ABSTRACT
Background:
Zika virus (ZIKV) sexual transmission and prolonged viral shedding in semen have been previously reported, suggesting a strong viral affinity for genital tissues. A transient impact of ZIKV on male fertility was shown in animal and human studies.
Methods:
Adult male patients with confirmed ZIKV infection diagnosed in the city of Araraquara, Brazil during the epidemic season of 2016 were invited one year after the acute infection to respond to a questionnaire of genital symptoms and to provide a semen sample for molecular ZIKV testing and spermogram analysis, as well as a serum sample for hormonal testing.
Results:
101 of 187 tested patients had positive ZIKV RT-PCR in plasma and/or urine samples (54%, 72 women and 29 men). Of 15 adult male participants for whom telephone contact was successful, 14 responded to the questionnaire of genital symptoms and six consented to provide a semen sample at a median of 12 months after the acute infection. We report abnormal spermogram results from patients one year after confirmed ZIKV infection.
Conclusions:
Our findings suggest a possible long-term detrimental effect of ZIKV infection on human male fertility that has to be further explored in well-characterized samples from cohort studies conducted in ZIKV-endemic areas.
KEYWORDS: Zika virus, Sexual transmission, Shedding, Semen, Spermogram, Fertility, Brazil
BACKGROUND
The Zika virus (ZIKV) infection outbreak in the Americas in the last few years was the largest in history, and knowledge on disease transmission routes, clinical spectrum and potential complications is still expanding. ZIKV is transmitted mainly through the bite of Aedes mosquitos 1 , but maternal-to-child transmission 2 , transmission through contaminated blood 3 and sexual intercourse 4 were also reported. Viral shedding in semen may be prolonged with documented shedding lasting up to six months 5 – 7 . The virus has been detected in semen from a vasectomized man 8 and has also been demonstrated in the head of spermatozoa by immunohistochemical fluorescence microscopy 9 , suggesting its affinity for different male genital tissues. Worryingly, two previous studies of ZIKV infection in mice treated with anti-Ifnar1 blocking monoclonal antibody have demonstrated impairment in male fertility, accompanied by testicular atrophy, lower serum testosterone and inhibin B levels, as well as oligospermia 10 , 11 . A recent study in a cohort of 15 ZIKV-infected men showed a transient reduction in sperm counts in the acute phase of infection, suggesting a potential impact of ZIKV on human male fertility 12 . However, ZIKV ability to cause long-term impairment on male fertility is unknown. From a cohort of 101 ZIKV-infected patients from Araraquara, Brazil, we assessed hormonal results from six ZIKV-infected men, of whom five underwent spermogram analysis in samples collected 12 months after the acute infection.
METHODS
Patients with confirmed ZIKV infection diagnosed at a single primary care unit during the epidemic season of 2016 in Araraquara, Sao Paulo State, Brazil, were identified for this study. All participants had a positive test by real-time reverse transcriptase polymerase chain reaction (RT-PCR) in urine and/or plasma samples obtained during the acute phase. Adult male patients were invited to respond to a short retrospective questionnaire of genital symptoms and to provide a semen sample for molecular ZIKV testing and spermogram analysis, as well as a serum sample for hormonal testing (FSH, LH, testosterone and inhibin B). Each participant was instructed to produce and collect the semen sample at home by masturbation and to bring the sample immediately, in room temperature, to the healthcare unit for analysis. The questionnaire of genital symptoms included questions on the presence of pain or burning when urinating, noticeable blood in semen or urine, testicular pain and any genital abnormality at the time of acute ZIKV infection or at the time the questionnaire was applied.
For RT-PCR testing, nucleic acid was extracted from a volume of 500 μL of total semen samples using the NucliSENS® easyMag® (bioMérieux, Durham, NC). Samples of total semen and seminal plasma were then reextracted using Qiagen® QIAamp Viral RNA mini kit 250 (QIAGEN, Hilden, Germany) according to manufacturer's recommendation. All RT-PCRs were performed with 10 μL of RNA samples by using the Taqman Fast virus OneStep Kit (Applied Biosystems, Forest City, CA) as previously described 13 and following the manufacturer's protocol.
Spermograms were all read fresh at the Araraquara clinic by a single experienced fertility clinic technician who was not blinded to the ZIKV status of the patients.
FSH and LH hormonal levels were measured using electrochemiluminometric assays, while testosterone and inhibin B were measured using serum samples chemiluminescence assays.
For comparison, spermogram results were compared with normal parameters from the World Health Organization (WHO) 14 and with parameters obtained from a consecutive sample of men without history of ZIKV symptoms and without past history of infertility who collected semen samples in the same region (State) and period.
All participants signed informed consent forms upon participation. The study was approved by the Ethics Review Board at University of Sao Paulo Medical School (committee's reference N° 2.554.861).
RESULTS
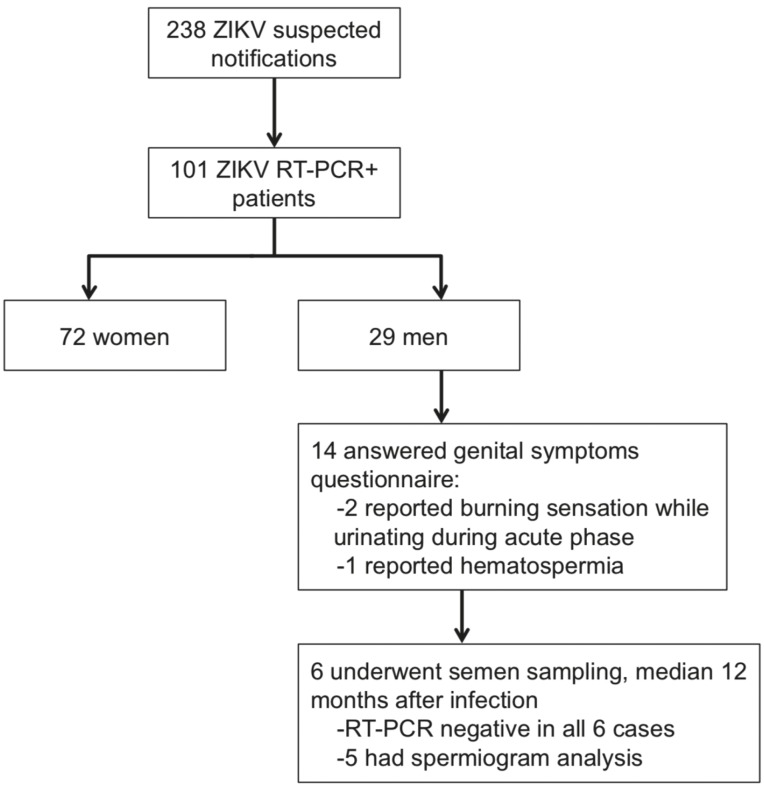
Between January and September 2016, 238 suspected ZIKV notifications were reported at Araraquara referent healthcare unit. Clinical definition of notified cases included patients presenting with maculopapular rash along with at least two of the following symptoms: fever, conjunctivitis, arthritis or periarticular edema. Of these patients, 187 were tested by RT-PCR and 101 (29 men) had positive ZIKV RT-PCR in plasma and/or urine samples. Adult male patients with confirmed ZIKV infections were invited to respond to a retrospective questionnaire of genital symptoms and to provide a semen sample for RT-PCR testing and spermogram analysis. Of 15 adult male participants for whom telephone contact was successful, 14 responded to the questionnaire of genital symptoms and six consented to provide a semen sample at a median of 12 months after the acute infection (range 11-12 months; Figure 1). All semen samples were delivered < 3 h after production. In the questionnaire of genital symptoms, two participants reported burning sensation when urinating at the time of acute ZIKV infection and one reported visible blood in urine; testicular pain or other abnormalities were not described and all participants were asymptomatic at the time the questionnaire was applied.
Figure 1. Selection of study participants.
ZIKV RT-PCR was negative in semen samples for all six participants. Spermogram analysis was performed for five participants, revealing abnormalities in seminal parameters in four of them, compared to WHO standards and local healthy men values. Although only one participant had sperm concentration below the lower reference limit (LRL) of 15 × 106/mL, two other participants had concentration near the LRL (17 and 26 × 106/mL). Progressive motility analysis was performed for three participants who delivered the semen sample less than 2 h after production; abnormal motility was seen in all three cases (2.5%, 14% and 22% progressive motility, respectively compared to expected LRL of 32%). Table 1 shows results for each ZIKV-infected participant, contrasted with LRL from WHO 14 and with median parameters of 17 local, healthy men without history of ZIKV symptoms or infertility antecedents. Of note, this comparison group was younger than ZIKV-infected patients included in the study. No ZIKV testing was performed in plasma, urine or semen specimens of this comparison group. Hormonal testing revealed no anomalies; values of testosterone (in patient 6) and inhibin B (in patient 1) were low, although still within the reference values.
Table 1. Spermogram and hormonal parameters for ZIKV-infected and asymptomatic individuals in Sao Paulo, Brazil, compared to international (WHO) reference standards.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Asymptomatic individuals* (N=17) | Reference values ** | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 61 | 70 | 44 | 38 | 49 | 66 | 33 (30-35) | |
| Months after ZIKV infection | 12 | 11 | 12 | 12 | 12 | NA | – | |
| Sperm concentration per ml | 8.9 × 106 | 17.0 × 106 | 60.0 × 106 | 43.0 × 106 | 26.0 × 106 | NA | 33 (22-60) | >15.0×106 |
| Progressive motility, % | 2.5 | NA | NA | 22 | 14 | NA | 42 (32-54) | >32% |
| Morphology strict criteria (Kruger), % | 2 | 3 | 4 | 2 | 3 | 3 | 2 (2-2) | >4% |
| Hormonal parameters | ||||||||
| FSH, IU/L | 8.1 | 3.5 | 4.5 | 3.1 | 3.3 | 2.3 | NT | <10.0 |
| LH, IU/L | NT | 3.4 | 3.7 | 3.3 | 6.0 | 2.4 | NT | <9.0 |
| Testosterone, ng/dL | 395 | 334 | 548 | 322 | 346 | 286 | NT | 240-816 |
| Inhibin B, pg/mL | 79 | 138 | 119 | 153 | 114 | 123 | NT | 47-308 |
Men without a history of ZIKV symptoms and without infertility antecedents who collected semen samples in the same region and period. Data for asymptomatic participants is presented as medians and interquartile ranges.
Reference values are WHO LRL (low reference limits) for spermiogram; and Brazilian laboratory own range for hormonal levels. FSH=follicle stimulating hormone; LH=luteinizing hormone; NT=not tested.
ZIKV-infected participants with abnormal spermogram results were referred for a free a consultation with a fertility specialist, but results from this evaluation were not available at the time of reporting.
DISCUSSION AND CONCLUSIONS
Despite methodological limitations, restricted number of cases and incomplete semen analysis for two cases, our findings suggest a possible detrimental effect of ZIKV infection on human male fertility detectable at approximately 12 months after the acute ZIKV infection. Since spermatogenesis process in humans takes less than three months, our results indicate a possible persistent impairment in male fertility following symptomatic ZIKV infection. However, the normality of hormonal profiles of these men may be reassuring. This association should be further explored in well-characterized samples from cohort studies conducted in ZIKV-endemic areas including both symptomatic and asymptomatic men. Our results in an extension of a cohort study of 15 ZIKV-infected men followed up in Guadeloupe over six months, who showed a temporary effect of ZIKV on spermatogenesis and hormonal profiles, correlated with persistence of ZIKV shedding in the semen 12 . Since approximately half a million Brazilian men may have been infected with ZIKV during the 2015-16 epidemic 15 , 16 , the long-term fertility sequelae may only appear now. ZIKV should be part of the investigations for men presenting to clinical services with fertility problems.
ACKNOWLEDGMENTS
We thank the study participants, the healthcare workers from Serviço Especial de Saúde de Araraquara and members of Laboratório de Virología LIM 52, Claudio Pannuti, José Eduardo Levi, Camila Malta Romano, Nathalia Souza, Alvina Clara Felix.
Footnotes
FUNDING
This work was partially supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES, process CSF-PVE-S-88887.122640/2016-00, Ministry of Education, Brazil; and the European Union's Horizon 2020 Research and Innovation Program under the ZIKAlliance Grant Agreement N° 734548.
REFERENCES
- 1.Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 2.Brasil P, Pereira JP, Jr, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musso D, Nhan T, Robin E, Roche C, Bierlaire D, Zisou K, et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.14.20761. 20761. [DOI] [PubMed] [Google Scholar]
- 4.Sherley M, Ong CW. Sexual transmission of Zika virus: a literature review. Sex Health. 2018;15:183–199. doi: 10.1071/SH17046. [DOI] [PubMed] [Google Scholar]
- 5.Matheron S, d’Ortenzio E, Leparc-Goffart I, Hubert B, de Lamballerie X, Yazdanpanah Y. Long lasting persistence of Zika virus in semen. Clin Infect Dis. 2016;63:1264–1264. doi: 10.1093/cid/ciw509. [DOI] [PubMed] [Google Scholar]
- 6.Turmel JM, Abgueguen P, Hubert B, Vandamme YM, Maquart M, Le Guillou-Guillemette H, et al. Late sexual transmission of Zika virus related to persistence in the semen. Lancet. 2016;387:2501–2501. doi: 10.1016/S0140-6736(16)30775-9. [DOI] [PubMed] [Google Scholar]
- 7.Nicastri E, Castilletti C, Liuzzi G, Iannetta M, Capobianchi MR, Ippolito G. Persistent detection of Zika virus RNA in semen for six months after symptom onset in a traveller returning from Haiti to Italy, February 2016. Euro Surveill. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.32.30314. 30314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arsuaga M, Bujalance SG, Díaz-Menéndez M, Vázquez A, Arribas JR. Probable sexual transmission of Zika virus from a vasectomised man. Lancet Infect Dis. 2016;16:1107–1107. doi: 10.1016/S1473-3099(16)30320-6. [DOI] [PubMed] [Google Scholar]
- 9.Mansuy JM, Suberbielle E, Chapuy-Regaud S, Mengelle C, Bujan L, Marchou B, et al. Zika virus in semen and spermatozoa. Lancet Infect Dis. 2016;16:1106–1107. doi: 10.1016/S1473-3099(16)30336-X. [DOI] [PubMed] [Google Scholar]
- 10.Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, et al. Zika virus infection damages the testes in mice. Nature. 2016;540:438–442. doi: 10.1038/nature20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uraki R, Hwang J, Jurado KA, Householder S, Yockey LJ, Hastings AK, et al. Zika virus causes testicular atrophy. Sci Adv. 2017;3:e1602899. doi: 10.1126/sciadv.1602899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joguet G, Mansuy JM, Matusali G, Hamdi S, Walschaerts M, Pavili L, et al. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: a prospective observational study. Lancet Infect Dis. 2017;17:1200–1208. doi: 10.1016/S1473-3099(17)30444-9. [DOI] [PubMed] [Google Scholar]
- 13.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization . WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: WHO; 2010. [[cited 2018 Oct 2]]. Available from: http://www.who.int/reproductivehealth/publications/infertility/9789241547789/en/ [Google Scholar]
- 15.Brasil. Ministério da Saúde Secretaria de Vigilância em Saúde. Monitoramento dos casos de dengue, febre de chikungunya e febre pelo vírus Zika até a semana epidemiológica 11, 2018. Bol Epidemiol. 2018;49:1–14. [Google Scholar]
- 16.Brasil. Ministério da Saúde . Protocolo de vigilância e resposta à ocorrência de microcefalia relacionada à infecção pelo vírus Zika: Plano Nacional de Enfrentamento à Microcefalia no Brasil: versão 1.2. Brasilia: Ministério da Saúde; 2015. [Google Scholar]