SUMMARY
Mycobacterial infection leads to the formation of characteristic immune aggregates called granulomas, a process accompanied by dramatic remodeling of the host vasculature. As granuloma angiogenesis favors the infecting mycobacteria, it may be actively promoted by bacterial determinants during infection. Using Mycobacterium marinum-infected zebrafish as a model, we identify the enzyme Proximal Cyclopropane Synthase of alpha-Mycolates (PcaA) as an important bacterial determinant of granuloma-associated angiogenesis. Cis-cyclopropanation of mycobacterial mycolic acids by pcaA drives the activation of host Vegf signaling within granuloma macrophages. Cyclopropanation of the mycobacterial cell wall glycolipid trehalose dimycolate (TDM) is both required and sufficient to induce robust host angiogenesis. Inducible genetic inhibition of angiogenesis and Vegf signaling during granuloma formation results in bacterial growth deficits. Together, these data reveal a mechanism by which PcaA-mediated cis-cyclopropanation of mycolic acids promotes bacterial growth and dissemination in vivo by eliciting granuloma vascularization and suggest potential approaches for host-directed therapies.
eTOC Blurb
Granuloma formation during tuberculosis is accompanied by remodeling of host vasculature. Walton et al. identify the mycobacterial enzyme PcaA, which catalyzes proximal cis-cyclopropanation of trehalose dimycolate, as an important determinant of granuloma-associated angiogenesis. This form of trehalose dimycolate induces Vegfa-mediated angiogenesis at the granuloma, promoting bacterial growth.
Graphical Abstract

INTRODUCTION
Upon entry into the lung, Mycobacterium tuberculosis (M. tuberculosis) is initially phagocytosed by host macrophages. Pathogenic mycobacteria evade the initial antimicrobial response through a variety of mechanisms, and infected and uninfected macrophages form characteristic aggregates that mature into tightly organized structures called granulomas (Ernst, 2012; Pagan and Ramakrishnan, 2018). The resulting environment provides a spatially restricted focus of infection that infecting mycobacteria have adapted to and can exploit (Pagan and Ramakrishnan, 2018; Rittershaus et al., 2013).
In addition to immune cell populations, stromal cells proximal to the granuloma undergo substantial reprogramming and remodeling. Analysis of human clinical specimens and a number of animal models revealed induction of Vegf family members by granuloma macrophages and extensive granuloma vascularization (Datta et al., 2015; Harding et al., 2015; Kumar et al., 2016; Oehlers et al., 2015; Polena et al., 2016). In tumor biology there is extensive interplay between angiogenesis and hypoxia, and, similarly, mycobacterial granulomas in both animal models and humans can develop hypoxic regions (Aly et al., 2007; Folkman, 2002; Oehlers et al., 2015; Tsai et al., 2006; Ulrichs et al., 2005). Notably, mycobacterially-induced angiogenesis at the granuloma promotes bacterial growth in vivo; nascent blood vessels associated with early granulomas may provide oxygen and nutrients, and can create a permissive replication niche for the bacteria within (Datta et al., 2015; Oehlers et al., 2017; Oehlers et al., 2015). Pharmacological limitation of granuloma-associated angiogenesis or vascular normalization results in improved host outcomes in animal models of infections (Oehlers et al., 2017; Oehlers et al., 2015; Polena et al., 2016). As granuloma angiogenesis favors the infecting mycobacteria, it is likely that this host response may be actively promoted, enhanced, or accelerated by bacterial determinants during infection.
Mycolic acids are present in the cell walls of certain Actinomycetes and have been associated with virulence for human and animal pathogens from genera such as Mycobacterium and Rhodococcus (Barry et al., 1998; Sydor et al., 2013). Of the mycolic acid-containing lipids, trehalose-6,6-dimycolate (TDM) is particularly abundant within the outer cell wall of M. tuberculosis (Brennan and Nikaido, 1995). TDM has been described as a potent adjuvant and is associated with a number of aspects of mycobacterial disease pathogenesis, including limitation of phagolysosome fusion and activation of matrix metalloproteases (Axelrod et al., 2008; Hunter et al., 2006; Indrigo et al., 2003; Middlebrook et al., 1947; Patin et al., 2017; Sakamoto et al., 2013). Studies of host recognition of TDM have implicated diverse cell surface proteins, including Toll-like receptors, lectins, and scavenger receptors (Bowdish et al., 2009; Lang, 2013; Miyake et al., 2015; Richardson and Williams, 2014). Thus, TDM may act through multiple signaling pathways as a major modulator of the host immune response to mycobacteria. To date, however, relatively little attention has been paid to the angiogenic potential of TDM, although TDM can induce angiogenesis in a rat corneal model (Saita et al., 2000).
TDM is essential for mycobacterial viability in vivo, but a number of chemical modifications that occur within the mycolic acid tails of TDM can be genetically targeted. The three major classes of mycolic acids (α, keto, and methoxy) incorporate double bonds or cyclopropyl groups into the proximal position of the meromycolate chain and either double bonds, cyclopropyl groups, ketones, or methoxy groups into the distal position (Brennan and Besra, 1997). These non-essential chemical modifications can be genetically altered to study in vivo infection phenotypes (Glickman et al., 2001; Glickman et al., 2000).
Notably, functionalization at the proximal position with a cyclopropyl group is associated with pathogenic species of mycobacteria; the mycolates from M. tuberculosis, M. avium, M. ulcerans, and M. marinum contain cylcopropyl groups at this position, while nonpathogenic species such as M. smegmatis and M. chelonae possess a double bond (Daffe et al., 1991; Liu et al., 1996). Additionally, an enzyme that catalyzes the addition of cis-cyclopropyl groups to the proximal end of mycolic acids of the α-mycolate class, PcaA, has been implicated in virulence (Glickman et al., 2000). In murine models, pcaA mutants display an in vivo growth defect at one week post-infection, concomitant with a reduced pro-inflammatory cytokine response from macrophages (Glickman et al., 2000; Rao et al., 2005). However, the influence of mycolic acid modifications on granuloma-associated angiogenesis has not been examined.
We have previously utilized the zebrafish/M. marinum model of infection to study angiogenesis during mycobacterial infection and granuloma formation (Oehlers et al., 2015). The zebrafish is a natural host to M. marinum, a close relative of the M. tuberculosis complex (Tobin and Ramakrishnan, 2008). Mycobacterial pathogenesis in zebrafish closely resembles that of human TB, from macrophage aggregation and vascularization to the formation of necrotic and hypoxic granulomas (Cronan et al., 2016; Davis et al., 2002; Oehlers et al., 2015; Swaim et al., 2006). Using M. marinum mutant strains, we demonstrate that PcaA-mediated modifications to TDM are required to elicit a robust angiogenic response during mycobacterial infection. We describe in vivo growth defects for pcaA mutants that coincide with the onset of granuloma vascularization. Furthermore, we demonstrate that the growth advantage of wildtype bacteria over pcaA mutants is Vegf dependent. Together, these data identify a role for TDM in angiogenesis-driven mycobacterial expansion via Vegf induction and identify the proximal cis-cyclopropyl modification of mycolic acid tails as crucial for TDM-mediated angiogenesis.
RESULTS
PcaA Deficiency Leads to Defects in Host Angiogenesis
To visualize vasculature within larval zebrafish, we used the Tg(kdrl:EGFP)s843 transgenic line in which EGFP expression is driven by the zebrafish kdrl promoter and labels all vasculature (Jin et al., 2005). Vasculature in the larval trunk forms a stereotypical pattern around the somites (Figure 1A). After dorsal infection with M. marinum, analysis of granulomas in the trunk provides a ready and quantifiable measure of angiogenesis in live animals (Figure 1B).
Figure 1. Mutations in pcaA Result in Angiogenesis-deficient Infection Foci.
A. Depiction of the M. marinum injection site within the trunk of a zebrafish larva. Brightfield and GFP channels (upper and lower panels, respectively) are shown for clarity. Dotted line indicates approximate area shown for images in (B). Scale bar, 500μm. B. Representative images of larvae at four days post-infection (dpi) infected with either wildtype, pcaATn28776, or complemented mutant M. marinum. Bacteria in red, vasculature in green (upper panels) or white (lower panels). Blue arrows denote infection-associated angiogenesis. Length of abnormal vasculature at 5 dpi for infections with pcaATn20324 (C) and 4 and 5 dpi for pcaATn28776 (D and E). Representative of more than three independent experiments for each mutant. Scale bars 100μm. For experiment involving pcaATn20324, ****p<0.0001; Student’s t-test. For all other statistics, *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001; One-way ANOVA with Tukey’s multiple comparison post-test.
As we had previously found that host angiogenesis benefits infecting mycobacteria, we hypothesized that specific bacterial factors directly promote angiogenesis. The lipid-rich mycobacterial cell wall is a key interface between pathogen and host, and a number of lipid species have been identified that modify the host immune response (Brennan, 2003). The most abundant of these is TDM, a lipid known to interact with diverse host immune pathways. Deletion of the M. tuberculosis pcaA gene results in bacteria with an atypical pool of cell wall mycolic acids. pcaA mutants lack proximal cis-cyclopropyl α-mycolates and exhibit reduced proportions overall of both free α-mycolates and TDM of the α-mycolate class (Glickman et al., 2000; Rao et al., 2005). Despite these alterations to the cell envelope, ΔpcaA M. tuberculosis remain viable during infection. We hypothesized that disruption of M. marinum pcaA gene would confer similar cell wall alterations without compromising pathogen viability, providing an opportunity to probe the importance of TDM in granuloma-associated angiogenesis.
Using a defined M. marinum transposon library, we isolated two transposon mutants in the M. marinum pcaA gene (C. Cosma and L. Ramakrishnan). The mutants, designated pcaATn28776 and pcaATn20324, possessed independent transposon insertions, in opposite orientations, ~10% of the way through the open reading frame. There were no significant differences in in vitro growth rates between wildtype M. marinum and the transposon mutants, with doubling times for wildtype, pcaATn28776, and pcaATn20324 of 11.3, 11.7, and 10.9 hours, respectively, under the conditions assayed.
We constructed fluorescent versions of each transposon strain to image both infection and angiogenesis in vivo. Both pcaA mutants exhibited severe defects in granuloma-associated vascularization (Figure 1B). Decreased angiogenesis in the pcaA mutants was observed from the outset of vascularization in paired wildtype infections, at approximately four days post-infection (dpi) (Figure 1B,C) and persisted at later timepoints (Figure 1E,F). This phenotype could be rescued through expression of PcaA using a constitutive promoter (Figure 1B,D,E). Granulomas formed normally in the pcaA mutants. Differences in granuloma-associated angiogenesis could be observed at 3 days post-infection, the earliest timepoint at which vascularization could be observed in wildtype infections, suggesting a potentially early and direct interaction of TDM with the host.
TDM is Sufficient to Induce Angiogenesis
Based on the strong genetic requirement for pcaA for robust granuloma vascularization, we asked whether TDM might directly induce angiogenesis. To perform TDM sufficiency experiments, we developed a method to administer highly hydrophobic TDM into the zebrafish trunk. We found we could inject mineral oil and surfactant (Incomplete Freund’s Adjuvant) at two days post-fertilization (dpf) to generate stable, persistent droplets within the larvae. We then injected vehicle control or purified alpha mycolate TDM from M. bovis dissolved in vehicle into the trunks of Tg(kdrl:EGFP) larvae at two days post-fertilization (dpf) (Figure 2A). By two days post-injection, there was little or no abnormal vasculature in the vehicle-injected animals (Figure 2B,C). In contrast, TDM-containing droplets induced substantial abnormal vasculature, which associated spatially with the TDM-loaded droplets (Figure 2B,C). These results suggested that TDM is sufficient to evoke robust angiogenesis and, combined with the genetic mutant data, suggested a direct role for TDM in granuloma-associated angiogenesis.
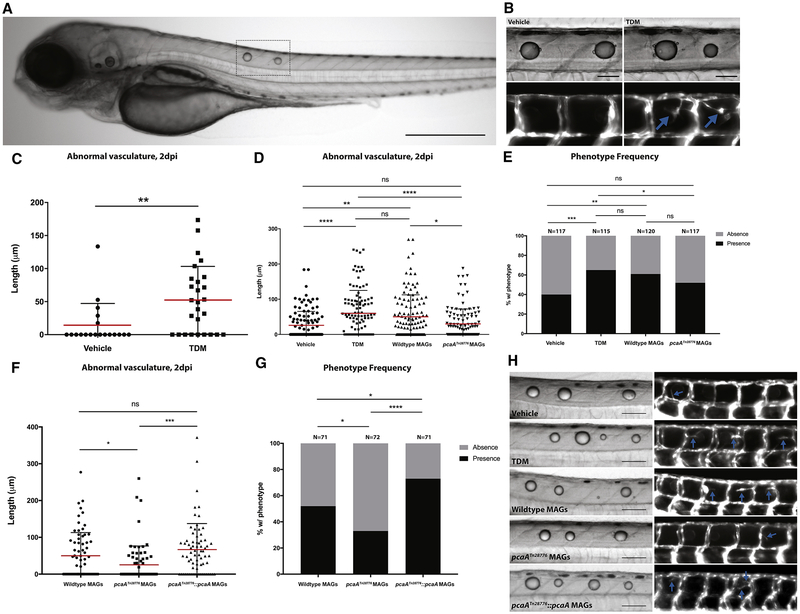
Figure 2. Purified TDM and Mycolic Acid Glycolipids from Wildtype Bacteria but not pcaA Mutants Elicit Robust Angiogenesis.
A. Representative image of two day post-injection larvae injected dorsally with TDM. Dotted line indicates injection site and droplet coalescence. Scale bar, 500μm. B. Representative images of injections of either vehicle (left) or TDM (right) into Tg(kdrl:EGFP) animals. Blue arrows indicate abnormal vasculature (lower panels). Scale bars, 100 μm. C. Quantification of abnormal vasculature induced by TDM or vehicle alone. Representative of 3 experiments. **p<0.01, Student’s t-test. D. Quantification of abnormal vasculature induced by MAGs isolated from wildtype or pcaA mutant M. marinum, with vehicle alone and TDM as negative and positive controls, respectively. Data from three experiments combined. *p<0.05; **p<0.01; ****p<0.0001; One-way ANOVA with Tukey’s multiple comparison post-test. E. Frequency of presence or absence of abnormal vasculature. *p<0.05; **p<0.01; ***p<0.001; Fisher’s exact test. F. Quantification of the length of abnormal vasculature induced by MAGs (5mg/ml) isolated from pcaATn28776::pcaA. MAGs from wildtype and pcaATn28776 included as positive and negative controls. Data from three experiments combined. *p<0.05; ***p<0.001; One-way ANOVA with Tukey’s multiple comparison post-test. G. Frequency of presence or absence of abnormal vasculature. *p<0.05; ****p<0.0001; Fisher’s exact test. H. Representative images of larvae injected with vehicle, TDM, wildtype MAGs, MAGs isolated from pcaATn28776, and MAGs isolated from pcaATn28776::pcaA. Blue arrows indicate abnormal vasculature. Scale bars, 100 μm.
Given the sufficiency of alpha mycolate TDM in inducing angiogenesis and the requirement of mycobacterial pcaA for in vivo infection-induced angiogenesis, we hypothesized that PcaA-modified mycolates must be incorporated into TDM to be proangiogenic in vivo. However, it has been shown previously that free mycolic acid methyl esters (MAMEs) are also modified by PcaA, leaving open the possibility that these too contribute to PcaA-dependent angiogenesis (Glickman et al., 2000). TLC analysis of the TDM used in the previous experiments contained only alpha mycolates (Figure S1A). Since oxygenated mycolates (methoxy- and keto-mycolates) have been previously identified as important for the virulence of M. tuberculosis (Dubnau et al., 2000), we wondered if these species would enhance or otherwise alter the angiogenic potential we observed for pure alpha-mycolate TDM.
To test these hypotheses, we compared the angiogenic effects of bulk TDM and bulk MAMEs isolated from M. tuberculosis, both of which possess mycolic acids modified by PcaA as well as the primary oxygenated mycolate species. We injected Tg(kdrl:EGFP) larvae with either TDM (2mg/ml) or MAMEs (2mg/ml) at 2 dpf and assessed abnormal vascularization at 4 dpf. TDM from M. tuberculosis induced robust angiogenesis (Figure S2A,B), achieving levels of vascularization comparable to TDM from M. bovis. The MAMEs, however, did not induce angiogenesis above vehicle alone (Figure S2C,D). Together, these data support the role of PcaA-modified TDM specifically, and not merely mycolic acids in general, as the critical inducer of PcaA-dependent angiogenesis. The sufficiency of pure alpha mycolate TDM in inducing angiogenesis along with a lack of enhancement of this phenotype when oxygenated mycolates are present strongly supports alpha mycolate TDM as the isoform responsible for driving vascularization.
The requirement that the cis-cyclopropyl alpha mycolates be incorporated into TDM in order to elicit angiogenesis suggested that host receptors for TDM might be involved in initiating this process. To begin to probe this mechanism, we focused on Myd88- and FcRγ-dependent signaling pathways, both of which have been implicated in host responses to TDM (Bowdish et al., 2009; Geisel et al., 2005; Lobato-Pascual et al., 2013; Miyake et al., 2013; Werninghaus et al., 2009). We generated CRISPR/Cas9 knockouts of Myd88 and the two zebrafish FcRγ paralogues to abrogate most Toll-like Receptor and Mincle/MCL signaling, respectively. Animals homozygous for a 22 bp deletion in Exon 1 of myd88 showed no differences in TDM-induced vascularization compared to heterozygous or wildtype siblings (Figure S3A–C). For the FcRγ experiments, we generated mosaic FcRγ knockout larvae in the Tg(kdrl:EGFP) background by CRISPR/Cas9 targeting of both FcRγ paralogues. Mosaic FcRγ knockout animals exhibited a significant decrease in abnormal vasculature in response to TDM compared to the control group (Figure S3D,E), suggesting that FcRγ contributes to TDM-induced angiogenesis.
Purified Mycolic Acid-containing Glycolipids from Wildtype but not pcaA Mutant Bacteria Induce Angiogenesis
We next sought to determine if the different TDM compositions present within the cell walls of wildtype and pcaA mutant M. marinum were responsible for differences in the in vivo angiogenic potential between strains. We isolated free mycolic acid-containing glycolipids (MAGs), which in mycobacteria consist primarily of TDM (Bloch et al., 1953), from wildtype and pcaA mutant M. marinum by overnight extraction with 2:1 chloroform:methanol followed by precipitation in acetone (Parish and Stoker, 2001).
Resolution of the TDM species in the pcaA mutant by thin layer chromatography (TLC) revealed a reduced proportion of alpha mycolate-containing TDM relative to ketoand methoxy-mycolate TDM in the pcaA mutant relative to wildtype (Figure S1A,B). Similarly, the M. tuberculosis pcaA knockout exhibits a large reduction in the proportion of alpha mycolate-containing TDM (Glickman et al., 2000; Rao et al., 2005).
To confirm that M. marinum PcaA possesses the same enzymatic function as the M. tuberculosis and M. bovis orthologues, we isolated MAMEs from wildtype and pcaA mutant M. marinum. Resolution of this fraction by TLC again revealed a reduced proportion of alpha mycolate MAMEs compared to the methoxy/keto fraction (Figure S1C,D). Importantly, 1H NMR on the alpha mycolate fractions showed a 12-fold reduction in cis-cyclopropanation for MAMEs isolated from the pcaA mutant (Figure S1E,F). These results indicate both that M. marinum PcaA performs the same function as in other mycobacteria and that the transposon insertion produces a loss-of-function allele.
We then compared in vivo angiogenic activity of wildtype purified MAGs with those isolated from the pcaA mutant and complemented mutant strains. Using the droplet administration method developed earlier, we injected equivalent doses of the purified MAGs from each strain into separate groups of larvae. MAGs from wildtype and complemented mutant bacteria consistently induced angiogenesis at a level equivalent to purified TDM, whereas this fraction from the pcaA mutant failed to induce angiogenesis above the vehicle control (Figure 2D–H). The TDM positive control, wildtype MAGs, and complemented mutant MAGs all also exhibited an increase in the frequency at which abnormal vascularization was observed compared to either vehicle alone or mutant MAGs (Figure 2E,G). Thus, loss of cis-cyclopropyl-modified mycolic acids abrogates the ability of these lipids to induce robust angiogenesis in vivo.
pcaA Mutants Fail to Induce Vegfa Expression
We have previously demonstrated that the Vegf receptor ligand Vegfaa is induced around sites of infection with wildtype M. marinum (Oehlers et al., 2015). VEGFA has been reported to be expressed in human granuloma macrophages, where extensive vascularization also occurs (Datta et al., 2015). Given the requirement and sufficiency of cyclopropanated TDM for granuloma-associated angiogenesis, we hypothesized that the non-modified TDM present in pcaA mutants might fail to induce Vegfaa. We utilized a transgenic line in which vegfaa regulatory sequences direct expression of an EGFP reporter transgene (TgBAC(vegfaa:EGFP)pd260 (Karra et al., 2018). During larval development the vegfaa reporter is driven in the developing vasculature at low levels in a stereotyped pattern throughout the animal (Karra et al., 2018). However, larvae infected with wildtype bacteria exhibited robust activation of this reporter at the granuloma (Figure 3A,B). In contrast, larvae infected with pcaA mutant bacteria largely failed to induce any robust signal above the developmental expression level, despite the formation of well-structured granulomas (Figure 3A,B). Larvae infected with the complemented mutant strain exhibited an intermediate phenotype, with ~3-fold greater reporter induction on average compared to the mutant strain (Figure 3A,B). These data implicate PcaA-dependent chemical modification of TDM in specifically promoting Vegfaa activation within the granuloma and suggested that TDM-dependent angiogenesis proceeds through engagement of the canonical pro-angiogenic Vegf signaling pathway.
Figure 3. pcaA Mutants Fail to Induce Host vegfaa Reporter Expression.
A. Representative images of TgBAC(vegfaa:EGFP) larvae 5 dpi infected with either wildtype (top), pcaA mutant (middle), or complemented pcaA mutant M. marinum (bottom). Blue arrows denote areas of GFP signal above normal developmental pattern. Scale bars, 500μm B. Quantification of GFP signal above baseline for wildtype, pcaA mutant, and complemented pcaA mutant larval infections. Data from three experiments combined. *p<0.05; **p<0.01; ****p<0.0001; One-way ANOVA with Tukey’s multiple comparison post-test. n=88 for wildtype infections; n=86 for pcaA mutant; n=86 for complemented mutant.
Previous characterization of the M. tuberculosis pcaA knockout strain in the murine model revealed a reduced host TNF response during early infection (Rao et al., 2005). As the timing of Vegf induction occurs near the timing of the murine host TNF deficit, we wondered whether a reduced TNF response might play a role in the lack of angiogenesis in pcaA mutant infections. We first utilized the tnfa transcriptional reporter transgenic line TgBAC(tnf:GFP)pd1028 (Marjoram et al., 2015). We infected these larvae with either wildtype, pcaA mutant, or complemented mutant M. marinum, and assessed bacterial burden and GFP signal for each larva at 3 dpi. We found that there were no differences on average in GFP signal or relative TNF transcript levels among each group (Figure S4A–D).
To test functionally whether TNF signaling plays a role in Vegf pathway activation during infection, we generated mosaic CRISPR/Cas9 knockouts of TNF Receptors 1 and 2 in TgBAC(vegfaa:EGFP) larvae. After infection with M. marinum we assessed bacterial burdens and GFP signal in the vegfaa reporter line at 5 dpi for each group. In TNFR edited animals, bacterial burden was increased (Figure S4F), consistent with previous reports (Clay et al., 2008) and suggesting that the edited animals were indeed functionally deficient in TNF signaling. However, no differences were observed in vegfaa reporter signal between the two groups (Figure S4E,G). Thus, the pcaA-dependent vegfaa induction appears to be independent of TNF signaling.
Genetically-encoded, Inducible Blockade of Vegf Signaling Abrogates TDM-induced Angiogenesis
We next tested whether TDM-induced angiogenesis was functionally dependent on Vegf signaling in vivo. We utilized a validated transgenic zebrafish line in which a heat shock inducible promoter drives a dominant-negative form of Vegfaa (Tg(hsp70l:dn-vegfaa)bns100), enabling temporally controlled inhibition of Vegf signaling (Marin-Juez et al., 2016). This dominant-negative form of Vegfaa functions by promoting the formation of an inactive heterodimer of native Vegfaa and dn-Vegfaa incapable of inducing Vegfr2 dimerization and subsequent pro-angiogenic signaling (Muller et al., 1997; Rossi et al., 2016).
To test a requirement for Vegf induction in TDM-induced angiogenesis, Tg(hsp70l:dn-vegfaa); Tg(kdrl:EGFP) larvae were heat shocked immediately prior to TDM injection at 2 dpf and heat shocked again at 3 dpf, followed by imaging at 4 dpf. Larvae were genotyped post hoc to distinguish dominant-negative vegfaa-expressing larvae from wildtype siblings. Animals expressing dominant-negative vegfaa showed dramatically reduced TDM-induced angiogenesis compared to heat-shocked wildtype siblings (Figure 4A,B). Thus, targeted genetic inhibition of Vegf is sufficient to abrogate TDM-induced angiogenesis.
Figure 4. Inducible and Pharmacological Blockade of Vegf Pathway Activation Abrogates TDM-induced Angiogenesis.
A. Quantification of abnormal vasculature at two dpi for larvae with and without the Tg(hsp70l:dn-vegfaa) transgene. ***p<0.001, Student’s t-test. B. Comparison of the frequency of the presence or absence of abnormal vasculature between larvae with or without the Tg(hsp70l:dn-vegfaa) transgene after blinded scoring. Data from three experiments combined. **p<0.01, Fisher’s exact test. C. Quantification of abnormal vascularization at two dpi for larvae treated with either the pan-Vegf receptor inhibitor Pazopanib (250nm) or vehicle (0.1% DMSO). ***p<0.001, Student’s t-test. D. Phenotype frequency for vehicle and Pazopanib treated groups. Data from three experiments combined. ***p<0.001, Fisher’s exact test.
To further validate these findings, we utilized a known small-molecule pan-Vegf receptor inhibitor, Pazopanib, that has been previously shown to effectively inhibit Vegf-dependent angiogenesis in humans and zebrafish (Oehlers et al., 2015; Podar et al., 2006). We injected Tg(kdrl:EGFP) larvae with TDM at 2 dpf, and randomly sorted half of the injected larvae into either E3 media containing vehicle (0.1% DMSO final concentration) or 250nM Pazopanib. Similar to the heat-shock-induced expression of dn-Vegfaa, treatment with Pazopanib abrogated the ability of TDM to induce angiogenesis in vivo (Figure 4C,D).
Wildtype M. marinum Growth Advantage Begins with the Onset of Angiogenesis
To examine the link between compromised angiogenesis and reduced bacterial growth, we temporally assessed pcaA mutant growth deficiency relative to wildtype infection. Larvae were infected with equal initial burdens of either wildtype, pcaA mutants, or the PcaA-complemented strain, and infection burdens were assessed at 1, 3, 4, and 5 dpi. Angiogenesis typically coincides with the beginning of granuloma formation at 3–4 dpi (data not shown). We observed identical in vivo growth among all three bacterial strains between days 1–3, suggesting that altered TDM does not lead to early differences in the ability of macrophages to restrict infection (Figure 5A,C). Instead, the pcaA mutant growth defect emerged only later, at 4 dpi, and became more pronounced at 5 dpi relative to wildtype (Figure 5A,C).
Figure 5. pcaA Mutant Bacteria Exhibit in vivo Growth Defects Coinciding with Onset of Granuloma Angiogenesis.
A. Five-day time course of larvae infected with wildtype, pcaA mutant, or complemented mutant M. marinum strains. Bacterial burdens were measured at 1, 3, 4, and 5 dpi. At 4 dpi pcaA mutants exhibit a reduced growth rate, which persists through 5 dpi. The complemented mutant strain exhibits wildtype levels of growth throughout. Representative of 3 independent experiments. *p<0.05, One-way ANOVA with Dunnett’s multiple comparison post-test. n=40 for each group. B. Quantification of abnormal vascularization. The timing of the growth defect correlates with the onset of robust vascularization for wildtype and complemented strain infections but a failure to extensively vascularize infection foci for pcaA mutant strain infections. ****p<0.0001, One-way ANOVA with Tukey’s multiple comparison post-test. n=40 for each group. C. Representative images of larvae at 5 dpi infected with each of the M. marinum strains. Wildtype, upper panel; pcaA mutant, middle; complemented mutant, lower. Scale bars, 500μm. D-F. Larvae possessing the Tg(kdrl:EGFP) transgene were co-infected with wildtype and PcaA mutant M. marinum. Bacterial burden (D) and length of abnormal vasculature (E) were quantified for each larva at 5 dpi. *p<0.05; **p<0.01;****p<0.0001; One-way ANOVA with Tukey’s multiple comparison post-test. Data shown represent an aggregate of two independent experiments. n=27 for wildtype infections alone; n=27 for pcaA mutant infections alone; n=28 for co-infection. F. Representative images of each infection. Upper panels: Merge of brightfield and fluorescent channels. Red depicts wildtype M. marinum; blue depicts pcaA mutant M. marinum; purple depicts co-localization between the two strains. Dotted lines denote the area shown in lower panels. Scale bars, 500μm. Middle panels: fluorescent channels alone, showing infection burden. Lower panels: vasculature. Blue arrows denote intersegmental vessel growth characteristic of granuloma angiogenesis. Scale bars, 100μm.
In addition, we used RT-qPCR to assess relative M. marinum 16S rRNA content from total RNA isolated from each group of larvae. The relative differences in burden among the three groups using this method were nearly identical to relative differences measured via fluorescent area (Figure S5G). This pcaA-dependent growth defect coincided with the observed decreased vascularization in the pcaA mutant at 4 dpi (Figure 5B). The burden and angiogenesis differences could be rescued by constitutive bacterial expression of PcaA (Figure 5A–C). Taken together, these findings suggest that the loss of pcaA-dependent angiogenesis at 4 dpi may underlie the growth defect observed in pcaA mutant mycobacteria.
While these data suggested that reduced angiogenesis preceded reduced burden in pcaA mutants, we next probed more definitively whether reduced angiogenesis underlies the burden decreases in pcaA mutants. Taking advantage of the natural variability in zebrafish larval infections, we had previously found that wildtype M. marinum infection burden and infection-induced vascularization are positively correlated (Oehlers et al., 2015). We observed the same correlation of burden and vascularization for infections with wildtype or complemented pcaA mutant bacteria (Figure S5A,C), but no correlation between burden and angiogenesis for the pcaA mutant strain (Figure S5B). These results suggested that even high pcaA mutant burdens do not trigger robust angiogenesis.
Similarly, we compared levels of angiogenesis within the subset of data for which pcaA mutants and wildtype bacteria could be burden matched. Even under these constraints, pcaA mutant infections were severely compromised for angiogenesis compared to both wildtype and complemented strains (Figure S5D–F). Together, these analyses support a cause-and-effect relationship in which the pcaA mutant in vivo growth defect is downstream of an inability to induce robust granuloma vascularization.
Bacterial pcaA Mutant Deficits are Non-Cell-Autonomous
To further probe the relationship between vascularization and burden, we reasoned that co-infection of differentially labeled wildtype and pcaA mutants would enable us to distinguish bacterium cell-autonomous effects of the mutation (for example, any compromised fitness arising from alterations of bacterial cell wall structure) from non-cell-autonomous effects relating to alterations in overall host environment. We co-infected animals with wildtype M. marinum in one color and pcaA mutants in a second color and monitored the growth of each strain independently. We found that wildtype M. marinum could rescue both the pcaA angiogenesis and burden deficits in co-infected granulomas (Figure 5D–F), further corroborating a principal role for non-cell-autonomous processes such as angiogenesis as the basis for the pcaA phenotype.
PcaA Mutants Exhibit a Growth Defect as a Result of a Failure to Engage the Vegf Pathway
Based on these data and in vivo imaging of the vegfaa reporter, we hypothesized that the loss of vascularization and restricted growth in the pcaA mutant resulted from a failure of Vegf-dependent vascularization. We infected Tg(kdrl:EGFP) and Tg(kdrl:EGFP); Tg(hsp70l:dn-vegfaa) larvae with wildtype or pcaA mutant M. marinum, heat shocked once a day at 3 and 4 dpi, and analyzed bacterial burden and vasculature at 5 dpi. In animals infected with wildtype M. marinum, we found that, as expected, there was a significant reduction in both angiogenesis (Figure 6B) and bacterial burden for the induced transgenic larvae (Figure 6A,C). Although small molecule-based and antibody-based manipulation of this pathway has been reported previously in mycobacterial infection (Datta et al., 2015; Oehlers et al., 2015), these results provide direct genetic evidence that inhibition of Vegf signaling is host-beneficial in early mycobacterial infection.
Figure 6. Vegf Pathway Blockade Inhibits in vivo Growth of Wildtype but not pcaA Mutants.
A. Bacterial burden of larvae with or without the Tg(hsp70l:dn-vegfaa) transgene at 5 dpi, infected with either wildtype or pcaA mutant M. marinum. Expression of the transgene induces a reduction in wildtype M. marinum burden compared to larvae without the transgene. Expression of the transgene had no effect on the bacterial burdens of larvae infected with the PcaA mutant strain. *p<0.05; **p<0.01; One-way ANOVA with Tukey’s multiple comparison post-test. n=31 and n=55 for wildtype infections in larvae without or with the Tg(hsp70l:dn-vegfaa) transgene, respectively; n=33 and n=39 for pcaA mutant infections in larvae without or with the Tg(hsp70l:dnvegfaa) transgene, respectively. B. Quantification of abnormal vasculature during wildtype infections for Tg(kdrl:EGFP)+ larvae with or without the Tg(hsp70l:dn-vegfaa) transgene. The double transgenic larvae were present as a subset of the cross between the Tg(kdrl:EGFP) and Tg(hsp70l:dn-vegfaa) transgenic lines. Induction of the Tg(hsp70l:dn-vegfaa) transgene results in a reduction in infection-induced angiogenesis. *p<0.05, Student’s t-test. n=16 and n=25 for larvae without or with the Tg(hsp70l:dn-vegfaa) transgene, respectively. C. Representative images of vasularization in larvae with the Tg(hsp70l:dn-vegfaa) transgene (right panels) and without (left panels). Upper panels: brightfield, with the dotted line depicting regions shown in the lower panels. Scale bars, 500μm. Middle panels: merge of vasculature and infection. Lower panels: Vasculature alone. Scale bars, 100μm. All quantitation performed blind to genotype.
In contrast, inducible inhibition of Vegfaa signaling in the pcaA mutants had no effect on burden (Figure 6A). Thus, in vivo, the entirety of physiologically relevant Vegf production during early infection is pcaA dependent. Consistent with the requirement for pcaA in Vegf induction, the infection burden in dn-Vegfaa larvae infected with wildtype bacteria was reduced to the level seen in the pcaA mutant infections. These data demonstrate that Vegf pathway activation is a bacteria-beneficial host process for pathogenic mycobacteria during early infection and provide additional evidence that the defects observed for the pcaA mutant derive from its inability to activate the Vegf pathway and vascularize infection foci.
DISCUSSION
Pathogenic mycobacteria are capable of both evading the host immune response and reprogramming host phagocytes in order to modify their environment. It is known that granulomas formed during M. tuberculosis infection initially become highly vascularized, and this angiogenesis has been shown to facilitate bacterial growth, likely by mitigating hypoxia and nutrient deprivation within the granuloma interior and promoting dissemination (Aly et al., 2007; Oehlers et al., 2015; Tsai et al., 2006; Ulrichs et al., 2005). Previous work found that granuloma angiogenesis is macrophage-dependent, and host-directed chemical inhibition of Vegf signaling abrogates vessel growth and reduces bacterial fitness (Oehlers et al., 2015), implying that pathogenic mycobacteria may drive or enhance this process within the host.
Here, we leveraged the unique advantages of the zebrafish model to demonstrate that specific chemical modifications to mycolic acids, a unique class of lipids constituting the major component of the outer cell wall of mycobacteria, are largely responsible for the early vascularization of infection foci. Specifically, we found that proximal cis-cyclopropanation of the mycolic acid tails of trehalose-6,6-dimycolate is the major angiogenic component of mycolic acid-containing cell wall lipids. This modification has previously been reported to play a role in interactions with innate immune cells, but its role in angiogenesis had not been examined (Glickman et al., 2000; Rao et al., 2005).
Loss of these cis-cyclopropanated lipids during infection leads to a reduced overall bacterial burden that coincides with the normal onset of granuloma vascularization. The in vivo pathogen growth deficiency during this period appears to be largely if not entirely driven by a defect in Vegf pathway activation after granuloma formation. VEGFA induction in granuloma macrophages has consistently been reported to be a feature of mycobacterial infection in non-human primates and in humans (Datta et al., 2015; Matsuyama et al., 2000; Polena et al., 2016). Similarly, in zebrafish, Vegfaa production arises in granuloma macrophages, and angiogenesis is macrophage dependent (Oehlers et al., 2015). Here, using transgenic lines, we found that Vegfaa production arises at the granuloma in a pcaA dependent fashion, and that selective genetic inhibition of Vegfaa signaling alone reduces bacterial burden to pcaA mutant levels.
These data suggest that vascularization of mycobacterial granulomas is not merely a passive phenomenon driven by the host response to dense, hypoxic cell aggregates. Rather, pathogenic mycobacteria actively promote or accelerate angiogenesis, expending energy by chemically modifying a cell wall lipid in a manner that, while not essential for viability, nonetheless creates a significant growth advantage in vivo.
TDM engages multiple host pathways that may promote mycobacterial pathogenesis and thus the molecule may induce pleiotropic effects (Axelrod et al., 2008; Hunter et al., 2006; Indrigo et al., 2003; Middlebrook et al., 1947; Patin et al., 2017; Sakamoto et al., 2013). Here, however, we find that one specific chemical modification drives TDM’s angiogenic activity via Vegfaa induction. Importantly, the pcaA mutants were not defective for initial in vivo growth within macrophages over the first three days of infection, and burden defects coincided with differences in angiogenesis. No additional burden defects emerged in the pcaA mutants upon genetic blockade of Vegfaa signaling, suggesting that the physiologically relevant effects of Vegfaa during early mycobacterial infection may be entirely pcaA dependent.
In cell culture models and in mice, M. tuberculosis pcaA mutants were shown to induce less initial TNF and to have a growth deficit at seven days post-infection, although there was recovery to wildtype bacterial numbers at later timepoints (Rao et al., 2005). However, the effects we have observed on angiogenesis are difficult to translate or probe in standard mouse models; C57Bl/6 mouse M. tuberculosis granulomas do not form the extensively structured hypoxic granulomas observed in human disease (Harper et al., 2012; Tsai et al., 2006).
The Vegf dependence of the wildtype growth advantage we observed in this study may be complementary to the previously observed TNF-dependent growth advantage (Rao et al., 2005). Pathogenic angiogenesis possesses a complex relationship with TNF. Short TNF pulses early in inflammation can prime endothelial cells for sprouting and proliferation while simultaneously blocking Vegf signaling, delaying but ultimately promoting angiogenesis (Sainson et al., 2008). In our model, however, TNF signaling does not appear to be crucial for early Vegf induction. Other reported activities of TDM may also influence vascularization at the mycobacterial granuloma; TDM can induce macrophage expression of MMP9, which has been implicated in angiogenesis via mobilization of VEGF as well as granuloma formation (Bergers et al., 2000; Sakamoto et al., 2013; Volkman et al., 2010).
Triggering host angiogenesis at the outset of infection may provide a mechanism by which mycobacteria maximize growth prior to subsequent restriction by host immunity. By initiating angiogenesis, the pathogen not only increases the delivery of oxygen and nutrients, but also generates conduits through which it may spread to distal tissue sites to establish a multi-focal infection (Oehlers et al., 2015; Polena et al., 2016). We have shown here and previously that interception of the Vegf and other proangiogenic pathways can be used to limit mycobacterial growth during infection, including in adult, established infections (Oehlers et al., 2017; Oehlers et al., 2015). Thus, host components engaged by cis-cyclopropanated TDM upstream of Vegfaa induction are attractive targets for further study. A fuller understanding of host interactions with this specific mycobacterial lipid may yield druggable targets in the host that have the potential to synergize with current anti-tubercular therapies via modulation of granuloma vasculature.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, David Tobin (david.tobin@duke.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Zebrafish (Danio rerio)
All zebrafish husbandry and experimental procedures were performed in accordance and compliance with policies approved by the Duke University Institutional Animal Care and Use Committee (protocol A122-17-05). Unless otherwise indicated, all zebrafish are derived from the wildtype AB strain.
Adult Zebrafish Housing
Adult zebrafish used to generate all the larvae used in this study were housed together by transgenic genotype in 3 or 10 L tanks. Water conditions are as follows: temperature: 28 °C; conductivity: 600–700 μS (maintained with Instant Ocean Sea Salt); pH: 7.0–7.3 (maintained with sodium bicarbonate; Arm & Hammer Pure Baking Soda).
Larval Zebrafish
All larval zebrafish used in this study were euthanized on or before 8 dpf. At these ages, sex is indeterminate for zebrafish (Liew and Orban, 2014; Uchida et al., 2002), and so no distinctions between male and female are made in the studies presented here.
Larvae were maintained at a temperature of 28.5 °C in 100 mm petri dishes in 40–50 ml of sterile E3 media (5 mM NaCl, 178 μM KCl, 328 μM CaCl2, 400 μM MgCl2) at a maximum density of 50 larvae per dish. In all cases, at 1 dpi larvae were transferred to E3 supplemented with 1-phenyl-2-thiourea (PTU, Sigma-Aldrich cat# P7629), final concentration 45 μg/ml) to arrest pigment development.
In all cases, anesthesia was performed with the addition of Tricaine (Sigma Aldrich, cat# E10521) to a final concentration of 160 μg/ml. Unless otherwise stated, larvae were anesthetized prior to any manipulation (i.e., moving between dishes, microinjection, live imaging, etc.). Time in anesthetic was limited to the minimum amount of time required to perform the desired manipulation.
Mycobacterium marinum
Wildtype
All strains used are derived from the M. marinum M strain. The wildtype M. marinum strain, expressing the tdTomato fluorescent protein and Hygromycin B resistance genes, has been described previously (Oehlers et al., 2015). Culture of wildtype bacteria was carried out on either 7H10 agar supplemented with Middlebrook OADC growth supplement (10% v/v; Sigma-Aldrich cat# M0678) and 50 μg/ml Hygromycin B or liquid 7H9 media supplemented with Middlebrook OADC growth supplement (10% v/v), 0.05% Tween 80 (Sigma-Aldrich cat# P1754), and 50 μg/ml Hygromycin B. Liquid 7H9 media containing OADC and Tween 80 is referred to hereafter as 7H9 Complete.
Transposon mutants
The two transposon mutants with disruptions in the pcaA ORF (pcaATn20324 and pcaATn28776) were identified from a sequenced library of M. marinum transposon mutants (C. Cosma and L. Ramakrishnan), and the previously identified insertion sites were confirmed by PCR and sequencing. Primers used were F primer (annealing upstream of the 5’ end of the pcaA ORF): 5’-AAGCCCTGTGGAACAGAAAG-3’; R primer (annealing downstream of the 3’ end of the pcaA ORF): 5’-ATCAAGCCAACACGCCTG-3’; and TnMarR3 (annealing within the transposon and directing amplification across the 3’ junction with genomic DNA): 5’-ACAACAAAGCTCTCACCAACCGTG-3’. The following primer pairs were used in separate reactions for each of the mutants as well as a no template control: F/TnMarR3; R/TnMarR3. Of the PCRs performed, amplicons were generated only from F/TnMarR3 using pcaATn28776 template and R/TnMarR3 using pcaATn20324 template. Sequencing of the amplicons using TnMarR3 confirmed disruptive insertions of the transposons into the pcaA ORFs of both strains.
Transposon mutants possessed the TnMar transposon, conferring resistance to Hygromycin B. Fluorescent transposon mutant strains were generated via electroporation (800 Ω, 25 μF, 2.5 kV, 0.2 cm gap) with plasmid msp12:mCerulean-KanR (a gift from Sunhee Lee, Duke University), resulting in the expression of the mCerulean fluorescent protein and conferring resistance to Kanamycin. Culture of transposon mutant strains was carried out using the same media as with wildtype bacteria except for the addition of 20 μg/ml Kanamycin to the media.
Complementation of the pcaATn28776 Strain
The pcaA open reading frame (ORF) was amplified from wildtype M. marinum genomic DNA (F primer: 5’-AATCACTTCGCAATGTCCGTCCAGCTCACG-3’; R primer: 5’-GTCGATCGTACGCTACTTCTCCAAAGTGAACTGA-3’). The pMV261-KanR plasmid (a gift from the Ramakrishnan lab) that contained the M. tuberculosis groEL promoter (referred to hereafter as hsp60) was amplified and linearized via inverse PCR (F primer: 5’-TAGCGTACGATCGACTGC-3’; R primer 5’-CATTGCGAAGTGATTCCTCC-3’). The two fragments were ligated together via InFusion (Clontech) producing a plasmid conferring resistance to Kanamycin with the pcaA ORF immediately downstream of the mycobacterial hsp60 promoter (referred to hereafter as pMV261-PcaA-KanR).
A fragment containing the hsp60 promoter and pcaA ORF was amplified from pMV261-PcaA-KanR (F primer: 5’-TCTCATCAACCGTGGAAATCTAGAGGTGACCACAACG-3’; R primer: 5’-TCCAGCCAGAAAGTGTTGTTGGCTAGCTGATCACC-3’). The entire msp12:mCerulean-KanR plasmid was amplified and linearized via inverse PCR (F primer: 5’- CACTTTCTGGCTGGATGATG-3’; R primer: 5’-CCACGGTTGATGAGAGCT-3’). The two fragments were ligated together via Infusion (Clontech) producing a plasmid conferring Kanamycin resistance as well as mycobacteria-specific constitutive expression of both the mCerulean fluorescent protein (msp12 promoter) and M. marinum PcaA (hsp60 promoter), referred to hereafter as pMCHP-KanR.
pMCHP-KanR was electroporated into pcaATn28776, resulting in a Kanamycin/Hygromycin B-resistant mutant strain overexpressing PcaA and mCerulean.
METHOD DETAILS
Microinjection of M. marinum
All strains were prepared for infection in the same manner, as described previously (Takaki et al., 2013). Bacteria were cultured at 33 °C in 50 ml 7H9 complete with the appropriate antibiotic(s) without shaking. At OD600 of 0.5–0.6, cultures were collected by centrifugation at 4000 × g for 20 min. Cultures were washed 3x with dH2O to remove all traces of antibiotic, and resuspended in 1 ml of Freezing 7H9 (7H9 supplemented with 10% v/v Middlebrook OADC). The concentrated suspension was centrifuged at 770 × g, and the unpelleted bacteria in the supernatant were transferred to a fresh tube. The pellet was then resuspended in 1 ml Freezing 7H9 using a syringe. Centrifugation, supernatant collection, and resuspension were repeated until all bacteria had been transferred to the new collection tube. The entire volume of collected supernatants was then filtered through a 5 μm nylon syringe filter, and centrifuged at 14,000 × g for 5 min. The pellet was resuspended in 0.5 ml of Freezing 7H9, and bacteria concentration was elucidated by averaging the count of fluorescent bacteria of several serial dilutions. The concentrate was adjusted to 5.0 × 108 bacteria/ml with Freezing 7H9, distributed into 5 μl aliquots, and stored at −80 °C.
Infections with either wildtype, transposon mutant, or complemented mutant M. marinum were performed as described (Oehlers et al., 2015). Briefly, larvae at 2 dpf were anesthetized with Tricaine and injected with 100–200 fluorescent bacteria dorsally near the rostral/caudal midline.
Microinjection of TDM, MAMEs, and MAGs
TDM (Sigma Aldrich, cat# T3034, purified from M. bovis; or BEI Resources cat# NR-14844, purified from M. tuberculosis H37Rv) at 2 mg/ml in Incomplete Freund’s Adjuvant (Sigma Aldrich, cat# F5506) was dissolved in 2:1 chloroform:methanol to generate stock solutions of 1 mg/ml and 250 μg/ml, respectively. MAMEs (BEI Resources cat# NR-14854, purified from M. tuberculosis H37Rv) was dissolved in 2:1 chloroform:methanol to generate a stock solution of 1mg/ml. MAGs purified from the various M. marinum strains used in this study were dissolved TDM and MAMEs were prepared for injection by evaporating a small volume of the stock solution under gentle airflow, and resuspending in and appropriate volume of IFA to achieve 2 mg/ml final concentration. Larvae at 2 dpf were anesthetized in Tricaine in E3 media and injected with approximately 10–20 nl of TDM/IFA or MAMEs/IFA (See Figure 1 for anatomical location). Control larvae were injected with the same volume of IFA alone. Larvae were then washed once in E3 and transferred to fresh E3 supplemented with PTU to continue arresting pigment development.
Purification of MAGs from M. marinum
Wildtype and pcaATn28776 M. marinum were first inoculated from freezer stocks into 3 ml of 7H9 Complete liquid media starter culture supplemented with 50 μg/ml Hygromycin B, and incubated at 33 °C in static culture until late log phase (OD600 of 0.8–21). The pcaATn28776::pcaA strain was grown under identical conditions except for the addition of 20 μg/ml Kanamycin. Cultures were spun down and resuspended in 1 ml fresh 7H9 Complete media, and added to 500 ml of 7H9 Complete with appropriate antibiotic(s). Cultures were grown at 33°C with gentle shaking until mid-log phase (OD600 of 0.5–0.7). Purification of mycolic acid-containing glycolipids was then performed separately for each culture, as described (Parish and Stoker, 2001). Briefly, bacteria were collected and washed 3x with dH2O, resuspended in 10 ml 2:1 chloroform:methanol, and incubated at room temperature overnight in a sealed container with vigorous stirring. The crude extract was collected, spun down to pellet insoluble debris, and slowly added to 500 ml acetone at −20°C and incubated overnight. Precipitate was recovered by spinning at 20,000x g for 15 min at 4°C, discarding the supernatant and resuspending in 2 ml 2:1 choloform:methanol. The solvent was evaporated under gentle airflow, and recovered material was weighed on an analytical balance. Each preparation was then resuspended at 5 mg/ml in 2:1 chloroform:methanol.
Purification of MAMEs from M. marinum
M. marinum strains were cultured as previously described for the MAG isolations. Alpha MAMEs were isolated by preparative TLC using EMD Millipore TLC Silica Gel 60 (Thermo Fisher Scientific cat# M1057150001) from crude MAMEs extracts. Briefly, 10 mg of each crude extract were developed in 94:6 petroleum ether:ethyl acetate. The alpha band was isolated by scraping and dissolved in deuterated chloroform (CDCl3).
Lipid Analysis
MAGs
TLC was performed on EMD Millipore TLC Silica Gel 60 (Thermo Fisher Scientific cat# M1057150001) as described (Pirson et al., 2012), with impregnation with 10% silver nitrate in water as described (Kennerly, 1986). Briefly, impregnation was carried out by submerging the TLC plate in the silver nitrate solution for 30 seconds. The plate was then allowed to dry at room temperature for 1 hour followed by baking at 50°C, 80°C, and 100°C in a stepwise fashion for 20min at the first two temperatures and 40 min at the last. Plates were then allowed to cool at room temperature for 20 min before use. 20 μg of lipid extract in methylene chloride was spotted on the plate and allowed to dry prior to running in a 100:14:0.8 chloroform:methanol:water solution. The plate was developed by submersion in a 10% sulfuric acid in methanol followed by charring. Plates were imaged using a Google Pixel camera.
MAMEs
TLC of the MAMEs isolates was performed in the same manner as for the MAG isolates, with the following exceptions. The isolates were developed 2x in 95:5 hexanes:ethyl acetate. 1H NMR was performed at 500 MHz with the sample dissolved in CDCl3.
Generating TNFR1/TNFR2 mosaic knockout larvae
sgRNA Synthesis
Target sites within the tnfrsfa and tnfrsfb loci were identified using the sgRNA prediction tool at www.crisprscan.org (Moreno-Mateos et al., 2015). Single stranded DNA oligos used to generate the sgRNA transcription templates for the tnfrsfa and tnfrsfb targets were 5’-taatacgactcactataGGGCTGGACGAGGTCCCTATgttttagagctagaa-3’ and 5’-taatacgactcactataGGTGCAATGCAGTACTGCTTgttttagagctagaa-3’, respectively. Double-stranded template assembly was performed via PCR using Q5 DNA Polymerase (NEB) with either of the above oligos and the constant tail oligo 5’-AAAAGCACCGACTCGGTGCCACTTTTTCAAGTTGATAACGGACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAAC-3’. Cycling parameters: 98°C, 1 min; 14 cycles of 98°C, 10s, 45°C, 30s; 72°C, 15s. 1 μg of template was used in an in vitro transcription reaction using the MEGAShortscript T7 Transcription Kit per the manufacturer’s instructions (Thermo Fisher Scientific cat# AM1354). sgRNAs were purified using RNeasy Mini Kit (Qiagen cat# 74104).
Injection into Single Cell Embryos
Single cell embryos of the TgBAC(vegfaa:EGFP) were injected with ~10nl of the following mixture: 800ng/ul Cas9 protein (IDT, cat#1074182), 300mM KCl, 265ng/μl sgRNA.
Assessment of Lesions at Target Loci
Genomic DNA was extracted from whole larvae at 5 dpi at the conclusion of the experiment in the same manner as described previously. 1ul of a 1/10 dilution of this extract was used as a template for HRMA using the MeltDoctor HRM Master Mix (Thermo Fisher Scientific) per the manufacturer’s instructions. Primers for TNFR1: Forward – 5’-CTGCTATTTGCAGTTCACGAG-3’; Reverse – 5’-GGTCAATGTTCTGCTCAGAAAC-3’. Primers for TNFR2: Forward – 5’-CTCTTCTAGGGACTCGCTTG-3’; Reverse – 5’-GGACACTTGAAACAATTGGGA-3’. PCR and melt profile were performed using the 7500 Fast Real-Time PCR System (Thermo Fisher Scientific). Genotyping analysis was performed by HRM Software 3.0 (Thermo Fisher Scientific) (Figure S6A,B).
Generating FcRγ mosaic knockout larvae
sgRNA Synthesis
Target sites within the fcer1g and fcer1gl loci were identified using crisprscan.org.
Single stranded DNA oligos used to generate the sgRNA transcription templates for the fcer1g and fcer1gl targets were 5’-taatacgactcactataGGCAGATGCGATGAGTCTGAgttttagagctagaa-3’ and 5’-taatacgactcactataGGCGGGATCCTGATCGTTTAgttttagagctagaa-3’, respectively. Templates were assembled in the same manner as for the TNFR sgRNA templates.
Injection into Single Cell Embryos
Tg(kdrl:EGFP) single cell embryos were injected with ~10nl of the following mixture: 800ng/ul Cas9 protein (IDT, cat#1074182), 300mM KCl, 265ng/μl sgRNA.
Assessment of Lesions at Target Loci
Genomic DNA was extracted from whole larvae at 2 dpi at the conclusion of the experiments in the manner previously described. HRMA was performed using the following primers: Primers for fcer1g: Forward – 5’-AACTTGTTGCTCAGTATGTTC-3’; Reverse – 5’-GACAGTGAGAACAATCCCATAG-3’. Primers for fcer1gl: Forward – 5’-GTTTCTCAGCTGCGCAACA-3’; Reverse – 5’-GATGCTCACCTTTAATCTGCAG-3’. PCR and melt profile were performed using the 7500 Fast Real-Time PCR System (Thermo Fisher Scientific). Genotyping analysis was performed by HRM Software 3.0 (Thermo Fisher Scientific) (Figure S6C,D).
Generating the myd88 knockout line
sgRNA Synthesis
Target sites within the myd88 locus were identified using crisprscan.org.
Single stranded DNA oligos used to generate the sgRNA transcription templates for the myd88 target was 5’-taatacgactcactataGGCGGCAGACTGGAGGACAGgttttagagctagaa-3’. Templates were assembled in the same manner as described previously.
Injection into Single Cell Embryos
Tg(kdrl:EGFP) single cell embryos were injected with ~10nl of the following mixture: 800ng/ul Cas9 protein (IDT, cat#1074182), 300mM KCl, 265ng/μl sgRNA.
Identification of frameshift alleles
Adult zebrafish derived from the injected embryos were crossed to wildtype fish, and the progeny were assessed via HRMA as described previously. F primer: 5’-CCGAAAGAAACTGGGTCTGTTCC-3’; R primer: 5’- ACGAGTTTCCCAGTCCGTCA-3’. Larvae exhibiting lesions at the myd88 target locus were further analyzed by sequencing, and an allele exhibiting a 22 base-pair deletion was identified. This deletion begins at amino acid 39 (of 284) and results in a frameshift and premature translation termination after 59 amino acids. F1 adult fish heterozygous for this allele were identified and pooled, and larvae used for the experiments requiring myd88 knockouts were generated by crossing these F1 adults. The resulting progeny exhibited Mendelian ratios of homozygous wildtype, heterozygous mutant, and homozygous mutant alleles via HRMA (See group N values, Figure S3C; HRMA output Figure S6E).
Use of Tg(hsp70l:dn-vegfaa) Larvae
Heat Shock Conditions
For experiments involving TDM: All larvae subjected to heat shock were incubated at 37°C for 75 min, occurring at −4 hrs and +20 hrs with respect to the time of TDM injection. For experiments involving infection: Heat shocks occurred at 3 and 4 dpi; 37°C for 75 min each time.
Genotyping
Genomic DNA was extracted individually from whole larvae as described previously. Extracts were diluted 1:10 and 1 μl from each larva was used as a template for PCR. Genotyping was carried out for up to 96 larvae in parallel using an Applied Biosystems 7500 Fast Real-Time PCR machine followed by High-Resolution Melt Analysis (HRMA) using MeltDoctor HRM Reagents (Thermo Fisher Scientific). A competitive pair of PCRs were performed concurrently, allowing expedited, automated identification of transgene-containing samples by melt curve analysis. Briefly, one forward primer (Primer F1; 5’-GAGAACGGTGTGACGGTAAC-3’) anneals to the 3’ end of Intron 2–3 in the endogenous vegfaa locus; another forward primer (Primer F2; 5’-CTGCCCACATACCCAAAGAAG-3’) anneals to the 5’ end of vegfaa Exon 2 in the endogenous locus and within the dn-vegfaa transgene; and a shared reverse primer (Primer R; 5’-GATGATGTCTACCAGCAGCTC-3’) anneals within Exon 3 of both the endogenous locus and within the dn-vegfaa transgene. The resulting reaction generates different, highly reproducible amplicon pools depending on the presence/absence of the transgene, which can be distinguished by HRMA (Figure S6F). HRMA patterns were initially assigned to genotypes based on analysis of a subset of samples using a simple presence/absence PCR for the transgene followed by Agarose Gel Electrophoresis (F primer: 5’-CATGTGGACTGCCTATGTTCATC-3’; R primer: 5’-CTTCTTTGGGTATGTGGGCAG-3’).
In vitro growth analysis
Starter cultures were generated by inoculating 1 ml 7H9 Complete with a stab of −80 °C freezer stock of each strain tested. Starter cultures were allowed to grow to stationary phase. Bacteria were pelleted by centrifugation, and resuspended in 1 ml fresh 7H9 Complete. Each strain was then diluted in triplicate to a starting OD600 of ~0.033 in 1 ml 7H9 Complete. Culture were incubated at 33 °C with vigorous shaking. OD600 readings were taken each day at ~24 hour intervals for ~72 hours, and the readings from each of three replicates was averaged for each timepoint per strain. Doubling times were calculated using GraphPad Prism 7. Briefly, a best-fit non-linear regression using an exponential growth model was applied to each data series, fixing y=0.033 for t=0.
RT-qPCR
RNA extractions were performed using the TRIzol Reagent (Thermo Fisher Scientific cat# 15596026) per manufacturer’s instructions. cDNA was generated for each group to be tested using the iScript cDNA Synthesis Kit (BioRad cat# 1708890) with an input of 1 μg of RNA.
M. marinum 16S burden analysis
M. marinum-specific 16S primers were designed based on 16S hypervariable regions identified as best able to distinguish between different Mycobacteria spp. (Chakravorty et al., 2007). F primer: 5’-CGATCTGCCCTGCACTTC-3’; R primer: 5’- CCACAGGACATGAATCCCGT-3’. RT-qPCR was performed using the Power SYBR Green PCR Master Mix (Thermo Fisher Scientific cat# 4367659) per the manufacturer’s instructions. β-actin transcript levels (zebrafish gene actb1) were used as the internal input control. F primer: 5’-CGAGCAGGAGATGGGAACC-3’; R primer: 5’-CAACGGAAACGCTCATTGC-3’.
Zebrafish tnfa induction analysis
Zebrafish tnfa cDNA was amplified using F primer: 5’-CAGGGCAATCAACAAGATGG-3’; R primer: 5’-TGGTCCTGGTCATCTCTCCA-3’. RT-qPCR was performed as described for M. marinum 16S cDNA. β-actin transcript levels were used as the internal input control.
Pazopanib treatment
Pazopanib (Sigma-Aldrich cat# CDS023580) was used as described (Oehlers et al., 2015). Briefly, larvae were randomly assigned to two 100 mm petri dishes containing 40 ml of E3 media immediately following injection with TDM. To one dish, 40 μl of DMSO was added to achieve a final concentration of 0.1% v/v DMSO in E3 (vehicle group). To the other dish, 40 ul of a 250 μM stock of Pazopanib in DMSO was added to achieve a final concentration of 250 nM Pazopanib, 0.1% v/v DMSO in E3 (treatment group). Animals were kept under these conditions continually for the duration of the experiment.
QUANTIFICATION AND STATISTICAL ANALYSIS
Image Analysis
Image analysis was performed using ImageJ (NIH). Abnormal angiogenesis was measured by tracing the path of vasculature present between intersegmental vessels. Bacterial burden for each larva was measured by flattening image stacks in the relevant fluorescent channel using a maximum intensity projection, setting a threshold for signal above background, and measuring total fluorescent area. Background thresholds were held constant across all larva for a given time point and fluorescent channel.
TNF reporter induction in the transgenic line TgBAC(tnf:GFP) was measured for each larva by flattening image stacks in the relevant fluorescent channel using a maximum intensity projection, setting a threshold for signal above background, and measuring total fluorescent area. Background thresholds were held constant across all larva for a given time point and fluorescent channel.
The transgenic line TgBAC(vegfaa:EGFP) possesses a high baseline signal due to normal activation of vegfaa during development. Larvae were collected from a cross of adults heterozygous for the transgene in order to generate the largest possible pool of transgene-positive larvae possible from the pool of adults on hand, and as a result the background signal varied by as much as two-fold from larva to larva (i.e., one or two copies of the transgene). Because of this, vegfaa induction was measured in a slightly modified manner: image stacks were first flattened using a maximum intensity projection. Background for a given larva was then measured by recording the maximum value of fluorescent signal within an area of three uninfected somites. The resulting value was used to set the threshold for that larva, and area of signal above the threshold was measured for the infected region. This process was repeated for each individual larva.
Statistics
All statistics were performed using GraphPad Prism 7, and are noted for each analysis in the respective figure legends. Unless otherwise noted in the figure legends, all comparisons are as follows: comparison of the average of two groups only utilized Student’s t-test; comparison of more than two groups utilized One-way ANOVA with Tukey’s multiple comparison post-test. Comparison of any pair of contingency table datasets (absence vs. presence of phenotype) utilized Fisher’s exact test. For clarity, contingency table data are displayed as percentage of total, with N=total number of animals listed above each bar. However, the statistical analyses were performed on the untransformed values. In all cases, minimum significance for rejecting the null hypothesis for a given comparison is p<0.05, denoted by *. ** denotes p<0.01; *** denotes p<0.001; and **** denotes p<0.0001.
Supplementary Material
Highlights.
Mycobacterial granuloma angiogenesis requires the cell wall glycolipid TDM
The mycobacterial enzyme PcaA promotes angiogenesis via cis-cyclopropanation of TDM
Cyclopropanated TDM induces granuloma vascularization through activating Vegfa signaling
pcaA mutation or Vegfa pathway blockade reduces bacterial growth in vivo
ACKNOWLEDGMENTS
We are grateful to S. Abraham, J. Coers, M. Kuehn, J. Rawls, and members of the Tobin laboratory for helpful discussions, C. Cosma and L. Ramakrishnan for the kind gift of the transposon mutants, and E. Hunt for fish care. This work was supported by an American Cancer Society Postdoctoral Fellowship PF-13-223-01-MPC (M.R.C.); a Damon Runyon Postdoctoral Fellowship (C.J.C.); NIH grants AI130236 and AI125517 (D.M.T.); AI051622 (C.R.B.); and HL081674 (K.D.P.); and the Max Planck Society (D.Y.R.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Aly S, Laskay T, Mages J, Malzan A, Lang R, and Ehlers S (2007). Interferon-gamma-dependent mechanisms of mycobacteria-induced pulmonary immunopathology: the role of angiostasis and CXCR3-targeted chemokines for granuloma necrosis. J Pathol 212, 295–305. [DOI] [PubMed] [Google Scholar]
- Axelrod S, Oschkinat H, Enders J, Schlegel B, Brinkmann V, Kaufmann SH, Haas A, and Schaible UE (2008). Delay of phagosome maturation by a mycobacterial lipid is reversed by nitric oxide. Cell Microbiol 10, 1530–1545. [DOI] [PubMed] [Google Scholar]
- Barry CE 3rd, Lee RE, Mdluli K, Sampson AE, Schroeder BG, Slayden RA, and Yuan Y (1998). Mycolic acids: structure, biosynthesis and physiological functions. Prog Lipid Res 37, 143–179. [DOI] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, et al. (2000). Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nature cell biology 2, 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch H, Sorkin E, and Erlenmeyer H (1953). A Toxic Lipid Component of the Tubercle Bacillus (Cord Factor) .1. Isolation from Petroleum Ether Extracts of Young Bacterial Cultures. Am Rev Tuberc Pulm 67, 629–643. [DOI] [PubMed] [Google Scholar]
- Bowdish DM, Sakamoto K, Kim MJ, Kroos M, Mukhopadhyay S, Leifer CA, Tryggvason K, Gordon S, and Russell DG (2009). MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog 5, e1000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PJ (2003). Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis (Edinb) 83, 91–97. [DOI] [PubMed] [Google Scholar]
- Brennan PJ, and Besra GS (1997). Structure, function and biogenesis of the mycobacterial cell wall. Biochem Soc Trans 25, 188–194. [DOI] [PubMed] [Google Scholar]
- Brennan PJ, and Nikaido H (1995). The envelope of mycobacteria. Annu Rev Biochem 64, 29–63. [DOI] [PubMed] [Google Scholar]
- Chakravorty S, Helb D, Burday M, Connell N, and Alland D (2007). A detailed analysis of 16S ribosomal RNA gene segments for the diagnosis of pathogenic bacteria. J Microbiol Methods 69, 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay H, Volkman HE, and Ramakrishnan L (2008). Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity 29, 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan MR, Beerman RW, Rosenberg AF, Saelens JW, Johnson MG, Oehlers SH, Sisk DM, Jurcic Smith KL, Medvitz NA, Miller SE, et al. (2016). Macrophage Epithelial Reprogramming Underlies Mycobacterial Granuloma Formation and Promotes Infection. Immunity 45, 861–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffe M, Laneelle MA, and Lacave C (1991). Structure and stereochemistry of mycolic acids of Mycobacterium marinum and Mycobacterium ulcerans. Res Microbiol 142, 397–403. [DOI] [PubMed] [Google Scholar]
- Datta M, Via LE, Kamoun WS, Liu C, Chen W, Seano G, Weiner DM, Schimel D, England K, Martin JD, et al. (2015). Anti-vascular endothelial growth factor treatment normalizes tuberculosis granuloma vasculature and improves small molecule delivery. Proceedings of the National Academy of Sciences of the United States of America 112, 1827–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Clay H, Lewis JL, Ghori N, Herbomel P, and Ramakrishnan L (2002). Real-time visualization of mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity 17, 693–702. [DOI] [PubMed] [Google Scholar]
- Dubnau E, Chan J, Raynaud C, Mohan VP, Laneelle MA, Yu KM, Quemard A, Smith I, and Daffe M (2000). Oxygenated mycolic acids are necessary for virulence of Mycobacterium tuberculosis in mice. Mol Microbiol 36, 630–637. [DOI] [PubMed] [Google Scholar]
- Ernst JD (2012). The immunological life cycle of tuberculosis. Nat Rev Immunol 12, 581–591. [DOI] [PubMed] [Google Scholar]
- Folkman J (2002). Role of angiogenesis in tumor growth and metastasis. Semin Oncol 29, 15–18. [DOI] [PubMed] [Google Scholar]
- Geisel RE, Sakamoto K, Russell DG, and Rhoades ER (2005). In vivo activity of released cell wall lipids of Mycobacterium bovis bacillus Calmette-Guerin is due principally to trehalose mycolates. J Immunol 174, 5007–5015. [DOI] [PubMed] [Google Scholar]
- Glickman MS, Cahill SM, and Jacobs WR Jr. (2001). The Mycobacterium tuberculosis cmaA2 gene encodes a mycolic acid trans-cyclopropane synthetase. J Biol Chem 276, 2228–2233. [DOI] [PubMed] [Google Scholar]
- Glickman MS, Cox JS, and Jacobs WR Jr. (2000). A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol Cell 5, 717–727. [DOI] [PubMed] [Google Scholar]
- Harding J, Ritter A, Rayasam A, Fabry Z, and Sandor M (2015). Lymphangiogenesis is induced by mycobacterial granulomas via vascular endothelial growth factor receptor-3 and supports systemic T-cell responses against mycobacterial antigen. The American journal of pathology 185, 432–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J, Skerry C, Davis SL, Tasneen R, Weir M, Kramnik I, Bishai WR, Pomper MG, Nuermberger EL, and Jain SK (2012). Mouse model of necrotic tuberculosis granulomas develops hypoxic lesions. J Infect Dis 205, 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RL, Olsen M, Jagannath C, and Actor JK (2006). Trehalose 6,6’-dimycolate and lipid in the pathogenesis of caseating granulomas of tuberculosis in mice. The American journal of pathology 168, 1249–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indrigo J, Hunter RL Jr., and Actor JK (2003). Cord factor trehalose 6,6’-dimycolate (TDM) mediates trafficking events during mycobacterial infection of murine macrophages. Microbiology 149, 2049–2059. [DOI] [PubMed] [Google Scholar]
- Jin SW, Beis D, Mitchell T, Chen JN, and Stainier DY (2005). Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development 132, 5199–5209. [DOI] [PubMed] [Google Scholar]
- Karra R, Foglia MJ, Choi WY, Belliveau C, DeBenedittis P, and Poss KD (2018). Vegfaa instructs cardiac muscle hyperplasia in adult zebrafish. Proceedings of the National Academy of Sciences of the United States of America In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerly DA (1986). Improved Analysis of Species of Phospholipids Using Argentation Thin-Layer Chromatography. J Chromatogr 363, 462–467. [Google Scholar]
- Kumar NP, Banurekha VV, Nair D, and Babu S (2016). Circulating Angiogenic Factors as Biomarkers of Disease Severity and Bacterial Burden in Pulmonary Tuberculosis. PloS one 11, e0146318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R (2013). Recognition of the mycobacterial cord factor by Mincle: relevance for granuloma formation and resistance to tuberculosis. Front Immunol 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew WC, and Orban L (2014). Zebrafish sex: a complicated affair. Brief Funct Genomics 13, 172–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Barry CE 3rd, Besra GS, and Nikaido H (1996). Mycolic acid structure determines the fluidity of the mycobacterial cell wall. J Biol Chem 271, 29545–29551. [DOI] [PubMed] [Google Scholar]
- Lobato-Pascual A, Saether PC, Fossum S, Dissen E, and Daws MR (2013). Mincle, the receptor for mycobacterial cord factor, forms a functional receptor complex with MCL and FcepsilonRI-gamma. Eur J Immunol 43, 3167–3174. [DOI] [PubMed] [Google Scholar]
- Marin-Juez R, Marass M, Gauvrit S, Rossi A, Lai SL, Materna SC, Black BL, and Stainier DY (2016). Fast revascularization of the injured area is essential to support zebrafish heart regeneration. Proceedings of the National Academy of Sciences of the United States of America 113, 11237–11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjoram L, Alvers A, Deerhake ME, Bagwell J, Mankiewicz J, Cocchiaro JL, Beerman RW, Willer J, Sumigray KD, Katsanis N, et al. (2015). Epigenetic control of intestinal barrier function and inflammation in zebrafish. Proceedings of the National Academy of Sciences of the United States of America 112, 2770–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama W, Hashiguchi T, Matsumuro K, Iwami F, Hirotsu Y, Kawabata M, Arimura K, and Osame M (2000). Increased serum level of vascular endothelial growth factor in pulmonary tuberculosis. Am J Respir Crit Care Med 162, 1120–1122. [DOI] [PubMed] [Google Scholar]
- Middlebrook G, Dubos RJ, and Pierce C (1947). Virulence and Morphological Characteristics of Mammalian Tubercle Bacilli. J Exp Med 86, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake Y, Masatsugu OH, and Yamasaki S (2015). C-Type Lectin Receptor MCL Facilitates Mincle Expression and Signaling through Complex Formation. J Immunol 194, 5366–5374. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Toyonaga K, Mori D, Kakuta S, Hoshino Y, Oyamada A, Yamada H, Ono K, Suyama M, Iwakura Y, et al. (2013). C-type lectin MCL is an FcRgamma-coupled receptor that mediates the adjuvanticity of mycobacterial cord factor. Immunity 38, 1050–1062. [DOI] [PubMed] [Google Scholar]
- Moreno-Mateos MA, Vejnar CE, Beaudoin JD, Fernandez JP, Mis EK, Khokha MK, and Giraldez AJ (2015). CRISPRscan: designing highly efficient sgRNAs for CRISPR-Cas9 targeting in vivo. Nat Methods 12, 982–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller YA, Li B, Christinger HW, Wells JA, Cunningham BC, and de Vos AM (1997). Vascular endothelial growth factor: crystal structure and functional mapping of the kinase domain receptor binding site. Proceedings of the National Academy of Sciences of the United States of America 94, 7192–7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehlers SH, Cronan MR, Beerman RW, Johnson MG, Huang J, Kontos CD, Stout JE, and Tobin DM (2017). Infection-Induced Vascular Permeability Aids Mycobacterial Growth. J Infect Dis 215, 813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehlers SH, Cronan MR, Scott NR, Thomas MI, Okuda KS, Walton EM, Beerman RW, Crosier PS, and Tobin DM (2015). Interception of host angiogenic signalling limits mycobacterial growth. Nature 517, 612–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagan AJ, and Ramakrishnan L (2018). The Formation and Function of Granulomas. Annual review of immunology. [DOI] [PubMed] [Google Scholar]
- Parish T, and Stoker NG (2001). Mycobacterium Tuberculosis Protocols. Methods in Molecular Medicine 54. [Google Scholar]
- Patin EC, Geffken AC, Willcocks S, Leschczyk C, Haas A, Nimmerjahn F, Lang R, Ward TH, and Schaible UE (2017). Trehalose dimycolate interferes with FcgammaR-mediated phagosome maturation through Mincle, SHP-1 and FcgammaRIIB signalling. PloS one 12, e0174973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirson C, Jones GJ, Steinbach S, Besra GS, and Vordermeier HM (2012). Differential effects of Mycobacterium bovis - derived polar and apolar lipid fractions on bovine innate immune cells. Vet Res 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podar K, Tonon G, Sattler M, Tai YT, Legouill S, Yasui H, Ishitsuka K, Kumar S, Kumar R, Pandite LN, et al. (2006). The small-molecule VEGF receptor inhibitor pazopanib (GW786034B) targets both tumor and endothelial cells in multiple myeloma. Proceedings of the National Academy of Sciences of the United States of America 103, 19478–19483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polena H, Boudou F, Tilleul S, Dubois-Colas N, Lecointe C, Rakotosamimanana N, Pelizzola M, Andriamandimby SF, Raharimanga V, Charles P, et al. (2016). Mycobacterium tuberculosis exploits the formation of new blood vessels for its dissemination. Scientific reports 6, 33162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V, Fujiwara N, Porcelli SA, and Glickman MS (2005). Mycobacterium tuberculosis controls host innate immune activation through cyclopropane modification of a glycolipid effector molecule. J Exp Med 201, 535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MB, and Williams SJ (2014). MCL and Mincle: C-Type Lectin Receptors That Sense Damaged Self and Pathogen-Associated Molecular Patterns. Front Immunol 5, 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittershaus ES, Baek SH, and Sassetti CM (2013). The normalcy of dormancy: common themes in microbial quiescence. Cell Host Microbe 13, 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Gauvrit S, Marass M, Pan L, Moens CB, and Stainier DYR (2016). Regulation of Vegf signaling by natural and synthetic ligands. Blood 128, 2359–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainson RCA, Johnston DA, Chu HC, Holderfield MT, Nakatsu MN, Crampton SP, Davis J, Conn E, and Hughes CCW (2008). TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood 111, 4997–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saita N, Fujiwara N, Yano I, Soejima K, and Kobayashi K (2000). Trehalose 6,6’-dimycolate (cord factor) of Mycobacterium tuberculosis induces corneal angiogenesis in rats. Infect Immun 68, 5991–5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Kim MJ, Rhoades ER, Allavena RE, Ehrt S, Wainwright HC, Russell DG, and Rohde KH (2013). Mycobacterial trehalose dimycolate reprograms macrophage global gene expression and activates matrix metalloproteinases. Infect Immun 81, 764–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaim LE, Connolly LE, Volkman HE, Humbert O, Born DE, and Ramakrishnan L (2006). Mycobacterium marinum infection of adult zebrafish causes caseating granulomatous tuberculosis and is moderated by adaptive immunity. Infect Immun 74, 6108–6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydor T, von Bargen K, Hsu FF, Huth G, Holst O, Wohlmann J, Becken U, Dykstra T, Sohl K, Lindner B, et al. (2013). Diversion of phagosome trafficking by pathogenic Rhodococcus equi depends on mycolic acid chain length. Cell Microbiol 15, 458–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki K, Davis JM, Winglee K, and Ramakrishnan L (2013). Evaluation of the pathogenesis and treatment of Mycobacterium marinum infection in zebrafish. Nature protocols 8, 1114–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin DM, and Ramakrishnan L (2008). Comparative pathogenesis of Mycobacterium marinum and Mycobacterium tuberculosis. Cell Microbiol 10, 1027–1039. [DOI] [PubMed] [Google Scholar]
- Tsai MC, Chakravarty S, Zhu G, Xu J, Tanaka K, Koch C, Tufariello J, Flynn J, and Chan J (2006). Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol 8, 218–232. [DOI] [PubMed] [Google Scholar]
- Uchida D, Yamashita M, Kitano T, and Iguchi T (2002). Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J Exp Biol 205, 711–718. [DOI] [PubMed] [Google Scholar]
- Ulrichs T, Kosmiadi GA, Jorg S, Pradl L, Titukhina M, Mishenko V, Gushina N, and Kaufmann SH (2005). Differential organization of the local immune response in patients with active cavitary tuberculosis or with nonprogressive tuberculoma. J Infect Dis 192, 89–97. [DOI] [PubMed] [Google Scholar]
- Volkman HE, Pozos TC, Zheng J, Davis JM, Rawls JF, and Ramakrishnan L (2010). Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science 327, 466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werninghaus K, Babiak A, Gross O, Holscher C, Dietrich H, Agger EM, Mages J, Mocsai A, Schoenen H, Finger K, et al. (2009). Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRgamma-Syk-Card9-dependent innate immune activation. J Exp Med 206, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.