [PubMed] [Google Scholar]
- 32.Murata A, Genda T, Ichida T, et al. Pretreatment AKR1B10 expression predicts the risk of hepatocellular carcinoma development after hepatitis C virus eradication. World J Gastroenterol. 2016;22:7569–78. doi: 10.3748/wjg.v22.i33.7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitz KJ, Sotiropoulos GC, Baba HA, et al. AKR1B10 expression is associated with less aggressive hepatocellular carcinoma: A clinicopathological study of 168 cases. Liver Int. 2011;31:810–16. doi: 10.1111/j.1478-3231.2011.02511.x. [DOI] [PubMed] [Google Scholar]
- 34.Ha SY, Song DH, Lee JJ, et al. High expression of aldo-keto reductase 1B10 is an independent predictor of favorable prognosis in patients with hepatocellular carcinoma. Gut Liver. 2014;8:648–54. doi: 10.5009/gnl13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edmondson HA, Steiner PE. Primary carcinoma of the liver: A study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 36.Trevisani F, D’Intino PE, Morselli-Labate AM, et al. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: Influence of HBsAg and anti-HCV status. J Hepatol. 2001;34:570–75. doi: 10.1016/s0168-8278(00)00053-2. [DOI] [PubMed] [Google Scholar]
- 37.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–22. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellissimo F, Pinzone MR, Cacopardo B, Nunnari G. Diagnostic and therapeutic management of hepatocellular carcinoma. World J Gastroenterol. 2015;21:12003–21. doi: 10.3748/wjg.v21.i42.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]