Abstract
Lifelong therapy with mineralocorticoid receptor antagonists (MRAs) or surgical adrenalectomy are the recommended treatments for primary aldosteronism (PA). Whether these treatments mitigate the risk for kidney disease remains unknown. We performed a retrospective cohort study of patients with PA treated with MRAs (N=400) or surgical adrenalectomy (N=120), and age- and estimated glomerular filtration rate (eGFR)-matched patients with essential hypertension (N=15,474) to determine risk for chronic kidney disease and longitudinal eGFR decline. Despite similar blood pressures, patients with PA treated with MRAs had a higher risk for incident chronic kidney disease compared with essential hypertension patients (adjusted HR 1.63 [95% CI 1.33, 1.99]). Correspondingly, the adjusted annual decline in eGFR was greater in PA patients treated with MRAs compared with essential hypertension patients (−1.6 [95% CI −1.4, −1.8] vs. −0.9 [95% CI −0.9, −1.0] mL/min/1.73 m2/year, p < 0.001). In contrast, patients with unilateral PA treated with surgical adrenalectomy had no significant difference in risk for incident chronic kidney disease or in annual decline in eGFR compared with essential hypertension patients. Among PA patients with diabetes treated with MRAs, there was a higher risk for incident albuminuria compared with essential hypertension (adjusted HR 2.52 [95% CI 1.28, 4.96]). MRA therapy in PA is associated with higher risk for developing chronic kidney disease, when compared with essential hypertension, and surgical adrenalectomy may mitigate this risk. When possible, curative surgical adrenalectomy may be superior to lifelong MRA therapy in preventing kidney disease in PA.
Keywords: Primary aldosteronism, chronic kidney disease, hypertension, glomerular filtration rate, mineralocorticoid receptor antagonists, adrenalectomy
Introduction
Primary aldosteronism (PA), a disorder of renin-independent aldosterone secretion,1, 2 is the most common cause of endocrine hypertension and increases the risk for cardiovascular and renal disease, independent of blood pressure, via excessive activation of the mineralocorticoid receptor (MR).3–8 Recent studies suggest that 4-19% of patients with hypertension9–11 and 3-14% of patients with normotension12, 13 may have PA, thus the global prevalence of patients with unrecognized PA who are at high risk for adverse cardiovascular and renal outcomes is considered to be high.6
Excessive aldosterone exposure is known to result in progressive renal injury by inducing renal fibrosis, vascular disease, and podocyte injury.14–16 Early in the disease process, excessive MR activation in primary aldosteronism results in increased intravascular volume and hyperfiltration as evidenced by an increased estimated glomerular filtration rate (eGFR).5, 17, 18 Cross-sectional studies suggest that this initial hyperfiltration is followed by a more rapid decline in eGFR and increased albuminuria due to the chronic effects of the aldosterone-MR interaction.5, 19 Although MR antagonists and surgical adrenalectomy are the expert consensus recommended therapies for PA,2 whether they adequately preserve GFR, and reduce the risk for developing chronic kidney disease (CKD) and albuminuria is not well understood. Prior human studies to evaluate the effect of PA therapies on renal outcomes have been small in size and observed no significant differences in long-term GFR decline or albuminuria progression, suggesting that medical and surgical therapies for PA may be similarly adequate at mitigating the risk for kidney disease in PA.18, 20
Due to the high prevalence and associated renal morbidity of PA, there is a vital need for large longitudinal studies to examine the comparative efficacies of recommended treatments for PA in reducing the risk for adverse renal outcomes. Recent large cohort studies have shown that even when PA patients are treated with MR antagonists to reduce blood pressure, they have a substantially higher risk for developing cardiovascular events and death when compared to similar patients with essential hypertension and PA patients who undergo surgical adrenalectomy.8, 21 Herein, we conducted a large cohort study to examine the risk for incident CKD, eGFR decline, and incident albuminuria in patients with PA treated with MR antagonists and surgical adrenalectomy compared with patients with essential hypertension who were of similar age and had comparable baseline eGFR and blood pressure.
Methods
The data and analytic methods that support the findings of this study can be requested from the corresponding author by qualified researchers trained in human subject confidentiality protocols and with institutional ethics board approval. The study procedures comply with the Declaration of Helsinki and were approved by the Partners Healthcare System Institutional Review Board Protocol.
Data Source and Study Cohort
The study cohort was derived from a research registry of all patients at Brigham and Women’s Hospital, Massachusetts General Hospital, and their affiliated partner hospitals (Figure 1). Eligibility for the study required that patients had to be seen between the years 1991-2016 and be ≥ 18 years of age. Entry into the study was defined as the date of the first follow-up visit 1-6 months after initiating an MR antagonist (for PA patients treated with MR antagonists), after undergoing surgical adrenalectomy (for PA patients treated surgically), or after the diagnosis of essential hypertension was first entered into the medical record (for essential hypertension patients).
Figure 1. Derivation of study cohort.
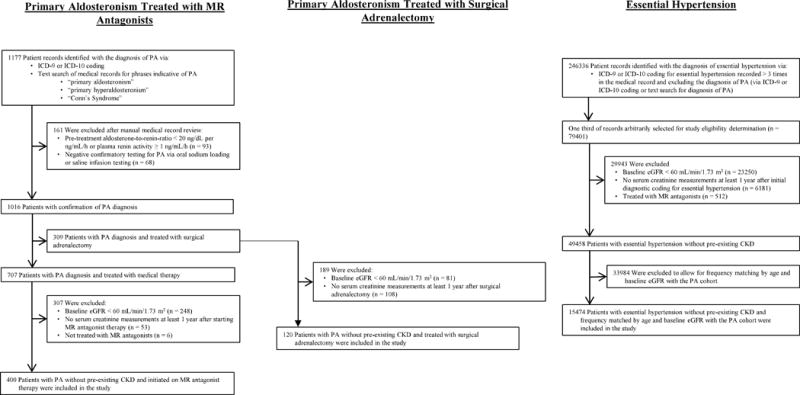
MR = mineralocorticoid receptor; PA = primary aldosteronism; ICD-9/10 = International Classification of Disease 9th or 10th Edition; eGFR = estimated glomerular filtration rate; CKD = chronic kidney disease.
The registry was searched for all potential patients with PA using International Classification of Disease, 9th and 10th Revisions (ICD-9 and ICD-10) codes as well as using the following text search terms: “primary aldosteronism”, “primary hyperaldosteronism”, and “Conn’s Syndrome”. Manual medical record review was performed by two experts (please see the online supplement at http://hyper.ahajournals.org) to exclude patients whose baseline laboratory criteria were inconsistent with the diagnosis of PA (Figure 1). Patients with PA were then categorized by whether they were treated with MR antagonists or surgical adrenalectomy as recommended by The Endocrine Society.2 PA patients treated with medical therapy were further excluded if they had an eGFR at study entry of < 60 mL/min/1.73 m2, had no serum creatinine measurements at least 1 year after initiating MR antagonist therapy, or were not treated with MR antagonists. PA patients treated with surgical adrenalectomy were excluded if they had an eGFR at study entry of < 60 mL/min/1.73 m2 or had no serum creatinine measurements at least 1 year after undergoing surgical adrenalectomy. There were 400 patients with PA treated with MR antagonist medications and 120 patients with PA treated with surgical adrenalectomy, all without pre-existing CKD, included in the study.
The registry was then searched for patients with essential hypertension using ICD-9 and ICD-10 codes while excluding any diagnosis of PA. Essential hypertension patients were excluded if they had an eGFR at study entry of < 60 mL/min/1.73 m2, had no serum creatinine measurements at least 1 year after initial ICD-9 or ICD-10 coding for essential hypertension, or were treated with MR antagonist medications. The essential hypertension population was frequency matched by decade of age and eGFR at study entry to the PA study population treated with MR antagonist therapy. There were 15474 patients with essential hypertension without pre-existing CKD included in the study (Figure 1).
Definition of Exposure
For the primary analysis, the main exposure was the diagnosis of PA subdivided by treatment with either MR antagonists or surgical adrenalectomy. The primary comparison group was patients with essential hypertension who were frequency matched by age and eGFR at study entry to the PA patients treated with MR antagonists. For more detailed explanation on exposure definitions, please see the online supplement at http://hyper.ahajournals.org.
Definition of Covariates
The following covariates at the time of study entry were considered as potential confounders: age, sex, race, history of diabetes mellitus, history of cardiovascular disease (defined as prior myocardial infarction/coronary revascularization, transient ischemic attack/stroke, or hospitalization for heart failure), eGFR, systolic blood pressure, and angiotensin-converting enzyme (ACE) inhibitor or angiotensin II receptor blocker use.
Definition of Outcomes
The primary outcome was incident CKD defined as the combination of a decrease in eGFR to < 60 mL/min/1.73 m2 in addition to an overall decline in eGFR of ≥ 15 mL/min/1.73 m2 from the value at study entry. eGFR values were calculated via the CKD-EPI equation22 using the serum creatinine measurements closest (+/− 3 months) to the following times after study entry: 1, 2, 3, 4, 5, and 10 years. Secondary outcomes were the longitudinal changes in eGFR and incident albuminuria. We defined albuminuria as a spot urine albumin-to-creatinine ratio (UACR) ≥ 30 mg/g. Since albuminuria was not routinely measured in all patients with essential hypertension, the albuminuria analysis was restricted to only patients with diabetes mellitus, where routine measure of albuminuria is standard of practice, and who had a UACR < 30 mg/g at study entry (N=26 for PA treated with MR antagonists, N=578 for essential hypertension). Patients with PA treated with surgical adrenalectomy were not included in the albuminuria analysis as there were a very small number of eligible patients with diabetes mellitus and a UACR < 30 mg/g at study entry (N=5).
Statistical Analysis
The primary analysis investigated whether patients treated for PA with MR antagonists or surgery had a higher risk of incident CKD compared with patients with essential hypertension. Secondary analyses investigated: 1) the longitudinal change in mean eGFR between these three populations; and 2) the risk for incident albuminuria among diabetic patients. We used adjusted Cox regression models (proc PHREG in SAS v9.4, Cary, NC) to estimate adjusted hazard ratios and 95% confidence intervals. The proportional hazard assumption was evaluated by testing significance of the exposure-time interactions in the Cox models. The standardized cumulative incidence of CKD was one minus a standardized survival function (proc PHREG baseline statement in SAS v9.4), which was estimated by averaging the estimated individual-specific survival curves based on the multivariate Cox models mentioned above over the distributions of the aforementioned covariates in the cohort. We used this model to calculate differences in standardized cumulative incidence of CKD at 2, 5, and 10 years of follow-up between patients with PA treated with MR antagonists, patients with PA treated with surgical adrenalectomy, and patients with essential hypertension.
For the secondary analysis investigating the longitudinal change in mean eGFR, we used multivariate repeated measures models (proc MIXED in SAS v9.4) to determine differences in the slope of eGFR decline between patients with PA and patients with essential hypertension.
Patients were censored on the date of the specified outcome occurrence or, if they did not develop the outcome, at the date of the final serum creatinine (for the CKD and eGFR analyses) or UACR (for the albuminuria analysis) measurement. All p-values are two-sided.
Results
Patient Characteristics
Compared with patients with essential hypertension, PA patients treated with MR antagonists had, by design, similar age and eGFR at study entry but were more likely to be black, have diabetes mellitus, have pre-existing cardiovascular disease, have lower serum potassium, and be prescribed potassium supplementation (Table 1). PA patients treated surgically were younger, had less pre-existing cardiovascular disease, and had a biochemically more severe PA phenotype (Table 1). At the time of study entry and throughout the duration of the study, the mean baseline blood pressures were comparable between patients with PA and essential hypertension (Figure 2). The number of prescribed antihypertensive medications, not including MR antagonists, was also similar amongst the groups; however, PA patients were less likely to be prescribed potassium-wasting diuretics and more likely to be prescribed potassium-sparing diuretics compared with patients with essential hypertension (Table 1). Dynamic confirmatory testing was employed in the majority of patients, whereas a minority had sufficiently suggestive screening diagnostics to confirm the diagnosis of PA (Table 1).2 Adrenal venous sampling was used to make treatment decisions in most surgical patients (83%), but only slightly more than half of medically treated PA patients (55%) (Table 1).
Table 1.
Baseline Characteristics of Study Cohort.
| Baseline Characteristics | Primary Aldosteronism – MR Antagonist (N = 400) |
Primary Aldosteronism - Surgery (N = 120) |
Essential Hypertension (N = 15474) |
|---|---|---|---|
| Age (yr) | 55.9 (11.6) | 49.6 (10.9) | 56.8 (11.4) |
| Sex – Female (N/%) | 182/46 | 51/42 | 7904/51 |
| Race or Ethnic Group (N/%) | |||
| White | 217/54 | 79/66 | 10072/65 |
| Black | 115/29 | 15/12 | 2466/16 |
| Hispanic | 36/9 | 13/11 | 1074/7 |
| Other* | 32/8 | 13/11 | 1862/12 |
| Serum Creatinine (mg/dL) | 0.95 (0.21) | 0.96 (0.20) | 0.92 (0.19) |
| Estimated Glomerular Filtration Rate (mL/min/1.73 m2) | 83.5 (16.6) | 85.5 (16.2) | 83.6 (16.7) |
| Diabetes Mellitus (N/%) | 105/26 | 14/12 | 3369/22 |
| Hemoglobin A1C (%) Patients with Diabetes | 6.0 (1.0) | 5.8 (0.7) | 6.0 (0.9) |
| Prior Cardiovascular Disease (N/%)† | 63/16 | 7/6 | 1976/13 |
| Follow-up Time (yr) | 7.5 (4.4) | 7.8 (5.5) | 8.7 (5.4) |
| Primary Aldosteronism Characteristics‡ | |||
| Serum Aldosterone (ng/dL) | 22.6 (17.0-33.0) | 29.0 (19.0-45.0) | – |
| Plasma Renin Activity (N/%)§ | |||
| ≤ 0.60 ng/mL/h | 360/90 | 109/91 | – |
| 0.61 – 0.99 ng/mL/h | 40/10 | 11/9 | – |
| ≥ 1.00 ng/mL/h | 0/0 | 0/0 | – |
| Aldosterone-to-Renin Ratio (ng/dL per ng/mL/h) | 60 (34-134) | 82 (46-190) | – |
| Serum Potassium (mmol/L) | 3.6 (0.5) | 3.5 (0.5) | 4.1 (0.5) |
| Potassium Supplementation (N/%) | 177/44 | 37/31 | 248/2 |
| Confirmatory Testing (N/%) | 286/72 | 103/86 | – |
| CT or MRI Imaging (N/%) | 385/96 | 120/100 | – |
| Unilateral Adrenal Abnormality (N/%) | 146/38 | 110/92 | – |
| Bilateral Adrenal Abnormalities (N/%) | 43/11 | 5/4 | – |
| Normal Appearing Adrenal Glands (N/%) | 196/51 | 5/4 | – |
| Adrenal Vein Sampling (N/%) | 219/55 | 100/83 | – |
| Unilateral PA (N/%) | 33/15 | 98/98 | – |
| Bilateral PA (N/%) | 153/70 | 0/0 | – |
| Unsatisfactory or Indeterminate (N/%) | 33/15 | 2/2 | – |
| Baseline Blood Pressure Prior to Study Entry‖ | |||
| Systolic Blood Pressure (mmHg) | 146 (16) | 143 (17) | 135 (18) |
| Diastolic Blood Pressure (mmHg) | 86 (11) | 87 (13) | 81 (11) |
| Baseline Blood Pressure at Time of Study Entry¶ | |||
| Systolic Blood Pressure (mmHg) | 140 (19) | 135 (19) | 137 (19) |
| Diastolic Blood Pressure (mmHg) | 83 (13) | 83 (12) | 80 (12) |
| Antihypertensive Medication Use | |||
| MR Antagonist Use (N/%) | 400/100 | 0/0 | 0/0 |
| Spironolactone (N/%) | 332/83 | – | – |
| Eplerenone (N/%) | 68/17 | – | – |
| Mean Spironolactone Total Daily Dose (mg) | 42 (28) | – | – |
| Mean Eplerenone Total Daily Dose (mg) | 52 (26) | – | – |
| Mean Number of Non-MR Antagonist Antihypertensive Medications | 2.8 (1.4) | 2.4 (1.3) | 2.6 (1.4) |
| ACE Inhibitor/Angiotensin II Receptor Blocker (N/%) | 238/60 | 68/57 | 9210/60 |
| Calcium Channel Blocker (N/%) | 271/68 | 74/62 | 6967/45 |
| Beta Blocker (N/%) | 247/62 | 68/57 | 9006/58 |
| Diuretic | |||
| Thiazide (N/%) | 161/40 | 25/21 | 8077/52 |
| Loop (N/%) | 24/6 | 1/1 | 2445/16 |
| Potassium-Sparing (Non-MR Antagonist) (N/%) | 72/18 | 20/17 | 1392/9 |
| Other (N/%)# | 88/22 | 28/23 | 2616/17 |
Unless otherwise specified, normally distributed continuous variables are reported as mean (SD); non-normally distributed continuous variables are reported as median (25th – 75th percentile IQR); categorical variables are reported as percentages. MR = mineralocorticoid receptor; ACE = angiotensin-converting enzyme.
Other race includes Asian, Native American, other, and unknown.
Defined as prior myocardial infarction/coronary revascularization, congestive heart failure hospitalization, or transient ischemic attack/stroke.
Laboratory values most recent prior to study entry.
For the majority of the study period, the hospital-affiliated laboratories reported a minimum plasma renin activity of < 0.60 ng/mL/h. For study purposes, these minimum values were recorded as 0.59 ng/mL/h.
Refers to the last blood pressure recorded prior to study entry (i.e. just prior to starting MR antagonist [PA – MR Antagonist], just prior to surgical adrenalectomy [PA – Surgery] or just prior to ICD-9/10 coding for essential hypertension [Essential Hypertension]).
Refers to the first blood pressure recorded 1-6 months after starting MR antagonist therapy [PA – MR Antagonist], undergoing surgical adrenalectomy [PA – Surgery], or initial ICD-9/10 coding for essential hypertension [Essential Hypertension].
Other antihypertensive medication includes hydralazine, clonidine, alpha blockers, nitrates, minoxidil, methyldopa, and direct renin inhibitors.
Figure 2. Blood pressure trends in study cohort.
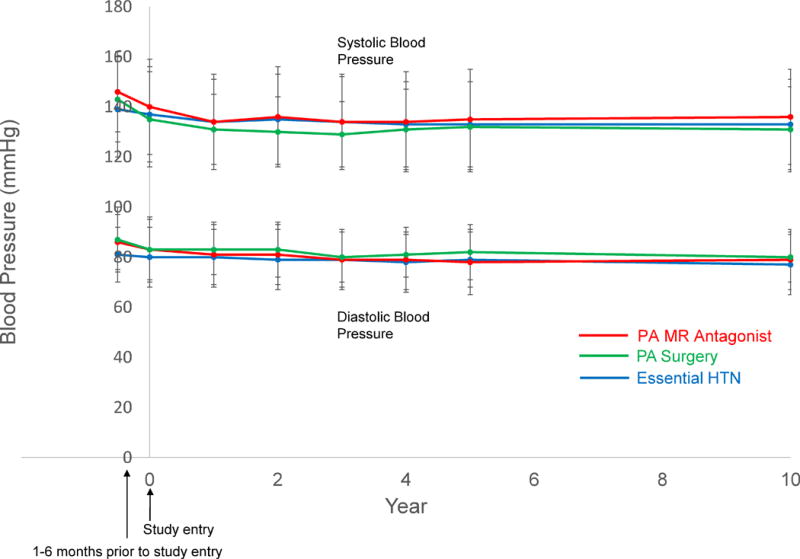
PA = primary aldosteronism; MR = mineralocorticoid receptor; HTN = hypertension.
Risk for Incident Chronic Kidney Disease
PA patients treated with MR antagonists had a higher rate of incident CKD compared with PA patients treated surgically and patients with essential hypertension (55.6 [95% CI 45.8, 66.8] vs. 16.7 [95% CI 8.3, 29.9] vs. 35.6 [34.4, 36.9] events per 1,000 person years). After accounting for blood pressure and other confounders, PA patients treated with MR antagonists had a higher risk for incident CKD compared with both PA patients treated surgically (adjusted HR 2.30 [95% CI 1.22, 4.35]) and patients with essential hypertension (adjusted HR 1.63 [95% CI 1.33, 1.99]) (Figure 3). There was no significant difference in risk for incident CKD comparing PA patients treated surgically with patients with essential hypertension (adjusted HR 0.71 [95% CI 0.39, 1.30]) (Figure 3). The adjusted 10-year cumulative incidence difference for developing CKD indicated that PA patients treated with MR antagonists had 17.9 (95% CI 6.5, 29.2) excess cases of CKD per 100 persons compared with PA patients treated surgically and 11.4 (95% CI 6.2, 16.5) excess cases of CKD per 100 persons compared with patients with essential hypertension (Table 2). These results did not materially change when the analysis was restricted to only PA patients with bilateral PA confirmed by adrenal vein sampling (AVS) and treated with MR antagonists (N=153) and only PA patients with unilateral PA confirmed by AVS and treated with surgery (N=98) (Figure S1, please see online supplement at http://hyper.ahajournals.org).
Figure 3. Standardized cumulative incidence curve of chronic kidney disease.
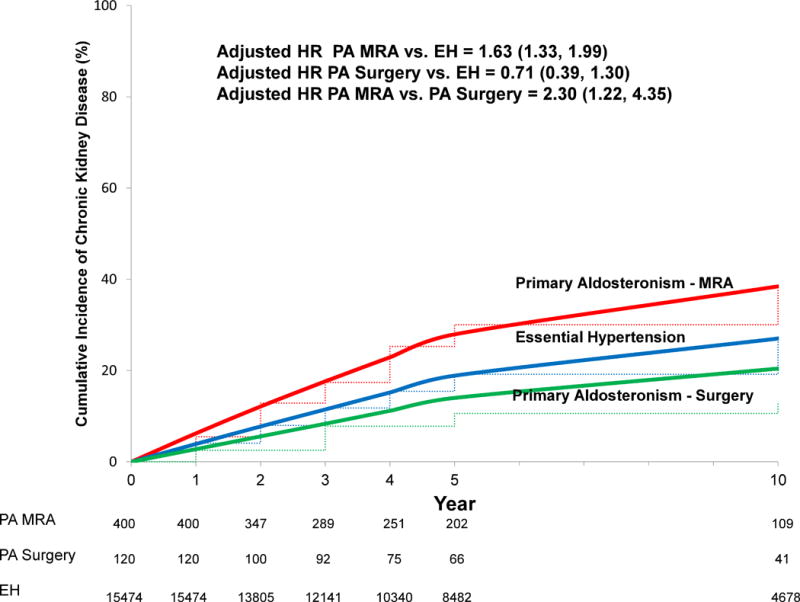
Solid lines = adjusted cumulative incidence; dashed lines = unadjusted cumulative incidence. Incident chronic kidney disease defined as the combination of a decrease in eGFR to < 60 mL/min/1.73 m2 in addition to an overall decline in eGFR of ≥ 15 mL/min/1.73 m2 from the value at study entry. HR adjusted for, and cumulative incidence curve standardized to the distribution of, the following variables at the time of study entry in our cohort: age, sex, race, diabetes mellitus, cardiovascular disease, eGFR, systolic blood pressure, and angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use. HR = hazard ratio; PA = primary aldosteronism; EH = essential hypertension; MRA = mineralocorticoid receptor antagonist.
Table 2.
Standardized Cumulative Incidence of CKD in Treated Primary Aldosteronism and Essential Hypertension.
| Cumulative Incidence Times | PA – MR Antagonists | PA – Surgical Adrenalectomy | Essential Hypertension | Difference in Incident CKD (PA MRA – PA Surgery) | Difference in Incident CKD (PA MRA – EH) | Difference in Incident CKD (EH – PA Surgery) |
|---|---|---|---|---|---|---|
| 2-Year Cumulative CKD Incidence (per 100 persons) | ||||||
| Unadjusted (95% CI) | 12.2 (9.9, 14.4) | 3.7 (1.5, 5.9) | 7.8 (7.4, 8.2) | 8.5 (5.4, 11.6) | 4.4 (2.1, 6.6) | 4.1 (1.9, 6.3) |
| Adjusted (95% CI)* | 12.1 (9.9, 14.2) | 5.6 (2.3, 8.7) | 7.7 (7.3, 8.1) | 6.5 (2.7, 10.4) | 4.3 (2.2, 6.5) | 2.2 (−1.1, 5.4) |
| 5-Year Cumulative CKD Incidence (per 100 persons) | ||||||
| Unadjusted (95% CI) | 28.3 (23.6, 32.8) | 9.3 (3.8, 14.4) | 18.9 (18.2, 19.5) | 19.1 (12.1, 26.1) | 9.5 (4.9, 14.1) | 9.6 (4.3, 14.9) |
| Adjusted (95% CI)* | 27.9 (23.6, 31.9) | 14.0 (6.2, 21.1) | 18.9 (18.2, 19.5) | 13.9 (5.3, 22.4) | 9.0 (4.8, 13.2) | 4.9 (−2.6, 12.3) |
| 10-Year Cumulative CKD Incidence (per 100 persons) | ||||||
| Unadjusted (95% CI) | 39.0 (33.0, 44.6) | 13.4 (5.6, 20.6) | 26.7 (25.8, 27.6) | 25.6 (16.2, 35.1) | 12.4 (6.5, 18.2) | 13.3 (5.7, 20.8) |
| Adjusted (95% CI)* | 38.3 (33.0, 43.2) | 20.4 (9.6, 30.0) | 26.9 (26.1, 27.8) | 17.9 (6.5, 29.2) | 11.4 (6.2, 16.5) | 6.5 (−3.7, 16.7) |
CKD = chronic kidney disease; PA = primary aldosteronism; MR = mineralocorticoid receptor; MRA = mineralocorticoid receptor antagonist; EH = essential hypertension.
Standardized to the distributions at the time of study entry in our cohort for the following variables: age, sex, race/ethnicity, diabetes mellitus, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker use, pre-existing cardiovascular disease, and systolic blood pressure.
Among the PA patients treated with MR antagonists, there was no significant difference in incident CKD (adjusted HR 1.07 [95% CI 0.53, 2.19]) between those whose first plasma renin activity level, measured at least one month after initiating MR antagonist therapy, became unsuppressed (≥ 1.0 ng/mL/h, N=43) compared with those whose plasma renin activity level remained suppressed (< 1.0 ng/mL/h, N=104).
Longitudinal Change in Mean Estimated Glomerular Filtration Rate
Although PA patients had an expected higher eGFR prior to initiation of any treatments, once therapy was initiated at the time of study entry, medical and surgical PA patients and those with essential hypertension had similar mean eGFR measurements (83.5 [16.6] vs. 85.5 [16.2] vs. 83.6 [16.7] mL/min/1.73 m2) (Figure 4). The adjusted mean annual decline in eGFR was greater in patients with PA treated with MR antagonists compared with patients with essential hypertension (−1.6 [95% CI −1.4, −1.8] vs. −0.9 [95% CI −0.9, −1.0] mL/min/1.73 m2/yr, p < 0.001). In contrast, there was no significant difference in adjusted mean annual decline in eGFR between patients with PA treated with surgical adrenalectomy and patients with essential hypertension (−0.8 [95% CI −0.5, −1.2] vs. −0.9 [95% CI −0.9, −1.0] mL/min/1.73 m2/yr, p = 0.53) (Figure 4).
Figure 4. Mean eGFR throughout study period.
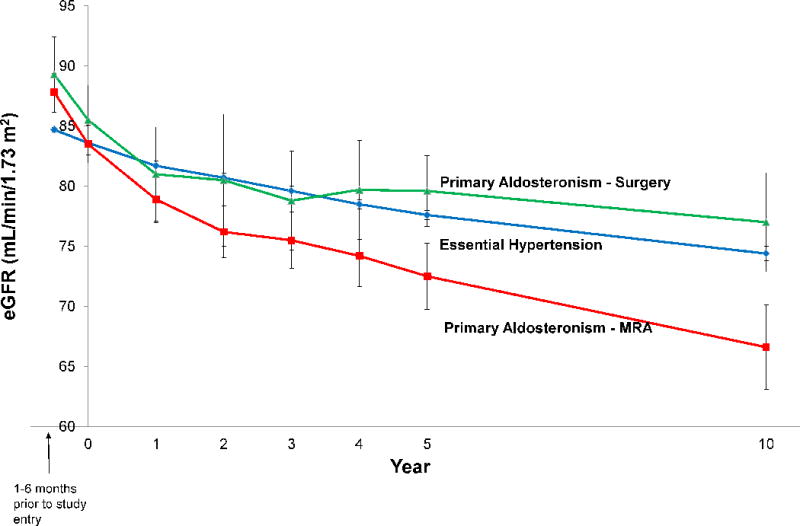
Error bars represent +/− 1.96*standard error of the mean. eGFR = estimated glomerular filtration rate.
Risk for Incident Albuminuria
There was a higher risk for developing incident albuminuria among diabetic PA patients treated with MR antagonists compared with diabetic patients with essential hypertension (adjusted HR 2.52 [95% CI 1.28, 4.96]) (Figure S2, please see online supplement at http://hyper.ahajournals.org).
Discussion
Although medical therapy with MR antagonists is generally assumed to be efficacious and is widely used to treat PA, the current study demonstrates that even when PA patients were treated with MR antagonists to achieve similar blood pressures as patients with essential hypertension, they had a 63% higher risk for incident CKD, a faster longitudinal decline in eGFR, and a higher rate of albuminuria among patients with diabetes. In contrast, when patients with unilateral PA were treated with surgical adrenalectomy, the decline in eGFR and risk for incident CKD were not significantly different from patients with essential hypertension. These findings suggest that the current, and officially recommended,2, 23 practice of MR antagonist therapy to block the effects of aldosterone in PA may not adequately mitigate the excess risk for adverse renal outcomes. In contrast, surgical therapy targeted to eliminate the source of autonomous aldosterone excess is associated with the same risk for kidney disease as comparable patients with essential hypertension.
There are several other clinical lessons that are suggested in these findings. First, the hyperfiltration in untreated PA may give clinicians the false security that their patients have normal kidney function; treatment of PA with MR antagonists or surgery rapidly unmasks underlying reductions in eGFR to reveal that many PA patients have much poorer kidney function than initially perceived (Figure 4). Second, since the medical treatment of PA with MR antagonists is associated with a risk for hyperkalemia,24 rapid longitudinal declines in eGFR may force clinicians to decrease MR antagonist doses to avoid hyperkalemia, thus resulting in a vicious cycle of inadequate aldosterone blockade and higher risk of MR-mediated kidney disease. Third, the current findings again highlight the deleterious effects of aldosterone excess and MR activation on the kidney at the patient-level.5, 14, 15, 17, 19 Finally, because these findings suggest that reliance on lifelong MR antagonist therapy in PA may not adequately prevent kidney disease, they should trigger a broader conversation on how to candidly counsel PA patients who choose to undergo medical therapy about the efficacy and future risk associated with this decision, and on the design of new studies and guidelines to address whether surgical adrenalectomy should be considered more frequently.
Prior to this study, there had been a paucity of literature on the effectiveness of MR antagonists and/or surgical adrenalectomy in reducing the incidence of adverse renal outcomes. Sechi et al. performed the most influential study in this regard,18 a prospective study of 50 patients with PA treated with either spironolactone or surgical adrenalectomy and 100 patients with essential hypertension with similar blood pressures and measured longitudinal trends in GFR and albuminuria.18 The study showed that GFR and albuminuria declined in the 6 months after treatment initiation, presumptively due to reversal of the hyperfiltration effect in untreated PA, with a subsequent rate of change in GFR and albuminuria that was not significantly different between patients with PA and patients with essential hypertension.18 Although one interpretation of these findings was that treatment of PA, with either MR antagonists or surgery, resulted in similar renal outcomes as comparable essential hypertensives, notable limitations included the small sample size and aggregation of medical and surgical treatments of PA rather than comparing their renal outcomes separately, as we did.
In this regard, our current findings represent a substantial advance from prior studies. The previous lack of large, robust prospective data assessing the effectiveness of MR antagonists and surgical adrenalectomy on renal outcomes in PA has limited the ability to make specific recommendations regarding the optimal treatment approach in PA. It has remained unclear whether, in patients with PA with disease amenable to surgical treatment, adrenalectomy improves long-term outcomes compared with MR antagonist use. Our prior study investigating cardiovascular outcomes in PA showed that surgical therapy for PA was associated with significantly lower risk of cardiovascular outcomes and death when compared with PA patients treated with MR antagonists.8 Collectively, these findings suggest that when a patient with PA has unilateral disease that is amenable to cure with surgery, and is healthy enough to undergo surgery, adrenalectomy is the optimal treatment to improve long-term health outcomes. In contrast, treatment of PA with MR antagonists may not adequately lower the risk of adverse renal outcomes when compared with similar patients with essential hypertension. Whether this is due to inadequate blockade of the MR with MR antagonist medications (e.g. inadequate dosing), inability to maximally titrate MR antagonists due to declining eGFR, lack of compliance with medications, or other reasons, could not be directly assessed in our study. In the context of these findings, it may be worth considering whether unilateral adrenalectomy to attenuate the severity of PA in patients with bilateral disease might be an effective approach; future studies dedicated to this objective would be needed to ascertain how best to mitigate renal risk in PA patients with bilateral adrenal disease.
It should be noted that in our prior study on cardiovascular outcomes in PA, achieving an unsuppressed renin activity level during MR antagonist therapy was associated with a substantially lower risk for adverse cardiovascular outcomes compared with having a persistently suppressed renin activity level, an observation suggesting that a rise in renin may serve as a biomarker for sufficient volume contraction and/or MR blockade.8 In contrast, the current study showed no association between renin activity and incident CKD. The most obvious explanation for this discrepancy is the intimate and paradoxical physiologic connection between the exposure of renin activity and the renal outcomes that relied on eGFR. Untreated PA results in volume expansion, renin suppression, hyperfiltration, and higher eGFR; in contrast, adequate treatment of aldosterone excess may abrogate the volume expansion and hyperfiltration and can result in a rise in renin but a decline in eGFR. Thus, the nature of this interdependent relationship, in addition to the small sample size of patients with longitudinal renin measurements and potential heterogeneity in MRA responses over time, may have limited the ability of renin activity to serve as a useful biomarker for renal outcomes.
Our results must be interpreted within the context of the study design. First, this study was observational. Although blood pressure, medication use, and other risk factors were similar at the time of study entry and our models adjusted for important confounders, there might still be residual confounding and misclassification. Second, our observational study design did not prospectively control blood pressure in each exposure group and could not completely account for the severity and duration of hypertension prior to study entry. However, upon initiation of either medical or surgical therapy at study entry, blood pressure control and use of non-MR antagonist anti-hypertensive medications were comparable between PA and essential hypertension patients, the longitudinal blood pressure control was comparable, and our models adjusted for the treated blood pressure at study entry. Third, the PA patients who received surgical therapy were on average 6 years younger than those with essential hypertension; however, other risk factors (including BMI, baseline eGFR and baseline blood pressure) were comparable and our models were adjusted for age. Fourth, eGFR is an imperfect marker of kidney disease as its magnitude is influenced by other variables such as hemodynamic effects from volume expansion/hyperfiltration which was demonstrated by the substantial initial decline in eGFR in PA patients after starting MR antagonist therapy or undergoing surgical adrenalectomy. However, PA patients did not enter into our study until after starting MR antagonist therapy or undergoing surgical adrenalectomy, and our data still demonstrate longitudinal and persistent differences in eGFR trends between patients with PA and essential hypertension. Fifth, a recent expert consensus study proposed criteria for classifying surgical cure of PA; however, our study did not have sufficient power, or consistent information throughout the study period, to evaluate the clinical relevance of these criteria on renal outcomes.25 Finally, the methodology of comparing treated PA patients with essential hypertension patients can be debated since this approach includes indication bias by treatment and different underlying pathologies. However, our study design was limited by the current standard of care practices dictated by expert guidelines,2 and prioritized the assessment of renal outcomes, independent of blood pressure, when treatment was directed to block aldosterone, versus eliminate the source of aldosterone, versus having no excess aldosterone. In this regard, our findings have direct implications for the current recommended practices in PA.
Supplementary Material
Perspectives.
PA is characterized by autonomous aldosterone secretion that results in excessive MR activation and earlier and more prominent renal damage compared with essential hypertension.5, 14, 15, 17, 19 MR antagonists and surgical adrenalectomy are the expert consensus recommended therapies for PA; however, this study demonstrates that even when patients with PA are treated with MR antagonists to achieve similar blood pressure control, they have a more rapid decline in eGFR, higher incidence of CKD, and an increased incidence of albuminuria among patients with diabetes, when compared with similar patients with essential hypertension. In contrast, surgical adrenalectomy to cure PA is associated with no significant differences in eGFR decline or CKD incidence compared with essential hypertension. When possible, surgical adrenalectomy to attenuate or cure hyperaldosteronism may be superior to MR antagonist therapy in mitigating the risk for kidney disease. Future studies should investigate new approaches to improving the efficacy of MR antagonist therapy and/or reconsidering the indications for using surgical adrenalectomy.
Novelty and Significance.
What is New?
This large cohort study evaluated whether the current guideline-recommended treatments for primary aldosteronism mitigate the risk for kidney disease.
What is Relevant?
- Patients with primary aldosteronism treated medically with mineralocorticoid receptor antagonists had:
- ○ 63% higher risk for incident chronic kidney disease compared with matched patients with essential hypertension
- ○ More than a two-fold higher risk for chronic kidney disease compared with primary aldosteronism patients treated surgically.
Primary aldosteronism patients treated with surgical adrenalectomy had no significant difference in risk for incident chronic kidney disease compared to patients with essential hypertension.
Summary
When possible, surgical adrenalectomy may be superior to mineralocorticoid receptor antagonist therapy in preventing kidney disease in primary aldosteronism.
Acknowledgments
We thank our funding sources.
Sources of Funding:
Gregory Hundemer was supported by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) of the National Institutes of Health (NIH) under Award Number F32 DK114953. Gary Curhan was supported by the NIDDK under Award Number K24 DK091417. Anand Vaidya was supported by the NIDDK under Award Numbers R01 DK115392 and R01 DK107407 and by the Doris Duke Charitable Foundation under Grant 2015085.
Footnotes
Conflicts of Interest/Disclosures:
None
References
- 1.Conn JW, Knopf RF, Nesbit RM. Clinical characteristics of primary aldosteronism from an analysis of 145 cases. Am J Surg. 1964;107:159–172. doi: 10.1016/0002-9610(64)90252-1. [DOI] [PubMed] [Google Scholar]
- 2.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF., Jr The management of primary aldosteronism: Case detection, diagnosis, and treatment: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–1916. doi: 10.1210/jc.2015-4061. [DOI] [PubMed] [Google Scholar]
- 3.Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, Sechi LA. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008;168:80–85. doi: 10.1001/archinternmed.2007.33. [DOI] [PubMed] [Google Scholar]
- 4.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Reincke M, Rump LC, Quinkler M, Hahner S, Diederich S, Lorenz R, Seufert J, Schirpenbach C, Beuschlein F, Bidlingmaier M, Meisinger C, Holle R, Endres S, Participants of German Conn’s R Risk factors associated with a low glomerular filtration rate in primary aldosteronism. J Clin Endocrinol Metab. 2009;94:869–875. doi: 10.1210/jc.2008-1851. [DOI] [PubMed] [Google Scholar]
- 6.Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, Mulatero P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50. doi: 10.1016/S2213-8587(17)30319-4. [DOI] [PubMed] [Google Scholar]
- 7.Hundemer GL, Baudrand R, Brown JM, Curhan G, Williams GH, Vaidya A. Renin phenotypes characterize vascular disease, autonomous aldosteronism, and mineralocorticoid receptor activity. J Clin Endocrinol Metab. 2017;102:1835–1843. doi: 10.1210/jc.2016-3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: A retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:51–59. doi: 10.1016/S2213-8587(17)30367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baudrand R, Guarda FJ, Torrey J, Williams G, Vaidya A. Dietary sodium restriction increases the risk of misinterpreting mild cases of primary aldosteronism. J Clin Endocrinol Metab. 2016;101:3989–3996. doi: 10.1210/jc.2016-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, Gabetti L, Mengozzi G, Williams TA, Rabbia F, Veglio F, Mulatero P. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69:1811–1820. doi: 10.1016/j.jacc.2017.01.052. [DOI] [PubMed] [Google Scholar]
- 11.Mosso L, Carvajal C, Gonzalez A, Barraza A, Avila F, Montero J, Huete A, Gederlini A, Fardella CE. Primary aldosteronism and hypertensive disease. Hypertension. 2003;42:161–165. doi: 10.1161/01.HYP.0000079505.25750.11. [DOI] [PubMed] [Google Scholar]
- 12.Markou A, Pappa T, Kaltsas G, Gouli A, Mitsakis K, Tsounas P, Prevoli A, Tsiavos V, Papanastasiou L, Zografos G, Chrousos GP, Piaditis GP. Evidence of primary aldosteronism in a predominantly female cohort of normotensive individuals: A very high odds ratio for progression into arterial hypertension. J Clin Endocrinol Metab. 2013;98:1409–1416. doi: 10.1210/jc.2012-3353. [DOI] [PubMed] [Google Scholar]
- 13.Baudrand R, Guarda FJ, Fardella C, Hundemer G, Brown J, Williams G, Vaidya A. Continuum of renin-independent aldosteronism in normotension. Hypertension. 2017;69:950–956. doi: 10.1161/HYPERTENSIONAHA.116.08952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greene EL, Kren S, Hostetter TH. Role of aldosterone in the remnant kidney model in the rat. J Clin Invest. 1996;98:1063–1068. doi: 10.1172/JCI118867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollenberg NK. Aldosterone in the development and progression of renal injury. Kidney Int. 2004;66:1–9. doi: 10.1111/j.1523-1755.2004.00701.x. [DOI] [PubMed] [Google Scholar]
- 16.Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T. Podocyte as the target for aldosterone: Roles of oxidative stress and sgk1. Hypertension. 2007;49:355–364. doi: 10.1161/01.HYP.0000255636.11931.a2. [DOI] [PubMed] [Google Scholar]
- 17.Ribstein J, Du Cailar G, Fesler P, Mimran A. Relative glomerular hyperfiltration in primary aldosteronism. J Am Soc Nephrol. 2005;16:1320–1325. doi: 10.1681/ASN.2004100878. [DOI] [PubMed] [Google Scholar]
- 18.Sechi LA, Novello M, Lapenna R, Baroselli S, Nadalini E, Colussi GL, Catena C. Long-term renal outcomes in patients with primary aldosteronism. JAMA. 2006;295:2638–2645. doi: 10.1001/jama.295.22.2638. [DOI] [PubMed] [Google Scholar]
- 19.Rossi GP, Bernini G, Desideri G, Fabris B, Ferri C, Giacchetti G, Letizia C, Maccario M, Mannelli M, Matterello MJ, Montemurro D, Palumbo G, Rizzoni D, Rossi E, Pessina AC, Mantero F, Participants PS. Renal damage in primary aldosteronism: Results of the papy study. Hypertension. 2006;48:232–238. doi: 10.1161/01.HYP.0000230444.01215.6a. [DOI] [PubMed] [Google Scholar]
- 20.Fourkiotis V, Vonend O, Diederich S, Fischer E, Lang K, Endres S, Beuschlein F, Willenberg HS, Rump LC, Allolio B, Reincke M, Quinkler M, Mephisto Study G Effectiveness of eplerenone or spironolactone treatment in preserving renal function in primary aldosteronism. Eur J Endocrinol. 2013;168:75–81. doi: 10.1530/EJE-12-0631. [DOI] [PubMed] [Google Scholar]
- 21.Rossi GP, Maiolino G, Flego A, Belfiore A, Bernini G, Fabris B, Ferri C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Muiesan ML, Mannelli M, Negro A, Palumbo G, Parenti G, Rossi E, Mantero F, Investigators PS Adrenalectomy lowers incident atrial fibrillation in primary aldosteronism patients at long term. Hypertension. 2018;71:585–591. doi: 10.1161/HYPERTENSIONAHA.117.10596. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaidya A, Malchoff CD, Auchus RJ, Committee AAS. An individualized approach to the evaluation and management of primary aldosteronism. Endocr Pract. 2017;23:680–689. doi: 10.4158/EP161717.RA. [DOI] [PubMed] [Google Scholar]
- 24.Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA. Rates of hyperkalemia after publication of the randomized aldactone evaluation study. N Engl J Med. 2004;351:543–551. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 25.Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, Satoh F, Amar L, Quinkler M, Deinum J, Beuschlein F, Kitamoto KK, Pham U, Morimoto R, Umakoshi H, Prejbisz A, Kocjan T, Naruse M, Stowasser M, Nishikawa T, Young WF, Jr, Gomez-Sanchez CE, Funder JW, Reincke M, Primary Aldosteronism Surgery Outcome i Outcomes after adrenalectomy for unilateral primary aldosteronism: An international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5:689–699. doi: 10.1016/S2213-8587(17)30135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


