Abstract
Enhanced activation of the endothelial mineralocorticoid receptor contributes to the development of arterial stiffness, which is an independent predictor of cardiovascular disease. Previously we showed that enhanced endothelium mineralocorticoid receptor signaling in female mice prompts expression and translocation of the alpha subunit of the epithelial sodium channel (ENaC) to the endothelial cell (EC) surface (EnNaC) inducing vascular fibrosis and stiffness. Further, amiloride, an ENaC antagonist, inhibits vascular fibrosis, remodeling, and stiffness induced by feeding a Western diet high in saturated fat and refined carbohydrates. However, how this occurs remains unknown. Thereby, we hypothesized that EC-specific EnNaC activation is necessary for aldosterone mediated endothelium stiffness. To address this notion EnNaC α subunit knock out (EnNaC−/−) and wild type littermate female mice were administrated aldosterone (250 μg/kg/day) via osmotic minipumps for 3 weeks beginning at 25–28 weeks of age. In isolated mouse ECs, inward sodium currents were significantly reduced in amiloride controls as well as in EnNaC−/−. Likewise, aldosterone-induced endothelium stiffness was increased and endothelium dependent relaxation less in EnNaC−/− versus wild type. Further, EnNaC−/− mice exhibited attenuated responses to aldosterone infusion including: aortic endoplasmic reticulum stress, endothelium nitric oxide synthase activation, endothelium permeability, expression of pro-inflammatory cytokines, oxidative stress, and aortic collagen 1 deposition, supporting the notion that αEnNaC subunit activation contributes to these vascular responses.
Keywords: Arterial stiffness, aldosterone, epithelial sodium channel, endothelial stiffness, inflammation
Increased arterial stiffness, characterized by increased pulse wave velocity (PWV), is associated with an increased risk for cardiovascular disease (CVD).1 In this context, a meta-analysis of 17 longitudinal studies in 15,877 persons showed that an increase of 1 m/s in PWV increased CVD events by 14%, CVD mortality by 15%, and all-cause mortality by 15%.2 Further, data from the Framingham Heart Study, which included 2232 persons, the presence of arterial stiffness was an independent predictor of CVD and associated morbidity and mortality.3 Recent data support the notion that aldosterone (Aldo), acting through the endothelial cell (EC) mineralocorticoid receptor (MR), promotes arterial stiffness and impairs endothelial mediated relaxation.4, 5 In this regard, Aldo, but not glucocorticoids, stimulates the ECMR, as the EC possesses the enzyme 11-beta hydroxysteroid dehydrogenase 2 which inactivates glucocorticoids.6, 7 On activation by Aldo, the ECMR translocates to the nucleus to modulate gene transcription and translation of proteins by binding to hormone/steroid response elements or negative response elements DNA sequences.8, 9 Aldo also exerts rapid non-genomic effects to affect increased cytosolic calcium levels, oxidative stress, endoplasmic reticulum (ER) stress, and cell death by activation of extracellular receptor kinase, Rho kinase, and protein kinase C.10–12 Classically, the epithelial sodium (Na+) channel (ENaC), located in the apical membrane of renal Aldo-responsive epithelia, plays an essential role in controlling Na+ balance and blood pressure.13 ENaC consists of three subunits, α, β and γ. While the α/β-subunit is essential for proper channel function, the γ-subunits act as amplifiers.13, 14 Recent data have also shown that ENaC exists in the endothelium (EnNaC) and regulates CV stiffness and function.15–17 Our recent work suggests that Aldo enhances ECMR signaling, in part, by inducing translocation of EnNaC to the plasma membrane of the aortic ECs.9 E3 ubiquitin ligase (Nedd4-2) and serum/glucocorticoid regulated kinase 1 (SGK1) were also shown to be involved in the Aldo-induced ENaC expression and activation.13, 18 In this regard, enhanced SGK1 inhibits ubiquinization by up-regulation of Nedd4-2, which acts as a negative role in controlling EnNaC cell surface expression, leading to increased membrane localization of this sodium channel and associated vascular stiffness.13, 18 Meanwhile, EnNaC activation is associated with an increase in inward Na+ currents and endothelial stiffening.9, 13 Here, we explore the notion that activated ECMR promotes endothelial stiffness through increased EnNaC synthesis/activation, enhanced EC membrane localization and endothelial stiffness.9, 13
Females with obesity and diabetes lose the CVD protection that is typically afforded by female sex and that this may be related to an increased propensity to develop CV stiffness. Our previous data found that C57BL6J mice fed a Western diet (WD), high in refined carbohydrates and saturated fat, became overweight and insulin resistant and there was a more rapid onset of aortic19 and cardiac stiffness20 in females compared to males. Additionally, we found higher plasma Aldo levels in females compared to males.20 Inhibition of EnNaC with very low doses non-pressor and non-sodium retaining doses of the ENaC antagonist, amiloride improved endothelial mediated relaxation and decreased arterial oxidative stress, fibrosis and stiffness without affecting blood pressure or Na+ retention,19 suggesting a specific role for EnNaC in promoting fibrosis and stiffness. Recent work also supports the concept that Aldo stimulation of ECMRs increases EnNaC membrane abundance in ECs, induces Na+ entry, and triggers the polymerization of G-actin to F-actin, resulting in reduction of endothelium nitric oxide (NO) synthase (eNOS) activity and NO production and thus excessive arterial stiffness.21–23 Thus, we hypothesized that EC-specific αEnNaC activation is necessary for Aldo mediated endothelium stiffness through increases in endothelial ER stress, which leads to reduced eNOS activity, increased endothelium permeability, expression of pro-inflammatory cytokines, and oxidative stress.
Methods
The data that support the findings in this study are available from the corresponding authors upon reasonable request. The αEnNaC subunit knockout (EnNaC−/−) mouse were generated and crossed with Tie 2 Cre+ mice and wild-type littermates as described before.17 A detailed description of experimental procedures including EC isolation, patch clamp technique, aortic stiffness measurement, endothelium permeability, gene expressions in western bot, real time PCR, as well as immunohistochemistry are available in the online-only Data Supplement. Results are reported as means ± SEM. Differences in outcomes were determined using one-way ANOVA multiple comparison analysis and Gabriel Students-Newman-Keuls post-test or paired t tests and were considered significant when p<0.05. All statistical analyses were performed using Sigma Plot (version 12) software (Systat Software).
Results
Effects of Aldo infusion on characteristics of mice
EnNaC−/− does not impact systolic blood pressure and heart rate under normal physiological conditions, as previously reported.17 There was no significant differences in body weight and fat mass between EnNaC−/− and their littermate controls (Table S1). Chronic Aldo infusion did not alter these parameters (Table S1). Of note, there were also no significant differences in serum albumin, serum Na+, fasting glucose, homeostatic model assessment-insulin resistance (HOMA-IR), plasma urea nitrogen (BUN), creatinine, urine protein, as well as potassium (K+) in all four experimental groups (Table S1). However, Aldo decreased urine Na+ that was not prevented in Aldo EnNaC−/− mice (Table S1).
EnNaC antagonist and EnNaC−/− represses inward Na+ currents in cultured ECs
Previous research has shown that plasma Aldo levels reach more than 2000 pMol in mice infused Aldo (288 μg/kg/d) for 4 weeks.24 On the basis of this, it was assumed that there were higher levels of Aldo in current study. Aldo increased MR mRNA expression in aortic tissues that was not prevented by the EnNaC−/− genotype (Fig. S2). To evaluate the effect of Aldo and EnNaC on Na+ currents in isolated ECs from these mice, Na+ currents were evaluated via patch clamp in the absence or presence of amiloride (1 μmol). Amiloride inhibited inward Na+ currents in the wild type cultured ECs (Fig. 1A–B). Following 3 weeks of Aldo infusion, the isolated ECs from EnNaC−/− mice exhibited less inward Na+ currents in comparison to those from EnNaC wild type (WT) mice (Fig. 1C–E), suggesting that EnNaC mediates Aldo-induced increase in Na+ currents.
Fig 1.
Effect of Aldo and ENaC on inward Na+ currents in ECs. (A) Na+ current tracings in ECs with or without amiloride stimulation. (B) Amiloride repressed inward Na+ currents in cultured ECs, n=4–5 cells. Na+ current tracings (C), group I–V curves of Na+ currents (D), and comparison of peak inward Na+ currents at −80 mV (E) in EnNaC WT and EnNaC−/− mice treated with Aldo, n=16 cells. *p<0.05 compared with control or EnNaC WT group in multiple comparison analysis.
αEnNaC mediates Aldo-induced aortic endothelium stiffness and impairment of vasodilation
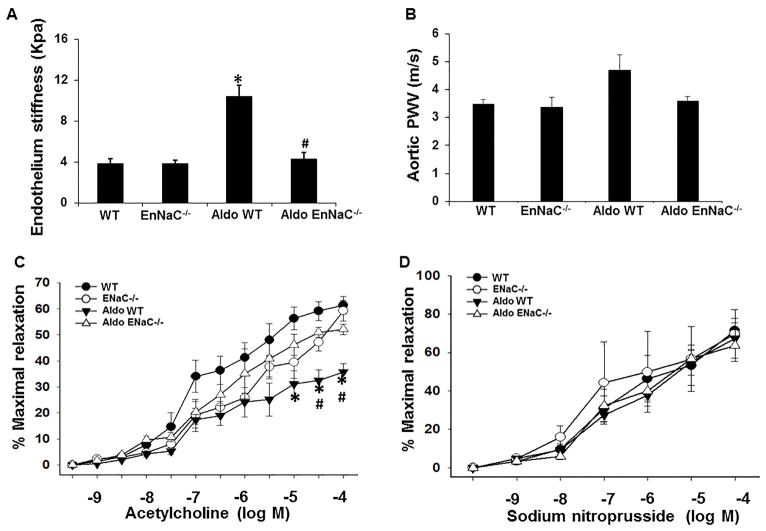
Three weeks of Aldo infusion induced an increase in ex vivo endothelium stiffness but not in in vivo PWV in EnNaC WT mice. The increased ex vivo endothelium stiffness in EnNaC WT mice was blunted in EnNaC−/− mice (Fig. 2A–B). Meanwhile, chronic Aldo infusion impaired endothelium vasodilatory responses to acetylcholine (Fig. 2C) but not to sodium nitroprusside (Fig. 2D). Further, αEnNaC inactivation prevented Aldo-induced impairment of aortic relaxation, suggesting that EnNaC activation promotes endothelial stiffness and decreases endothelial mediated relaxation upon chronic Aldo stimulation.
Fig 2.
EnNaC−/− mice prevented Aldo infusion-induced endothelium stiffness and aortic relaxation dysfunction. (A) The ex vivo measurement of endothelium stiffness by using atomic force microscopy, n=4–5. (B) Aortic pulse wave velocity measured in vivo, n=3–6. (C) Vasodilator responses of isolated aortic rings to the endothelium-dependent dilators, acetylcholine (C) and to the endothelium-independent vasodilator, sodium nitroprusside (D), n=4. *p<0.05 compared with EnNaC WT group; #p<0.05 compared with Aldo in EnNaC WT group in multiple comparison analysis.
αEnNaC mediates Aldo-induced aortic ER stress and attenuation of eNOS activation
In response to Aldo stimulation, glucose regulated protein (GRP78), a major chaperone in ER, controlling the folding and assembly of proteins, was increased in EnNaC WT mice (Fig. 3). This change was associated with reduced eNOS activity. Likewise, EnNaC−/− prevented chronic Aldo infusion-induced increases in GRP78 expression and reduction of eNOS activity. The chronic Aldo infusion-induced increases in GRP78 expression and reduction of eNOS activity were not observed in EnNaC−/ (Fig. 3). Thus, αEnNaC mediated Aldo-induced ER stress is associated with reduced eNOS activity.
Fig 3.
EnNaC−/− mice prevented Aldo infusion-induced endoplasmic reticulum stress and reduction of eNOS activity. (A) The protein abundance of GRP78 and eNOS in aortic tissues were performed with immunoblotting. Quantitative analysis of protein abundance in GRP78 (B) and p-eNOS (C). *p<0.05 compared with EnNaC WT group; #p<0.05 compared with Aldo in EnNaC WT group in multiple comparison analysis.
αEnNaC mediates Aldo-induced expression of endothelium adhesion molecules, permeability, and pro-inflammatory cytokines
Endothelial stiffness promotes EC cell expression of adhesion molecules including intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), which, in turn, induces leukocyte/macrophage migration, adhesion and infiltration.25 Chronic Aldo infusion increased expression of ICAM-1 and VCAM-1 (Fig. 4A). Aldo also significantly increased endothelium permeability (Fig. 4B) and CD68 expression (Fig. 4C), which is highly expressed in monocytes and tissue macrophages.26 Meanwhile, Aldo also induced expression of interleukin 1 (IL1) and IL6 (Fig. 4D–E). However, These Aldo infusion induced increases in expression of ICAM-1, VCAM-1, CD68, IL1, IL6, as well as increased endothelial permeability were not observed in EnNaC−/− aortic tissues (Fig. 4).
Fig 4.
EnNaC−/− mice prevented Aldo-induced expression of endothelium adhesion molecules, permeability, and pro-inflammatory cytokines. (A) The protein abundance of ICAM-1 and VCAM-1 in aortic tissues. (B) ex vivo aortic endothelium permeability assay, n=5. mRNA levels of CD68 (C), IL1 (D) and IL6 (E) in aortic tissues, n=4. *p<0.05 compared with EnNaC WT group; #p<0.05 compared with Aldo in EnNaC WT in multiple comparison analysis.
αEnNaC mediates Aldo-induced aortic oxidative stress and remodeling
To determine the role of enhanced αEnNaC activation on oxidative stress, structural parameters, and vascular dysfunction, we evaluated aortic oxidative stress and fibrosis by 3-nitrotyrosine (3-NT), nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 2 (NOX2), and collagen 1 immunostaining, respectively. Chronic Aldo stimulation increased aortic 3-NT, NOX2, and oxidative stress (Fig. 5), that was accompanied by increased collagen 1 expression (Fig. 5). Importantly, αEnNaC inactivation attenuated the Aldo- increased aortic immunostaining for 3-NT, NOX2, as well as collagen 1 expression (Fig. 5).
Fig 5.
EnNaC−/− mice prevented Aldo infusion-induced aortic oxidative stress and remodeling. (A) Representative images of aortic sections stained for 3-NT, a marker of oxidant stress from accumulation of oxidant peroxynitrite (ONOO−). (B) Representative image immunostaining for NOX2 with corresponding measures of average gray scale intensities (C–D). Representative image immunostaining for collagen 1 (E) with corresponding measures of average gray scale intensities (F). Scale bar = 50 μm. n=5–6 per group. *p<0.05 compared with EnNaC WT group; #p<0.05 compared with Aldo in EnNaC WT in multiple comparison analysis.
Discussion
This investigation demonstrates that EC specific deletion of the αEnNaC prevents the development of chronic Aldo infusion-induced endothelium stiffness and impaired endothelial mediated relaxation. EnNaC−/− inactivation also reduced inward EC Na+ currents as measured by patch clamp and this reduction in Na+ current was associated with reduction of Aldo-induced endothelium stiffness and associated impairment of endothelium dependent relaxation. Aldo infusion led to increased aortic ER stress, reduction of eNOS activation/relaxation, endothelium permeability, expression of pro-inflammatory cytokines, and oxidative stress that were involved in an increase in tissue collagen 1 and remodeling. These profibrotic responses to Aldo were significantly attenuated in EnNaC−/− mice.
Generally, the α/β-subunit is essential for proper channel function while the γ-subunit acts as an amplifier, thus each subunit plays an important role in the maintaining ENaC activity and function.13, 14 However, one study has reported that αENaC is not required for β and γENaC to form a channel.27 meanwhile, βENaC and γENaC appear to be the predominant while αENaC is rare in vascular smooth muscle cells.28, 29 In Sprague-Dawley rats, vasopressin infusion increases mRNA and protein abundance of ENaC β- and γ-subunits in kidney epithelial cells.30, 31 Recent studies showed that the expression and activity of ENaC is regulated by flow-induced shear stress in the epithelium of cortical collecting duct,32 suggesting a role for ENaC in detection and transduction of mechanical forces. Further emphasizing this point, ENaC subunits are molecular components of the arterial baroreceptor complex.33 Related to this, one study has shown the β/γ-ENaC subunits are expressed in nodose neuronal cell bodies and aortic baroreceptor nerve terminals whereas the αENaC subunit is undetectable.34 Meanwhile, the expression of β/γ-ENaC subunits are reduced in aortic baroreceptor nerve terminals in a chronic heart failure rat model.34 Further, pressure-induced constriction of cerebral arteries is inhibited in a mouse model of reduced β-ENaC,35 suggesting that ENaC is a molecular component of the arterial baroreceptor mechano-transducer. Moreover, increased EC specific EnNaC expression and activity significantly links EC stiffness in both cultured human ECs and Liddle syndrome mouse model.13 Very recent data has also shown that endothelial αEnNaC plays a critical role in regulation of shear-stress sensing and flow-mediated vascular dilation.17 Collectively, these studies provide strong support for ENaC being involved in vasoregulation, particularly that involving mechanotransduction.
Inhibition of EnNaC with a low dose of amiloride or EC specific αEnNaC−/− does not affect blood pressure in normal or obese mice.17, 36 Our previous studies9 and those of others37 have shown that ECMR mediated Aldo-induced EnNaC expression and accumulation on EC membranes is mediated by activation of the EnNaC promoter, increased expression of EnNaC, as well as increases of SGK 1 activity with reduction in ubiquitination of surface EnNaC. The data from the current investigation further supports the notion that αEnNaC subunit activation mediates Aldo-increased Na+ channel activity, inward Na+ currents, and endothelial stiffness. To this point, increased Na+ entry into EC triggers the polymerization of G-actin to F-actin and the resultant endothelial cortical polymerization and stiffening of the endothelium.22, 38 Further, gene deletion of Aldo synthase Cyp11b239 and inhibition of ENaC with amiloride19 attenuates WD -induced upregulation of EnNaC, endothelium, and associated aortic stiffness.
In this investigation, Aldo infusion did not significantly induce an increase in PWV and aortic stiffness. A potential explanation is that Aldo infusion did not cause dysfunction of vascular smooth muscle cells since Aldo infusion and αEnNaC deletion did not affect sodium nitroprusside-induced aortic vasodilation. Indeed, increased PWV in aortic stiffness involves the dysfunction of EC, vascular smooth muscle cells, and extracellular matrix.40, 41 Aldo stimulation did induce endothelium dysfunction by upregulation of aortic ER stress as characterized by an increase expression of the critical ER stress protein GRP78. Consistent with this, Aldo has been found to increase intracellular reactive oxygen species production, ER stress, GRP78 expression, as well as cell apoptosis in renal tubular epithelial cells42 and vascular ECs.43 Thus, αEnNaC potentially links ER stress and cell function. In this regard, increased Na+ currents affects calcium homeostasis, increases ER stress and cell death in pancreas β cells as well.44 Also, increases EnNaC mediated Na currents leads to actin polymerization45 which further induces calcium-mediated actin resetting that mediates cell adaptations, ER stress, and EC dysfunction with decreased eNOS activity and NO production.46, 47 Moreover, ER stress further increases oxidative stress (NOX2/4 mRNA levels and increased NADPH oxidase activity) causes decreases in eNOS promoter activity, eNOS expression, phosphorylation, and thus eNOS activity.48 These data suggest that αEnNaC activation mediates Aldo infusion-induced increases in GRP78 expression and reduction of eNOS activity. Consistent with this concept, another study found that increased GRP78 and ER stress promotes expression of ICAM-1 and tumor necrosis factor-α and EC dysfunction through NF-κB activation,49 suggesting that ER stress plays an important role in promoting VCAM-1 expression and monocyte recruitment,50 which are early inflammatory events in the events leading to increased endothelium stiffness and impaired endothelial eNOS activation.
In this investigation we also observed that enhanced ECMR signaling and EnNaC activation promoted increased expression of ICAM-1 and VCAM-1, which have previously been associated with increased leukocyte adhesion in human ECs.51, 52 Increased EC leukocyte adhesion and transmigration have also been shown to be associated with endothelial stiffness.53 Indeed, endothelial stiffness increases endothelial monolayer tension and permeability and thus is regarded as an important factor in impairment of endothelial barrier function.54 Also consistent with this concept, our previous work demonstrated that WD-induced vascular stiffness decreased arterial tight junction proteins including claudin-5 and occludin and resulted in macrophage recruitment and cardiac fibrosis that were prevented by very low dose amiloride.36 The current research shows that αEnNaC mediates Aldo-increased endothelium permeability and increased expression of CD68, IL1 and IL6. Since CD68 is highly expressed in monocytes and tissue macrophages,26 decreased CD 68 in EnNaC−/− in the current study suggested αEnNaC mediates monocytes/macrophages infiltration and migration. Meanwhile, both IL-1 and IL6 are important mediators in the regulation of immune responses and inflammation,55 and thus increased expression of IL1 and IL6 may have resulted from activated aortic tissue macrophages.
Increased oxidative stress is associated with increased ENaC activity and salt-sensitive hypertension. Studies have found that reactive oxygen species production mediates ENaC expression and activation.16, 56 Recent studies have indicated that increased ENaC also promotes oxidative stress in dendritic cells.57 To this point, Na+ enters cells, initiates calcium influx through α/γ-ENaC and the sodium hydrogen exchanger 1, resulting in activation of protein kinase C, phosphorylation of p47phox, association of p47phox with gp91phox, and hypertension.57 Thus, there is an interaction between ENaC, inward Na+ currents and cellular oxidative stress. Interestingly, endothelial oxidative stress and NOX2 are related to vascular fibrosis and stiffening through pro-inflammatory effects in angiotensin II-infused transgenic mice.58 The resultant increase in oxidative stress is associated with activation of profibrotic signaling transforming growth factor beta 1 thereby promoting vascular fibrosis.59 Our previous data has shown that WD induced increased cardiac NOX2, but not NOX4, expression is mediated by an EnNaC-dependent mechanism.36 These pathophysiological changes contribute to aortic oxidative stress, remodeling, and fibrosis and observed increases in expression of 3-NT, NOX2, and collagen 1 in the aorta. Thus, activation of αEnNaC in the setting of Aldo-induced vascular dysfunction is associated with increased EC cell permeability, inflammation, and oxidative stress, all of which promote increased endothelial stiffness.
In conclusion, we have extended our previous observations regarding the role of ECMR mediated EnNaC activation in promoting arterial stiffness.19, 36 Current data indicate that Aldo increases EnNaC membrane abundance and activity in ECs which promotes Na+ entry into the endothelium, resulting in increases in GRP78, inflammation, 3-NT and NOX2 and a reduction of eNOS activity and NO production and a subsequent increase in vascular stiffness, suggesting an important role of ECMR specific αEnNaC activation in increasing endothelial stiffness (Fig. 6). These pathophysiological changes are associated with increases in EC inward Na+ currents, ER stress, reduction of eNOS activity, increased endothelium adhesion and permeability, and increased expression of pro-inflammatory molecules and oxidative stress.
Fig 6.
EnNaC mediates Aldo-induced endothelium stiffness and aortic dysfunction. Aldo increases EnNaC membrane abundance in ECs which promotes Na+ entry into the endothelium, resulting in increases in GRP78, inflammation, 3-NT and NOX2 and a reduction of eNOS activity and NO production and a subsequent increase in vascular stiffness.
Perspectives
The findings in this investigation have important implications in EC function and vascular biology. Increased EC specific EnNaC expression and activity is associated with EC stiffness and arterial dysfunction. For the first time, we show evidence for an important role of αEnNaC on endothelium permeability, reduction of eNOS activity, inflammation, oxidative stress, as well as endothelium stiffness, thereby providing a potential therapeutic strategy in prevention of excessive arterial stiffness and associated CVD.
Supplementary Material
Novelty and Significance.
What is new?
Endothelium specific αEnNaC knock out prevents EnNaC activation, endothelium stiffness, endothelium permeability, ER stress, reduction of eNOS activity, inflammation, oxidative stress, and associated aortic remodeling.
What is relevant?
Endothelium stiffness is a risk factor for CVD including hypertension, coronary heart disease, and renal disease and is highly predictive of clinical events, morbidity, and mortality.
Inhibition of EnNaC represents a potential therapeutic strategy in prevention of arterial stiffness and associated CVD.
Summary
Enhanced EnNaC signaling plays an important in the development of endothelium stiffness and vascular dysfunction. These pathophysiological changes are associated with increases in endothelium permeability, ER stress, reduction of eNOS activity, inflammation, oxidative stress, and aortic remodeling.
Acknowledgments
We acknowledge the work by Dr. Vincent G. DeMarco, who did and analyzed PWV data. We also acknowledge Dr. Ravi Nistala for his help in analyzing urine data, Matthew B. Martin and Dongqing Chen for their help in animal experiments and Ernesto Martinez-Martinez for providing EnNaC−/− and littermate mice. This work was supported with resources and the use of facilities at the Harry S Truman Memorial Veterans Hospital in Columbia, MO.
Sources of Funding
JRS receives funding from the Veterans Affairs Merit System (2 I01 BX001981-05A1) and NIH (R01 HL73101-01A and R01 HL107910-01). Dr. Jia receives funding from American Diabetes Association (Innovative Basic Science Award #1-17-IBS-201). Dr. Whaley-Connell receives funding from the Veterans Affairs Merit System (BX003391). Dr. Hill receives funding from NIH (RO1HL085119).
Footnotes
Disclosures
None.
References
- 1.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T American Heart Association Council on H. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–27. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–11. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barhoumi T, Fraulob-Aquino JC, Mian MOR, Ouerd S, Idris-Khodja N, Huo KG, Rehman A, Caillon A, Dancose-Giambattisto B, Ebrahimian T, Lehoux S, Paradis P, Schiffrin EL. Matrix metalloproteinase-2 knockout prevents angiotensin II-induced vascular injury. Cardiovasc Res. 2017;113:1753–1762. doi: 10.1093/cvr/cvx115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen Dinh Cat A, Callera GE, Friederich-Persson M, Sanchez A, Dulak-Lis MG, Tsiropoulou S, Montezano AC, He Y, Briones AM, Jaisser F, Touyz RM. Vascular dysfunction in obese diabetic db/db mice involves the interplay between aldosterone/mineralocorticoid receptor and Rho kinase signaling. Sci Rep. 2018;8:2952. doi: 10.1038/s41598-018-21087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jimenez-Canino R, Lorenzo-Diaz F, Odermatt A, Bailey MA, Livingstone DEW, Jaisser F, Farman N, Alvarez de la Rosa D. 11beta-HSD2 SUMOylation Modulates Cortisol-Induced Mineralocorticoid Receptor Nuclear Translocation Independently of Effects on Transactivation. Endocrinology. 2017;158:4047–4063. doi: 10.1210/en.2017-00440. [DOI] [PubMed] [Google Scholar]
- 7.Bauersachs J, Jaisser F, Toto R. Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension. 2015;65:257–63. doi: 10.1161/HYPERTENSIONAHA.114.04488. [DOI] [PubMed] [Google Scholar]
- 8.Meinel S, Ruhs S, Schumann K, Stratz N, Trenkmann K, Schreier B, Grosse I, Keilwagen J, Gekle M, Grossmann C. Mineralocorticoid receptor interaction with SP1 generates a new response element for pathophysiologically relevant gene expression. Nucleic Acids Res. 2013;41:8045–60. doi: 10.1093/nar/gkt581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, DeMarco VG, Ramirez-Perez FI, Sun Z, Hayden MR, Meininger GA, Mueller KB, Jaffe IZ, Sowers JR. Endothelial Mineralocorticoid Receptor Mediates Diet-Induced Aortic Stiffness in Females. Circ Res. 2016;118:935–943. doi: 10.1161/CIRCRESAHA.115.308269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ong GS, Young MJ. Mineralocorticoid regulation of cell function: the role of rapid signalling and gene transcription pathways. J Mol Endocrinol. 2017;58:R33–R57. doi: 10.1530/JME-15-0318. [DOI] [PubMed] [Google Scholar]
- 11.Schiffrin EL. Effects of aldosterone on the vasculature. Hypertension. 2006;47:312–8. doi: 10.1161/01.HYP.0000201443.63240.a7. [DOI] [PubMed] [Google Scholar]
- 12.Rautureau Y, Paradis P, Schiffrin EL. Cross-talk between aldosterone and angiotensin signaling in vascular smooth muscle cells. Steroids. 2011;76:834–9. doi: 10.1016/j.steroids.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Jeggle P, Callies C, Tarjus A, Fassot C, Fels J, Oberleithner H, Jaisser F, Kusche-Vihrog K. Epithelial sodium channel stiffens the vascular endothelium in vitro and in Liddle mice. Hypertension. 2013;61:1053–9. doi: 10.1161/HYPERTENSIONAHA.111.199455. [DOI] [PubMed] [Google Scholar]
- 14.Shi S, Kleyman TR. Gamma subunit second transmembrane domain contributes to epithelial sodium channel gating and amiloride block. Am J Physiol Renal Physiol. 2013;305:F1585–92. doi: 10.1152/ajprenal.00337.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang ZR, Liu HB, Sun YY, Hu QQ, Li YX, Zheng WW, Yu CJ, Li XY, Wu MM, Song BL, Mu JJ, Yuan ZY, Zhang ZR, Ma HP. Dietary salt blunts vasodilation by stimulating epithelial sodium channels in endothelial cells from salt-sensitive Dahl rats. Br J Pharmacol. 2018;175:1305–1317. doi: 10.1111/bph.13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Downs CA, Johnson NM, Coca C, Helms MN. Angiotensin II regulates delta-ENaC in human umbilical vein endothelial cells. Microvasc Res. 2018;116:26–33. doi: 10.1016/j.mvr.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Tarjus A, Maase M, Jeggle P, Martinez-Martinez E, Fassot C, Loufrani L, Henrion D, Hansen PBL, Kusche-Vihrog K, Jaisser F. The endothelial alphaENaC contributes to vascular endothelial function in vivo. PLoS One. 2017;12:e0185319. doi: 10.1371/journal.pone.0185319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galiana-Simal A, Olivares-Alvaro E, Klett-Mingo M, Ruiz-Roso MB, Ballesteros S, de Las Heras N, Fuller PJ, Lahera V, Martin-Fernandez B. Proanthocyanidins block aldosterone-dependent up-regulation of cardiac gamma ENaC and Nedd4-2 inactivation via SGK1. J Nutr Biochem. 2016;37:13–19. doi: 10.1016/j.jnutbio.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Lemus LA, Aroor AR, Ramirez-Perez FI, Jia G, Habibi J, DeMarco VG, Barron B, Whaley-Connell A, Nistala R, Sowers JR. Amiloride Improves Endothelial Function and Reduces Vascular Stiffness in Female Mice Fed a Western Diet. Front Physiol. 2017;8:456. doi: 10.3389/fphys.2017.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manrique C, DeMarco VG, Aroor AR, Mugerfeld I, Garro M, Habibi J, Hayden MR, Sowers JR. Obesity and insulin resistance induce early development of diastolic dysfunction in young female mice fed a Western diet. Endocrinology. 2013;154:3632–42. doi: 10.1210/en.2013-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Druppel V, Kusche-Vihrog K, Grossmann C, Gekle M, Kasprzak B, Brand E, Pavenstadt H, Oberleithner H, Kliche K. Long-term application of the aldosterone antagonist spironolactone prevents stiff endothelial cell syndrome. FASEB J. 2013;27:3652–9. doi: 10.1096/fj.13-228312. [DOI] [PubMed] [Google Scholar]
- 22.Fels J, Jeggle P, Kusche-Vihrog K, Oberleithner H. Cortical actin nanodynamics determines nitric oxide release in vascular endothelium. PLoS One. 2012;7:e41520. doi: 10.1371/journal.pone.0041520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng Y, Waters M, Andrews A, Honarmandi P, Ebong EE, Rizzo V, Tarbell JM. Fluid shear stress induces the clustering of heparan sulfate via mobility of glypican-1 in lipid rafts. Am J Physiol Heart Circ Physiol. 2013;305:H811–20. doi: 10.1152/ajpheart.00764.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cassis LA, Helton MJ, Howatt DA, King VL, Daugherty A. Aldosterone does not mediate angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Br J Pharmacol. 2005;144:443–8. doi: 10.1038/sj.bjp.0706098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo Y, Feng J, Xu Q, Wang W, Wang X. NSun2 Deficiency Protects Endothelium From Inflammation via mRNA Methylation of ICAM-1. Circ Res. 2016;118:944–56. doi: 10.1161/CIRCRESAHA.115.307674. [DOI] [PubMed] [Google Scholar]
- 26.McNeill E, Iqbal AJ, Jones D, Patel J, Coutinho P, Taylor L, Greaves DR, Channon KM. Tracking Monocyte Recruitment and Macrophage Accumulation in Atherosclerotic Plaque Progression Using a Novel hCD68GFP/ApoE−/− Reporter Mouse-Brief Report. Arterioscler Thromb Vasc Biol. 2017;37:258–263. doi: 10.1161/ATVBAHA.116.308367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonny O, Chraibi A, Loffing J, Jaeger NF, Grunder S, Horisberger JD, Rossier BC. Functional expression of a pseudohypoaldosteronism type I mutated epithelial Na+ channel lacking the pore-forming region of its alpha subunit. J Clin Invest. 1999;104:967–74. doi: 10.1172/JCI6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drummond HA, Jernigan NL, Grifoni SC. Sensing tension: epithelial sodium channel/acid-sensing ion channel proteins in cardiovascular homeostasis. Hypertension. 2008;51:1265–71. doi: 10.1161/HYPERTENSIONAHA.107.093401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavlov TS, Staruschenko A. Involvement of ENaC in the development of salt-sensitive hypertension. Am J Physiol Renal Physiol. 2017;313:F135–F140. doi: 10.1152/ajprenal.00427.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicco C, Wittner M, DiStefano A, Jounier S, Bankir L, Bouby N. Chronic exposure to vasopressin upregulates ENaC and sodium transport in the rat renal collecting duct and lung. Hypertension. 2001;38:1143–9. doi: 10.1161/hy1001.092641. [DOI] [PubMed] [Google Scholar]
- 31.Ecelbarger CA, Kim GH, Terris J, Masilamani S, Mitchell C, Reyes I, Verbalis JG, Knepper MA. Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am J Physiol Renal Physiol. 2000;279:F46–53. doi: 10.1152/ajprenal.2000.279.1.F46. [DOI] [PubMed] [Google Scholar]
- 32.Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na(+) channels are regulated by flow. Am J Physiol Renal Physiol. 2001;280:F1010–8. doi: 10.1152/ajprenal.2001.280.6.F1010. [DOI] [PubMed] [Google Scholar]
- 33.Drummond HA, Welsh MJ, Abboud FM. ENaC subunits are molecular components of the arterial baroreceptor complex. Ann N Y Acad Sci. 2001;940:42–7. doi: 10.1111/j.1749-6632.2001.tb03665.x. [DOI] [PubMed] [Google Scholar]
- 34.Li YL, Zhang D, Tu H, Muelleman RL. Altered ENaC is Associated With Aortic Baroreceptor Dysfunction in Chronic Heart Failure. Am J Hypertens. 2016;29:582–9. doi: 10.1093/ajh/hpv141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.VanLandingham LG, Gannon KP, Drummond HA. Pressure-induced constriction is inhibited in a mouse model of reduced betaENaC. Am J Physiol Regul Integr Comp Physiol. 2009;297:R723–8. doi: 10.1152/ajpregu.00212.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jia G, Habibi J, Aroor AR, Hill MA, DeMarco VG, Lee LE, Ma L, Barron BJ, Whaley-Connell A, Sowers JR. Enhanced endothelium epithelial sodium channel signaling prompts left ventricular diastolic dysfunction in obese female mice. Metabolism. 2018;78:69–79. doi: 10.1016/j.metabol.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Mansley MK, Korbmacher C, Bertog M. Inhibitors of the proteasome stimulate the epithelial sodium channel (ENaC) through SGK1 and mimic the effect of aldosterone. Pflugers Arch. 2018;470:295–304. doi: 10.1007/s00424-017-2060-5. [DOI] [PubMed] [Google Scholar]
- 38.Callies C, Fels J, Liashkovich I, Kliche K, Jeggle P, Kusche-Vihrog K, Oberleithner H. Membrane potential depolarization decreases the stiffness of vascular endothelial cells. J Cell Sci. 2011;124:1936–42. doi: 10.1242/jcs.084657. [DOI] [PubMed] [Google Scholar]
- 39.Jeggle P, Hofschroer V, Maase M, Bertog M, Kusche-Vihrog K. Aldosterone synthase knockout mouse as a model for sodium-induced endothelial sodium channel up-regulation in vascular endothelium. FASEB J. 2016;30:45–53. doi: 10.1096/fj.14-259606. [DOI] [PubMed] [Google Scholar]
- 40.Avendano MS, Martinez-Revelles S, Aguado A, Simoes MR, Gonzalez-Amor M, Palacios R, Guillem-Llobat P, Vassallo DV, Vila L, Garcia-Puig J, Beltran LM, Alonso MJ, Cachofeiro MV, Salaices M, Briones AM. Role of COX-2-derived PGE2 on vascular stiffness and function in hypertension. Br J Pharmacol. 2016;173:1541–55. doi: 10.1111/bph.13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim SK, McCurley AT, DuPont JJ, Aronovitz M, Moss ME, Stillman IE, Karumanchi SA, Christou DD, Jaffe IZ. Smooth Muscle Cell-Mineralocorticoid Receptor as a Mediator of Cardiovascular Stiffness With Aging. Hypertension. 2018;71:609–621. doi: 10.1161/HYPERTENSIONAHA.117.10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding W, Yang L, Zhang M, Gu Y. Reactive oxygen species-mediated endoplasmic reticulum stress contributes to aldosterone-induced apoptosis in tubular epithelial cells. Biochem Biophys Res Commun. 2012;418:451–6. doi: 10.1016/j.bbrc.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 43.Lu JP, Li X, Jin YL, Chen MX. Endoplasmic reticulum stress-mediated aldosterone-induced apoptosis in vascular endothelial cells. J Huazhong Univ Sci Technolog Med Sci. 2014;34:821–4. doi: 10.1007/s11596-014-1359-0. [DOI] [PubMed] [Google Scholar]
- 44.Yang YH, Vilin YY, Roberge M, Kurata HT, Johnson JD. Multiparameter screening reveals a role for Na+ channels in cytokine-induced beta-cell death. Mol Endocrinol. 2014;28:406–17. doi: 10.1210/me.2013-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wales P, Schuberth CE, Aufschnaiter R, Fels J, Garcia-Aguilar I, Janning A, Dlugos CP, Schafer-Herte M, Klingner C, Walte M, Kuhlmann J, Menis E, Hockaday Kang L, Maier KC, Hou W, Russo A, Higgs HN, Pavenstadt H, Vogl T, Roth J, Qualmann B, Kessels MM, Martin DE, Mulder B, Wedlich-Soldner R. Calcium-mediated actin reset (CaAR) mediates acute cell adaptations. Elife. 2016;5:e19850. doi: 10.7554/eLife.19850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choy KW, Mustafa MR, Lau YS, Liu J, Murugan D, Lau CW, Wang L, Zhao L, Huang Y. Paeonol protects against endoplasmic reticulum stress-induced endothelial dysfunction via AMPK/PPARdelta signaling pathway. Biochem Pharmacol. 2016;116:51–62. doi: 10.1016/j.bcp.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 47.Mohanan Nair M, Zhao R, Xie X, Shen GX. Impact of glycated LDL on endothelial nitric oxide synthase in vascular endothelial cells: involvement of transmembrane signaling and endoplasmic reticulum stress. J Diabetes Complications. 2016;30:391–7. doi: 10.1016/j.jdiacomp.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 48.Galan M, Kassan M, Kadowitz PJ, Trebak M, Belmadani S, Matrougui K. Mechanism of endoplasmic reticulum stress-induced vascular endothelial dysfunction. Biochim Biophys Acta. 2014;1843:1063–75. doi: 10.1016/j.bbamcr.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu S, Gao X, Yang S, Meng M, Yang X, Ge B. The role of endoplasmic reticulum stress in endothelial dysfunction induced by homocysteine thiolactone. Fundam Clin Pharmacol. 2015;29:252–9. doi: 10.1111/fcp.12101. [DOI] [PubMed] [Google Scholar]
- 50.Bailey KA, Haj FG, Simon SI, Passerini AG. Atherosusceptible Shear Stress Activates Endoplasmic Reticulum Stress to Promote Endothelial Inflammation. Sci Rep. 2017;7:8196. doi: 10.1038/s41598-017-08417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marzolla V, Armani A, Mammi C, Moss ME, Pagliarini V, Pontecorvo L, Antelmi A, Fabbri A, Rosano G, Jaffe IZ, Caprio M. Essential role of ICAM-1 in aldosterone-induced atherosclerosis. Int J Cardiol. 2017;232:233–242. doi: 10.1016/j.ijcard.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caprio M, Newfell BG, la Sala A, Baur W, Fabbri A, Rosano G, Mendelsohn ME, Jaffe IZ. Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res. 2008;102:1359–67. doi: 10.1161/CIRCRESAHA.108.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Bussel BC, Henry RM, Schalkwijk CG, Dekker JM, Nijpels G, Stehouwer CD. Low-grade inflammation, but not endothelial dysfunction, is associated with greater carotid stiffness in the elderly: the Hoorn Study. J Hypertens. 2012;30:744–52. doi: 10.1097/HJH.0b013e328350a487. [DOI] [PubMed] [Google Scholar]
- 54.Urbano RL, Furia C, Basehore S, Clyne AM. Stiff Substrates Increase Inflammation-Induced Endothelial Monolayer Tension and Permeability. Biophys J. 2017;113:645–655. doi: 10.1016/j.bpj.2017.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feder LS, Todaro JA, Laskin DL. Characterization of interleukin-1 and interleukin-6 production by hepatic endothelial cells and macrophages. J Leukoc Biol. 1993;53:126–32. doi: 10.1002/jlb.53.2.126. [DOI] [PubMed] [Google Scholar]
- 56.Ilatovskaya DV, Pavlov TS, Levchenko V, Staruschenko A. ROS production as a common mechanism of ENaC regulation by EGF, insulin, and IGF-1. Am J Physiol Cell Physiol. 2013;304:C102–11. doi: 10.1152/ajpcell.00231.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barbaro NR, Foss JD, Kryshtal DO, Tsyba N, Kumaresan S, Xiao L, Mernaugh RL, Itani HA, Loperena R, Chen W, Dikalov S, Titze JM, Knollmann BC, Harrison DG, Kirabo A. Dendritic Cell Amiloride-Sensitive Channels Mediate Sodium-Induced Inflammation and Hypertension. Cell Rep. 2017;21:1009–1020. doi: 10.1016/j.celrep.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murdoch CE, Chaubey S, Zeng L, Yu B, Ivetic A, Walker SJ, Vanhoutte D, Heymans S, Grieve DJ, Cave AC, Brewer AC, Zhang M, Shah AM. Endothelial NADPH oxidase-2 promotes interstitial cardiac fibrosis and diastolic dysfunction through proinflammatory effects and endothelial-mesenchymal transition. Journal of the American College of Cardiology. 2014;63:2734–41. doi: 10.1016/j.jacc.2014.02.572. [DOI] [PubMed] [Google Scholar]
- 59.Lee SJ, Kang JG, Ryu OH, Kim CS, Ihm SH, Choi MG, Yoo HJ, Kim DS, Kim TW. Effects of alpha-lipoic acid on transforming growth factor beta1-p38 mitogen-activated protein kinase-fibronectin pathway in diabetic nephropathy. Metabolism. 2009;58:616–23. doi: 10.1016/j.metabol.2008.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.