ABSTRACT
Background: There is a lack of knowledge on the association between daily physical activity and lung function in patients with asthma.
Objective: This study aims to examine the association between daily physical activity and asthma control, lung function, and lung function decline in patients with adult-onset asthma.
Design: This study is part of Seinäjoki Adult Asthma Study (SAAS), where 201 patients were followed for 12 years after asthma diagnosis. Daily physical activity was assessed at follow-up by a structured questionnaire and used to classify the population into subgroups of low (≤240 min) or high (>240 min) physical activity. Three spirometry evaluation points were used: 1. diagnosis, 2. the maximum lung function during the first 2.5 years after diagnosis (Max0-2.5), 3. follow-up at 12 years.
Results: High physical activity group had slower annual FEV1 (p<0.001) and FVC (p<0.018) decline. Additionally, the high physical activity group had higher FEV1 values at follow-up, and higher FEV1/FVC ratios at follow-up and diagnosis. There was no difference in BMI, smoking, medication, or frequency of physical exercise between high and low physical activity groups. Differences remained significant after adjustments for possible confounding factors.
Conclusion: This is the first demonstration of an association between long-term FEV1 decline and daily physical activity in clinical asthma. Low physical activity is independently associated with faster decline in lung function. Daily physical activity should be recommended in treatment guidelines in asthma.
KEYWORDS: Asthma, adult, adult-onset, FEV1 decline, lung function decline, physical activity, systemic inflammation
Introduction
Physical activity has been shown to have several health benefits such as reducing the risk of ischemic heart disease, diabetes and colon cancer [1]. Most of the previous research on physical activity has focused on moderate to vigorous intensity activities such as running whereas less is known on the low intensity everyday movement which constitutes the majority of the physical activity accumulated throughout the day. A structured exercise program is often used as an intervention in studies, but the rest of the daily physical activity is frequently overlooked. Caspersen et al. [2] defined physical activity as ‘any bodily movement produced by skeletal muscles that requires energy expenditure’ and physical exercise as ‘a subset of physical activity that is planned, structured, and repetitive and has as a final or an intermediate objective the improvement or maintenance of physical fitness’. These definitions are currently used by the World Health Organization (WHO) [3]. Physical activity thus includes all human movement whereas physical exercise is high or moderate intensity movement conducted with a purpose for shorter periods of time. It is important to differentiate between these two terms since different intensities of physical activity have different effects on the body [4]. Thus, it is crucial to study physical activity as a whole instead of focusing solely on the moderate to vigorous intensity activities. In the context of this study the term physical activity includes all physical activities from lounging around the shopping mall to going for a jog and the term exercise only means moderate to high intensity activities.
Asthma is a chronic inflammatory disease of the airways. Over the past years researchers have identified several phenotypes of asthma revealing the heterogeneity of the disease [5,6]. Age of onset of asthma has been found as a key differentiating factor between the phenotypes. It has been shown recently that the adult-onset phenotypes differ vastly from childhood-onset asthma [5,6]. Childhood asthma is often an atopic disease characterized by high numbers of bronchial eosinophils and high serum levels of IgE with a good response to glucocorticoid treatment. In contrast, patients with disease onset at adulthood have often more severe asthma that rarely remits [7]. Recently, asthma diagnosed at adult-age has been shown to constitute a majority among all new cases of asthma [8], and it seems that several lifestyle factors such as cigarette smoking and obesity are major factors in adult-onset asthma [6,9]. Since the concept of adult-onset phenotypes is rather new, only few studies exist on it and there is no clear consensus on the best therapy for these patients. Occasionally, adult-onset asthma may also be difficult to distinguish from chronic obstructive pulmonary disease (COPD) due to the overlap of symptoms and risk factors [10].
Physical exercise has been shown to be a beneficial factor in the control of asthma [11]. Physical exercise reduces systemic and bronchial inflammation, and increases maximal oxygen uptake [12,13]. Most research on daily physical activity in asthma focuses either on the risk of developing asthma or exercise-induced bronchoconstriction. There are no published long-term studies on daily physical activity in patients with clinical asthma. Daily physical activity has been studied extensively in COPD where higher levels of physical activity decelerate lung function decline and improve the quality of life [14,15]. Previous reports have shown that high physical activity is associated with better lung function, slower lung function decline, and lower mortality also in the general population [16,17]. However, so far there are no previous studies on the association between physical activity and lung function change over several years in patients with clinical asthma.
The aim of this study was to examine the association between daily physical activity and asthma control, lung function, or lung function decline in patients with adult-onset asthma.
Materials and methods
Study population and design
Seinäjoki Adult Asthma Study (SAAS) is a single-center (Department of Respiratory Medicine, Seinäjoki Central Hospital, Seinäjoki, Finland) 12-year follow-up study. The study protocol and the inclusion and exclusion criteria have been previously published [18]. Study population consists of 260 consecutive patients who were diagnosed with adult-onset asthma in 1999–2002 by a respiratory physician (Figure 1). Patients were recruited from the diagnostic visit. Diagnosis was based on typical symptoms, and it was confirmed by objective lung function measurements [18]. Smokers (current or ex-) and patients with co-morbidities were included in the study. The patients were treated and monitored according to Finnish Asthma Program guidelines [19] either in the specialized care or in primary care. The collection of research data has been previously reported [7,9,10,20]. At diagnosis, data was collected on lung function, blood eosinophils, the initial pharmacotherapy, and asthma symptoms by Airways Questionnaire 20 (AQ20) [21]. Atopy was defined as at least one positive response (≥3 mm) in skin prick toward common aeroallergens. After 12 years patients were invited to a re-evaluation (2012–2013), and 201 (77%) patients arrived to the follow-up visit. At follow-up information was collected on asthma status, medication, and co-morbidities by structured questionnaire. Lung function, blood eosinophils and neutrophils, IgE levels, fraction of exhaled nitric oxide (FeNO), interleukin-6 (IL-6), and high sensitivity C-reactive protein (hsCRP) were measured. Patients also filled Asthma Control Test (ACT) [22], AQ20, and a structured lifestyle questionnaire. Specifically trained research nurse reviewed all questionnaires with the patients to limit misunderstandings. A written informed consent was obtained to a study protocol approved by the Ethics committee of Tampere University Hospital, Tampere, Finland (R12122).
Figure 1.
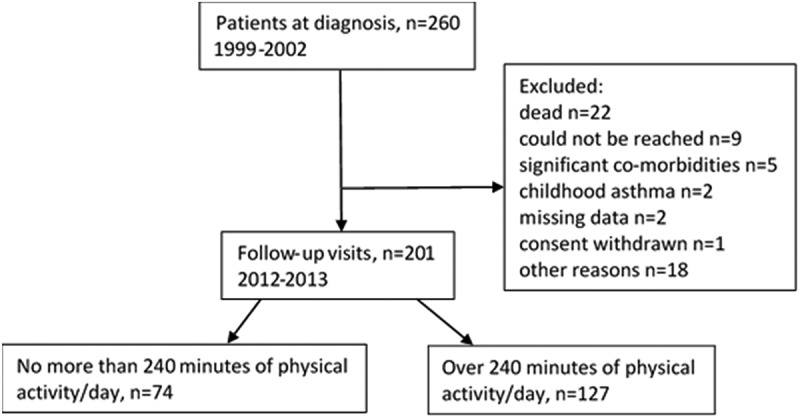
Flow chart of the study.
Determination of physical activity
The level of physical activity was assessed at follow-up by a structured questionnaire. Overall daily physical activity was assessed by an open question: ‘How many minutes in a day do you spend moving?’ After pre-evaluation, patients were divided into high and low daily physical activity groups. Cut off-point was established at 240 min of daily physical activity per day. The frequency of physical exercise was estimated by the following question: ‘How often do you take part in leisure time activities for at least half an hour so that you are at least somewhat breathless and sweaty?’ This question had eight choices ranging from never to everyday. In this study, physical activity time and physical exercise frequency are assessed as their own variables and are not to be confused with each other.
Determination of lung function
Lung function was assessed using a spirometer (Vmax Encore 22, Viasys Healthcare, Palm Springs, CA, USA) according to international recommendations [23] and Finnish reference values [24]. Post-bronchodilator measurements were taken 15 min after inhalation of salbutamol (400 µg). After the initiation of asthma therapy, only pre-bronchodilator spirometry was measured on most of the patients, and therefore we evaluated the changes in pre-bronchodilator spirometry values throughout the study. Lung function measurement points were: (1) baseline (i.e. time of asthma diagnosis), (2) the maximum lung function (Max0-2.5) during the first 2.5 years after diagnosis (i.e. after start of anti-inflammatory therapy) based on the highest pre-bronchodilator FEV1% predicted, and (3) after 12 years of follow-up (Figure 2) [9].
Figure 2.

Representation of the three lung function measurement points.
Statistical analyses
Continuous data is expressed as mean (SD) for variables with normal distribution and as median and interquartile range for variables with skewed distribution. To assess differences between high and low physical activity groups, we used Mann-Whitney test for continuous variables with skewed distributions, independent samples t-test for continuous variables with normal distribution, and Chi-Square test for categorized variables.
Multiple linear regression analysis was performed to analyze factors associated with FEV1 decline from point of Max0-2.5 to the follow-up visit. To avoid multicollinearity, explanatory variables were checked for strong (>0.7) mutual correlation. Patients whose BMI differed over 3 SD (n = 4) or whose FEV1 decline differed over 3.3 SD (n = 3) from mean were removed from the linear regression analysis as outliers to ensure homoscedasticity. Forward, backward and enter methods were used to improve model fit. Statistical analyses were performed using SPSS software, version 23 (IBM SPSS, Chicago, IL, USA). A p-value <0.05 was regarded as statistically significant.
Results
Patient characteristics
Characteristics of the patients who attended the follow-up visit are shown at eTable 1. The median age of the study population was 45 (SD 14) years at diagnosis and 58% were females. Of the patients, 53% had smoking history. The proportion of daily inhaled glucocorticoid users was 8% at diagnosis, 96% at Max0-2.5, and 76% at follow-up. Patients reported a median of 360 (interquartile range 180–540) min of daily physical activity.
When patients were divided into two groups based on the level of daily physical activity (≤240 min vs. >240 min) the group with the higher amount of physical activity was younger and they had earlier asthma-onset (Table 1). The high physical activity group included a higher percentage of females than the low physical activity group. The high physical activity group also included less patients with post-bronchodilator FEV1/FVC<0.7 and a history of smoking for at least 10 pack years (Table 1), characteristics which may indicate an asthma-COPD overlap syndrome (ACOS) [25]. Notably, there were no differences in BMI, smoking status, medication for asthma, or frequency of physical exercise between the two groups (Table 1).
Table 1.
Basic subject characteristics by physical activity groups.
| ≤240 min of physical activity/day | >240 min of physical activity/day | p-value* | |
|---|---|---|---|
| Number of patients | 74 | 127 | |
| Age (years) | 61 (13) | 57 (14) | 0.036 |
| Age of onset (years) | 49 (13) | 44 (14) | 0.029 |
| Males | 39 (53%) | 45 (35%) | 0.018 |
| Duration of daily physical activity (min) | 135 (90–180) | 480 (360–600) | <0.001 |
| Patients exercising at least 3 times per week | 43 (58%) | 69 (54%) | 0.660 |
| BMI (kg/m2) | 28.7 (5.2) | 28.4 (5.8) | 0.807 |
| Ex or current smoker | 38 (51%) | 68 (54%) | 0.772 |
| Pack years (of ex- and current smokers) | 20 (10–32) | 15 (4–27) | 0.067 |
| Post-brochodilator FEV1/FVC <0.7 and at least 10 pack years | 17 (24%) | 16 (13%) | 0.050 |
| Patients with at least one co-morbidity | 51 (70%) | 76 (60%) | 0.172 |
| Number of co-morbidities, COPD included | 1 (0–3) | 1 (0–2) | 0.091 |
| Daily ICS users | 60 (81%) | 93 (73%) | 0.233 |
| ICS dose (μg budesonide equivalent) | 800 (400–1000) | 800 (280–1000) | 0.393 |
| Patients who received oral corticosteroids | 30 (41%) | 35 (28%) | 0.062 |
| Daily add-on medication (includes LABA) | 39 (53%) | 62 (49%) | 0.661 |
| Atopy | 18 (27%) | 49 (42%) | 0.055 |
*Statistical significances were evaluated using Mann-Whitney test, independent samples t-test or Chi-square test, respectively. Results are displayed as median (interquartile range), mean (SD), or n (%). BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; COPD: chronic obstructive pulmonary disease; ICS: inhaled corticosteroid; LABA: long acting beta-adrenoceptor agonist.
Association between physical activity and lung function
Lung function was significantly different between the high and low physical activity groups. The high physical activity group had higher pre- and post-bronchodilator FEV1-values at follow-up (Table 2) but only pre-bronchodilator FEV1-value was higher at diagnosis (eTable 2). FEV1/FVC-ratios (pre- and post-BD) were significantly different at diagnosis and at follow-up between the groups (Table 2, eTable 2). After removal of patients with post-bronchodilator FEV1/FVC<0.7 and a smoking history of at least 10 pack years (eTable 3), which may indicate the presence of ACOS [25], post-bronchodilator FEV1/FVC-ratio at follow-up remained significantly lower in the low activity group (p = 0.032). This indicates that possible ACOS is not explaining the results.
Table 2.
Lung function at follow-up by physical activity groups.
| ≤240 min of physical activity/day | >240 min of physical activity/day | p-value* | |
|---|---|---|---|
| FVC (%ref) pre-BD | 95 (16) | 98 (15) | 0,222 |
| FVC (%ref) post-BD | 97 (16) | 99 (14) | 0,269 |
| FEV1 (%ref) pre-BD | 81 (19) | 88 (16) | 0,004 |
| FEV1 (%ref) post-BD | 84 (18) | 91 (16) | 0,004 |
| FEV1/FVC pre-BD | 0.71 (0.63–0.78) | 0.75 (0.69–0.79) | 0,002 |
| FEV1/FVC post-BD | 0.72 (0.65–0.79) | 0.77 (0.71–0.81) | 0,001 |
*Statistical significances were evaluated using Mann-Whitney test or independent samples t-test. Results are displayed as median (interquartile range) or mean (SD).FVC: forced vital capacity; BD: bronchodilator; FEV1: forced expiratory volume in 1 s.
Association between physical activity and lung function decline
When evaluating lung function decline from Max0-2.5 to follow-up visit in the high and low physical activity groups, a more rapid decline in FEV1 (ml and % of reference) and FVC (ml) in the group with less daily physical activity was observed (Table 3, Figure 3). The measurement point of Max0-2.5 was chosen because the patients had untreated asthma at diagnosis, and their lung function improved significantly during the following months due to start of asthma therapy. Therefore, comparisons between diagnostic and follow-up values would be comparisons between untreated and treated asthma. The differences between the groups remained significant when patients with post-bronchodilator FEV1/FVC<0.7 and a smoking history of at least 10 pack years were removed from the cohort (eTable 4). The frequency of physical exercise had no effect on lung function decline (data not shown).
Table 3.
Annual pre-BD lung function decline from Max0-2.5 to follow-up by physical activity group.
| ≤240 min of physical activity/day | >240 min of physical activity/day | p-value* | |
|---|---|---|---|
| ΔFVC/year, pre-BD (ml) | −43.6 (42.1) | −29.3 (39.7) | 0.018 |
| ΔFVC/year, pre-BD (%) | −0.12 (1.14) | 0.03 (0.95) | 0.325 |
| ΔFEV1/year, pre-BD (ml) | −58.8 (37.3) | −41.4 (34.2) | 0.001 |
| ΔFEV1/year, pre-BD (%) | −0.83 (1.13) | −0.39 (0.97) | 0.005 |
| ΔFEV1/ΔFVC -ratio/year, pre-BD | −0.0052 (−0.0101 to −0.0019) | −0.0041 (−0.0075 to −0.0016) | 0.062 |
*Statistical significances were evaluated using Mann-Whitney test or independent samples t-test. Results are displayed as median (interquartile range) or mean (SD). FVC: forced vital capacity, BD: bronchodilator, FEV1: forced expiratory volume in 1 s.
Figure 3.
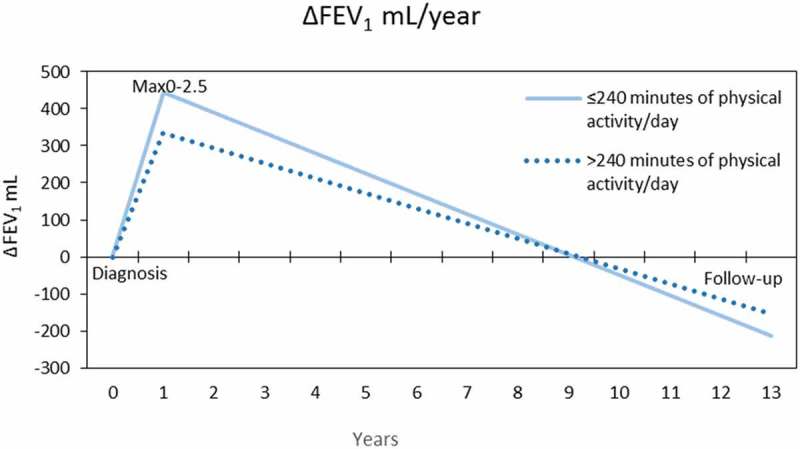
Changes in mean Pre-BD FEV1 (mL) during 12 years of follow-up in the groups of <240 or ≥240 min of daily physical activity.
Association between daily physical activity, asthma control, and symptoms of asthma
The high and low physical activity groups had no significant difference in asthma control or symptoms when measured by using AQ20 overall scores (eTable 5) or ACT (Table 4). However, when analyzing the separate questions in ACT and AQ20 questionnaires, differences were found. The low physical activity group had more shortness of breath and a worse self-perceived asthma control (ACT questions 2 and 5; Table 4). In AQ20 questionnaire the low physical activity group reported more breathlessness in questions 3 (breathlessness during gardening) (p = 0.002), 13 (breathlessness during housework) (p = 0.020) and 10 (difficulties in getting around the house due to chest problems) (p = 0.028) (eTable 5).
Table 4.
Asthma control and symptoms at follow-up.
| ≤240 min of physical activity/day | >240 min of physical activity/day | p-value* | |
|---|---|---|---|
| Uncontrolled asthma** | 27 (36.5) | 32 (25.2) | 0.234 |
| ACT score at follow-up | 22 (17–24) | 22 (20–24) | 0.159 |
| ACT Q2 shortness of breath at least 3–6 times/week | 22 (29.7) | 16 (12.6) | 0.005 |
| ACT Q5 asthma somewhat controlled, poorly controlled, not at all controlled | 25 (33.8) | 25 (19.7) | 0.029 |
*Statistical significances were evaluated using Chi-square test or Mann-Whitney test. Results are displayed as n (%) or median (interquartile range). ACT: asthma control test.
**As assessed according to GINA 2010 as previously described [18].
Association of daily physical activity, systemic and airway inflammatory parameters
Blood neutrophils, eosinophils, FeNO, IL-6, and hsCRP were measured. No differences were observed in any of these biomarkers of inflammation between the high and low activity groups (eTable 6).
Factors associated with lung function decline
To assess whether lower physical activity remains statistically significant factor associated with more rapid lung function decline in patients with adult-onset asthma after adjusting for age, sex, smoking, BMI, atopy, and use of inhaled glucocorticoid, we carried out multiple linear regression analysis. Daily physical activity ≤240 min remained statistically significantly associated with more rapid decline in FEV1 in adjusted analysis. Other explanatory factors of faster lung function decline were sex (male), atopy, and a higher BMI. (Table 5) During the building of this linear regression model other variables were tried but inclusion of these variables gave the best model.
Table 5.
Association of explanatory factors with lung function decline from Max0-2.5 to follow-up in multiple linear regression.
| Variable | Estimate (∆ml) | 95% Confidence Interval | p-value |
|---|---|---|---|
| (Constant) | −22.39 | −69.16 to 24.39 | 0.346 |
| Physical activity over 240 min/day | 17.90 | 8.00 to 27.80 | <0.001 |
| Sex (female) | 13.12 | 3.08 to 23.16 | 0.011 |
| Age at follow-up | −0.23 | −0.59 to 0.13 | 0.209 |
| BMI at follow-up | −1.01 | −2.02 to −0.01 | 0.048 |
| Not a daily ICS user at follow-up | 1.40 | −10.15 to 12.95 | 0.811 |
| Atopy | −11.38 | −21.72 to −1.04 | 0.031 |
| Ever smoker at follow-up | −1.97 | −11.70 to 7.76 | 0.690 |
n = 173 (outliers: n = 7, missing data: n = 21), BMI: body mass index, ICS: inhaled corticosteroid.
Discussion
The aim of this study was to examine the significance of daily physical activity in adult-onset asthma. Our data indicates that higher daily physical activity is associated with slower decline in lung function even when adjusting for sex, age, BMI, atopy, medication, and smoking. High and low physical activity groups in this study had no difference in BMI, smoking, medication, interleukin-6, hs-CRP, IgE, blood neutrophil or eosinophil counts, FeNO levels, or the frequency of exercise. The fact that there was no difference in BMI was surprising since high BMI has been linked to low physical activity [26]. The low cut-off point of 240 min could explain the absence of differences in inflammatory markers. Also, differences in dietary habits and energy expenditure can explain the absence of BMI difference. High physical activity group had a higher percentage of females, which suggests that females do more low intensity physical activities in this set up. There was no difference in biomarkers of systemic inflammation between the groups with lower and higher daily physical activity even though it has been shown that regular physical exercise can reduce systemic inflammation [27]. The frequency of exercise was not associated with lung function decline in this study, which was expected since there were no significant differences in the frequency of exercise between the two groups of high and low levels of daily physical activity.
To our knowledge this study is the first to examine the association between daily physical activity and FEV1 decline in clinical asthma. Our finding is supported by a similar association recently reported between leisure time activity and lung function change in persons having self-reported asthma in a population based cohort of the Nord-Trøndelag Health Study [28]. Our study has several strengths. All asthmatics were carefully diagnosed by a respiratory physician, and the diagnosis was verified with spirometry, serial peak flow measures, or measures of bronchial hyperreactivity. Most patients were treated with at least inhaled glucocorticoids between Max0-2.5 and follow-up. Smokers and patients with co-morbidities were not excluded making this a real life study. During 1999–2002 a considerable proportion of novel asthma diagnoses in the region were made at Seinäjoki Central Hospital and >94% of the patients obtaining diagnosis of asthma in this hospital at the study period took part in the study [18]. Therefore, this study population well represents a primary care population with asthma. However, patients with very mild or seasonal asthma may have been excluded since patients had to fill the strict lung function criteria [18].
The cut-off point for high and low levels of daily physical activity in this study was chosen at 240 min of physical activity per day. The cut-off point divided the cohort into the lowest one third against the two highest thirds. The patients were made well aware of what the question meant so that all misunderstandings could be minimized. Patients in this study had a median of 360 min of daily physical activity, which is similar to levels measured by accelerometers in patients with chronic diseases (range 308–395 min) [29–31]. The cut-off point of 240 min of physical activity is a relatively low level considering that even older care home residents in the UK [32] and aged (73–98 year-olds) Icelandic people [33] accumulated 122–190 min of daily physical activity. The cut-off point was intentionally set at this relatively low level to allow examinations of the effects of very low amounts of physical activity.
The most significant limitation of this study is the absence of spirometric post-bronchodilator values at Max0-2.5. This is due to the fact that the patients were treated according to the Finnish asthma guidelines where systematic recording of post-bronchodilatory values was not recommended at that time. Even though the patients’ estimates on their physical activity are close to measured values on similar cohorts, it would have been optimal to record daily physical activity using accelerometers. In addition, physical activity was assessed only at follow-up visit. However, it has been shown that most of our health related habits are formed during childhood or early adulthood and decline slowly or remain constant during adulthood. Significant changes in health related habits are unlikely to happen without a comprehensive lifestyle intervention [34,35]. Therefore, it can be assumed that the high physical activity group had higher activity levels also at diagnosis.
Similar findings to our study have been previously obtained in patients with COPD and in general population [14,16]. During an 11-year follow-up in patients with COPD, low physical activity group had a faster decline in lung function (−4.8 ml/year faster drop of FEV1, −7.7 ml/year faster drop of FVC) when compared to high physical activity group [14]. Slower FEV1 decline with higher physical activity has been detected on general population also [16]. In our study FEV1 declined 17.4 ml/year faster, and FVC declined 14.3 ml/year faster in the low activity group. Sex (male), high frequency of disease exacerbations, old age, absence of glucocorticoid treatment, and smoking have been found to accelerate FEV1 decline in asthmatics [9,36,37]. After adjusting for these factors with multiple linear regression analysis, the association of physical activity with FEV1 decline remained significant. Exacerbations were not included in the linear regression, but there was no difference in the proportion of those patients receiving oral corticosteroid treatment during the follow-up period between the physical activity groups suggesting that there was no difference in exacerbations.
The findings are clinically significant, since remission rate in adult onset asthma is low [7] and most patients suffer from asthma for decades. The linearity of the effect of physical activity on lung function remains unclear. We found one daily physical activity intervention study in pediatric patients with asthma [38], and no change in FEV1 was registered during a one-week follow-up. Also, in a recent cross-sectional study [39] daily physical activity had no effect on spirometric values of healthy adolescents. In patients with childhood-onset asthma there is a positive association between physical activity and lung function during a 3 week period [40], but this could be due to several acute bronchodilations or patients unconsciously adjusting their daily activity according to their lung function earlier that day. In light of this evidence it seems likely that daily physical activity does not cause long term improvements in FEV1, but that it slows down FEV1 decline. However, as the data on physical activity was collected at follow-up visit, the relation between lung function decline and physical activity level may also reflect that those with more rapid lung function decline are prone to decrease their level of physical activity.
Conclusion
This study highlights the importance of active lifestyle and the perils of sedentary habits. Modern physical activity guidelines focus on higher intensity activities, but it would be important to include low intensity activities to these guidelines for more holistic approach. More studies with a precise analysis of the activity intensity are needed to further explore the effect of physical activity on patients with asthma.
Biographies
Juho Loponen, BM. Currently a medical student at University of Tampere. Academic interests include physical activity, inflammation and asthma.
Pinja Ilmarinen, PhD. Researcher at Seinäjoki Central Hospital, Finland. Her research focuses on inflammatory lung diseases and especially phenotypes and mediators of adult-onset asthma.
Leena E Tuomisto, MD, PhD. Senior respiratory specialist at Seinäjoki Central Hospital. Her research interests include adult-onset asthma from diagnosis to prognosis.
Onni Niemelä, MD, Ph.D. Professor of Laboratory Medicine at Seinäjoki Central Hospital and University of Tampere. Research interests include liver diseases, asthma, exercise physiology and inflammation.
Minna Tommola, MD. Consultant in respiratory medicine in Central Finland Central Hospital, Jyväskylä, Finland. Research interests include smoking and asthma.
Pentti Nieminen, PhD. Currently an associate professor in medical informatics and data-analysis at the University of Oulu. Research interests include scientific communication, biostatistics and medical education.
Lauri Lehtimäki, MD, PhD. Consultant in respiratory medicine at Allergy Centre, Tampere University Hospital, Finland. He also works as associate professor at Faculty of Medicine and Life Sciences at University of Tampere. His main clinical interest is severe asthma and his research focuses on inflammatory lung diseases.
Hannu Kankaanranta, MD, PhD. Head of Respiratory Medicine at Seinäjoki Central Hospital, Seinäjoki, Finland. He also works as a professor of respiratory medicine at the Faculty of Medicine and Life Sciences at University of Tampere. His main research interest is adult-onset asthma.
Funding Statement
This study was sponsored by Tampere Tuberculosis Foundation (Tampere, Finland), the Finnish Anti-Tuberculosis Association Foundation (Helsinki, Finland), Jalmari and Rauha Ahokas Foundation (Helsinki, Finland), the Competitive State Research Financing of the Expert Responsibility Area of Tampere University Hospital (VTR, Tampere, Finland), and the Medical Research Fund of Seinäjoki Central Hospital (Seinäjoki, Finland) [Grant number 1717/6044]. None of the sponsors participated in the planning, execution, drafting, or writing of this study.
Acknowledgments
Aino Sepponen (Dept of Respiratory Medicine, Seinäjoki Central Hospital, Seinäjoki, Finland) is gratefully acknowledged for her help through all the stages of this work.
Contributors
JL analyzed and interpreted the data, and wrote the manuscript. HK, LL, ON and LET designed the study. PI contributed to the study design, interpretation of the data, and writing of the manuscript. MT contributed to the planning of lung function decline analyses. PN provided statistical advice and commented on drafts of the manuscript. HK, PI, LL, ON, LET and MT commented on drafts of the manuscript. All authors accept full responsibility for the conduct of the study, had access to the data, and controlled the decision to publish. JL is the guarantor.
Competing interests
We have read and understood European Clinical Respiratory Journal policy on declaration of interests and declare the following. JL, ON and PN have nothing to disclose. PI reports personal fees from Mundipharma, personal fees from Orion, personal fees from AstraZeneca, outside the submitted work. LT reports other from Takeda, Chiesi and Orion, other from TEVA, other from Filha ry, outside the submitted work. MT reports personal fees from Astra Zeneca, personal fees from Boehringer Ingelheim, personal fees from Filha ry, personal fees from GlaxoSmithKline, outside the submitted work. LL reports personal fees from Boehringer-Ingelheim Finland, personal fees from Orion Pharma, personal fees from GSK, personal fees from Chiesi, personal fees from Mundipharma, personal fees from Astra Zeneca, personal fees and non-financial support from Novartis, personal fees and non-financial support from Teva, personal fees from ALK, outside the submitted work. HK reports personal fees and non-financial support from Almirall, grants, personal fees and non-financial support from AstraZeneca, personal fees from Chiesi Pharma AB, personal fees from GlaxoSmithKline, personal fees and non-financial support from Boehringer-Ingelheim, personal fees from Leiras-Takeda, personal fees from MSD, personal fees from Novartis, personal fees from Mundipharma, personal fees from Medith, personal fees from Resmed Finland, non-financial support from Intermune, personal fees from Roche, personal fees from Orion Pharma, outside the submitted work.
Supplementary material
Supplementary data for this article can be accessed here.
References
- [1]. Kyu HH, Bachman VF, Alexander LT, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ. 2016;354:3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Caspersen CJ, Powell KE, Christenson GM.. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–9. [PMC free article] [PubMed] [Google Scholar]
- [3]. World Health Organization WHO health topics: physical activity; 2016. [cited 2017 March27]. Available from: http://www.who.int/topics/physical_activity/en/
- [4]. Goto C, Higashi Y, Kimura M, et al. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation. 2003;108:530–535. [DOI] [PubMed] [Google Scholar]
- [5]. Amelink M, de Nijs SB, de Groot JC, et al. Three phenotypes of adult-onset asthma. Allergy. 2013;68:674–680. [DOI] [PubMed] [Google Scholar]
- [6]. Ilmarinen P, Tuomisto LE, Kankaanranta H. Phenotypes, Risk Factors, and Mechanisms of Adult-Onset Asthma. Mediators Inflamm. 2015;2015:514868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Tuomisto LE, Ilmarinen P, Niemelä O, et al. 12-year prognosis of adult-onset asthma: Seinäjoki Adult Asthma Study. Respir Med. 2016;117:223–229. [DOI] [PubMed] [Google Scholar]
- [8]. Kankaanranta H, Tuomisto LE, Ilmarinen P. Age-specific incidence of new asthma diagnoses in Finland. J Allergy Clin Immunol Pract. 2017;5:189–191. [DOI] [PubMed] [Google Scholar]
- [9]. Tommola M, Ilmarinen P, Tuomisto LE, et al. The effect of smoking on lung function: a clinical study on adult-onset asthma. Eur Respir J. 2016;5:1298–1306. [DOI] [PubMed] [Google Scholar]
- [10]. Tommola M, Ilmarinen P, Tuomisto LE, et al. Differences between asthma-COPD overlap syndrome and adult-onset asthma. Eur Respir J. 2017;49:1602383. [DOI] [PubMed] [Google Scholar]
- [11]. Franca-Pinto A, Mendes FAR, de Carvalho-Pinto RM, et al. Aerobic training decreases bronchial hyperresponsiveness and systemic inflammation in patients with moderate or severe asthma: a randomised controlled trial. Thorax. 2015;70:732–739. [DOI] [PubMed] [Google Scholar]
- [12]. Carson KV, Chandratilleke MG, Picot J, et al. Physical training for asthma. Cochrane Database Syst Rev. 2013;9:001116. [DOI] [PubMed] [Google Scholar]
- [13]. Pakhale S, Luks V, Burkett A, et al. Effect of physical training on airway inflammation in bronchial asthma: a systematic review. BMC Pulm Med. 2013;13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Garcia-Aymerich J, Lange P, Benet M, et al. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med. 2007;175:458–463. [DOI] [PubMed] [Google Scholar]
- [15]. McGlone S, Venn A, Walters EH, et al. Physical activity, spirometry and quality-of-life in chronic obstructive pulmonary disease. Copd. 2006;3:83–88. [DOI] [PubMed] [Google Scholar]
- [16]. Jakes RW, Day NE, Patel B, et al Physical inactivity is associated with lower forced expiratory volume in 1 second: european Prospective Investigation into Cancer-Norfolk Prospective Population Study. Am J Epidemiol. 2002;156:139–147. [DOI] [PubMed] [Google Scholar]
- [17]. Pelkonen M, Notkola I, Lakka T, et al. Delaying decline in pulmonary function with physical activity: a 25-year follow-up. Am J Respir Crit Care Med. 2003;168:494–499. [DOI] [PubMed] [Google Scholar]
- [18]. Kankaanranta H, Ilmarinen P, Kankaanranta T, et al. Seinajoki Adult Asthma Study (SAAS): a protocol for a 12-year real-life follow-up study of new-onset asthma diagnosed at adult age and treated in primary and specialised care. NPJ Prim Care Respir Med. 2015;25:15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Haahtela T, Tuomisto LE, Pietinalho A, et al. A 10 year asthma programme in Finland: major change for the better. Thorax. 2006;61:663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Ilmarinen P, Tuomisto LE, Niemela O, et al. Comorbidities and elevated IL-6 associate with negative outcome in adult-onset asthma. Eur Respir J. 2016;48:1052–1062. [DOI] [PubMed] [Google Scholar]
- [21]. Win T, Pearce L, Nathan J, et al. Use of the Airway Questionnaire 20 to detect changes in quality of life in asthmatic patients and its association with the St George’s Respiratory Questionnaire and clinical parameters. Can Respir J. 2008;15:133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Schatz M, Sorkness CA, Li JT, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117:549–556. [DOI] [PubMed] [Google Scholar]
- [23]. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. [DOI] [PubMed] [Google Scholar]
- [24]. Viljanen AA, Halttunen PK, Kreus KE, et al. Spirometric studies in non-smoking, healthy adults. Scand J Clin Lab Invest Suppl. 1982;159:5–20. [PubMed] [Google Scholar]
- [25]. Global Initiative for Asthma and Global Initiative for COPD Global Iniative for Asthma (GINA): 2015 Asthma, COPD and Asthma-COPD Overlap Syndrome (ACOS); 2015. [cited 2016 October19]. Available from: http://www.ginasthma.org
- [26]. World Health Organization World Health Organization: global strategy on diet, physical activity and health 2004; 2004. [cited 2017 March27]. Available from: http://www.who.int/dietphysicalactivity/strategy/eb11344/strategy_english_web.pdf?ua=1
- [27]. Adamopoulos S, Parissis J, Kroupis C, et al. Physical training reduces peripheral markers of inflammation in patients with chronic heart failure. Eur Heart J. 2001;22:791–797. [DOI] [PubMed] [Google Scholar]
- [28]. Brumpton BM, Langhammer A, Henriksen AH, et al. Physical activity and lung function decline in adults with asthma: the HUNT Study. Respirology. 2017;22:278–283. [DOI] [PubMed] [Google Scholar]
- [29]. Booth JN, Bromley LE, Darukhanavala AP, et al. Reduced physical activity in adults at risk for type 2 diabetes who curtail their sleep. Obesity (Silver Spring). 2012;20:278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Fastenau A, Ocp VS, Gosselink R, et al. Discrepancy between functional exercise capacity and daily physical activity: a cross-sectional study in patients with mild to moderate COPD. Prim Care Respir J. 2013;22:425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Ross R, McGuire KA. Incidental physical activity is positively associated with cardiorespiratory fitness. Med Sci Sports Exerc. 2011;43:2189–2194. [DOI] [PubMed] [Google Scholar]
- [32]. Barber SE, Forster A, Birch KM. Levels and patterns of daily physical activity and sedentary behavior measured objectively in older care home residents in the UK. J Aging Phys Act. 2015;23:133–143. [DOI] [PubMed] [Google Scholar]
- [33]. Arnardottir NY, Koster A, Van Domelen DR, et al. Objective measurements of daily physical activity patterns and sedentary behaviour in older adults: age, Gene/Environment Susceptibility-Reykjavik Study. Age Ageing. 2013;42:222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Aarts H, Paulussen T, Schaalma H. Physical exercise habit: on the conceptualization and formation of habitual health behaviours. Health Educ Res. 1997;12:363–374. [DOI] [PubMed] [Google Scholar]
- [35]. Breslow L, Enstrom JE. Persistence of health habits and their relationship to mortality. Prev Med. 1980;9:469–483. [DOI] [PubMed] [Google Scholar]
- [36]. O’Byrne PM, Pedersen S, Lamm CJ, et al. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179:19–24. [DOI] [PubMed] [Google Scholar]
- [37]. Dijkstra A, Vonk JM, Jongepier H, et al. Lung function decline in asthma: association with inhaled corticosteroids, smoking and sex. Thorax. 2006;61:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Walders-Abramson N, Wamboldt FS, Curran-Everett D, et al. Encouraging physical activity in pediatric asthma: a case-control study of the wonders of walking (WOW) program. Pediatr Pulmonol. 2009;44:909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Smith MP, von Berg A, Berdel D, et al. Physical activity is not associated with spirometric indices in lung-healthy German youth. Eur Respir J. 2016;48:428. [DOI] [PubMed] [Google Scholar]
- [40]. Ritz T, Rosenfield D, Steptoe A. Physical activity, lung function, and shortness of breath in the daily life of individuals with asthma. Chest. 2010;138:913–918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


