Abstract
Study question
Are higher overall and central adiposity associated with reduced fecundability, measured by time-to-pregnancy (TTP), in Asian women?
Summary answer
Higher overall adiposity, but not central adiposity, was associated with longer TTP in Asian women.
What is known already
High body mass index (BMI) has been associated with a longer TTP, although the associations of body composition and distribution with TTP are less clear. There are no previous studies of TTP in Asian women, who have a relatively higher percentage of body fat and abdominal fat at relatively lower BMI.
Study design, size, duration
Prospective preconception cohort using data from 477 Asian (Chinese, Malay and Indian) women who were planning to conceive and enrolled in the Singapore PREconception Study of long-Term maternal and child Outcomes (S-PRESTO) study, 2015-2017.
Participants/materials, setting, methods
Women’s mean age was 30.7 years. Overall adiposity was assessed by BMI, sum of 4-site skinfold thicknesses (SFT) and total body fat percentage (TBF%, measured using air displacement plethysmography); central adiposity was assessed by waist circumference (WC), waist-to-hip ratio (WHR), waist-to-height ratio (WHtR) and A body Shape Index (ABSI). Pregnancy occurring within one year from recruitment was ascertained by ultrasonography. Those who did not conceive within one year of recruitment, were lost to follow-up, or initiated fertility treatment were censored. TTP was measured in cycles. Discrete-time proportional hazards models were used to estimate the fecundability ratio (FR) and 95% confidence interval (CI) for each anthropometric measure in association with fecundability, adjusting for confounders.
Main results and the role of chance
Compared to women with a normal BMI of 18.5-22.9 kg/m2, women with higher BMI of 23-27.4 and ≥27.5 kg/m2 showed lower FR of 0.66 (95% CI 0.45, 0.97) and 0.53 (0.31, 0.89), respectively. Compared to women in the lowest quartile of SFT (25-52.9mm), those in the highest quartile of ≥90.1 mm showed lower FR of 0.58 (95% CI 0.36, 0.95). Compared to women in the lowest quartile of TBF% (13.6-27.2%), those in the upper two quartiles of 33.0-39.7% and ≥39.8% showed lower FR of 0.56 (95% CI 0.32, 0.98) and 0.43 (0.24, 0.80), respectively. Association of high BMI with reduced fecundability was particularly evident among nulliparous women. Measures of central adiposity (WC, WHR, WHtR, ABSI) were not associated with fecundability.
Limitations, reasons for caution
Small sample size could restrict power of analysis.The analysis was confined to planned pregnancies, which could limit generalizability of findings to non-planned pregnancies, estimated at around 44% in Singapore. Information on the date of last menstrual period for each month was not available, hence the accuracy of self-reported menstrual cycle length could not be validated, potentially introducing error into TTP estimation. Measures of exposures and covariates such as cycle length were not performed repeatedly over time; cycle length might have changed during the period before getting pregnant.
Wider implications of the findings
Other than using BMI as the surrogate measure of body fat, we provide additional evidence showing that higher amounts of subcutaneous fat that based on the measure of SFT at the sites of biceps, triceps, suprailiac and subscapular, and TBF% are associated with longer TTP. Achieving optimal weight and reducing total percentage body fat may be a potential intervention target to improve female fertility. The null results observed between central adiposity and TTP requires confirmation in further studies.
Study funding/competing interest(s)
This research is supported by Singapore National Research Foundation under its Translational and Clinical Research Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council, (NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014). Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), Singapore. Y.S.C., K.M.G., F.Y. and Y.S.L. have received reimbursement to speak at conferences sponsored by companies selling nutritional products. Y.S.C., K.M.G. and S.Y.C. are part of an academic consortium that has received research funding from Abbott, Nutrition, Nestle and Danone. Other authors declared no conflicts of interest.
Trial registration number
N/A.
Keywords: adiposity, body mass index, obesity, fertility, preconception cohort
Introduction
The falling trend in global fertility has been an issue of concern in many settings, imposing significant economic and social implications on a pre-existing ageing demographic (United Nations, 2015). Compounding this problem is the ongoing rise of worldwide overweight and obesity rates, particularly among women of childbearing age (NCD Risk Factor Collaboration, 2016). In Singapore, proportion of overweight people is projected to increase from 24.6% in 1990 to 38.6% by 2050; while those obese are predicted to almost quadruple from 4.3% in 1990 to 15.9% by 2050 (Phan et al., 2014). Overweight and obesity, as measured by body mass index (BMI), has been associated with lower female fecundability - the cycle probability of conception (Wise et al., 2010; Wise et al., 2013; McKinnon et al., 2016). To a lesser extent, underweight has also been related to reduced fecundability (Hassan and Killick, 2004; Gesink Law et al., 2007). Both extremes of BMI are associated with reproductive dysfunctions through increasing risk of anovulation (Davies, 2006), alterations in levels of various hormones and energy metabolism (Talmor et al., 2015; Fontana and Torre, 2016).
BMI, however, is a crude measure of body fat and does not consider body composition and shape. Excess fat deposition in the abdominal region has been associated with oligomenorrhea (De Pergola et al., 2009) and increased androgenicity of women (Diamanti-Kandarakis and Bergiele, 2001). Evidence has been limited and inconsistent regarding the extent to which female fecundability is influenced by central adiposity and body fat distribution, as often measured by waist circumference (WC) or waist-to-hip ratio (WHR) (Wise et al., 2010; Wise et al., 2013; McKinnon et al., 2016). Nevertheless, the use of WC or WHR as a proxy for central adiposity is subject to key limitations. Both indicators are sensitive to body size (weight and height) and highly correlated with BMI, which make it difficult to disentangle the impacts of body shape and of body size (Krakauer and Krakauer, 2012; Vikram et al., 2016). Also, WHR has limited validity in practical risk management when changes in both WC and hip circumference (HC) are in a similar direction (Vikram et al., 2016). Evidence suggests that the waist-to-height ratio (WHtR) and A Body Shape Index (ABSI =WC (m)/[BMI (kg/m2)2/3 x Height (m)1/2]) may be better proxies of central adiposity and better predictors of health risks (Krakauer and Krakauer, 2012; Vikram et al., 2016).
Previous studies assessing adiposity in relation to fecundability were conducted among white/black women (Wise et al., 2010; Wise et al., 2013; McKinnon et al., 2016), but have not been performed among Asian women. Asian populations have a relatively higher percentage of body fat and abdominal fat at relatively lower BMI (Wang et al., 1994). To clarify the role of body weight and body fat distribution on fecundability in Asian women, we examined these associations among Chinese, Malay and Indian women enrolled in a prospectively studied preconception cohort in Singapore. Using Asian specific cut-offs, we assessed BMI, WC and WHR in relation to fecundability, as measured by time-to-pregnancy (TTP) in cycles (Weinberg et al., 1989). To address existing gaps in the literature, we additionally assessed subcutaneous fat deposition based on multiple sites of skinfold thicknesses (SFT), and total body fat percentage (TBF%) using air displacement plethysmography. We examined WHtR and ABSI as indicators of central adiposity. We hypothesized that increased overall adiposity as measured by BMI, SFT and TBF%, and increased central adiposity as measured by WC, WHR, WHtR and ABSI were associated with reduced fecundability.
Materials and methods
Ethical approval
The study was conducted according the guidelines laid down in the Declaration of Helsinki. Ethical approval was obtained from the Singhealth Centralised Institute Review Board (reference 2014/692/D). Written informed consent was obtained from all women.
Study population
Data were drawn from the Singapore PREconception Study of long-Term maternal and child Outcomes (S-PRESTO) prospective cohort study, designed to examine the influences of events prior to and in early pregnancy on metabolic and mental health outcomes for both mother and offspring in later life.
Asian women of Chinese, Malay or Indian ethnicity planning to conceive and between ages of 18-45 years were enrolled from the general population of Singapore. Women were excluded if they reported actively trying to conceive for more than 18 months, had been diagnosed with type 1 or type 2 diabetes, had been taken anticonvulsant medication, oral steroids or received assisted fertility treatment in the past one month.
Study procedure
During the recruitment visit (first visit), face-to-face interviews and detailed measurements were conducted by trained research staff in the clinic at the KK Women’s and Children’s hospital (KKH). Baseline information including socio-demography, health history, menstrual characteristics, lifestyle habits and anthropometric measurements were collected.
Women were followed for up to 12 months of trying for pregnancy. Instructions were given to perform pregnancy testing if their period was 3-4 days late or in 2 weeks after unprotected intercourse. Women informed research staff if they had a positive result on the provided urinary pregnancy test kit, sensitive to 25 mIU/ml hCG (Biotron Diagnostics, USA). When pregnancy was reported, the last menstruation period (LMP) was documented and women were scheduled for an ultrasound scan. In the absence of any update within 6, 9 and 12 months from the recruitment visit, research staff conducted a follow-up survey by telephone to track participants’ pregnancy status.
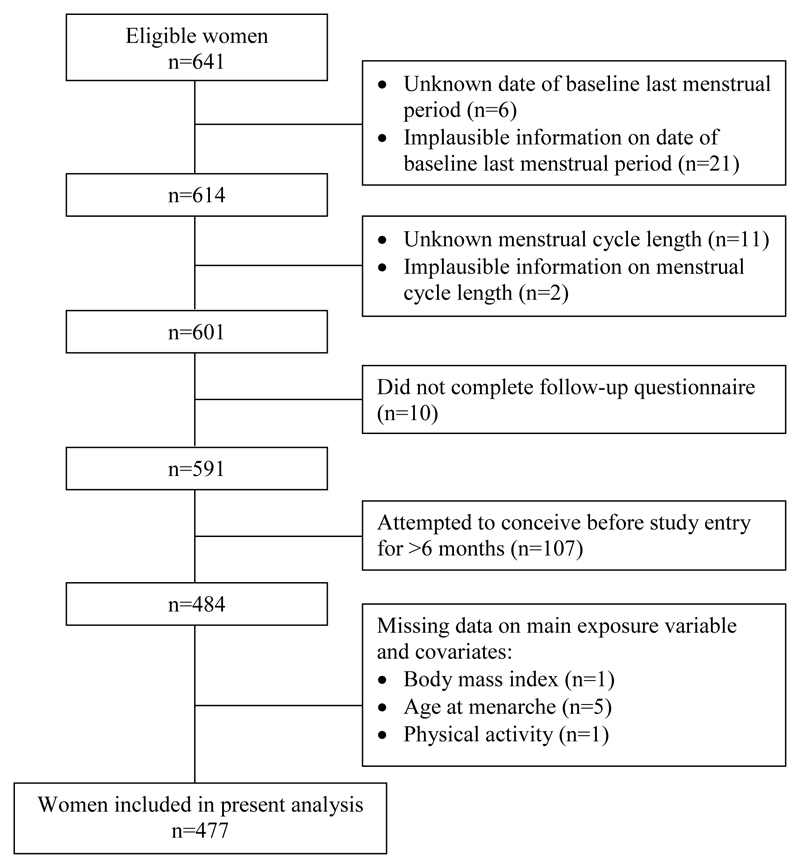
Of the 641 eligible women enrolled from February 2015 to July 2017, we excluded women from this analysis based on the following criteria: unknown or implausible date of LMP at recruitment (n=27); unknown or implausible menstrual cycle length (n=13); did not complete follow-up questionnaire (n=10); or had been trying to conceive for >6 months before study entry (n=107). It has been suggested that women who had been trying to conceive for a longer period at study entry might have adjusted their behavior (McKinnon et al., 2016), which could introduce bias in the associations.
Assessment of anthropometry
We measured woman’s weight to the nearest 0.1 kg using a SECA 803 weighing machine (Hamburg, Germany); height was measured to the nearest 0.1 cm using a SECA 213 Portable Stadiometer (Hamburg, Germany). We measured WC and HC to the nearest 0.1 cm using a non-stretchable measuring tape. WC was measured at the uppermost lateral border of the ilium (Centers for Disease Control and Prevention, 2007), while HC was measured at the maximal gluteal protuberance at the level of symphysis (Centers for Disease Control and Prevention, 1988). All measurements were taken in duplicate and averaged. We calculated BMI as weight (kg)/height (m)2; WHR as WC (cm)/HC (cm); WHtR as WC (cm)/height (cm); ABSI as WC (m)/[BMI (kg/m2)2/3 x Height (m)1/2] (Krakauer and Krakauer, 2012).
We measured biceps, triceps, suprailiac and subscapular skinfold thicknesses at the right side of the body using a Holtain skinfold caliper (Holtain Ltd, Crymych, UK). The measures were taken in triplicate to the nearest 0.2mm and averaged. The sum of 4-site SFT was derived. We used BOD POD Air Displacement Plethysmography version 5.2.0 (Cosmed, Rome, Italy) to assess fat mass and fat free mass based on whole body densitometric principles.
We categorized all anthropometric variables according to clinical thresholds for Asian populations whenever available. BMI status was hence categorized as <18.5, 18.5-22.9, 23-27.4 and ≥27.5kg/m2 (World Health Organization, 2004). WC was categorized as ≤74, 75-80, 81-86 and ≥87cm (World Health Orgzanition, 2008). WHR was categorized as <0.80, 0.80-0.84 and ≥0.85 (World Health Orgzanition, 2008). A cut-off value of >0.5, which has been used in previous studies, was defined as abnormal for WHtR (Li et al., 2013; Vikram et al., 2016). SFT, TBF% and ABSI were categorized into quartiles.
Assessment of pregnancy and cycles at risk
The event of interest was based on the first report of pregnancy as confirmed by ultrasound scan, regardless of pregnancy outcome.Total waiting time was calculated as the interval between the date of LMP obtained at recruitment and before conception (pregnant within 1 year) or last follow-up call (if not pregnant). The interval was then converted to cycles at risk by dividing with the average cycle length. Menstrual cycle length was obtained at recruitment by asking women about their range of usual cycles between the start of one period and the start of the next period. Women were also asked about the number of months of attempting to conceive at study entry. The total number of cycles at risk were calculated as follows: (months of attempting to conceive at study entry/average cycle length) + [(date of LMP before conception or the most recent follow-up) - (date of LMP at recruitment)]/average cycle length. For women who became pregnant, one more conception cycle was added (Zhang et al., 2017).
Assessment of covariates
We identified potential confounders a priori from the literature (Gesink Law et al., 2007; Wise et al., 2010; Wise et al., 2013; McKinnon et al., 2016) and by using a directed acyclic graph.
These included age (<30, 30-34, ≥35 years), ethnicity (Chinese, Malay, Indian or any mix between these three ethnicities), education (non-tertiary, tertiary), parity (nulliparous, parous), cycle length [<28, 28-30,>30days], cycle regularity (regular, irregular), age at menarche (<12, 12-13, >13 years), alcohol intake (no, yes), smoking exposure (no, yes) and physical activity [inactive, minimally active, HEPA active (health enhancing physical activity; a high active category)]. We asked women if their menstrual periods were regular or if it varied by more than 5 days between periods in the past 6 months, which we classified it as irregular cycle. Smoking exposure was defined as any active or passive cigarette smoking. Physical activity was assessed using the International Physical Activity Questionnaire (IPAQ) (IPAQ Research Committee, 2005). We tested whether these potential confounders were associated with the anthropometric variables and time-to-pregnancy. We only controlled for potential confounders that changed the multivariable fecundability ratio (FR) by 10% or more relative to the unadjusted FR. Based on these criteria, the selected confounders comprised age, ethnicity, education, parity and cycle length.
Statistical analysis
We used Fisher’s exact tests to assess differences in covariates across BMI categories; while Kaplan–Meier methodology was used to estimate the cumulative probability of pregnancy. We used discrete-time proportional hazards models to estimate FRs and 95% confidence intervals (CIs) for women’s anthropometric measures in association with fecundability as measured by TTP, in cycles (Weinberg et al., 1989). The FR represents the cycle-specific probability of conception among exposed women, relative to unexposed women. A FR <1 indicating decreased fecundability (longer TTP), while a FR >1 indicating increased fecundability (shorter TTP) (Wise et al., 2010). To account for left truncation, we based risk sets only on observed cycles at risk, i.e. attempt time to conceive while participating in the study (Jenkins, 2005; Wise et al., 2010). For example, if a woman had been trying to conceive for three cycles before entering the study and then reported a pregnancy after six cycles of total attempt time, we only included three cycles in the analysis starting at cycle 4 when she was first observed in the study (i.e. cycles 4 through 6). Censoring was applied when a woman (i) had not conceived after 12 months, which is the typical length of time after which couples seek medical assistance for infertility, (ii) was lost to follow up, (iii) reported no longer trying to conceive or (iv) initiated fertility treatment, whichever occurred first. Separate models were run for individual anthropometric measure with TTP. We performed crude (Model 1) and adjusted analyses (Model 2). Potential confounders were indicated as above. To assess the independent contribution of overall and central adiposity, we conducted additional models (Model 3) that simultaneously adjusted for BMI and WHR (Wise et al., 2013). We did not conduct separate analysis that further controlled for polycystic ovarian syndrome (PCOS) which could be a potential causal intermediate (Wise et al., 2013), but only one woman self-reported having the syndrome. We tested the possibility of a non-linear relationship between each anthropometric measure and fecundability using restricted cubic splines. As it has been reported that the association between body size and fecundability might vary by parity (Wise et al., 2010), we performed interaction test of anthropometry measure with parity on fecundability using Model 3. The results were stratified by parity. Statistical analyses were conducted using SPSS Statistics Version 19.0 (IBM Corp, Armonk, NY, USA) or Stata Statistical Software, Release 13 (StataCorp, College Station, TX, USA).
Results
We included 477 (74.4%) women with complete dataset for the present analysis (Figure 1). In comparison to excluded women (n=164, 25.6%), those included were more likely to attain tertiary education (70.4 versus 59.1%) and to report a regular menstrual cycle (68.3 versus 57.9%) (Supplementary Information Table S1).
Figure 1.
Flowchart of the study.
The 477 women in the study contributed to 4069 cycles and 168 pregnancies. Period of attempting to conceive before study entry was at the median of 0.97 cycles (interquartile range 0-2.99). After 6 and 12 cycles of pregnancy attempts, 30% and 45% women reported a pregnancy, respectively. Across BMI categories, women with BMI ≥27.5 kg/m2 were more often of Malay and Indian ethnicities, had attained lower education, and were likely to be parous, to have reached menarche at age <12 years and to have been exposed to cigarette smoke and less likely to consume alcohol than the other BMI categories (Table I). The mean values for anthropometry variables are presented in Table II.
Table I.
Characteristics of women by body mass index, S-PRESTO study, 2015-2017 (n=477)
| Body mass index, kg/m2 |
||||||
|---|---|---|---|---|---|---|
| Characteristics | Total (n=477) | <18.5 (n=43) | 18.5-22.9 (n=230) | 23-27.4 (n=130) | ≥27.5 (n=74) | pa |
| Age (years), n (%) | 0.378 | |||||
| <30 | 176 (36.9) | 18 (41.9) | 85 (37.0) | 46 (35.4) | 27 (36.5) | |
| 30-34 | 239 (50.1) | 23 (53.5) | 118 (51.3) | 60 (46.2) | 38 (51.4) | |
| ≥35 | 62 (13.0) | 2 (4.7) | 27 (11.7) | 24 (18.5) | 9 (12.2) | |
| Ethnicity, n (%) | <0.001 | |||||
| Chinese | 362 (75.9) | 38 (88.4) | 196 (85.2) | 93 (71.5) | 35 (47.3) | |
| Malay | 54 (11.3) | 2 (4.7) | 13 (5.7) | 20 (15.4) | 19 (25.7) | |
| Indian | 50 (10.5) | 3 (7.0) | 16 (7.0) | 15 (11.5) | 16 (21.6) | |
| Mix | 11 (2.3) | 0 | 5 (2.2) | 2 (1.5) | 4 (5.4) | |
| Highest education level, n (%) | <0.001 | |||||
| Non-tertiaty | 141 (29.6) | 5 (11.6) | 51 (22.2) | 38 (29.2) | 47 (63.5) | |
| Tertiary | 336 (70.4) | 38 (88.4) | 179 (77.8) | 92 (70.8) | 27 (36.5) | |
| Parity, n (%) | 0.005 | |||||
| Nulliparous | 301 (63.1) | 30 (69.8) | 159 (69.1) | 77 (59.2) | 35 (47.3) | |
| Parous | 176 (36.9) | 13 (30.2) | 71 (30.9) | 53 (40.8) | 39 (52.7) | |
| Cycle regularity,b n (%) | 0.155 | |||||
| Regular | 326 (68.3) | 32 (74.4) | 164 (71.3) | 87 (66.9) | 43 (58.1) | |
| Irregular | 151 (31.7) | 11 (25.6) | 66 (28.7) | 43 (33.1) | 31 (41.9) | |
| Cycle length,c n (%) | 0.162 | |||||
| <28 days | 72 (15.1) | 6 (14.0) | 36 (15.7) | 24 (18.5) | 6 (8.1) | |
| 28-30 days | 243 (50.9) | 25 (58.1) | 124 (53.9) | 59 (45.4) | 35 (47.3) | |
| >30 days | 162 (34.0) | 12 (27.9) | 70 (30.4) | 47 (36.2) | 33 (44.6) | |
| Age at menarche, n (%) | <0.001 | |||||
| <12 years | 134 (28.1) | 5 (11.6) | 52 (22.6) | 43 (33.1) | 34 (45.9) | |
| 12-13 years | 254 (53.2) | 25 (58.1) | 126 (54.8) | 70 (53.8) | 33 (44.6) | |
| >13 years | 89 (18.7) | 13 (30.2) | 52 (22.6) | 17 (13.1) | 7 (9.5) | |
| Alcohol intake, n (%) | 0.014 | |||||
| No | 234 (49.1) | 16 (37.2) | 106 (46.1) | 64 (49.2) | 48 (64.9) | |
| Yes | 243 (50.9) | 27 (62.8) | 124 (53.9) | 66 (50.8) | 26 (35.1) | |
| Smoking exposure, n (%) | 0.004 | |||||
| No | 383 (80.3) | 41 (95.3) | 181 (78.7) | 109 (83.8) | 52 (70.3) | |
| Yes | 94 (19.7) | 2 (4.7) | 49 (21.3) | 21 (16.2) | 22 (29.7) | |
| Physical activity,d n (%) | 0.910 | |||||
| Inactive | 68 (14.3) | 5 (11.6) | 37 (16.1) | 18 (13.8) | 8 (10.8) | |
| Minimally active | 247 (51.8) | 24 (55.8) | 118 (51.3) | 68 (52.3) | 37 (50.0) | |
| HEPA active | 162 (34.0) | 14 (32.6) | 75 (32.6) | 44 (33.8) | 29 (39.2) | |
S-PRESTO, Singapore PREconception Study of long-Term maternal and child Outcomes; HEPA, health enhancing physical activity.
p based on Fisher's exact
Based on self-reported data where irregular cycle was defined as varing by more than 5 days between periods in the past 6 months.
Based on self-reported data at baseline where the average cycle length was computed from the range of usual cycles between the start of one period and the start of the next period.
Classified according to the International Physical Activity Questionnaire guidelines for data processing and analysis.
Table II.
Anthropometric measures of participants, S-PRESTO study, 2015-2017
| Anthropometric measures | n | Mean (SD) |
|---|---|---|
| Body mass index (kg/m2) | 477 | 23.42 (4.96) |
| Sum of skinfold thickness (mm) | 471 | 72.56 (24.43) |
| Total body fat (%) | 386 | 33.61 (8.75) |
| Waist circumference (cm) | 475 | 82.56 (10.94) |
| Waist-to-hip ratio | 475 | 0.86 (0.06) |
| Waist-to-height ratio | 475 | 0.52 (0.07) |
| A Body Shape Index (m11/6 /kg2/3) | 475 | 0.080 (0.005) |
S-PRESTO, Singapore PREconception Study of long-Term maternal and child Outcomes; SD, standard deviation
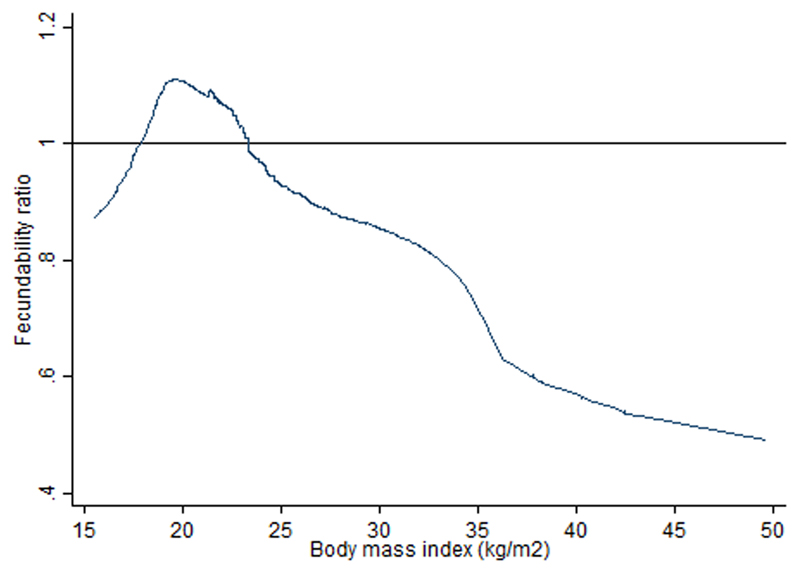
As presented in Table III (Model 2), compared to women with a normal BMI of 18.5-22.9 kg/m2, women with BMI of <18.5, 23-27.4 and ≥27.5 kg/m2 showed FR of 0.60 (95% CI 0.34, 1.06), 0.66 (0.45, 0.97) and 0.53 (0.31, 0.89), respectively. Compared to women in the lowest quartile of SFT (25.0-52.9mm), those in the upper quartiles of 53.0-70.0, 70.1-90.0 and ≥90.1 mm showed FR of 1.11 (0.73, 1.67), 0.96 (0.62, 1.46) and 0.58 (0.36, 0.95), respectively. Reduced fecundability was observed in women with higher TBF% of 33.0-39.7% [FR 0.56 (0.32, 0.98)] and ≥39.8% [FR 0.43 (0.24, 0.80)] compared to TBF% of 13.6-27.2%. Compared with Model 2, inclusion of both BMI and WHR simultaneously in the same model (Model 3) showed similar trends of associations between both models. Figure 2 displays the restricted cubic spline curve for the association between BMI and fecundability. Association of high BMI with reduced fecundability was particularly evident among nulliparous women (Supplementary Information Table S2). No associations were found for WC, WHR, WHtR and ABSI with fecundability before (Table III) and after parity stratification (Supplementary Information Table S2).
Table III.
Associations between anthropometric measures and time-to-pregnancy in women from the S-PRESTO study, 2015-2017
| Anthropometric measures | n | Pregnancies | Cycles | Model 1a |
Model 2b |
Model 3c |
|||
|---|---|---|---|---|---|---|---|---|---|
| FR | 95% CI | FR | 95% CI | FR | 95% CI | ||||
| Body mass index (kg/m2) | |||||||||
| <18.5 | 43 | 14 | 80 | 0.67 | 0.38, 1.17 | 0.60 | 0.34, 1.06 | 0.59 | 0.33, 1.06 |
| 18.5-22.9 | 230 | 95 | 539 | 1.00 | (ref.) | 1.00 | (ref.) | 1.00 | (ref.) |
| 23-27.4 | 130 | 38 | 215 | 0.70 | 0.48, 1.03 | 0.66 | 0.45, 0.97 | 0.64 | 0.44, 0.95 |
| ≥27.5 | 74 | 21 | 102 | 0.66 | 0.41, 1.06 | 0.53 | 0.31, 0.89 | 0.54 | 0.31, 0.91 |
| Sum of skinfold thickness (mm) | |||||||||
| Q1 (25.0-52.9) | 117 | 46 | 254 | 1.00 | (ref.) | 1.00 | (ref.) | 1.00 | (ref.) |
| Q2 (53.0-70.0) | 118 | 49 | 284 | 1.09 | 0.73, 1.62 | 1.11 | 0.73, 1.67 | 1.03 | 0.67, 1.57 |
| Q3 (70.1-90.0) | 118 | 42 | 232 | 0.91 | 0.60, 1.39 | 0.96 | 0.62, 1.46 | 0.91 | 0.56, 1.50 |
| Q4 (90.1-151.8) | 118 | 30 | 149 | 0.62 | 0.39, 0.98 | 0.58 | 0.36, 0.95 | 0.60 | 0.30, 1.20 |
| Total body fat (%) | |||||||||
| Q1 (13.6-27.2) | 96 | 39 | 290 | 1.00 | (ref.) | 1.00 | (ref.) | 1.00 | (ref.) |
| Q2 (27.3-32.9) | 97 | 36 | 192 | 0.81 | 0.45, 1.44 | 0.83 | 0.52, 1.32 | 0.74 | 0.46, 1.20 |
| Q3 (33.0-39.7) | 97 | 25 | 200 | 0.62 | 0.31, 1.22 | 0.56 | 0.32, 0.98 | 0.50 | 0.25, 0.97 |
| Q4 (39.8-61.3) | 96 | 23 | 144 | 0.54 | 0.26, 1.13 | 0.43 | 0.24, 0.80 | 0.40 | 0.16, 0.97 |
| Waist circumference (cm) | |||||||||
| ≤74 | 110 | 40 | 205 | 1.00 | (ref.) | 1.00 | (ref.) | 1.00 | (ref.) |
| 75-80 | 130 | 50 | 235 | 0.97 | 0.64, 1.47 | 0.94 | 0.59, 1.48 | 0.76 | 0.48, 1.22 |
| 81-86 | 100 | 37 | 160 | 0.94 | 0.60, 1.48 | 1.01 | 0.63, 1.62 | 0.88 | 0.52, 1.50 |
| ≥87 | 135 | 40 | 169 | 0.75 | 0.49, 1.17 | 0.66 | 0.40, 1.08 | 0.74 | 0.37, 1.49 |
| Waist-to-hip ratio | |||||||||
| <0.80 | 74 | 27 | 137 | 1.00 | (ref.) | 1.00 | (ref.) | 1.00 | (ref.) |
| 0.80-0.84 | 131 | 39 | 188 | 0.81 | 0.56, 1.18 | 0.79 | 0.53, 1.18 | 0.76 | 0.52, 1.11 |
| ≥0.85 | 270 | 101 | 604 | 1.18 | 0.77, 1.80 | 1.19 | 0.75, 1.87 | 1.16 | 0.73, 1.83 |
| Waist-to-height ratio | |||||||||
| ≤0.5 | 217 | 78 | 416 | 1.00 | (ref.) | 1.00 | (ref.) | 1.00 | (ref.) |
| >0.5 | 258 | 89 | 513 | 0.93 | 0.69, 1.26 | 0.92 | 0.66, 1.27 | 0.92 | 0.66, 1.27 |
| A body shape index (m11/6 /kg2/3) | |||||||||
| Q1 (0.064-0.077) | 118 | 38 | 170 | 1.00 | (ref.) | 1.00 | (ref.) | 1.00 | (ref.) |
| Q2 (0.078-0.080) | 119 | 45 | 246 | 0.97 | 0.63, 1.49 | 0.94 | 0.61, 1.45 | 0.94 | 0.61, 1.45 |
| Q3 (0.081-0.084) | 119 | 42 | 279 | 0.91 | 0.59, 1.41 | 0.85 | 0.55, 1.32 | 0.85 | 0.55, 1.32 |
| Q4 (0.085-0.094) | 119 | 42 | 234 | 0.89 | 0.57, 1.38 | 0.90 | 0.58, 1.40 | 0.90 | 0.58, 1.40 |
Data were analyzed using discrete-time proportional hazards models. S-PRESTO, Singapore PREconception Study of long-Term maternal and child Outcomes; FR, fecundability ratio; CI, confidence interval
Crude model
Adjusted for age, ethnicity, education, parity and cycle length
Adjusted for model 2, plus waist-to-hip ratio (in models for body mass index) and body mass index (in all models except waist-to-height ratio and A body shape index models)
Figure 2.
Association between body mass index and fecundability ratio, fitted by restricted cubic spline. Reference level for fecundability ratio is a BMI of 22 kg/m2. The curve is adjusted for age, ethnicity, education, parity, cycle length and waist-to-hip ratio.
Discussion
This prospective cohort study examined how adiposity and fat distribution were associated with fecundability, as measured by TTP among multiethnic Asian women planning a pregnancy. Reduced fecundability was observed among women with higher BMI, SFT and TBF%. The association of high BMI with reduced fecundability was particularly evident among nulliparous women. Central adiposity, as indicated by measures of WC, WHR, WHtR and ABSI, was not associated with fecundability. These findings suggest that increased overall adiposity contributes to delayed conception among Asian women.
Our findings agree with previous studies that have reported reduced fecundability in women with high BMI (Wise et al., 2010; Wise et al., 2013; McKinnon et al., 2016). The finding of effect modification by parity is supported by previous reports (Gesink Law et al., 2007; Wise et al., 2010; Wise et al., 2013) showing a stronger association between high BMI and reduced fecundability among nulliparous women. The literature on fecundability of underweight women has reported mixed findings. Studies reported that underweight women had longer TTP (Hassan and Killick, 2004; Gesink Law et al., 2007), but others have shown no association (Wise et al., 2013; McKinnon et al., 2016). In our study, we could not confirm this association but there was some suggestion of decreased fecundability among underweight women as shown by the effect estimate of FR<1. Other than using BMI as the surrogate measure of body fat, we provide new evidence showing that higher levels of subcutaneous fat and TBF% were associated with longer TTP.
The association between high adiposity and reduced fecundability has biological plausibility. Excess body fat has profound effects on reproductive hormone secretion and metabolism (Talmor et al., 2015; Fontana and Torre, 2016). Increased adiposity may elevate luteinising hormone levels, raise the androgen-to-oestrogen ratio and alter endocrine milieu, which in turn impairs folliculogenesis and follicular atresia (Talmor et al., 2015). An abnormal lipoprotein metabolism, high levels of fat and inflammation in the fluid surrounding oocytes of obese women can also adversely affect oocyte development and quality, as well as uterine receptivity (Talmor et al., 2015; Fontana and Torre, 2016).
Only few studies have investigated the association between central obesity and fecundability, and results are inconsistent across studies (Wise et al., 2010; Wise et al., 2013; McKinnon et al., 2016). A prospective cohort among black women reported that larger WC (≥84 cm) or WHR (≥0.85), independent of BMI, was associated with decreased fecundability (Wise et al., 2013). Another American prospective cohort found that the inverse associations of both WC and WHR with fecundability were attenuated after adjustment for BMI (McKinnon et al., 2016). In contrast, a Danish population-based prospective cohort showed no associations for WC and WHR with fecundability before BMI adjustment, but observed unexpected positive associations after adjustment for BMI (Wise et al., 2010). In this Asian cohort, we applied WHtR and ABSI that take body size into account.We consistently found no evidence of association for WC, WHR, WHtR or ABSI with fecundability. However, whether the present findings differ from other Asian populations should be studied further. It is possible that abdominal adipocytes are not similarly hormonally active across populations.
Conception rates in this study were lower than in other studies of natural conception, where about 50% and 70% women have reported a pregnancy after 6 and 12 cycles of pregnancy attempts, respectively (Wise et al., 2010; McKinnon et al., 2016). This finding may be explained by factors such as lower coital frequency in the local population despite intending to get pregnant, or that we did not exclude women and couples who had subfertlity issues. Most women (63.3%) had tried to conceive before entering the study. Some entered the study after months of unprotected intercourse without achieving a successful pregnancy, suggesting tendency to have had impaired fertility, whether due to themselves or their spouse.
Our results may be imprecise due to a small sample size, but were consistent with those of prior literature reporting on BMI and fecundability. The present analysis was confined to planned pregnancies, which could limit generalizability of findings to non-planned pregnancies, estimated at around 44% in Singapore (Cheng et al., 2016). This may result in bias if pregnancy intention was related to both exposures studied (i.e. weight status and adiposity) and fecundability. We had no information for the date of LMP on a monthly basis to validate the accuracy of reported menstrual cycle length. The assessment of cycles at risk was based on the participants’ recall of cycle length at baseline, which might introduce error for TTP estimation. Measures of exposures and covariates such as cycle length were not performed repeatedly over time; cycle length might have changed during the period before getting pregnant. We also did not collect data on ovulation monitoring, timing and frequency of intercourse, therefore not able to do the adjustment in the analysis. Though timed intercourse may increase conception rates, ovulation prediction is challenging and has been associated with stress (Pfeifer et al., 2017). In addition, we had no information on paternal age and BMI. It has been suggested that by controlling maternal age, which is strongly correlated with their partner’s age (Mutsaerts et al., 2012), it should be able to reduce the extent of confounding (Wise et al., 2013). Increasing paternal BMI has been shown to negatively affect fertility (Liu and Ding, 2017). Lack of controlling for this variable might potentially overestimate the association between maternal BMI and fecundability in this study. Nonetheless, several studies have indicated no associations between paternal BMI and fecundability (Wise et al., 2010; Mutsaerts et al., 2011). The proportion of women who had been excluded tended to have lower educational level and irregular cycle. We controlled for education but not cycle regularity in the analysis as it did not substantially change the association between anthropometry and TTP.
The main strength of this study was the prospective study design, where the anthropometric and covariate data were collected before the occurrence of pregnancy, thereby reducing potential for differential exposure misclassification. Detailed measurements of anthropometry and body composition especially air displacement plethysmography (BOD POD) were performed in this study, which enabled us to have a more comprehensive examination of body fat with fecundability. Most women were enrolled at the beginning of their attempts to conceive, with 65% of women having less than 3 months of attempt time at entry into the study. This helps to reduce potential reverse causality, i.e. weight loss effort made by women who had more pregnancy attempts at study entry, to improving their fertility as risk factor. However, it has been reported that BMI tends to remain stable within a 6-month interval (McKinnon et al., 2016), as supported by previous studies showing consistent findings between weight status and fecundability across categories (≤2 and 3-6 cycles) of attempt time at study entry (Wise et al., 2010; McKinnon et al., 2016).
In conclusion, in this Asian prospective cohort, we found an association between increased overall adiposity and delayed TTP among women, particularly evident for nulliparous women. This finding is especially relevant in Singapore where the population is facing an obesity epidemic (Phan et al., 2014) and fertility problems (Singapore Department of Statistics, 2017). Attaining optimal weight or achieving meaningful weight loss/fat loss preconceptually may be a targeted intervention to improve female fertility (Talmor et al., 2015). This represents one of the great challenges that requires persistent and sustainable efforts to be taken up at population and individual levels.
Supplementary Material
Acknowledgements
We thank the staff and participants of the S-PRESTO study. The S-PRESTO study group includes Anne Eng Neo Goh, Anne Rifkin-Graboi, AnqiQiu, Bee Wah Lee, Bobby Cheon, Christiani Jeyakumar Henry, Ciaran Gerard Forde, Doris Fok, Elaine Quah, Elizabeth Tham, Evelyn Chung Ning Law, Evelyn Xiu Ling Loo, Faidon Magkos, Falk Mueller-Riemenschneider, George Seow Heong Yeo, Helen Yu Chen, Hugo P S van Bever, Joanne Yoong, Joao N. Ferreira., Jonathan Tze Liang Choo, Kenneth Kwek, Kuan Jin Lee, Lieng Hsi Ling, Ling Wei Chen, Lourdes Mary Daniel, Marielle V. Fortier, Mary Foong-Fong Chong, Mei Chien Chua, Melvin Leow, Michael Meaney, Neerja Karnani, Oon Hoe Teoh, Peter D Gluckman, Queenie Ling Jun Li, Sendhil Velan, Seng Bin Ang, Shephali Tagore, Shirong Cai, Sok Bee Lim, Stella Tsotsi, Stephen Chin-Ying Hsu, Sue Anne Toh, Teng Hong Tan, Tong Wei Yew, Victor Samuel Rajadurai, Wee Meng Han, Wei Wei Pang, Yiong Huak Chan.
Funding
This research is supported by the Singapore National Research Foundation (NRF) under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore-NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), Singapore. Y.B.C. is supported by the Singapore NRF under its Clinician Scientist Award administered by the Singapore Ministry of Health’s NMRC (NMRC/CSA/0039/2012). K.M.G. is supported by the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre and the European Union's Seventh Framework Programme (FP7/2007-2013), project EarlyNutrition (n°289346). N.L. is supported by the NMRC Transition Award (NMRC/TA/0037/2015). J.K.Y.C. is supported by the NMRC Clinician Scientist Award (NMRC/CSA(SI)/008/2016).
Footnotes
Authors’ roles
YSC, KMG, LPCS, SYC and JKYC designed and led the S-PRESTO cohort study. SLL and JKYC designed the present study. SLL, SES, SN, MTT, IMA and JYB contributed to data management and cleaning. YBC advised on the statistical analysis. SLL performed data analysis. SLL, YBC, SES, SN, MTT, IMA, JYB, KMG, KHT, YSL, HHT, BSMC, NL, FY, SYC, CC and JKYC interpreted the findings and revised drafts of the paper. SLL wrote the paper. All authors read and approved the final manuscript.
Conflict of interest
Y.S.C., K.M.G., F.Y. and Y.S.L. have received reimbursement to speak at conferences sponsored by companies selling nutritional products. Y.S.C., K.M.G. and S.Y.C. are part of an academic consortium that has received research funding from Abbott, Nutrition, Nestle and Danone. Other authors declared no conflicts of interest.
References
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Study (NHANES): Anthropometry Procedures Manual. 2007.
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey III. Body Measurements (Anthropometry) Westat, Inc.; Rockville, US: 1988. pp. 3–16. [Google Scholar]
- Cheng TS, Loy SL, Cheung YB, Godfrey KM, Gluckman PD, Kwek K, Saw SM, Chong YS, Lee YS, Yap F, et al. Demographic Characteristics, Health Behaviors Before and During Pregnancy, and Pregnancy and Birth Outcomes in Mothers with Different Pregnancy Planning Status. Prev Sci. 2016;17:960–969. doi: 10.1007/s11121-016-0694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MJ. Evidence for effects of weight on reproduction in women. Reprod Biomed Online. 2006;12:552–561. doi: 10.1016/s1472-6483(10)61180-7. [DOI] [PubMed] [Google Scholar]
- De Pergola G, Tartagni M, d'Angelo F, Centoducati C, Guida P, Giorgino R. Abdominal fat accumulation, and not insulin resistance, is associated to oligomenorrhea in non-hyperandrogenic overweight/obese women. J Endocrinol Invest. 2009;32:98–101. doi: 10.1007/BF03345694. [DOI] [PubMed] [Google Scholar]
- Singapore Department of Statistics. Population trends: Fertility. Singapore: Department of Statistics, Ministry of Trade & Industry; 2017. [Google Scholar]
- Diamanti-Kandarakis E, Bergiele A. The influence of obesity on hyperandrogenism and infertility in the female. Obes Rev. 2001;2:231–238. doi: 10.1046/j.1467-789x.2001.00041.x. [DOI] [PubMed] [Google Scholar]
- Fontana R, Della Torre S. The Deep Correlation between Energy Metabolism and Reproduction: A View on the Effects of Nutrition for Women Fertility. Nutrients. 2016;8:87. doi: 10.3390/nu8020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22:414–420. doi: 10.1093/humrep/del400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MA, Killick SR. Negative lifestyle is associated with a significant reduction in fecundity. Fertil Steril. 2004;81:384–392. doi: 10.1016/j.fertnstert.2003.06.027. [DOI] [PubMed] [Google Scholar]
- IPAQ Research Committee. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ) 2005.
- Jenkins SP. Survival Analysis. Institute of Social and Economic Research, University of Essex; Colchester, UK: Discrete time multivariate models; pp. 85–86. [Google Scholar]
- Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One. 2012;7:e39504. doi: 10.1371/journal.pone.0039504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WC, Chen IC, Chang YC, Loke SS, Wang SH, Hsiao KY. Waist-to-height ratio, waist circumference, and body mass index as indices of cardiometabolic risk among 36,642 Taiwanese adults. Eur J Nutr. 2013;52:57–65. doi: 10.1007/s00394-011-0286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ding Z. Obesity, a serious etiologic factor for male subfertility in modern society. Reproduction. 2017;154:R123–R131. doi: 10.1530/REP-17-0161. [DOI] [PubMed] [Google Scholar]
- McKinnon CJ, Hatch EE, Rothman KJ, Mikkelsen EM, Wesselink AK, Hahn KA, Wise LA. Body mass index, physical activity and fecundability in a North American preconception cohort study. FertilSteril. 2016;106:451–459. doi: 10.1016/j.fertnstert.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Mutsaerts MA, Groen H, Huiting HG, Kuchenbecker WK, Sauer PJ, Land JA, Stolk RP, Hoek A. The influence of maternal and paternal factors on time to pregnancy--a Dutch population-based birth-cohort study: the GECKO Drenthe study. Hum Reprod. 2012;27:583–593. doi: 10.1093/humrep/der429. [DOI] [PubMed] [Google Scholar]
- NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer S, Butts S, Fossum G, Gracia C, La Barbera A, Mersereau J, Odem R, Paulson R, Penzias A, Pisarska M, et al. Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. FertilSteril. 2017;107:52–58. [Google Scholar]
- Phan TP, Alkema L, Tai ES, Tan KH, Yang Q, Lim WY, Teo YY, Cheng CY, Wang X, Wong TY, et al. Forecasting the burden of type 2 diabetes in Singapore using a demographic epidemiological model of Singapore. BMJ Open Diabetes Res Care. 2014;2:e000012. doi: 10.1136/bmjdrc-2013-000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmor A, Dunphy B. Female obesity and infertility. Best Pract Res Clin Obstet Gynaecol. 2015;29:498–506. doi: 10.1016/j.bpobgyn.2014.10.014. [DOI] [PubMed] [Google Scholar]
- United Nations. World Fertility Patterns 2015 - Data Booklet (ST/ESA/SER.A/370) Department of Economic and Social Affairs, Population Division; 2015. [Google Scholar]
- Vikram NK, Latifi AN, Misra A, Luthra K, Bhatt SP, Guleria R, Pandey RM. Waist-to-Height Ratio Compared to Standard Obesity Measures as Predictor of Cardiometabolic Risk Factors in Asian Indians in North India. Metab Syndr Relat Disord. 2016;14:492–499. doi: 10.1089/met.2016.0041. [DOI] [PubMed] [Google Scholar]
- Wang J, Thornton JC, Russell M, Burastero S, Heymsfield S, Pierson RN., Jr Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. Am J ClinNutr. 1994;60:23–28. doi: 10.1093/ajcn/60.1.23. [DOI] [PubMed] [Google Scholar]
- Weinberg CR, Wilcox AJ, Baird DD. Reduced fecundability in women with prenatal exposure to cigarette smoking. Am J Epidemiol. 1989;129:1072–1078. doi: 10.1093/oxfordjournals.aje.a115211. [DOI] [PubMed] [Google Scholar]
- Wise LA, Palmer JR, Rosenberg L. Body size and time-to-pregnancy in black women. Hum Reprod. 2013;28:2856–2864. doi: 10.1093/humrep/det333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LA, Rothman KJ, Mikkelsen EM, Sorensen HT, Riis A, Hatch EE. An internet-based prospective study of body size and time-to-pregnancy. Hum Reprod. 2010;25:253–264. doi: 10.1093/humrep/dep360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Waist circumference and waist-hip ratio: Report of a WHO Expert Consultation. Geneva: Department of Nutrition for Health and Development, World Health Organization; 2008. [Google Scholar]
- Zhang Q, Wang YY, Zhang Y, Zhang HG, Yang Y, He Y, Xu JH, Zhao J, Peng ZQ, Ma X. The influence of age at menarche, menstrual cycle length and bleeding duration on time to pregnancy: a large prospective cohort study among rural Chinese women. BJOG. 2017;124:1654–1662. doi: 10.1111/1471-0528.14469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.