Abstract
Rheumatoid arthritis (RA) is a commonly occurring autoimmune disease. Its defining pathological characteristic is the excessive proliferation of fibroblast-like synoviocytes (FLS), which is similar to tumor cells and results in a range of clinical problems. As a commonly used antipyretic, analgesic and anti-inflammatory drug, aspirin is the first-line treatment for RA. However, its mechanism of action has not been well explained. The goal is to investigate the biological effects of aspirin on primary RA-FLS and its underlying mechanisms. In this experiment we treated cells with various concentrations of aspirin (0, DMSO, 1, 2, 5, 10 mM). Cell proliferation activity was detected with CCK-8 assays. Apoptosis and cell cycle distribution were detected via flow cytometry. Apoptosis and cell cycle-associated proteins (Bcl-2, Bax, PRAP1, Cyclin D1, P21), as well as the key proteins and their phosphorylation levels of the NF-κB and JAK/STAT3 signaling pathways, were detected via western blot analysis. Bioinformatics prediction revealed that aspirin was closely associated with cell proliferation and apoptosis, including the p53 and NF-κB signaling pathways. By stimulating with aspirin, cell viability decreased, while the proportion of apoptotic cells increased, and the number of cells arrested in the G0/G1 phase increased in a dose-dependent manner. The expression of Bax increased with aspirin stimulation, while the levels of Bcl-2, PRAP1, Cyclin D1 and P21 decreased; p-STAT3, p-P65 and p-50 levels also decreased while STAT3, P65, P50, p-P105 and P105 remained unchanged. From our data, it can be concluded that aspirin is able to promote apoptosis and inhibit the proliferation of RA-FLS through blocking the JAK/STAT3 and NF-κB signaling pathways.
Keywords: aspirin, fibroblast-like synoviocytes, apoptosis, cell cycle, proliferation, NF-κB, JAK/STAT3
Introduction
Rheumatoid arthritis (RA) is the most common chronic inflammatory joint disease, with a worldwide incidence of 0.5-1%. It is characterized by synovial inflammation of the joints. Repeated synovitis can cause joint pain, joint compulsion/stiffness and swelling, leading to the destruction of articular cartilage and bone. Furthermore, it may gradually cause joint deformity and dysfunction (1). Fibroblast-like synoviocytes (FLS) are an important component of the synovium and serve a major role in the pathological process of RA. One of the major pathological features of RA is the abnormal proliferation of FLS, manifesting as hyperplasia of synovial tissue (2). This is similar to the proliferation of tumor cells and is termed neoplastic. Reports showed that insufficient apoptosis and abnormal proliferation of FLS are observed in RA, and an excess of FLS lead to the various pathological processes of RA (3). Thus, promoting apoptosis of FLS and inhibiting proliferation is the primary focus of current studies surrounding RA treatment.
Aspirin is a nonsteroidal anti-inflammatory drug (NSAID), and is also known as acetylsalicylic acid (ASA). It functions as a cyclooxygenase (COX) inhibitor that has been widely used in anti-oxidation, anti-microbial and anti-inflammatory treatments, especially for RA. Aspirin has also been utilized for its function of anti-platelet aggregation and anti-inflammatory analgesia by inhibiting COX, which ultimately results in the decreased production of prostaglandins (4). In recent years, numerous studies have found that aspirin can also inhibit the proliferation of tumor cells and promote their apoptosis in a concentration- and time-dependent manner (5,6). These findings will be helpful in developing the wider use of aspirin and may explain, from a new perspective, the mechanism of aspirin as pertains to the treatment of RA.
STAT3 is an important transcription factor that is stimulated by exogenous signals such as IFN, IL-6 and other growth factors. The persistent activation of STAT3 can be detected in RA and the majority of tumor (7,8). Downstream targets of STAT3 are involved in cell proliferation, differentiation and apoptosis. It has also been shown that aberrant activation of STAT3 increases proliferation and tumorigenesis. The IL6/JAK/STAT3 pathway is also important in the pathogenesis of RA. NF-κB is an intracellular transcription factor that controls and participates in cell growth, survival, apoptosis, inflammatory responses and oncogenesis. It not only serves an important role in the whole pathological process of RA, but also in the excessive proliferation and decreased apoptosis of RA-FLS (9).
So far, and to the best of our knowledge, there have been no reports on the direct effect of aspirin on RA-FLS and its potential mechanism. In our study, it was found that one of the ways in which aspirin functions is via promoting apoptosis as well as inhibiting proliferation of RA-FLS, in a dose-dependent manner. Thus, a possible mechanism has been proposed herein, by which aspirin functions to inhibit JAK/STAT3 and NF-κB signaling pathways in RA-FLS.
Materials and methods
Drugs and reagents
Aspirin was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany; A2093). It was dissolved in dimethyl sulfoxide (DMSO); the pH was adjusted to 7.0 by 1 mol/l NaOH and kept at −20°C for later use. Antibodies against Cyclin D1, P21, STAT3, p-STAT3, P50 were purchased from Abcam (Cambridge, UK). Secondary antibodies against GAPDH, Bax, PARP1 were from Proteintech Group (Wuhan, China). Bcl-2 was from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). P65, p-P65 were from Cell Signaling Technology, Inc. (Danvers, MA, USA). p-P50 was from Affinity Company (Changzhou, China).
Cell culture
Primary human RA-FLS were obtained from the BeNa Culture Collection Company (BNCC340230; Beijing, China). Dulbecco’s modified Eagle’s medium (DMEM), penicillin/streptomycin and fetal bovine serum (FBS) were purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The cells were cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (penicillin: 10,000 U/ml, streptomycin: 10,000 µg/ml), at 37°C in a humidified atmosphere of 95% air and 5% CO2. In this experiment RA-FLS cells were treated with different concentrations of aspirin (0, DMSO, 1, 2, 5, 10 mM) in vitro.
Cell proliferation assay
The cells were harvested and then seeded in 96-well plates (1x104 cells/well) with a total volume of 200 µl culture medium. Cells were divided into 6 groups: Control group, DMSO-treated group and aspirin-treated groups (1, 2, 5 and 10 mM). Then, cells were incubated for 12, 24 and 48 h at 37°C. After the cells were treated for indicated times, a Cell Counting Kit-8 (CCK-8; MedChem Express, China) was used (20 µl per well) and cells were incubated for 3 h at 37°C. Subsequently, the plate absorbance was read using an automated microplate spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 450 nm. Each experiment was repeated at least three times.
Cell apoptosis flow cytometry analysis
Aspirin-induced apoptosis was detected using an Annexin V-FITC Apoptosis Assay Kit (Hanbio, Shanghai, China). Treated cells (0, DMSO; 1, 2, 5 and 10 mM aspirin for 24 h) were harvested. Annexin V-FITC and propidium iodide (PI) were used based on the manufacturer’s instructions. Annexin V specifically binds to the phosphatidyl serine (PS) residues on the cell membrane, and FITC is a marker of Annexin V; while PI binds to DNA once the cell membrane becomes permeable. The cells were stained, and the data were analyzed using BD Accuri C6 Plus (Becton-Dickinson, San Jose, CA, USA) software. Each experiment was repeated at least three times.
Cell cycle analysis
Cells were plated in parallel in 35-mm2 culture plates at a concentration of 1x106 cells/plate. After 24 h of serum starvation, cells were exposed to aspirin for various durations (0, DMSO; 1, 2, 5 and 10 mM) and then were harvested by trypsinization, washed twice in cool PBS and placed in 75% ethanol overnight at 4°C. Following this, cells were incubated in solution with the DNA-binding dye propidium iodide (PI) and RNase A (KeyGEN Biotech, Nanjing, China) for 30 min at 37°C in the dark. Finally, red fluorescence from 488 mm laser-excited PI in every cell was analyzed using a flow cytometer (Becton-Dickinson) using a peak fluorescence gate to discriminate aggregates. The percentage of cells in the G0/G1, S and G2/M phases was determined from DNA content histograms created with BD Accuri C6 Plus software. Each experiment was repeated at least three times.
Western blot analysis
The 6 groups of cells (2x106) were washed in PBS and then lysed with RIPA lysis buffer (Beyotime Institute of Biotechnology, Beijing, China). Cell debris was removed by centrifugation (Thermo Fisher Scientific, Inc.) at 10,000 x g for 15 min at 4°C, followed by protein concentration measurement using a BCA assay kit (Beyotime Institute of Biotechnology). Proteins were denatured by boiling for 5 min prior to electrophoresis, so that complete depolymerization of proteins could be achieved and a negative charge added; then, equivalent amounts of protein (30-40 µg) samples were separated via 10-15% SDS-PAGE and transferred to immobilon polyvinylidene difluoride membranes. The membranes were blocked with 5% BSA in TBS-T for 1 h, and then incubated with rabbit anti-Bax (1:2,000 dilution, Proteintech Group; #S0599-2-lg), anti-Bcl-2 (1:1,000 dilution, Santa Cruz Biotechnology, Inc.; #sc-7382), anti-PARP1 (1:2,000 dilution, Proteintech Group; #13371-1-AP), anti-P21 (1:1,000 dilution, #EPR3993), anti-cyclin D1 (1:10,000 dilution, #EPR2241; both from Abcam), anti-P65 (1:2,000 dilution; #8242), anti-p-P65 (1:2,000 dilution; #3033; both from Cell Signaling Technology, Inc.), anti-P50/P105 (1:1,000 dilution, Abcam: #E381), anti-p-P50/105 (1:1,000 dilution, Affinity Company: #AF3219), anti-STAT3 (1:2,000 dilution, #EPR787Y) and anti-p-STAT3 (1:200,000 dilution, #EP2147Y; both from Abcam) antibodies for 2 h at room temperature. The membranes were then washed three times with TBST for 10 min each time, and incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies at a dilution of 1:5,000 for 1 h. After three washes with TBST, the immunoreactive bands were visualized using an ECL detection system (SmartChemi 420, Beijing, China). GAPDH served as the loading control. Each experiment was repeated at least three times.
Drug-target and direct protein targets (DPT)-associated genes search
The DrugBank 5.0.11 (https://www.DrugBank.ca/) is a database that collects detailed information about various drugs and associated materials (10). In the present study, 11 drug targets for aspirin were found using the DrugBank database. Information was collected on these 11 targets and the gene names entered into the ‘Multiple Proteins by Names/Identifiers’ search box in the STRING database (https://STRING-db.org/cgi/input.pl) (11) for primary DPT-associated genes. Parameters were set in minimum required interaction score: Highest confidence 0.900, then 300 secondary DPT-interacting proteins. Finally, 311 genes were collected, and data down-loaded for the next analysis.
Network generation/visualization, founding hub gene and functional annotation
Associations between the 311 genes downloaded from the STRING database were identified using Cytoscape. APP-Cytohubba in Cytoscape was used to find the top 10 hub genes of the network by degree. The Database for Annotation Visualization and Integrated Discovery (DAVID) online tool (https://david.ncifcrf.gov/) was used to conduct functional and pathway enrichment analyses in the present study (12,13). Additional GO and KEGG pathway enrichment analyses were performed to detect the potential biological functions and pathways of the 311 genes.
Statistical analysis
All experiments were performed at least three times, and the data (bar graphs) are presented as the means ± standard deviations (SD). SPSS v.23.0 software (IBM Corp., Armonk, NY, USA) was used to analyze the data. One-way ANOVA was performed for multiple group comparisons, and the mean values of different groups were compared using the Student-Newman-Keuls (SNK) test. P<0.05 was considered to indicate statistically significant differences.
Results
Visualization of aspirin-linkage networks by Cytoscape
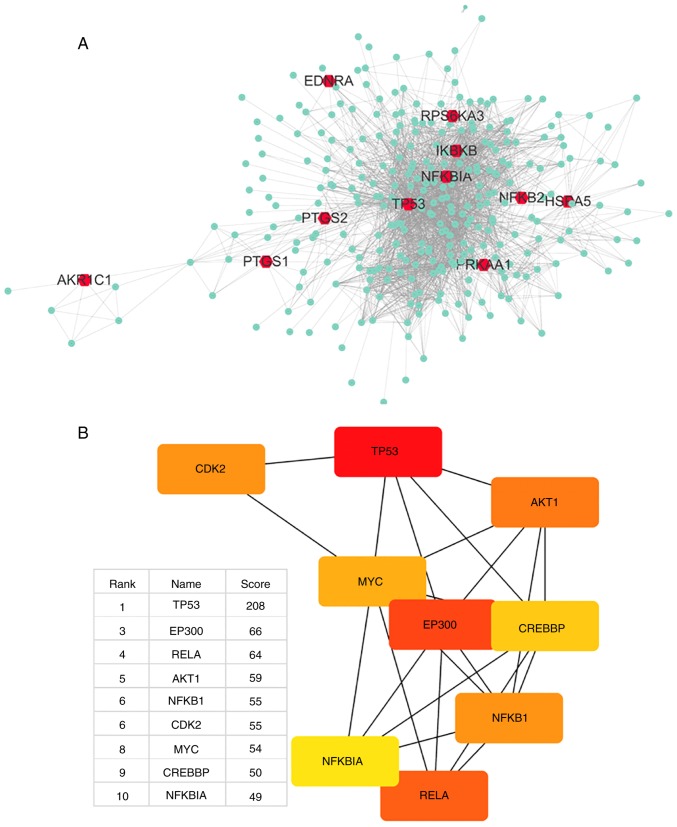
Aspirin was input to DrugBank, which output DB00945-aspirin with 11 targets. Aspirin can inhibit PTGS1, PTGS2 and AKR1C1, activate PRKAA1, acetylate TP53, bind to HSPA5, and antagonize NFKB2; the actions of EDNRA, IKBKB, RPS6KA3 and NFKBIA were not mentioned but were closely associated with aspirin. Table I presents detailed information on the 11 primary DPTs of aspirin. Using the STRING database, a total of 300 proteins were identified that were associated with aspirin and its 11 primary DPTs, and a network of aspirin target-protein interactions was created (Fig. 1A). The 11 primary DPTs and their secondary DPT-associated proteins are presented in Fig. 1A. Next, Cytohubba in Cytoscape was used to analyze the top 10 hub genes in this network by degree, and these are as follows: TP53, UBC, EP300, RELA, AKT1, NFKB1, CDK2, MYC, CREBBP and NFKBIA (Fig. 1B).
Table I.
Identification of direct targets of aspirin using DrugBank.
| Target | Full name | Uniprot ID | Actions | Related papers (PMID) |
|---|---|---|---|---|
| PTGS1 | Prostaglandin-endoperoxide synthase 1 | P23219 | Inhibitor | 17030227 |
| 17078596 | ||||
| 17131625 | ||||
| 17259075 | ||||
| 17319904 | ||||
| 11752352 | ||||
| PTGS2 | Prostaglandin-endoperoxide synthase 2 | P35354 | Inhibitor | 17033106 |
| 17037745 | ||||
| 17181859 | ||||
| 17258197 | ||||
| 17301265 | ||||
| 11752352 | ||||
| AKR1C1 | Aldoketo reductase family 1 member C1 | Q04828 | Inhibitor | 18045204 |
| PRKAA1 | Protein kinase AMP-activated catalytic subunit alpha 1 | Q13131 | Activator | 22406476 |
| 22517326 | ||||
| EDNRA | Endothelin receptor type A | P25101 | – | 10727528 |
| IKBKB | Inhibitor of nuclear factor κB kinase subunit beta | O14920 | – | 9817203 |
| TP53 | Tumor protein p53 | P04637 | Acetylation | 21475861 |
| HSPA5 | Heat shock protein family A (Hsp70) member 5 | P11021 | Binding | 11689471 |
| RPS6KA3 | Ribosomal protein S6 kinase A3 | P51812 | – | 10553090 |
| NFκBIA | NF-κB inhibitor alpha | P25963 | – | 10553090 |
| NFκB2 | Nuclear factor κB subunit 2 | Q00653 | Antagonist | 8052854 |
Figure 1.
Network of drug-target genes and DPT-associated genes and their enrichment analysis. (A) DrugBank was used to find 11 primary and DPTs of aspirin (red); STRING was used to find 300 secondary DPT-interacting proteins (green); Cytoscape was used to create a PPI network of all 311 genes. (B) Cytohubba was used to find network hub genes by degree, and the top 10 hub genes are displayed (red, high score; yellow, low score). The second hub gene-UBC was not suitable as a hub gene, thus was removed. DPT, direct protein target; STRING, Search Tool for the Retrieval of Interacting Genes/Proteins; PPI, protein-protein interaction. Network of drug-target genes and DPT-associated genes and their enrichment analysis. (C) DAVID was used to analyze GO annotations (biological process) of all 311 genes. The top 12 terms were selected according to P-value. (D) The top 12 KEGG pathways enriched for all 311 genes were identified using the aforementioned method. DAVID, Database for Annotation, Visualization and Integrated Discovery; KEGG, Kyoto Encyclopedia of Genes and Genomes; GO, gene ontology.
The gene UBC encodes a ubiquitin precursor protein often found in the interactome database, as almost all proteins are ubiquitinated and degraded via the proteasome. Thus, it was considered that UBC is not a specific interaction hub molecule and was removed from the list of top 10 hub genes. The central position of the TP53 gene suggests that it may largely be involved in the biological mechanism of aspirin.
Enrichment analysis of GO functions and pathways using DAVID
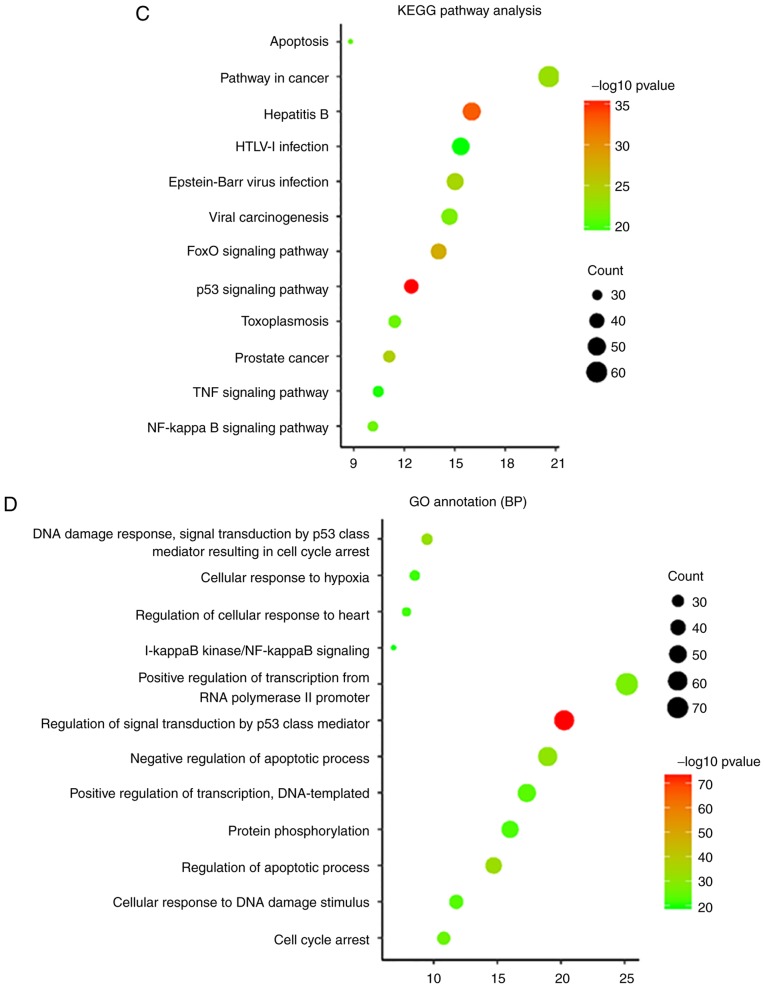
To assess the functional features of aspirin-mediated gene sets, GO annotations of all 311 genes were performed. The top 12 terms were selected according to P-value. Top 12 KEGG pathways enrichment was also performed (Fig. 1C and D). A few interesting terms were selected interested us from the 12 KEGG terms and 12 GO (BP) terms (Table II). Functional analysis suggests that aspirin-associated genes are mainly linked to apoptosis and proliferation (cell cycle). Cytohubba results showed that TP53 serves the most important role in the network. Additionally, genes involved in the NF-κB signaling pathway were revealed to be among the top 10 hub genes (Fig. 1B). The aforementioned evidence demonstrates a direct association of the biological effects of aspirin with the p53 axis and NF-κB signaling pathway. Furthermore, aspirin can directly affect multiple key genes in the NF-κB signaling pathway, including IKBKB, NFKBIA and NFKB2.
Table II.
Notable pathway/biological process enrichment terms from the 12 significant KEGG/GO terms.
| KEGG term | P-value | Count | Genes |
|---|---|---|---|
| hsa04115:p53 signaling pathway | 2.99E-36 | 38 | CHEK1, CHEK2, SFN, PMAIP1, RRM2B, CCNG1, SESN2, PTEN, SESN1, CCNE2, CCNE1, CASP3, TP53I3, CDKN2A, CASP8, SERPINE1, FAS, RCHY1, TP53AIP1, TP53, ATR, ATM, CDK2, CCNB1, CCND1, CDKN1A, SERPINB5, BBC3, CD82, BAX, TSC2, DDB2, MDM2, APAF1, MDM4, PERP, GADD45A, IGFBP3 |
| hsa04068:FoxO signaling pathway | 1.51E-28 | 43 | PRKAG3, USP7, HRAS, STK11, PRKAG1, PRKAG2, FOXO1, FOXO3, PTEN, AKT1, PRMT1, PDPK1, PIK3CA, BCL6, PRKAA1, PRKAA2, CHUK, EGFR, CREBBP, PRKAB2, SKP2, PRKAB1, SIRT1, IRS1, ATM, CDK2, CCNB1, MAPK1, CCND1, CDKN1A, PLK3, EP300, CDKN1B, PLK2, CSNK1E, MAPK14, MAPK3, MDM2, SETD7, MAPK9, MAPK8, IKBKB, GADD45A |
| hsa04064:NF-kappa B signaling pathway | 6.09E-22 | 31 | TRAF2, TNF, PTGS2, NFKBIA, NFKB1, TLR4, NFKB2, BCL2L1, MAP3K7, CSNK2A2, TNFRSF1A, MYD88, CSNK2A1, BCL2, TRAF6, CHUK, IRAK1, BCL10, RELA, RELB, UBE2I, MALT1, BIRC3, TAB1, TAB2, BIRC2, ATM, LCK, IKBKG, ERC1, IKBKB |
| hsa04210:Apoptosis | 1.06E-21 | 27 | TRAF2, TNF, NFKBIA, NFKB1, BCL2L1, AKT1, TNFRSF1A, CASP6, CASP3, BCL2, CASP8, PIK3CA, FAS, CHUK, RELA, TP53, BIRC3, BIRC2, ATM, TNFRSF10A, TNFRSF10C, TNFRSF10B, TNFRSF10D, BAX, IKBKG, APAF1, IKBKB |
|
| |||
| GO term (BP) | P-value | Count | Genes |
|
| |||
| GO:0042981~regulation of apoptotic process | 6.81E-74 | 45 | TRAF2, MCL1, BCL2L1, PMAIP1, CALR, GLS2, CASP6, TNFRSF1A, TRIAP1, TP53I3, CASP8, BCL3, BCL6, NDRG1, FAS, CASP1, TP53AIP1, BCL10, TP53BP2, CREBBP, TP53, SKP2, MALT1, BIRC5, WRN, BIRC3, CDK5, BIRC2, BRCA1, ATM, TNFRSF10A, TNFRSF10C, TNFRSF10B, DUSP1, TNFRSF10D, BBC3, BAX, BNIP3L, JAK2, PPP1R13B, APAF1, GDF15, PERP, IGFBP3, TP53INP1 |
| GO:0006977~DNA damage response, signal transduction by p53 class mediator resulting in cell cycle arrest | 1.02E-32 | 29 | E2F4, PML, AURKA, CHEK2, SFN, TRIAP1, PRMT1, PCBP4, NPM1, TP53, ARID3A, CDC25C, ATM, CDK2, CCNB1, CDKN1A, CDKN1B, EP300, PLK3, BTG2, PLK2, BAX, RGCC, PCNA, UBC, MDM2, MDM4, CARM1, GADD45A |
| GO:0043066~negative regulation of apoptotic process | 1.21E-31 | 58 | FOXO1, NFKB1, AURKA, PTEN, AKT1, TRIAP1, CASP3, MYD88, SIN3A, FAS, MYC, EGFR, IRAK1, RELA, TP53, HMGA2, TNFRSF10D, UBC, MDM2, MAPK8, MDM4, YWHAZ, MCL1, FHL2, NFKBIA, PRKDC, BCL2L1, SRC, BCL2, NPM1, DNAJA1, BCL3, TAF9, PRKAA1, HSPA5, PRKAA2, TRAF6, MALT1, BIRC5, RPS6, BIRC3, SIRT1, BIRC2, RPS6KA3, CDKN1A, HDAC3, HSP90B1, PLK3, CDKN1B, HDAC2, DUSP1, HDAC1, PLK2, PSMD10, GSK3B, BNIP3L, IKBKB, BARD1 |
| GO:0007050~cell cycle arrest | 3.12E-26 | 33 | PRKAG3, HRAS, STK11, PRKAG1, PRKAG2, PML, CALR, CDKN2A, PCBP4, PRKAA1, CAB39, PRKAA2, MYC, CUL1, KAT2B, MSH2, PRKAB2, TP53, PRKAB1, RB1, RPTOR, ATM, JMY, CDKN1A, CDKN1B, TSC1, TSC2, ERN1, RHEB, MTOR, GADD45A, TP53INP1, BARD1 |
| GO:0006974~cellular response to DNA damage stimulus | 6.03E-24 | 36 | BLM, STK11, FOXO1, CHEK1, PMAIP1, CHEK2, AKT1, BCL2, BCL3, BCL6, TAF9, DYRK2, TRAF6, MYC, TAF1, TP53BP1, YY1, TP53, TOPBP1, ATR, WRN, SIRT1, BRCA1, ATM, RAD50, MAPK1, CCND1, CDKN1A, PLK3, BTG2, OTUB1, BBC3, IKBKG, MAPK3, SETD7, BARD1 |
Aspirin inhibits RA-FLS cell proliferation
The effect of aspirin on cell proliferation was examined by using a CCK-8 to test cell viability. After administering aspirin, proliferation in each tested group was significantly and dose-dependently inhibited when compared with the control. Fig. 2A-C shows the effects of different concentrations of aspirin on cell viability at 12, 24 and 48 h. It was observed that cell viability gradually decreased with increasing concentrations of aspirin, and that DMSO had a slight effect on cell viability, but the effect is not statistically significant. Fig. 2D shows the percentage reduction in cell proliferation at each of the three time periods. These results indicate that aspirin can reduce cell viability and inhibit proliferation in a concentration-dependent manner.
Figure 2.
The CCK-8 assay results are presented as bar graphs for the antiproliferative effects of aspirin on RA-FLS. (A-C) RA-FLS were treated with (0, DMSO, 1, 2, 5 and 10 mM) aspirin for 12, 24 and 48 h. At each time interval, cell viability was determined by CCK-8 analysis. Data are presented as the means ± SD (error bars) from three independent experiments. Aspirin inhibited the growth of RA-FLS in a dose-dependent manner. (D) Cell proliferation decreased at 12, 24 and 48 h. Data are presented as the means ± SD (error bars) from three independent experiments. *P<0.05, **P<0.01, ***P<0.001 vs. DMSO group. CCK-8: Cell-Counting Kit-8; RA-FLS, rheumatoid arthritis-fibroblast-like synoviocytes; DMSO, dimethyl sulfoxide; SD, standard deviation.
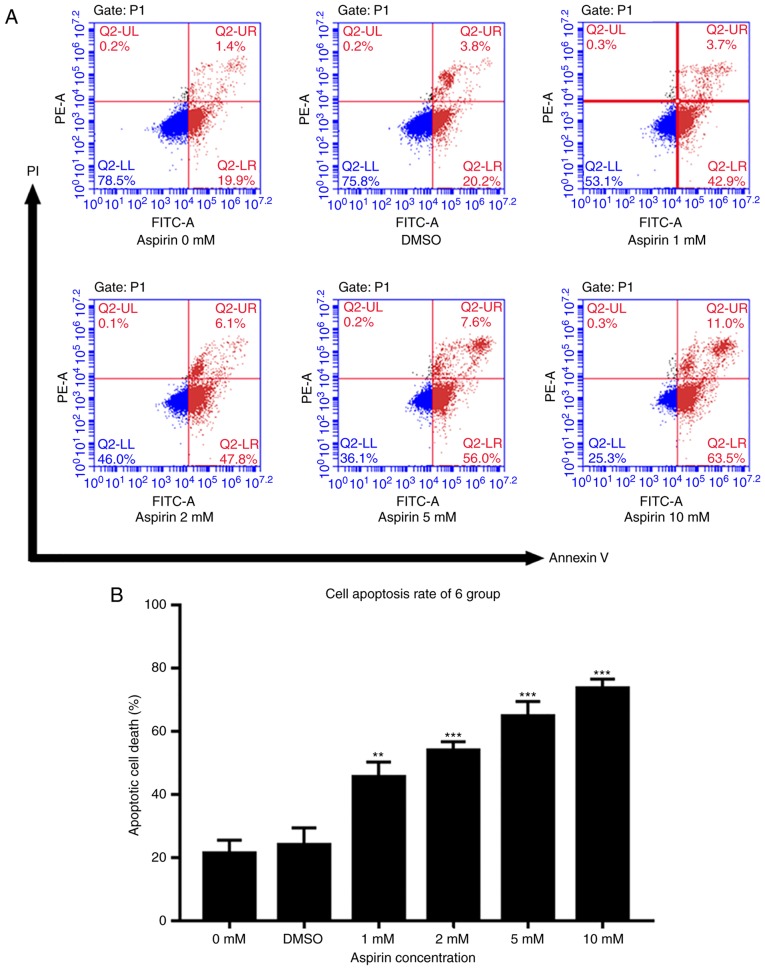
Aspirin triggers RA-FLS cell apoptosis
An Annexin V- FITC/PI assay and flow cytometry were used to test the effects of aspirin on cell apoptosis. As described in Fig. 3A, the percentage of apoptotic cells in treated groups increased gradually with the drug concentration. DMSO groups showed a slightly higher percentage of apoptosis than the control group, but this was not statistically significant. Fig. 3B shows a histogram of the apoptotic proportions in three independent experiments. These results suggest that aspirin can induce apoptosis in a concentration-dependent manner.
Figure 3.
Aspirin induces apoptosis of RA-FLS. (A) Cells were exposed to various concentrations of aspirin for 24 h, and then harvested for apoptosis analysis via flow cytometry and Annexin V-FITC/PI assay. The figures show the apoptosis of RA-FLS induced by aspirin. (B) Bar graph showing the rates of RA-FLS apoptosis induced by various concentrations of aspirin for 24 h; this increased with higher concentrations of aspirin, thus is concentration-dependent. Data are presented as the means ± SD (error bars) from three independent experiments. **P<0.01, ***P<0.001 vs. DMSO group; FITC, fluorescein isothiocyanate; PI, propidium iodide; DMSO, dimethyl sulfoxide; RA-FLS, rheumatoid arthritis-fibroblast-like synoviocytes.
Aspirin induces cell cycle arrest at the G0/G1 phase and decreases the S phase fraction in RA-FLS
To further under-stand the role of aspirin in inhibiting proliferation and to explore its mechanism, flow cytometry was used to detect changes in cell cycle distribution. As presented in Fig. 4A, after aspirin administration, the S phase fraction decreased, while cells in the G0/G1 phase increased in a dose-dependent manner. Therefore, it is hypothesized that aspirin may arrest cells in the G0/G1 phase, preventing them from entering the S phase and thus decreasing proliferation.
Figure 4.
Graphs showing the effects of aspirin on cell cycle distribution in RA-FLS. (A) RA-FLS were treated with aspirin at various concentrations (0, DMSO; 1, 2, 5 and 10 mM) and the cell cycle distribution was detected by flow cytometry after 24 h. (B) Percentages of cells in the different phases are shown in the bar graph. With increased aspirin concentration, the number of cells in the G0/G1 phase increased while those in the S phase decreased. Aspirin blocks cells in the G0/G1 phase to prohibit progression into the S phase. Data are presented as the means ± SD (error bars) from three independent experiments. *P<0.05, **P<0.01, ***P<0.001 vs. DMSO group. DMSO, dimethyl sulfoxide; RA-FLS, rheumatoid arthritis-fibroblast-like synoviocytes.
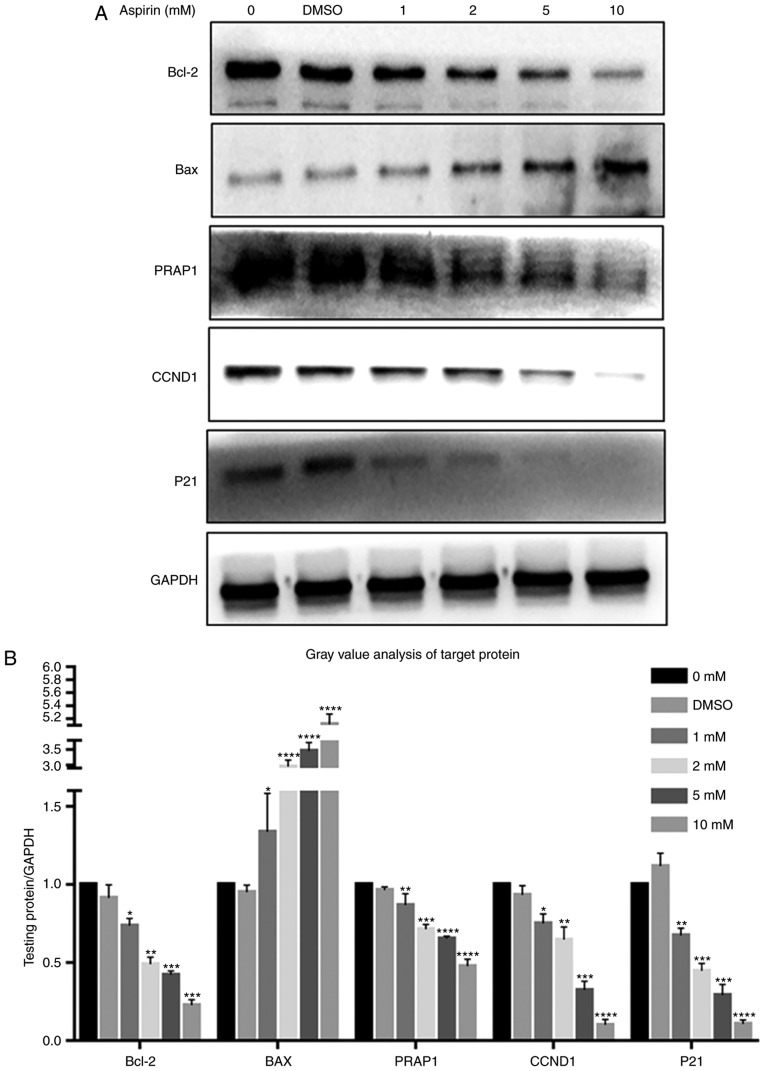
Aspirin treatment regulates the expression of Bcl-2, Bax, PARP1, Cyclin D1 and P21 in RA-FLS
As indicated in Fig. 5, Bcl-2 levels decreased after the addition of aspirin, in a dose-dependent manner (Fig. 5A). Simultaneously, Bax levels increased with the addition of aspirin (Fig. 5A). Intracellular PARP1 content also decreased with increasing aspirin concentration (Fig. 5A). Cyclin D1, which promotes progression in the cell cycle, exhibited decreased intracellular levels after the addition of aspirin, in a concentration-dependent manner (Fig. 5A). P21 was also decreased after the addition of aspirin, contrary to expectation (Fig. 5A). This suggests that P21 may not serve a role in this process. The results showed no significant differences between the DMSO and control groups. Fig. 5B depicts a bar graph with the gray value analysis for Bcl-2, Bax, PARP1, Cyclin D1 and P21.
Figure 5.
Effects of aspirin on Bcl-2, BAX, PARP1, Cyclin D1 and P21 expression in RA-FLS. (A) After RA-FLS were exposed to various concentration of aspirin for 24 h, western blotting was used to determine Bcl-2, BAX, PARP1, Cyclin D1 and P21 protein levels. The corresponding internal control was GADPH. Changes in Bcl-2, BAX, PARP1, Cyclin D1 and P21 protein expression in RA-FLS treated with aspirin are shown: Bcl-2, PARP1, Cyclin D1 and P21 were decreased while BAX was increased, each with increasing aspirin concentration. (B) Bar graph shows the gray value analysis of Bcl-2, BAX, PARP1, Cyclin D1 and P21. Data are presented as the means ± SD (error bars) from three independent experiments. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 vs. DMSO group. We set the value as target protein vs. GAPDH, and set the value of control group as 1. CCND1, Cyclin D1; RA-FLS, rheumatoid arthritis-fibroblast-like synoviocytes; Bcl-2, B-cell lymphoma-2; BAX, Bcl-2-associated X protein; PARP1, poly (ADP-ribose) polymerase 1.
Aspirin downregulates JAK/STAT3 and NF-κB signaling by inhibiting STAT3 and P65, P50 phosphorylation in RA-FLS
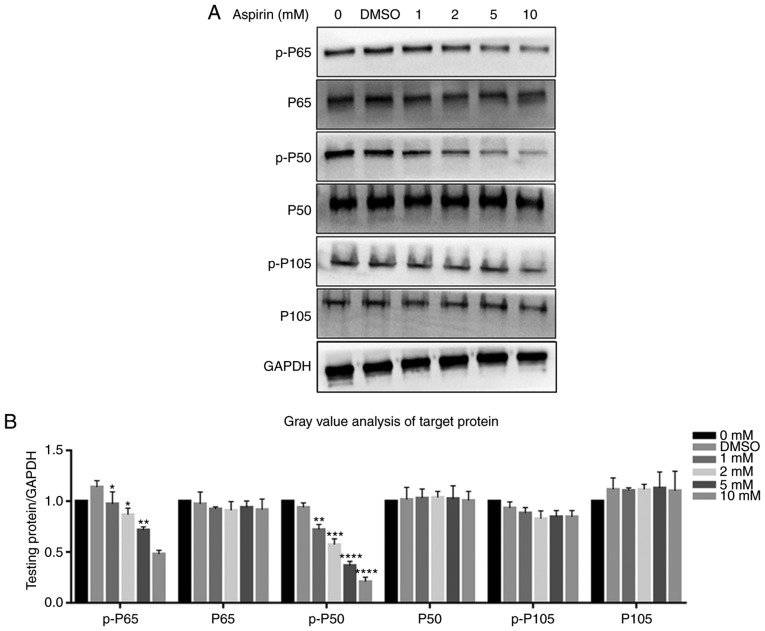
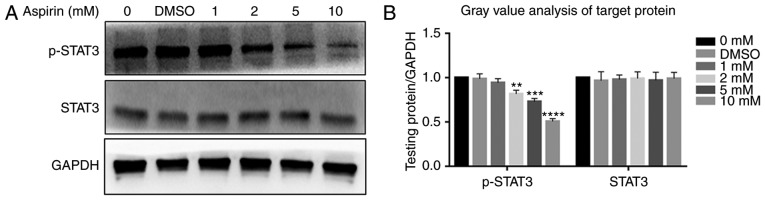
Protein levels of STAT3, p-STAT3, P65, p-P65, P50, p-P50, P105 and p-P105 were investigated via western blotting. It was observed that the JAK/STAT3 and NF-κB signaling pathways were each inhibited after the addition of aspirin. p-P65, p-P50 and p-STAT3 levels decreased gradually with an increase in aspirin concentration, while the P65, P50, P105 and p-P105 levels were not affected (Fig. 6A). Fig. 6B depicts a bar graph representing protein expression. STAT3 was not affected, while p-STAT3 levels decreased (Fig. 7A); Fig. 7B shows the protein expression in a bar plot. This indicates that aspirin inhibits the phosphorylation of P65, P50 and STAT3, thereby inhibiting JAK/STAT3 and NF-κB signal transduction; this inhibitory effect of phosphorylation was concentration-dependent. The DMSO group was similar to the control, and the differences between them were not statistically significant.
Figure 6.
Effects of aspirin on the NF-κB signaling pathway. It was observed that aspirin significantly affects the phosphorylation levels of P65 and P50. (A) Cells were treated with various concentrations of aspirin for 24 h, and then whole cell lysates were obtained and subjected to western blotting to detect p-P65, P65, p-P50, P50, p-P105 and P105. GAPDH served as the loading control. The levels of p-P65 and p-P50 decreased, whilst P65, P50, p-P105 and P105 remained the same. Phosphorylation of P65 and P50 was inhibited to varying degrees by aspirin. (B) Bar graph shows p-P65, P65, p-P50, P50, p-P105 and P105 protein expression, which was analyzed relative to GAPDH expression by densitometry. Data are presented as the means ± SD (error bars) from three independent experiments. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 vs. the DMSO group. We set the value as target protein vs. GAPDH, and set the value of control group as 1. RA-FLS, rheumatoid arthritis-fibroblast-like synoviocytes; NF-κB, nuclear transcription factor-κB; DMSO, dimethyl sulfoxide; p-, phosphorylated.
Figure 7.
Effects of aspirin on the JAK/STAT3 signaling pathway. Aspirin significantly affects the phosphorylation level of STAT3. (A) Cells were treated with various concentrations of aspirin for 24 h, after which whole cell lysates were obtained and subjected to western blotting to detect p-STAT3 and STAT3. GAPDH served as the loading control. Results showed a decrease in p-STAT3, whilst STAT3 remained unchanged. Phosphorylation of STAT3 was inhibited to varying degrees by aspirin. (B) Bar graph represents the expression of p-PSTAT3 and STAT3, which were analyzed relative to GAPDH expression by densitometry. Data are presented as the means ± SD (error bars) from three independent experiments. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 vs. DMSO group. We set the value as target protein vs. GAPDH and set the value of control group as 1. RA-FLS, rheumatoid arthritis-fibroblast-like synoviocytes; STAT3, signal transducer and activator of transcription 3; DMSO, dimethyl sulfoxide; p-, phosphorylated.
Discussion
Aspirin and other NSAIDs are commonly used as anti- inflammatory and analgesic drugs (14). As research progresses, other functions of aspirin are being discovered, such as its potential in cancer prevention (15,16). In vivo, low doses (75-150 mg/day) of aspirin can prevent and treat thrombosis, though higher doses of aspirin (3,000-6,000 mg/day) are required for treating RA. High doses of aspirin can cause serious side effects, while low doses may be less effective; reports have shown that for long-term use, 50-160 mg/day is optimal (17). In vitro experiments demonstrated that the maximum effective dose of aspirin varies between different cells, but generally remains in the 5-10 mM range; it may even reach 20 mM in cervical cancer cells (18). Therefore, four doses, 1, 2, 5 and 10 mM, were selected using the literature and preliminary experiments. The majority of in vivo experiments regarding aspirin in RA have been about its anti-inflammatory effects on FLS and immunocytes, resulting in a reduction in inflammatory factors (19). In RA, abnormal excessive inflammatory factors lead to abnormal FLS proliferation; aspirin has anti-inflammation effects that reduce these inflammation factors and block the associated regulation of FLS, resulting in decreased abnormal proliferation. However, the direct effects of aspirin on FLS have not yet been studied, thus the current study aimed to investigate the antitumor effects of aspirin, particularly the mechanisms by which aspirin can inhibit proliferation and promote the apoptosis of multiple tumor cell types. Bioinformatics analyses were used to investigate biological processes and signaling pathways in which aspirin is involved. Functional/activity network (FAN) analysis of gene-phenotype connectivity liaised by aspirin (20) was used, producing 10 genes: TP53, EP300, RELA, AKT1, NFKB1, CDK2, MYC, CREBBP and NFKBIA. Many of these genes are associated with cell proliferation and apoptosis. TP53 is an important gene involved in proliferation (21), indicating that aspirin affects cell proliferation (22). Furthermore, RELA, NFKB1 and NFKBIA are important molecules in the NF-κB signaling pathway. Therefore, we hypothesized that the NF-κB signaling pathway is significantly regulated by aspirin, which has been confirmed in multiple myeloma cells using in vivo and in vitro experiments (23). Enrichment analysis results revealed that many biological processes and pathways affected by aspirin are associated with cell proliferation (cell cycle) and apoptosis (Table II). These results indicate that aspirin has a significant regulatory effect on cell proliferation and apoptosis. In both GO (BP) and KEGG, signals associated with the NF-κB cascade were evident, suggesting that aspirin may regulate cells through this pathway (24). According to these bioinformatics results, it was initially concluded that aspirin is able to regulate cell proliferation and apoptosis, mainly through the p53 and NF-κB signaling pathways (25).
FLS are similar to cancer cells without the restriction of excessive proliferation (26,27). Apoptotic defects are another important cause of synovial hyperproliferation (28). Aspirin is a first-line treatment drug in RA, working to prevent the conversion of arachidonic acid to prostaglandin by inhibiting COX (4). In other words, it serves the role of an anti-inflammatory analgesic drug. Among the emerging biotherapeutic approaches to RA is cell-based therapy, which targets synovial cells. Therefore, it is essential to understand whether and how aspirin is able to directly affect FLS. Data from the present study indicate that the activity of RA-FLS decreases significantly after the addition of aspirin. Decreased RA-FLS was also noted to persist as the concentration of aspirin increased, indicating it was concentration-dependent. This result is similar to the effect of aspirin on glandular tumors (29). Flow cytometry revealed that apoptosis occurred irrespective of aspirin use; however, after adding aspirin the degree of apoptosis increased. With increased aspirin concentration, the level of apoptosis increased relatively, regardless of whether cells were in the early or late apoptotic stage. It was speculated that this effect is concentration-dependent. In colon cancer, aspirin can induce tumor cell apoptosis (30). There are two known pathways of apoptosis: The death receptor pathway and the intrinsic mitochondrial pathway. The intrinsic mitochondrial pathway is considered to be the more critical pathway of the two in terms of the mechanism of apoptosis (31-34). Mitochondrial pathway-mediated apoptosis is largely regulated by the Bcl-2 protein family. Bax exerts pro-apoptotic effects while Bcl-2 exerts inhibitory effects on apoptosis (33). In this study, the expression of Bax protein was significantly increased in FLS treated with aspirin, while the expression of Bcl-2 protein was significantly decreased. This resulted in an increased Bax/Bcl-2 protein ratio, which is observed in many other cell types during external stimulus-induced apoptosis (23). That aspirin treatment significantly increases the expression of PARP1 protein, relative to the control, suggests that aspirin-induced FLS apoptosis may be mediated by the caspase-mitochondrial apoptotic pathway.
We examined the distribution of the cell cycle by flow cytometry and the expression of cell cycle-associated proteins was measured via western blotting. According to the results, as aspirin concentration increased, the G0/G1 phase fraction increased, indicating that aspirin can prevent cells entering the S phase thus arresting cells in the G0/G1 phase and affecting proliferation (35). The molecular mechanisms of the cell cycle regulation involve cyclin, cyclin-dependent kinase (CDK) and CDK inhibitor (CKI) interactions (34,36). After adding aspirin, Cyclin D1 decreased in a concentration-dependent manner, demonstrating that aspirin causes cell cycle arrest in the G0/G1 phase, potentially via the regulation of Cyclin D1 affecting the normal cell cycle, thus inhibiting proliferation. This is similar to results obtained with numerous other tumor cell types (36). The P21 gene is a cyclin-dependent kinase inhibitor able to arrest cells in the G1 phase (37-40). The results showing the gradual decrease of P21 following the addition of aspirin are inconsistent with our expectations, and contrary to those obtained from other experiments on inhibiting the cell cycle (29). Therefore, there are significant differences between cell types. We hypothesized that aspirin might inhibit the cell cycle in RA-FLS not through P21, but via other molecules such as P27, though further experiments are required for specificity and for verification.
The IL-6/JAK/STAT3 pathway serves a key role in RA (41); there are many genes regulating the cell cycle and apoptosis downstream of the JAK/STAT3 pathway. Therefore, further study of JAK/STAT3 signaling pathway may reveal the mechanism of aspirin on FLS (41). In the present study, the content of STAT3 and p-STAT3 was compared using western blotting before and after the addition of aspirin, revealing that the content of STAT3 was not affected by the addition of aspirin though p-STAT3 levels decreased with increasing aspirin concentration. These results suggest that aspirin likely affects the phosphorylation of STAT3, leading to JAK/STAT3 signaling pathway inhibition. These results are concordant with those in other tumor cells, in which aspirin decreased STAT3 phosphorylation by blocking the formation of STAT3 phosphodiesterase (42). Aspirin inhibits the proliferation of RA-FLS and promotes apoptosis. This may be associated with blockade of the JAK/STAT3 signaling pathway, leading to decreased downstream anti-apoptotic and cell cycle regulatory gene expression.
The NF-κB signaling pathway also serves a key role in the pathogenesis of RA. NF-κB is an important nuclear transcription factor in the pathogenesis of RA, which has two principal mechanisms: Firstly, NF-κB activation can increase the transcription of inflammatory mediators, and these inflammatory mediators can, in turn, activate NF-κB expression; both constitute a positive feedback mechanism leading to the inflammatory response of RA (43). Secondly, it is able to block synovial cell apoptosis, resulting in synovial cell hyperplasia (44). The transcription factor NF-κB regulates the expression of anti-apoptosis proteins and antagonizes the TNF-induced apoptosis of FLS (45). Studies have shown that aspirin can inhibit NF-κB activity and prevent its transfer to the nucleus (46). According to previous bioinformatics results, aspirin may affect the NF-κB signaling pathway by affecting P65 and P50. Our results showed that the content of P65, P50, P105 and p-P105 did not change after the addition of aspirin, while p-P65 and p-P50 levels gradually decreased with increased aspirin concentration. This indicates that aspirin affects P65 and P50 phosphorylation, resulting in NF-κB pathway inhibition. There are anti-apoptotic genes and cell cycle-promoting genes downstream of the NF-κB pathway (47); therefore, it may be considered that aspirin can inhibit the phosphorylation of P65 and P50 to inhibit NF-κB, thus affecting apoptosis and proliferation. This is consistent with results obtained in osteoclast cells (48).
In summary, our results indicate that aspirin is capable of promoting apoptosis in RA-FLS, and of inhibiting proliferation by blocking the cell cycle, in a concentration-dependent manner. Aspirin was found to reduce STAT3, P65 and P50 phosphorylation, thereby inhibiting the JAK/STAT3 and NF-κB signaling pathways. Aspirin promoting apoptosis and inhibiting proliferation may be achieved by blocking both pathways. This direct effect of aspirin on RA-FLS, which has not been previously reported to the best of our knowledge, may provide a novel perspective for revealing the underlying molecular mechanisms and exploring new therapeutic targets for RA. However, many problems remain unsolved that require further study, including the in vivo effects of aspirin on FLS for which we intend to use an RA rat model to investigate.
Funding
This study was supported by the National Nature Science Foundation of China (grant nos. 81470719 and 81611140133).
Availability of data and materials
All data generated or analyzed during this study are included in this published article, and are available from the corresponding author on reasonable request.
Authors’ contributions
ML, TH and NA conceived and designed the study; XZ and HF analyzed the data; JS and DL created the figures and tables; XZ, HF, JD, JS and DL conducted the experiments; XZ wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
Not applicable.
References
- 1.Cooles FA, Isaacs JD. Pathophysiology of rheumatoid arthritis. Curr Opin Rheumatol. 2011;23:233–240. doi: 10.1097/BOR.0b013e32834518a3. [DOI] [PubMed] [Google Scholar]
- 2.Müller-Ladner U, Pap T. Pathogenesis of RA: More than just immune cells. Z Rheumatol. 2005;64:396–401. doi: 10.1007/s00393-005-0772-y. In German. [DOI] [PubMed] [Google Scholar]
- 3.Pope RM. Apoptosis as a therapeutic tool in rheumatoid arthritis. Nat Rev Immunol. 2002;2:527–535. doi: 10.1038/nri846. [DOI] [PubMed] [Google Scholar]
- 4.Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003;110:255–258. doi: 10.1016/S0049-3848(03)00379-7. [DOI] [PubMed] [Google Scholar]
- 5.Yao RS, Rioux N, Castonguay A, You M. Inhibition of COX-2 and induction of apoptosis: Two determinants of nonsteroidal anti-inflammatory drugs’ chemopreventive efficacies in mouse lung tumorigenesis. Exp Lung Res. 2000;26:731–742. doi: 10.1080/01902140150216783. [DOI] [PubMed] [Google Scholar]
- 6.Richter M, Weiss M, Weinberger I, Fürstenberger G, Marian B. Growth inhibition and induction of apoptosis in colorectal tumor cells by cyclooxygenase inhibitors. Carcinogenesis. 2001;22:17–25. doi: 10.1093/carcin/22.1.17. [DOI] [PubMed] [Google Scholar]
- 7.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krause A, Scaletta N, Ji JD, Ivashkiv LB. Rheumatoid arthritis synoviocyte survival is dependent on Stat3. J Immunol. 2002;169:6610–6616. doi: 10.4049/jimmunol.169.11.6610. [DOI] [PubMed] [Google Scholar]
- 9.Makarov SS. NF-kappa B in rheumatoid arthritis: A pivotal regulator of inflammation, hyperplasia and tissue destruction. Arthritis Res. 2001;3:200–206. doi: 10.1186/ar300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46:D1074–D1082. doi: 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 13.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kodela R, Chattopadhyay M, Goswami S, Gan ZY, Rao PP, Nia KV, Velázquez-Martínez CA, Kashfi K. Positional isomers of aspirin are equally potent in inhibiting colon cancer cell growth: Differences in mode of cyclooxygenase inhibition. J Pharmacol Exp Ther. 2013;345:85–94. doi: 10.1124/jpet.112.201970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh CC, Hernández-Ledesma B, de Lumen B. Lunasin, a novel seed peptide, sensitizes human breast cancer MDA-MB-231 cells to aspirin-arrested cell cycle and induced apoptosis. Chem Biol Interact. 2010;186:127–134. doi: 10.1016/j.cbi.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 16.De Luna-Bertos E, Ramos-Torrecillas J, García-Martínez O, Díaz-Rodríguez L, Ruiz C. Effect of aspirin on cell growth of human MG-63 osteosarcoma line. Sci World J. 2012 doi: 10.1100/2012/834246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuzick J, Thorat MA, Bosetti C, Brown PH, Burn J, Cook NR, Ford LG, Jacobs EJ, Jankowski JA, La Vecchia C, et al. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann Oncol. 2015;26:47–57. doi: 10.1093/annonc/mdu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiang S, Sun Z, He Q, Yan F, Wang Y, Zhang J. Aspirin inhibits ErbB2 to induce apoptosis in cervical cancer cells. Med Oncol. 2010;27:379–387. doi: 10.1007/s12032-009-9221-0. [DOI] [PubMed] [Google Scholar]
- 19.Fries JF, Ramey DR, Singh G, Morfeld D, Bloch DA, Raynauld JP. A reevaluation of aspirin therapy in rheumatoid arthritis. Arch Intern Med. 1993;153:2465–2471. doi: 10.1001/archinte.1993.00410210093010. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh TC, Wu ST, Bennett DJ, Doonan BB, Wu E, Wu JM. lFunctional/activity network (FAN) analysis of gene-phenotype connectivity liaised by grape polyphenol resveratrol. Oncotarget. 2016;7:38670–38680. doi: 10.18632/oncotarget.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alshatwi AA. Catechin hydrate suppresses MCF-7 proliferation through TP53/Caspase-mediated apoptosis. J Exp Clin Canc Res. 2010;29:167. doi: 10.1186/1756-9966-29-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clària J, Lee MH, Serhan CN. Aspirin-triggered lipoxins (15-epi-LX) are generated by the human lung adenocarcinoma cell line (A549)-neutrophil interactions and are potent inhibitors of cell proliferation. Mol Med. 1996;2:583–596. doi: 10.1007/BF03401642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding JH, Yuan LY, Huang RB, Chen GA. Aspirin inhibits proliferation and induces apoptosis of multiple myeloma cells through regulation of Bcl-2 and Bax and suppression of VEGF. Eur J Haematol. 2014;93:329–339. doi: 10.1111/ejh.12352. [DOI] [PubMed] [Google Scholar]
- 24.Kopp E, Ghosh S. Inhibition of Nf-kappa B by sodium-salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 25.Shao J, Fujiwara T, Kadowaki Y, Fukazawa T, Waku T, Itoshima T, Yamatsuji T, Nishizaki M, Roth JA, Tanaka N. Overexpression of the wild-type p53 gene inhibits NF-kappaB activity and synergizes with aspirin to induce apoptosis in human colon cancer cells. Oncogene. 2000;19:726–736. doi: 10.1038/sj.onc.1203383. [DOI] [PubMed] [Google Scholar]
- 26.Gay S, Gay RE, Koopman WJ. Molecular and cellular mechanisms of joint destruction in rheumatoid-arthritis: Two cellular mechanisms explain joint destruction. Ann Rheum Dis. 1993;52:S39–S47. doi: 10.1136/ard.52.Suppl_1.S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huber LC, Distler O, Tarner I, Gay RE, Gay S, Pap T. Synovial fibroblasts: Key serveers in rheumatoid arthritis. Rheumatology (Oxford) 2006;45:669–675. doi: 10.1093/rheumatology/kel065. [DOI] [PubMed] [Google Scholar]
- 28.Pattacini L, Boiardi L, Casali B, Salvarani C. Differential effects of anti-TNF-alpha drugs on fibroblast-like synoviocyte apoptosis. Rheumatology (Oxford) 2010;49:480–489. doi: 10.1093/rheumatology/kep358. [DOI] [PubMed] [Google Scholar]
- 29.Yang C, Liu J, Wang YX, Tong JJ, Wu YH, Liu Y. Aspirin inhibits the proliferation of canine mammary gland tumor cells in vitro and in vivo. Transl Cancer Res. 2017;6:188–197. doi: 10.21037/tcr.2017.01.07. [DOI] [Google Scholar]
- 30.Stark LA, Din FVN, Zwacka RM, Dunlop MG. Aspirin-induced activation of the NF-kappaB signaling pathway: A novel mechanism for aspirin-mediated apoptosis in colon cancer cells. Faseb J. 2001;15:1273–1275. doi: 10.1096/fj.00-0529fje. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Yuan L, Xiao HF, Xiao CX, Wang YT, Liu XB. Momordin Ic induces HepG2 cell apoptosis through MAPK and PI3K/Akt-mediated mitochondrial pathways. Apoptosis. 2013;18:751–765. doi: 10.1007/s10495-013-0820-z. [DOI] [PubMed] [Google Scholar]
- 32.Fulda S, Debatin KM. Extrinsic vs. intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Li X, Wang XL, Lau W, Wang Y, Xing Y, Zhang X, Ma X, Gao F. Ginsenoside Rd attenuates myocardial ischemia/reperfusion injury via Akt/GSK-3β signaling and inhi-bition of the mitochondria-dependent apoptotic pathway. PLoS One. 2013;8:e70956. doi: 10.1371/journal.pone.0070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunter T. Braking the cycle. Cell. 1993;75:839–841. doi: 10.1016/0092-8674(93)90528-X. [DOI] [PubMed] [Google Scholar]
- 35.Fan WS, Li JH, Chen JF, Zhu L, Wang Y, Sun B, Hua B, Guo C, Yan Z. Aspirin inhibits the proliferation of synovium-derived mesenchymal stem cells by arresting the cell cycle in the G0/G1 phase. Am J Transl Res. 2017;9:5056–5062. [PMC free article] [PubMed] [Google Scholar]
- 36.Ou YQ, Zhu WB, Li Y, Qiu PX, Huang YJ, Xie J, He SM, Zheng XK, Leng TD, Xu D, Yan GM. Aspirin inhibits proliferation of gemcitabine-resistant human pancreatic cancer cells and augments gemcitabine-induced cytotoxicity. Acta Pharmacol Sin. 2010;31:73–80. doi: 10.1038/aps.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Martindale JL, Gorospe M, Holbrook NJ. Regulation of p21WAF1/CIP1 expression through mitogen-activated protein kinase signaling pathway. Cancer Res. 1996;56:31–35. [PubMed] [Google Scholar]
- 38.Lloyd RV, Erickson LA, Jin L, Kulig E, Qian X, Cheville JC, Scheithauer BW. p27kip1: A multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol. 1999;154:313–323. doi: 10.1016/S0002-9440(10)65277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherr CJ. The Pezcoller lecture: Cancer cell cycles revisited. Cancer Res. 2000;60:3689–3695. [PubMed] [Google Scholar]
- 40.Agus DB, Cordon-Cardo C, Fox W, Drobnjak M, Koff A, Golde DW, Scher HI. Prostate cancer cell cycle regulators: Response to androgen withdrawal and development of androgen independence. J Natl Cancer Inst. 1999;91:1869–1876. doi: 10.1093/jnci/91.21.1869. [DOI] [PubMed] [Google Scholar]
- 41.Liu JX, Fei D, Xing J, Du J. MicroRNA-29a inhibits proliferation and induces apoptosis in rheumatoid arthritis fibroblast-like synoviocytes by repressing STAT3. Biomed Pharmacother. 2017;96:173–181. doi: 10.1016/j.biopha.2017.09.120. [DOI] [PubMed] [Google Scholar]
- 42.Guo H, Liu J, Ben Q, Qu Y, Li M, Wang Y, Chen W, Zhang J. The aspirin-induced long non-coding RNA OLA1P2 blocks phosphorylated STAT3 homodimer formation. Genome Biol. 2016;17:24. doi: 10.1186/s13059-016-0892-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang W, Sun W, Jin L. Caffeic acid alleviates inflammatory response in rheumatoid arthritis fibroblast-like synoviocytes by inhibiting phosphorylation of IκB kinase α/β and IκBα. Int Immunopharmacol. 2017;48:61–66. doi: 10.1016/j.intimp.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 44.Ni S, Miao K, Zhou X, Xu N, Li C, Zhu R, Sun R, Wang Y. The involvement of follistatin-like protein 1 in osteoarthritis by elevating NF-κB-mediated inflammatory cytokines and enhancing fibroblast like synoviocyte proliferation. Arthritis Res Ther. 2015;17:91. doi: 10.1186/s13075-015-0605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim SK, Park KY, Yoon WC, Park SH, Park KK, Yoo DH, Choe JY. Melittin enhances apoptosis through suppression of IL-6/sIL-6R complex-induced NF-κB and STAT3 activation and Bcl-2 expression for human fibroblast-like synoviocytes in rheumatoid arthritis. Joint Bone Spine. 2011;78:471–477. doi: 10.1016/j.jbspin.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Chen JY, Stark LA. Aspirin prevention of colorectal cancer: Focus on NF-κB signalling and the nucleolus. Biomedicines. 2017;5:E43. doi: 10.3390/biomedicines5030043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guesmi F, Prasad S, Tyagi AK, Landoulsi A. Antinflammatory and anticancer effects of terpenes from oily fractions of Teucruim alopecurus, blocker of IκBα kinase, through downregulation of NF-κB activation, potentiation of apoptosis and suppression of NF-κB-regulated gene expression. Biomed Pharmacother. 2017;95:1876–1885. doi: 10.1016/j.biopha.2017.09.115. [DOI] [PubMed] [Google Scholar]
- 48.Zhang P, Wu C, Huang XH, Shen CL, Li L, Zhang W, Yao CZ. Aspirin suppresses TNF-α-induced MMP-9 expression via NF-κB and MAPK signaling pathways in RAW264.7 cells. Exp Ther Med. 2017;14:5597–5604. doi: 10.3892/etm.2017.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article, and are available from the corresponding author on reasonable request.