Abstract
While there is no doubt that regional lymph node metastases are an enormously important factor in melanoma staging and treatment, the biology behind this significance and its precise implications for treatment planning have been a leading controversy in melanoma and other solid tumors for over a century. Recent clinical data, including data from prospective randomized clinical trials have refined our understanding of the process of nodal metastases and the advantages and disadvantages of different clinical management strategies. This review presents two points of view in this debate and discusses the results of new data analyses as well as pivotal clinical trials informing the discussion.
Keywords: Melanoma, Sentinel lymph node, Clinical trial, lymphatic mapping, surgical oncology
Overall Introduction
Mark B. Faries
In melanoma, the presence or absence of nodal metastases has been recognized as a critically important factor in treating patients for generations. However, the nature of that importance has been the subject of intense debate. Two camps have developed among those who care for patients with the disease: those who believe lymph nodes are an incubator for sequential progression and metastasis, and those who believe lymph nodes represent only a marker of any melanoma’s metastatic potential. The debate has had significant practical implications in treatment, particularly with regard to the management of regional nodes in the absence of clinically-apparent disease at the time of diagnosis. This review presents two sides of the debate in its first two sections. Although the authors’ actual views on this topic may be more nuanced, each side of the debate is forcefully presented in the hopes of allowing the reader to grasp the depth of conviction of those holding that position. In reality, each point of view likely has some validity, with variable applicability to each individual patient.
Both sides of the debate recognize the staging value of regional lymph nodes. This review goes on to provide additional data analysis to refine this prognostic understanding. Nodal stage has been categorized by the method of detection of nodal metastases (i.e. clinically occult vs. clinically apparent metastases), but there may also be qualitative differences between lymph nodes in the regional basin. This is discussed by Dr. Reintgen and colleagues, who demonstrate that the presence of metastases in non-sentinel lymph nodes results in a markedly worse prognosis. This effect appears more important than the absolute number of involved lymph nodes, which is the standard indicator used in the current staging system. They go on to show that the prognostic impact of nodal metastases changes during follow up as well. When examined at the time of diagnosis, nodal metastases have an enormous impact on the risk of metastasis and death. The longer patients go without recurrence, though, this added risk becomes smaller and smaller so that after 4 years disease-free, their risk approaches that of node-negative patients.
Finally, even in at a time when a “debate” can argue the merits of incubators and markers, the practical aspects of management of regional lymph nodes has become much more standardized than was previously the case. This is only possible through extensive work done through clinical trials. Dr. Caracò reviews these trials and their implications to treatment.
“Sentinel lymph nodes represent incubators for metastasis”
Mark B. Faries
“It is essential to remove, whenever possible, those lymph glands which first receive the infective protoplasm, and bar its entrance into the blood, before they have undergone increase in bulk. This is ‘Anticipatory Gland-Excision’, a simple common-sense measure, adding nothing to the gravity of a surgical operation, while most materially enhancing its efficacy.”1
This assertion, made in The Lancet in 1892 by Herbert Snow, a surgeon at the London Cancer Hospital, is the opening salvo in a battle that has raged in various forms for over a century. The debate has affected not only melanoma treatment, but that of breast cancer, lung cancer, esophageal cancer and many other solid tumors. Through the accumulation of clinical data, we have been able to refine our understanding of the issues surrounding the debate and improve our treatment of patients. However, controversy remains and the medical community continues to work toward a comprehensive understanding of staging, surgical treatment and tumor biology. In this section, the position supporting Snow’s point of view will be presented.
Why sequential nodal disease progression is intuitive
For as long as melanoma has been recognized as a disease, its ability to spread to regional lymph nodes has been observed.2 The tendency for melanoma and many other solid tumors to spread first and often exclusively to regional lymph nodes was clear historically and remains clinically apparent today. Examining Surveillance Epidemiology and End Results data from 2007–2013 among patients presenting with melanoma metastases, the ratio of regional nodal disease to distant metastases was 2.3: 1.3 This suggests that there is a strong tendency for malignant progression first to lymph nodes and only subsequently to distant sites. The ratios are even more biased in the case of breast cancer, thyroid cancer, oral cancers, cervical cancer and others. So it was natural to presume early removal of metastases in lymph nodes would improve outcomes by interrupting the metastatic cascade. So elective removal of lymph nodes even prior to patients developing clinically apparent metastases became a standard treatment recommendation in many instances.
However, as time went on, several things became apparent. First, in most cases of primary melanoma, the regional lymph nodes are normal. Second, complete dissection of regional nodes carries the risk of morbidity including wound complications, nerve injury and lymphedema. Finally, laboratory experiments demonstrated that lymph nodes do not function as mechanical filters, based on the observation that tumor cells injected into lymphatic channels could be rapidly detected in efferent lymph fluid, having passed through a draining lymph node.4 These findings led some to challenge the concept of elective node dissection and practice observation of regional nodal basins with intervention only when disease became clinically apparent. In melanoma, there were several trials conducted to examine the value of elective node dissection, and the overall results of these trials did not identify a statistically significant survival advantage for early dissection, leading some to conclude that the marker hypothesis was correct.
Why were the initial assumptions not borne out?
As it turns out, the biological question at the root of this debate was not as simple as was initially assumed. Indeed, lymph nodes are not mechanical filters for cancer cells, but this is not required for the incubator hypothesis to be correct. What is needed is for lymph nodes to provide a relatively favorable environment for the persistence, growth and metastatic development of malignant cells, and these conditions do appear to be present. Lymph nodes draining a primary melanoma site are prepared for the arrival of melanoma cells through induction of lymphangiogenesis there, and for the survival of melanoma cells through selective immunosuppression.5–7 Such a safe haven is often the only site of disease after excision of the primary tumor and allowing this disease to persist and grow in that location for 1–2 years prior to clinical detection is likely to allow more distant metastases that could have been prevented by early removal. This sequential spread is apparent from data from the first Multicenter Selective Lymphadenectomy Trial (MSLT-I) in which patients were randomly assigned to immediate nodal staging by sentinel node biopsy or to observation.8 Observed patients who developed clinically apparent nodal disease had an average number of involved nodes that was more than twice the number seen with immediate removal. This clearly demonstrates the potential for melanoma to spread at least from node to node, and probably to distant sites as well and suggests that, despite the lack of filtration, incubation of tumor cells is likely occurring in the regional nodal basin.
What about the randomized clinical trials that are frequently described as “negative” and proving no difference in outcome based on early nodal surgery? In fact, there are three aspects of these trials that are generally overlooked by proponents of the marker hypothesis, which should change our conclusions. First, the trials did not have adequate statistical power to truly answer the question. Second, the trials do not, in fact, ask a question of nodal treatment versus no nodal treatment. They only ask about early nodal treatment versus later nodal treatment. Third, there are trials in other malignancies that show significant overall or disease-specific survivals based on early (elective) or more extensive nodal treatment. Finally, observations of consistent benefit in certain patient populations are discounted out of hand as of no value.
The sample size of reported randomized trials of elective lymph node dissection in melanoma and other tumor types was frequently too small to give a reasonable possibility of showing a significant benefit.9–14 Most patients in these studies had pathologically negative lymph nodes, limiting the fraction of patients who could possibly derive a survival benefit. The overall small sample sizes precluded statistical significance even when a clinically significant difference in outcomes was observed. For the melanoma trials, this was true in every instance, with survival benefits for dissection in every case, but never to a statistically significant degree. P-values of 0.11 (WHO #14), 0.12 (Intergroup) and 0.18 (MSLT-I), all in favor of early treatment suggest that the increased survival may not be random and certainly preclude any definitive conclusion that the two treatments are equal.
From a biological perspective, the only way to determine if the incubator or marker hypothesis is correct would be to randomize patients to nodal treatment or no nodal treatment. In fact, none of the trials do this. They randomize patients to early treatment vs. late treatment (e.g. melanoma elective node trials), more treatment versus less treatment (e.g. gastric D1 vs. D215, esophageal trials16, lung trials17) or surgical treatment versus other treatment modalities (e.g. ACoSOG Z011).18 While these are appropriate randomizations to ask the relevant clinical questions, they do not provide definitive insights for the biological question.
It is also important to note that there are examples of statistically and clinically significant improvements in either overall- or disease-specific survival based on earlier or more nodal surgery. These include a randomized trial in oral cancer in which patients were randomized to either immediate, elective node dissection or to observation with dissection in the event of nodal recurrence.19 This trial showed significantly improved overall survival for the elective dissection group (80.0% versus 67.5% at 3-years). The Dutch gastric cancer trial randomized patients to either limited (D1) or extended (D2) lymphadenectomy.15 Although an excess of operative mortality in the D2 group prevented an overall survival benefit, examination of gastric cancer-specific mortality showed a significant advantage for the more extensive dissection group (HR 0.74 95%CI 0.59–0.93, p=0.01). This indicates there was a biological effect of dissection, though it was counteracted clinically by increased operative mortality.
The final, and perhaps most important issue is that of subgroups. In general, analyses of subgroups within clinical trials is viewed with suspicion as an effort to pull a positive result from a negative trial. While this is a valid concern, in the case of nodal treatment in melanoma, it may be appropriate to examine the issue of subgroups if we are to understand the underlying biology of the disease and treat our patients appropriately. Since we have already seen that the currently available evidence is not able to definitively end debate, identification of consistent and biologically plausible subgroups who do or do not seem to benefit from early treatment is a reasonable approach. One of the best such analyses involves primary melanoma thickness, which influences the potential benefit of intervention. For the thinnest melanomas, the risk of nodal metastasis is very small. This means that no reasonable trial could be done to include enough patients to demonstrate a statistical benefit. In thick melanomas, the risk of distant metastasis at the time of diagnosis is high, so even in the presence of nodal metastases, a survival benefit is impossible. It is only in the intermediate thickness group that the probability of nodal metastasis without distant metastasis is high enough to show benefit. Examining the clinical trial results consistently shows this to be true.
In the WHO#1 trial, the WHO#14 trial, the Intergroup trial and MSLT-1, there was strong evidence, statistically significant in some cases, of a survival benefit for early treatment when the intermediate thickness group was examined (Table 1).8, 9,12–14, 20 There was no hint of a benefit in any of the trials for thicker melanomas. This finding is remarkably consistent among these multiple, independent prospective data sets. Indeed, a metaanalysis on this subject revealed no overall significant therapeutic effect, but a highly significant benefit (p<0.0001) for patients with intermediate-thickness melanomas (0.76–3.99mm).21
Table 1.
5-year survival based on treatment assignment and tumor thickness in randomized trials
| Intermediate | Thick | All intermediate/thick | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Trial | Early | Observation | Δ | Early | Observation | Δ | Early | Observation | Δ |
| WHO#1 | 78.5% | 69.7% | 8.8% | 52.9% | 51.7% | 1.2% | 71% | 65% | 4% |
| WHO#14 | 68% | 49% | 19% | 66% | 63% | 3% | 61.7% | 51.3% | 10.4% |
| Intergroup | 89% | 84% | 5% | 57% | 59% | −2% | 86% | 82% | 4% |
| MSLT-I* | 69.8% | 57.5% | 12.3% | 60.8% | 53.8% | 7% | NR | NR | NR |
Intermediate thickness: WHO#1: 1.6–4.5mm, WHO#14 1.5–4.0 mm, Intergroup: 1–2 mm, MSLT-I: 1.2–3.5mm
Thick: WHO#1: >4.5mm, WHO#14: >4mm, Intergroup 3.1–4mm, MSLT-I: >3.5 mm
Node positive only
WHO: World Health Organization, MSLT: Multicenter Selective Lymphadenectomy Trial, NR: Not Reported
Conclusion
Overall it appears the incubator hypothesis is well supported by available information. The most basic observations of the metastatic behavior of most solid tumors indicates that the lymphatic route is the preferred initial mode of tumor dissemination. Although lymph nodes do not function as mechanical filters for tumor cells, the relative ease of arrival and protection from immune-mediated destruction there make regional nodes ideally suited to incubate tumor cells while their metastatic potential increases. Finally, a mature examination of the results of randomized clinical trials in this area shows that the data are very consistent with a therapeutic effect of lymph node dissection in biologically-appropriate subgroups.
“Sentinel lymph nodes represent a marker for metastasis”
Dale Han
Introduction
The clinical implications of nodal metastasis have been long debated, and over the past century, several theories were developed to explain the biology of cancer spread.22–26 Within this background, Morton reported on sentinel lymph node biopsy (SLNB) in 1992 as a less invasive way to evaluate nodal status in patients with melanoma.27 SLNB was revolutionary, but the technique heightened the debate on the biology of cancer metastasis. This is because two theories emerged to explain the biology of SLN metastasis.28 The first was the “incubator hypothesis” which was an extension of the “spectrum theory” of metastatic spread.23, 28 In the incubator hypothesis, it was theorized that the SLN functioned as an incubator, allowing for deposits of melanoma within the node to develop further metastatic potential, thereby increasing the risk for subsequent and sequential distant spread. The incubator hypothesis implied that treatment of nodal disease had the potential to halt distant metastases and improve survival by preventing disease incubation in the nodal basin. Therefore, locoregional therapy had a potentially important role in the incubator hypothesis to prevent disease persistence and decrease opportunities for cancer cells to develop into metastatic lesions.
In contrast, the second theory describing the biology of SLN metastasis, known as the “marker hypothesis”, promoted the ideas that nodal metastasis simply indicated distant microscopic spread of disease and that systemic therapy was needed to positively affect outcomes in patients with SLN disease.28 The marker hypothesis was based on the “systemic theory” of cancer spread.22 In the “systemic theory” of metastasis, clinically-evident localized primary cancer is only an indicator of early distant metastasis. Lymph node disease is simply a marker of clinically-occult distant metastasis, and cancer is fundamentally a systemic disease. Therefore, locoregional therapy, including lymphadenectomy, has limited efficacy and systemic therapy is needed to affect survival in patients with cancer.
The validity of each theory of SLN biology has been extensively debated, and there is no consensus as to which theory is correct. However, data is now emerging which appears to favor one of these theories. A central tenant for the incubator hypothesis is that treatment of nodal metastasis can prevent the development of distant disease and thereby improve survival. Yet recent clinical trials, specifically MSLT (multicenter selective lymphadenectomy trial)-II and DeCOG-SLT, have shown that early elimination of nodal metastases, and therefore decreasing incubation time, does not provide a survival benefit.29,30 These large clinical trials provide evidence that the presence of nodal metastases simply provides a prognostic marker to indicate patients who are at higher risk for developing distant disease. Therefore, removal of nodal disease has limited benefit, and primary tumor biology may essentially drive the development of distant metastasis.
Primary tumor biology drives the development of distant metastases
There is data to suggest that the biology of the primary tumor predominantly drives prognosis and that nodal status is simply a marker of the aggressiveness of the primary melanoma. Thickness and ulceration are validated prognostic primary tumor markers that have been incorporated into American Joint Cancer Committee (AJCC) staging system. In patients with localized disease (stage I and II), survival appears similar to the survival seen in patients with N1a and N2a disease in the 7th edition AJCC staging system for melanoma.31 This would imply that primary tumor biology appears to drive prognosis and the development of distant metastasis as much as the presence of microscopic nodal disease. In fact, the overriding importance of primary tumor characteristics in determining prognosis is evidenced by the fact that the new 8th edition AJCC staging system for melanoma now incorporates thickness in addition to ulceration status to define stage III disease.32 This would again imply that the biology of the primary tumor appears to drive prognosis as much as the presence of nodal disease.
Furthermore, in looking at MSLT-I data, specifically rates of developing distant disease as first site as stratified by thickness and nodal status, one sees the predominance of primary tumor characteristics in driving the development of distant metastases.14 In negative SLN patients, as primary tumor thickness increases, the rate of developing distant disease increases. For instance, in patients with intermediate thickness melanomas, 12% of negative SLNB patients (77 distant metastases as first site in 643 negative SLN patients) developed distant disease as first site which increased to 24.1% in patients with thick melanoma (28 distant metastases as first site in 116 negative SLN patients).14 This shows that in patients with no microscopic nodal disease, thickness is one of the primary factors driving the development of distant disease. However, in patients with a positive SLNB, distant metastases as first site was 24.6% in patients with intermediate thickness melanoma and was similar at 26.3% in patients with thick melanoma.14 Although the rate of distant metastasis as first site was higher in positive versus negative SLN patients in the intermediate thickness group, the rate of distant metastasis as first site was essentially the same between positive and negative SLN patients in the thick group (26.3% and 24.1%, respectively). This would suggest that regardless of nodal status, primary tumor thickness may ultimately drive the development of distant disease and that detection of SLN metastasis simply selects out patients who have disease with the propensity to metastasize in the intermediate thickness group.
Does treating nodal metastases halt the progression of disease?
The incubator hypothesis theorizes that treatment of nodal disease through lymph node dissection has the potential to control and stop the progression of disease, thereby improving survival. Therefore, the incubator hypothesis implies that treatment of the smallest volume of SLN metastases has the potential for the greatest effect. Several clinical trials have evaluated the effect of lymphadenectomy on outcomes in melanoma patients, specifically in patients with micrometastatic nodal disease. The benefit of nodal dissection was first questioned by studies evaluating elective lymph node dissection (ELND). Overall, four large clinical trials assessed ELND in clinically node-negative melanoma patients and showed that performance of ELND to control micrometastatic nodal disease did not improve survival.33,34 Only the Intergroup Melanoma Trial showed improved survival with ELND in very specific subgroups, such as in patients with tumors of 1–2 mm thickness or with non-ulcerated tumors.33,34
SLNB was then developed as a less extensive means to determine nodal status. MSLT-I showed that detection of nodal micrometastases was prognostic and was the most powerful predictor for recurrence and death from melanoma in patients with intermediate thickness melanoma.14 However, performance of SLNB with subsequent completion lymph node dissection (CLND) for patients with a positive SLN was not shown to improve melanoma-specific survival when compared with patients who were observed and had a subsequent therapeutic lymphadenectomy for a nodal recurrence (HR, 1.12, 95% CI, 0.76–1.67; P=0.56).14
It should be noted that MSLT-I was not powered to show a survival difference between the treatment arms, and a subsequent study, MSLT-II, was conducted to determine if CLND for a positive SLNB provided a benefit. Retrospective data had already suggested that there was no survival difference between positive SLN patients who did and did not have a CLND.35 In MSLT-II, positive SLN patients were randomized to either immediate CLND or observation with subsequent lymphadenectomy for a nodal recurrence.29 The results of MSLT-II were recently published and showed that performance of CLND and early treatment of nodal metastases was not associated with a melanoma-specific survival benefit (HR, 1.08, 95% CI, 0.88–1.34; P=0.42).29 Furthermore, a study from Germany, DeCOG-SLT, also randomized positive SLN patients to either nodal observation or immediate CLND.30 The results of this trial again failed to demonstrate an overall survival benefit for CLND (HR, 0.96, 90% CI, 0.67–1.38; P=0.87) and also reported that in patients with the smallest size of metastatic melanoma in lymph nodes (≤0.1 mm), no difference was seen in distant metastasis-free survival between patients who did and did not have a CLND.30 Therefore, collectively these results show that control of nodal disease does not prevent the development of distant disease and ultimately death from melanoma. However, although the performance of CLND does not influence survival, the presence of additional nodal disease in the CLND specimen is prognostic for worse survival as reported in MSLT-II (HR, 1.78, 95% CI, 1.19–2.67; P=0.005).29 All of these data point to the fact that detection of nodal disease portends worse survival, but treatment of the nodal metastases does not prevent distant metastases nor does it affect survival as shown in the ELND, SLNB and CLND studies.
Conclusions
Two models have been hypothesized to explain the biology of SLN metastases. The incubator hypothesis theorizes that treatment of nodal disease has the ability to potentially prevent distant metastasis and thereby improve survival. In particular, treatment of smaller deposits of nodal metastases, such as micrometastatic disease, has the potential to provide the greatest benefit since there is less time for disease incubation. However, clinical data clearly shows that treatment of nodal disease whether as ELND, SLNB or CLND does not prevent the development of distant disease nor does it improve survival. Furthermore, treatment of nodal metastases at the lowest volume of disease (nodal micrometastases ≤0.1 mm) does not prevent the development of distant metastases. In contrast, it appears that primary tumor biology predominantly determines the risk for the development of distant disease and that the presence of nodal disease serves as only a marker for micrometastatic disease elsewhere. A preponderance of data is now accumulating which appears to support the marker hypothesis to explain the biology of SLN metastasis, although it is possible that both the incubator and marker hypotheses may be correct in specific subsets of patients.
Nodal Staging and Conditional Survival in Patients with Melanoma
Michael Reintgen, Lauren Kerivan, Douglas Reintgen
The definition of a sentinel lymph node (SLN) in patients with malignant melanoma is any node in the regional basin that has a direct connection to the primary site via an afferent lymphatic.36 This purely anatomical definition explains how lymphatic mapping works by injecting small particle agents (vital blue dye and radiocolloid) around the primary site to be taken up by afferent lymphatics that flow into the regional basin and are trapped in the first nodes encountered, the SLNs. Very little of the mapping agents pass through the SLN and the same can be said of metastatic melanoma cells. Likewise, once metastatic melanoma cells enter a SLN, the SLN does a remarkable job limiting the disease spread in the regional basin. Evidence for this is derived from the fact that if patients have nodal spread (Stage III), 85% of the time it is confined to the SLN. Only 15% of patients with a positive SLN have disease beyond the SLN.37
The hypotheses that the disease status of NSLNs will have an important impact on recurrence and overall survival in melanoma patients with a positive SLN was investigated. Does a patient with only SLN involvement with metastatic disease have a better prognosis than a patient with SLN and NSLN metastatic disease regardless of the number of nodes involved?
The melanoma database at USF was established in 1989 and currently has registered over 15,000 patients with the disease with relatively complete pathologic and follow-up data. The database was queried to abstract all patients with clinical negative exams in their nodal basin who were later found to have metastatic disease in their SLN with the lymphatic mapping technique. Patients were eligible for the study if they had the following characteristics: 1) a documented diagnosis of invasive melanoma, 2) received a SLN biopsy with at least one SLN positive for metastatic disease by routine pathology (H&E with or without immunohistochemistry), 3) received a completion lymph node dissection (CLND) within 3 months from the date of SLN biopsy, 4) had no evidence of distant metastases (Stage IV disease) at the time of CLND.
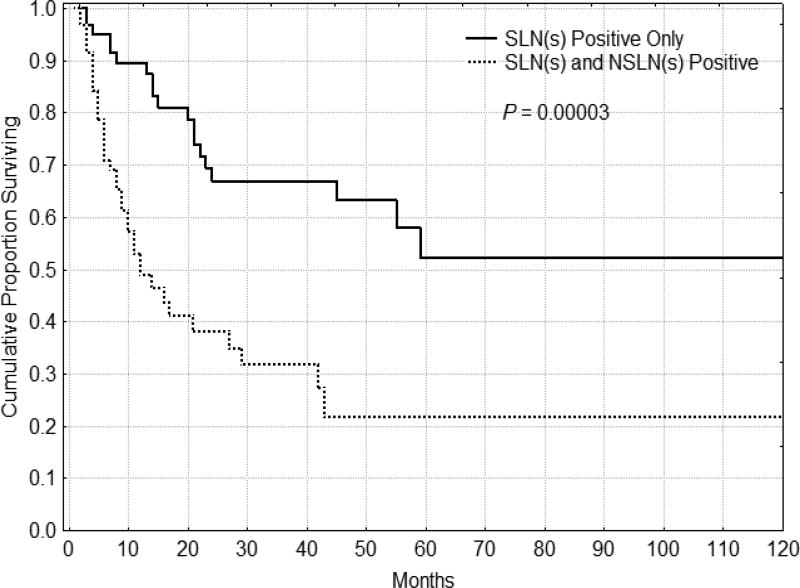
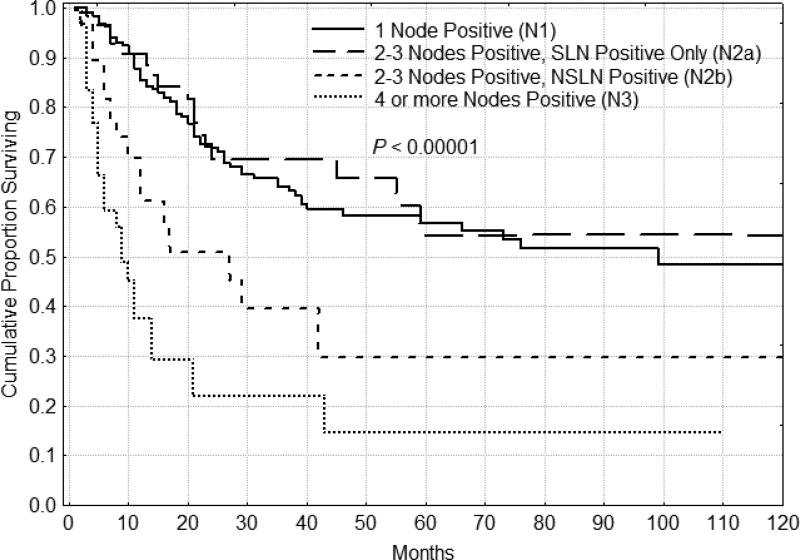
The 5 year DFS for the 1 node positive, 2–3 nodes positive and 4 or more nodes positive groups were 58%, 47% and 15% respectively, confirming the validity of the current nodal staging system for melanoma. If disease is confined to the SLNs, prognosis is improved compared to those patients with positive SLNs and NSLNs. Figure 1 shows all patients who have their metastatic disease limited to the SLN having a significant DFS advantage over patients with SLN and NSLN positive disease (p = 0.00003). These results hold despite the total number of nodes involved with disease. Figure 2 illustrates DFS for patients with N2 disease (those with 2–3 nodes positive). If the disease is confined to the SLNs, patients had a better DFS (p=0.00001), regardless of total number of nodes positive. In fact, when patients with N2 disease (2–3 nodes positive) were distinguished by the type of nodes positive, those with only SLNs positive had survival that was similar to those patients with only 1 node positive (N1), while those with at least one NSLN positive had a significantly worse prognosis. Patients with one, two and three nodes positive with SLN-only disease had a similar 5 year DFS.
Figure 1.
Disease-free survival for patients (n=125) with more than 1 node positive (N2–N3). Patients who had only sentinel nodes positive (n=64) had significantly better disease-free and overall (not shown, P = 0.00003) survival than those patients with a positive non-sentinel node (n=61).
Figure 2.
Disease-free survival for patients (n=93) with 2–3 nodes positive (N2). Patients who had only sentinel nodes positive (n=62) had significantly better disease-free and overall (not shown, P = 0.00356) survival than those patients with a positive non-sentinel node (n=31).
Patients with Stage III metastatic melanoma, which is disease that has spread to the regional basin, are a heterogeneous group. The AJCC melanoma staging database examined 2,313 patients with Stage III disease and found that 81% had micrometastases.38,39 The 5-year OS was 67% for patients with nodal micrometastases vs. 43% for those with macro-metastases (palpable, clinically evident disease). Multivariate analysis demonstrated that for patients with micrometastases, number of tumor-containing lymph nodes, primary tumor thickness, patient age, ulceration and anatomic site of the primary independently predicted survival (all P < 0.01). The nodal metastatic distribution between SLN and NSLN involvement was not in the analysis.
The concept of lymphatic mapping and SLN biopsy has revolutionized the surgical treatment for the nodal staging of patients with melanoma and breast cancer who may have microscopic nodal involvement. Very quickly, this technique became the standard of care since it resulted in a less morbid procedure that improved the accuracy of staging by performing a more detailed histologic examination of the SLN, a win-win situation for patients with melanoma. There does appear to be an orderly progression of nodal metastatic disease, initially involving the SLNs. Little has been written about the distribution of metastases in the regional basin.
Does tumor progression beyond the SLN imply a larger quantity of disease reaching the regional basin and overwhelming the trapping mechanisms of the SLN so that higher echelon nodes become involved? One potential explanation for the poor survival in the NSLN-positive patients may be influenced by the method of detection. The patients with metastatic melanoma in their NSLN had disease detected by H&E staining alone, perhaps representing a group of patients with significant tumor burden. In contrast, SLNs are examined with more sectioning and immunostaining, detecting more disease. Or, do these findings suggest an aggressive tumor biology with metastatic cells that have a more malignant phenotype that allows low volume disease to escape the immunologic barriers in the first node encountered, the SLN. It will be important to examine the characteristics of the metastatic cells in the SLNs vs. NSLNs to determine if distinct genetic or immunologic differences exist that allow the melanoma cells to escape beyond the SLNs.
The main factor in the current AJCC N-staging system for patients with malignant melanoma is number of nodes positive with metastatic disease. There is emerging data that suggests that actually the distribution of metastases in the regional basin is important, in regards to SLN only vs. SLN and NSLN disease. The study from USF concluded that the pattern of regional node involvement in patients with malignant melanoma is a powerful predictor of outcome independent of the total number of nodes positive.40 In this report, DFS remained the same despite an increasing number of SLNs involved with disease and it was only when the disease spread beyond the SLN to neighboring NSLN that prognosis decreased. The findings of this study and others in the literature have implications for the N-staging system for melanoma.
Conditional Survival in Melanoma
Malignant melanoma is epidemic in the State of Florida and the cause of the majority of skin cancer deaths. Providing an accurate prognosis to patients diagnosed with cancer is an important role of the oncologist. Although traditional survival estimates for patients with melanoma at 5 and 10 years from the time of diagnosis have been reported in the literature, there is a lack of current data predicting future survival for patients who survive a number of years after diagnosis. A patient’s risk profile changes with time, such that the prognosis for a patient several years post-diagnosis or treatment without a recurrence will differ from the estimate provided upon initial diagnosis. Conditional survival (CS) and conditional disease free survival (CDFS) estimates, or future disease-free and survival assessments based on the time a patient has already survived, have been proposed as a better way to predict long-term prognoses for cancer survivors and is more meaningful information for patients and their families.41,42 CS and CDFS estimates account for the dynamic nature of hazard rates for patients with cancer and, thus, have been increasingly used to provide more relevant risk predictions for patients with primary malignancies. CS calculations have also been used in the medical/legal world to determine the merit of cases and in the defense of physician practices in the United States and in the insurance industry to determine rates for coverage. Reliable data can only be generated from large databases of patients with long term follow-up in place, such as the USF Melanoma Database.
There were a total of 7531 melanoma patients in the study who received their care at the University of South Florida. The distribution of patients according to the Stage of Disease at diagnosis was 53.4%, 32.5%, 11.1% and 3%, for Stage I, II, III and IV melanoma respectively. For all stages of disease, as patients with melanoma were followed without recurrence or death, the prognosis improved. For Stage I patients, the 5-year DFS calculated from diagnosis increased from 61.7% to 93.2% if patients survive up to 4 years without recurrence. Similar trends were noted for Stage II –IV disease. For Stage I patients, 5-year OS from date of diagnosis also increased from 88.8% to 98.6% if patients survived 4 years. 5-year OS at any period of time during the recurrence free follow-up period was similar for patients with Stage I (88.8%, 87.2%, 86.2%, 86.4%, 86.2%) and II (66.7%, 62%, 62.8%, 64.2%, 66.1%) disease but improved significantly for Stage III (50.7%, 50.2%, 56.5%, 59.8%, 71.4% and IV patients (27.6%, 34.9%, 48.3%, 50.0%, 68.7%) as patients survived 1,2,3 and 4 years from diagnosis. For example, if Stage III patients survive 4 years, the 5-year OS increases from 50.7% to 71.4%. The best prognostic group, or those patients with Stage I melanoma who survive without recurrence for the first 4 years of follow-up, continued to have an increased death rate compared to the normal population. The conditional survival data for patients with Stage I, II and III melanoma do not substantiate any medical/legal causation arguments as long as patients do not have a recurrence. This study showed that prognosis improves for melanoma patients if they survive during the follow-up period without recurrence. This was observed for all stages of disease. These data provide more meaningful recurrence and survival information for patients and their families, for clinicians, for lawyers in the medical/legal system, and for the insurance industry.43
Role of sentinel lymph node staging in melanoma clinical trials
Corrado Caraco
INTRODUCTION
Since the introduction of this procedure in 1992, many trials were conducted to assess its staging and therapeutic role. Initially the procedure was analyzed to test the safety and efficacy in the short and long term outcome, if it is at least as effective and/or safe as existing proven techniques and to verify the prognostic role and the prognostic factors correlated to the outcome. The first Multicenter Selective Lymphadenectomy Trial (MSLT-I), comparing the procedure followed by immediate complete lymph node dissection in positive patients versus observation only, and final results showed that, for intermediate-thickness or thick primary melanomas, sentinel biopsy provided important prognostic information and permitted to identify patients with nodal metastases who may benefit from immediate complete lymphadenectomy.14
The value of completion lymph node dissection (CLND) for patients with SLN metastases remain unclear and two randomized trials were designed to clarified it: the MSLT-II and the DeCOG trial.29,30 In both trials sentinel node positive patients were randomized to compare completion lymph node dissection (CLND) to observation with frequent nodal ultrasonography and melanoma specific survival or distant metastases-free survival as primary endpoints. Both trials showed no benefit on CLND on overall survival, but many limitations need to be considered in the making decision and in the discussion with patients about risks and benefits of each approach.
RESULTS FROM DECOG TRIAL
DeCOG-SLT is a multicenter, randomized, phase 3 trial comparing survival of sentinel lymph node biopsy positive patients with melanoma with and without complete lymph node dissection recruited from 41 German skin cancer centers. 483 patients were randomly assigned to either the complete lymph node dissection group (242 patients) or the observation group (241 patients). The primary endpoint was distant metastasis-free survival, calculated from the date of randomization to the date of diagnosis of first distant metastases, date of latest follow-up visit, or date of death by any cause.
The study was closed early due to projected time to complete accrual being much longer than planned, and the event rate being lower than expected, leading to the trial being underpowered. Follow-up compliance was similar in the two treatment groups with a median follow-up time of 35.5 months in the observation group and 33.0 months in the complete lymph node dissection group and is very low considering that in the MSLT II trial, in a larger group of patients, it was necessary a mean follow-up time of about 43 months to evaluate final results.
The results showed a 3-year overall survival of 81,7% in the observation group and 81.2% in the complete lymph node dissection group while 3-year recurrence-free survival was 66.8% in the complete lymph node dissection group and 67.4% in the observation group. The multivariate proportional hazards regression analysis of survival outcomes in the intention-to-treat population showed that tumor load in the sentinel node and primary tumor thickness were the most important prognostic factors both for overall survival and recurrence-free survival.
The conclusion of the trial was that complete lymph node dissection should not be recommended in patients with melanoma with lymph node micrometastases of at least a diameter of 1 mm or smaller. There are some limitations to be considered in the analysis of these data:
Most enrolled cases were T3–T4 with a lower impact of nodal status on the outcome, due to a higher risk of systemic disease;
In 55% of cases were removed 2 to 3 sentinel node like a mini nodal dissection, reducing the possibility of further positive non-sentinel node in the treated lymphatic basin;
More than 90% of cases had one positive sentinel node and about 70% of them with a tumor burden within sentinel node less than 1 mm in size. This mean a minimal risk of residual nodal disease;
About 30% of cases had positive non sentinel node in the completion lymph node dissection, but no information was available about the number of positive lymph nodes after therapeutic nodal dissection in the observation group.
In the observation group were observed more regional lymph node recurrences than in the completion lymph node dissection group and were 15% vs 8% respectively. Despite the impact on overall survival, the regional control might lead to improve the number of involved non-sentinel nodes and change the prognosis, especially in a follow-up not perfectly controlled as in a clinical trial.
Furthermore, the considerations about the differences in morbidity in the two groups seems not evaluable in so different surgical procedures as well the absence of data on the number of positive lymph node in recurred patients in observation group, limits the overall evaluation of the delay of surgery.
RESULTS FROM MSLT-II TRIAL
MSLT-II is an international, multicenter, randomized, phase III trial to evaluate the usefulness of completion lymph-node dissection in patients with melanoma and positive sentinel node. The primary endpoint was melanoma-specific survival, secondary endpoints included overall survival, disease-free survival, survival without regional nodal metastases, distant metastases free-survival and the extent of nodal involvement. From December 2004 and March 2014, in 63 centers, 1939 patients were randomized.
At 3-years of follow-up melanoma specific survival was not statistically different between the dissection group and the observation group, also after adjustment for other prognostic factors. The analysis based on sentinel tumor-burden did not reveal any significant melanoma specific survival benefit from completion lymph node dissection. The 3-year disease-free survival was slightly higher in the dissection group than in observation group and means an increase in the rate of disease control in the regional nodes in the dissection group compared to observation group and was 92% and 77% respectively. The conclusions were that completion lymph node dissection increased the rate of regional disease control and provided prognostic information but did not increase melanoma specific survival.
The analysis of the enrolled population leads to some consideration about possible bias that might underpowered the trial.
Most patients in the trial population had a low volume nodal tumor burden and were likely to have non-sentinel node metastases. This aspect reduced the potential value on overall survival of completion lymph node dissection and limited statistical confidence due a dilution of its therapeutic effect because three quarters of the population had metastases only in the sentinel nodes.
Avoiding completion lymph node dissection means an accurate ultrasound follow-up. Are these patients able to perform frequent visits of follow-up and accurate nodal ultrasound everywhere? A delay in the diagnosis of nodal recurrence might be influence the increasing number of metastatic lymph nodes and impact on prognosis.
Avoiding completion lymph node dissection create a lack of the information about the number of involved nodes and may impede the appropriate risk stratification and selection for adjuvant therapy. All Stage III adjuvant trials enrolled positive sentinel node cases after completion lymph node dissection and seems unbelievable to adopt adjuvant treatment without completion lymph node dissection or to replace it.
DISCUSSION
The value of completion lymph node dissection in patients with positive sentinel node remain controversial because most cases have all nodal disease removed with sentinel biopsy and no additional nodal disease.44 Nodal status remains an important prognostic factor completing the staging for adjuvant therapy purpose and the removal of non-sentinel node disease means an increased rate of regional disease control.
Data from an Italian multicenter retrospective analysis on 1220 patients with positive sentinel node submitted to completion lymph node dissection permitted to design a predictive model to be used for patient risk stratification and decision making.45 The multivariate analysis of this series showed that the risk of harboring metastatic non-sentinel node was higher when a) the primary melanoma is thicker (median 3.6 mm); b) the primary sited in the trunk/head & neck compared to the limbs; c) fewer sentinel nodes are excised (less than 2 sentinel nodes removed); d) more than one positive sentinel node removed; e) the sentinel node metastases is extensive and deeper compared to subcapsular location only.
NCCN as well Italian National AIOM guidelines suggest to discuss with the patient the opportunity of completion lymph node dissection or an intensive follow-up: both are options for patients with low-risk micrometastatic disease, with due consideration of clinicopathological factors. The nomogram proposed by Italian retrospective analysis may be a predictive tool that enable the physician to discuss with the patient the likelihood of metastases in the non-sentinel node, and thus the convenience of performing further surgery after a positive sentinel node.
Abbreviations
- ACoSOG
American College of Surgeons Oncology Group
- AIOM
Associazione Italiana Di Oncologia Medica
- AJCC
American Joint Commission on Cancer
- CDFS
Conditional Disease-Free Survival
- CLND
Completion Lymph Node Dissection
- DeCOG SLT
German Oncology Cooperative Group, Selective Lymphadenectomy Trial
- DFS
Disease-Free Survival
- ELND
Elective Lymph Node Dissection
- H&E
Hematoxylin and Eosin
- HR
Hazard Ratio
- MSLT
Multicenter Selective Lymphadenectomy Trial
- NSLN
Non-sentinel Lymph Node
- SLN
Sentinel Lymph Node
- SLNB
Sentinel Lymph Node Biopsy
- USF
University of South Florida
- WHO
World Health Organization
Contributor Information
Mark B. Faries, The Angeles Clinic and Research Institute, Los Angeles, CA, Cedars Sinai Medical Center, Los Angeles, CA, 11818 Wilshire Blvd, Suite 200, Los Angeles, CA 90025
Dale Han, Section of Surgical Oncology, Department of Surgery, Yale University School of Medicine, New Haven, CT.
Michael Reintgen, Morsani School of Medicine, University of South Florida, Tampa FL.
Lauren Kerivan, Morsani School of Medicine, University of South Florida, Tampa FL.
Douglas Reintgen, Morsani School of Medicine, University of South Florida, Tampa FL.
Corrado Caracò, Istituto Nazionale Tumori “Fondazione Pascale”, Naples, Italy.
References
- 1.Snow HM. Melanotic Cancerous Disease. Lancet. 1892;140:869–922. [Google Scholar]
- 2.Bodenham DC. A study of 650 observed malignant melanomas in the South-West region. Ann R Coll Surg Engl. 1968;43:218–39. [PMC free article] [PubMed] [Google Scholar]
- 3. [accessed on 1/2/2018];SEER Cancer Stat Facts. https://seer.cancer.gov/statfacts/html/melan.html.
- 4.Fisher B, Fisher ER. Transmigration of lymph nodes by tumor cells. Science. 1966;152:1397–8. doi: 10.1126/science.152.3727.1397. [DOI] [PubMed] [Google Scholar]
- 5.Dadras SS, Lange-Asschenfeldt B, Velasco P, et al. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol. 2005;18:1232–42. doi: 10.1038/modpathol.3800410. [DOI] [PubMed] [Google Scholar]
- 6.Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am J Pathol. 2007;170:774–86. doi: 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cochran AJ, Morton DL, Stern S, et al. Sentinel lymph nodes show profound downregulation of antigen-presenting cells of the paracortex: implications for tumor biology and treatment. Mod Pathol. 2001;14:604–8. doi: 10.1038/modpathol.3880358. [DOI] [PubMed] [Google Scholar]
- 8.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–17. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 9.Veronesi U, Adamus J, Bandiera DC, et al. Inefficacy of immediate node dissection in stage 1 melanoma of the limbs. The New England journal of medicine. 1977;297:627–30. doi: 10.1056/NEJM197709222971202. [DOI] [PubMed] [Google Scholar]
- 10.Veronesi U, Adamus J, Bandiera DC, et al. Delayed regional lymph node dissection in stage I melanoma of the skin of the lower extremities. Cancer. 1982;49:2420–30. doi: 10.1002/1097-0142(19820601)49:11<2420::aid-cncr2820491133>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Sim FH, Taylor WF, Pritchard DJ, et al. Lymphadenectomy in the management of stage I malignant melanoma: a prospective randomized study. Mayo Clinic proceedings. Mayo Clinic. 1986;61:697–705. doi: 10.1016/s0025-6196(12)62768-2. [DOI] [PubMed] [Google Scholar]
- 12.Cascinelli N, Morabito A, Santinami M, et al. Immediate or delayed dissection of regional nodes in patients with melanoma of the trunk: a randomised trial. WHO Melanoma Programme. Lancet. 1998;351:793–6. doi: 10.1016/s0140-6736(97)08260-3. [DOI] [PubMed] [Google Scholar]
- 13.Balch C, Soong S, Ross M, et al. Long-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0–4.0 mm) Ann Surg Oncol. 2000;7:87–97. doi: 10.1007/s10434-000-0087-9. [DOI] [PubMed] [Google Scholar]
- 14.Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370:599–609. doi: 10.1056/NEJMoa1310460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–49. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 16.Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–9. doi: 10.1056/NEJMoa022343. [DOI] [PubMed] [Google Scholar]
- 17.Izbicki JR, Passlick B, Pantel K, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: results of a prospective randomized trial. Ann Surg. 1998;227:138–44. doi: 10.1097/00000658-199801000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giuliano AE, Ballman KV, McCall L, et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA. 2017;318:918–926. doi: 10.1001/jama.2017.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Cruz AK, Vaish R, Kapre N, et al. Elective versus Therapeutic Neck Dissection in Node-Negative Oral Cancer. N Engl J Med. 2015;373:521–9. doi: 10.1056/NEJMoa1506007. [DOI] [PubMed] [Google Scholar]
- 20.Balch CM, Cascinelli N, Sim FH. Elective Lymph Node Dissection: Results of Prospective Randomized Surgical Trials. In: Balch CM, Houghton AN, Sober AJ, et al., editors. Cutaneous Melanoma. Saint Louis, MO: Quality Medical Publishing; 2003. pp. 379–95. [Google Scholar]
- 21.Hein DW, Moy RL. Elective lymph node dissection in stage I malignant melanoma: a meta-analysis. Melanoma Res. 1992;2:273–7. doi: 10.1097/00008390-199211000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Fisher B. Laboratory and clinical research in breast cancer--a personal adventure: the David A. Karnofsky memorial lecture. Cancer Res. 1980 Nov;40(11):3863–3874. [PubMed] [Google Scholar]
- 23.Hellman S. Karnofsky Memorial Lecture. Natural history of small breast cancers. J Clin Oncol. 1994 Oct;12(10):2229–2234. doi: 10.1200/JCO.1994.12.10.2229. [DOI] [PubMed] [Google Scholar]
- 24.Halsted WS. I. The Results of Operations for the Cure of Cancer of the Breast Performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann Surg. 1894 Nov;20(5):497–555. doi: 10.1097/00000658-189407000-00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leong SP, Tseng WW. Micrometastatic cancer cells in lymph nodes, bone marrow, and blood: Clinical significance and biologic implications. CA Cancer J Clin. 2014 May-Jun;64(3):195–206. doi: 10.3322/caac.21217. [DOI] [PubMed] [Google Scholar]
- 26.Cady B. Basic principles in surgical oncology. Arch Surg. 1997 Apr;132(4):338–346. doi: 10.1001/archsurg.1997.01430280012001. [DOI] [PubMed] [Google Scholar]
- 27.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992 Apr;127(4):392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 28.Morton DL, Hoon DS, Cochran AJ, et al. Lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: therapeutic utility and implications of nodal microanatomy and molecular staging for improving the accuracy of detection of nodal micrometastases. Ann Surg. 2003 Oct;238(4):538–549. doi: 10.1097/01.sla.0000086543.45557.cb. discussion 549–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faries MB, Thompson JF, Cochran AJ, et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N Engl J Med. 2017 Jun 8;376(23):2211–2222. doi: 10.1056/NEJMoa1613210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leiter U, Stadler R, Mauch C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016 Jun;17(6):757–767. doi: 10.1016/S1470-2045(16)00141-8. [DOI] [PubMed] [Google Scholar]
- 31.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009 Dec 20;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of skin. In: Amin MB, Edge S, Greene F, editors. AJCC Cancer Staging Manual. 8. New York, NY: Springer; 2017. [Google Scholar]
- 33.Essner R. Surgical treatment of malignant melanoma. Surg Clin North Am. 2003 Feb;83(1):109–156. doi: 10.1016/S0039-6109(02)00205-0. [DOI] [PubMed] [Google Scholar]
- 34.Lens MB, Dawes M, Goodacre T, Newton-Bishop JA. Elective lymph node dissection in patients with melanoma: systematic review and meta-analysis of randomized controlled trials. Arch Surg. 2002 Apr;137(4):458–461. doi: 10.1001/archsurg.137.4.458. [DOI] [PubMed] [Google Scholar]
- 35.Wong SL, Morton DL, Thompson JF, et al. Melanoma patients with positive sentinel nodes who did not undergo completion lymphadenectomy: a multi-institutional study. Ann Surg Oncol. 2006 Jun;13(6):809–816. doi: 10.1245/ASO.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 36.Ross MI, Reintgen DS, Balch CM. Selective Lymphadenectomy: The Emerging Role of Lymphatic Mapping and Sentinel Node Biopsy in the Management of Early Stage Melanoma. Seminars in Surgical Oncology. 1993;9:219–223. [PubMed] [Google Scholar]
- 37.Miliotes G, Albertini J, Berman C, Messina J, Glass F, Cruse CW, Rapaport D, Puleo C, Fenske N, Petsoglou C, DeConti R, Lyman G, Reintgen DS. The tumor biology of melanoma nodal metastases. American Surgeon. 1996;62:81–88. [PubMed] [Google Scholar]
- 38.Balch C, Gershenwald JE, Soong S-J, Thompson J, Byrd D, Cascinelli N, Cochran AJ, Coit D, Eggermont A, Johnson T, Kirkwood J, Leong S, McMasters K, Mihn M, Morton D, Ross M, Sondak V. Multivariate analysis of prognostic factors among 2313 patients with stage III melanoma: Comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28:2452–2459. doi: 10.1200/JCO.2009.27.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American Joint Committee on Cancer Staging Manuel. (7) [Google Scholar]
- 40.Reintgen M, Murray L, Akman K, Giuliano R, Loznicki A, Shivers S, Reintgen DS. Evidence for a better nodal staging system for melanoma: The clinical relevance of metastatic disease confined to the sentinel lymph nodes. Ann Surg Oncol. 2013;20:668–674. doi: 10.1245/s10434-012-2652-4. [DOI] [PubMed] [Google Scholar]
- 41.Ries LAG, Reichman ME, Lewis DR, Hankey BF, Edwards BK. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. The oncologist. 2003;8(6):541–552. doi: 10.1634/theoncologist.8-6-541. [DOI] [PubMed] [Google Scholar]
- 42.Merrill RM, Hunter BD. Conditional survival among cancer patients in the United States. The oncologist. 2010;15(8):873–882. doi: 10.1634/theoncologist.2009-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kerivan L, Reintgen M, Shivers S, Reintgen E, Reintgen DS. Conditional Survival in Melanoma: Meaningful Prognostic Information for Patients and Physicians; Presented at the Society of Surgical Oncology Meeting; March, 2017; Seattle, Washington. [Google Scholar]
- 44.Faries MB. Completing the Dissection in Melanoma: Increasing Decision Precision. Ann Surg Oncol. 2018 Jan 4; doi: 10.1245/s10434-017-6330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossi CR, Mocellin S, Campana LG, et al. Prediction of Non-sentinel Node Status in Patients with Melanoma and Positive Sentinel Node Biopsy: An Italian Melanoma Intergroup (IMI) Study. Ann Surg Oncol. 2018;25:271–279. doi: 10.1245/s10434-017-6143-5. [DOI] [PubMed] [Google Scholar]