Abstract
Several studies have proposed that fibrillary aggregates of tau and other amyloidogenic proteins are neurotoxic and result in numerous neurodegenerative diseases. However, these studies usually involve sonication or extrusion through needles before experimentation. As a consequence, these methods may fragment large aggregates producing a mixture of aggregated species rather than intact fibrils. Therefore, the results of these experiments may be reflective of other amyloidogenic species, such as oligomers and/or protofibrils/short fibrils. To investigate the effects of sonication on the aggregation of tau and other amyloidogenic proteins, fibrils were prepared and well characterized, then sonicated and evaluated by various biochemical and biophysical methods to identify the aggregated species present. We found that indeed a mixture of aggregated species was present along with short fibrils indicating that sonication leads to impure fibril samples and should be analyzed with caution. Our results corroborate the previous studies showing that sonication of prion and Aβ fibrils leads to the formation of toxic, soluble aggregates. We also show that the oligomeric forms are the most toxic species although it is unclear how sonication causes oligomer formation. Recent results suggest that these small toxic oligomers produced by sonication, rather than the stable fibrillar structures, are prion‐like in nature displaying seeding and cross‐seeding behavior.
Keywords: tau oligomers, tauopathies, sonication, amyloid
Introduction
Misfolding and aggregation of tau into neurofibrillary tangles (NFTs) is one of the pathological hallmarks occurring in tauopathies including Alzheimer's disease (AD) and other neurodegenerative diseases.1, 2 Although NFTs have long been considered the neurotoxic species responsible for neuronal death observed in the case of tauopathies, recent studies have shown otherwise. Studies with Htau mice (transgenic mice expressing wild‐type human tau) have shown that there was no correlation between the presence of tau tangles in aged mice and neuronal death.3 Several other studies also corroborated the finding that disease‐associated synaptic and mitochondrial dysfunction, cell death, and neuronal loss occurred before NFT formation.4, 5, 6, 7 Another study showed that neurons bearing tangles survive in spite of the loss of membrane integrity indicating minimal role of tau fibrils in inducing cytotoxic effects.8 Congruently, several studies have shown that prefibrillar soluble aggregates or oligomers and protofibrils of tau correlate with cognitive deficits.7, 9, 10, 11, 12, 13 Therefore, tau oligomers have emerged as a potential target for therapeutic development, especially, targets for immunotherapy.14, 15, 16
In spite of the overwhelming evidence supporting the critical role played by tau oligomers in initiation and progression of the disease phenotype, there is still some controversy around their role and some studies argue that tau fibrils are the primary toxic agents.17, 18, 19 These studies, however, subject the insoluble tau fibrils to sonication before the treatment of the cells. Sonication may result in fragmenting of the tau fibrils into a mixture of small aggregates and/or short fibrils and therefore would raise a question about the nature of true propagating and infectious agents. Studies with amyloid‐β and prion proteins have shown that sonication or extrusion through needles resulted in formation of soluble aggregates which were more potent in seeding as compared to mature fibrils.20, 21, 22, 23 Thus, it is quite clear that sonication converts insoluble tau fibrils in to soluble small aggregates or oligomers. Moreover, sonication of tau fibrils has been shown to cause shearing of filaments, particularly those in paired helical filament (PHF) form.24, 25, 26 All the evidence supports the argument that sonication can result in a breakdown of the intact fibrils into a mixture of small aggregates or oligomers and short fibrils and that propagation and spreading of sonicated fibrils could actually be due to such short oligomers and not insoluble fibrils. This could be important in teasing out the true “infectious agent” responsible for propagating the disease phenotype.
In this report, we show that when sonicated, tau and α‐Synuclein fibrils result in the formation of a mixture of small aggregates and short fibrils. These small aggregates were T22‐ and Syn33‐positive (tau &α‐Synuclein oligomer‐specific antibodies) indicating that the mixture did contain oligomers. Interestingly, these aggregates showed prion‐like behavior and underwent seeding and cross‐seeding. Moreover, as expected, this mixture of aggregates or oligomers and short fibrils generated by sonication of tau fibrils were toxic in SH‐SY5Y neuroblastoma cells. These results are significant in establishing that the smaller soluble aggregates of tau, and not mature fibrils, are more potent seeding agents and are toxic.
Results
Sonication generates tau oligomer‐specific antibody, T22, positive aggregates
Both tau and α‐Synuclein were expressed recombinantly in Escherichia coli as described previously27, 28 and characterized by immunoblotting and MALDI ToF/ToF Matrix Assisted Laser Desorption/Ionization, Time of Flight/Time of Flight (MALDI ToF/ToF) which shows that both proteins were monomeric and pure (Fig. S1(a,b)). To investigate if sonication lead to the fragmentation of fibrils and formation of a mixture of aggregates/oligomers and short fibrils, tau fibrils were prepared from tau monomers (TauM) by incubating with heparin as detailed in Materials and Methods section. The fibrils were then sonicated as depicted in the schematic (Fig. 1). To confirm if the mixture of aggregates and short fibrils generated via sonication of tau fibrils were oligomeric in nature, the samples were blotted on to a nitrocellulose membrane using the filter trap assembly. The membranes were then probed with T22, antibody specific for tau oligomers which does not recognize and bind to either the soluble monomeric tau or insoluble fibrils.11 When probed with T22, an intense band was observed for the tau aggregate mixture generated via sonication abbreviated as sTauO (Fig. 2(a), sTauO). In contrast, the band for tau fibrils, abbreviated as TauF, was faint suggesting the presence of some tau oligomers in the fibril sample, which, perhaps, did not form fibrils and remained oligomeric (Fig. 2(a), TauF). As expected, no bands were observed for TauM and PBS (phosphate buffer saline). The recombinant oligomers labeled as TauO were used as a positive control (Fig. 2(a), TauO). All proteins were loaded at identical concentration of 2 μg. Additionally, the presence of tau in all the samples was confirmed using a commercially available pan anti‐tau antibody, Tau 13. Similar results were obtained for α‐Synuclein fibrils and oligomers generated via sonication using Syn33,29 an oligomer‐specific antibody and 4D6, a pan anti‐Synuclein antibody. These results confirm that sonication, indeed, resulted in the formation of a mixture of aggregates/oligomers and short fibrils. Next, to further confirm that the fibrils were fragmented into an ensemble of aggregates/oligomers and short fibrils postsonicated, all the samples described above were probed with a conformation‐specific antibody that recognizes only fibrillar conformations, OC.30 An intense band was observed for unsonicated samples for both, tau and α‐Synuclein (Fig. S2(a,b), TauF and α‐SynF), whereas a diffused band was observed for sonicated ensemble (Fig. S2(a,b), sTauO and sα‐SynO) indicating sparse presence, if not complete absence, of large fibrils in the sonicated ensemble.
Figure 1.
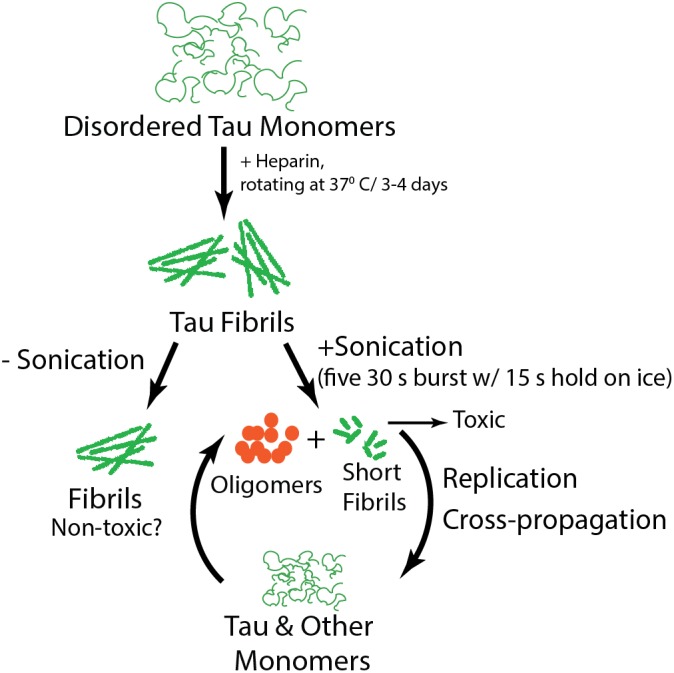
Schematic of tau fibril formation and sonication.
Figure 2.
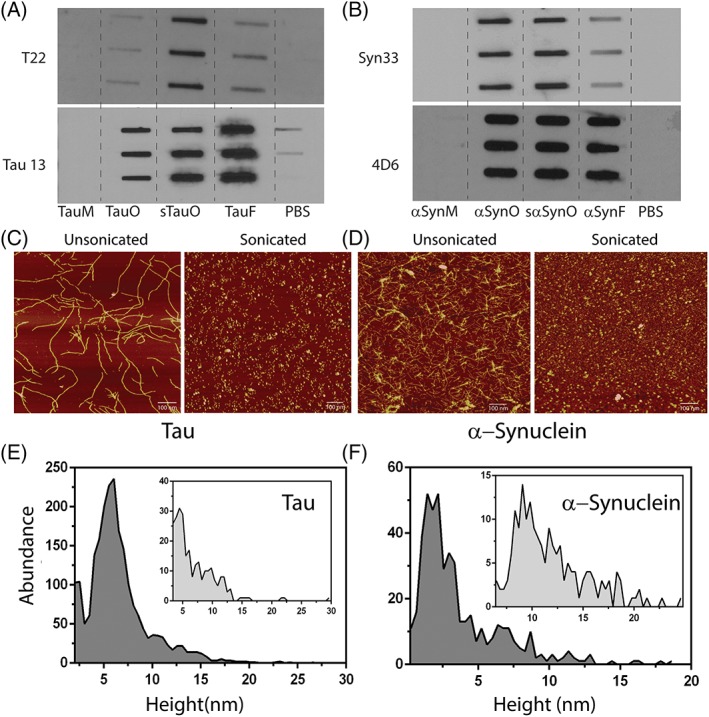
Sonication generates T22‐positive aggregates/oligomers. (a, b) Filter trap analyses of tau and α‐Synuclein monomers (TauM/αSynM); tau and α‐Synuclein oligomers from diseased brain tissues (TauO/αSynO); tau and α‐Synuclein oligomers generated via sonication (sTauO/sα‐SynO); and tau and α‐Synuclein fibrils (TauF/αSynF) probed using T22 or Syn33 (antibodies specific for tau and α‐Synuclein oligomers only, respectively), and Tau 13 or 4D6 (pan anti‐tau or anti‐α‐Synuclein antibodies). The triplicates represent n = 3. (c, d) Atomic force microscopy images showing unsonicated and sonicated tau and α‐Synuclein fibrils and oligomers. Images are representative of multiple repeats. (e, f) Height distribution of sonicated ensemble and unsonicated fibrils (inset) of tau and α‐Synuclein analyzed using particle analysis tool of NanoScope Analysis software.
Furthermore, the formation of fibrils was monitored using atomic force microscopy (AFM). TauMs, 0.5–1 mg, incubated with heparin in a 4:1 molar ratio of heparin to monomer formed typical fibrils with a height of about 15–17 nm and a periodic helicity as observed for fibrils induced from recombinant monomers using heparin (Fig. 2(c), unsonicated).31 Similarly, recombinantly expressed α‐Synuclein monomers, 1–4 mg, were incubated with physiological salt concentration at 37°C with constant stirring to induce fibril formation and was monitored using AFM. The α‐Synuclein fibrils were linear and short with an average height of 10–12 nm (Fig. 2(d), unsonicated). As expected, sonication of fibrils of both the proteins resulted in their fragmentation forming a mixture of aggregates/oligomers and short fibrils in the case of tau and primarily short fibrils in the case of α‐Synuclein (Fig. 2(c,d), sonicated, respectively). The average height of the sonicated ensemble consisting of tau aggregates/oligomers and short fibrils was ~4–6 nm, whereas the average height of unsonicated fibrils was in the range of 11–15 nm. The average height of sonicated α‐Synuclein ensemble was ~5–7 nm and that of unsonicated fibrils was in the range of 9–10 nm. Additionally, the abundance or distribution of different species (monomer, oligomers, and/or fibrils) in the sonicated ensemble and unsonicated fibrils was estimated by plotting the height against the abundance from the AFM images using the particle analysis tool available in the NanoScope Analysis v1.20r1 AFM data processing software. The distribution indicated that the majority of the species in the sonicated ensemble image (Fig. 2(c), sonicated) have heights ranging from 5 to 7 nm (Fig. 2(e)). As expected, the abundance of species above the height of 10 nm was marginal in the sonicated ensemble image. In comparison, particle analysis of the fibril image (Fig. 2(c), unsonicated) showed species with heights ranging from 5 to 15 nm suggesting heterogeneity in the sample (Fig. 2(e), inset). A similar profile was observed for the sonicated ensemble of α‐Synuclein and α‐Synuclein fibrils where the sonicated ensemble showed a distribution of species with heights ranging from 2 to 5 nm (Fig. 2(f)), whereas the fibrils (unsonicated) showed species ranging from 7 to 20 nm (Fig. 2(f), inset). Moreover, the dynamic light scattering of the sonicated ensemble and the unsonicated fibrils of tau showed distinct size differences; the sonicated ensemble showed a single peak distribution with an average size of 59.4 nm (Fig. S3, sonicated), whereas the unsonicated fibrils showed much higher size distribution at 843.8 nm (Supporting Information Fig. S3, unsonicated).
These results collectively indicate that sonication results in fragmentation of tau and α‐Synuclein fibrils resulting in the formation of an ensemble of small aggregates/oligomers and short fibrils.
Oligomers generated via sonication undergo a secondary structure transition and propagate
To investigate if the fibrils simply break apart during sonication or undergo some sort of structural transition, which can explain their toxic nature detailed further, the secondary structures of unsonicated and sonicated fibrils were monitored using far‐UV circular dichroism. The CD spectrum of unsonicated fibrils, prepared from recombinant TauMs, showed a broad minimum at ~223 nm and a minimum at ~208 nm observed typically for synthetic fibrils prepared in the presence of heparin (Fig. 3(a), solid line).31 In comparison, the sonicated fibrils showed a single sharp minimum at ~205 nm (Fig. 3(a), dashed line), which is reminiscent of more disorder or flexibility in the structure indicating a structural transition caused by sonication. For α‐Synuclein, the change in the secondary structure presonication (Fig. 3(b), solid line) and postsonication (Fig. 3(b), dashed line) was not as prominent as observed for tau as both pre‐ and postsonicated α‐Synuclein fibrils showed largely disordered structures suggesting that sonication did not induce any structural changes.
Figure 3.
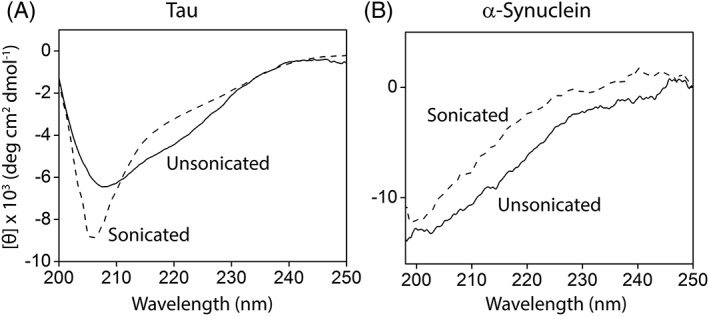
Secondary structure analysis of (a) sonicated (dashed) and unsonicated (solid) tau fibrils and (b) sonicated (dashed) and unsonicated (solid) α‐Synuclein fibrils. The image is a representative of three or more repeats.
Furthermore, the self‐ and cross‐propagative nature of these aggregates and short fibrils, generated via sonication, was investigated by seeding and cross‐seeding reactions. Briefly, Tau and α‐Synuclein monomers were incubated with tau and α‐Synuclein oligomers generated via sonication in the ratio of 100:1 for 2 h at 37°C with constant rotation at 30 rpm. The reactions were monitored using AFM. The tau oligomeric seed incubated with tau or α‐Synuclein monomers converted the respective monomers into similar oligomers with an average height of 3–5 nm (Fig. 4(a)). Similar results were obtained for α‐Synuclein oligomeric seed incubated with α‐Synuclein or TauMs (Fig. 4(b)).
Figure 4.
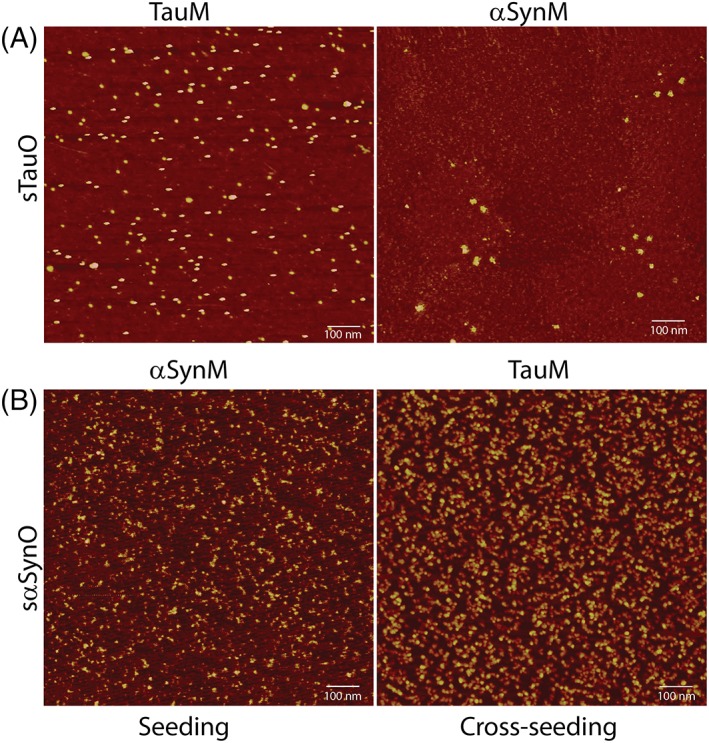
Seeding and cross‐seeding of tau aggregates. (a) sTauO added as seeds to tau or α‐Synuclein monomers. (b) sα‐SynO added as seeds to α‐Synuclein or TauM. The images are representative of three consistent repeats.
The toxicity of sonicated fibrils was significantly reduced after preincubated with excess amounts of affinity‐purified tau oligomer antibody T22
Finally, the toxicity of the aggregates/oligomers generated via sonication was tested. In addition to unsonicated fibrils, sonicated fibrils, recombinant tau oligomers, and the vehicle (PBS), cells were treated with sonicated fibrils preincubated with affinity‐purified tau oligomer‐specific antibody T22 for 45 min. There was a significant (P < 0.01) decrease in the viability for the cells incubated with sonicated fibrils as compared to those incubated with unsonicated fibrils (Fig. 5). In fact, the toxicity of sonicated fibrils (Tau Fib [Son]) was comparable to that of recombinant tau oligomers. Furthermore, the toxicity of sonicated fibrils (Tau Fib [Son]) was reduced with increasing amounts of preincubated T22 antibody, and cells treated with sonicated fibrils preincubated with T22 antibody in 1:4 ratio had viability comparable to those treated with unsonicated fibrils indicating that the oligomeric ensemble generated postsonication is the toxic species.
Figure 5.
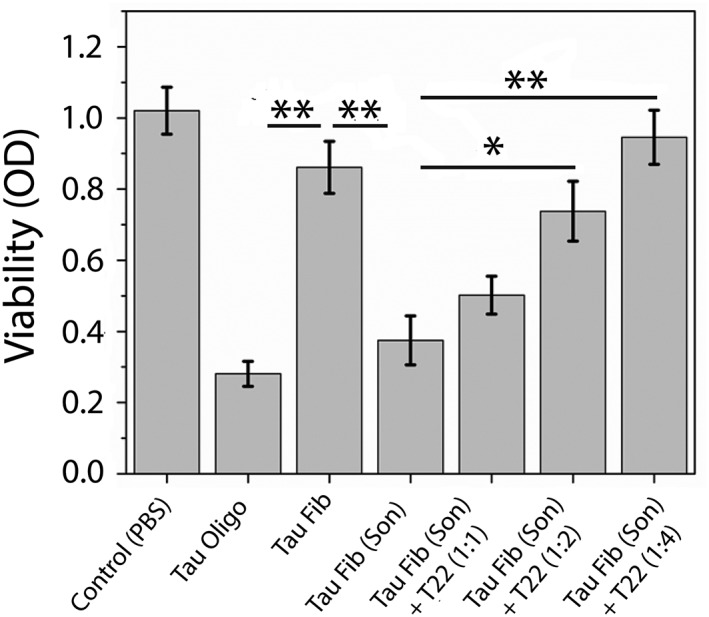
Cell viability assay of SH‐SY5Y cells treated with 2 μM of different preparations: tau oligomers, tau fibrils and sonicated tau fibrils, and sonicated tau fibrils preincubated with T22 antibody at 1:1, 1:2, and 1:4 ratios, respectively (sonicated tau fibrils: T22 antibody). The data demonstrate that oligomers generated by sonication of preformed fibrils are highly toxic to cells. The toxicity of sonicated fibrils significantly reduced upon preincubating them with excess amount of affinity‐purified T22 antibody. Bars and error bars represent mean values and standard deviations, respectively (* P < 0.05). (** P < 0.01).
Discussion and Conclusions
Tau oligomers, and not the visible meta‐stable tau aggregates including NFTs, have emerged as the main pathogenic species in different tauopathies.10, 11, 12, 13, 32, 33, 34, 35, 36 In this report, we corroborate this known fact that soluble oligomers of tau and other amyloidogenic proteins like α‐Synuclein and Aβ are the primary toxic species in the disease phenotype and act like the prion seeds that have the potential to propagate the disease phenotype. The aim of this report was twofold: first to demonstrate that toxic oligomeric seeds can be generated from fibrils through a simple method of sonication, and second to caution about interpreting the results obtained from using the ensemble species generated via sonication. Several studies have shown that tau fibril‐treated cells exhibited seeding and propagation of aggregates via endocytosis.17, 18, 19 However, most of these studies use fibrils that were sonicated before cell treatment. We argue that treatments like sonication or even extrusion through needles do not actually yield intact fibrils but an ensemble of small aggregates/oligomers and short fibrils are responsible for the activity observed in the particular experiment. This could be important in understanding the actual toxic species. We show that sonication induced the fragmentation of tau and α‐Synuclein fibrils in to a mixture or ensemble of aggregates/oligomers and short fibrils. When tested against T22 and Syn33, a tau and α‐Synuclein oligomer‐specific antibodies, respectively, this mixture showed a positive signal confirming the presence of oligomers. Additionally, when probed with a fibril‐specific antibody, OC, the ensemble showed a diffuse band as compared to unsonicated fibrils indicating the sparse presence, if not complete absence, of large fibrillar species. Although it has been shown earlier that sonication of Aβ fibrils also leads to formation of a mixture of toxic small aggregates,37, 38 similar results were obtained when Aβ 40 or 42 fibrils were sonicated and tested against A11, Αβ oligomer‐specific antibody (Supporting Information Fig. S4). The particle size analyses of the ensemble for both tau and α‐Synuclein yielded a distribution of species with heights ranging from 5 to 7 nm and 2 to 5 nm, respectively, which corresponds to the average height of oligomers of both the proteins.12, 39 In comparison, the unsonicated fibrils for tau and α‐Synuclein showed a wide distribution in height ranging from 5 to 15 nm suggesting the presence of fibrils and other species with high heterogeneity.40 The secondary structure analysis of sonicated and unsonicated tau fibrils showed a distinct structural transition from an ordered structure, in case of unsonicated fibrils, to a more disordered structure of the sonicated ensemble. However, a similar structural transition was not observed in the case of α‐Synuclein; this can be attributed to the heterogeneity of unsonicated fibrils. Moreover, the sonicated ensemble, when added as a seed to tau or α‐Synuclein monomers, templated the corresponding monomers into similar oligomers showing the “prion‐like” behavior as observed previously.41 It can be speculated that the structural change observed for sonicated tau fibrils, perhaps, could be the reason for these aggregates to undergo seeding and cross‐seeding. Lastly, a simple cell viability assay, irrevocably, showed that the sonicated ensemble and not the fibrils were toxic further bolstering the idea of oligomers being the primary toxic species. Interestingly, when the sonicated ensemble was preincubated with increasing amount of an oligomer‐specific antibody, T22, their toxicity was reduced further confirming that the oligomers are, indeed, the primary toxic species. The enhanced toxicity of the oligomer can be explained by their easy internalization via bulk endocytosis and is responsible for disease propagation and spread.42 Thus, we have a strong evidence to believe that these small aggregates/oligomers and short fibrils formed via sonication of insoluble tau or α‐Synuclein fibrils, which appear to undergo seeding and cross‐seeding, may be responsible for the propagation of misfolded proteins making them “prion‐like.” These findings have been corroborated by several other studies as well.34, 38, 41, 43 Further evidence to support this idea can be adapted from the prion field where it has been proven that nonfibrillar PrP particles are the most efficient seeds that convert the nonpathogenic PrP to protease‐resistant PrP and thus possibly initiates the prion disease.22, 44
We speculate two possible pathways of fibril breakdown via sonication. One possibility may be that sonicated fibrils are fragmented into short fibrils and oligomers, and as they fragment, they undergo a structural transition. Alternatively, fibrils formed from monomers may dissemble into monomers that, in the presence of continuous sonication, subsequently assemble into oligomers. The evidence for the first hypothesis comes from the simple fact that these fibrils are likely formed from oligomers and thus break back down into their building blocks. Moreover, we have carried out experiments where aliquots from all five cycles of sonication as well as aliquots after 0, 5, 15, 20, and 30 min of incubation postsonication were probed using T22 and were seen to be T22‐positive indicating that oligomers are formed immediately, even after the first cycle of sonication (data not shown). The second hypothesis is supported in part by data that suggest sonication of monomeric proteins results in oligomeric aggregates, as sonication generates high local temperatures that destabilize proteins and thus promote their misfolding and amyloid formation.
Such observations are important for amyloid diseases in which the oligomeric species is observed and believed to be the most toxic. Both tau and α‐Synuclein are natively unfolded proteins in their monomeric form which lacks an ordered structure under physiological conditions.45, 46 Like Aβ, both tau and α‐Synuclein undergo templated aggregation or fibril formation. Misfolded TauMs that are primarily disordered aggregate to form fibrils showing β‐sheet rich structures in the presence of negatively charged cofactors such as RNA and heparin. The soluble oligomers show a structure that is intermediate between disordered monomers and β‐sheet‐rich NFTs with sizes varying accordingly.47 Two theories have been proposed to model tau aggregation, and in either model tau undergoes disorder to order transition as it aggregates sequentially forming soluble oligomers, paired helical filaments, and eventually NFTs.48 In the initial phases of aggregation, α‐Synuclein partially folds into premolten globule‐like conformations which show a high aggregation propensity.46 These early conformations form the nonfibrillar soluble oligomers or aggregates which are known to have spheroidal morphology with heights ranging from 2 to 6 nm and largely disordered and partial α‐helical structures.49 These oligomers further aggregate, rather rapidly, to form extended protofilaments or protofibrils which have a height of 5–6 nm and undergo a significant disordered to order transition into β‐sheet‐rich structures.50 The seeding capability of oligomers, and not the fibrils, may explain how disease progresses from one region of the brain to the next. Physiologically, there could be certain processes in cellular environment that can create sonication‐like conditions that can cause fibrils to fragment into smaller aggregates.25 Sonication is known to induce free radical formation via sonolysis of water.25 The ˙OH radicals can further initiate formation of reactive oxygen species which are known to disrupt protein structure via oxidative damage.51 Oxidative stress and formation of reactive oxygen species is a common feature of neurodegenerative diseases including AD and has even been speculated as an early event during disease progression causing aggregation of amyloid proteins.52, 53 Therefore, one can speculate that the high level of oxidative stress and damage prevalent in AD pathology can create sonication‐like conditions causing fragmentation and breakdown of fibrils. Therefore, we should not discount the presence of oligomers in these mixtures produced via sonication and ought to be cautious when utilizing such techniques, which at the same time can be useful for reverse engineering different fibril preparations into smaller toxic aggregate with different conformations.
Materials and Methods
Preparation of tau/α‐Synuclein fibrils
The human tau‐441 isoform (2N4R) was expressed recombinantly in E. coli BL21 (DE3) cells and purified as detailed previously.27 Aliquots of a monomeric tau solution (1 mg/mL) were prepared in sterile water with 1× PBS (final concentration) and incubated with heparin (15 kDa) in 1:4 ratio of heparin to TauM11 at 37°C for 3–5 days. The tubes were rotated constantly using a rotary shaker at a speed of 30 rpm.
Human α‐Synuclein was expressed recombinantly as reported previously.28 The α‐Synuclein monomers were incubated with physiological salt concentration in water and stirred at 37°C for 6–7 days. Sodium azide was added to the reaction at 0.01% to prevent bacterial growth.
Sonication of fibrils
The fibrils were sonicated using a Fisher Scientific Sonic Dismembrator Model 100. The samples were subjected to five 30‐s bursts on ice with 15 s intervals. The sonication intensity was maintained between 30% and 50% to avoid foaming of the sample. After the fifth burst, the samples were incubated on ice for 30 min before being used in any experiments.
Seeding and cross‐seeding
Sonicated fibrils of either tau or α‐Synuclein were incubated with aggregate‐free monomers of either tau or α‐Synuclein in 1:100 ratio of seed to monomers for 2 h at 37°C on a rotary shaker set at 30 rpm as reported previously.54
Atomic force microscopy
Atomic force microscopic images were obtained using a Bruker MultiMode 8 microscope. The samples were diluted in the range of 0.03–0.05 μg/μL. A 12 mm mica attached to a 15‐mm metallic disc was freshly cleaved to obtain a uniform surface. The 10‐μL diluted sample was added on the freshly cleaved mica. The sample was allowed to be adsorbed on the surface for at least 30 min or overnight. The mica was then washed three times with 150 μL of sterile water, and the surface was dried. The images were procured using the Bruker ScanAssyst mode.
For calculating distribution, three different fields were chosen from the regions of interest. Using the particle analysis tool of the NanoScope Analysis v1.20r1 AFM data processing software, the height of the particles was extrapolated. The results were exported to Graphpad, averaged, and plotted.
Filter‐trap assay
A piece of nitrocellulose membrane was cut to fit the slot blot chamber and soaked in 1X Tris‐buffered saline low‐tween (TBST) for 5 min.55 The samples of tau and α‐Synuclein were loaded in triplicates in the wells of the slot blot apparatus. The amount of protein loaded across all the chambers was maintained constant at 2 μg. After the samples were transferred on to the membrane, it was removed from the cassette and blocked with 10% milk solution prepared in 1X TBST at 4 °C overnight or for 1 h at room temperature (RT). The membranes with tau samples were then probed with T22 (1:250) and Tau 13, a pan tau antibody (1:25,000; BioLegend (San Diego, CA, USA)). Membranes with α‐Synuclein samples were probed with Syn33,29 an antibody for α‐Synuclein oligomers (1:1000) and 4D6, a pan α‐Synuclein antibody (1:10,000; BioLegend (San Diego, CA, USA)) diluted in 5% milk solution and incubated for 1 h at RT. The membranes were washed three times for 10 min in 1X TBST and then incubated with their respective secondary antibodies for 1 h at RT. Immunoreactivity for T22 and Syn33 antibodies was detected by using an horse raddish peroxidase (HRP)‐conjugated anti‐rabbit IgG antibody (1:10,000) diluted in 5% milk. Tau 13 and 4D6 immunoreactivity was detected by using HRP‐conjugated anti‐mouse IgG antibody (1:10,000) diluted in 5% milk. After washing for three times as described above, detection substrate was added to the membrane and developed immediately on X‐ray autoradiography films.
Cell viability assay
SH‐SY5Y neuroblastoma cells were maintained in Dulbecco's Modified Eagle Medium (DMEM) with 10 mM HEPES ((4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid)), 10% fetal bovine serum, 4 mM glutamine, penicillin (200 U/mL), and streptomycin (200 μg/mL) in 5% CO2 at 37°C. The medium was replaced every 2 days, and cells were plated at 10,000 cells per well in 96‐well plates and grown overnight. The medium was removed and 2 μM of preparations (tau oligomers, tau fibrils, and sonicated tau fibrils) were added to the cells. After incubation for 90 min at 37°C, cells were subjected to MTT (3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide) colorimetric assay using Cell Proliferation I (MTT) assay kit (Roche), according to the manufacturer's directions. Separately sonicated tau fibrils were also preincubated with affinity‐purified T22 antibody for 45 min in 1:1, 1:2, and 1:4 ratio (sonicated tau fibrils:T22 antibody) before the treatment of the cells. This treatment was also followed by MTT colorimetric assay to assess the viability of the cells treated with preincubated samples. Each treatment was performed in triplicates, and one‐way analysis of variance was used to get the statistical significance.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Appendix S1: Supporting Information.
Acknowledgments
We thank the Kayed lab members for helpful discussions. We are grateful for financial support from the National Institutes of Health (R01AG054025, R01NS094557, RF1AG055771), Mitchell Center for Neurodegenerative Diseases, and the Gillson Longenbaugh Foundation.
References
- 1. Ballatore C, Lee VM‐Y, Trojanowski JQ (2007) Tau‐mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci 8:663–672. [DOI] [PubMed] [Google Scholar]
- 2. Gendron TF, Petrucelli L (2009) The role of tau in neurodegeneration. Mol Neurodegener 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andorfer C, Kress Y, Espinoza M, De Silva R, Tucker KL, Barde YA, Duff K, Davies P (2003) Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem 86:582–590. [DOI] [PubMed] [Google Scholar]
- 4. Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM (2007) Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53:337–351. [DOI] [PubMed] [Google Scholar]
- 5. de Calignon A, Fox LM, Pitstick R, Carlson GA, Bacskai BJ, Spires‐Jones TL, Hyman BT (2010) Caspase activation precedes and leads to tangles. Nature 464:1201–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spires TL, Orne JD, Santa Cruz K, Pitstick R, Carlson GA, Ashe KH, Hyman BT (2006) Region‐specific dissociation of neuronal loss and neurofibrillary pathology in a mouse model of tauopathy. Am J Pathol 168:1598–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lasagna‐Reeves CA, Castillo‐Carranza DL, Sengupta U, Clos AL, Jackson GR, Kayed R (2011) Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild‐type mice. Mol Neurodegener 6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Calignon A, Spires‐Jones TL, Pitstick R, Carlson GA, Hyman BT (2009) Tangle‐bearing neurons survive despite disruption of membrane integrity in a mouse model of tauopathy. J Neuropathol Exp Neurol 68:757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sydow A, Van der Jeugd A, Zheng F, Ahmed T, Balschun D, Petrova O, Drexler D, Zhou L, Rune G, Mandelkow E (2011) Tau‐induced defects in synaptic plasticity, learning, and memory are reversible in transgenic mice after switching off the toxic Tau mutant. J Neurosci 31:2511–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300:486–489. [DOI] [PubMed] [Google Scholar]
- 11. Lasagna‐Reeves CA, Castillo‐Carranza DL, Sengupta U, Sarmiento J, Troncoso J, Jackson GR, Kayed R (2012) Identification of oligomers at early stages of tau aggregation in Alzheimer's disease. FASEB J 26:1946–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lasagna‐Reeves CA, Castillo‐Carranza DL, Sengupta U, Guerrero‐Munoz MJ, Kiritoshi T, Neugebauer V, Jackson GR, Kayed R (2012) Alzheimer brain‐derived tau oligomers propagate pathology from endogenous tau. Sci Rep 2:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patterson KR, Remmers C, Fu Y, Brooker S, Kanaan NM, Vana L, Ward S, Reyes JF, Philibert K, Glucksman MJ, Binder LI (2011) Characterization of prefibrillar tau oligomers in vitro and in Alzheimers disease. J Biol Chem 286:23063–23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Castillo‐Carranza DL, Lasagna‐Reeves CA, Kayed R (2013) Tau aggregates as immunotherapeutic targets. Front Biosci 5:426–438. [DOI] [PubMed] [Google Scholar]
- 15. Castillo‐Carranza DL, Gerson JE, Sengupta U, Guerrero‐Muñoz MJ, Lasagna‐Reeves CA, Kayed R (2014) Specific targeting of tau oligomers in Htau mice prevents cognitive impairment and tau toxicity following injection with brain‐derived tau oligomeric seeds. J Alzheimers Dis 40:S97–S111. [DOI] [PubMed] [Google Scholar]
- 16. Castillo‐Carranza DL, Sengupta U, Guerrero‐Muñoz MJ, Lasagna‐Reeves CA, Gerson JE, Singh G, Estes DM, Barrett AD, Dineley KT, Jackson GR (2014) Passive immunization with Tau oligomer monoclonal antibody reverses tauopathy phenotypes without affecting hyperphosphorylated neurofibrillary tangles. J Neurosci 34:4260–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iba M, Guo JL, McBride JD, Zhang B, Trojanowski JQ, Lee VM‐Y (2013) Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer's‐like tauopathy. J Neurosci 33:1024–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Narasimhan S, Guo JL, Changolkar L, Stieber A, McBride JD, Silva LV, He Z, Zhang B, Gathagan RJ, Trojanowski JQ (2017) Pathological tau strains from human brains recapitulate the diversity of tauopathies in nontransgenic mouse brain. J Neurosci 37:11406–11423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo JL, Lee VM‐Y (2011) Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer‐like tangles. J Biol Chem 286:15317–15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Langer F, Eisele YS, Fritschi SK, Staufenbiel M, Walker LC, Jucker M (2011) Soluble Aβ seeds are potent inducers of cerebral β‐amyloid deposition. J Neurosci 31:14488–14495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Silveira JR, Raymond GJ, Hughson AG, Race RE, Sim VL, Hayes SF, Caughey B (2005) The most infectious prion protein particles. Nature 437:257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cho KR, Huang Y, Yu S, Yin S, Plomp M, Qiu SR, Lakshminarayanan R, Moradian‐Oldak J, Sy M‐S, De Yoreo JJ (2011) A multistage pathway for human prion protein aggregation in vitro: from multimeric seeds to β‐oligomers and nonfibrillar structures. J Am Chem Soc 133:8586–8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ayrolles‐Torro A, Imberdis T, Torrent J, Toupet K, Baskakov IV, Poncet‐Montange G, Grégoire C, Roquet‐Baneres F, Lehmann S, Rognan D (2011) Oligomeric‐induced activity by thienyl pyrimidine compounds traps prion infectivity. J Neurosci 31:14882–14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frost B, Diamond MI (2010) Prion‐like mechanisms in neurodegenerative diseases. Nat Rev Neurosci 11:155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stathopulos PB, Scholz GA, Hwang YM, Rumfeldt JA, Lepock JR, Meiering EM (2004) Sonication of proteins causes formation of aggregates that resemble amyloid. Protein Sci 13:3017–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Umemoto A, Yagi H, So M, Goto Y (2014) High‐throughput analysis of ultrasonication‐forced amyloid fibrillation reveals the mechanism underlying the large fluctuation in the lag time. J Biol Chem 289:27290–27299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Margittai M, Langen R (2004) Template‐assisted filament growth by parallel stacking of tau. Proc Natl Acad Sci USA 101:10278–10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Planchard MS, Exley SE, Morgan SE, Rangachari V (2014) Dopamine‐induced α‐synuclein oligomers show self‐and cross‐propagation properties. Protein Sci 23:1369–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sengupta U, Guerrero‐Muñoz MJ, Castillo‐Carranza DL, Lasagna‐Reeves CA, Gerson JE, Paulucci‐Holthauzen AA, Krishnamurthy S, Farhed M, Jackson GR, Kayed R (2015) Pathological interface between oligomeric alpha‐synuclein and tau in synucleinopathies. Biol Psychiatry 78:672–683. [DOI] [PubMed] [Google Scholar]
- 30. Kayed R, Head E, Sarsoza F, Saing T, Cotman CW, Necula M, Margol L, Wu J, Breydo L, Thompson JL (2007) Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener 2:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morozova OA, March ZM, Robinson AS, Colby DW (2013) Conformational features of tau fibrils from Alzheimer's disease brain are faithfully propagated by unmodified recombinant protein. Biochemistry 52:6960–6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta‐peptide. Nat Rev Mol Cell Biol 8:101–112. [DOI] [PubMed] [Google Scholar]
- 33. Caughey B, Baron GS, Chesebro B, Jeffrey M (2009) Getting a grip on prions: oligomers, amyloids, and pathological membrane interactions. Annu Rev Biochem 78:177–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lasagna‐Reeves CA, Castillo‐Carranza DL, Jackson GR, Kayed R (2011) Tau oligomers as potential targets for immunotherapy for Alzheimer's disease and tauopathies. Curr Alzheimer Res 8:659–665. [DOI] [PubMed] [Google Scholar]
- 35. Cowan CM, Quraishe S, Mudher A (2012) What is the pathological significance of tau oligomers? Biochem Soc Trans 40:693–697. [DOI] [PubMed] [Google Scholar]
- 36. Fox LM, William CM, Adamowicz DH, Pitstick R, Carlson GA, Spires‐Jones TL, Hyman BT (2011) Soluble tau species, not neurofibrillary aggregates, disrupt neural system integration in a tau transgenic model. J Neuropathol Exp Neurol 70:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sengupta U, Nilson AN, Kayed R (2016) The role of amyloid‐β oligomers in toxicity, propagation, and immunotherapy. EBioMedicine 6:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ohhashi Y, Kihara M, Naiki H, Goto Y (2005) Ultrasonication‐induced amyloid fibril formation of {beta}2‐microglobulin. J Biol Chem 280:32843–32848. [DOI] [PubMed] [Google Scholar]
- 39. Yanamandra K, Gruden MA, Casaite V, Meskys R, Forsgren L, Morozova‐Roche LA (2011) α‐Synuclein reactive antibodies as diagnostic biomarkers in blood sera of Parkinson's disease patients. PLoS One 6:e18513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wegmann S, Jung YJ, Chinnathambi S, Mandelkow E‐M, Mandelkow E, Muller DJ (2010) Human Tau isoforms assemble into ribbon‐like fibrils that display polymorphic structure and stability. J Biol Chem 285:27302–27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Farmer K, Gerson JE, Kayed R (2017) Oligomer formation and cross‐seeding: the new frontier. Israel J Chem 57:665–673. [Google Scholar]
- 42. Wu JW, Herman M, Liu L, Simoes S, Acker CM, Figueroa H, Steinberg JI, Margittai M, Kayed R, Zurzolo C (2013) Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J Biol Chem 288:1856–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jackson SJ, Kerridge C, Cooper J, Cavallini A, Falcon B, Cella CV, Landi A, Szekeres PG, Murray TK, Ahmed Z (2016) Short fibrils constitute the major species of seed‐competent tau in the brains of mice transgenic for human P301S tau. J Neurosci 36:762–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Silveira JR, Raymond GJ, Hughson AG, Race RE, Sim VL, Hayes SF, Caughey B (2005) The most infectious prion protein particles. Nature 437:257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goedert M, Jakes R, Spillantini M, Hasegawa M, Smith M, Crowther R (1996) Assembly of microtubule‐associated protein tau into Alzheimer‐like filaments induced by sulphated glycosaminoglycans. Nature 383:550–553. [DOI] [PubMed] [Google Scholar]
- 46. Uversky VN, Li J, Fink AL (2001) Evidence for a partially folded intermediate in α‐synuclein fibril formation. J Biol Chem 276:10737–10744. [DOI] [PubMed] [Google Scholar]
- 47. Maeda S, Sahara N, Saito Y, Murayama M, Yoshiike Y, Kim H, Miyasaka T, Murayama S, Ikai A, Takashima A (2007) Granular tau oligomers as intermediates of tau filaments. Biochemistry 46:3856–3861. [DOI] [PubMed] [Google Scholar]
- 48. Barghorn S, Mandelkow E (2002) Toward a unified scheme for the aggregation of tau into Alzheimer paired helical filaments. Biochemistry 41:14885–14896. [DOI] [PubMed] [Google Scholar]
- 49. Ding TT, Lee S‐J, Rochet J‐C, Lansbury PT (2002) Annular α‐synuclein protofibrils are produced when spherical protofibrils are incubated in solution or bound to brain‐derived membranes. Biochemistry 41:10209–10217. [DOI] [PubMed] [Google Scholar]
- 50. Apetri MM, Maiti NC, Zagorski MG, Carey PR, Anderson VE (2006) Secondary structure of α‐synuclein oligomers: characterization by raman and atomic force microscopy. J Mol Biol 355:63–71. [DOI] [PubMed] [Google Scholar]
- 51. Hawkins CL, Davies MJ (2001) Generation and propagation of radical reactions on proteins. Biochim Biophys Acta Bioenergetics 1504:196–219. [DOI] [PubMed] [Google Scholar]
- 52. Luque‐Contreras D, Carvajal K, Toral‐Rios D, Franco‐Bocanegra D, Campos‐Pena V (2014) Oxidative stress and metabolic syndrome: cause or consequence of Alzheimer's disease? Oxid Med Cell Longevity 2014:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhao Y, Zhao B (2013) Oxidative stress and the pathogenesis of Alzheimer's disease. Oxid Med Cell Longevity 2013:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sengupta U, Carretero‐Murillo M, Kayed R (2018) Preparation and characterization of tau oligomer strains. Methods Mol Biol 1779:113–146. [DOI] [PubMed] [Google Scholar]
- 55. Lo Cascio F, Kayed R (2018) Azure C targets and modulates toxic tau oligomers. ACS Chem Neurosci 9:1317–1326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information.


