Abstract
microRNA (miRNA)-26a-loaded liposomes were prepared in the present study for effective treatment of leukemia. The results demonstrated that miRNA-26a reduced the viability of chronic lymphocytic leukemia (CLL) cells in a concentration-dependent manner. Cells treated with miRNA-26a-loaded liposomes exhibited increased rates of apoptosis, as determined by flow cytometry and Hoechst 33342 staining. Western blot analysis revealed an increased apoptotic effect of miRNA-26a-loaded liposomes compared with control. Treatment with these liposomes resulted in significant downregulation of the expression of the miRNA-26a target genes, myeloid cell leukemia 1 and cyclin-dependent kinase 6. Taken together, the results of the present study indicate that miRNA-26a exerts apoptosis-inducing and anticancer effects on leukemia cells, suggesting therapeutic potential. This approach may be possible to extrapolate to other neoplasms, including lymphomas and acute myeloid leukemia.
Keywords: leukemia, apoptosis, miRNA-26a, anticancer effect, liposome
Introduction
Chronic lymphocytic leukemia (CLL) is a type of blood cancer that is heterogeneous at the clinical and cellular levels (1,2), and which arises due to uncontrolled proliferation of lymphocytes that accumulate in the blood and bone marrow. These immature cells can permeate other organs, including the liver, kidney and central nervous system, which can ultimately result in fatality (2,3). Patients with acute myeloid leukemia (AML) often exhibit characteristic mutations and dysregulated gene expression, both of which contribute to the generally poor prognosis of the disease (4). Despite significant advances in our understanding of the biology of leukemia, an optimal treatment regime for CLL is lacking (5-7). Current treatment regimens rely on chemotherapy, which involves systemic drug administration. However, the efficacy of chemotherapy is limited due to immediate clearance of the drugs from blood circulation. In addition, the development of resistance to chemotherapy drugs is a major reason for the poor prognosis of leukemia (8). The development of resistance is attributed to the mechanism of action of chemotherapeutic drugs, which activate intrinsic apoptosis pathways (9). In the case of leukemia, however, apoptosis pathways are inhibited by Abl expression and a lack of FAS receptors. Generally, these can be reversed by administration of high doses of anticancer drugs, but this is accompanied by marked systemic toxic effects in healthy tissues (10). Therefore, there is a requirement to identify non-chemotherapeutic treatments for leukemia with improved efficacy and reduced side effects.
MicroRNAs (miRNAs) are short noncoding RNAs that are involved in regulating the expression of their target genes (11). Certain miRNAs have been reported to be directly responsible for leukemogenesis and are involved in the prognosis of cancer. miRNAs regulate the expression of genes at the posttranscriptional level by altering messenger RNA (mRNA), thereby modifying associated biological processes or pathways (12,13). Thus, miRNAs can act as oncogenes or tumor inhibitors. For example, silencing of miR-15a/16-1 in an animal model was reported to result in the development of an indolent form of leukemia (14). Bcl-2 is an miRNA target, and its interaction with miRNA eliminates the expression of Bcl-2, resulting in cell death. The anticancer drug, venetoclax, which inhibits Bcl-2, was recently approved for use in CLL (15). Other miRNAs have also been demonstrated to be useful in the regulation of leukemia, with miRNA-34a reported to downregulate 17p-CLL and halt disease progression (16,17). These studies lay the groundwork for the development of miRNA-based therapies. The present study was performed to examine the effects of miRNA-26a on leukemia cells.
One of the most important concerns in miRNA-based therapy is the delivery strategy. Due to their very low stability in the body, miRNAs require a carrier to elicit their pharmacological therapeutic effects. The development of nanoscale biomaterials is a popular topic of interest for targeted treatment of cancer (18). An ideal delivery system would deliver the maximum amount of drug to the target site without being toxic itself (19). Liposomes are ideal carriers for systemic applications and have been studied in detail in clinical trials (20). Liposomes are biocompatible and of an appropriate size for accumulation in tumor tissue. The long half-life of liposomes in the circulation allows for accumulation of the carrier in leukemia cells (21).
The aim of the present study was to design an miRNA-loaded delivery system for the effective treatment of leukemia. miRNA-26a was physically loaded into liposomes and the various biological properties, including cell viability, apoptosis and morphological changes, were studied in vitro.
Materials and methods
Materials
Cholesterol, 1,2-dioleyl-3-trimethylam monium-propane (DODAP), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), and N-palmitoyl-sphingosine-1-[succinyl(polyethylene glycol)]2000 (DSPE-PEG) were purchased from Avanti Polar Lipids (Alabaster, AL, USA). All other chemicals were of reagent grade and were used without further purification.
Preparation of miRNA-loaded liposomes
Liposomes were prepared using the thin-film hydration method. In brief, DODAP:CHOL:DSPC:DSPE-PEG were dissolved at a molar ratio of 25:50:23:2 in a mixture of 1 ml of chloroform (100%) and methanol (100%) (4:1). The organic solvent was agitated such that all lipids were dissolved, and removed from the rotary evaporator at 60°C. Thereafter, the lipid film was hydrated using distilled water at 60°C for 15 min and extruded using a mini-extruder for 21 cycles through a polycarbonate membrane with a pore size of 100 nm. The liposomes thus formed were purified by dialysis for 24 h. Liposomes were stored in glass vials, and 10 µg miRNA-26a (3-UCG GAU AGG ACC UAA UGA ACU U-5) was added and incubated for 12 h under constant agitation. The miRNA-loaded liposomes were stored at 4°C until further use. The miRNA was complexed with Lipofectamine 2000 (Thermo Fisher Scientific, Inc., Waltham, MA, USA) as a transfection agent and incubated with the cancer cells.
Characterization of liposomes
The miRNA-loaded liposomes were evaluated in terms of particle size and particle-size distribution using a Zetasizer Nano ZS (Malvern Instruments, Worcestershire, UK). The liposomes (dispersed in 0.1X PBS, density, 1 kg/m3) were diluted appropriately in ultra-pure water (density, 997 kg/m3) and experiments were performed in triplicate at room temperature. The morphology of the liposomes was examined by transmission electron microscopy (TEM; Tecnai G2 12 TWIN TEM; FEI; Thermo Fisher Scientific, Inc.). The samples were mixed with 2% phosphotungistic acid as a counterstain solution for 4 min and placed in a drop onto a copper grid prior to drying. The samples were then analyzed by TEM (×10,000, magnification).
Gel electrophoresis
Physical entrapment of miRNA in liposomes was evaluated by electrophoresis through 2% agarose gels in Tris-acetate-ethylenediaminetetraacetic acid (EDTA) buffer containing 0.5 µg/ml of GelRED (Biotium, Inc., Fremont, CA, USA). The free miRNA and miRNA-loaded liposomes were mixed with 10% glycerin, 1% bromothymol blue and 2% SDS, subjected to electrophoresis at 120 V for 20 min, and then photographed using a gel imaging system (ChemiDocTM; Bio-Rad Laboratories, Inc.).
Cytotoxicity assay
CLL cells (American Type Culture Collection; ATCC; Manassas, VA, USA) were maintained in RPMI medium supplemented with 10% FBS (Lonza Group, Ltd., Basel, Switzerland) and 1% penicillin-streptomycin antibiotic mixture in an atmosphere of 65% humidity with 5% CO2 and 37°C. The cells were seeded in 96-well plates at a density of 1.2×104 cells per well and incubated for 24 h. The cells were then treated with miRNA-loaded liposomes or blank liposomes (0.1, 1, 10, 50 and 100 µM) and incubated for a further 24 h. The cells were treated for 24 h with increasing concentrations of miRNA-26a (25, 50 and 100 µM) to study concentration-dependent cytotoxicity against leukemia cells. Untreated cells were maintained throughout the study period. The cells were treated with 100 µl 1.25 mg/ml MTT solution and incubated for 4 h at 37°C, after which the formazan crystals were dissolved in DMSO. The absorbance was determined at 570 nm using a microplate reader (Infinite M200 reader; Tecan, Männedorf, Switzerland). For comparison, NIH-3T3 cells (ATCC) was purchased and grown in RPMI medium supplemented with 10% FBS (Lonza Group, Ltd.) and 1% penicillin-streptomycin antibiotic mixture in a humidified atmosphere (65%) with 5% CO2 and 37°C. The same protocol was followed for MTT assay of these cells.
Hoechst 33342 assay
The cells were seeded in 6-well plates at a density of 3×105 cells per well and incubated for 24 h. The cells were then treated with miRNA-loaded liposomes (MRL) or blank liposomes (25, 50 and 100 µM), and incubated for a further 24 h. The cells were treated for 24 h with increasing concentrations of the miRNA-26a (25, 50 and 100 µM) to examine concentration-dependent effects on apoptosis. The following day, the cells were washed carefully with ultrapure water and stained with 10 µg/ml Hoechst 22242 for 15 min at 37°C. The cells were then fixed with 4% paraformaldehyde and washed again with PBS. Apoptosis was qualitatively assessed by fluorescence microscopy.
Apoptosis
The cells were seeded in 12-well plates at a density of 2×105 cells per well and incubated for 24 h, then treated with miRNA-loaded liposomes or blank liposomes (25, 50 and 100 µM), and incubated for a further 24 h. The cells were treated with 200 µM MRL and incubated for an additional 24 h. The following day, the cells were washed carefully with PBS and centrifuged at 1,300 x g at 4°C. The cell pellets were resus-pended in binding buffer (BD Biosciences, Franklin Lakes, NJ, USA) and incubated with 2.5 µl Annexin V and 2.5 µl propidium iodide (PI) (BD Biosciences) for 15 min, followed by flow cytometric analysis (FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
Cells were treated with blank liposome and miR-26a-loaded liposomes (25, 50 and 100 µM), harvested after 24 h and lysed using radioimmunoprecipitation assay lysis buffer for 15 min at 24°C. The lysed cells were centrifuged at 12,000 x g for 15 min at 4°C. The supernatant was collected and the protein concentration was determined using a BCA protein assay kit (Thermo Fischer Scientific, Inc.). A total of 25 µg protein per lane was separated by 10% SDS-PAGE and transferred onto polyvinylidene fluoride membranes (Bio-Rad Laboratories, Hercules, CA, USA). The membranes were then blocked with 5% skimmed milk for 1 h, followed by incubation with the following primary antibodies at 4°C overnight: Anti-cyclin-dependent kinase 6 (CDK6; cat. no. 3136; 1:1,000), anti-MCL1 (cat. no. 4572; 1:1,000), BCL2 family apoptosis regulator (BCL2 cat. no. 2870; 1:1,000), anti-poly (ADP-Ribose) polymerase (PARP; cat. no. 9542; 1:1,000) and anti-GAPDH (dilution, 1:1,000; cat. no. 2118; all from Cell Signaling Technology, Inc., Danvers, MA, USA). The following day, the membranes were incubated with horseradish peroxidase-conjugated anti-mouse IgG secondary antibodies (cat. no. 7076; 1:3,000). Images of the blots were obtained using an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA) and quantified using ImageJ software (version 7.0; National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
The data are presented as the mean ± standard deviation. All analyses were performed with SPSS software (version 17; SPSS, Inc., Chicago, IL, USA). Comparisons between groups were assessed by one-way analysis of variance. In instances of multiple comparisons, analysis of variance was performed, followed by the Scheffé post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results and Discussion
Preparation and characterization of miRNA-loaded liposomes
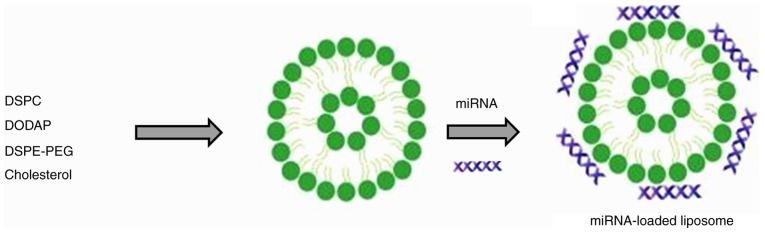
Despite significant advances in our understanding of the biology of leukemia, there remains no optimal treatment for CLL. Current treatment options involve chemotherapy, however, they have poor efficacy due to the immediate clearance of drugs from the circulation and the development of drug resistance. Although miRNAs serve important roles in the pathogenesis of cancer, they can also function as tumor suppressors (22). The more established small interfering RNAs (siRNAs) silence the expression of a single gene, while miRNAs can silence multiple genes simultaneously (23). Therefore, miRNA-based therapy has potential in the treatment of various types of cancer. In the present study, miRNA-26a was selected for the treatment of leukemia cells. Delivery strategy remains an important concern in miRNA-based therapy, as the stability of miRNA in vivo is low. Liposomes are among the most well-studied carrier systems in clinical trials, are highly biocompatible and of a size appropriate for accumulation in tumor tissue (24). The negatively charged miR-26a forms an electrostatic complex with the positively charged surfaces of the liposomes (Fig. 1) (25).
Figure 1.
Schematic representation of the preparation of miRNA-26a-loaded liposomes. Cationic liposomes were prepared and electrostatically conjugated with miRNA-26a. miRNA, microRNA.
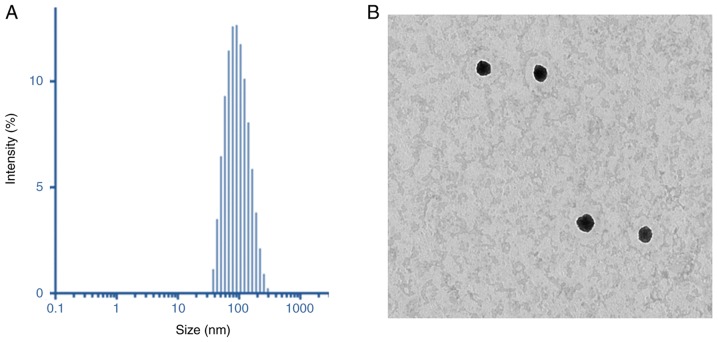
Dynamic light scattering (DLS) analysis was performed to characterize the final particle size and size distributions of the liposome preparations. As demonstrated in Fig. 3A, the particles were 110 nm in size, with a uniform dispersity index of 0.15. The particle size of MRL was small enough for cancer-targeting applications (26). The surface charge of MRL was +21.5±1.25 mV, indicating the presence of cationic charged liposomes. The dried particles were spherical in shape and dispersed. The particle size observed from TEM was consistent with that determined by DLS analysis (Fig. 3A). The positively charged liposomes were predicted to be internalized into the cancer cells, thereby further enhancing the efficacy of cancer treatment. The morphology of the particles was analyzed by TEM (Fig. 3B).
Figure 3.
Physicochemical analysis. (A) Dynamic light scattering analysis of miRNA-loaded liposomes. (B) Particle morphology analysis by transmission electron microscopy revealed a spherical nanoparticle dimension.
Gel electrophoresis
The loading of miR-26a into liposomes was confirmed by gel electrophoresis (Fig. 2). Free miRNA was electrophoresed to the opposite end of the gel, while loading of miRNA into liposomes prevented its release and migration in a concentration-dependent manner. The results indicated the ability of liposomes to withhold the encapsulated miRNA and thereby improve its stability and therapeutic efficacy.
Figure 2.
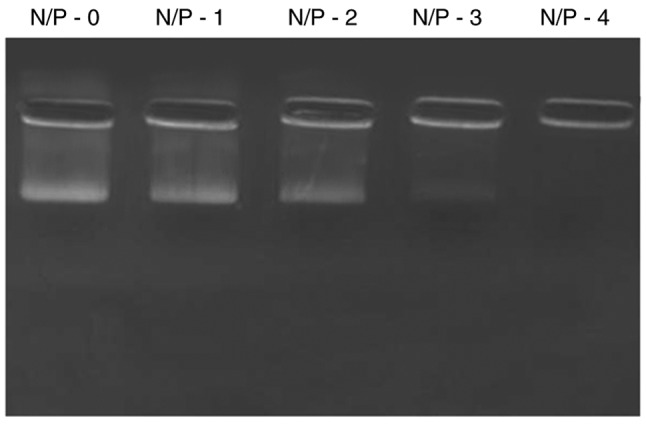
Gel electrophoresis of miRNA. The miRNA-loaded liposomes were evaluated for stability and degradation by gel electrophoresis. Increased N/P ratio indicates increased stability of miRNA. N, nitrogen; P, phosphorous.
In vitro cell viability
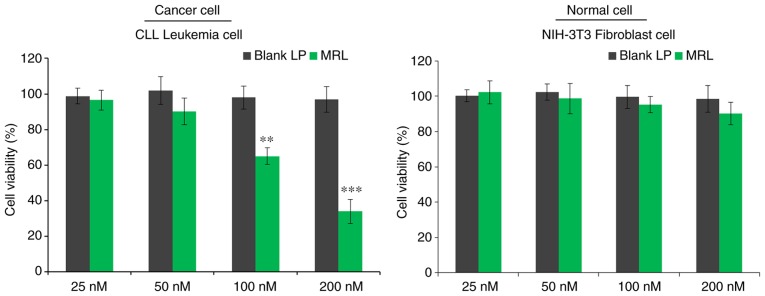
The viability of CLL cells in vitro was evaluated by MTT assay (Fig. 4). Briefly, cells were treated with blank liposomes or miRNA-loaded liposomes and incubated for 24 h. The control blank liposomes had no effect on the viability of cancer cells, indicating that the liposomes were non-toxic and biocompatible vectors. As expected, miRNA-26a-loaded liposomes decreased the viability of cancer cells in a concentration-dependent manner (P<0.001), with >60% cells killed when treated with the highest concentration (200 nM). The results indicated the anticancer effect of miRNA-26a against leukemia cells.
Figure 4.
The cytotoxic potential of the miRNA-loaded liposomes was analyzed by MTT assay in CLL cancer cells treated with different concentrations of miRNA-26a. **P<0.01, ***P<0.001 vs. blank liposome. CLL, chronic lymphocytic leukemia; miRNA, microRNA; LP, liposome; MRL, miRNA-loaded liposome.
Hoechst 33342 assay
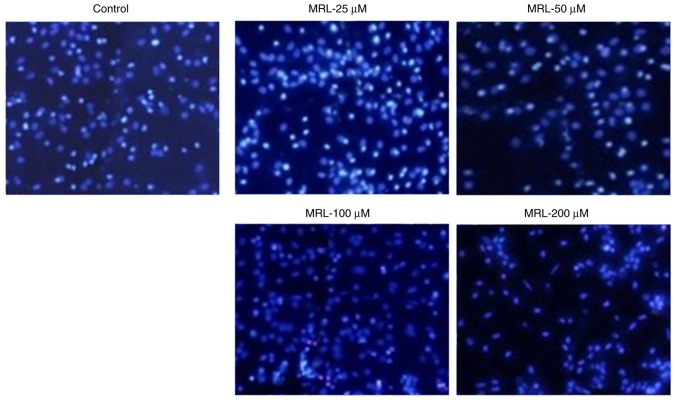
The anticancer effect of miRNA-26a was further evaluated by Hoechst 33342 staining (Fig. 5). Untreated cells maintained their typical morphology and dispersal on the plate, whereas treatment with miRNA-26a induced apoptosis in a concentration-dependent manner. Apoptosis of cancer cells was more evident with increasing concentrations of miRNA-26a (Fig. 5). For example, cells treated with 200 µM miRNA exhibited typical apoptotic morphology, including condensation of chromatin, breakdown of the nuclear membrane, and apoptotic body formation. Taken together, these observations indicated the anticancer potential of miRNA-26a loaded within a stable nanocarrier.
Figure 5.
Apoptosis was qualitatively analyzed by Hoechst 33342 staining and fluorescence microscopy. Apoptotic body formation was observed in MRL-treated cells. MRL, miRNA-loaded liposome.
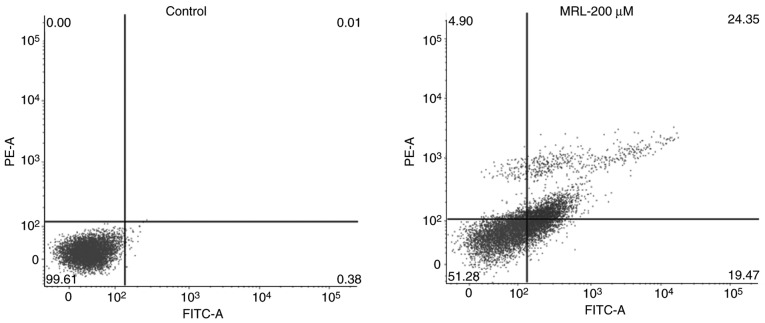
Apoptotic rate, determined by flow cytometry
Quantitative analysis of apoptosis was performed by Annexin V/PI staining and flow cytometry (Fig. 6). Cancer cells were treated with 200 µM miRNA for 24 h. A significant (P<0.01) increase in apoptosis was observed compared with the control group. Approximately 25% of the total cells were in the late apoptotic stage, while 20% were in the early apoptosis stage, indicating the potent anticancer effect of the MRL formulation.
Figure 6.
Quantitative analysis of apoptosis was performed by flow cytometry. The percentage distributions of cells in early and late apoptosis are indicated. MRL, miRNA-loaded liposome.
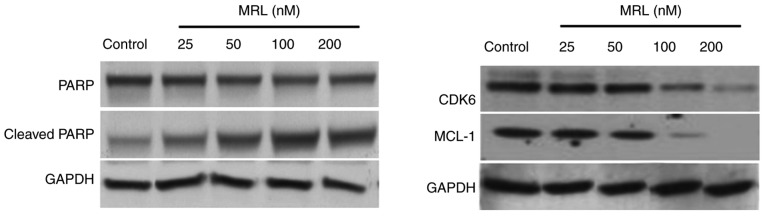
Western blot analysis
The mechanism of action of miRNA-26a was examined by western blotting (Fig. 7). The protein expression levels of target genes, cyclin-dependent kinase 6 (Cdk6) and myeloid cell leukemia 1 (Mcl-1), and the marker of apoptosis, PARP, were analyzed. The results indicated that high concentrations of miRNA-26a decreased the protein expression level of PARP and increased that of cleaved PARP, indicating the apoptosis-inducing potential of miRNA-26a. Seeing as miRNA-26a-loaded liposomes significantly downregulated the expression of the target genes, Mcl-1 and Cdk6, this may be the mechanism of action by which it mediates apoptotic effect. Numerous studies have demonstrated that miR-26a can target and downregulate a number of protein-coding gene targets, including CDK6, cyclin D2 and E2, and Mcl-1, in different types of cancer cells (27,28).
Figure 7.
Western blotting analysis of the mechanism of action of miRNA-26q. The protein expression levels of apoptotic proteins and miRNA-26a targets were analyzed. MRL, miRNA-loaded liposome; PARP, poly (ADP-Ribose) polymerase; CDK6, cyclin-dependent kinase 6; MCL-1, MCL1, BCL2 family apoptosis regulator.
In conclusion, miRNA-26a-loaded liposomes successfully prepared for the effective treatment of leukemia cells. It was demonstrated that 200 nM miRNA-26a significantly decreased the viability of CLL cells compared with control. The miRNA-26a-loaded liposomes exerted a marked apoptosis-inducing effect, as demonstrated by flow cytometry and Hoechst 33342 staining. Western blot analysis revealed a superior apoptosis-inducing effect of miRNA-26a-loaded liposomes compared with free miRNA-26a. miRNA-26a significantly downregulated the expression of its target genes, Mcl-1 and Cdk6. The results of the present study indicate that miRNA-26a-loaded liposomes exert apoptosis-inducing and anticancer effects on leukemia cells, suggesting their possible utility in future therapies. This approach has the potential for extrapolation to other types of neoplasms, including lymphomas and AML.
Acknowledgments
Not applicable.
Funding
The present study was supported by Shandong Blood Center (Shangdon, China).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JL and CKS contributed equally to all research. CKS was responsible for the writing of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kwan JM, Fialho AM, Kundu M, Thomas J, Hong CS, Das Gupta TK, Chakrabarty AM. Bacterial proteins as potential drugs in the treatment of leukemia. Leuk Res. 2009;33:1392–1399. doi: 10.1016/j.leukres.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 2.Bhojwani D, Yang JJ, Pui CH. Biology of childhood acute lymphoblastic leukemia. Pediatr Clin North Am. 2015;62:47–60. doi: 10.1016/j.pcl.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Randhawa JK, Ferrajoli A. A review of supportive care and recommended preventive approaches for patients with chronic lymphocytic leukemia. Expert Rev Hematol. 2016;9:235–244. doi: 10.1586/17474086.2016.1129893. [DOI] [PubMed] [Google Scholar]
- 4.Morabito F, Gentile M, Seymour JF, Polliack A. Ibrutinib, idelalisib and obinutuzumab for the treatment of patients with chronic lymphocytic leukemia: Three new arrows aiming at the target. Leuk Lymphoma. 2015;56:3250–3256. doi: 10.3109/10428194.2015.1061193. [DOI] [PubMed] [Google Scholar]
- 5.Fabbri G, Dalla-Favera R. The molecular pathogenesis of chronic lymphocytic leukaemia. Nat Rev Cancer. 2016;16:145–162. doi: 10.1038/nrc.2016.8. [DOI] [PubMed] [Google Scholar]
- 6.Nimmanapalli R, Bhalla K. Novel targeted therapies for Bcr-Abl positive acute leukemias: Beyond STI571. Oncogene. 2002;21:8584–8590. doi: 10.1038/sj.onc.1206086. [DOI] [PubMed] [Google Scholar]
- 7.Soni G, Yadav KS. Applications of nanoparticles in treatment and diagnosis of leukemia. Mater Sci Eng C. 2015;47:156–164. doi: 10.1016/j.msec.2014.10.043. [DOI] [PubMed] [Google Scholar]
- 8.Terme M, Borg C, Guilhot F, Masurier C, Flament C, Wagner EF, Caillat-Zucman S, Bernheim A, Turhan AG, Caignard A, Zitvogel L. BCR/ABL promotes dendritic cell-mediated natural killer cell activation. Cancer Res. 2005;65:6409–6417. doi: 10.1158/0008-5472.CAN-04-2675. [DOI] [PubMed] [Google Scholar]
- 9.Nagata Y, Todokoro K. Requirement of activation of JNK and p38 for environmental stress-induced erythroid differentiation and apoptosis and of inhibition of ERK for apoptosis. Blood. 1999;94:853–863. [PubMed] [Google Scholar]
- 10.Jia L, Patwari Y, Kelsey SM, Newland AC. Trail-induced apoptosis in Type I leukemic cells is not enhanced by overexpression of bax. Biochem Biophys Res Commun. 2001;283:1037–1045. doi: 10.1006/bbrc.2001.4895. [DOI] [PubMed] [Google Scholar]
- 11.Negrini M, Ferracin M, Sabbioni S, Croce CM. MicroRNAs in human cancer: From research to therapy. J Cell Sci. 2007;120:1833–1840. doi: 10.1242/jcs.03450. [DOI] [PubMed] [Google Scholar]
- 12.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al. Frequent deletions and downregulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein U, Lia M, Crespo M, Siegel R, Shen Q, Mo T, Ambesi-Impiombato A, Califano A, Migliazza A, Bhagat G, Dalla-Favera R. The DLEU2/miR-15a/161 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell. 2010;17:28–40. doi: 10.1016/j.ccr.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, Kipps TJ, Anderson MA, Brown JR, Gressick L, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:311–322. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, et al. A microRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353:1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 17.Fabbri M, Bottoni A, Shimizu M, Spizzo R, Nicoloso MS, Rossi S, Barbarotto E, Cimmino A, Adair B, Wojcik SE, et al. Association of a microRNA/TP53 feedback circuitry with pathogenesis and outcome of B-cell chronic lymphocytic leukemia. JAMA. 2011;305:59–67. doi: 10.1001/jama.2010.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramasamy T, Ruttala HB, Gupta B, Poudel BK, Choi HG, Yong CS, Kim JO. Smart chemistry-based nanosized drug delivery systems for systemic applications: A comprehensive review. J Control Release. 2017;258:226–253. doi: 10.1016/j.jconrel.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 19.Choi JY, Ramasamy T, Tran TH, Ku SK, Shin BS, Choi HG, Yong CS, Kim JO. Systemic delivery of axitinib with nano-hybrid liposomal nanoparticles inhibits hypoxic tumor growth. J Mater Chem B. 2015;3:408–416. doi: 10.1039/C4TB01442A. [DOI] [PubMed] [Google Scholar]
- 20.Ramasamy T, Ruttala HB, Sundaramoorthy P, Poudel BK, Youn YS, Ku SK, Choi HG, Yong CS, Kim JO. Multimodal selenium nanoshell-capped Au@mSiO2 nanoplatform for NIR-responsive chemophotothermal therapy against metastatic breast cancer. NPG Asia Mater. 2018;10:197–216. doi: 10.1038/s41427-018-0034-5. [DOI] [Google Scholar]
- 21.Ramasamy T, Haidar ZS, Tran TH, Choi JY, Choi HG, Jeong JH, Shin BS, Choi HG, Yong CS, Kim JO. Layer-by-layer assembly of liposomal nanoparticles with PEGylated polyelectrolytes enhances systemic delivery of multiple anticancer drugs. Acta Biomater. 2014;10:5116–5127. doi: 10.1016/j.actbio.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Gao DY, Huang L. In vivo delivery of miRNAs for cancer therapy: Challenges and strategies. Adv Drug Deliv Rev. 2015;81:128–141. doi: 10.1016/j.addr.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam JK, Chow MY, Zhang Y, Leung SW. siRNA versus miRNA as therapeutics for gene silencing. Mol Ther Nucleic Acids. 2015;4:e252. doi: 10.1038/mtna.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruttala HB, Ramasamy T, Madeshwaran T, Hiep TT, Kandasamy U, Oh KT, Choi HG, Yong CS, Kim JO. Emerging potential of stimulus-responsive nanosized anticancer drug delivery systems for systemic applications. Arch Pharm Res. 2018;41:111–129. doi: 10.1007/s12272-017-0995-x. [DOI] [PubMed] [Google Scholar]
- 25.Ahmadzada T, Reid G, McKenzie DR. Fundamentals of siRNA and miRNA therapeutics and a review of targeted nanoparticle delivery systems in breast cancer. Biophys Rev. 2018;10:69–86. doi: 10.1007/s12551-017-0392-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganju A, Khan S, Hafeez BB, Behrman SW, Yallapu MM, Chauhan SC, Jaggi MM. miRNA nanotherapeutics for cancer. Drug Discov Today. 2017;22:424–432. doi: 10.1016/j.drudis.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, Liang L, Zhang XF, Jia HL, Qin Y, Zhu XC, Gao XM, Qiao P, Zheng Y, Sheng YY, et al. MicroRNA-26a suppresses tumor growth and metastasis of human hepatocellular carcinoma by targeting interleukin-6-Stat3 pathway. Hepatology. 2013;58:158–170. doi: 10.1002/hep.26305. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Y, Lu Y, Zhang Q, Liu JJ, Li TJ, Yang JR, Zeng C, Zhuang SM. MicroRNA-26a/b and their host genes cooperate to inhibit the G1/S transition by activating the pRb protein. Nucleic Acids Res. 2012;40:4615–4625. doi: 10.1093/nar/gkr1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.