Abstract
Nuclear receptors (NRs) are a family of transcription factors that regulate numerous physiological processes such as metabolism, reproduction, inflammation, as well as the circadian rhythm. NRs sense changes in lipid metabolite levels to drive differential gene expression, producing distinct physiologic effects. This is an allosteric process whereby binding a cognate ligand and specific DNA sequences drives the recruitment of diverse transcriptional co‐regulators at chromatin and ultimately transactivation or transrepression of target genes. Dysregulation of NR signaling leads to various malignances, metabolic disorders, and inflammatory disease. Given their important role in physiology and ability to respond to small lipophilic ligands, NRs have emerged as valuable therapeutic targets. Here, we summarize and discuss the recent progress on understanding the complex mechanism of action of NRs, primarily from a structural perspective. Finally, we suggest future studies to improve our understanding of NR signaling and better design drugs by integrating multiple structural and biophysical approaches.
Keywords: nuclear receptor, ligand binding domain, DNA binding domain, co‐regulator, transactivation, transrepression
Abbreviations
- AF
activation function
- cryo‐EM
cryo‐electron microscopy
- DBD
DNA binding domain
- ER
estrogen receptor
- FXR
farnesoid X receptor
- GR
glucocorticoid receptor
- HDX‐MS
hydrogen/ deuterium exchange coupled with mass spectrometry
- H12
helix 12
- LBD
ligand binding domain
- LXR
liver X receptor
- NR
nuclear receptor
- NTD
N‐terminal domain
- RE
response element
- SMT
single‐molecule tracking
- SR
steroid receptor
- TR
thyroid hormone receptor
- TF
transcription factor
Introduction
The nuclear receptor (NR) superfamily is composed of a family of transcription factors (TFs) that play an important role in a number of biological processes including metabolism, reproduction, and inflammation.1, 2 The first member of this family was cloned in 1985, but today the family has expanded to include 48 members in humans.3, 4 Most NRs are regulated endogenously by small lipophilic ligands such as steroids, retinoids, and phospholipids, but this protein family also contains “orphan” members for which no ligand has yet been identified.5 Ligand binding induces conformational changes within the receptor, which in turn binds specific DNA sequences throughout the genome.6, 7 Once DNA‐bound, co‐regulator proteins, chromatin remodelers, and the general transcriptional machinery are recruited to the DNA in order to activate or repress target gene expression.8, 9, 10 Since NRs are responsible for regulating thousands of genes, their activity is tightly controlled.11, 12 If left unchecked, aberrant NR activity can underlie numerous diseases such as cancer, diabetes, and chronic inflammation.13, 14
Our knowledge of the NR family has drastically expanded within the last decade due to advancements in genome‐wide methodologies, structural studies of receptor domains and full‐length complexes, and identification of new co‐regulator proteins that modulate receptor activity.15 This work has laid the foundation for pharmaceutical companies and academic researchers to develop synthetic ligands that target these receptors.16, 17 Yet, due to the diverse array of genes regulated by these proteins, along with the fact that many drugs are not explicitly specific for one receptor, drugs that target NRs tend to have unwanted side effects.16, 18 For this reason, more research is required to understand all the mechanisms that guide NR regulation. Improving our understanding of NR regulation could pave the way for future therapeutics. Here, we introduce this protein family and focus on the structural mechanisms governing NR action.
Nuclear Receptor Superfamily Classification
NRs are divided into seven subfamilies.19, 20 A list of receptors, subfamilies, and their ligands are shown in Table 1.
Table 1.
Nuclear Receptor Superfamily
| Family | Common name | Abbreviation | Gene name | Ligand |
|---|---|---|---|---|
| 0B | Dosage‐sensitive sex reversal‐adrenal hypoplasia congenital critical region on the X chromosome, Gene 1 | DAX1 | NR0B1 | Orphan |
| Short heterodimeric partner | SHP | NR0B2 | Orphan | |
| 1A | Thyroid hormone receptor‐α | TRα | THRA | Thyroid hormones |
| Thyroid hormone receptor‐β | TRβ | THRB | Thyroid hormones | |
| 1B | Retinoic acid receptor‐α | RARα | RARA | Retinoic acids |
| Retinoic acid receptor‐β | RARβ | RARB | Retinoic acids | |
| Retinoic acid receptor‐γ | RARγ | RARG | Retinoic acids | |
| 1C | Peroxisome proliferator‐activated receptor‐α | PPARα | PPARA | Fatty acids |
| Peroxisome proliferator‐activated receptor‐β | PPARβ | PPARD | Fatty acids | |
| Peroxisome proliferator‐activated receptor‐γ | PPARγ | PPARG | Fatty acids | |
| 1D | Reverse‐Erb‐α | REV‐ERBα | NR1D1 | Heme |
| Reverse‐Erb‐β | REV‐ERBβ | NR1D2 | Heme | |
| 1F | Retinoic acid‐related orphan‐α | RORα | RORA | Sterols |
| Retinoic acid‐related orphan‐β | RORβ | RORB | Sterols | |
| Retinoic acid‐related orphan‐γ | RORγ | RORC | Sterols | |
| 1H | Farnesoid X receptor | FXRα | NR1H4 | Bile Acids |
| Farnesoid X receptor‐β | FXRβ | NR1H5P | Orphan | |
| Liver X receptor‐α | LXRα | NR1H3 | Oxysterols | |
| Liver X receptor‐β | LXRβ | NR1H2 | Oxysterols | |
| 1I | Vitamin D receptor | VDR | VDR | 1α,25‐dihydroxyvitamin D3 |
| Pregnane X receptor | PXR | NR1I2 | Endobiotics and xenobiotics | |
| Constitutive androstane receptor | NR1I3 | Xenobiotics | ||
| 2A | Hepatocyte nuclear Factor‐4‐α | HNF4α | HNF4A | Fatty acids |
| Hepatocyte nuclear Factor‐4‐γ | HNF4γ | HNF4G | Fatty acids | |
| 2B | Retinoid X receptor‐α | RXRα | RXRA | 9‐Cis retinoic acid |
| Retinoid X receptor‐β | RXRβ | RXRB | 9‐Cis retinoic acid | |
| Retinoid X receptor‐γ | RXRγ | RXRG | 9‐Cis retinoic acid | |
| 2C | Testicular Receptor 2 | TR2 | NR2C1 | Orphan |
| Testicular Receptor 4 | TR4 | NR2C2 | Orphan | |
| 2E | Tailless homolog orphan receptor | TLX | NR2E1 | Orphan |
| Photoreceptor‐cell‐specific nuclear receptor | PNR | NR2E3 | Orphan | |
| 2F | Chicken ovalbumin upstream promoter‐transcription factor α | COUP‐TFα | NR2F1 | Orphan |
| Chicken ovalbumin upstream promoter‐transcription factor β | COUP‐TFβ | NR2F2 | Orphan | |
| Chicken ovalbumin upstream promoter‐transcription factor γ | COUP‐TFγ | NR2F6 | Orphan | |
| 3A | Estrogen receptor‐α | ERα | ESR1 | Estrogens |
| Estrogen receptor‐β | ERβ | ESR2 | Estrogens | |
| 3B | Estrogen‐related receptor‐α | ERRα | ESRRA | Orphan |
| Estrogen‐related receptor‐β | ERRβ | ESRRB | Orphan | |
| Estrogen‐related receptor‐γ | ERRγ | ESRRG | Orphan | |
| 3C | Androgen receptor | AR | AR | Androgens |
| Glucocorticoid receptor | GR | NR3C1 | Glucocorticoids | |
| Mineralocorticoid receptor | MR | NR3C2 | Mineralocorticoids and glucocorticoids | |
| Progesterone receptor | PR | PGR | Progesterone | |
| 4A | Nerve growth Factor 1B | NGF1‐B | NR4A1 | Orphan |
| Nurr‐related Factor 1 | NURR1 | NR4A2 | Unsaturated fatty acids | |
| Neuron‐derived orphan Receptor 1 | NOR‐1 | NR4A3 | Orphan | |
| 5A | Steroidogenic Factor 1 | SF‐1 | NR5A1 | Phospholipids |
| Liver receptor Homolog‐1 | LRH‐1 | NR5A2 | Phospholipids | |
| 6A | Germ cell nuclear factor | GCNF | NR6A1 | Orphan |
Table of human nuclear receptors, gene name, and their activating ligands.
Subgroup 0: This group includes the atypical NRs, dosage‐sensitive sex reversal‐adrenal hypoplasia congenital critical region on the X chromosome, Gene 1 (DAX) and small heterodimer partner (SHP).21, 22 These two proteins are unique in their structures and contain only a ligand‐binding domain (LBD) that folds in a manner consistent with the rest of the family.23, 24, 25 Their LBDs also contain motifs that are commonly seen in NR coactivators.26 These motifs interact with other NR LBDs to regulate transcription.27, 28, 29, 30, 31
Subgroup 1: This large family is formed by thyroid hormone receptors (TR),32 retinoic acid receptors (RAR),33 peroxisome proliferator activated receptors (PPAR),34 reverse‐Erb receptors (REV‐ERB,35 retinoic acid related receptors (ROR),35 farnesoid X receptors (FXR),36 liver X receptors (LXR),37 and vitamin D receptors (VDR).38 These receptors are regulated by a variety of lipophilic signaling molecules including thyroid hormone, fatty acids, bile acids, and sterols.
Subgroup 2: This subfamily contains orphan receptors such as the retinoid X receptors (RXR),39 chicken ovalbumin upstream promoter transcription factors (COUP‐TF),40 and hepatocyte nuclear Factor 4 (HNF4).41 All of these orphans have been shown to bind fatty acids via structural studies. However, it is unclear whether these ligands play a role in dynamic ligand‐driven regulation, as seen in other NR classes. RXR is of particular importance as it forms heterodimeric complexes with many NRs and is the only receptor in the group with a known activating ligand, 9‐cis retinoic acid.42
Subgroup 3: This group comprises the steroid receptors (SRs), which are key regulators of a host of metabolic, reproductive, and developmental processes.43 The SR family includes the androgen receptor (AR),44 progesterone receptor (PR),45 glucocorticoid receptor (GR),46 mineralocorticoid receptor (MR),47 and two closely related estrogen receptors (ERα and ERβ).48 Cholesterol‐derived hormones, like cortisol and estrogen, regulate SRs through direct binding.
Subgroup 4: This group contains the orphan nuclear receptors nerve growth Factor 1B (NGF1‐B), nurr‐related Factor‐1 (NURR1), and neuron‐derived orphan Receptor‐1 (NOR‐1). These proteins are required for neuron development and maintenance.49
Subgroup 5: This group contains steroidogenic Factor 1 (SF‐1)50 and liver receptor Homolog‐1 (LRH‐1).51 Although generally still classified as orphan receptors, evidence suggests these proteins are regulated by phospholipids.27, 52 LRH‐1 and SF‐1 are vital for development and metabolism.51, 53
Subgroup 6: This group contains only one receptor, germ cell nuclear factor (GCNF),54 an orphan receptor that has a critical role in development.55 This protein remains in its own category due to a critical difference in its LBD; it does not contain an activator function HR (AF‐H) and is known to drive gene silencing.56
Structural Insight into Nuclear Receptor Action
X‐ray crystal structures of nuclear receptors, both full‐length and discrete domains, have provided critical information on how ligands and DNA response elements are recognized, how they dimerize, and interact with co‐regulators.
Overall architecture
Despite diversity in the size, shape, and charges of activating ligands, almost all members of the nuclear receptor superfamily share a common modular domain structure.15, 57 Except for the atypical receptors SHP and DAX, the overall architecture is composed of five domains: A–E [Fig. 1(A)]. Each of these subdomains plays a specific role in receptor biology.58 The mass of NRs can vary but they are generally between 66 and 100 kD [Fig. 1(B)].
Figure 1.
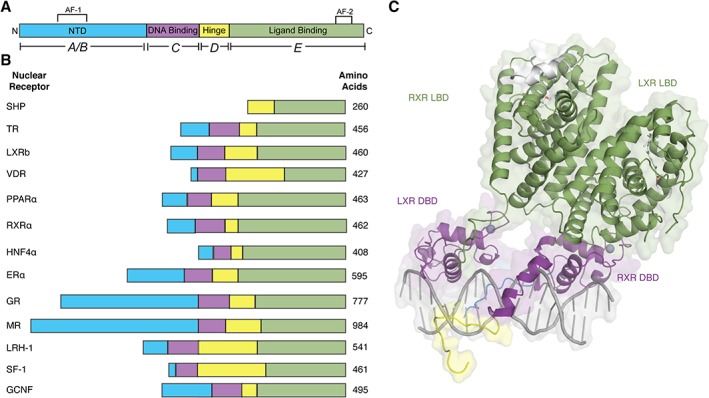
Modular domain structure of NRs. (A) Basic modular domain structure of NRs is composed of an unstructured NTD that contains the Activation Function 1 (AF‐1) surface, a zinc finger DBD, a flexible hinge region, and a LBD that binds to ligands and interacts with co‐regulator proteins through the Activation Function 2 (AF‐2) surface. (B) General domain size and amino acid length of a variety of NRs. The DBD and LBDs are the most conserved regions where as the other domains are more variable in length and sequence composition. (C) Example of a full‐length NR structure shows LXR‐RXR heterodimer (PDB: http://firstglance.jmol.org/fg.htm?mol=4NQA) (DBD colored purple, hinge region in yellow, and LBD in green).
A/B: N‐terminal domain (NTD): The NTD is a highly disordered domain, which explains why the NTD is not amenable to structural analysis. Additionally, there is little sequence conservation between NR NTDs and there is a large disparity in the size of this domain [Fig. 1(B)].
The NTD contains the activator Function‐1 region (AF‐1), which interacts with a variety of co‐regulator proteins in a cell‐ and promoter‐specific manner.59 For all NRs, the majority of the domain is disordered. However, the GR NTD can adopt a more alpha‐helical structure when co‐regulators are bound.60 This region also gives rise to multiple isoforms through alternative splicing, as seen in TR and GR.46 Finally, the NTD is the target for numerous post‐translational modifications including phosphorylation, SUMOylation, and acetylation.61 These modifications have varying effects, both driving and repressing transcription.
C: DNA binding domain (DBD): This region is the most conserved among all nuclear receptor domains.62 The DBD has two subdomains that each contains four cysteine residues that co‐ordinate a zinc ion to create the canonical DNA‐binding zinc finger motif [Fig. 2(A) and (B)].63 Each zinc finger is then followed by an amphipathic helix and a peptide loop.64, 65 The first subdomain contains the DNA‐reading helix, which interacts with the major groove to make base‐specific interactions with the DNA.66 The second subdomain helix makes non‐specific contacts with the DNA backbone. The peptide loop in this subdomain contains the distal box, or “D box,” that contains residues for receptor dimerization.67, 68, 69 Some NRs, like LRH‐1 and GCNF, contain a DBD C‐terminal extension (CTE) that makes additional base‐specific contacts within the DNA minor groove.70, 71
Figure 2.
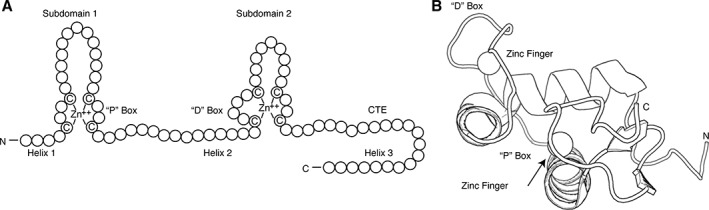
NR DNA binding domains. (A) Cartoon representation of NR DBDs indicating important motifs. This domain contains two subdomains, each containing one zinc finger. The first subdomain residues interact with the DNA major groove to make base‐specific interactions on genomic response elements. The second subdomain participates in DBD dimerization and makes non‐specific contacts with the DNA backbone. Some NRs, like LRH‐1 and GCNF, also contain C‐terminal extensions (CTEs) that make base‐specific contacts with the minor groove. (B) Cartoon representation of folded GR DBD highlighting the important regions (PDB: http://firstglance.jmol.org/fg.htm?mol=3FYL). Zinc atoms are represented as spheres.
D: Hinge Region: The hinge region is a short, flexible linker between the DBD and the LBD.58 This region has the least sequence and size conservation between nuclear receptors. Like the NTD, this region is also a site for regulatory PTMs. The hinge can also contain a nuclear localization signal.61, 72
E: Ligand binding domain (LBD): The LBD is a complex allosteric signaling domain that not only binds to ligands but also interacts directly with co‐regulator proteins.73, 74 This structurally conserved domain commonly contains 11 α‐helices and four β‐strands that fold into three parallel layers to form an alpha helical sandwich (Fig. 3).75 This folding creates a hydrophobic ligand‐binding pocket (LBP) at the base of the receptor.73, 76, 77 Superposition of NR LBD structures reveals that the top part of the receptor is most similar whereas the base, which contains the LBP, is more variable.15, 75 This variability across NRs at the ligand‐binding region allows NRs to recognize a diverse cadre of ligands.
Figure 3.
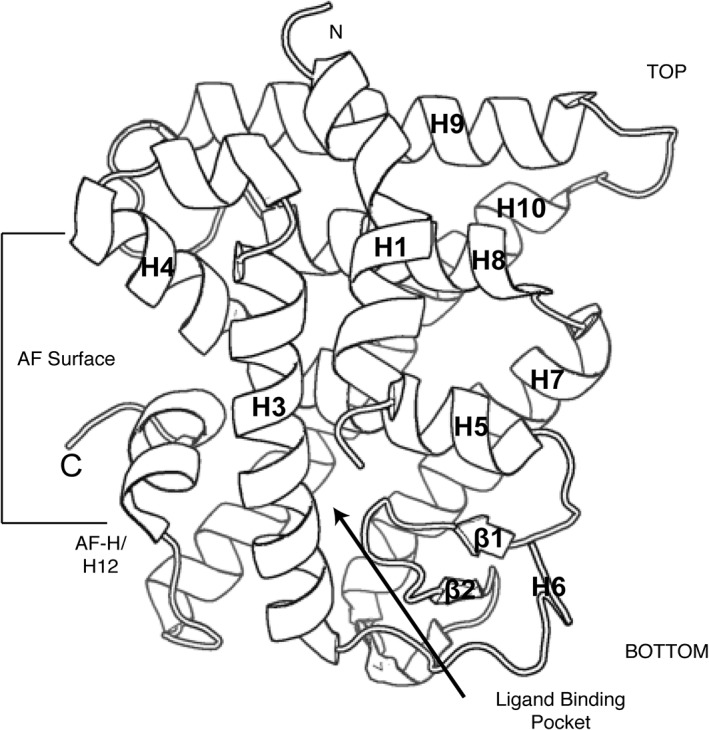
NR ligand binding domains. Cartoon representation of the structurally conserved NR LBD. This domain is composed of 11 α‐helices and 4 β‐strands that fold into three layers of a helical sandwich bundle. This fold creates a hydrophobic ligand binding pocket at the bottom of the receptor. This domain also contains the AF surface, composed of H3, H4, and the AF‐H, which interacts with co‐regulator proteins (PDB: http://firstglance.jmol.org/fg.htm?mol=1PZL).
The LBD contains another activation function surface (AF‐2), which is composed of helices 3, 4, and 12. Helix 12, or the activation function helix (AF‐H) has been shown to be conformationally dynamic upon ligand binding, altering the orientation of AF‐2 to facilitate interaction with different co‐regulator proteins.73, 75
NR–ligand interactions
Nuclear receptors bind directly to a variety of small, lipophilic ligands such as steroids, thyroid hormone, retinoids, and lipids that either diffuse or are transported across the cell membrane.5 Of the 48 human NRs, 24 have known ligands and the remaining 24 are classified as “orphans” or “adopted orphans,” meaning that a likely ligand has been identified. In the absence of ligand, NRs tend to be unstable, explaining the dearth of apo‐NR LBD structures.75, 78, 79 Ligand binding greatly increases the stability of the LBD, evidenced by changes in NMR spectra between liganded and unliganded PPARs and less proteolytic cleavage seen in the ER ligand‐bound versus apo state.77, 78, 80, 81 This stabilization, among other factors, facilitates co‐regulator binding.82
Ligands bind the receptor within the LBP at the base of the LBD. This pocket is composed of ~75% hydrophobic residues, but also contains critical polar residues that make key hydrogen bonding interactions to the ligand.75 These hydrogen bonds help position the ligand in the correct orientation. For example, endogenous SR ligands are composed of a rigid fused 4‐ring scaffold that positions various H‐bond donors and acceptors to interact with the receptor [Fig. 4(A) and (B)]. SRs use a conserved glutamine on H3 and arginine on H5 to lock the ligand's A ring in place [Fig. 4(A) and (B)].83, 84 A striking example of the importance of these hydrogen bond networks in the LBP is seen in FXR and LXR ligands; although similar, these ligands are bound in completely opposite orientations due to the available hydrogen bonding network within the LBP [Fig. 4(C) and (D)].85, 86 These differences ensure the natural ligands are bound by the correct receptor. Ligand selection is further achieved by a dramatic difference in the size of ligand binding pockets across NRs. For example, SR LBP pocket volumes tend to be 400–600 Å3 and 700–850 Å3 for FXR and LXR, and almost 1300 Å3 for PPARs [Fig. 4(E)].83, 85, 87 The volume of the pocket generally corresponds to the size of the ligand suggesting significant component of ligand selection stems from steric selection.
Figure 4.
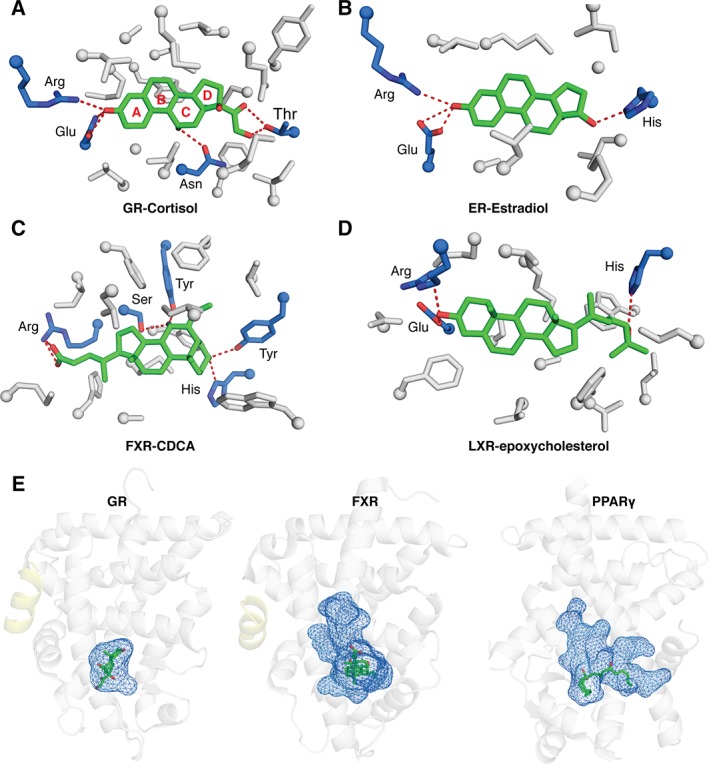
NR ligand interactions. Close up view of SR LBPs showing that (A) GR LBD‐cortisol (PDB: http://firstglance.jmol.org/fg.htm?mol=4P6X) and (B) ER LBD‐estradiol (PDB: http://firstglance.jmol.org/fg.htm?mol=1ERE) use conserved Glu and Arg residues (blue sticks) to make hydrogen bonding interactions (red) with steroid ligands. These interactions help orient the ligand within the pocket. (C) Close up views of FXR LBD‐CDCA (PDB: http://firstglance.jmol.org/fg.htm?mol=1OT7) and (D) LXR LBD‐epoxycholesterol (PDB: http://firstglance.jmol.org/fg.htm?mol=1P8D) show, despite similar ligands, the receptors orient them in opposite directions. This allows natural ligands to discriminate between NRs whose LBDs are highly conserved (E) Comparisons of ligand cavity sizes between GR (PDB: http://firstglance.jmol.org/fg.htm?mol=4P6X), FXR (PDB: http://firstglance.jmol.org/fg.htm?mol=1OT7), and PPAR (PDB: http://firstglance.jmol.org/fg.htm?mol=5AZV).
NR–DNA interactions
Nuclear receptor DBDs bind to a variety of DNA response elements (REs) whose nucleotide sequences can take the form of a palindrome, direct repeat, or extended monomeric sites (Fig. 5).63, 67 The SRs bind palindromic repeats [Fig. 5(A)]. These palindromes contain two AGGACA repeats that can be separated by a spacer region that varies in length. The length of this spacer has been shown to allosterically modulate SRs, resulting in varied transcriptional outputs.88, 89, 90 However, the most common spacer length is 3 bp.68, 91, 92 Receptors that bind direct repeats include the RXR‐RAR heterodimer, GCNF, and VDR [Fig. 5(B)].93, 94, 95 These sequences are composed of two AGGTCA sites separated by a spacer sequence from 0 to 5 bp long. Finally, LRH‐1 and SF‐1 are examples of receptors that bind extended monomeric sequences [Fig. 5(C)].71, 96 These REs contain one AGGTCA site as well an A/T rich sequence directly upstream.
Figure 5.
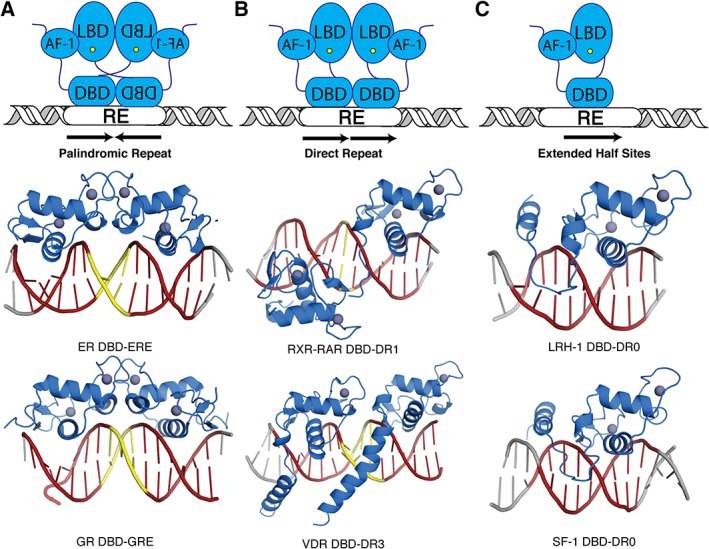
Genomic response elements. Nuclear receptors bind to genomic response elements (RE) that come in a variety of forms. (A) Members of the SR subfamily bind to palindromic repeats (shown as red DNA cartoon). These repeats are separated by different spacer lengths (shown as yellow DNA cartoon). As examples, the ER DBD – estrogen response elements (ERE) and GR DBD – glucocorticoid response element (GRE) crystal structures are shown. (B) Most other NRs bind to direct repeats, which can also be separated by spacers from 0 to 5 bp. The structures of the RXR‐RAR DBD heterodimer is shown in complex with a DR with 1 bp spacer (DR1) and the VDR homodimer DBD is shown in complex with a DR with 3 bp spacer (DR3). (C) Although rare, some NRs bind to DNA as a monomer to extended half site sequences. Examples include LRH‐1 DBD and SF‐1 DBD (PDBs, from left to right: top row – http://firstglance.jmol.org/fg.htm?mol=4AA6, http://firstglance.jmol.org/fg.htm?mol=1DSZ, and http://firstglance.jmol.org/fg.htm?mol=5L0M; bottom row – http://firstglance.jmol.org/fg.htm?mol=3FYL, http://firstglance.jmol.org/fg.htm?mol=1KB4, and http://firstglance.jmol.org/fg.htm?mol=2FF0).
NRs function as monomers, homodimers, or heterodimers
NRs are generally found as monomers in solution but upon DNA binding can form higher order complexes. NRs can be monomeric on DNA but are more often found as homodimers or in heterodimeric complexes with RXR.3 This increases overall size and complexity of NRs, allowing new surfaces to be accessed for PTMs or co‐regulator binding.46
LRH‐1, NGF1‐B, and SF‐1 are among the few NRs that bind DNA as monomers.71, 96 These receptors utilize the CTE within their DBDs to facilitate additional DNA contacts within the minor groove, expanding their DNA footprint. Members of the SR subfamily commonly form homodimers. The ER LBD structure shows H8, H9, H10, and Loops 8–9 from each monomer interact to form a homodimer [Fig. 6(A)].84 This is in contrast with the GR dimer, which showed a unique dimer interface not seen in other NR structures [Fig. 6(B)].97 Finally, the rest of the NR superfamily commonly forms heterodimers with RXR.3, 98 Similar to the ER structure, the dimer interface is formed among H7, H9, H10, H11, and Loops 8 and 9 [Fig. 6(C)].99
Figure 6.
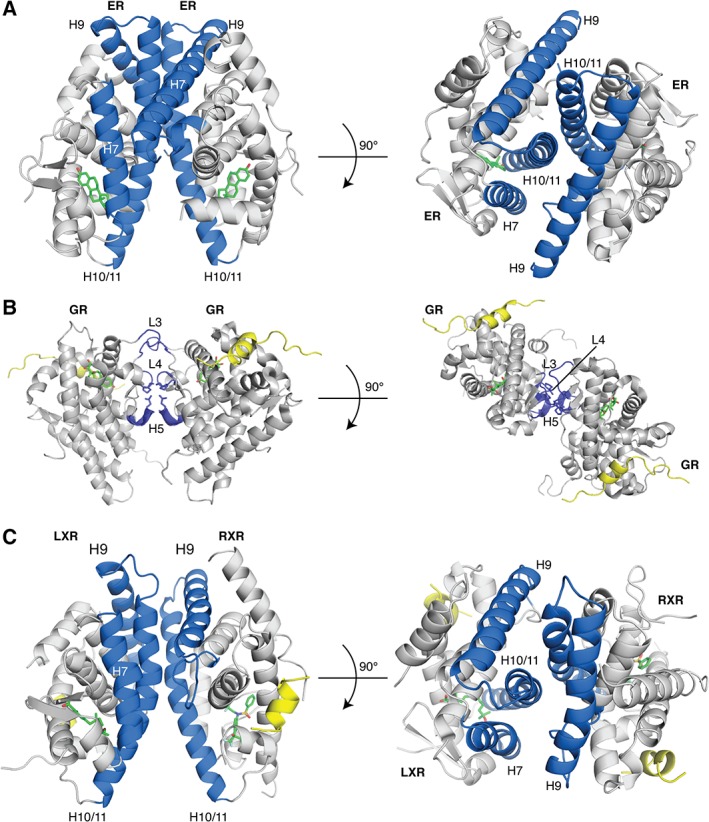
NR dimerization interfaces. Many NRs utilize the H10/H11 surface to form homodimers or heterodimers. (A) ER LBD – estrogen homodimeric complex shows dimerization occurs between H7, H9, H10/11 (PDB: http://firstglance.jmol.org/fg.htm?mol=1ERE). (B) The LXR‐RXR LBD heterodimer shows a similar dimerization interface (PDB: http://firstglance.jmol.org/fg.htm?mol=1UHL). (C) Unlike the other two, the GR LBD homodimer structure revealed a novel dimerization interface (PDB: http://firstglance.jmol.org/fg.htm?mol=1M2Z). The dimerization interface is colored blue, ligands are shown as sticks (green) and co‐regulator peptides are colored yellow.
NR–co‐regulator interactions
After DNA binding, NRs recruit a variety of proteins collectively known as co‐regulators.8, 99 To date, there are approximately 200 different co‐regulator proteins, which fall into two main categories: co‐activators and co‐repressors.8, 9 These interact directly with NRs at the AF‐1 and AF‐2 surfaces.59 Since the AF‐1 lies within the unstructured NTD, we have not been able to obtain structural information about these interactions.58, 60 However, almost all NR LBD structures are co‐crystallized with fragments of co‐regulator NR‐interaction domains at the AF‐2 surface.59
Co‐activator proteins interact with NRs via an alpha‐helix containing a short LXXLL motif (L‐ leucine, X‐ any amino acid).26, 82 This motif interacts with the NR AF‐2 surface. The co‐regulator's leucine residues lie within the hydrophobic groove of the AF‐2 surface and the ends of the helical peptide are generally held in place by a charge clamp formed by a lysine on the NR's H3 and a glutamate on H12 that cap the helix dipole [Fig. 7(A)].82 Co‐repressors contain conserved (L/I)XX(I/V)I or LXXX(I/L)XXX(I/L) motif (referred to as CoRNR box) (L‐ leucine, I‐ isoleucine, X‐ any amino acid).100, 101 These extended motifs interact at the same hydrophobic AF surface but their length inhibits the canonical charge clamp formation [Fig. 7(B)].102, 103
Figure 7.
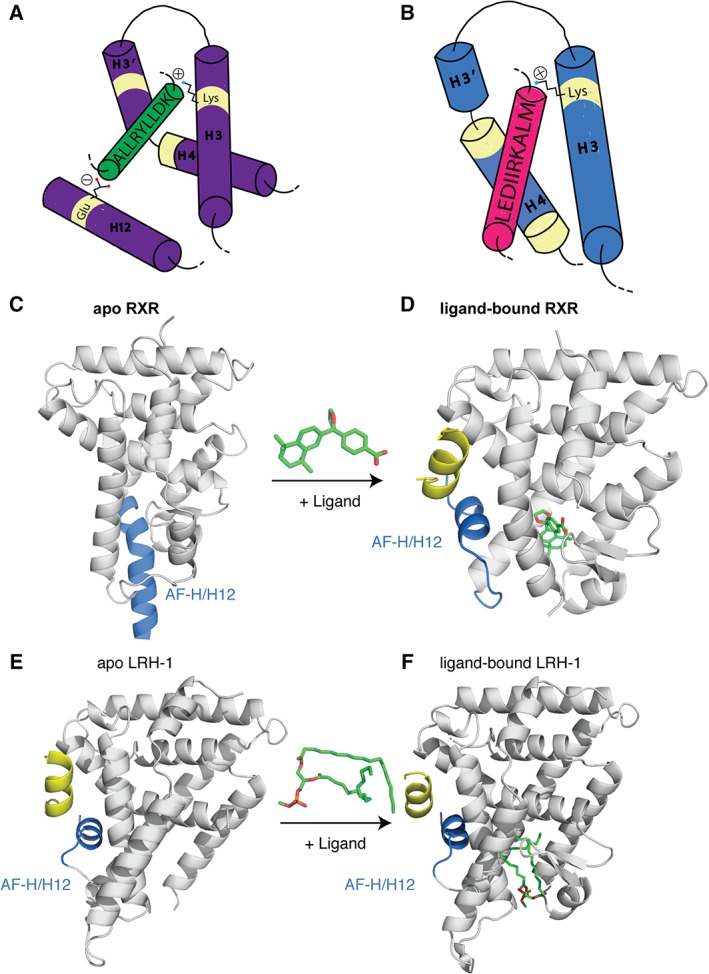
NR co‐regulator interactions. (A) Cartoon representation of the co‐regulator LXXLL peptide (green) interacting with the AF surface (purple). The peptide is held in place by a conserved charge clamp interaction formed by a glutamate on H12 and a lysine on H3. (B) Cartoon representation of co‐repressor peptides (pink) interacting with the AF surface (blue). Co‐repressors contain extended (L/I)XX(I/V)I or LXXX(I/L)XXX(I/L) motifs that do not allow for the charge clamp formation. The basis of the “mouse‐trap” model was made by comparing the apo (C) and ligand bound (D) structures of RXR. Upon ligand binding a large rearrangement of H12 is seen (PDBs: http://firstglance.jmol.org/fg.htm?mol=1LBD, http://firstglance.jmol.org/fg.htm?mol=1MVC). (E,F) The more favored “dynamic stabilization” model of NR activation suggests H12 does not undergo such a large conformational change, but instead H12 flexible and ligand binding simply stabilizes the helix. This model was proposed after other apo NR structures, did not show H12 displaced and, upon ligand binding, there was little change in the location of this helix (PDBs: http://firstglance.jmol.org/fg.htm?mol=4DOR, http://firstglance.jmol.org/fg.htm?mol=4PLE). Co‐regulator peptides are colored blue and ligands are shown as sticks (green).
The discrimination between either co‐activator or co‐repressor binding has been linked to the conformational flexibility of H12.6, 75 Originally, the “mouse‐trap” model was proposed. This model was based on the structures of apo RXR and ligand‐bound RAR [Fig. 7(C) and (D)].104, 105, 106 It was posited that upon agonist binding, there was a large structural rearrangement of H12, causing it to snap shut. However, this phenomenon was only observed for a few proteins.84 Other NR LBD structures, like LRH‐1 in both the apo and the ligand bound state, did not demonstrate large movements in H12.107 This suggested another model was possible. The current favored model is the “dynamic stabilization model,” which suggests that H12 is not in one fixed position, but rather is dynamic.79 Ligand binding stabilizes the helix into a more fixed conformation [Fig. 7(E) and (F)]. Methods that measure dynamics of H12 have been pivotal in providing evidence to support this model.77, 81 In addition, other LBD surfaces are stabilized upon ligand binding and appear to communicate with the AF‐2 surface to modulate receptor activation. Examples include LRH‐1, PPAR, ER, and GR.108, 109
Nuclear Receptor Signaling
Nuclear receptor mechanism of action
NRs have been classified as into four mechanistic Subtypes I–IV (Fig. 8):
Figure 8.
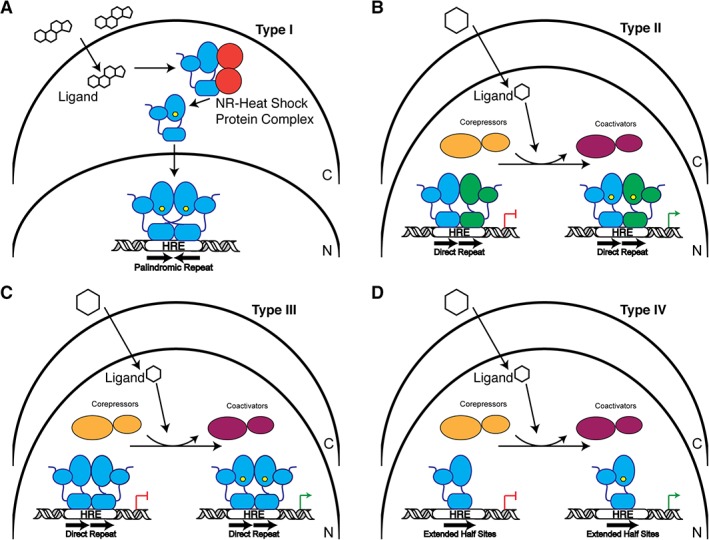
Schematic of NR signaling mechanisms. (A) Type I receptors reside in the cytoplasm (C) in complex with chaperone proteins. Upon ligand binding (hexagon), the receptor is released from this complex and is trafficked into the nucleus (N) where they typically bind to palindromic hormone response elements (HREs) as a homodimer to regulate transcription. (B) Type II receptors are localized in the nucleus. In their unliganded state, they interact with co‐repressor proteins, but upon ligand binding are exchanged for co‐activators. NRs in this group generally form heterodimeric complexes with RXR. (C) Similar to Type II receptors, Type III receptors reside in the nucleus and exchange bound co‐repressors and co‐activators. These receptors bind to direct repeat HREs as homodimers. (D) Type IV receptors are almost identical to Type III except they bind HREs that are extended half sites as monomers.
Type I Nuclear Receptors: Receptors of this group are SRs and are activated by cholesterol‐derived steroidal hormones such as estrogens, androgens, progestagens, and corticoids.43 These receptors are sequestered to the cytoplasm bound to chaperone proteins but upon ligand activation, they exchange their chaperone proteins and undergo nuclear translocation. In the nucleus, SRs generally bind as homodimers to DNA REs that consist of two inverted repeats [Fig. 8(A)].110, 111
Type II Nuclear Receptors: Receptors of this type, such as RAR and LXR, are often retained in the nucleus, regardless of the presence of activating ligand.10 Upon ligand binding, the receptor is released from a co‐repressor complex and swapped for co‐activators and the transcriptional machinery. These receptors commonly form heterodimers with RXR on direct repeat DNA REs [Fig. 8(B)].3
Type III Nuclear Receptors: This type of NR, such as VDR, has a similar mechanism of action to Type II NRs but instead form homodimers on their REs, which are direct repeat sequences [Fig. 8(C)].63
Type IV Nuclear Receptors: This type of NR has a similar mechanism of action to Type II NRs but instead bind to DNA as a monomer and recognize extended half‐sites within REs [Fig. 8(D)].71, 96 Examples of Type IV include LRH‐1 and SF‐1.
Transactivation and transrepression
NRs modulate transcription through many distinct mechanisms that ultimately result in either activation or repression of specific gene programs. As stated above, transcriptional activation is achieved by ligand binding stabilizing an active state.7 In this state, NRs recruit co‐activator proteins, which are typically scaffolds that initiate the formation of large protein complexes that harbor histone modifying enzymes such as histone acetyltransferases (HATs) or histone methyltransferases (HMTs).112, 113 These activities facilitate the opening of chromatin, making it accessible to additional regulatory proteins. Finally, the general transcriptional machinery and RNA Polymerase II are recruited to drive transcription [Fig. 9(A)].114
Figure 9.
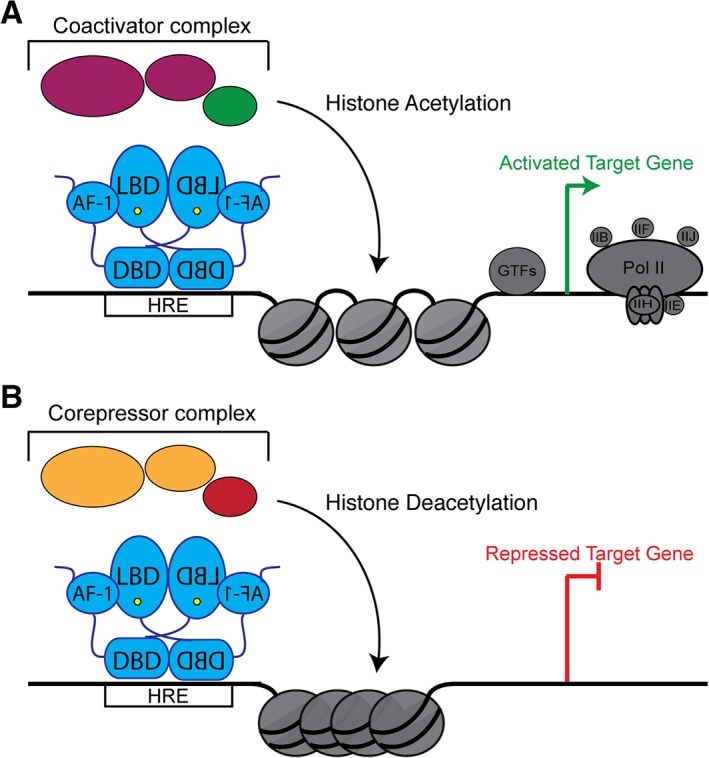
NRs both activate and repress transcription. (A) To activate gene expression, NRs (blue) interact with their DNA response elements. DNA‐bound NRs recruit co‐activator proteins (magenta), which in turn recruit histone‐modifying enzymes. These histone‐modifying enzymes are commonly histone acetylases (green), which acetylate histone tails. This modification is a mark of active chromatin. Ultimately, the general transcriptional machinery and RNA Polymerase Pol II (gray) are recruited to drive gene expression. (B) To repress transcription, NRs recruit co‐repressor proteins (orange). These proteins recruit other histone deacetylases (red) that reverse histone acetylation and restrict chromatin accessibility. This condensation prevents the transcriptional machinery from accessing the DNA, thus repressing gene expression.
Conversely, NRs can repress transcription by two different mechanisms.115 First, NRs can bind to co‐repressors in their apo state as shown in Type II–IV receptors.115 These co‐repressor proteins recruit histone modifying enzymes such as histone deacetylases (HDACs),8 which act in opposition of HATs to restrict chromatin and block the transcriptional machinery from accessing the DNA [Fig. 9(B)].115, 116 Second, NRs can interact with “negative DNA response elements.”117, 118 Binding to these elements results in NRs adopting different conformations than when bound to “positive” DNA response elements and favors co‐repressor recruitment to block transcription.119
Nuclear Receptors as Critical Pharmaceutical Targets
Aberrant nuclear receptor signaling pathways contribute to numerous disease states such as cancer, diabetes, obesity, and others.14, 17 For this reason, NRs are major pharmaceutical targets. Initial ligand design has been quite simple as NR LBPs are enclosed and are amenable to binding a variety of ligands.75 However, due to the breadth and complexity of NR biology, designing ligands with limited cross‐reactivity or partial agonism has proven quite difficult. Despite these issues, NR‐targeting ligands make up 10–20% of current FDA‐approved drugs have a worldwide market of 30 billion dollars per year.120
Historically, there have been two main approaches for identifying NR ligands. First, NR ligands were isolated from human tissue extracts.121 For example, the study of the adrenal gland led to the discovery of a compound effective at blocking inflammation. This compound was later discovered to be cortisol, the endogenous ligand for GR.121 Later, synthesis of cortisol sparked the development of the synthetic compounds dexamethasone and prednisolone.122 Second, compounds were identified by connecting ligand effects with protein biology.17 For example, thiazolidinediones showed promise in treating diabetes.123 These effects were later linked to PPARγ signaling.123 The newest generation of NR ligands are termed “selective nuclear receptor modulators,” which are designed against a single NR to partially or selectively activate a subset of signaling pathways. These idea is to separate the beneficial outcomes of treatment from the less desirable side effects.124 Such ligands would be highly beneficial for targeting ER, AR, and GR.125, 126 Due to the complexity of NR signaling, these compounds have been largely unsuccessful thus far.
Future Perspectives
Insights into allostery
Significant advances in understanding the mechanism of action of NR LBDs have been made by imaging static structural features of LBDs with distinct ligands and relatively short peptides derived from co‐regulators. However, this approach does not capture conformational and allosteric effects driven by other domains within the receptors (e.g. DNA binding domain) and other effectors (e.g. DNA). We also have a limited number of apo NR structures, although typically only a few conformational populations are captured in a crystalline lattice.
Solution NMR techniques are ideal for quantitatively dissecting the dynamic motions of proteins in distinct time scales, however this technique has seen limited use in studying NR LBDs. Since intrinsic dynamics has been proposed as the “carrier” for allosteric communication,127, 128 solution studies would greatly further our understanding of NR activation. For example, NMR studies of the PPARγ LBD showed half of the expected resonances in the spectrum.77 These missing resonances stem from line broadening of specific regions, including the AF‐2, suggesting microsecond (μs) to millisecond (ms) timescale dynamics in these regions. Ligand binding rigidified these motions, rendering their resonances observable. Hydrogen/ deuterium exchange coupled with mass spectrometry (HDX‐MS) is another powerful technique used to experimentally characterize the conformational dynamics of NR LBDs. Similar patterns of conformational dynamics in apo and various ligand‐bound states in PPARγ were observed by HDX‐MS, consistent with solution NMR results. HDX‐MS analysis also detected different dynamical patterns in PPARγ between full and partial agonist‐bound states.109 Molecular dynamics (MD) simulations are also powerful tools used to characterize LBD conformational dynamics, especially when structural information is available. A MD study revealed that the distinct allosteric communications in LRH‐1 drive differential co‐activator recruitment preferences (i.e. Tif2 and PCG1α), despite the same agonist being present. Moreover, these simulation data agreed with experimental HDX‐MS data, providing cross‐platform confirmation of different co‐regulator recruitment in LRH‐1.108
Different biophysical techniques may also be integrated to fully understand the conformational plasticity and intrinsic allosteric/dynamic communication pathway utilized by NR LBDs. For example, RXRα is known to form a heterodimer with either PPARγ or most of the Type II NRs, such as TR. Intriguingly, the RXRα‐PPARγ heterodimer, but not the RXRα‐TR heterodimer, can be activated by retinoic acid. This TR‐mediated allosteric silencing signal is, therefore, critical for controlling the RXRα‐driven response. Integrative studies using x‐ray crystallography, NMR, and HDX‐MS showed the allosteric pathway initiated from the middle of dimer interface, then propagated to the core of LBD, ultimately to Helix 12 and AF‐2 to control ligand binding.129 Therefore, LBD dynamics are an important component in defining the complex NR signaling code. Moreover, understanding dynamical differences within the same structural ensemble strengthens a structure–activity relationship pipeline in drug development. This combined approach has been used in drug discovery for PPARγ and should be used to better guide design of ‘selective nuclear receptor modulators’ targeting specific LBDs in the future.130, 131
Full length structures
Nuclear receptors contain no intrinsic activity; rather, they nucleate the formation of large transcriptional complexes that modulate gene expression. Imaging these complexes, which contain dozens to hundreds of individual proteins, would shed tremendous light on NR function.
So far, there are only three such crystal structures available: PPARγ–RXRα heterodimer, HNF‐4α, and RXRα–LXRβ heterodimer.132, 133, 134 These structures provide information about the inter‐domain interactions between NR dimers and organization of each domain in full‐length NRs when bound to DNA response elements. The small number of available crystal structures reflects the challenge of obtaining crystal structures. Inspection of these structures shows that HNF‐4α, PPARγ, RXRα, and LXRβ all have relatively short A/B and hinge regions [Fig. 1(B)], which are known to be highly disordered and disturb crystal packing. For this reason, crystal structures of intact NRs with longer A/B region (such as MR and GR) or hinge region (such as SF‐1 and LRH‐1) would be extremely challenging. Indeed, the A/B region in PPARγ is highly dynamic based on HDX‐MS analysis and cannot be visualized in any three solved structures with different ligands.132 The A/B regions were not included in the construct design for HNF‐4α, RXRα, and LXRβ used in the crystallization study.
To bypass the crystallization hurdles associated with full‐length structures, cryo‐electron microscopy (cryo‐EM) and small angle X‐ray scattering (SAXS) have been used. With recent advances in the direct electron detection devices, single particle cryo‐EM can achieve atomic resolution and is currently well‐poised to determine large complex structures.135, 136 To date, three cryo‐EM studies of human NRs, focusing on ER137, 138 and RXR/VDR heterodimer,139 have been reported. To obtain a large complex for cryo‐EM studies, full‐length co‐regulators rather than short peptides can be utilized. Therefore, conformation of full‐length co‐regulators with NRs will be visualized in atomic detail.137 Using different co‐activators, a recent cryo‐EM study revealed the recruitment order of co‐activators and how this controlled epigenetic regulation on histones.138 Likewise, orthologous proteins of human NRs have also been studied. For instance, cryo‐EM structure of USP and EcR heterodimer, the insect https://en.wikipedia.org/wiki/Orthologs of the https://en.wikipedia.org/wiki/Mammalian RXR and FXR, respectively, has been determined providing first insight into the orientation of LBD on an inverted repeat DNA sequence.140 Interestingly, the A/B regions and most of the hinge regions are omitted in the constructs used in these cryo‐EM studies due to their intrinsic disorder.139, 140 Given that most human transcription factors contain a significant fraction of unstructured regions, this remains a major hurdle in their structural characterization. This further reinforces the importance of including co‐regulatory proteins to help order otherwise disordered structural elements in the context of transcriptional complexes.
Single‐molecule studies
Another central question is how to combine high‐resolution structural and dynamical information to advance our understanding of the biophysical basis that permits NRs (and other TFs) to control gene expression. Studies have linked DNA affinity or receptor dimerization to transcriptional output but given the complex landscape of a transcribing promoter it is still challenging to link these in vitro observations to the direction and magnitude of gene expression. Can we have a more continuous picture of NR function in vivo, capturing both association with DNA and recruitment of co‐regulators, beyond the discrete structural snapshots we currently have?
Recent technological advances in live‐cell microscopy and fluorescent labeling are now being leveraged to study NRs as TFs in real‐time.141 By combining fluorescence correlation spectroscopy, fluorescence recovery after photobleaching, and single‐molecule microscopes, two unique binding events were found in the AR‐DNA recognition process.142 The first binding event spans only hundreds of μs and is characterized by brief, stochastic DNA interaction, whereas the second event spans several seconds indicating longer, sequence‐specific DNA association. This study provided the first glimpse of NR action, following ligand activation, dynamically associating and dissociating with DNA to search for the target sequence. Rather than integrating different complementary methods, single‐molecule tracking (SMT)‐based direct measurement permits the quantification of both the dwell time and the fraction of NR molecules on target DNA in live cells.143, 144 By utilizing GFP‐labeled polymerase II, only a small fraction of GR (~10%) was found to reside at sites with active transcription. The dwell time of GR at these sites were ~10 s.143 SMT microscopy also permits characterizing highly dynamic interactions of ER, GR and their pioneer factors, such as FoxA1, with chromatin.145 Interestingly, FoxA1 does not present a DNAase footprint, reinforcing the advantage of monitoring fast and transient interactions by SMT. A recent SMT report focusing on GR and various co‐factors further corroborated these studies and showed that GR‐chromatin association was dominated by transient interactions characterized by low populations (5–10%) of the receptor on chromatin for only short times (<ms).146
SMT is technically challenging as there is a trade‐off between delivering enough photons over time to permit accurate measurements and capturing inherently fast (μs) binding events.141 Even with this challenge, single‐molecule experiments hold the potential to revolutionize how we define TF–chromatin interactions. For example, conventional studies performed by ensemble biochemistry (such as in vivo ChIP‐seq collected via millions of cells), give the impression of widespread NR‐chromatin occupancy with long residence time (min–hr timescale). Single‐molecule experiments revealed that only a small fraction of NRs are functionally bound to their response elements in a given cell with rather short residence time (μs–s timescale). Therefore, single‐molecule studies support the notion of dynamic and stochastic assembly of transcriptional complexes and offers a new paradigm of our mechanistic understanding of transcription initiation mediated by NRs.147 One important question that remains to be addressed is what portion of sequence‐specific DNA binding results in transcriptional activation. This requires imaging multiple factors at a single‐copy of a specific promoter. With advanced super resolution microscopes, improved image acquisition techniques and better statistical algorithms, single‐molecule studies in live cells will simultaneously track the 3D spatial distribution of NRs over time and monitor 3D enhancer organization. This requires multi‐fluorescence channel SMT images and provides 5D trajectories of NRs during transcription. This has tremendous potential to uncover the particularly dynamic interactions of NRs with their co‐regulators and chromatin at a spatiotemporal resolution to understand the detailed mechanism of NRs in controlling gene expression.141, 148, 149
Acknowledgments
This work was supported by National Institutes of Health [R01DK095750], American Heart Association [Grant 14GRNT20460124] and W.M. Keck Foundation Medical Research Grant to E. A. O. E. R. W. was supported by a predoctoral F31 fellowship awarded by National Institutes of Health General Medical Sciences [1F31GM113397‐01A1]. X. L. was supported by an American Heart Association postdoctoral fellowship [17POST33660110].
References
- 1. Kininis M, Kraus WL (2008) A global view of transcriptional regulation by nuclear receptors: gene expression, factor localization, and DNA sequence analysis. Nucl Receptor Signal 6:e005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gustafsson JA (2016) Historical overview of nuclear receptors. J Steroid Biochem Mol Biol 157:3–6. [DOI] [PubMed] [Google Scholar]
- 3. Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM (1995) The nuclear receptor superfamily: the second decade. Cell 83:835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Govindan MV, Devic M, Green S, Gronemeyer H, Chambon P (1985) Cloning of the human glucocorticoid receptor cDNA. Nucleic Acids Res 13:8293–8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu EH, Lambert MH (2003) Structural insights into regulation of nuclear receptors by ligands. Nucl Recept Signal 1:e004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagy L, Schwabe JW (2004) Mechanism of the nuclear receptor molecular switch. Trends Biochem Sci 29:317–324. [DOI] [PubMed] [Google Scholar]
- 7. Li Y, Lambert MH, Xu HE (2003) Activation of nuclear receptors: a perspective from structural genomics. Structure 11:741–746. [DOI] [PubMed] [Google Scholar]
- 8. Lonard DM, O'Malley BW (2007) Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell 27:691–700. [DOI] [PubMed] [Google Scholar]
- 9. McKenna NJ, Lanz RB, O'Malley BW (1999) Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev 20:321–344. [DOI] [PubMed] [Google Scholar]
- 10. Tata JR (2002) Signalling through nuclear receptors. Nat Rev Mol Cell Biol 3:702–710. [DOI] [PubMed] [Google Scholar]
- 11. Aranda A, Pascual A (2001) Nuclear hormone receptors and gene expression. Physiol Rev 81:1269–1304. [DOI] [PubMed] [Google Scholar]
- 12. Meier CA (1997) Regulation of gene expression by nuclear hormone receptors. J Recept Signal Transduct Res 17:319–335. [DOI] [PubMed] [Google Scholar]
- 13. Tenbaum S, Baniahmad A (1997) Nuclear receptors: structure, function and involvement in disease. Int J Biochem Cell Biol 29:1325–1341. [DOI] [PubMed] [Google Scholar]
- 14. Resche‐Rigon M, Gronemeyer H (1998) Therapeutic potential of selective modulators of nuclear receptor action. Curr Opin Chem Biol 2:501–507. [DOI] [PubMed] [Google Scholar]
- 15. Huang P, Chandra V, Rastinejad F (2010) Structural overview of the nuclear receptor superfamily: insights into physiology and therapeutics. Annu Rev Physiol 72:247–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu HE (2015) Family reunion of nuclear hormone receptors: structures, diseases, and drug discovery. Acta Pharmacol Sin 36:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gronemeyer H, Gustafsson JA, Laudet V (2004) Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov 3:950–964. [DOI] [PubMed] [Google Scholar]
- 18. Moore JT, Collins JL, Pearce KH (2006) The nuclear receptor superfamily and drug discovery. ChemMedChem 1:504–523. [DOI] [PubMed] [Google Scholar]
- 19. Germain P, Staels B, Dacquet C, Spedding M, Laudet V (2006) Overview of nomenclature of nuclear receptors. Pharmacol Rev 58:685–704. [DOI] [PubMed] [Google Scholar]
- 20. Owen GI, Zelent A (2000) Origins and evolutionary diversification of the nuclear receptor superfamily. Cell Mol Life Sci 57:809–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suntharalingham JP, Buonocore F, Duncan AJ, Achermann JC (2015) DAX‐1 (NR0B1) and steroidogenic factor‐1 (SF‐1, NR5A1) in human disease. Best Pract Res Clin Endocrinol Metab 29:607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crawford PA, Dorn C, Sadovsky Y, Milbrandt J (1998) Nuclear receptor DAX‐1 recruits nuclear receptor corepressor N‐CoR to steroidogenic factor 1. Mol Cell Biol 18:2949–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee YS, Chanda D, Sim J, Park YY, Choi HS (2007) Structure and function of the atypical orphan nuclear receptor small heterodimer partner. Int Rev Cytol 261:117–158. [DOI] [PubMed] [Google Scholar]
- 24. Seol W, Choi HS, Moore DD (1996) An orphan nuclear hormone receptor that lacks a DNA binding domain and heterodimerizes with other receptors. Science 272:1336–1339. [DOI] [PubMed] [Google Scholar]
- 25. Zhi X, Zhou XE, He Y, Zechner C, Suino‐Powell KM, Kliewer SA, Melcher K, Mangelsdorf DJ, Xu HE (2014) Structural insights into gene repression by the orphan nuclear receptor SHP. Proc Natl Acad Sci USA 111:839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heery DM, Kalkhoven E, Hoare S, Parker MG (1997) A signature motif in transcriptional co‐activators mediates binding to nuclear receptors. Nature 387:733–736. [DOI] [PubMed] [Google Scholar]
- 27. Ortlund EA, Lee Y, Solomon IH, Hager JM, Safi R, Choi Y, Guan Z, Tripathy A, Raetz CR, McDonnell DP, Moore DD, Redinbo MR (2005) Modulation of human nuclear receptor LRH‐1 activity by phospholipids and SHP. Nat Struct Mol Biol 12:357–363. [DOI] [PubMed] [Google Scholar]
- 28. Borgius LJ, Steffensen KR, Gustafsson JA, Treuter E (2002) Glucocorticoid signaling is perturbed by the atypical orphan receptor and corepressor SHP. J Biol Chem 277:49761–49766. [DOI] [PubMed] [Google Scholar]
- 29. Nedumaran B, Kim GS, Hong S, Yoon YS, Kim YH, Lee CH, Lee YC, Koo SH, Choi HS (2010) Orphan nuclear receptor DAX‐1 acts as a novel corepressor of liver X receptor alpha and inhibits hepatic lipogenesis. J Biol Chem 285:9221–9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johansson L, Bavner A, Thomsen JS, Farnegardh M, Gustafsson JA, Treuter E (2000) The orphan nuclear receptor SHP utilizes conserved LXXLL‐related motifs for interactions with ligand‐activated estrogen receptors. Mol Cell Biol 20:1124–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang H, Slewa A, Janssen E, Skaland I, Yu Y, Gudlaugsson E, Feng W, Kjellevold K, Soiland H, Baak JP (2011) The prognostic value of the orphan nuclear receptor DAX‐1 (NROB1) in node‐negative breast cancer. Anticancer Res 31:443–449. [PubMed] [Google Scholar]
- 32. Brent GA (2012) Mechanisms of thyroid hormone action. J Clin Invest 122:3035–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tang XH, Gudas LJ (2011) Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol 6:345–364. [DOI] [PubMed] [Google Scholar]
- 34. Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S (2011) The peroxisome proliferator‐activated receptor: A family of nuclear receptors role in various diseases. J Adv Pharm Technol Res 2:236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kojetin DJ, Burris TP (2014) REV‐ERB and ROR nuclear receptors as drug targets. Nature Rev Drug Discovery 13:197–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li G, LG G (2015) Farnesoid X receptor, the bile acid sensing nuclear receptor, in liver regeneration. Acta Pharm Sin B 5:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hong C, Tontonoz P (2014) Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat Rev Drug Discov 13:433–444. [DOI] [PubMed] [Google Scholar]
- 38. Haussler MR (1986) Vitamin D receptors: nature and function. Annu Rev Nutr 6:527–562. [DOI] [PubMed] [Google Scholar]
- 39. Evans RM, Mangelsdorf DJ (2014) Nuclear receptors, RXR, and the big bang. Cell 157:255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Park JI, Tsai SY, Tsai MJ (2003) Molecular mechanism of chicken ovalbumin upstream promoter‐transcription factor (COUP‐TF) actions. Keio J Med 52:174–181. [DOI] [PubMed] [Google Scholar]
- 41. Watt AJ, Garrison WD, Duncan SA (2003) HNF4: a central regulator of hepatocyte differentiation and function. Hepatology 37:1249–1253. [DOI] [PubMed] [Google Scholar]
- 42. Mangelsdorf DJ, Evans RM (1995) The RXR heterodimers and orphan receptors. Cell 83:841–850. [DOI] [PubMed] [Google Scholar]
- 43. Fuller PJ (1991) The steroid receptor superfamily: mechanisms of diversity. FASEB J 5:3092–3099. [DOI] [PubMed] [Google Scholar]
- 44. Gao W, Bohl CE, Dalton JT (2005) Chemistry and structural biology of androgen receptor. Chem Rev 105:3352–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jacobsen BM, Horwitz KB (2012) Progesterone receptors, their isoforms and progesterone regulated transcription. Mol Cell Endocrinol 357:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weikum ER, Knuesel MT, Ortlund EA, Yamamoto KR (2017) Glucocorticoid receptor control of transcription: precision and plasticity via allostery. Nat Rev Mol Cell Biol 18:159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gomez‐Sanchez E, Gomez‐Sanchez CE (2014) The multifaceted mineralocorticoid receptor. Compr Physiol 4:965–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA (2007) Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87:905–931. [DOI] [PubMed] [Google Scholar]
- 49. Maruyama K, Tsukada T, Ohkura N, Bandoh S, Hosono T, Yamaguchi K (1998) The NGFI‐B subfamily of the nuclear receptor superfamily (review). Int J Oncol 12:1237–1243. [DOI] [PubMed] [Google Scholar]
- 50. Hoivik EA, Lewis AE, Aumo L, Bakke M (2010) Molecular aspects of steroidogenic factor 1 (SF‐1). Mol Cell Endocrinol 315:27–39. [DOI] [PubMed] [Google Scholar]
- 51. Fayard E, Auwerx J, Schoonjans K (2004) LRH‐1: an orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol 14:250–260. [DOI] [PubMed] [Google Scholar]
- 52. Sablin EP, Blind RD, Krylova IN, Ingraham JG, Cai F, Williams JD, Fletterick RJ, Ingraham HA (2009) Structure of SF‐1 bound by different phospholipids: evidence for regulatory ligands. Mol Endocrinol 23:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Val P, Lefrancois‐Martinez AM, Veyssiere G, Martinez A (2003) SF‐1 a key player in the development and differentiation of steroidogenic tissues. Nucl Recept 1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zechel C (2005) The germ cell nuclear factor (GCNF). Mol Reprod Dev 72:550–556. [DOI] [PubMed] [Google Scholar]
- 55. Wang Q, Cooney AJ (2013) Revisiting the role of GCNF in embryonic development. Semin Cell Dev Biol 24:679–686. [DOI] [PubMed] [Google Scholar]
- 56. Okumura LM, Lesch BJ, Page DC (2013) The ligand binding domain of GCNF is not required for repression of pluripotency genes in mouse fetal ovarian germ cells. PLoS One 8:e66062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rastinejad F, Huang P, Chandra V, Khorasanizadeh S (2013) Understanding nuclear receptor form and function using structural biology. J Mol Endocrinol 51:T1–T21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pawlak M, Lefebvre P, Staels B (2012) General molecular biology and architecture of nuclear receptors. Curr Top Med Chem 12:486–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kumar R, Thompson EB (2003) Transactivation functions of the N‐terminal domains of nuclear hormone receptors: protein folding and coactivator interactions. Mol Endocrinol 17:1–10. [DOI] [PubMed] [Google Scholar]
- 60. Lavery DN, McEwan IJ (2005) Structure and function of steroid receptor AF1 transactivation domains: induction of active conformations. Biochem J 391:449–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Anbalagan M, Huderson B, Murphy L, Rowan BG (2012) Post‐translational modifications of nuclear receptors and human disease. Nucl Recept Signal 10:e001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Danielsen M (2001) Bioinformatics of nuclear receptors. Methods Mol Biol 176:3–22. [DOI] [PubMed] [Google Scholar]
- 63. Gronemeyer H, Moras D (1995) Nuclear receptors. How to finger DNA. Nature 375:190–191. [DOI] [PubMed] [Google Scholar]
- 64. Helsen C, Kerkhofs S, Clinckemalie L, Spans L, Laurent M, Boonen S, Vanderschueren D, Claessens F (2012) Structural basis for nuclear hormone receptor DNA binding. Mol Cell Endocrinol 348:411–417. [DOI] [PubMed] [Google Scholar]
- 65. Hard T, Kellenbach E, Boelens R, Maler BA, Dahlman K, Freedman LP, Carlstedt‐Duke J, Yamamoto KR, Gustafsson JA, Kaptein R (1990) Solution structure of the glucocorticoid receptor DNA‐binding domain. Science 249:157–160. [DOI] [PubMed] [Google Scholar]
- 66. Schwabe JW, Chapman L, Finch JT, Rhodes D (1993) The crystal structure of the estrogen receptor DNA‐binding domain bound to DNA: how receptors discriminate between their response elements. Cell 75:567–578. [DOI] [PubMed] [Google Scholar]
- 67. Khorasanizadeh S, Rastinejad F (2001) Nuclear‐receptor interactions on DNA‐response elements. Trends Biochem Sci 26:384–390. [DOI] [PubMed] [Google Scholar]
- 68. Luisi BF, Xu WX, Otwinowski Z, Freedman LP, Yamamoto KR, Sigler PB (1991) Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature 352:497–505. [DOI] [PubMed] [Google Scholar]
- 69. Watson LC, Kuchenbecker KM, Schiller BJ, Gross JD, Pufall MA, Yamamoto KR (2013) The glucocorticoid receptor dimer interface allosterically transmits sequence‐specific DNA signals. Nat Struct Mol Biol 20:876–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Solomon IH, Hager JM, Safi R, McDonnell DP, Redinbo MR, Ortlund EA (2005) Crystal structure of the human LRH‐1 DBD‐DNA complex reveals Ftz‐F1 domain positioning is required for receptor activity. J Mol Biol 354:1091–1102. [DOI] [PubMed] [Google Scholar]
- 71. Weikum ER, Tuntland ML, Murphy MN, Ortlund EA (2016) A structural investigation into Oct4 regulation by orphan nuclear receptors, Germ Cell Nuclear Factor (GCNF), and Liver Receptor Homolog‐1 (LRH‐1). J Mol Biol 428:4981–4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Haelens A, Tanner T, Denayer S, Callewaert L, Claessens F (2007) The hinge region regulates DNA binding, nuclear translocation, and transactivation of the androgen receptor. Cancer Res 67:4514–4523. [DOI] [PubMed] [Google Scholar]
- 73. Moras D, Gronemeyer H (1998) The nuclear receptor ligand‐binding domain: structure and function. Curr Opin Cell Biol 10:384–391. [DOI] [PubMed] [Google Scholar]
- 74. Weatherman RV, Fletterick RJ, Scanlan TS (1999) Nuclear‐receptor ligands and ligand‐binding domains. Annu Rev Biochem 68:559–581. [DOI] [PubMed] [Google Scholar]
- 75. Wurtz JM, Bourguet W, Renaud JP, Vivat V, Chambon P, Moras D, Gronemeyer H (1996) A canonical structure for the ligand‐binding domain of nuclear receptors. Nat Struct Biol 3:87–94. [DOI] [PubMed] [Google Scholar]
- 76. Nolte RT, Wisely GB, Westin S, Cobb JE, Lambert MH, Kurokawa R, Rosenfeld MG, Willson TM, Glass CK, Milburn MV (1998) Ligand binding and co‐activator assembly of the peroxisome proliferator‐activated receptor‐gamma. Nature 395:137–143. [DOI] [PubMed] [Google Scholar]
- 77. Johnson BA, Wilson EM, Li Y, Moller DE, Smith RG, Zhou G (2000) Ligand‐induced stabilization of PPARgamma monitored by NMR spectroscopy: implications for nuclear receptor activation. J Mol Biol 298:187–194. [DOI] [PubMed] [Google Scholar]
- 78. Keidel S, LeMotte P, Apfel C (1994) Different agonist‐ and antagonist‐induced conformational changes in retinoic acid receptors analyzed by protease mapping. Mol Cell Biol 14:287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pissios P, Tzameli I, Kushner P, Moore DD (2000) Dynamic stabilization of nuclear receptor ligand binding domains by hormone or corepressor binding. Mol Cell 6:245–253. [DOI] [PubMed] [Google Scholar]
- 80. Gee AC, Katzenellenbogen JA (2001) Probing conformational changes in the estrogen receptor: evidence for a partially unfolded intermediate facilitating ligand binding and release. Mol Endocrinol 15:421–428. [DOI] [PubMed] [Google Scholar]
- 81. Kallenberger BC, Love JD, Chatterjee VK, Schwabe JW (2003) A dynamic mechanism of nuclear receptor activation and its perturbation in a human disease. Nat Struct Biol 10:136–140. [DOI] [PubMed] [Google Scholar]
- 82. Darimont BD, Wagner RL, Apriletti JW, Stallcup MR, Kushner PJ, Baxter JD, Fletterick RJ, Yamamoto KR (1998) Structure and specificity of nuclear receptor‐coactivator interactions. Genes Dev 12:3343–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. He Y, Yi W, Suino‐Powell K, Zhou XE, Tolbert WD, Tang X, Yang J, Yang H, Shi J, Hou L, Jiang H, Melcher K, Xu HE (2014) Structures and mechanism for the design of highly potent glucocorticoids. Cell Res 24:713–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, Ohman L, Greene GL, Gustafsson JA, Carlquist M (1997) Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389:753–758. [DOI] [PubMed] [Google Scholar]
- 85. Mi LZ, Devarakonda S, Harp JM, Han Q, Pellicciari R, Willson TM, Khorasanizadeh S, Rastinejad F (2003) Structural basis for bile acid binding and activation of the nuclear receptor FXR. Mol Cell 11:1093–1100. [DOI] [PubMed] [Google Scholar]
- 86. Williams S, Bledsoe RK, Collins JL, Boggs S, Lambert MH, Miller AB, Moore J, McKee DD, Moore L, Nichols J, Parks D, Watson M, Wisely B, Willson TM (2003) X‐ray crystal structure of the liver X receptor beta ligand binding domain: regulation by a histidine‐tryptophan switch. J Biol Chem 278:27138–27143. [DOI] [PubMed] [Google Scholar]
- 87. Egawa D, Itoh T, Akiyama Y, Saito T, Yamamoto K (2016) 17‐OxoDHA is a PPARalpha/gamma dual covalent modifier and agonist. ACS Chem Biol 11:2447–2455. [DOI] [PubMed] [Google Scholar]
- 88. Schwabe JW, Rhodes D (1991) Beyond zinc fingers: steroid hormone receptors have a novel structural motif for DNA recognition. Trends Biochem Sci 16:291–296. [DOI] [PubMed] [Google Scholar]
- 89. Lefstin JA, Thomas JR, Yamamoto KR (1994) Influence of a steroid receptor DNA‐binding domain on transcriptional regulatory functions. Genes Dev 8:2842–2856. [DOI] [PubMed] [Google Scholar]
- 90. Schwabe JW, Chapman L, Rhodes D (1995) The oestrogen receptor recognizes an imperfectly palindromic response element through an alternative side‐chain conformation. Structure 3:201–213. [DOI] [PubMed] [Google Scholar]
- 91. Lefstin JA, Yamamoto KR (1998) Allosteric effects of DNA on transcriptional regulators. Nature 392:885–888. [DOI] [PubMed] [Google Scholar]
- 92. Meijsing SH, Pufall MA, So AY, Bates DL, Chen L, Yamamoto KR (2009) DNA binding site sequence directs glucocorticoid receptor structure and activity. Science 324:407–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rastinejad F, Wagner T, Zhao Q, Khorasanizadeh S (2000) Structure of the RXR‐RAR DNA‐binding complex on the retinoic acid response element DR1. EMBO J 19:1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rastinejad F, Perlmann T, Evans RM, Sigler PB (1995) Structural determinants of nuclear receptor assembly on DNA direct repeats. Nature 375:203–211. [DOI] [PubMed] [Google Scholar]
- 95. Shaffer PL, Gewirth DT (2002) Structural basis of VDR‐DNA interactions on direct repeat response elements. EMBO J 21:2242–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Little TH, Zhang Y, Matulis CK, Weck J, Zhang Z, Ramachandran A, Mayo KE, Radhakrishnan I (2006) Sequence‐specific deoxyribonucleic acid (DNA) recognition by steroidogenic factor 1: a helix at the carboxy terminus of the DNA binding domain is necessary for complex stability. Mol Endocrinol 20:831–843. [DOI] [PubMed] [Google Scholar]
- 97. Bledsoe RK, Montana VG, Stanley TB, Delves CJ, Apolito CJ, McKee DD, Consler TG, Parks DJ, Stewart EL, Willson TM, Lambert MH, Moore JT, Pearce KH, Xu HE (2002) Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell 110:93–105. [DOI] [PubMed] [Google Scholar]
- 98. Svensson S, Ostberg T, Jacobsson M, Norstrom C, Stefansson K, Hallen D, Johansson IC, Zachrisson K, Ogg D, Jendeberg L (2003) Crystal structure of the heterodimeric complex of LXRalpha and RXRbeta ligand‐binding domains in a fully agonistic conformation. EMBO J 22:4625–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Millard CJ, Watson PJ, Fairall L, Schwabe JW (2013) An evolving understanding of nuclear receptor coregulator proteins. J Mol Endocrinol 51:T23–T36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hu X, Lazar MA (1999) The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature 402:93–96. [DOI] [PubMed] [Google Scholar]
- 101. Webb P, Anderson CM, Valentine C, Nguyen P, Marimuthu A, West BL, Baxter JD, Kushner PJ (2000) The nuclear receptor corepressor (N‐CoR) contains three isoleucine motifs (I/LXXII) that serve as receptor interaction domains (IDs). Mol Endocrinol 14:1976–1985. [DOI] [PubMed] [Google Scholar]
- 102. Nagy L, Kao HY, Love JD, Li C, Banayo E, Gooch JT, Krishna V, Chatterjee K, Evans RM, Schwabe JW (1999) Mechanism of corepressor binding and release from nuclear hormone receptors. Genes Dev 13:3209–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Perissi V, Staszewski LM, McInerney EM, Kurokawa R, Krones A, Rose DW, Lambert MH, Milburn MV, Glass CK, Rosenfeld MG (1999) Molecular determinants of nuclear receptor‐corepressor interaction. Genes Dev 13:3198–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Egea PF, Mitschler A, Moras D (2002) Molecular recognition of agonist ligands by RXRs. Mol Endocrinol 16:987–997. [DOI] [PubMed] [Google Scholar]
- 105. Bourguet W, Ruff M, Chambon P, Gronemeyer H, Moras D (1995) Crystal structure of the ligand‐binding domain of the human nuclear receptor RXR‐alpha. Nature 375:377–382. [DOI] [PubMed] [Google Scholar]
- 106. Renaud JP, Rochel N, Ruff M, Vivat V, Chambon P, Gronemeyer H, Moras D (1995) Crystal structure of the RAR‐gamma ligand‐binding domain bound to all‐trans retinoic acid. Nature 378:681–689. [DOI] [PubMed] [Google Scholar]
- 107. Musille PM, Kossmann BR, Kohn JA, Ivanov I, Ortlund EA (2016) Unexpected allosteric network contributes to LRH‐1 co‐regulator selectivity. J Biol Chem 291:1411–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mays SG, Okafor CD, Tuntland ML, Whitby RJ, Dharmarajan V, Stec J, Griffin PR, Ortlund EA (2017) Structure and dynamics of the liver receptor homolog 1‐PGC1alpha complex. Mol Pharmacol 92:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hughes TS, Chalmers MJ, Novick S, Kuruvilla DS, Chang MR, Kamenecka TM, Rance M, Johnson BA, Burris TP, Griffin PR, Kojetin DJ (2012) Ligand and receptor dynamics contribute to the mechanism of graded PPARgamma agonism. Structure 20:139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. O'Malley BW, Tsai MJ (1992) Molecular pathways of steroid receptor action. Biol Reprod 46:163–167. [DOI] [PubMed] [Google Scholar]
- 111. Pratt WB, Galigniana MD, Morishima Y, Murphy PJ (2004) Role of molecular chaperones in steroid receptor action. Essays Biochem 40:41–58. [DOI] [PubMed] [Google Scholar]
- 112. Bulynko YA, O'Malley BW (2011) Nuclear receptor coactivators: structural and functional biochemistry. Biochemistry 50:313–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gadaleta RM, Magnani L (2014) Nuclear receptors and chromatin: an inducible couple. J Mol Endocrinol 52:R137–R149. [DOI] [PubMed] [Google Scholar]
- 114. Acevedo ML, Kraus WL (2004) Transcriptional activation by nuclear receptors. Essays Biochem 40:73–88. [DOI] [PubMed] [Google Scholar]
- 115. Glass CK, Rosenfeld MG (2000) The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev 14:121–141. [PubMed] [Google Scholar]
- 116. Tetel MJ, Auger AP, Charlier TD (2009) Who's in charge? Nuclear receptor coactivator and corepressor function in brain and behavior. Front Neuroendocrinol 30:328–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hudson WH, Youn C, Ortlund EA (2013) The structural basis of direct glucocorticoid‐mediated transrepression. Nat Struct Mol Biol 20:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Surjit M, Ganti KP, Mukherji A, Ye T, Hua G, Metzger D, Li M, Chambon P (2011) Widespread negative response elements mediate direct repression by agonist‐liganded glucocorticoid receptor. Cell 145:224–241. [DOI] [PubMed] [Google Scholar]
- 119. Hua G, Ganti KP, Chambon P (2016) Glucocorticoid‐induced tethered transrepression requires SUMOylation of GR and formation of a SUMO‐SMRT/NCoR1‐HDAC3 repressing complex. Proc Natl Acad Sci USA 113:E635–E643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sladek FM (2003) Nuclear receptors as drug targets: new developments in coregulators, orphan receptors and major therapeutic areas. Expert Opin Ther Targets 7:679–684. [DOI] [PubMed] [Google Scholar]
- 121. Kendall EC (1951) The development of cortisone as a therapeutic agent. Antibiot Chemother 1:7–15. [PubMed] [Google Scholar]
- 122. Becker DE (2013) Basic and clinical pharmacology of glucocorticosteroids. Anesth Prog 60:25–31. quiz 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bermudez V, Finol F, Parra N, Parra M, Perez A, Penaranda L, Vilchez D, Rojas J, Arraiz N, Velasco M (2010) PPAR‐gamma agonists and their role in type 2 diabetes mellitus management. Am J Ther 17:274–283. [DOI] [PubMed] [Google Scholar]
- 124. Burris TP, Solt LA, Wang Y, Crumbley C, Banerjee S, Griffett K, Lundasen T, Hughes T, Kojetin DJ (2013) Nuclear receptors and their selective pharmacologic modulators. Pharmacol Rev 65:710–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Clark AR, Belvisi MG (2012) Maps and legends: the quest for dissociated ligands of the glucocorticoid receptor. Pharmacol Ther 134:54–67. [DOI] [PubMed] [Google Scholar]
- 126. Diez‐Perez A (2006) Selective estrogen receptor modulators (SERMS). Arq Bras Endocrinol Metabol 50:720–734. [DOI] [PubMed] [Google Scholar]
- 127. Kern D, Zuiderweg ER (2003) The role of dynamics in allosteric regulation. Curr Opin Struct Biol 13:748–757. [DOI] [PubMed] [Google Scholar]
- 128. Tzeng SR, Kalodimos CG (2011) Protein dynamics and allostery: an NMR view. Curr Opin Struct Biol 21:62–67. [DOI] [PubMed] [Google Scholar]
- 129. Kojetin DJ, Matta‐Camacho E, Hughes TS, Srinivasan S, Nwachukwu JC, Cavett V, Nowak J, Chalmers MJ, Marciano DP, Kamenecka TM, Shulman AI, Rance M, Griffin PR, Bruning JB, Nettles KW (2015) Structural mechanism for signal transduction in RXR nuclear receptor heterodimers. Nat Commun 6:8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Choi JH, Banks AS, Estall JL, Kajimura S, Bostrom P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Bluher M, Griffin PR, Spiegelman BM (2010) Anti‐diabetic drugs inhibit obesity‐linked phosphorylation of PPARgamma by Cdk5. Nature 466:451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kojetin DJ, Burris TP (2013) Small molecule modulation of nuclear receptor conformational dynamics: implications for function and drug discovery. Mol Pharmacol 83:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Chandra V, Huang P, Hamuro Y, Raghuram S, Wang Y, Burris TP, Rastinejad F (2008) Structure of the intact PPAR‐gamma‐RXR‐ nuclear receptor complex on DNA. Nature 456:350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chandra V, Huang P, Potluri N, Wu D, Kim Y, Rastinejad F (2013) Multidomain integration in the structure of the HNF‐4alpha nuclear receptor complex. Nature 495:394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lou X, Toresson G, Benod C, Suh JH, Philips KJ, Webb P, Gustafsson JA (2014) Structure of the retinoid X receptor alpha‐liver X receptor beta (RXRalpha‐LXRbeta) heterodimer on DNA. Nat Struct Mol Biol 21:277–281. [DOI] [PubMed] [Google Scholar]
- 135. Cheng Y (2015) Single‐particle cryo‐EM at crystallographic resolution. Cell 161:450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bai XC, McMullan G, Scheres SH (2015) How cryo‐EM is revolutionizing structural biology. Trends Biochem Sci 40:49–57. [DOI] [PubMed] [Google Scholar]
- 137. Yi P, Wang Z, Feng Q, Pintilie GD, Foulds CE, Lanz RB, Ludtke SJ, Schmid MF, Chiu W, O'Malley BW (2015) Structure of a biologically active estrogen receptor‐coactivator complex on DNA. Mol Cell 57:1047–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Yi P, Wang Z, Feng Q, Chou CK, Pintilie GD, Shen H, Foulds CE, Fan G, Serysheva I, Ludtke SJ, Schmid MF, Hung MC, Chiu W, O'Malley BW (2017) Structural and functional impacts of ER coactivator sequential recruitment. Mol Cell 67(733–743):e734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Orlov I, Rochel N, Moras D, Klaholz BP (2012) Structure of the full human RXR/VDR nuclear receptor heterodimer complex with its DR3 target DNA. EMBO J 31:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Maletta M, Orlov I, Roblin P, Beck Y, Moras D, Billas IM, Klaholz BP (2014) The palindromic DNA‐bound USP/EcR nuclear receptor adopts an asymmetric organization with allosteric domain positioning. Nat Commun 5:4139. [DOI] [PubMed] [Google Scholar]
- 141. Liu Z, Lavis LD, Betzig E (2015) Imaging live‐cell dynamics and structure at the single‐molecule level. Mol Cell 58:644–659. [DOI] [PubMed] [Google Scholar]
- 142. Van Royen ME, van Cappellen WA, Geverts B, Schmidt T, Houtsmuller AB, Schaaf MJ (2014) Androgen receptor complexes probe DNA for recognition sequences by short random interactions. J Cell Sci 127:1406–1416. [DOI] [PubMed] [Google Scholar]
- 143. Morisaki T, Muller WG, Golob N, Mazza D, McNally JG (2014) Single‐molecule analysis of transcription factor binding at transcription sites in live cells. Nat Commun 5:4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Presman DM, Ball DA, Paakinaho V, Grimm JB, Lavis LD, Karpova TS, Hager GL (2017) Quantifying transcription factor binding dynamics at the single‐molecule level in live cells. Methods 123:76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Swinstead EE, Miranda TB, Paakinaho V, Baek S, Goldstein I, Hawkins M, Karpova TS, Ball D, Mazza D, Lavis LD, Grimm JB, Morisaki T, Grontved L, Presman DM, Hager GL (2016) Steroid receptors reprogram FoxA1 occupancy through dynamic chromatin transitions. Cell 165:593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Paakinaho V, Presman DM, Ball DA, Johnson TA, Schiltz RL, Levitt P, Mazza D, Morisaki T, Karpova TS, Hager GL (2017) Single‐molecule analysis of steroid receptor and cofactor action in living cells. Nat Commun 8:15896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Stasevich TJ, McNally JG (2011) Assembly of the transcription machinery: ordered and stable, random and dynamic, or both? Chromosoma 120:533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lenstra TL, Rodriguez J, Chen H, Larson DR (2016) Transcription dynamics in living cells. Annu Rev Biophys 45:25–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Vera M, Biswas J, Senecal A, Singer RH, Park HY (2016) Single‐cell and single‐molecule analysis of gene expression regulation. Annu Rev Genet 50:267–291. [DOI] [PMC free article] [PubMed] [Google Scholar]


