Abstract
Adverse systemic effect caused by betel nut had been reported for decades. Our aim was to determine whether betel nut had detrimental impact on the development of colorectal polyps in general population. Participants who attended health examinations at the Tri-Service General Hospital (TSGH) from 2010 to 2016 were included in the study. The habit of betel nut chewing was obtained from a self-reported questionnaire. Colorectal polyps were diagnosed by colonoscopies operated by experienced physicians. A logistic regression model was used for the association between betel nut chewing with the presence of colorectal polyps. After adjustment for pertinent information such as age, gender, biochemistry data and personal history, the odd ratios (ORs) of colorectal polyps among betel nut chewers was 1.49 (95%CI: 1.14–1.94). Besides, betel nut chewers in the higher percentage body fat (PBF) group had higher risk for developing colorectal polyps with ORs of 2.07 (95%CI:1.23–3.47). Subjects with habit of betel nut chewing were associated with an increased risk of colorectal polyps in Taiwanese general population. Screening for betel nut chewing history and encouraging cessation might offer improved quality of life. A further research for this association was warranted.
Introduction
Betel nut is regarded as one of the frequently used addictive substance worldwide and betel nut chewers were prevalent in Asian countries more than 10% world’s population[1, 2]. Traditionally, people often chew betel nuts in combination with additional elements such as P. betle and lime in Taiwan[3]. Arecal alkaloid, which is the main components of betel nut, is absorbed in human body during chewing[2]. Arecoline, arecaidine, guvacoline and guvacine were the main alkaloids of betel nut and caused several systemic effects including nervous, cardiovascular, gastrointestinal and endocrine system[4–7]. Growing evidence had already proposed that these components contributed to various systemic diseases including obesity, diabetes mellitus, and metabolic syndrome[8–10]. Additionally, betel nut chewing could increase the risk of cardiovascular diseases and development of oral, esophagus and hepatocellular carcinoma[11–13].
In Taiwan, the incidence rates of colon and rectum cancer were 9299/106 and 5559/106, respectively in 2012[14]. According to the statistical data of Taiwan Health Promotion Administration of the Ministry of Health and Welfare in 2016[15], the mortality cases of colorectal cancer were 5772 and the mortality rates were 14.6/106. The prevalence of colorectal polyps in asymptomatic subjects was 27.4%[16]. Accumulated literatures indicated that most colorectal cancers were originated from a precursor benign polyp[17]. Race, gender, obesity and metabolic syndrome were common factors that increased risk of developing colorectal polyps[18, 19]. Besides, lifestyle including cigarette smoking, alcoholic consumption, and lack of physical activity were also reported to accelerate the neoplastic process[20–22].
To date, the association of consumption of betel nut and colorectal polyps had not been reported in previous study. The main goal of our study was to determine the effect of betel nut on the progress of colorectal polyps by using a large population-based cross-sectional analysis in a general population in Taiwan.
Methods
Study design and participants selection
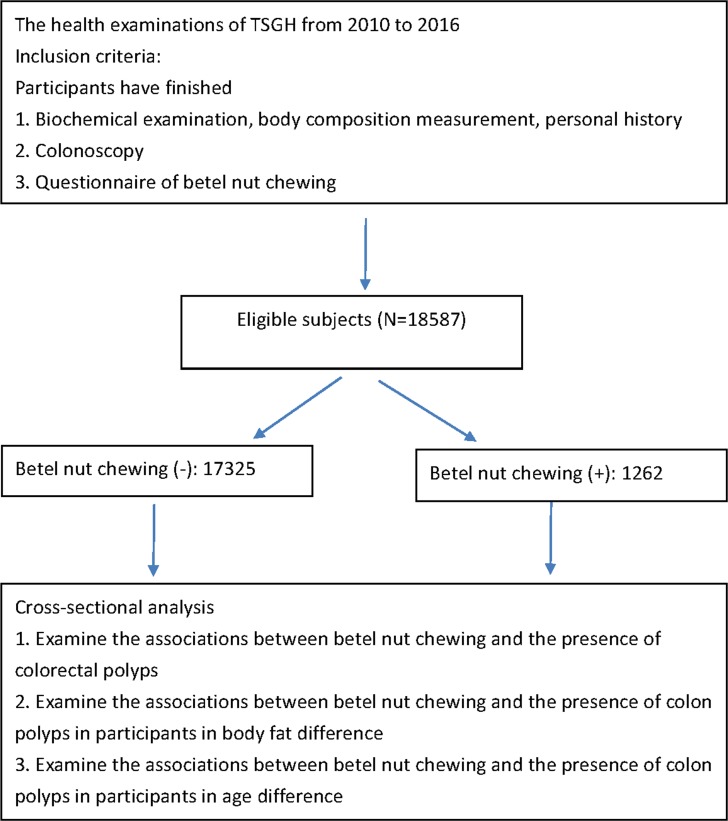
The health examinations at Tri-Service General Hospital (TSGH) were consisted of comprehensive medical records including laboratory biochemistry data, body composition measurement and self-reported personal history. 69226 participants aged more than 20 years underwent health examinations from 2010 to 2016. All protocol was approved by the institutional review board of the Tri-Service General Hospital (TSGH) with patient written consent given. The study was conducted according to the Helsinki Declaration. The TSGH Institutional Review Board waived the need to obtain individual informed consent because the data were analyzed anonymously. Based on the inclusion criteria presented in Fig 1, participants who finished biochemical examination, body composition measurement, colonoscopy, and questionnaire of betel nut chewing were included. 18587 eligible individuals with and without habits of betel nut chewing were analyzed in the next step. First, we explored whether betel nut chewing was associated with the risk of developing colorectal polyps. Next, we investigated the relationship between betel-chewing behavior and the presence of colorectal polyps in body fat and age difference.
Fig 1. Flow chart which represented the steps of analysis performed in the study.
Diagnosis of colorectal polyps
Colonoscopic examinations were operated by trained physicians. Digital of rectum before the endoscope was the routine in the examination. Participants took a laxative on the night before the examination and in the morning of the day for colonoscopy. All visualized lesions were biopsied and histologically assessed by experienced pathologists. For the purpose of our analysis, participants were categorized into two groups according to the presence of colorectal polyps.
Measurement of body composition
Percentage body fat (PBF) is measured by bioelectrical impedance analysis (BIA) (InBody720, Biospace, Inc., Cerritos, CA, USA), a useful method for assessing body composition[23]. The procedure of BIA is simple and noninvasive, and the results were reproducible and rapidly obtained.
Covariates measurement
The pertinent information of study sample is collected by a self-report questionnaire consisted of age, sex, history of cigarette smoking and alcoholic consumption. Body mass index (BMI) is defined as the body weight divided by the square of the body height (kg/m2) of a participant. Latex nephelometry is used for analyzed the concentrations of highly sensitive C-reactive protein (hsCRP). Serum low-density lipoprotein cholesterol (LDL-C) is quantified by an enzymatic colorimetric method.
Statistical analysis
Multivariable models were adjusted as follows: Model 1 was adjusted as for age and gender. Model 2 was adjusted as for model 1 plus proteinuria, LDL-C, uric acid (UA), aspartate aminotransferase (AST), creatinine (Cr), hsCRP. Model 3 was adjusted as for Model 2 plus history of smoking and alcoholic drinking. All statistical analyses in the present study were analyzed by the SPSS Statistics software package (version 18.0, SPSS Inc., Chicago, IL, USA) for Windows. A two-sided P-value of < 0.05 was considered statistically significant. Multiple logistic regression was performed for the association of betel-chewing behavior on the presence of colorectal polyps.
Results
Characteristics of study sample
All characteristics of the study sample were listed in Table 1. The mean age of the non-users and betel nut chewers was 46.04±13.11 and 46.89±11.54 years old. Betel nut chewers had significantly higher BMI, LDL-C, UA, Cr, AST, albumin, and hsCRP than non-users.
Table 1. Characteristics of study sample with or without betel nut chewing.
| Variables | Betel nut chewing (-) (N = 17325) |
Betel nut chewing (+) (N = 1262) |
P Value |
|---|---|---|---|
| Continuous Variables, mean (SD) | |||
| Age (years) | 46.04 (13.11) | 46.89 (11.54) | <0.05 |
| BMI (kg/m2) | 24.02 (3.72) | 25.97 (3.62) | <0.01 |
| LDL-C (mg/dL) | 117.44 (31.86) | 119.83 (35.16) | <0.05 |
| Uric acid (mg/dL) | 5.75 (1.49) | 6.61 (1.52) | <0.01 |
| Creatinine (mg/dL) | 0.84 (0.31) | 0.96 (0.26) | <0.01 |
| AST (U/L) | 21.05 (11.66) | 23.07 (11.88) | <0.01 |
| Albumin (g/dL) | 4.46 (0.28) | 4.49 (0.28) | <0.01 |
| hsCRP (mg/dL) | 0.22 (0.46) | 0.32 (0.68) | <0.01 |
| TSH (IU/mL) | 2.25 (1.62) | 2.18 (2.19) | 0.18 |
| Category Variables, (%) | |||
| Gender (male) | 9002 (58.2) | 824 (98.3) | <0.01 |
| Proteinuria | 4297 (27.3) | 311 (34.7) | <0.01 |
| Smoking | 4499 (25.6) | 877 (89.9) | <0.01 |
| Drinking | 7921 (45.1) | 827 (85.5) | <0.01 |
BMI, body mass index; LDL-C, low density lipoprotein cholesterol; UA, uric acid; Cr, creatinine; AST, aspartate aminotransferase; hsCRP, high sensitive C-reactive protein; TSH, thyroid stimulating hormone.
Betel-chewing behavior and colorectal polyps
As shown in Table 2, the univariate logistic regression model revealed the odds ratios (ORs) of each covariable for the presence of colorectal polyps. The ORs of betel nut chewing were 2.39 (confidence interval (CI): 1.97–2.89). All covariables were significantly associated with the presence of colorectal polyps, except albumin.
Table 2. Univariate regression analyses for the presence of colorectal polyps.
| Variables | OR (95%CI) | P-value |
|---|---|---|
| Continuous Variables | ||
| Age (years) | 1.06 (1.05–1.06) | <0.01 |
| BMI (kg/m2) | 1.07 (1.05–1.08) | <0.01 |
| PBF | 1.02 (1.01–1.02) | <0.01 |
| LDL-C (mg/dL) | 1.01 (1.01–1.01) | <0.01 |
| Uric acid (mg/dL) | 1.11 (1.07–1.15) | <0.01 |
| Creatinine (mg/dL) | 1.27 (1.12–1.44) | <0.01 |
| AST (U/L) | 1.01 (1.01–1.02) | <0.01 |
| Albumin (g/dL) | 0.92 (0.75–1.13) | 0.44 |
| hsCRP (mg/dL) | 1.16 (1.05–1.29) | <0.01 |
| Category Variables | ||
| Betel nut chewing | 2.39 (1.97–2.89) | <0.01 |
| Gender | 1.76 (1.53–2.02) | <0.01 |
| Proteinuria | 1.48 (1.30–1.67) | <0.01 |
| Smoking | 2.59 (2.31–2.90) | <0.01 |
| Drinking | 1.48 (1.32–1.66) | <0.01 |
The association of betel nut chewing on the presence of colorectal polyps was analyzed by a multiple logistic regression model shown in Table 3. The ORs in the Model 1 for colorectal polyps among subjects with betel nut chewing was 2.11 (95% CI:1.63–2.72). Slight attenuate in this relationship was noted but remained significant after adjustment for biochemical data and personal history in Model 2 and Model 3 with ORs of 2.00 (95%CI: 1.55–2.59) and 1.49 (95%CI: 1.14–1.94), respectively.
Table 3. Multivariate regression analyses for association between betel nut chewing and the presence of colon polyps.
| Models | Model a 1 OR (95% CI) |
P Value |
bR2 | Model a 2 OR (95% CI) |
P Value |
bR2 | Model a 3 OR (95% CI) |
P Value |
bR2 |
|---|---|---|---|---|---|---|---|---|---|
| Variables | Colorectal polyps | ||||||||
|
Betel nut chewing (N = 1262) |
2.11 (1.63–2.72) | <0.01 | 0.11 | 2.00 (1.55–2.59) | <0.01 | 0.13 | 1.49 (1.14–1.94) | <0.01 | 0.15 |
a Adjusted covariates:
Model 1 = age + gender
Model 2 = Model 1 + proteinuria, LDL-C, UA, Cr AST, hsCRP
Model 3 = Model 2 + history of smoking, drinking
b Nagelkerke R squared
Betel nut chewing and colorectal polyps in different body fat percentage
In Table 4, we categorized betel nut chewers as PBF tertiles. After fully adjusting for pertinent covariables, betel nut chewing was significantly associated with the presence of colorectal polyps in T1 and T3 with ORs of 1.58 (95%CI: 1.00–2.49) and 2.07 (95%CI: 1.23–3.47), respectively. However, no significant association between T2 and the presence of colorectal polyps in the fully-adjusted model.
Table 4. Association between betel nut chewing and the presence of colorectal polyps categorized by the tertiles of PBF.
| Models | PBF Group |
Model a 1 OR (95% CI) |
P Value |
bR2 | Model a 2 OR (95% CI) |
P Value |
bR2 | Model a 3 OR (95% CI) |
P Value |
bR2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Colorectal polyps | |||||||||
| Betel nut chewing | Tertile 1 | 2.21 (1.43–3.42) | <0.01 | 0.09 | 2.21 (1.42–3.43) | <0.01 | 0.11 | 1.58 (1.00–2.49) | <0.05 | 0.13 |
| Tertile 2 | 1.29 (0.83–1.99) | 0.26 | 0.10 | 1.25 (0.80–1.94) | 0.33 | 0.12 | 0.95 (0.60–1.50) | 0.83 | 0.13 | |
| Tertile 3 | 2.56 (1.55–4.21) | <0.01 | 0.10 | 2.51 (1.52–4.14) | <0.01 | 0.10 | 2.07 (1.23–3.47) | <0.01 | 0.11 | |
a Adjusted covariates:
Model 1 = age + gender
Model 2 = Model 1 + proteinuria, LDL-C, UA, Cr AST, hsCRP
Model 3 = Model 2 + history of smoking, drinking
b Nagelkerke R squared.
Different age groups in association between betel nut chewing and the presence of colorectal polyps
As shown in Table 5, we categorized betel nut chewers as different age groups (20–29 years, 30–39 years, 40–49 years, 50–59 years, and ≧60 years). After fully adjusting for covariables, betel nut chewing was significantly associated with the presence of colorectal polyps in 40–49 and ≧60 age groups with ORs of 1.77 (95%CI: 1.06–2.95) and 1.84 (95%CI: 1.00–3.38), respectively.
Table 5. Different age groups in association between betel nut chewing and the presence of colon polyps.
| Models | Age Group |
Model a 1 OR (95% CI) |
P Value |
bR2 | Model a 2 OR (95% CI) |
P Value |
bR2 | Model a 3 OR (95% CI) |
P Value |
bR2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Colorectal polyps | |||||||||
| Betel nut chewing | 20–29 | Reference | - | 0.10 | Reference | - | 0.34 | Reference | - | 0.36 |
| 30–39 | 2.97 (1.41–6.23) | <0.01 | 0.05 | 2.61 (1.21–5.62) | <0.05 | 0.09 | 1.99 (0.89–4.45) | 0.09 | 0.10 | |
| 40–49 | 2.66 (1.65–4.30) | <0.01 | 0.05 | 2.49 (1.52–4.088) | <0.01 | 0.08 | 1.77 (1.06–2.95) | <0.05 | 0.10 | |
| 50–59 | 1.40 (0.93–2.11) | 0.11 | 0.05 | 1.33 (0.88–2.01) | 0.18 | 0.07 | 1.06 (0.69–1.62) | 0.79 | 0.09 | |
| ≧60 | 2.27 (1.27–4.04) | <0.01 | 0.06 | 2.31 (1.28–4.17) | <0.01 | 0.08 | 1.84 (1.00–3.38) | <0.05 | 0.09 | |
a Adjusted covariates:
Model 1 = age + gender
Model 2 = Model 1 + proteinuria, LDL-C, UA, Cr AST, hsCRP
Model 3 = Model 2 + history of smoking, drinking
b Nagelkerke R squared
Discussion
Our findings highlighted the detrimental association between betel nut chewing and colorectal polyps in a cross-sectional analysis. Interestingly, betel nut chewers with increased PBF were positively associated with the risk of colorectal polyps, but those with reduced PBF were not. To the best of our knowledge, our study was the first to examine the impact of betel nut chewing on the process of colorectal polyps and observed the opposite findings in difference of body fat distribution.
To date, no published literature had addressed the relationship between betel nut chewing and colorectal polyps. There were several factors that increase risk of colorectal polyps including race, gender, smoking, and obesity[18]. The key factor jointed the obesity and the development of colorectal polyps was insulin resistance[24]. It contributed to several metabolic abnormalities such as excess glucose levels and dyslipidemia leading to alterations in cell signaling and oxidative stress[25]. Trabulo et al. had demonstrated that metabolic syndrome was significantly associated with the development of colorectal adenomas and cancer[26]. A meta-analysis had supported the harmful impact of metabolic syndrome in process of colorectal neoplasms[19]. Arecoline, arecaidine, guvacoline and guvacine were the main alkaloids of betel nut and caused several systemic effects including nervous, cardiovascular, gastrointestinal and endocrine system[4–7]. The metabolism of arecoline was hydrolysis to arecaidine and N-oxidation combined with double bond reduction of the arecaidine[27]. Chung et al. had reported that chronic betel nut chewing was an important factor for developing metabolic syndrome[28]. In a population-based prospective study, betel nut chewing was significant associated with metabolic syndrome and it had increased risks of metabolic syndrome component such as central obesity, hypertension, hyperglycemia and dyslipidemia[10]. Taking above researches together, metabolic dysfunction might be a potential mechanism between betel nut chewing and the presence of colorectal polyps, which was the main finding in our study.
Although the underlying mechanism of how betel nut causing metabolic syndrome remained unclear, various possible pathways had been suggested earlier. Betel nut chewing was considered to be related to increased levels of prostanoids, interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α)[29]. These inflammatory mediators induced a state of low-grade chronic inflammation and were associated with insulin resistance and metabolic alternation[30, 31]. Hsu et al. had indicated that arecoline led to the development of insulin resistance and metabolic diseases by inhibiting adipogenic differentiation such as inducing adenylyl cyclase-dependent lipolysis and interfering insulin-induced glucose uptake[32]. One of the potential mechanisms of the interaction between metabolic syndrome with colonic neoplasms was oxidative stress. DNA damage caused by increased reactive oxygen species was found in those with metabolic syndrome and thus placed subjects at risk for neoplasm process[33]. Another plausible mechanisms was dysregulation of cytokines and growth signals including insulin growth factor-1 (IGF-1), insulin and adipokines, which might contribute to cancer-related process[34–36]. Inflammatory cytokines such as IL-6 and TNF-α were responsible to activation of signal transducers and activators of transcription factors[35, 37]. IGF-1 and hyperinsulinemia were reported to induce PI3K/Akt activity that regulated downstream targets.[38]. All these molecules were responsible for cell proliferation, tumor growth, and changes from normal colonic mucosa to adenoma and adenocarcinoma[39].
Obesity was considered to have adverse impact on the development of colorectal neoplasms and visceral obesity was associated with higher risk for colorectal cancer than BMI[40, 41]. Accumulation of visceral adipose tissue was associated with insulin resistance, increased circulating levels of leptin and reduced adipokine[42]. Epidemiological and clinical researches had indicated that altered concentrations of leptin and adipokine might lead to the presence of colorectal adenoma and carcinoma[43, 44]. Above studies could support our finding that betel nut chewers in the higher PBF group had increased risk of developing colorectal polyps.
Despite the strength of a large population-based design, our study still had some potential limitations. First, casual inference between betel nut chewing and colorectal polyps was no assessible in the present study because it was a cross-sectional design. A longitudinal survey was suggested to be examined in further studies. Second, the health examinations only recorded the personal history of betel nut chewing that were lacking detailed information about how many betel nut uses per day and the duration of usage. Thus, the cumulative exposure of betel nut could not be accessed in the current study. Next, study sample was composed of healthy general population who underwent health examinations but not from nationally representative individuals. External validation was necessary in further studies. Last, all results of colonoscopic examinations in the present study were obtained from health examinations at the Health Management Center of TSGH. The different types of colorectal polyps are not available in our dataset.
Conclusion
While the systemic effect of betel nut chewing had been known for decades, detrimental impact on development of colorectal polyps was first reported among a general population in Taiwan of our study. Screening personal history of betel nut chewing in clinical practices and encouraging cessation might offer an improved quality of life for individuals who were at risk of systemic diseases by betel nut use. Furthermore, attention on this risk factor through public health programs and further investigation in association between betel nut chewing and colorectal diseases were necessary.
Data Availability
The data set is owned by the Institutional Review Board (IRB) of Tri-Service General Hospital (TSGH). TSGH IRB only approved the data analysis in our study and did not approve data sharing. Therefore, we do not have permission to share the data set. Interested researchers can submit data access requests to the Tri-Service General Hospital IRB using the following email address: tsghirb@ndmctsgh.edu.tw. Others would be able to access these data in the same manner as the authors and the authors also did not have any special access privileges.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Gupta PC, Warnakulasuriya S. Global epidemiology of areca nut usage. Addiction biology. 2002;7(1):77–83. 10.1080/13556210020091437 . [DOI] [PubMed] [Google Scholar]
- 2.Boucher BJ. Paan without tobacco: an independent risk factor for oral cancer. International journal of cancer. 2001;91(4):592–3. . [DOI] [PubMed] [Google Scholar]
- 3.Lan TY, Chang WC, Tsai YJ, Chuang YL, Lin HS, Tai TY. Areca nut chewing and mortality in an elderly cohort study. American journal of epidemiology. 2007;165(6):677–83. 10.1093/aje/kwk056 . [DOI] [PubMed] [Google Scholar]
- 4.Chu NS. Neurological aspects of areca and betel chewing. Addiction biology. 2002;7(1):111–4. 10.1080/13556210120091473 . [DOI] [PubMed] [Google Scholar]
- 5.Park YB, Jeon SM, Byun SJ, Kim HS, Choi MS. Absorption of intestinal free cholesterol is lowered by supplementation of Areca catechu L. extract in rats. Life sciences. 2002;70(16):1849–59. . [DOI] [PubMed] [Google Scholar]
- 6.Boucher BJ, Mannan N. Metabolic effects of the consumption of Areca catechu. Addiction biology. 2002;7(1):103–10. 10.1080/13556210120091464 . [DOI] [PubMed] [Google Scholar]
- 7.Dasgupta R, Chatterji U, Nag TC, Chaudhuri-Sengupta S, Nag D, Maiti BR. Ultrastructural and hormonal modulations of the thyroid gland following arecoline treatment in albino mice. Molecular and cellular endocrinology. 2010;319(1–2):1–7. 10.1016/j.mce.2010.01.005 . [DOI] [PubMed] [Google Scholar]
- 8.Javed F, Bello Correra FO, Chotai M, Tappuni AR, Almas K. Systemic conditions associated with areca nut usage: a literature review. Scandinavian journal of public health. 2010;38(8):838–44. 10.1177/1403494810379291 . [DOI] [PubMed] [Google Scholar]
- 9.Benjamin AL. Community screening for diabetes in the National Capital District, Papua New Guinea: is betelnut chewing a risk factor for diabetes? Papua and New Guinea medical journal. 2001;44(3–4):101–7. . [PubMed] [Google Scholar]
- 10.Shafique K, Zafar M, Ahmed Z, Khan NA, Mughal MA, Imtiaz F. Areca nut chewing and metabolic syndrome: evidence of a harmful relationship. Nutrition Journal. 2013;12:67–. 10.1186/1475-2891-12-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin W-Y, Chiu T-Y, Lee L-T, Lin C-C, Huang C-Y, Huang K-C. Betel nut chewing is associated with increased risk of cardiovascular disease and all-cause mortality in Taiwanese men. The American journal of clinical nutrition. 2008;87(5):1204–11. 10.1093/ajcn/87.5.1204 [DOI] [PubMed] [Google Scholar]
- 12.Wollina U, Verma S, Parikh D, Parikh A. [Oral and extraoral disease due to betel nut chewing]. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete. 2002;53(12):795–7. 10.1007/s00105-002-0413-1 . [DOI] [PubMed] [Google Scholar]
- 13.Hsiao TJ, Liao HW, Hsieh PS, Wong RH. Risk of betel quid chewing on the development of liver cirrhosis: a community-based case-control study. Annals of epidemiology. 2007;17(6):479–85. 10.1016/j.annepidem.2006.12.007 . [DOI] [PubMed] [Google Scholar]
- 14.Chiang CJ, Lo WC, Yang YW, You SL, Chen CJ, Lai MS. Incidence and survival of adult cancer patients in Taiwan, 2002–2012. Journal of the Formosan Medical Association = Taiwan yi zhi. 2016;115(12):1076–88. 10.1016/j.jfma.2015.10.011 . [DOI] [PubMed] [Google Scholar]
- 15.Health. NDo. Prevention of colorectal cancer. Available from: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=615&pid=1126.
- 16.Wang F-W, Hsu P-I, Chuang H-Y, Tu M-S, Mar G-Y, King T-M, et al. Prevalence and Risk Factors of Asymptomatic Colorectal Polyps in Taiwan. Gastroenterology Research and Practice. 2014;2014:985205 10.1155/2014/985205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez ME, Baron JA, Lieberman DA, Schatzkin A, Lanza E, Winawer SJ, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136(3):832–41. 10.1053/j.gastro.2008.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grahn SW, Varma MG. Factors that Increase Risk of Colon Polyps. Clinics in Colon and Rectal Surgery. 2008;21(4):247–55. 10.1055/s-0028-1089939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jinjuvadia R, Lohia P, Jinjuvadia C, Montoya S, Liangpunsakul S. The Association between Metabolic Syndrome and Colorectal Neoplasm: Systemic review and Meta-analysis. Journal of clinical gastroenterology. 2013;47(1):33–44. 10.1097/MCG.0b013e3182688c15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shrubsole MJ, Wu H, Ness RM, Shyr Y, Smalley WE, Zheng W. Alcohol drinking, cigarette smoking, and risk of colorectal adenomatous and hyperplastic polyps. American journal of epidemiology. 2008;167(9):1050–8. 10.1093/aje/kwm400 . [DOI] [PubMed] [Google Scholar]
- 21.Fu Z, Shrubsole MJ, Smalley WE, Wu H, Chen Z, Shyr Y, et al. Lifestyle factors and their combined impact on the risk of colorectal polyps. American journal of epidemiology. 2012;176(9):766–76. 10.1093/aje/kws157 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burnett-Hartman AN, Newcomb PA, Mandelson MT, Adams SV, Wernli KJ, Shadman M, et al. Colorectal polyp type and the association with charred meat consumption, smoking, and microsomal epoxide hydrolase polymorphisms. Nutrition and cancer. 2011;63(4):583–92. 10.1080/01635581.2011.553021 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sergi G, De Rui M, Stubbs B, Veronese N, Manzato E. Measurement of lean body mass using bioelectrical impedance analysis: a consideration of the pros and cons. Aging clinical and experimental research. 2017;29(4):591–7. 10.1007/s40520-016-0622-6 . [DOI] [PubMed] [Google Scholar]
- 24.Tripkovic I, Tripkovic A, Ivanisevic Z, Capkun V, Zekan L. Insulin increase in colon cancerogenesis: a case-control study. Archives of medical research. 2004;35(3):215–9. 10.1016/j.arcmed.2003.12.003 . [DOI] [PubMed] [Google Scholar]
- 25.Gunter MJ, Leitzmann MF. Obesity and colorectal cancer: epidemiology, mechanisms and candidate genes. The Journal of nutritional biochemistry. 2006;17(3):145–56. 10.1016/j.jnutbio.2005.06.011 . [DOI] [PubMed] [Google Scholar]
- 26.Trabulo D, Ribeiro S, Martins C, Teixeira C, Cardoso C, Mangualde J, et al. Metabolic syndrome and colorectal neoplasms: An ominous association. World Journal of Gastroenterology: WJG. 2015;21(17):5320–7. 10.3748/wjg.v21.i17.5320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giri S, Idle JR, Chen C, Zabriskie TM, Krausz KW, Gonzalez FJ. A metabolomic approach to the metabolism of the areca nut alkaloids arecoline and arecaidine in the mouse. Chemical research in toxicology. 2006;19(6):818–27. 10.1021/tx0600402 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung F-M, Chang D-M, Chen M-P, Tsai JC-R, Yang Y-H, Shieh T-Y, et al. Areca Nut Chewing Is Associated With Metabolic Syndrome. Role of tumor necrosis factor-α, leptin, and white blood cell count in betel nut chewing–related metabolic derangements. 2006;29(7):1714–. 10.2337/dc06-0628 [DOI] [PubMed] [Google Scholar]
- 29.Jeng JH, Wang YJ, Chiang BL, Lee PH, Chan CP, Ho YS, et al. Roles of keratinocyte inflammation in oral cancer: regulating the prostaglandin E2, interleukin-6 and TNF-alpha production of oral epithelial cells by areca nut extract and arecoline. Carcinogenesis. 2003;24(8):1301–15. 10.1093/carcin/bgg083 . [DOI] [PubMed] [Google Scholar]
- 30.Ling PR, Bistrian BR, Mendez B, Istfan NW. Effects of systemic infusions of endotoxin, tumor necrosis factor, and interleukin-1 on glucose metabolism in the rat: relationship to endogenous glucose production and peripheral tissue glucose uptake. Metabolism: clinical and experimental. 1994;43(3):279–84. . [DOI] [PubMed] [Google Scholar]
- 31.Marsland AL, McCaffery JM, Muldoon MF, Manuck SB. Systemic inflammation and the metabolic syndrome among middle-aged community volunteers. Metabolism: clinical and experimental. 2010;59(12):1801–8. 10.1016/j.metabol.2010.05.015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu HF, Tsou TC, Chao HR, Shy CG, Kuo YT, Tsai FY, et al. Effects of arecoline on adipogenesis, lipolysis, and glucose uptake of adipocytes-A possible role of betel-quid chewing in metabolic syndrome. Toxicology and applied pharmacology. 2010;245(3):370–7. 10.1016/j.taap.2010.04.008 . [DOI] [PubMed] [Google Scholar]
- 33.Cowey S, Hardy RW. The metabolic syndrome: A high-risk state for cancer? The American journal of pathology. 2006;169(5):1505–22. 10.2353/ajpath.2006.051090 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. The American journal of clinical nutrition. 2007;86(3):s836–42. 10.1093/ajcn/86.3.836S . [DOI] [PubMed] [Google Scholar]
- 35.Leu CM, Wong FH, Chang C, Huang SF, Hu CP. Interleukin-6 acts as an antiapoptotic factor in human esophageal carcinoma cells through the activation of both STAT3 and mitogen-activated protein kinase pathways. Oncogene. 2003;22(49):7809–18. 10.1038/sj.onc.1207084 . [DOI] [PubMed] [Google Scholar]
- 36.Hursting SD, Hursting MJ. Growth signals, inflammation, and vascular perturbations: mechanistic links between obesity, metabolic syndrome, and cancer. Arteriosclerosis, thrombosis, and vascular biology. 2012;32(8):1766–70. 10.1161/ATVBAHA.111.241927 . [DOI] [PubMed] [Google Scholar]
- 37.Balkwill F. Tumour necrosis factor and cancer. Nature reviews Cancer. 2009;9(5):361–71. 10.1038/nrc2628 . [DOI] [PubMed] [Google Scholar]
- 38.Huang XF, Chen JZ. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes Rev. 2009;10(6):610–6. 10.1111/j.1467-789X.2009.00607.x . [DOI] [PubMed] [Google Scholar]
- 39.Riondino S, Roselli M, Palmirotta R, Della-Morte D, Ferroni P, Guadagni F. Obesity and colorectal cancer: role of adipokines in tumor initiation and progression. World J Gastroenterol. 2014;20(18):5177–90. 10.3748/wjg.v20.i18.5177 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harima S, Hashimoto S, Shibata H, Matsunaga T, Tanabe R, Terai S, et al. Correlations between obesity/metabolic syndrome-related factors and risk of developing colorectal tumors. Hepato-gastroenterology. 2013;60(124):733–7. 10.5754/hge12895 . [DOI] [PubMed] [Google Scholar]
- 41.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. The American journal of clinical nutrition. 2007;86(3):556–65. 10.1093/ajcn/86.3.556 . [DOI] [PubMed] [Google Scholar]
- 42.Giorgino F, Laviola L, Eriksson JW. Regional differences of insulin action in adipose tissue: insights from in vivo and in vitro studies. Acta physiologica Scandinavica. 2005;183(1):13–30. 10.1111/j.1365-201X.2004.01385.x . [DOI] [PubMed] [Google Scholar]
- 43.Endo H, Hosono K, Uchiyama T, Sakai E, Sugiyama M, Takahashi H, et al. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut. 2011;60(10):1363–71. 10.1136/gut.2010.235754 . [DOI] [PubMed] [Google Scholar]
- 44.An W, Bai Y, Deng SX, Gao J, Ben QW, Cai QC, et al. Adiponectin levels in patients with colorectal cancer and adenoma: a meta-analysis. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation (ECP). 2012;21(2):126–33. 10.1097/CEJ.0b013e32834c9b55 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set is owned by the Institutional Review Board (IRB) of Tri-Service General Hospital (TSGH). TSGH IRB only approved the data analysis in our study and did not approve data sharing. Therefore, we do not have permission to share the data set. Interested researchers can submit data access requests to the Tri-Service General Hospital IRB using the following email address: tsghirb@ndmctsgh.edu.tw. Others would be able to access these data in the same manner as the authors and the authors also did not have any special access privileges.