Abstract
BACKGROUND
Incident heart failure (HF) is increased in persons with human immunodeficiency virus (PHIV). Protease inhibitors (PIs) are associated with adverse cardiac remodeling and vascular events; however, there are no data on the use of PIs in PHIV with HF.
OBJECTIVES
This study sought to compare characteristics, cardiac structure, and outcomes in PHIV with HF who were receiving PI-based versus non-PI (NPI) therapy.
METHODS
This was a retrospective single-center study of all 394 antiretroviral therapy–treated PHIV who were hospitalized with HF in 2011, stratified by PI and NPI. The primary outcome was cardiovascular (CV) mortality, and the secondary outcome was 30-day HF readmission rate.
RESULTS
Of the 394 PHIV with HF (47% female, mean age 60 ± 9.5 years, CD4 count 292 ± 206 cells/mm3),145 (37%) were prescribed a PI, whereas 249 (63%) were prescribed NPI regimens. All PI-based antiretroviral therapy contained boosted-dose ritonavir. PHIV who were receiving a PI had higher rates of hyperlipidemia, diabetes mellitus, and coronary artery disease (CAD); higher pulmonary artery systolic pressure (PASP); and lower left ventricular ejection fraction. In follow-up, PI use was associated with increased CV mortality (35% vs. 17%; p < 0.001) and 30-day HF readmission (68% vs. 34%; p < 0.001), effects seen in all HF types. Predictors of CV mortality included PI use, CAD, PASP, and immunosuppression. Overall, PIs were associated with a 2-fold increased risk of CV mortality.
CONCLUSIONS
PI-based regimens in PHIV with HF are associated with dyslipidemia, diabetes, CAD, a lower left ventricular ejection fraction, and a higher PASP. In follow-up, PHIV with HF who are receiving a PI have increased CV mortality and 30-day HF readmission.
Keywords: antiretroviral therapy, heart failure, heart failure readmission, human immunodeficiency virus
The long-term survival of persons with human immunodeficiency virus (HIV) (PHIV) has improved (1). This improved survival in large part reflects the use of effective and tolerable antiretroviral therapy (ART) (1,2). Classes of ART include nucleoside reverse transcriptase inhibitors, nonnucleoside reverse transcriptase inhibitors, protease inhibitors (PIs), integrase inhibitors, and fusion inhibitors (3). Previous analyses have demonstrated associations between the use of some PIs and vascular events such as stroke and myocardial infarction (4–6).
In animal models, PIs are associated with an increase in transforming growth factor beta-1, leading to myocardial fibrosis and impaired cardiac function (7,8). Furthermore, echocardiographic studies in PHIV without known cardiovascular (CV) disease have shown that PIs are associated with left ventricular hypertrophy (9). It is therefore plausible that the use of PI-based ART may be associated with adverse outcomes in PHIV with heart failure (HF). Determination of whether PI-based ART regimens affect HF outcomes may be important for the following reasons: 1) the risk of incident HF is increased more than 2-fold among PHIV (10,11) and, as the group of PHIV ages, rates of incident HF are projected to increase dramatically (12); and 2) once HF is established in HIV infection, there is a 4-fold increased risk of being admitted for decompensated HF and a 3-fold increase in CV mortality (13). Therefore, we aimed to study the association between PI use and CV outcomes in PHIV with HF. We hypothesized that PI-based therapy would be associated with adverse CV changes and adverse outcomes in HF.
METHODS
STUDY DESIGN AND PATIENT GROUP.
After obtaining Institutional Board Review approval, we retrospectively analyzed the data on 2,578 patients admitted to a U.S. tertiary care hospital (Bronx-Lebanon Hospital Center of Icahn School of Medicine at Mount Sinai, Bronx, New York) in 2011 with a primary diagnosis of acute decompensated HF. HF admission was defined according to the American College of Cardiology and American Heart Association key data ele ments and definitions for CV endpoint events: a hospital admission with a primary diagnosis of HF and length of stay of at least 24 h, with new or worsening symptoms of HF on presentation, objective evidence of new or worsening HF, and initiation or intensification of treatment specifically for HF (14).Individuals who had a recent (≤3 months) history of cardiac surgery (n = 72), were excluded from the study. We further excluded patients without HIV infection (n = 1,838) and PHIV who were not prescribed ART (n = 24) (Figure 1), thus resulting in a final study group of 394 PHIV with HF. The cohort was further stratified by HF with reduced ejection fraction (HFrEF) (left ventricular ejection fraction [LVEF] <40%), HF with borderline ejection fraction (HFbEF) (LVEF 40% to 49%), and HF with preserved ejection fraction (HFpEF) (LVEF ≥50%) (Figure 1). The diagnoses of HIV infection and HF, as well as other clinically relevant variables, were ascertained in each patient through manual review of each of individual electronic health record (EHR).
FIGURE 1. Consort Diagram for the Study.
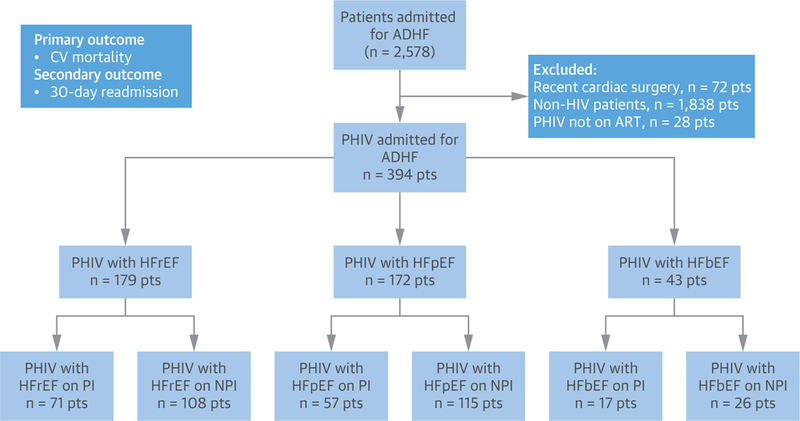
ADHF = acute decompensated heart failure; CV = cardiovascular; HFbEF = heart failure with borderline ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HIV = human immunodeficiency virus; NPI = non–protease inhibitor antiretroviral therapy; PHIV = persons with human immunodeficiency virus; PI = protease inhibitor (ritonavir boosted); Pts = patients.
COVARIATES.
Through EHR review, data were collected on traditional HF risk factors (including hypertension, dyslipidemia, diabetes mellitus, coronary artery disease [CAD], family history of CAD, body mass index, previous or active cigarette smoking, and previous or active cocaine use). During EHR review, data were collected on LVEF and medication use at the time of discharge from the index HF hospitalization. Additional data included information on socioeconomic status (education level and employment status). For the purposes of this study, assignment to a PI regimen was made by reviewing the medications at the time of discharge. Details on HIV-specific parameters (CD4, viral load [VL]) were recorded from those available closest to the time of discharge from the index HF hospitalization (all were within 1 month of the date of discharge).
OUTCOMES.
Our primary outcome was CV mortality, defined as death resulting from HF, sudden cardiac death, arrhythmias, and/or acute ischemic events(15). Death was determined through the Social Security Death Index (SSDI), and cause of death was confirmed by physician-adjudicated individual EHR review. The secondary outcome was 30-day HF hospital readmission rate, defined as described earlier and ascertained through physician-adjudicated individual EHR review. All outcomes were adjudicated by a physician blinded to all other variables including HIV drug class. The follow-up period began on the date of discharge from the first HF hospitalization in 2011.
STATISTICAL ANALYSIS.
Continuous variables are presented as mean ± SD or median (interquartile range [IQR]), as appropriate on the basis of normality, and categorical variables are presented as percentages. Continuous data were compared with the use of unpaired Student’s t-tests or Wilcoxon rank sum tests, as appropriate. Categorical data were compared using the chi-square or the Fisher exact test. Clinical parameters at index HF hospitalization and subsequent outcomes were compared among the following groups: 1) PHIV with HF stratified by PI versus NPI;2) PHIV with HFrEF stratified by PI versus NPI;3) PHIV with HFpEF stratified by PI versus NPI; and4) PHIV with HFbEF stratified by PI versus NPI. Survival curves were plotted using Kaplan-Meier curves.
Univariate and multivariate regression analyses were performed to determine the association between baseline covariates and the CV mortality rate. Multivariate Cox proportional hazard regression analyses for CV mortality rate were constructed using a p <0.01 on the univariate analysis for entry. Otherwise, statistical significance was defined using a 2-tailed p value ≤0.05. Both VL and CD4 count were not included in the multivariate model together because of the overlap between those individuals with a low CD4 count and those with a high VL. Statistical analyses were performed using SPSS software version 24 (IBM Corp., Armonk, New York).
RESULTS
BASELINE CHARACTERISTICS. PHIV with HF.
Among PHIV receiving ART and hospitalized with HF, the median duration of prescribed ART was 8.5 years (IQR: 4 to 16 years), the mean CD4 count was 295 cells/mm3, and the median VL was 274 copies/ml (range <50 to 3,000,457 copies/ml). A total of 58% (228 of 394) of patients had a CD4 count of ≥200 cells/mm3, and 62% (244 of 394) had a VL <200 copies/ml. Of the 394 PHIV taking ART, 145 patients (37%) had a PI-based regimen, whereas 249 patients (63%) had an NPI-based regimen. All PI-based regimens were ritonavir boosted. PI regimens prescribed in our cohort are listed in Online Table 1. When groups were stratified by a PI-based ART regimen versus a NPI-based regimen, those patients with a PI-based ART regimen were more likely to have hyperlipidemia (52% vs. 35%; p < 0.001), diabetes mellitus (44% vs. 31%; p = 0.012), CAD (52% vs. 33%; p < 0.001), higher pulmonary artery pressure (PASP) (48 ± 9.8 mm Hg vs. 43 ± 9.0 mm Hg; p < 0.001), and lower LVEF (44 ± 14.0% vs. 49 ± 12.0%; p = 0.003) (Table 1).
TABLE 1.
Baseline Characteristics
| PHIV (n = 394) | PI (n = 145) | NPI (n = 249) | p Value | |
|---|---|---|---|---|
| Female | 185 (47) | 65 (45) | 120 (48) | 0.519 |
| BMI, kg/m2 | 27.0± 5.8 | 28.0± 5.8 | 26.0± 5.8 | 0.011 |
| Age, yrs | 60.0± 9.5 | 59.0± 10.0 | 60.0± 9.2 | 0.314 |
| Race | ||||
| Hispanic | 126 (32) | 51 (35) | 77 (31) | 0.572 |
| African American | 169 (43) | 62 (43) | 107 (43) | |
| Others | 99 (25) | 32 (22) | 65 (26) | |
| CV risk factors | ||||
| Diabetes | 142 (36) | 64 (44) | 78 (31) | 0.012 |
| Hypertension | 241 (61) | 101 (69) | 140 (56) | 0.008 |
| Hyperlipidemia | 162 (41) | 76 (52) | 86 (35) | <0.001 |
| Smoking | 193 (49) | 69 (47) | 124 (50) | 0.671 |
| CAD | 158 (40) | 75 (52) | 83 (33) | <0.001 |
| Myocarditis | 47 (12) | 19 (13) | 28 (11) | 0.583 |
| ICD | 40 (10) | 22 (15) | 18(7) | 0.011 |
| Cocaine use | 131 (33) | 52 (36) | 79 (32) | 0.400 |
| SBP, mm Hg | 143.0± 27.7 | 144.0± 28.2 | 142.0± 27.8 | 0.494 |
| DBP, mmHg | 77.0± 18.2 | 79.0± 19.6 | 76.0± 20.1 | 0.150 |
| Heart rate, beats/min | 84.0± 21.4 | 82.0± 23.2 | 85.0± 22.4 | 0.207 |
| PASP, mm Hg | 45.0± 9.5 | 48.0± 9.8 | 43.0± 9.0 | <0.001 |
| LVEF, % | 47.0± 12.7 | 44.0± 14.3 | 48.0± 12.0 | 0.003 |
| SA | 113 (29) | 39 (27) | 74 (30) | 0.550 |
| HCV | 87 (22) | 35 (24) | 52 (21) | 0.452 |
| HIV parameters | ||||
| CD4 count at 1st HF admission, cells/mm3 | 295± 207 | 284± 213 | 295± 201 | 0.609 |
| Nadir CD4 count, cells/mm3 | 245±197 | 213± 184 | 276± 212 | 0.003 |
| VL <200 copies/ml | 244 (62) | 87 (60) | 157 (63) | 0.547 |
| Duration of ART, yrs | 8 (4–16) | 8 (4–16) | 9 (4–16) | 0.217 |
| Duration of PI, yrs | – | 6 (4–11) | – | – |
| Duration of HIV* | 8 (4–16) | 8 (4–16) | 9 (4–16) | 0.217 |
| Duration of untreated HIV† | 75 (48–107) | 76 (48–107) | 74 (47–104) | 0.659 |
| Socioeconomic parameters | ||||
| High school or GED completion | 252 (64) | 96 (66) | 156 (62) | 0.478 |
| Unemployment | 47 (12) | 19 (13) | 28 (11) | 0.583 |
| ART medications | ||||
| NRTIs | 394 (100) | 145 (100) | 249 (100) | >0.05 |
| INSTIs | 106 (27) | 6(4) | 92 (37) | <0.001 |
| NNRTIs | 165 (42) | 6(4) | 157 (63) | <0.001 |
| HF medications | ||||
| Beta-blocker | 351 (89) | 128 (88) | 223 (89) | 0.738 |
| ACE inhibitor or ARB | 302 (86) | 108 (85) | 194 (87) | 0.437 |
| Spironolactone | 36 (9) | 17(12) | 19 (8) | 0.174 |
| Furosemide | 311 (79) | 105 (72) | 206 (83) | 0.015 |
Values are n (%), mean± SD, or median (interquartile range). Bold p values are statistically significant.
Duration of HIV is in years.
Duration of untreated HIV is the duration before starting ART after the diagnosis in days.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; ART = antiretroviral therapy; BMI = body mass index; CAD = coronary artery disease; CV = cardiovascular; DBP = diastolic blood pressure; GED = general equivalency diploma; HCV = hepatitis C virus infection; HIV = human immunodeficiency virus; ICD = implantable cardioverter-defibrillator; INSTI = integrase inhibitor; LVEF = left ventricular ejection fraction; NNRTI = non-nucleoside reverse transcriptase inhibitor; NPI = non–protease inhibitor antiretroviral therapy; NRTI = nucleoside reverse transcriptase inhibitor; PASP = pulmonary artery systolic pressure; PHIV = persons with human immunodeficiency virus; PI = ritonavir-based protease inhibitor; SA = sleep apnea; SBP = systolic blood pressure; VL = viral load.
PHIV with HFrEF.
There were 179 PHIV with HFrEF who were receiving ART (median duration 8.5 years [IQR: 4 to 16 years]). Among PHIV with HFrEF and ART, the mean CD4 count was 299 cells/mm3, and the median VL was 273 copies/ml (range <50 to 3,000,457 copies/ml). A total of 57% (102 of 179) of patients had a CD4 count of ≥200 cells/mm3, and 60% (108 of 179) had VL <200 copies/ml. Of the 179 PHIV with HFrEF, 71 patients (40%) had a PI-based ART regimen, whereas 108 (60%) had an NPI-based regimen. When PHIV with HFrEF were stratified by PI-based ART versus NPI, findings were similar to those in the overall cohort (Table 2).
TABLE 2.
Baseline Characteristics (PHIV With HFrEF)
| HFrEF on PIs (n = 71) | HFrEF on NPI (n = 108) | p VaLue | |
|---|---|---|---|
| Female | 32 (45) | 54 (50) | 0.518 |
| BMI, kg/m2 | 27.0± 6.0 | 26.0± 5.6 | 0.257 |
| Age, yrs | 58.0± 10.0 | 60.0± 8.3 | 0.148 |
| Race | |||
| Hispanic | 26 (37) | 36 (33) | 0.899 |
| African American | 29 (41) | 47 (43) | |
| Others | 16 (22) | 25 (23) | |
| CV risk factors | |||
| Diabetes | 29 (41) | 30 (28) | 0.070 |
| Hypertension | 50 (70) | 61 (57) | 0.060 |
| Hyperlipidemia | 34 (48) | 33 (31) | 0.020 |
| Smoking | 39 (54) | 48 (45) | 0.169 |
| CAD | 41 (57) | 35 (34) | <0.001 |
| Myocarditis | 9(12) | 14(13) | 0.956 |
| ICD | 24 (34) | 16 (15) | 0.003 |
| Cocaine | 21 (30) | 39 (36) | 0.365 |
| SBP, mm Hg | 143.0± 26.2 | 141.0± 27.4 | 0.628 |
| DBP, mm Hg | 80.0± 19.2 | 78.0± 19.8 | 0.504 |
| Heart rate, beats/min | 83.0± 22.0 | 81.0± 21.1 | 0.542 |
| PASP, mm Hg | 50.0± 9.5 | 46.0± 8.4 | 0.003 |
| LVEF, % | 31.0± 7.1 | 39.0± 7.8 | <0.001 |
| SA | 18 (25) | 30 (28) | 0.720 |
| HCV | 20 (29) | 24 (23) | 0.366 |
| HIV parameters | |||
| CD4 count at 1st HF admission, cells/mm3 | 288± 215 | 310± 213 | 0.502 |
| Nadir CD4 count, cells/mm3 | 215± 182 | 281± 241 | 0.050 |
| VL <200 copies/ml | 42 (59) | 66 (63) | 0.793 |
| Duration of ART, yrs | 8 (4–16) | 9(4–16) | 0.111 |
| Duration of PI, yrs | 6 (4–11) | – | – |
| Duration of HIV* | 8 (4–16) | 9(4–16) | 0.111 |
| Duration of untreated HIV† | 76 (48–107) | 74 (46–106) | 0.689 |
| Socioeconomic parameters | |||
| High school or GED completion | 43 (60) | 68 (63) | 0.746 |
| Unemployment | 11 (16) | 15 (14) | 0.765 |
| ART medications | |||
| NRTIs | 71 (100) | 108(100) | >0.05 |
| INSTIs | 3(4) | 42 (39) | <0.001 |
| NNRTIs | 2(3) | 64 (59) | <0.001 |
| HF medications | |||
| Beta-blocker | 64 (90) | 95 (88) | 0.809 |
| ACE inhibitor or ARB | 63 (89) | 94 (87) | 0.818 |
| Spironolactone | 18 (25) | 18 (17) | 0.156 |
| Furosemide | 55 (78) | 93 (86) | 0.135 |
Values are n (%), mean± SD, or median (interquartile range). Bold p values are statistically significant.
Duration of HIV is in years.
Duration of untreated HIV is the duration before starting ART after the diagnosis in days.
HFrEF = heart failure with reduced ejection fraction; other abbreviations as in Table 1.
PHIV with HFpEF.
There were 172 PHIV with HFpEF who were receiving ART (median duration 8 years [IQR: 4 to 14 years]). In this group, the mean CD4 count was 291 cells/mm3, and the median VL was 307 copies/ml (range <50 to 3,000,457 copies/ml). A total of 60% (98 of 172) of patients had a CD4 count of ≥200 cells/mm3, and 63% (106 of 172) had VL <200 copies/ml. Of the 172 PHIV with HFpEF, 57 patients (33%) had a PI-based ART regimen, whereas 115 patients (67%) had an NPI. When PHIV with HFpEF were stratified by PI-based ART regimen versus NPI, findings were comparable with those of the overall cohort and the HFrEF group (Table 3).
TABLE 3.
Baseline Characteristics (PHIV With HFpEF)
| HfpEF on PI (n = 57) |
HfpEF on NPI (n = 115) |
p Value | |
|---|---|---|---|
| Female | 24 (43) | 56 (49) | 0.415 |
| BMI, kg/m2 | 28.0± 5.6 | 27.0± 5.6 | 0.272 |
| Age, yrs | 60.0± 9.0 | 60.0± 9.4 | 0.994 |
| Race | |||
| Hispanic | 19 (34) | 35 (30) | 0.701 |
| African American | 26 (46) | 49 (43) | |
| Others | 12 (21) | 31 (27) | |
| CV risk factors | |||
| Diabetes | 26 (45) | 40 (35) | 0.169 |
| Hypertension | 36 (64) | 64 (56) | 0.348 |
| Hyperlipidemia | 31 (54) | 40 (35) | 0.014 |
| Smoking | 20 (35) | 59 (51) | 0.044 |
| CAD | 28 (49) | 33 (29) | 0.008 |
| Myocarditis | 8 (14) | 13 (11) | 0.626 |
| Cocaine | 26 (46) | 32 (28) | 0.020 |
| SBP, mm Hg | 144.0± 25.8 | 141.0± 26.6 | 0.483 |
| DBP, mm Hg | 78± 18.8 | 79± 18.2 | 0.737 |
| Heart rate, beats/min | 80.0± 23.2 | 82.0± 22.7 | 0.590 |
| PASP, mm Hg | 47.0± 9.9 | 40.0± 8.1 | <0.001 |
| LVEF, % | 57.0± 5.3 | 56.0± 5.0 | 0.231 |
| SA | 16 (28) | 36 (31) | 0.663 |
| HCV | 11 (20) | 25 (22) | 0.711 |
| HIV parameters | |||
| CD4 count at 1st HF admission, cells/mm3 | 276± 215 | 291± 193 | 0.645 |
| Nadir CD4 count, cells/mm3 | 218± 185 | 272± 191 | 0.080 |
| VL <200 copies/ml | 34 (60) | 72 (63) | 0.707 |
| Duration of ART, yrs | 8 (4–14) | 9 (4–14) | 0.842 |
| Duration of PI, yrs | 6 (4–11) | – | – |
| Duration of HIV* | 8 (4–14) | 9 (4–14) | 0.842 |
| Duration of untreated HIV† | 75 (46–104) | 74 (46–106) | 0.632 |
| Socioeconomic parameters | |||
| High school or GED completion | 38 (66) | 72 (63) | 0.601 |
| Unemployment | 7(13) | 14 (12) | 0.983 |
| ART medications | |||
| NRTIs | 57 (100) | 115 (100) | >0.05 |
| INSTIs | 3(5) | 44 (38) | <0.001 |
| NNRTIs | 4(7) | 71 (62) | <0.001 |
| HF medications | |||
| Beta-blocker | 48 (85) | 102 (89) | 0.468 |
| ACE inhibitor or ARB | 47 (83) | 99 (86) | 0.651 |
| Spironolactone | 0(0) | 0(0) | |
| Furosemide | 39 (69) | 92 (80) | 0.093 |
Values are n (%), mean± SD, or median (interquartile range). Bold p values are statistically significant.
Duration of HIV is in years.
Duration of untreated HIV is the duration before starting ART after the diagnosis in days.
HFpEF = heart failure with preserved ejection fraction; other abbreviations as in Table 1.
PHIV with HFbEF.
There were 43 PHIV with HFbEF who were receiving ART (median duration 9.0 years [IQR: 4 to 16 years]). In this group, the mean CD4 count was 281 cells/mm3, and the median VL was 296 copies/ml (range <50 to 3,000,457 copies/ml). A total of 53% (23 of 43) of patients had a CD4 count of ≥200 cells/mm3, and 63% (33 of 43) had VL <200 copies/ml. Of the 43 PHIV with HFbEF, 17 patients (40%) had a PI, whereas 26 patients (60%) had an NPI.
OUTCOMES. CV mortality.
In the entire cohort of 394 patients, there were 93 CV deaths (23%) over 2 years of follow-up. The CV mortality rate was higher among PHIV hospitalized with HF who were taking a PI versus an NPI (35% vs. 17%; p < 0.001) (Figure 2A). Similar findings of increased CV mortality were noted when the cohort was stratified by the type of HF (HFrEF, HFpEF, and HFbEF). Specifically, among the PHIV hospitalized with HFrEF, the CV mortality rate was higher among the individuals who were taking a PI versus an NPI (36% vs. 21%; p = 0.021) (Figure 3A); among the PHIV hospitalized with HFpEF, the CV mortality rate was higher in the individuals taking a PI versus an NPI (33% vs. 15%; p = 0.004) (Figure 4A); and among the PHIV hospitalized with HFbEF, the CV mortality rate was also higher in the individuals who were taking a PI versus an NPI (35% vs. 4%; p = 0.01) (Figure 5A). There was an interaction present between the type of HF and the use of a PI-based regimen; however, PI use remained an independent predictor of CV mortality (Online Table 2). There was also no difference in CV mortality when different types of PIs were compared (Online Table 3). Saquinavir causes QT and PR interval prolongation and is no longer recommended as first-line for ART (16); therefore, the association between PI use and adverse outcomes was retested after the exclusion of patients taking saquinavir, and the finding remained unchanged (Online Tables 4 and 5). Among PHIV with HF, factors associated with CV mortality on univariate analysis included the following: the use of a PI-based regimen; traditional HF risk factors or measures of CV disease (a history of CAD, increased PASP, and low LVEF); HIV-specific parameters (low nadir CD4 count or at index hospitalization and high VL); and low education level (Table 4). In a multivariable model, the following parameters remained independently associated with CV mortality rate: use of a PI-based regimen; history of CAD; increased PASP; low nadir CD4 count or at index hospitalization (or high VL); and low education level (Table 5, Online Tables 6 and 7). Similar findings were noted when analogous analyses were performed among groups stratified by HFpEF and HFrEF (Tables 6 to 9). For example, in PHIV hospitalized with HFrEF, factors associated with CV mortality on univariate analysis included use of a PI-based regimen, traditional HF risk factors or measures of CV disease (a history of CAD, increased PASP, and low LVEF), HIV-specific parameters (low CD4 count and high VL), and low education level (Table 6). In a multivariable model among PHIV hospitalized with HFrEF, the following parameters remained independently associated with CV mortality rate: use of a PI-based regimen; history of CAD; increased PASP; decreased LVEF; low CD4 count (or high VL); and low education level (Table 7).
FIGURE 2. Kaplan-Meier Survival Curves: PHIV With HF.
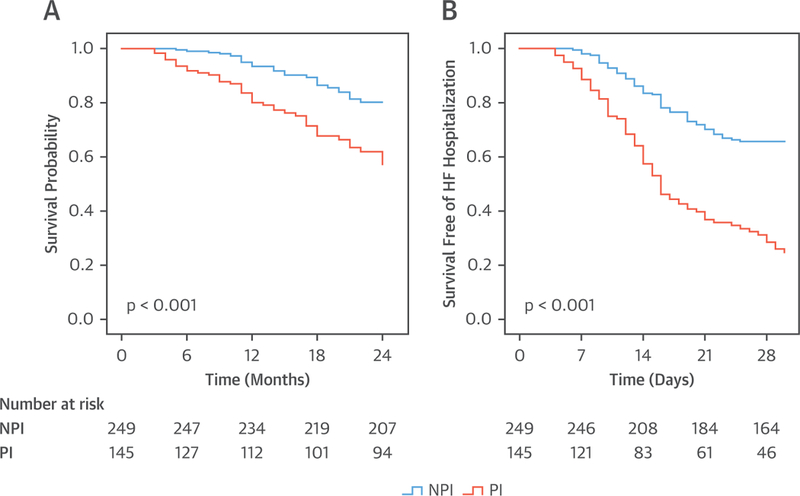
Kaplan-Meier survival curves comparing (A) cardiovascular mortality and (B) 30-day readmission in persons living with HIV who were admitted with heart failure (HFrEF, HFpEF, or HFbEF) and who were taking a PI versus NPI antiretroviral therapy (ART). Abbreviations as in Figure 1.
FIGURE 3. Kaplan-Meier Survival Curves: PHIV With HFrEF.
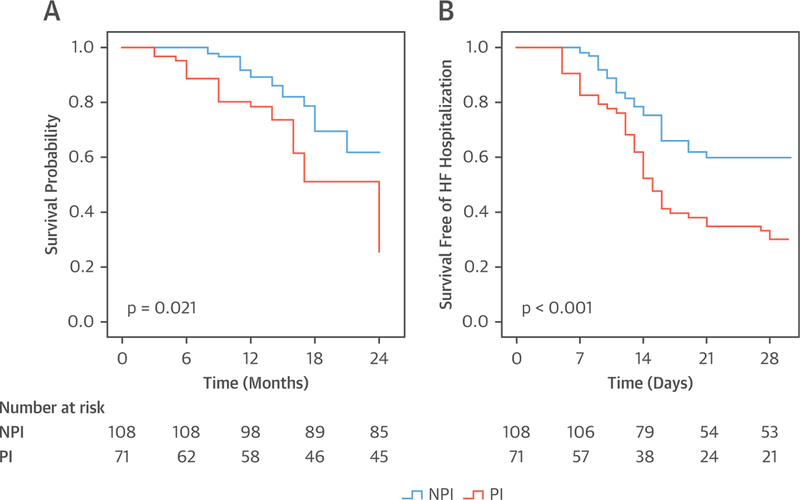
Kaplan-Meier survival curves comparing (A) cardiovascular mortality and (B) 30-day readmission in persons living with HIV who were admitted with HFrEF and who were taking a PI versus NPI ART. Abbreviations as in Figures 1 and 2.
FIGURE 4. Kaplan-Meier Survival Curves: PHIV With HFpEF.
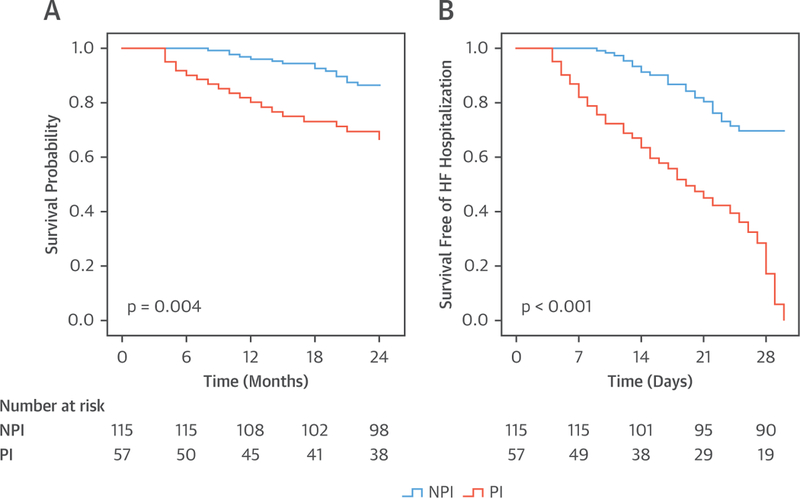
Kaplan-Meier survival curves comparing (A) cardiovascular mortality and (B) 30-day readmission in persons living with HIV who were admitted with HFpEF and who were taking a PI versus NPI ART. Abbreviations as in Figures 1 and 2.
FIGURE 5. Kaplan-Meier Survival Curves: PHIV With HFbEF.
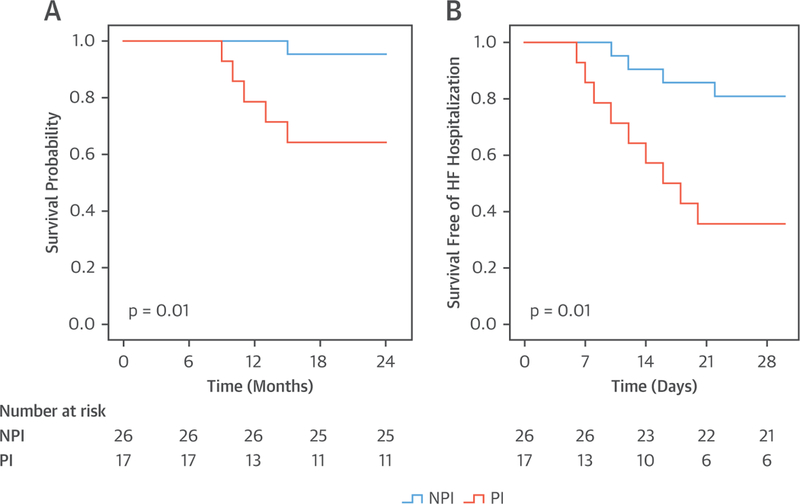
Kaplan-Meier survival curves comparing (A) cardiovascular mortality and (B) 30-day readmission in persons living with HIV who were admitted with HFbEF and who were taking a PI versus NPI ART. Abbreviations as in Figures 1 and 2.
TABLE 4.
Univariate Analysis (PHIV Total Cohort, N = 394): Outcome CV Mortality
| 95% CI | ||||
|---|---|---|---|---|
| Hazard Ratio | Lower | Upper | p Value | |
| Sex | 1.017 | 0.761 | 1.276 | 0.913 |
| Age | 0.954 | 0.920 | 1.017 | 0.324 |
| BMI | 0.946 | 0.902 | 1.012 | 0.112 |
| Diabetes | 1.234 | 1.015 | 1.579 | 0.037 |
| Hypertension | 1.078 | 0.842 | 1.329 | 0.591 |
| Hyperlipidemia | 1.202 | 0.955 | 1.583 | 0.080 |
| Smoking | 1.142 | 0.875 | 1.527 | 0.235 |
| History of CAD | 1.735 | 1.311 | 2.329 | <0.001* |
| ICD | 1.171 | 1.042 | 1.343 | <0.001* |
| Cocaine | 1.165 | 0.963 | 1.493 | 0.063 |
| PASP | 1.142 | 1.068 | 1.172 | <0.001* |
| LVEF | 0.953 | 0.934 | 0.973 | <0.001* |
| OSA | 1.014 | 0.679 | 1.518 | 0.963 |
| CD4 count ≥200 | 0.993 | 0.991 | 0.995 | <0.001* |
| Nadir CD4 count | 0.897 | 0.871 | 0.951 | <0.001* |
| VL >200 | 3.212 | 1.911 | 5.861 | <0.001* |
| ART duration | 0.973 | 0.919 | 1.054 | 0.658 |
| HIV duration | 1.011 | 0.928 | 1.098 | 0.682 |
| Untreated HIV duration | 1.171 | 0.883 | 1.361 | 0.731 |
| Education† | 0.721 | 0.521 | 0.977 | 0.006* |
| Unemployment | 1.027 | 0.953 | 1.657 | 0.341 |
| Abacavir | 0.761 | 0.581 | 1.057 | 0.657 |
| Beta blocker | 0.608 | 0.335 | 1.105 | 0.106 |
| ACE inhibitor or ARB | 0.580 | 0.333 | 1.008 | 0.056 |
| Spironolactone | 1.216 | 1.074 | 1.377 | 0.001* |
| Furosemide | 0.725 | 0.466 | 1.128 | 0.162 |
| PI | 1.598 | 1.219 | 2.095 | <0.001* |
Bold p values are statistically significant.
p < 0.01.
High school diploma, GED, or higher.
CI = confidence interval; other abbreviations as in Table 1.
TABLE 5.
Multivariate Analysis (PHIV Total Cohort, N = 394): Outcome CV Mortality*
| 95% CI | ||||
|---|---|---|---|---|
| Hazard Ratio | Lower | Upper | p Value | |
| PI | 1.797 | 1.257 | 2.567 | 0.001 |
| CD4 count | 0.991 | 0.987 | 0.995 | <0.001 |
| History of CAD | 2.113 | 1.512 | 2.971 | <0.001 |
| ICD | 1.125 | 0.737 | 2.433 | 0.513 |
| PASP | 1.083 | 1.053 | 1.179 | <0.001 |
| LVEF | 1.016 | 0.971 | 1.074 | 0.568 |
| Spironolactone | 1.632 | 0.956 | 3.048 | 0.110 |
| Education† | 0.763 | 0.531 | 0.877 | 0.023 |
Bold p values are statistically significant.
Cox proportional hazard regression for multivariate analysis for primary outcome (CV mortality). This model included all the covariates with p < 0.01 on univariate analysis (Table 4) except for VL. Both VL and CD4 count were not included in the multivariate model together because of the overlap between those individuals with a low CD4 count and a high VL. VL was included in the multivariate model (Online Table 3).
High school diploma, GED, or higher.
TABLE 6.
Univariate Analysis (PHIV With HFrEF, n = 179): Outcome CV Mortality
| 95% CI | ||||
|---|---|---|---|---|
| Hazard Ratio | Lower | Upper | p Value | |
| Sex | 1.219 | 0.821 | 1.842 | 0.288 |
| Age | 0.972 | 0.934 | 1.024 | 0.264 |
| BMI | 0.934 | 0.879 | 1.018 | 0.138 |
| Diabetes | 1.278 | 0.956 | 1.703 | 0.095 |
| Hypertension | 1.147 | 0.843 | 1.560 | 0.326 |
| Hyperlipidemia | 1.271 | 0.826 | 1.742 | 0.116 |
| Smoking | 1.344 | 0.869 | 2.076 | 0.143 |
| History of CAD | 1.644 | 1.122 | 2.344 | 0.002* |
| ICD | 1.419 | 1.078 | 1.866 | 0.001* |
| Cocaine | 1.163 | 0.542 | 2.421 | 0.632 |
| PASP | 1.062 | 1.014 | 1.133 | 0.003* |
| LVEF | 0.882 | 0.842 | 0.924 | <0.001* |
| OSA | 1.032 | 0.827 | 1.242 | 0.847 |
| CD4 count $200 | 0.996 | 0.994 | 0.998 | 0.002* |
| Nadir CD4 count | 0.868 | 0.764 | 0.878 | 0.004* |
| VL >200 | 1.852 | 1.265 | 3.402 | 0.005* |
| ART duration | 0.932 | 0.823 | 1.068 | 0.327 |
| HIV duration | 1.023 | 0.801 | 1.174 | 0.561 |
| Untreated HIV duration | 1.186 | 0.762 | 1.414 | 0.672 |
| Education† | 0.677 | 0.539 | 0.934 | 0.007* |
| Unemployment | 1.056 | 0.857 | 1.341 | 0.341 |
| Abacavir | 0.801 | 0.573 | 1.135 | 0.523 |
| Beta blocker | 0.864 | 0.782 | 0.946 | 0.031 |
| ACE inhibitor or ARB | 0.833 | 0.747 | 0.921 | 0.021 |
| Spironolactone | 1.477 | 1.135 | 1.983 | 0.002* |
| Furosemide | 0.915 | 0.781 | 1.167 | 0.436 |
| PI | 1.521 | 1.019 | 2.126 | 0.006* |
TABLE 7.
Multivariate Analysis (PHIV With HFrEF, n = 179): Outcome CV Mortality*
| 95% CI | ||||
|---|---|---|---|---|
| Hazard Ratio | Lower | Upper | p Value | |
| PI | 1.755 | 1.063 | 2.741 | 0.009 |
| CD4 count | 0.992 | 0.990 | 0.995 | <0.001 |
| History of CAD | 1.565 | 1.248 | 1.762 | 0.005 |
| ICD | 1.615 | 0.758 | 3.246 | 0.235 |
| PASP | 1.126 | 1.012 | 1.316 | 0.021 |
| LVEF | 0.941 | 0.912 | 0.978 | 0.003 |
| Spironolactone | 1.286 | 0.343 | 2.217 | 0.681 |
| Education† | 0.725 | 0.643 | 0.976 | 0.012 |
Bold p values are statistically significant.
Cox proportional hazard regression for multivariate analysis for primary outcome (CV mortality). This model included all the covariates with p < 0.01 on univariate analysis (Table 6) except for VL.
High school diploma, GED, or higher.
30-day HF readmission rates.
In the entire cohort of 394 patients, 46% were readmitted with decompensated HF within 30 days of discharge from the incident HF hospitalization. The use of a PI-based regimen was associated with a 2-fold increased risk of readmission for HF. Specifically, the 30-day hospital readmission rate was higher among PHIV with HF who were taking a PI versus an NPI (68% vs. 34%; p < 0.001) (Figure 2B). Similar findings of an increased 30-day HF readmission rate with PIs were noted when groups were analyzed according to the type of HF: HFrEF with a PI versus HFrEF not with a PI; (70% vs. 55%; p < 0.001) (Figure 3B); HFpEF with a PI versus HFpEF with an NPI (66% vs 22%; p < 0.001) (Figure 4B); and HFbEF with a PI versus HFbEF with an NPI (65% vs. 23%; p = 0.01) (Figure 5B). Additionally, no difference in 30-day readmission rate was noted among the different types of PIs (data not shown). Factors associated with 30-day HF read-mission on univariate analysis included use of a PI-based regimen, traditional HF risk factors or measures of CV disease (a history of CAD, cocaine use, increased PASP), HIV-specific parameters (low CD4 count and high VL), and socioeconomic parameters (low education level and unemployment) (Online Table 8). In a multivariable model in PHIV hospitalized with HFrEF, the following parameters remained independently associated with CV mortality rate: use of a PI-based regimen; history of CAD; cocaine use; increased PASP; low CD4 count (or high VL); low education level; and unemployment (Online Table 9).
TABLE 8.
Univariate Analysis (PHIV With HFpEF, n = 172): Outcome CV Mortality
| 95% CI | ||||
|---|---|---|---|---|
| Hazard Ratio | Lower | Upper | p Value | |
| Sex | 1.232 | 0.824 | 1.863 | 0.264 |
| Age | 0.965 | 0.947 | 1.024 | 0.812 |
| BMI | 0.952 | 0.879 | 1.062 | 0.438 |
| Diabetes | 1.201 | 0.863 | 1.782 | 0.217 |
| Hypertension | 1.057 | 0.581 | 1.548 | 0.942 |
| Hyperlipidemia | 1.182 | 0.812 | 1.737 | 0.331 |
| Smoking | 1.013 | 0.711 | 1.437 | 0.968 |
| History of CAD | 1.824 | 1.167 | 2.835 | <0.001* |
| Cocaine | 1.322 | 1.003 | 1.983 | 0.031 |
| PASP | 1.215 | 1.123 | 1.311 | <0.001* |
| LVEF | 0.975 | 0.924 | 1.025 | 0.862 |
| OSA | 1.042 | 0.571 | 1.823 | 0.883 |
| CD4 count $200 | 0.968 | 0.955 | 0.987 | <0.001* |
| Nadir CD4 count | 0.801 | 0.628 | 0.966 | 0.002* |
| VL >200 | 1.332 | 1.121 | 1.635 | <0.001* |
| ART duration | 1.031 | 0.910 | 1.241 | 0.543 |
| HIV duration | 1.104 | 0.817 | 1.305 | 0.621 |
| Untreated HIV duration | 1.117 | 0.786 | 1.422 | 0.672 |
| Education† | 0.701 | 0.523 | 0.961 | 0.005* |
| Unemployment | 1.211 | 0.765 | 1.451 | 0.681 |
| Abacavir | 0.671 | 0.374 | 1.058 | 0.764 |
| Beta blocker | 0.236 | 0.113 | 0.473 | <0.001* |
| ACE inhibitor or ARB | 0.248 | 0.127 | 0.524 | <0.001* |
| Furosemide | 0.485 | 0.263 | 0.746 | 0.003* |
| PI | 1.642 | 1.143 | 2.321 | 0.002* |
TABLE 9.
Multivariate Analysis (PHIV With HFpEF, n = 172): Outcome CV Mortality*
| 95% CI | ||||
|---|---|---|---|---|
| Hazard Ratio | Lower | Upper | p Value | |
| PI | 2.013 | 1.107 | 3.234 | 0.009 |
| CD4 count | 0.972 | 0.964 | 0.986 | <0.001 |
| History of CAD | 2.176 | 1.263 | 3.548 | 0.003 |
| Cocaine use | 1.234 | 0.676 | 2.316 | 0.548 |
| PASP | 1.112 | 1.053 | 1.236 | <0.001 |
| Beta blocker | 0.622 | 0.234 | 2.821 | 0.552 |
| ACE inhibitor or ARB | 0.372 | 0.114 | 1.381 | 0.118 |
| Furosemide | 0.834 | 0.351 | 1.824 | 0.671 |
| Education† | 0.545 | 0.342 | 0.823 | 0.023 |
Bold p values are statistically significant.
Cox proportional hazard regression for multivariate analysis for primary outcome (CV mortality). This model included all the covariates with p < 0.01 on univariate analysis (Table 8) except for VL.
High school diploma, GED, or higher.
DISCUSSION
We tested the associations between ritonavir-boosted PI regimens and cardiac structure and outcomes among PHIV with HF (Central Illustration). We report 4 key findings of broad clinical relevance to the care of patients with HIV and HF in our study: 1) 36% of the cohort of PHIV were receiving a PI-based regimen, as compared with a non–PI-based regimen, and these patients had higher rates of hyperlipidemia, diabetes mellitus, and CAD; 2) patients taking a PI had a higher PASP and lower LVEF; 3) in follow-up, PHIV with HF who had a PI-based regimen had higher rates of CV death; and 4) increased 30-day HF hospitalization for those patients who had PI-based ART compared with those who had NPI-based ART. To our knowledge, these findings are the first data linking PI-based ART to adverse cardiac structural changes and outcomes among PHIV with HF.
CENTRAL ILLUSTRATION. Cardiovascular Outcomes Associated With Protease Inhibitors.
In this study, ritonavir-boosted protease inhibitors were associated with increased cardiovascular mortality and 30-day heart failure (HF) readmission rates among persons with human immunodeficiency virus (HIV) with HF.
Previous studies have shown an association between some PI-based ART regimens and vascular events (6,17–19); for example, data from the (DAD) Data Collection on Adverse Events of Anti-HIV Drugs Study) cohort from subjects enrolled from 1999 through 2005 and from 2009 through 2016 have shown that some PI-based regimens were associated with an increase in a composite vascular endpoint (death, stroke, myocardial infarction, and revascularization) (4,6). There are no data on the association between PI-based regimens and outcomes specifically among PHIV with HF, a group at high risk of adverse events. Chen et al. (20), in a study involving 21,435 PHIV, demonstrated an association between current use of tenofovir (a nucleoside reverse transcriptase inhibitor) and an approximately 30% to 40% lower risk of incident HF compared with past users or never users of this drug. In that study, patients with HF were excluded, and the effect of PI regimens was not the focus; however, a significant proportion of the comparator group was prescribed a PI-based ART regimen.
As compared with uninfected controls, there is a marked increase in CV death among PHIV (13,21,22). For instance, Tseng et al. (22) completed a study of >2,800 individuals with HIV that showed a 4.5-fold greater risk of sudden cardiac death among PHIV. In that cohort, those patients who died had a higher prevalence of CAD, cardiomyopathy, and HF. Indeed, the prevalence of HF in that group was 30% in patients who died as compared with 9% in those patients with HIV who did not die (22). There are limited data on CV mortality in PHIV with HF (10,13). Janjua et al. (13) reported increased CV mortality in women living with HIV; however, analyses testing the association of factors such as ART classes with CV outcomes were limited by the small sample and number of outcomes. We found that PI-based regimens were associated with an increase in CV mortality among PHIV with HF. The mechanism for this increase in CV mortality related to PI use is unclear, but several possibilities exist, including the following: 1) patients prescribed PIs had an increased 30-day HF readmission rate, which is closely linked to mortality in broad groups of patients with HF (23);2) patients receiving ART had a higher PASP and a lower LVEF, both strong predictors of adverse outcomes, including mortality, in HF (24,25); and 3) PHIV with HF who were taking a PI had increased rates of diabetes and CAD, both independent risk factors for mortality in HF (26,27). Additionally, other factors not addressed in this study may have played a role. Specifically, among PHIV, the increased CV risk is, in part, independent of traditional CV risk factors and is probably related to a persistent and heightened state of inflammation (28–30). Inflammation is associated with worse outcomes in HF and CV disease, and the persistent inflammation in HIV may not be addressed using ART alone. For example, Zanni et al. (30) reported an increase in arterial inflammation after 6 months of ART treatment, a finding suggesting that additional therapies, beyond ART, may be needed to control inflammation among PHIV.
In this study, PHIV with HF who had a ritonavir-boosted PI had higher rates of hyperlipidemia and diabetes. The mechanisms involved in the increased rates of hyperlipidemia and diabetes seen with PI-based regimens and how the increase contributes to the heightened adverse outcomes noted in our cohort are active areas of research and may involve an increase in intramyocardial fat or cardiac steatosis (31–34). PHIV have a 3-fold elevation in intramyocardial fat and a relationship among the increase in intramyocardial fat, serum lipids, visceral fat, and impairment in measures of both diastolic and systolic function (34). In support of this, PHIV with HF who received PI-based ART in our study had a lower LVEF and a higher PASP as compared with patients who had NPI-based regimens. Additionally, the findings of a higher PASP and a lower LVEF were consistent across all types of HF. PI-based ARTs have been linked to an increase in PASP in other cohorts with HIV without HF (9). For example, in a prospective observational study of 322 PHIV without HF who underwent an echocardiogram, Mondy et al. (9) reported an elevated PASP in 23% and an association between PI use (ritonavir boosted) and the increased PASP. Additionally, emerging data suggest that ritonavir-boosted PIs may increase myocardial fibrosis, in turn reducing systolic and diastolic function (7,35,36). Another example, by Laurence et al. (7) in a mouse model using boosting doses of ritonavir, reported that PIs were associated with an increase expression of transforming growth factor beta-1, leading to an increase in myocardial fibrosis and a lower LVEF.
There was an association between socioeconomic parameters (e.g., education level and employment status) and CV outcomes among PHIV with HF. Lower education level (high school or GED status) and employment status have been shown to be associated with poor HF outcomes among HIV-uninfected individuals (37,38). However, there are few data linking socioeconomic parameters with HF outcomes among PHIV. The present study demonstrated an inverse association between lower education level and CV mortality and 30-day HF readmission and a positive association between unemployment and 30-day HF readmission. Future studies will need to focus on whether additional teaching and early post-discharge follow-up may have positive impacts on CV outcomes in this cohort.
STUDY LIMITATIONS.
This was a retrospective cohort study in a single U.S. urban tertiary care center of PHIV hospitalized with HF and thus a high-risk group. We do not have a denominator for the number of patients seen with HF in an outpatient setting, with and without HIV, and it is unclear whether PI use is associated with a difference in outcomes among PHIV with HF who were not hospitalized. Despite an ART being prescribed in 90% of the cohort, viral suppression was noted in 62%. The level of viral suppression may be inferior to that noted in more updated registries and clinical trials. However, this level of viral suppression is comparable to that reported in other contemporary observational clinical cohort studies (56% to 62%) (11,20,22), and the viral suppression reported in our study is similar to that reported in a study by Hanna et al. (39) involving more than 7,196 PHIV from the same geographic location during the same time period (66%). Therefore, a potential explanation for the worse outcomes in PHIV is poor adherence to treatment for either HIV or HF. In this retrospective study, adherence could not be assessed. However, we did test whether other surrogates of adherence were different between groups. In patients with HF who do not take HF medications, both heart rate and blood pressure are increased (40,41). In our study cohort, there was no difference in blood pressure and heart rate between the PHIV who had a PI as compared with the PHIV who had a non–PI-based regimen, a finding suggesting that compliance with HF medications may be similar between groups. Finally, even though we have applied the covariates in different multivariate models for independent prediction of outcomes, the possibility of residual confounding persists.
CONCLUSIONS
Being prescribed a ritonavir-boosted PI-based ART regimen was associated with dyslipidemia, a higher prevalence of CAD, increased PASP, and lower LVEF. In follow-up, PHIV with HF who were prescribed a PI-based ART regimen were noted to be at twice the risk for 30-day HF admission and CV mortality, an effect independent of HIV control. Further research is needed to determine whether PI-based regimens, either individual regimens or as a class effect, contribute pathophysiologically to processes leading to worse outcomes in HF (e.g., myocardial fat and fibrosis) and whether these findings can be replicated in prospective cohorts.
Supplementary Material
PERSPECTIVES
COMPETENCY IN MEDICAL KNOWLEDGE:
Ritonavir-boosted PI therapy is associated with hyperlipidemia, diabetes, lower LVEF, higher PASP, and adverse CV outcomes in PHIV with HF.
TRANSLATIONAL OUTLOOK:
Additional studies in this cohort are needed to determine the pathophysiological mechanisms responsible for these adverse effects.
Acknowledgments
Dr. Alvi was supported by the National Institutes of Health/National Heart, Lung, and Blood Institute (5T32HL076136). Dr. Zanni was supported in part through the National Institutes of Health/National Heart, Lung, and Blood Institute (1R01HL137562–01A1), the National Institutes of Health/Harvard Center for AIDS Research (P30-AI060354), and the Nutrition Obesity Research Center at Harvard (P30-DK040561). Dr. A.M. Neilan was supported by the Eleanor and Miles Shore Scholars in Medicine Fellowship, the Harvard University Center for AIDS Research (P30AI060354), and the International Maternal Pediatric AIDS Clinical Trials Network Early Investigator Award (UM1AI068632). Dr. Triant was supported in part through the National Institutes of Health/National Heart, Lung, and Blood Institute (1R01HL132786–01A1). Dr. T.G. Neilan was supported in part through the Kohlberg Foundation, an American Heart Association Fellow to Faculty Award (12FTF12060588), the National Institutes of Health/National Heart, Lung, and Blood Institute (1R01HL130539–01A1; 1R01HL137562–01A1; K24HL113128–06), and the National Institutes of Health/Harvard Center for AIDS Research (P30 AI060354); and has served on the advisory board for Takeda.All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- ART
antiretroviral therapy
- CV
cardiovascular
- EHR
electronic health record
- HF
heart failure
- HFbEF
heart failure with borderline ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HIV
human immunodeficiency virus
- IQR
interquartile range
- LVEF
left ventricular fraction
- PHIV
persons with human immunodeficiency virus
- PI
ritonavir-based protease inhibitors
- VL
viral load
Footnotes
APPENDIX For supplemental tables, please see the online version of this paper.
REFERENCES
- 1.Lohse N, Obel N. Update of survival for persons with HIV infection in Denmark. Ann Intern Med 2016;165:749–50. [DOI] [PubMed] [Google Scholar]
- 2.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013;8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volberding PA, Deeks SG. Antiretroviral therapy and management of HIV infection. Lancet 2010; 376:49–62. [DOI] [PubMed] [Google Scholar]
- 4.Sabin CA, Ryom L, d’Arminio Monforte A, et al. Abacavir use and risk of recurrent myocardial infarction: the D: A: D Study. AIDS 2018;32:79–88. [DOI] [PubMed] [Google Scholar]
- 5.Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis 2010;201:318–30. [DOI] [PubMed] [Google Scholar]
- 6.DAD Study Group, Friis-Moller N, Reiss P, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007;356: 1723–35. [DOI] [PubMed] [Google Scholar]
- 7.Laurence J, Elhadad S, Robison T, et al. HIV protease inhibitor-induced cardiac dysfunction and fibrosis is mediated by platelet-derived TGF-beta1 and can be suppressed by exogenous carbon monoxide. PLoS One 2017;12:e0187185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahamed J, Terry H, Choi ME, Laurence J. Transforming growth factor-beta1-mediated cardiac fibrosis: potential role in HIV and HIV/antiretroviral therapy-linked cardiovascular disease. AIDS 2016;30:535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mondy KE, Gottdiener J, Overton ET, et al. High Prevalence of echocardiographic abnormalities among HIV-infected persons in the era of highly active antiretroviral therapy. Clin Infect Dis 2011; 52:378–86. [DOI] [PubMed] [Google Scholar]
- 10.Freiberg MS, Chang CH, Skanderson M, et al. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging Cohort Study. JAMA Cardiol 2017;2:536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butt AA, Chang CC, Kuller L, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med 2011;171:737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remick J, Georgiopoulou V, Marti C, et al. Heart failure in patients with human immunodeficiency virus infection: epidemiology, pathophysiology, treatment, and future research. Circulation 2014; 129:1781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janjua SA, Triant VA, Addison D, et al. HIV Infection and heart failure outcomes in women. J Am Coll Cardiol 2017;69:107–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol 2015;66:403–69. [DOI] [PubMed] [Google Scholar]
- 15.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011;123: 2736–47. [DOI] [PubMed] [Google Scholar]
- 16.Food and Drug Administration. FDA Drug Safety Communication: Invirase (Saquinavir) Labels Now Contain Updated Risk Information on Abnormal Heart Rhythms. 2010. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm230096.htm. Accessed June 6, 2018.
- 17.Iloeje UH, Yuan Y, L’Italien G, et al. Protease inhibitor exposure and increased risk of cardiovascular disease in HIV-infected patients. HIV Med 2005;6:37–44. [DOI] [PubMed] [Google Scholar]
- 18.Friis-Moller N, Ryom L, Smith C, et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: the Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study. Eur J Prev Cardiol 2016;23:214–23. [DOI] [PubMed] [Google Scholar]
- 19.Mary-Krause M, Cotte L, Simon A, Partisani M, Costagliola D. Clinical Epidemiology Group from the French Hospital Database. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. AIDS 2003; 17:2479–86. [DOI] [PubMed] [Google Scholar]
- 20.Chen R, Scherzer R, Hsue PY, et al. association of tenofovir use with risk of incident heart failure in HIV-infected patients. J Am Heart Assoc 2017;6: e005387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feinstein MJ, Bahiru E, Achenbach C, et al. Patterns of cardiovascular mortality for HIV-infected adults in the United States: 1999 to 2013. Am J Cardiol 2016;117:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tseng ZH, Secemsky EA, Dowdy D, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol 2012;59:1891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol 2013;61:391–403. [DOI] [PubMed] [Google Scholar]
- 24.Kalogeropoulos AP, Fonarow GC, Georgiopoulou V, et al. Characteristics and outcomes of adult outpatients with heart failure and improved or recovered ejection fraction. JAMA Cardiol 2016;1:510–8. [DOI] [PubMed] [Google Scholar]
- 25.Maron BA, Hess E, Maddox TM, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. Circulation 2016;133:1240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 2015;385:2067–76. [DOI] [PubMed] [Google Scholar]
- 27.Kober L, Thune JJ, Nielsen JC, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016;375: 1221–30. [DOI] [PubMed] [Google Scholar]
- 28.Luetkens JA, Doerner J, Schwarze-Zander C, et al. Cardiac magnetic resonance reveals signs of subclinical myocardial inflammation in asymptomatic HIV-infected patients. Circ Cardiovasc Imaging 2016;9:e004091. [DOI] [PubMed] [Google Scholar]
- 29.Hsue PY, Tawakol A. Inflammation and fibrosis in HIV: getting to the heart of the matter. Circ Cardiovasc Imaging 2016;9:e004427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanni MV, Toribio M, Robbins GK, et al. Effects of antiretroviral therapy on immune function and arterial inflammation in treatment-naive patients with human immunodeficiency virus infection. JAMA Cardiol 2016;1:474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thiara DK, Liu CY, Raman F, et al. Abnormal myocardial function is related to myocardial steatosis and diffuse myocardial fibrosis in HIV-infected adults. J Infect Dis 2015;212:1544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holloway CJ, Ntusi N, Suttie J, et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation 2013;128: 814–22. [DOI] [PubMed] [Google Scholar]
- 33.Diaz-Zamudio M, Dey D, LaBounty T, et al. Increased pericardial fat accumulation is associated with increased intramyocardial lipid content and duration of highly active antiretroviral therapy exposure in patients infected with human immunodeficiency virus: a 3T cardiovascular magnetic resonance feasibility study. J Cardiovasc Magn Reson 2015;17:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson MD, Szczepaniak LS, LaBounty TM, et al. Cardiac steatosis and left ventricular dysfunction in HIV-infected patients treated with highly active antiretroviral therapy. J Am Coll Cardiol Img 2014;7:1175–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mak IT, Kramer JH, Chen X, Chmielinska JJ, Spurney CF, Weglicki WB. Mg supplementation attenuates ritonavir-induced hyperlipidemia, oxidative stress, and cardiac dysfunction in rats. Am J Physiol Regul Integr Comp Physiol 2013;305: R1102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reyskens KM, Fisher TL, Schisler JC, et al. Cardio-metabolic effectsof HIV protease inhibitors (lopinavir/ritonavir). PLoS One 2013;8: e73347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eapen ZJ, McCoy LA, Fonarow GC, et al. Utility of socioeconomic status in predicting 30-day outcomes after heart failure hospitalization. Circ Heart Fail 2015;8:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rorth R, Fosbol EL, Mogensen UM, et al. Employment status at time of first hospitalization for heart failure is associated with a higher risk of death and rehospitalization for heart failure. Eur J Heart Fail 2018;20:240–7. [DOI] [PubMed] [Google Scholar]
- 39.Hanna DB, Felsen UR, Ginsberg MS, et al. Increased antiretroviral therapy use and virologic suppression in the Bronx in the context of multiple HIV prevention strategies. AIDS Res Hum Retro-viruses 2016;32:955–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kapoor JR, Kapoor R, Ju C, et al. Precipitating clinical factors, heart failure characterization, and outcomes in patients hospitalized with heart failure with reduced, borderline, and preserved ejection fraction. J Am Coll Cardiol HF 2016;4:464–72. [DOI] [PubMed] [Google Scholar]
- 41.van der Wal MH, Jaarsma T, van Veldhuisen DJ. Non-compliance in patients with heart failure; how can we manage it? Eur J Heart Fail 2005;7:5–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


