Abstract
Background
This study aimed to determine whether plasma nesfatin-1, cortisol, and inflammatory cytokines could be used as novel noninvasive biomarkers for the diagnosis of moderate and severe depressive disorder (MSDD).
Materials and methods
A total of 70 patients with MSDD and 70 healthy subjects were assessed. Patients with MSDD were selected from Hefei Fourth People’s Hospital, Anhui Mental Health Center, and subjects in the control group were selected from healthy volunteers. Hamilton Depression Rating Scale-17 (HAMD-17) was used to evaluate the two groups. ELISA was used for the measurement of plasma nesfatin-1, cortisol, IL-6, C-reactive protein (CRP), and tumor necrosis factor-α (TNF-α) levels. The diagnostic value of plasma nesfatin-1, cortisol, IL-6, CRP, and TNF-α for MSDD was assessed.
Results
Compared to healthy controls, the HAMD-17 scores and average nesfatin-1, cortisol, IL-6, and CRP levels in patients with MSDD were significantly increased. Moreover, multivariate linear regression analysis showed that HAMD-17 score was positively associated with plasma nesfatin-1 and cortisol. Furthermore, the results of the receiver operating characteristic (ROC) curve analysis revealed an area under curve (AUC) of 0.985 with 94.3% sensitivity and 97.1% specificity of nesfatin-1, and an AUC of 0.957 with 91.4% sensitivity and 85.7% specificity of cortisol in discriminating patients with MSDD from healthy volunteers. A combined ROC analysis using nesfatin-1 and cortisol revealed an AUC of 0.993 with a sensitivity of 97.1% and a specificity of 98.6% in separating patients with MSDD from healthy volunteers.
Conclusion
These results suggest that plasma nesfatin-1 and cortisol might be potential novel biomarkers for the diagnosis of MSDD.
Keywords: C-reactive protein, cortisol, IL-6, depression, nesfatin-1, tumor necrosis factor-α
Introduction
Depression, a serious debilitating mental disease, is among the leading causes of disability leading to a high rate of suicide and economic burden and affecting up to 120 million people worldwide.1 The current diagnosis of depression is mainly based on patient interviews and symptoms, with uncertainties as high as 30% as a consequence.2 Thus, it is important to find an objective examination method to improve the diagnostic accuracy of depression.
The stress response and the resulting hyperactivity of the hypothalamic pituitary adrenal (HPA) axis are considered to be one of the causes of depressive episodes.3,4 Preclinical studies have shown that hyperfunctioning of the HPA axis and elevated serum corticosterone levels could be observed in animal models of depression.5 Con sistent with this finding, clinical studies have suggested that HPA axis dysfunction, such as excessive secretion of cortisol, occurs in patients with depression.6 Therefore, the plasma concentration of cortisol was considered to be a reliable independent marker for diagnosing patients with moderate and severe depressive disorder (MSDD) in the present study.
Anorexigenic and orexigenic peptides have been considered to be associated with the pathogenesis of depression.7,8 Nesfatin-1 is a new anorexigenic peptide that plays an important role in the integration of food intake, energy expenditure, and glucose homeostasis.9 Recently, a link may have been discovered between nesfatin-1 and depression due to the findings that intracerebroventricular injection of nesfatin-1 could activate the HPA axis in rats and induce fear and anxiety-like behavioral changes.10 Moreover, our recent studies have shown that peripheral injection of nesfatin-1 could also induce depressive behavioral changes and activate the HPA axis in rats.11 Furthermore, higher plasma nesfatin-1 level was found in patients with major depressive disorder (MDD) than in healthy controls.12 Taken together, the fact that plasma nesfatin-1 level is associated with the severity of depression,13 it is rational to assume that nesfatin-1 might act as a potential diagnostic biomarker in depression.
Numerous studies support the notion that the occurrence of depression is often accompanied by activation of the inflammatory system, and overexpression of pro-inflammatory factors is thought to play a role in the pathogenesis of depression.14,15 Meta-analysis shows that the most closely related inflammatory factors of depression are IL-6, C-reactive protein (CRP), and tumor necrosis factor-alpha (TNF-α).16 In addition, more and more studies have shown that elevated levels of IL-6 and CRP in plasma are believed to increase the risk of depression and even predict the following symptoms of depression.17 Therefore, whether these inflammatory factors could serve as objective and reliable biological indicators for the diagnosis of depression was evaluated in the present study.
Because of the dysfunction of the HPA axis, with the important role of nesfatin-1 and inflammatory markers in the pathophysiology of depression, the aim of the present study was to investigate whether plasma nesfatin-1, cortisol, and inflammatory cytokines could be used as novel noninvasive biomarkers for MSDD.
Materials and methods
Subjects
This study was conducted at Hefei Fourth People’s Hospital, Anhui Mental Health Center, between July 2016 and April 2018. A total of 1,489 subjects with depression were screened via psychiatric interviews by an experienced researcher in accordance with the guidelines of the structured clinical interview according to the Diagnostic and Statistical Manual for Psychiatric Disorders-Fourth Version (DSM-IV), and 70 patients with MSDD were selected from the above patients. The criteria for inclusion in the group are as follows: 1) age 18–65 years; 2) Hamilton Depression Rating Scale-17 (HAMD-17) scores >17; and 3) currently not receiving any medication (Blood was collected when the patient with MSDD was just admitted to the hospital for treatment). Exclusion criteria were as follows: 1) suffering from other mental disorders; 2) diagnosed with alcohol or other substance dependent diseases; and 3) suffering from a major neurological or medical illness. Seventy healthy volunteers were included in the control group. The recruitment process is summarized in Figure 1. In accordance with the principles of the Declaration of Helsinki, all subjects provided informed written consent prior to participation. The Ethics Committee of Hefei Fourth People’s Hospital, Anhui Mental Health Center, approved this study.
Figure 1.
Flowchart showing the recruitment of participant.
Measurements
The weight, height, and body mass index (BMI) of all subjects were measured. The blood sample was taken from the participants’ vein between 8:00 A.M. and 9:00 A.M. All subjects were fasting while giving blood. Tubes containing EDTA were used for collecting the samples. The blood samples were immediately centrifuged at 3,000 rpm for 5 minutes at 4°C. Extract supernatant as plasma sample was collected. The extracted plasma was stored at −80°C until detection. Commercially available ELISA kits were used to measure the concentrations of nesfatin-1 (Cusabio Biotech. Co., LTD, Wuhan, Hubei, China), cortisol (Enzo Life Sciences, Inc., Farmingdale, NY, USA), IL-6, CRP, and TNF-α (Yuanye Biotech.Co., LTD, Shanghai, China) according to the manufacturer’s instructions.
Statistical analysis
Data were analyzed using SPSS version 16.0 statistical analysis software (SPSS, Chicago, IL, USA). The differences between groups (age, BMI, HAMD-17 scores, and concentrations of nesfatin-1, cortisol, IL-6, CRP, and TNF-α) were evaluated by Student’s t-test. The sex differences between the groups were analyzed by chi-squared test. Relationships between HAMD-17 scores and other variables were analyzed by Pearson correlation tests and independent relationships were determined by multivariate linear regression analysis. The receiver operating characteristic (ROC) curve analysis was used to determine the area under curve (AUC) and cut-off values of plasma nesfatin-1 and cortisol. A P-value of <0.05 was considered statistically significant.
Results
Demographic values were not significantly different between the MSDD patients and healthy controls. As shown in Table 1, there were no significant differences in age, BMI, or sex between the two groups. The mean HAMD-17 scores were significantly higher in the MSDD group than in the control group (t=−14.144, P<0.01, Table 1). Similarly, the MSDD patients showed elevated nesfatin-1 (t=−19.349, P<0.01, Table 1) and cortisol (t=−14.694, P<0.01, Table 1) levels when compared with the healthy subjects. Additionally, the results of covariance analysis showed that the differences of plasma nesfatin-1 and cortisol between the MSDD groups and healthy control groups were not influenced by age and sex (Supplemental materials, Tables S1–S4). For inflammatory cytokines, the plasma concentrations of IL-6 (t=−4.037, P<0.01) and CRP (t=−3.87, P<0.01) were significantly higher than those in the healthy control group, whereas no statistical difference was observed in TNF-α levels (t=−0.445, P=0.657) between the two groups.
Table 1.
Comparison of age, gender, BMI, HAMD-17 scores, plasma nesfatin-1, cortisol, IL-6, CRP, and TNF-α of MSDD group and control group (mean ± SEM)
| Variables | Control group | MSDD group | Statistics (t or c2) | P-value |
|---|---|---|---|---|
| Age | 34.81±12.19 | 35.86±15.78 | −0.438 | 0.662 |
| Gender (female/male) | 39/31 | 35/35 | 0.459a | 0.498 |
| BMI (kg/m2) | 23.34±1.67 | 23.29±1.44 | 0.206 | 0.837 |
| HAM-D | 6.60±3.10 | 27.41±11.92 | −14.144 | <0.01b |
| Nesfatin-1 (ng/mL) | 0.83±0.16 | 1.61±0.29 | −19.349 | <0.01b |
| Cortisol (nmol/L) | 196.78±102.76 | 431.55±85.50 | −14.694 | <0.01b |
| IL-6 (pg/mL) | 59.50±13.17 | 66.65±6.78 | −4.037 | <0.01b |
| CRP (mg/L) | 2.48±0.29 | 2.66±0.25 | −3.87 | <0.01b |
| TNF-α (ng/L) | 167.15±27.82 | 168.92±18.29 | −0.445 | 0.657 |
Notes:
Shows χ2 score.
P<0.01 was considered statistically significant.
Abbreviations: BMI, body mass index; CRP, C-reactive protein; HAM-D, Hamilton Depression Rating Scale; MSDD, moderate and severe depressive disorder; TNF-α, tumor necrosis factor-α.
As shown in Table 2, the results of the Pearson correlation analysis showed that the HAMD-17 scores were positively correlated with plasma nesfatin-1 (r=0.647, P<0.01), cortisol (r=0.566, P<0.01), IL-6 (r=0.236, P=0.005), and CRP (r=0.291, P<0.01) concentrations. However, no correlation between HAMD-17 scores and TNF-α (r=0.012, P=0.887) was observed.
Table 2.
Correlation between plasma HAMD-17 scores and plasma nesfatin-1, cortisol, IL-6, CRP, and TNF-α (Pearson correlation analysis)
| Variables | r | P-value |
|---|---|---|
|
| ||
| Nesfatin-1 | 0.647 | <0.01a |
| Cortisol | 0.566 | <0.01a |
| IL-6 | 0.236 | 0.005a |
| CRP | 0.291 | <0.01a |
| TNF-α | 0.012 | 0.887 |
Note:
P<0.01 was considered statistically significant.
Abbreviations: CRP, C-reactive protein; HAM-D, Hamilton Depression Rating Scale; TNF-α, tumor necrosis factor-α.
Multivariate linear regression analysis showed that the HAMD-17 score was positively associated with plasma nesfatin-1 and cortisol (Table 3).
Table 3.
Multiple linear regression analysis between HAMD-17 scores (dependent variable) and plasma nesfatin-1, cortisol, IL-6, CRP, and TNF-α (independent variables)
| Variables | B | t | P-value | 95% CI
|
|
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
|
| |||||
| Constant | −18.293 | −2.043 | 0.043 | −35.997 | −0.589 |
| Nesfatin-1 | 13.921 | 5.186 | <0.01a | 8.612 | 19.230 |
| Cortisol | 0.020 | 2.556 | 0.012b | 0.005 | 0.036 |
| IL-6 | −0.004 | −0.046 | 0.963 | −0.169 | 0.161 |
| CRP | 4.755 | 1.511 | 0.133 | −1.467 | 10.976 |
Note:
P<0.01,
P<0.05 was considered statistically significant.
Abbreviations: CRP, C-reactive protein; HAM-D, Hamilton Depression Rating Scale; TNF-α, tumor necrosis factor-α.
Additionally, the ROC curve analysis indicated the potential diagnostic values of plasma nesfatin-1 and cortisol (Table 4): The AUCs for nesfatin-1 and cortisol were 0.985 (95% CI: 0.959–1.000) and 0.957 (95% CI: 0.927–0.986), respectively. At a cut-off point of 1.135 ng/mL for nesfatin-1, the sensitivity and specificity were 94.3% and 97.1% for separating patients with MSDD from healthy volunteers. At a cut-off point of 325.245 nmol/L for cortisol, the sensitivity and the specificity were 91.4% and 85.7%, respectively. When the test results for nesfatin-1 and cortisol were considered jointly, the ROC analysis revealed an AUC of 0.993 with a sensitivity of 97.1% and specificity 98.6% in discriminating patients with MSDD from healthy volunteers, indicating that the combination of nesfatin-1 and cortisol could improve the accuracy of diagnosis of MSDD (Figure 2).
Table 4.
ROC analysis of various indicators for diagnosis of MSDD
| Variables | AUC | Cut-off | Sensitivity | Specificity | |
|---|---|---|---|---|---|
|
| |||||
| Nesfatin-1 | 0.985 | 1.135 | 0.943 | 0.971 | |
| Cortisol | 0.957 | 325.245 | 0.914 | 0.857 | |
| Nesfatin-1+ | 0.993 | 0.971 | 0.986 | ||
| Cortisol | 0.679 | ||||
| IL-6 CRP | 0.672 | ||||
| TNF-α | 0.535 | ||||
Abbreviations: AUC, area under curve; CRP, C-reactive protein; MSDD, moderate and severe depressive disorder; ROC, receiver operating characteristic; TNF-α, tumor necrosis factor-α.
Figure 2.
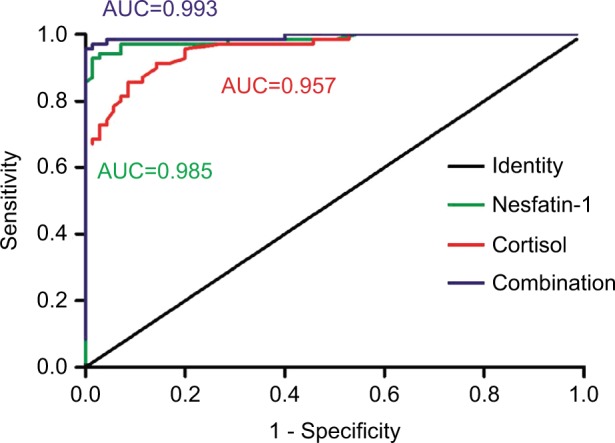
ROC curve of plasma nesfatin-1 and cortisol in identification of the patients with MSDD.
Abbreviations: AUC, area under curve; MSDD, moderate and severe depressive disorder; ROC, Receiver operating characteristic.
Discussion
In the present study, we demonstrated that the HAMD-17 score and the plasma nesfatin-1, cortisol, IL-6, and CRP concentrations were significantly higher in MSDD patients than in healthy controls. Moreover, multivariate linear regression analysis showed that HAMD-17 score was positively associated with plasma nesfatin-1 and cortisol. Furthermore, the results of the ROC analysis revealed an AUC of 0.985 with 94.3% sensitivity and 97.1% specificity of nesfatin-1, and an AUC of 0.957 with 91.4% sensitivity and 85.7% specificity of cortisol in discriminating patients with MSDD from healthy controls, suggesting that plasma nesfatin-1 and cortisol might be potential novel biomarkers for the diagnosis of MSDD.
Stress and the subsequent hyperactivity of the HPA axis have been involved in the development of depression.18 Numerous studies have confirmed that disturbance in the HPA axis function, indicated by elevated plasma corticosterone concentrations, was observed in a rat model of depression (chronic unpredictable mild stress,19 restraint stress,20 or water avoidance stress).21 Similar dysfunction in the HPA axis is also observed in depressed patients.22 In line with these results, in the present study, patients with MSDD showed higher plasma cortisol levels than healthy control subjects. Recently, numerous studies have indicated that biomarker panels that monitor changes in cortisol, as well as other HPA axis factors, could provide important information for the diagnosis of depression.23 Based on the ROC analysis, the plasma cortisol cut-off point of 325.245 nmol/L showed 91.4% sensitivity and 85.7% specificity, indicating that plasma cortisol has a superior diagnostic value (AUC=0.957) of MSDD.
Nesfatin-1, a novel satiety molecule, has been reported to be involved in a variety of affective disorders, including anxiety disorder,24 depression,13 and psychosis.25 Clinical studies have shown that the average nesfatin-1 level in MDD patients is statistically higher than that in the control group.12 Similarly, plasma nesfatin-1 levels were also increased in rat subjected to avoiding stress, an acute depression model.26 Consistent with these findings, the MSDD patients showed elevated nesfatin-1 levels when compared with the healthy subjects in the present study. On the other hand, the intra-peritoneal administration of nesfatin-1, in a single dose or multiple doses, led to despair behavior and HPA axis hyperactivity in rats.11 These results provide more information on the link between nesfatin-1 and depression. Recently, another study has demonstrated that the plasma nesfatin-1 level is positively correlated with the severity of depression.13 Consistently, a positive correlation between the nesfatin-1 levels and HAMD-17 scores was found in the present study. As mentioned above, it is rational to hypothesize that plasma nesfatin-1 might be a potential biomarker for the diagnosis of MSDD. The results of ROC curve analysis showed that the AUC for nesfatin-1 was 0.985 and the cut-off point of 1.135 ng/mL showed 94.3% sensitivity and 97.1% specificity in separating patients with MSDD from healthy controls. Thus, nesfatin-1 may be a useful biomarker for detection of MSDD due to its high sensitivity and specificity.
Combining the detection of different biomarkers has been reported to be a useful strategy in several studies, for the reason that it can increase the sensitivity and specificity of each biomarker. In the present study, both nesfatin-1 and cortisol exhibited high specificity and sensitivity. In a combined ROC analysis using the two markers, both sensitivity and specificity reached a relatively high value in discriminating patients with MSDD from healthy controls. To date, no reliable independent biomarkers have been established for MSDD. Our results suggest that the combination of nesfatin-1 and cortisol plasma assays may offer a useful tool for the detection of MSDD.
Accumulated data support a certain link between depression and inflammatory response. It has been indicated that depressed patients27 and animals28 showed increased concentrations of IL-6 and peripheral injection of IL-6 can induce depressive behavioral changes in mice.29 In addition, elevated CRP levels were observed in patients with depression and high CRP levels were considered to be positively associated with the degree of depression.30 Consistently, the present study showed that plasma levels of IL-6 and CRP were significantly increased in the patients with MSDD, which again suggests that IL-6 and CRP may play an important role in the development of depression. Although Pearson correlation analysis showed positive correlations were found between the HAMD-17 scores and the plasma IL-6 and CRP concentrations, these correlations were not observed by multivariate linear correlation analysis and the AUCs of IL-6 and CRP were less than 0.7. These results suggest that inflammatory factors (IL-6 and CRP) are closely related to depression, but may not be suitable as a biomarker for the diagnosis of MSDD.
There are several limitations in the present study. Firstly, this study is a single-center study and the sample size is relatively small. Secondly, we measured the plasma concentration of nesfatin-1 in only those patients who were not taking medication within 2 weeks; the long-term effects of previous drugs on plasma nesfatin-1 remain unknown. Thirdly, when collecting the general data of subjects, the patients’ smoking status should be included, for the reason that smoking status may potentially affect their inflammatory factor levels.
Conclusion
In conclusion, the detection of plasma nesfatin-1 and cortisol may prove to be of clinical value for the diagnosis of MSDD. Multicentric and longitudinal studies are clearly required to validate the potential of nesfatin-1 and cortisol as novel biomarkers for MSDD.
Supplementary materials
Tables S1 and S2 show the results of analysis of covariance with age and sex as covariates (dependent variable: nesfatin-1), respectively. Tables S3 and S4 show the results of analysis of covariance with age and sex as covariates (dependent variable: cortisol), respectively.
The results of covariance analysis show that the differences of plasma nesfatin-1 and corticosterone between the MSDD groups and healthy control groups are not influenced by age and sex.
Table S1.
Tests of between-subjects effects Dependent variable: nesfatin-1
| Source | Type III sum of squares | df | Mean square | F | P-value |
|---|---|---|---|---|---|
|
| |||||
| Corrected model | 21.132a | 7 | 3.019 | 52.342 | 0.000 |
| Intercept | 133.913 | 1 | 133.913 | 2321.814 | 0.000 |
| Age | 0.013 | 3 | 0.004 | 0.074 | 0.974 |
| Group | 12.721 | 1 | 12.721 | 220.562 | 0.000 |
| Age × group | 0.113 | 3 | 0.038 | 0.653 | 0.582 |
| Error | 7.613 | 132 | 0.058 | ||
| Total | 237.758 | 140 | |||
| Corrected total | 28.746 | 139 | |||
Notes: R2=0.735 (Adjusted R2=0.721). The differences in plasma nesfatin-1 between the MSDD groups and healthy control groups are not influenced by age (P>0.05).
Table S2.
Tests of between-subjects effects Dependent variable: nesfatin-1
| Source | Type III sum of squares | df | Mean square | F | P-value |
|---|---|---|---|---|---|
|
| |||||
| Corrected model | 21.055a | 3 | 7.018 | 124.110 | 0.000 |
| Intercept | 208.025 | 1 | 208.025 | 3678.658 | 0.000 |
| Gender | 0.044 | 1 | 0.044 | 0.784 | 0.377 |
| Group | 20.749 | 1 | 20.749 | 366.928 | 0.000 |
| Gender × group | 0.001 | 1 | 0.001 | 0.011 | 0.918 |
| Error | 7.691 | 136 | 0.057 | ||
| Total | 237.758 | 140 | |||
| Corrected total | 28.746 | 139 | |||
Notes: R2=0.732 (Adjusted R2=0.727). The differences in plasma nesfatin-1 between the MSDD groups and healthy control groups are not influenced by gender (P>0.05).
Table S3.
Tests of between-subjects effects Dependent variable: cortisol
| Source | Type III sum of squares | df | Mean square | F | P-value |
|---|---|---|---|---|---|
|
| |||||
| Corrected model | 1.950E6 | 7 | 278560.062 | 30.335 | 0.000 |
| Intercept | 9146742.680 | 1 | 9146742.680 | 996.090 | 0.000 |
| Age | 10032.684 | 3 | 3344.228 | 0.364 | 0.779 |
| Group | 1077392.415 | 1 | 1077392.415 | 117.329 | 0.000 |
| Age × group | 14107.698 | 3 | 4702.566 | 0.512 | 0.675 |
| Error | 1212109.577 | 132 | 9182.648 | ||
| Total | 1.698E7 | 140 | |||
| Corrected total | 3162030.007 | 139 | |||
Notes: R2=0.617 (Adjusted R2=0.586). The differences in plasma cortisol between the MSDD groups and healthy control groups are not influenced by age (P>0.05).
Table S4.
Tests of between-subjects effects Dependent variable: cortisol
| Source | Type III sum of squares | df | Mean square | F | P-value |
|---|---|---|---|---|---|
|
| |||||
| Corrected model | 1.941E6 | 3 | 647137.777 | 72.104 | 0.000 |
| Intercept | 1.377E7 | 1 | 1.377E7 | 1533.734 | 0.000 |
| Gender | 366.221 | 1 | 366.221 | 0.041 | 0.840 |
| Group | 1902079.346 | 1 | 1902079.346 | 211.928 | 0.000 |
| Gender × group | 11989.871 | 1 | 11989.871 | 1.336 | 0.250 |
| Error | 1220616.678 | 136 | 8975.123 | ||
| Total | 1.698E7 | 140 | |||
| Corrected total | 3162030.007 | 139 | |||
Notes: R2=0.614 (Adjusted R2=0.605). The differences in plasma cortisol between the MSDD groups and healthy control groups are not influenced by gender (P>0.05).
Acknowledgments
This project was supported by the Research projects of Hefei Fourth People’s Hospital (2018ZD10).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Tirado Muñoz J, Farré A, Mestre-Pintó J, Szerman N, Torrens M. Dual diagnosis in Depression: treatment recommendations. Adicciones. 2018;30(1):66–76. doi: 10.20882/adicciones.868. [DOI] [PubMed] [Google Scholar]
- 2.Beekman ATF, Spijker J. Personalised diagnosis and treatment of depression. Tijdschr Psychiatr. 2018;60(3):156–160. [PubMed] [Google Scholar]
- 3.Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4(2):141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Yang XH, Song SQ, Xu Y. Resveratrol ameliorates chronic unpredictable mild stress-induced depression-like behavior: involvement of the HPA axis, inflammatory markers, BDNF, and Wnt/β-catenin pathway in rats. Neuropsychiatr Dis Treat. 2017;13:2727–2736. doi: 10.2147/NDT.S150028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maghsoudi N, Ghasemi R, Ghaempanah Z, et al. Effect of Chronic Restraint Stress on HPA Axis Activity and Expression of BDNF and Trkb in the Hippocampus of Pregnant Rats: Possible Contribution in Depression during Pregnancy and Postpartum Period. Basic Clin Neurosci. 2014;5(2):131–137. [PMC free article] [PubMed] [Google Scholar]
- 6.Pirnia B, Givi F, Roshan R, Pirnia K, Soleimani AA. The cortisol level and its relationship with depression, stress and anxiety indices in chronic methamphetamine-dependent patients and normal individuals undergoing inguinal hernia surgery. Med J Islam Repub Iran. 2016;30:395. [PMC free article] [PubMed] [Google Scholar]
- 7.Hassan AM, Mancano G, Kashofer K, et al. Nutr Neurosci. 2018. Apr 26, High-fat diet induces depression-like behaviour in mice associated with changes in micro-biome, neuropeptide Y, and brain metabolome. Epub. [DOI] [PubMed] [Google Scholar]
- 8.Kokare DM, Dandekar MP, Singru PS, Gupta GL, Subhedar NK. Involvement of alpha-MSH in the social isolation induced anxiety- and depression-like behaviors in rat. Neuropharmacology. 2010;58(7):1009–1018. doi: 10.1016/j.neuropharm.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Zhai T, Li SZ, Fan XT, et al. Circulating Nesfatin-1 Levels and Type 2 Diabetes: A Systematic Review and Meta-Analysis. J Diabetes Res. 2017;2017:7687098–8. doi: 10.1155/2017/7687098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merali Z, Cayer C, Kent P, Anisman H. Nesfatin-1 increases anxiety- and fear-related behaviors in the rat. Psychopharmacology. 2008;201(1):115–123. doi: 10.1007/s00213-008-1252-2. [DOI] [PubMed] [Google Scholar]
- 11.Ge JF, Xu YY, Qin G, et al. Depression-like Behavior Induced by Nesfatin-1 in Rats: Involvement of Increased Immune Activation and Imbalance of Synaptic Vesicle Proteins. Front Neurosci. 2015;9:429. doi: 10.3389/fnins.2015.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ari M, Ozturk OH, Bez Y, Oktar S, Erduran D. High plasma nesfatin-1 level in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(2):497–500. doi: 10.1016/j.pnpbp.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Xiao MM, Li JB, Jiang LL, Shao H, Wang BL. Plasma nesfatin-1 level is associated with severity of depression in Chinese depressive patients. BMC Psychiatry. 2018;18(1):88. doi: 10.1186/s12888-018-1672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farooq RK, Asghar K, Kanwal S, Zulqernain A. Role of inflammatory cytokines in depression: Focus on interleukin-1β. Biomed Rep. 2017;6(1):15–20. doi: 10.3892/br.2016.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou W, Feng R, Yang Y. Changes in the serum levels of inflammatory cytokines in antidepressant drug-naïve patients with major depression. PLoS One. 2018;13(6):e0197267. doi: 10.1371/journal.pone.0197267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 17.Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2013;150(3):736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Bangsgaard EO, Ottesen JT. Patient specific modeling of the HPA axis related to clinical diagnosis of depression. Math Biosci. 2017;287:24–35. doi: 10.1016/j.mbs.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Dubey VK, Ansari F, Vohora D, Khanam R. Possible involvement of corticosterone and serotonin in antidepressant and antianxiety effects of chromium picolinate in chronic unpredictable mild stress induced depression and anxiety in rats. J Trace Elem Med Biol. 2015;29:222–226. doi: 10.1016/j.jtemb.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Thakare VN, Dhakane VD, Patel BM. Potential antidepressant-like activity of silymarin in the acute restraint stress in mice: Modulation of corticosterone and oxidative stress response in cerebral cortex and hippocampus. Pharmacol Rep. 2016;68(5):1020–1027. doi: 10.1016/j.pharep.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Emmerzaal TL, Kozicz T. Nesfatin-1; implication in stress and stress-associated anxiety and depression. Curr Pharm Des. 2013;19(39):6941–6948. doi: 10.2174/138161281939131127125042. [DOI] [PubMed] [Google Scholar]
- 22.Xia QR, Liang J, Cao Y, et al. Increased plasma nesfatin-1 levels may be associated with corticosterone, IL-6, and CRP levels in patients with major depressive disorder. Clin Chim Acta. 2018;480:107–111. doi: 10.1016/j.cca.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt HD, Shelton RC, Duman RS. Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacology. 2011;36(12):2375–2394. doi: 10.1038/npp.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pałasz A, Janas-Kozik M, Borrow A, Arias-Carrión O, Worthington JJ. The potential role of the novel hypothalamic neuropeptides nesfatin-1, phoenixin, spexin and kisspeptin in the pathogenesis of anxiety and anorexia nervosa. Neurochem Int. 2018;113:120–136. doi: 10.1016/j.neuint.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Sahpolat M, Ari M. Plasma nesfatin 1 level in patients with first attack psychosis. Bratisl Lek Listy. 2017;118(2):77–79. doi: 10.4149/BLL_2017_015. [DOI] [PubMed] [Google Scholar]
- 26.Xu YY, Ge JF, Qin G, et al. Acute, but not chronic, stress increased the plasma concentration and hypothalamic mRNA expression of NUCB2/nesfatin-1 in rats. Neuropeptides. 2015;54:47–53. doi: 10.1016/j.npep.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Liu H, Zhang Y, Gao Y, Zhang Z. Elevated levels of Hs-CRP and IL-6 after delivery are associated with depression during the 6 months post partum. Psychiatry Res. 2016;243:43–48. doi: 10.1016/j.psychres.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 28.Yang C, Hashimoto K. Peripheral IL-6 signaling: a promising therapeutic target for depression? Expert Opin Investig Drugs. 2015;24(7):989–990. doi: 10.1517/13543784.2015.1055669. [DOI] [PubMed] [Google Scholar]
- 29.Kurosawa N, Shimizu K, Seki K. The development of depression-like behavior is consolidated by IL-6-induced activation of locus coeruleus neurons and IL-1β-induced elevated leptin levels in mice. Psychopharmacology. 2016;233(9):1725–1737. doi: 10.1007/s00213-015-4084-x. [DOI] [PubMed] [Google Scholar]
- 30.Köhler-Forsberg O, Buttenschøn HN, Tansey KE, et al. Association between C-reactive protein (CRP) with depression symptom severity and specific depressive symptoms in major depression. Brain Behav Immun. 2017;62:344–350. doi: 10.1016/j.bbi.2017.02.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Tests of between-subjects effects Dependent variable: nesfatin-1
| Source | Type III sum of squares | df | Mean square | F | P-value |
|---|---|---|---|---|---|
|
| |||||
| Corrected model | 21.132a | 7 | 3.019 | 52.342 | 0.000 |
| Intercept | 133.913 | 1 | 133.913 | 2321.814 | 0.000 |
| Age | 0.013 | 3 | 0.004 | 0.074 | 0.974 |
| Group | 12.721 | 1 | 12.721 | 220.562 | 0.000 |
| Age × group | 0.113 | 3 | 0.038 | 0.653 | 0.582 |
| Error | 7.613 | 132 | 0.058 | ||
| Total | 237.758 | 140 | |||
| Corrected total | 28.746 | 139 | |||
Notes: R2=0.735 (Adjusted R2=0.721). The differences in plasma nesfatin-1 between the MSDD groups and healthy control groups are not influenced by age (P>0.05).
Table S2.
Tests of between-subjects effects Dependent variable: nesfatin-1
| Source | Type III sum of squares | df | Mean square | F | P-value |
|---|---|---|---|---|---|
|
| |||||
| Corrected model | 21.055a | 3 | 7.018 | 124.110 | 0.000 |
| Intercept | 208.025 | 1 | 208.025 | 3678.658 | 0.000 |
| Gender | 0.044 | 1 | 0.044 | 0.784 | 0.377 |
| Group | 20.749 | 1 | 20.749 | 366.928 | 0.000 |
| Gender × group | 0.001 | 1 | 0.001 | 0.011 | 0.918 |
| Error | 7.691 | 136 | 0.057 | ||
| Total | 237.758 | 140 | |||
| Corrected total | 28.746 | 139 | |||
Notes: R2=0.732 (Adjusted R2=0.727). The differences in plasma nesfatin-1 between the MSDD groups and healthy control groups are not influenced by gender (P>0.05).
Table S3.
Tests of between-subjects effects Dependent variable: cortisol
| Source | Type III sum of squares | df | Mean square | F | P-value |
|---|---|---|---|---|---|
|
| |||||
| Corrected model | 1.950E6 | 7 | 278560.062 | 30.335 | 0.000 |
| Intercept | 9146742.680 | 1 | 9146742.680 | 996.090 | 0.000 |
| Age | 10032.684 | 3 | 3344.228 | 0.364 | 0.779 |
| Group | 1077392.415 | 1 | 1077392.415 | 117.329 | 0.000 |
| Age × group | 14107.698 | 3 | 4702.566 | 0.512 | 0.675 |
| Error | 1212109.577 | 132 | 9182.648 | ||
| Total | 1.698E7 | 140 | |||
| Corrected total | 3162030.007 | 139 | |||
Notes: R2=0.617 (Adjusted R2=0.586). The differences in plasma cortisol between the MSDD groups and healthy control groups are not influenced by age (P>0.05).
Table S4.
Tests of between-subjects effects Dependent variable: cortisol
| Source | Type III sum of squares | df | Mean square | F | P-value |
|---|---|---|---|---|---|
|
| |||||
| Corrected model | 1.941E6 | 3 | 647137.777 | 72.104 | 0.000 |
| Intercept | 1.377E7 | 1 | 1.377E7 | 1533.734 | 0.000 |
| Gender | 366.221 | 1 | 366.221 | 0.041 | 0.840 |
| Group | 1902079.346 | 1 | 1902079.346 | 211.928 | 0.000 |
| Gender × group | 11989.871 | 1 | 11989.871 | 1.336 | 0.250 |
| Error | 1220616.678 | 136 | 8975.123 | ||
| Total | 1.698E7 | 140 | |||
| Corrected total | 3162030.007 | 139 | |||
Notes: R2=0.614 (Adjusted R2=0.605). The differences in plasma cortisol between the MSDD groups and healthy control groups are not influenced by gender (P>0.05).