Abstract
Background:
Tuberculosis is a major cause of mortality among HIV-infected inpatients, and the World Health Organization (WHO) recommends an algorithm to improve diagnosis. The urine lateral flow lipoarabinomannan (LAM) and sputum Xpert MTB/RIF tests are promising tools, but the optimal diagnostic algorithm is unclear.
Methods:
This prospective cohort study enrolled HIV-positive inpatients with cough and WHO danger signs. The Xpert MTB/RIF test and mycobacterial culture were performed on sputum using sputum induction when necessary, and the LAM test was performed on stored urine. Tuberculosis was diagnosed by culture from any site. The diagnostic accuracy and costs of testing were determined for single and combined tests.
Results:
Tuberculosis was confirmed in 169 of 332 patients (50.9%). The yield of LAM, Xpert MTB/RIF on spontaneous sputum (Xpert Spot), and Xpert MTB/RIF on spontaneous or induced sputum (Xpert SI) was 35.5%, 23.1%, and 90.5%, respectively. When LAM was placed before Xpert Spot and Xpert SI in an algorithm, the yield was 50.9% and 92.3%, respectively. Adding culture to Xpert MTB/RIF only increased the yield by 1.2% and 2.7%, respectively. Use of the LAM test reduced costs.
Conclusions:
Sputum induction is important to increase the yield of Xpert MTB/RIF for seriously ill patients with HIV and cough. LAM testing has little effect on yield when sputum induction is available, but reduces costs and may have other benefits.
Keywords: Tuberculosis, Diagnosis, LAM, Xpert MTB/RIF, Sputum induction
Introduction
Undiagnosed tuberculosis is a major cause of death among hospitalized HIV-infected patients in Africa (Gupta et al., 2015). Smear-negative and extrapulmonary tuberculosis, which can be difficult to diagnose, occur with a higher prevalence in HIV-infected patients. The World Health Organization (WHO) strongly recommends the use of the Xpert MTB/RIF test (Cepheid, Sunnyvale, CA, USA) rather than conventional microscopy, culture, and drug susceptibility testing (DST) as the initial sputum diagnostic test in adults suspected of having HIV-associated tuberculosis (WHO, 2016). This advice is based on a systematic review and meta-analysis of Xpert MTB/RIF for the diagnosis of tuberculosis in patients with HIV, which found a pooled sensitivity of 79% and pooled specificity of 98% (Steingart et al., 2014). When patients are sputum-scarce, the yield of the Xpert MTB/RIF test may be improved by sputum induction with nebulized hypertonic saline (Gonzalez-Angulo et al., 2012).
Currently, the WHO also conditionally recommends the use of the urine lateral flow lipoarabinomannan test (Alere Determine TB LAM Ag; Alere Inc., Waltham, MA, USA) (LAM) to assist in the diagnosis of tuberculosis in HIV-infected adult inpatients with signs and symptoms of tuberculosis who have a CD4 cell count of ≤100 cells/µ1, and in HIV-infected patients who are seriously ill regardless of their CD4 count (WHO, 2016). A systematic review and meta-analysis of the LAM assay for the diagnosis of tuberculosis showed a pooled sensitivity and specificity of 45% and 92%, respectively, and that sensitivity is improved in patients with a CD4 count <100 cells/µl (Shah et al., 2016). Following the announcement of the WHO recommendation, a randomized controlled trial showed that the LAM test reduced inpatient mortality by 17% (Peter et al., 2016). Recent evidence has shown that routine testing in HIV-infected inpatients using the LAM assay provides major incremental diagnostic yield with high specificity (Lawn et al., 2017), even in patients with CD4 counts >100 cells/µ1, and that LAM rapidly identifies individuals with a poor prognosis.
In 2016, the WHO published updated guidelines, including an algorithm to improve the diagnosis of tuberculosis among seriously ill adult patients suspected of having tuberculosis and at least one danger sign (respiratory rate >30 per minute, temperature >39 °C, heart rate >120 beats per minute, and unable to walk unaided) (WHO, 2016). The WHO algorithm utilizes the Xpert MTB/RIF test, parenteral antibiotics, and chest X-ray, and a footnote suggests that the LAM test may be used to assist in the diagnosis of tuberculosis regardless of the CD4 count. It appears that no study has yet assessed the diagnostic performance and incremental yield of the urine LAM assay in patients fulfilling the criteria for the WHO seriously ill algorithm.
This study determined the diagnostic accuracy and incremental yield of the LAM test, Xpert MTB/RIF with and without sputum induction, and sputum culture in a cohort of HIV-infected patients fulfilling the criteria for the WHO seriously ill algorithm in Cape Town, South Africa. A series of algorithmic approaches to diagnosing tuberculosis using these tests was then simulated and the diagnostic accuracy and cost compared.
Methods
The full methods of the parent study have been described elsewhere (Griesel et al., 2018). In summary, a prospective cohort study of consecutively recruited adults with HIV, any duration of cough, and WHO danger signs was conducted at two secondary level hospitals serving communities with a high burden of HIV and tuberculosis in Cape Town, South Africa, from November 2011 to October 2014: GF Jooste Hospital (November 2011 to February 2013) and Khayelitsha District Hospital (March 2013 to October 2014). Inclusion criteria were HIV-infected, ≥18 years, admitted within 24 hours, coughing for any duration, and one or more WHO danger signs. Exclusion criteria were anti-tuberculosis therapy, either current or completed in the previous month, ordefaultedwithinthe past6 months, anexacerbation of cardiac failure or chronic obstructive pulmonary disease, and an inability to produce a spontaneous or induced sputum sample. Participants were followed up at 28 and 56 days post-discharge.
Participants received a standardized work-up for their respiratory illness including a chest radiograph and CD4 count, unless a result was available within 6 months prior to admission. Sputum samples were taken from all participants: one sample for Gram stain, culture, and sensitivity testing and two samples for smear examination with auramine staining for acid-fast bacilli (AFB) and mycobacterial culture. The Xpert MTB/RIF assay was performed on one sample sent for mycobacterial culture. Sputum was induced using an ultrasonic nebulizer and hypertonic saline (5%) when participants were unable to produce sputum spontaneously. This was generally done because of lack of a productive cough, but weakness to produce a sample was also a reason (this was not recorded). Use of sputum induction, side-effects, and complications were not recorded. A mycobacterial blood culture (BacT/Alert specimen bottle) wasperformed and urine collectionwas started in June 2012 when extra funding became available, with later batch testing using a LAM assay (Alere Determine TB LAM Ag; Alere Inc., Waltham, MA, USA). Results were read independently by two experienced technicians who were blinded to the other results and were deemed positive if both readers scored ≥2+ using the preJanuary 2014 manufacturer’s reference card. Further investigations for tuberculosis were at the discretion of the admitting medical team. Confirmed tuberculosis was defined as any positive culture during the patient admission.
For the purposes of the present study, only patients with a urine LAM result were included. Diagnostic accuracy was determined for the LAM and Xpert MTB/RIF tests when sputum was spontaneously expectorated and when sputum induction was available for patients unable to spontaneously produce sputum. Various algorithms for using LAM, Xpert MTB/RIF with and without sputum induction, and a single sputum culture were modelled to determine yield and diagnostic accuracy.
The cost of investigations per patient was determined by calculating the proportion of patients who would need each test using a given diagnostic algorithm. The cost of DST using a line probe assay when sputum culture was positive was included. Assumed costs were $32 for Xpert MTB/RIF (Meyer-Rath et al., 2012), $12 for culture with no growth(Meyer-Rathetal.,2012), $40 for culture with growth and line probe assay (Meyer-Rath et al., 2012), $3.50 for LAM (Sun et al., 2013), and $15 for sputum induction (Harris et al., 2011).
Statistical analysis
The sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) were calculated for the urine LAM and sputum (induced and spontaneous) Xpert tests using culture-positive tuberculosis as the reference standard for confirmed tuberculosis.
Ethical approval
The University of Cape Town Human Research Ethics Committee approved the study protocol. All enrolled participants signed an informed consent agreement. Confused participants were enrolled and given the option to continue with participation once they were orientated.
Results
Three hundred and thirty-two patients had LAM, Xpert MTB/ RIF, and tuberculosis culture results available and were included in this analysis; 169 of 332 (50.9%) had confirmed tuberculosis. Baseline characteristics are shown in Table 1. Median age was 36 years and 66% were female. The median CD4 count was 107 cells/µ1 and 39% were currently using antiretroviral therapy (ART). The most common WHO danger sign was tachycardia (77%) and most had at least one tuberculosis symptom in addition to cough. No patient was excluded due to an inability to produce urine, although from the original cohort, 26 were excluded as they were unable to produce sputum even after induction. The median CD4 count among those with induced sputum samples was 97 cells/µ1 (interquartile range (IQR) 35–228) and among those not induced was 129 cells/µ1 (IQR 55–219).
Table 1.
Baseline characteristics of 332 patients with urine LAM and sputum Xpert MTB/RIF results.
| Variables | Missing | ||
|---|---|---|---|
| Baseline variables | |||
| Age | Median (IQR) | 36.2 (29.7–42.1) | |
| Sex (female) | n (%) | 220 (66.3) | |
| BMI (kg/m2) | Median (IQR) | 20.6 (18.4–25.0) | 8 |
| CD4 (cells/µ1) | Median (IQR) | 107 (38–228) | 1 |
| Cough duration (days) | Median (IQR) | 14 (7–21) | 1 |
| Cough duration ≥14 days | n (%) | 203 (61.3) | 1 |
| Hypotensive | n (%) | 81 (24.4) | |
| Confused | n (%) | 58 (17.5) | |
| Using ART | n (%) | 128 (38.6) | |
| Duration on ART (days) | Median (IQR) | 998 (178–1928) | 7 |
|
WHO danger signs | |||
| Respiratory rate >30/min | n (%) | 203 (63.2) | 11 |
| Heart rate >120/min | n (%) | 256 (77.1) | |
| Temp >39 °C | n (%) | 45 (13.6) | |
| Unable to walk unaided | n (%) | 157 (48.9) | 11 |
|
Tuberculosis symptoms | |||
| Fever | n (%) | 272 (81.9) | |
| Night sweats | n (%) | 222 (67.1) | 1 |
| Weight loss | n (%) | 313 (94.3) | |
|
Laboratory investigations | |||
| Haemoglobin (g/dl) | Median (IQR) | 9.9 (8.1–11.5) | 1 |
| WCC (× 109/l) | Median (IQR) | 9.2 (5.8–13.5) | 1 |
LAM, lateral flow lipoarabinomannan test; IQR, interquartile range; BMI, body mass index; ART, antiretroviral therapy; WHO, World Health Organization; WCC, white cell count.
The LAM test was positive in 60 of 169 cases (yield 35.5%). With regard to diagnostic accuracy, sensitivity was 35.5%, specificity 93.3%, PPV 84.5%, and NPV 58.2% (Tables 2 and 3). The sensitivity of the LAM assay was 51.1% (95% confidence interval (CI) 40.4–61.7%) in those with a CD4 count <100 cells/µ1 compared to 17.1% (95% CI 9.4–27.57%) in those with a CD4 count >100 cells/µ1 (17%).
Table 2.
Diagnostic accuracy of LAM in a cohort of 332 seriously ill HIV-infected patients.
| Confirmed TB | Not TB | Total | |
|---|---|---|---|
| LAM positive | 60 | 11 | 71 |
| LAM negative | 109 | 152 | 261 |
| Total | 169 | 163 | 332 |
LAM, urine lateral flow lipoarabinomannan test; TB, tuberculosis.
Table 3.
Diagnostic accuracy of LAM, Xpert Spot, and Xpert SI in a cohort of 332 seriously ill HIV-infected patients with a prevalence of tuberculosis of 51%.
| Test | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| LAM | 35.5 | 93.3 | 84.5 | 58.2 |
| Xpert Spota | 92.9 | 97.8 | 97.5 | 93.8 |
| Xpert SIb | 90.5 | 94.5 | 94.4 | 90.6 |
LAM, urine lateral flow lipoarabinomannan test; PPV, positive predictive value; NPV, negative predictive value.
Xpert Spot: test only performed if the patient could produce sputum spontaneously, for 88 patients who were able to produce sputum spontaneously.
Xpert SI: includes spontaneously produced sputum and induced sputum.
Spontaneously produced sputum was available from 88 of 332 (26.5%) patients, and the Xpert MTB/RIF on these specimens (Xpert Spot) was positive in 39 of 169 cases (yield 23.1%), with sensitivity and specificity, based on the 88 patients who produced spontaneous sputum, of 92.9% and 97.8%, respectively. The remaining 244 patients produced sputum following sputum induction, and Xpert MTB/RIF on these specimens yielded a further 114 cases, giving a total yield of Xpert MTB/RIF on spontaneous and induced specimens (Xpert SI) of 153/169 (90.5%). With regard to diagnostic accuracy of Xpert SI, sensitivity was 90.5%, specificity 94.5%, PPV 94.4%, and NPV 90.6% (Table 3).
The incremental yield of LAM, Xpert Spot, Xpert SI, and a single sputum for mycobacterial culture was determined in this cohort (Figure 1). In nine cases, two sputum cultures taken on the same day gave discordant results and it was not possible to determine whether the first or second was positive. For the purposes of evaluation, it was assumed that there was a 50% chance that the first sample was positive in each case. Performing the LAM test prior to Xpert Spot increased the yield from 23.1% to 50.9%, an incremental yield of 27.8%. Performing LAM prior to Xpert SI increased the yield from 90.5% to 92.3%, an incremental yield of 1.8%. The incremental yield of culture was low, ranging from 1.2% to 4.5% depending on the initial testing strategy (Table 4). Patient flow for the whole cohort with and without the LAM test is shown in Figures 2 and 3.
Figure 1.
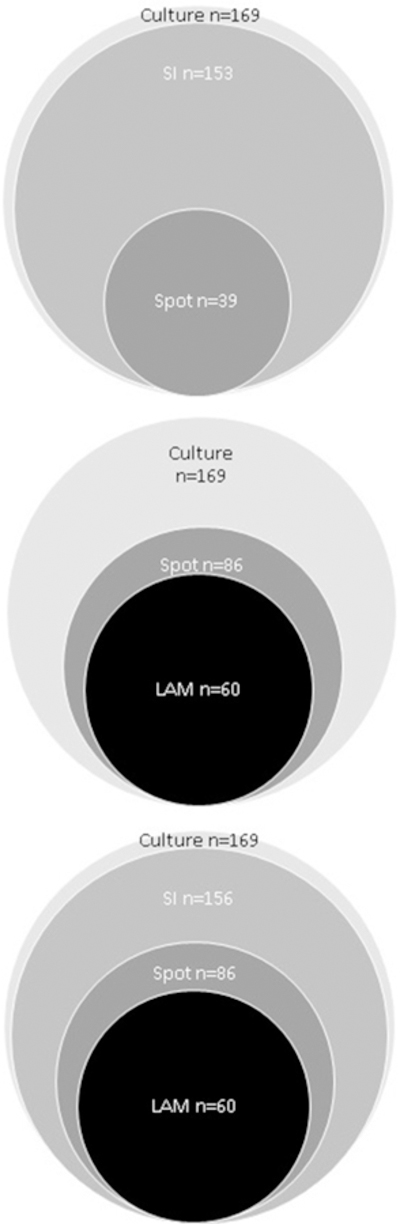
Diagram showing the incremental yield of tuberculosis diagnoses according to the sequence of test performance for 332 patients with HIV, cough, and World Health Organization danger signs.
LAM = urine lipoarabinomannan.
Xpert Spot = test only performed if the patient could produce sputum spontaneously.
Xpert SI = includes spontaneously produced sputum and induced sputum.
Table 4.
Yield number of tests, and cost per patient according to the sequence of tests for 332 patients suspected of having tuberculosis.a
| Tests | Yield | Incremental yield of culture (%) |
Xpert, n (%) | LAM, n (%) | Induction of sputum, n (%) |
Mycobacterial culture, n (%) |
DST, n (%) | Cost/patient ($US) |
|---|---|---|---|---|---|---|---|---|
| Xpert Spotb Culture |
24.3 | 1.2 | 88 (26.5) | 0 (0) | 0 (0) | 48 (14.5) | 2 (0.6) | 10.5 |
| LAM Xpert Spotb |
50.9 | N/A | 73 (22.0) | 332 (100) | 0 (0) | 0 (0) | 0 (0) | 10.5 |
| LAM Xpert Spotb Culture |
52.1 | 1.2 | 73 (22.0) | 332 (100) | 0 (0) | 47 (14.2) | 2 (0.6) | 12.5 |
| LAM Xpert SIc |
92.3 | N/A | 261 (78.6) | 332 (100) | 188 (56.7) | 0 (0) | 0 (0) | 37.2 |
| Xpert SIc Culture |
95.0 | 4.5 | 332 (100) | 0 (0) | 244 (73.5) | 170 (51.2) | 7.5d (2.3) | 49.6 |
| LAM Xpert SIc Culture |
95.9 | 3.6 | 261 (78.6) | 332 (100) | 188 (56.7) | 160 (48.2) | 6d (1.8) | 42.0 |
LAM, urine lateral flow lipoarabinomannan test; DST, drug susceptibility testing by line probe assay; N/A, not applicable.
Where multiple tests are shown, it is assumed that testing is performed in series and terminates when a test is positive. Columns represent the proportion of patients who would receive each test if the sequence was followed in this way. Total costs per patient are calculated based on these proportions and the costs of each test.
Xpert Spot = test only performed if the patient could produce sputum spontaneously.
Xpert SI = includes spontaneously produced sputum and induced sputum.
Includes patients where two sputum cultures taken on the same day gave discordant results and it was not possible to determine whether the first or second was positive. For the purposes of evaluation, it was assumed that there was a 50% chance that the first sample was positive in each case.
Figure 2.
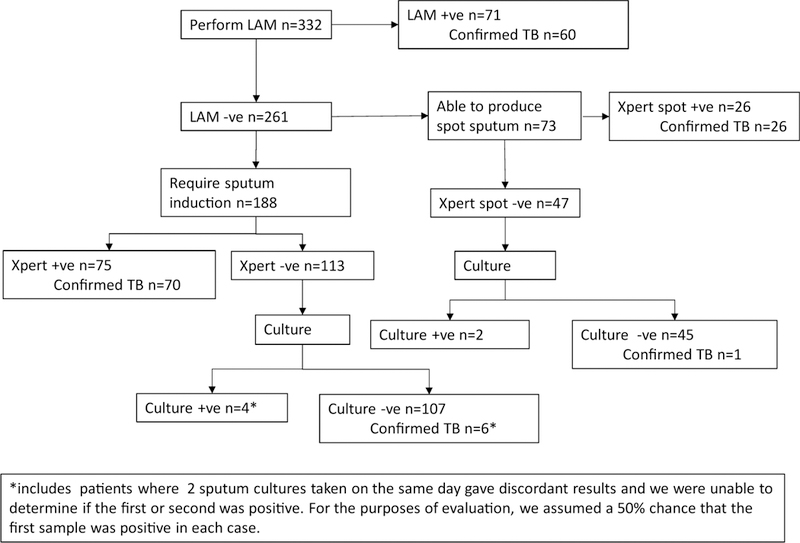
Flow of patients according to test results, beginning with urine LAM, for 332 patients with HIV, cough and WHO danger signs.
Figure 3.
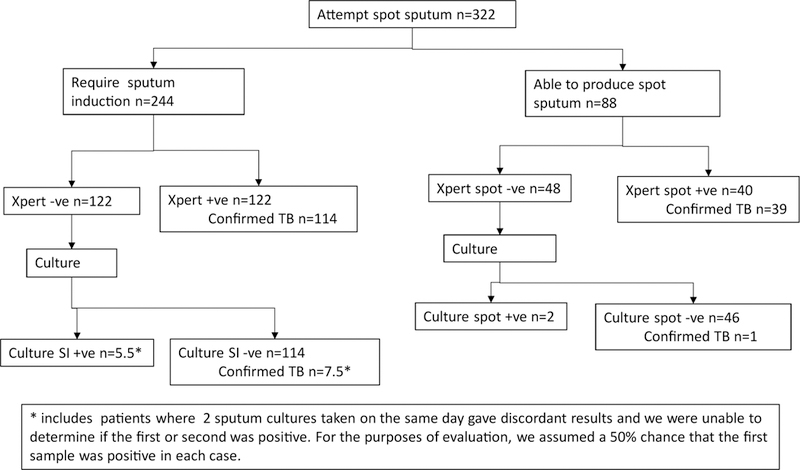
Flow of patients according to test results, beginning with self-expectorated sputum, for 332 patients with HIV, cough, and World Health Organization danger signs.
Algorithms based on performing tests in sequence and only moving to the next test if the previous one is negative were simulated. The number of tests and cost per patient for each algorithm are shown in Table 4. A trend towards costs increasing as the yield of the algorithm increases was seen. Of the three algorithms with a yield >92%, the cheapest was LAM and Xpert SI at $37.20 per patient and the most expensive was Xpert SI and culture at $49.60 per patient. The best performing algorithm of LAM, Xpert SI, and culture cost $42 per patient.
Discussion
This study of inpatients fulfilling the criteria of the 2016 WHO algorithm for seriously ill HIV-infected patients showed that the yield of the LAM test was 35.5% and the yield of the Xpert Spot was low at 23.1%, but could be improved to 90.5% if sputum induction was used. LAM increased the yield of Xpert Spot to 50.9% (incremental yield 27.8%) and Xpert SI to 92.3% (incremental yield 1.8%). Performing a single mycobacterial culture on sputum when the Xpert MTB/RIF is negative only had a small incremental yield. Diagnostic algorithms including Xpert SI had excellent diagnostic accuracy with PPV ranging from 90.7% to 96.4% and NPV from 91.9% to 95.5%. There were small incremental benefits of diagnostic accuracy by including LAM and culture, but the inclusion of LAM reduced costs.
A systematic review and meta-analysis of the LAM assay for the diagnosis of tuberculosis using a grade 2 cut-off and (pre-January 2014 manufacturer’s reference card) a microbiological reference standard, showed median pooled sensitivity and specificity of 45% (credible interval (CrI) 29–63%) and 92% (CrI 80–97%), respectively (Hepple et al., 2012); pooled sensitivity was higher in participants with a CD4 count ≤100 cells/µl . It has been suggested that the specificity of the LAM assay was underestimated in this meta-analysis due to the use of inappropriate reference standards in several studies (Lawn et al., 2015a). The present study data are consistent with those of the meta-analysis by showing the sensitivity and specificity of LAM to be 35.5% and 93.3%, respectively, in patients with a median CD4 count of 107 cells/µ1 . The sensitivity of the LAM assay is at the lower end of the expected range, as has been found previously in studies in which patients who were unable to produce sputum were excluded (Hepple et al., 2012).
Two studies have reported the incremental yield of LAM compared to Xpert MTB/RIF. Lawn et al. (2017) recently found the yield of LAM in unselected HIV-infected patients admitted to hospital in Cape Town (median CD4 count of 80 cells/µ1) to be 38.1%. They found a much lower yield of Xpert SI at 26.6%, which could be explained by the inclusion of patients regardless of symptoms, only 37% of whom could produce a sputum sample after induction. Consequently, the incremental yield of LAM compared to Xpert SI was higher than found in the present study at 25.7%. Peter et al. (2015) included outpatients suspected of having tuberculosis who could self-expectorate sputum with a median CD4 count of 210 cells/µ1. Yield was 22.7% for LAM and 76.1% for Xpert MTB/RIF; the incremental yield of LAM compared to Xpert MTB/RIF was low at 2.1%.
A systematic review and meta-analysis of Xpert MTB/RIF for the diagnosis of tuberculosis in patients with HIV found a pooled sensitivity of 79% (95% CrI 70–86%) and pooled specificity of 98% (CrI 96–99%) (Steingart et al., 2014). The present study found a higher sensitivity (90.5%) and a lower specificity (94.5%). One possible explanation for the high sensitivity in the present study is the exclusion of patients who were unable to provide a spontaneous or induced sample of sputum. In some studies, such patients have been considered sputum Xpert MTB/RIF-negative for the purpose of defining diagnostic accuracy (Lawn et al., 2015b), resulting in a reduction in reported sensitivity. Another reason for improved sensitivity of Xpert MTB/RIF in the present study was the use of sputum induction, which has been shown to increase yield (Gonzalez-Angulo et al., 2012).
A model of cost-effectiveness has suggested that the addition of LAM to a strategy including Xpert MTB/RIF would result in an incremental cost of $731 per disability-adjusted life year (DALY) averted in South Africa (95% uncertainty range: $234–$12 397) (Sun et al., 2013). However, this model did not consider the strategy of only performing Xpert MTB/RIF when LAM is negative and therefore overestimated costs. The present study suggests a cost saving on tests of $7.50 per patient when LAM is performed before Xpert MTB/RIF in a setting where sputum induction is available and culture is used. This is likely to be an underestimate of the total cost saving in clinical practice, as additional savings may come from the reduced need for other tests to diagnose tuberculosis, such as abdominal ultrasound scans.
This study suggests that there is a large incremental yield of sputum induction in seriously ill patients with cough and that LAM provides a cost saving. Previous data suggest that the incremental yield of LAM is higher in unselected seriously ill patients who might not be able to produce sputum and that LAM rapidly identifies individuals with a poor prognosis (Lawn et al., 2017). An update to the WHO algorithm for seriously ill HIV-infected patients is proposed based on these findings (Figure 4). It is suggested that the LAM assay be used as the first test, followed by immediate anti-tuberculosis therapy when positive. Xpert Ultra, which has been developed as a more sensitive next-generation assay, has been found in a multicentre diagnostic accuracy study to be significantly more sensitive but less specific than Xpert MTB/RIF, particularly in sputum smear-negative patients with HIV (Dorman et al., 2018). Xpert Ultra is likely to replace Xpert MTB/RIF as the initial sputum test for patients with HIV who are suspected of having tuberculosis. It is therefore suggested that Xpert Ultra be performed on sputum with induction where necessary in all seriously ill patients with negative LAM results. Due to the enhanced sensitivity of Xpert Ultra, it is highly unlikely that patients with a negative LAM and Xpert Ultra have pulmonary tuberculosis, so it is suggested that additional mycobacterial culture of sputum is not necessary, although it is acknowledged that tuberculosis can be present despite multiple negative cultures.
Figure 4.
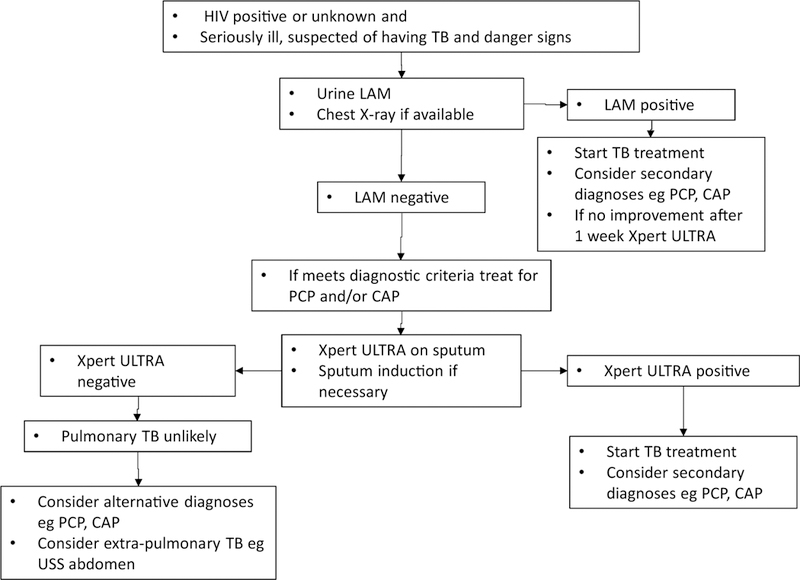
Proposed algorithm for the investigation and treatment of seriously ill HIV-patients with World Health Organization danger signs.
A potential disadvantage of the LAM assay compared to Xpert MTB/RIF or mycobacterial culture and susceptibility testing is that it does not determine drug resistance. In this study, four of 60 patients with positive LAM results and confirmed tuberculosis had rifampicin resistance. This has the potential to delay the initiation of appropriate treatment, although the duration of delay and clinical significance is unclear. It is suggested that in resource-poor settings, the Xpert test should only be performed when LAM is negative. In practice, testing for rifampicin resistance using Xpert Ultra in any patient with a positive LAM result who has not responded appropriately to therapy after 1 week is suggested. The clinical outcomes and cost-effectiveness of this strategy are yet to be determined.
This study has several limitations. First, the findings may not be generalizable to settings with a lower prevalence of tuberculosis. Second, all participants had a cough, which is no longer an inclusion criterion for the WHO algorithm for seriously ill HIV-infected patients. Third, patients who were unable to produce a sputum sample after induction were excluded, and these may reflect sicker patients with a higher prevalence of tuberculosis. Strengths of this study are its prospective design, the use of multiple cultures, and sputum induction in participants unable to produce sputum in order to establish a reference standard with high sensitivity.
In conclusion, in patients with cough meeting inclusion criteria for the 2016 WHO algorithm for seriously ill HIV-infected patients, sputum induction leads to a large increase in the yield of tuberculosis diagnosed by Xpert MTB/RIF. Access to sputum induction is therefore an important part of strategies to improve tuberculosis diagnosis in seriously ill patients. The LAM assay gives a small incremental yield when sputum induction is available, but has other advantages including cost saving and identification of patients with a poor prognosis. The WHO algorithm for seriously ill patients with HIV should begin with LAM and emphasize the importance of sputum induction.
Acknowledgments
Funding
This work was supported by the National Institutes of Health (grant number R01 AI 96735–01 IRIDA).
Footnotes
Ethics statement
The University of Cape Town Human Research Ethics Committee approved the study protocol. All enrolled participants signed informed consent. Confused participants were enrolled and given the option to continue with participation once they were orientated.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Dorman SE, Schumacher SG, Alland D, Nabeta P, Armstrong DT, King B, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis 2018;18(1):76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Angulo Y, Wiysonge CS, Geldenhuys H, Hanekom W, Mahomed H, Hussey G, et al. Sputum induction for the diagnosis of pulmonary tuberculosis: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis 2012;31 (7):1619–30. [DOI] [PubMed] [Google Scholar]
- Griesel R, Stewart A, van der Plas H, Sikhondze W, Rangaka MX, Nicol MP, et al. Optimizing tuberculosis diagnosis in human immunodeficiency virus-infected inpatients meeting the criteria of seriously ill in the World Health Organization algorithm. Clin Infect Dis 2018;66(9):1419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in postmortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS 2015;29(15):1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JR, Marston BJ, Sangrujee N, DuPlessis D, Park B. Cost-effectiveness analysis of diagnostic options for pneumocystis pneumonia (PCP). PLoS One 2011;6(8) e23158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepple P, Ford N, McNerney R. Microscopy compared to culture for the diagnosis of tuberculosis in induced sputum samples: a systematic review. Int J Tuberc Lung Dis 2012;16(5):579–88. [DOI] [PubMed] [Google Scholar]
- Lawn SD, Kerkhoff AD, Nicol MP, Meintjes G. Underestimation of the true specificity of the urine lipoarabinomannan point-of-care diagnostic assay for HIV-associated tuberculosis. J Acquir Immune Defic Syndr 2015a;69(4):e144–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn SD, Kerkhoff AD, Burton R, Schutz C, van Wyk G, Vogt M, et al. Rapid microbiological screening for tuberculosis in HIV-positive patients on the first day of acute hospital admission by systematic testing of urine samples using Xpert MTB/RIF: a prospective cohort in South Africa. BMC Med 2015b;13:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn SD, Kerkhoff AD, Burton R, Schutz C, Boulle A, Vogt M, et al. Diagnostic accuracy, incremental yield and prognostic value of Determine TB-LAM for routine diagnostic testing for tuberculosis in HIV-infected patients requiring acute hospital admission in South Africa: a prospective cohort. BMC Med 2017;15(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Rath G, Schnippel K, Long L, MacLeod W, Sanne I, Stevens W, et al. The impact and cost of scaling up GeneXpert MTB/RIF in South Africa. PLoS One 2012;7(5) e36966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter J, Theron G, Chanda D, Clowes P, Rachow A, Lesosky M, et al. Test characteristics and potential impact of the urine LAM lateral flow assay in HIV-infected outpatients under investigation for TB and able to self-expectorate sputum for diagnostic testing. BMC Infect Dis 2015;15:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter JG, Zijenah LS, Chanda D, Clowes P, Lesosky M, Gina P, et al. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: a pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet 2016;387 (10024):1187–97. [DOI] [PubMed] [Google Scholar]
- Shah M, Hanrahan C, Wang ZY, Dendukuri N, Lawn SD, Denkinger CM, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults. Cochrane Database Syst Rev 2016;Cd011420. [DOI] [PMC free article] [PubMed]
- Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2014;Cd009593. [DOI] [PMC free article] [PubMed]
- Sun D, Dorman S, Shah M, Manabe YC, Moodley VM, Nicol MP, et al. Cost utility of lateral-flow urine lipoarabinomannan for tuberculosis diagnosis in HIV-infected African adults. Int J Tuberc Lung Dis 2013;17(4):552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach 2016. Available from: http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf?ua=1. [PubMed]


