Abstract
Renal denervation (RDN) has been shown to restore endogenous neuronal nitric oxide synthase (nNOS) in the paraventricular nucleus (PVN), and reduce sympathetic drive during chronic heart failure (CHF). The purpose of the present study was to assess the contribution of afferent renal nerves (ARN) to the nNOS mediated sympathetic outflow within the PVN in rats with CHF. CHF was induced in rats by ligation of the left coronary artery. Four weeks after surgery, selective afferent RDN (A-RDN) was performed by bilateral perivascular application of capsaicin on the renal arteries. Seven days after intervention, nNOS protein expression, nNOS immunostaining signaling, and diaphorase positive stained cells were significantly decreased in the PVN of CHF rats, changes that were reversed by A-RDN. A-RDN reduced basal lumbar sympathetic nerve activity (LSNA) in rats with CHF (8.5 ± 0.5 vs 17.0 ± 1.2 % of Max). Microinjection of nNOS inhibitor L-NMMA into the PVN produced a blunted increase in LSNA in rats with CHF. This response was significantly improved after A-RDN (ΔLSNA: 25.7 ± 2.4 vs 11.2 ± 0.9%). Resting ARN activity was substantially increased in CHF compared to sham rats (56.3 ± 2.4 vs 33.0 ± 4.7 %). These results suggest that intact ARN contribute to the reduction of nNOS in the PVN. A-RDN restores nNOS and thus attenuates the sympathoexcitation. Also, resting ARN activity is elevated in CHF rats, which may highlight a crucial neural mechanism arising from the kidney in the maintenance of enhanced sympathetic drive in CHF.
Keywords: renal afferent nerve, sympathetic nerve activity, central nervous system, chronic heart failure, nitric oxide synthase
Introduction
Enhanced sympathetic nerve activity has been identified as a major contributor to the complex pathophysiology of chronic heart failure (CHF) both in experimental models and patients 1, 2. Although most therapeutic pharmaceutical strategies target the peripheral symptoms of the disease, they may not influence the enhanced sympathetic nerve activity. The mechanisms for the enhanced sympathoexcitation observed in CHF are not fully understood. The paraventricular nucleus (PVN) of the hypothalamus is an important site that integrates and responds to a variety of neural and humoral signals regulating sympathetic drive and extracellular fluid volume status 3, 4. In the PVN, PVN neurons projecting to the rostral ventrolateral medulla (RVLM) are more active in rats with CHF suggesting that preautonomic neurons within the PVN are activated and contribute importantly in initiating sympathoexcitation in CHF 5. Our previous studies have also shown that excitatory neurotransmission is enhanced whereas inhibitory neurotransmission via nitric oxide (NO) is reduced in rats with CHF 6–9.
The kidneys are positioned to be the origin as well as target of sympathetic nervous system activation 10. The discharge frequency of putative vasopressinergic magnocellular neurosecretory neurons in the PVN is increased during stimulation of afferent renal nerves (ARN) and during the activation of specific renal receptors 11. Recently we have shown that ARN stimulation activates the PVN neurons projecting to the RVLM 12. Stimulation of ARN also increases sympathetic activity and arterial pressure 12, 13. These observations suggest that afferent information from the kidney is important in the coordination of neural and hormonal activity concerned with body fluid balance and the regulation of arterial blood pressure in normal and disease conditions 14–18. CHF is associated with a number of symptoms that would be expected to stimulate renal afferent activity such as reduced perfusion pressure, increased venous pressure, increased inflammation, increased oxidative stress 19. It is possible that a pathological positive feedback signal from the level of the kidney may exist, thereby causing an increase in overall sympathetic tone.
Renal denervation (RDN) has been shown to reduce arterial pressure and sympathetic outflow in patients with resistant hypertension and various animal models 20–22. Transection of aortic depressor nerve produced a chronic neurogenic hypertension in normotensive rats 23–28, although it failed to raise blood pressure in spontaneously hypertensive rats 29. RDN has been shown to decrease sympathetic activity and arterial pressure in this neurogenic hypertensive animal model 30. Our previous study also shows noradrenergic activity is altered in the hypothalamus after RDN, suggesting that the sympathoinhibitory effects of RDN are mediated centrally at the level of the hypothalamus 31. Our recent study has shown that RDN restores endogenous neuronal NO synthase (nNOS) in the PVN, and at the same time reduce sympathetic drive during CHF 32. However, these studies were based on total RDN, where both afferent and efferent renal nerves were ablated. The present study was conducted to assess the role of afferent renal nerves on altered nNOS within the PVN with concomitant increase in sympathetic outflow in rats with CHF. We hypothesized that the effects of afferent RDN (A-RDN) normalized endogenous nNOS in the PVN as well as restored sympathetic outflow in rats with CHF. Resting ARN activity was substantially increased in CHF compared to sham rats. We proposed that enhanced ARN activity led to the enhanced activation of neurons in the PVN that translated ultimately to the activation of sympathetic nervous system in CHF.
Methods
The authors declare that all supporting data are available within the article and its online supplementary files.
Animals
All procedures used for this study were approved by University of Nebraska Medical Center Institutional Animal Care and Use Committee and conducted according to the National Institutes of Health guide for the Care and Use of Laboratory Animals, Eighth Edition, National Academies Press, 2011. Male Sprague-Dawley rats weighing 220 to 250 g were purchased from Sasco Breeding laboratories (Omaha, NE). Animals were housed with a 12-hour light-dark cycle at ambient 22°C 30–40% relative humidity. Laboratory chow and tap water were available ad libitum. After acclimatization for 1 week, rats were assigned randomly to one of four groups: sham, CHF, sham+capsaicin (CAP), CHF+CAP (n = 12–18/group).
Surgical preparations
Rats were randomly assigned to either a sham-operated control group or a CHF group. CHF was produced by left coronary artery ligation, as previously described 33. Four weeks after ligation surgery, rats underwent afferent RDN (A-RDN) under anesthesia (ketamine, 48 mg/kg; xylazine 12 mg/kg, ip). The experiments were performed at 1 week after A-RDN (5 weeks after the coronary ligation).
Statistical analysis
Data were subjected to a two-way ANOVA followed by a Multiple Range (for multiple comparisons) or Student-Newman Keuls test. P < 0.05 were considered to indicate statistical significance.
Specific Methods
Specific methods are available in the Online-only Data Supplement.
Results
General characteristics
Online supplement Table S1 presents morphological characteristics and left ventricular function parameters among the four experimental groups, sham, CHF, sham+capsaicin (CAP), CHF+CAP. The body weight and heart weight were significantly increased in CHF group compared to the sham group. A-RDN had no significant effects on the body weight and heart weight in sham and CHF groups. CHF rats had > 30% infarcts of the left ventricular wall while sham rats had no visible myocardial damage. A-RDN did not change infarct size in CHF rats. Left ventricular end-diastolic pressure (LVEDP) was significantly increased in the CHF rats compared to both sham groups and CHF+CAP group. Both +dP/dt and –dP/dt were significantly decreased in the both CHF and CHF+CAP rats compared to both sham groups. LVEDP was partially reduced by A-RDN while +dP/dt and –dP/dt were not significantly affected by A-RDN.
Validation of afferent renal denervation by immunohistochemistry and Western blot
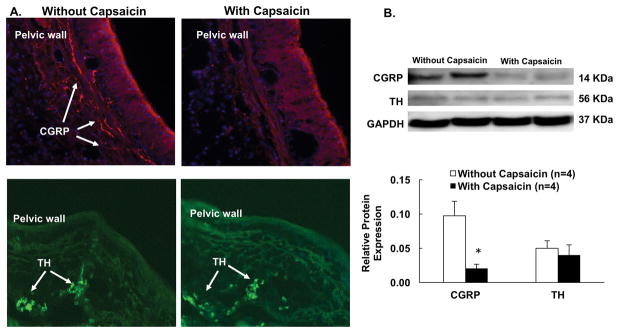
Figure 1A shows the immunohistochemistry images of one kidney without capsaicin and one kidney with capsaicin treatment. The kidney without capsaicin has significant amount of calcitonin gene-related peptide (CGRP) labeling in the renal pelvic wall while the capsaicin treated kidney has little CGRP labeling. Furthermore, Western blot data showed similar pattern for reduction of CGRP protein in the renal pelvic wall in the capsaicin treated kidney. CGRP protein expression was decreased 78% in capsaicin treated rats (Figure 1B) compared to the rats without capsaicin treatment. There was no significant difference in tyrosine hydroxylase (TH) labeling and protein expression in the renal pelvic wall after capsaicin between the groups.
Figure 1.
A. Representative photomicrograph of renal pelvic wall with calcitonin gene-related peptide (CGRP) and tyrosine hydroxylase (TH) immunoreactive staining in the rats without and with capsaicin treatment (magnification, X400). B. Relative CGRP and TH protein expression in the renal pelvic wall in the two groups of rats. Data are presented as mean ± SE. *P < 0.05 vs. Control.
Serum norepinephrine concentration measurements
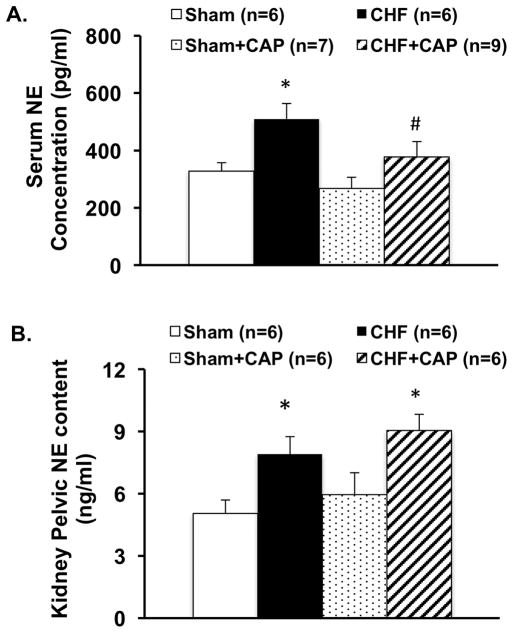
Serum norepinephrine (NE) concentration used as an index of overall sympathetic activation was significantly greater in CHF rats compared to sham operated controls. A-RDN by capsaicin reduced the serum concentration of NE in rats with CHF (377 ± 54 CHF+CAP vs. 509 ± 54 pg/mL CHF, P < 0.05). There was no significant change in the sham rats with A-RDN suggesting that the A-RDN did not change the NE concentration in control conditions (Figure 2A).
Figure 2.
Serum norepinephrine (NE) concentration (A) and renal pelvic NE content in sham and CHF rats with/without capsaicin. Data are presented as mean ± SE. * P < 0.05 vs. sham; # P < 0.05 vs. corresponding group without capsaicin.
Renal pelvic NE content was significantly greater in CHF rats compared to sham operated controls (7.9 ± 0.9 vs. 5.1 ± 0.6 ng/g, P < 0.05). A-RDN has no significant effects on the renal pelvic content of NE in both sham and CHF rats (Figure 2B).
NOS activity (diaphorase staining) and expression of nNOS (immunohistochemistry and protein) in the PVN
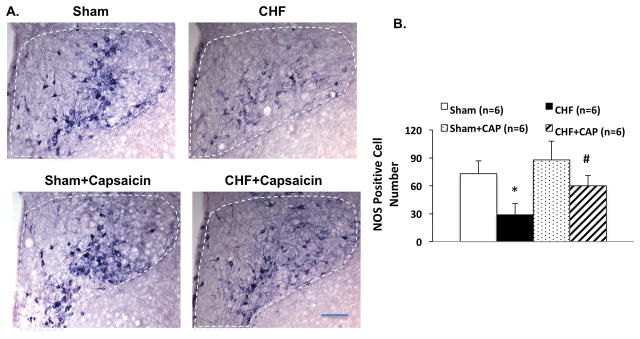
The number of positive cells for the NADPH-diaphorase activity (as a marker of NOS activity) in the PVN was significantly decreased in CHF compared to the sham group (73 ± 14 vs. 29 ± 12, P < 0.05). This reduction in NOS activity was attenuated in the CHF rats after A-RDN (60 ± 11 CHF+CAP vs. 29 ± 12 CHF, P < 0.05) (Figure 3). There was no significant difference in the number of NADPH positive cells in the PVN after A-RDN between the sham and CHF groups.
Figure 3.
The effect of afferent renal denervation (A-RDN) on NADPH-diaphorase in the paraventricular nucleus (PVN) of rats with CHF. Representative photomicrograph of PVN with NADPH diaphorase (nitric oxide synthase; NOS) positive immunohistological staining (A) in four groups of rats, sham, CHF, sham+CAP and CHF+CAP. Bar = 100 μm. (B) Mean values of NOS positive cells in the PVN. Data are presented as mean ± SE. *P < 0.05 vs. sham; #P < 0.05 vs. without capsaicin.
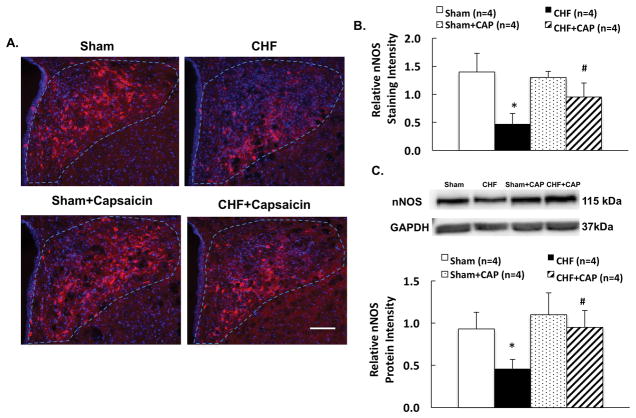
Immunostaining signal for nNOS in the PVN was decreased in CHF compare to the sham group. This reduction in nNOS immunoreactivity was also attenuated in the CHF rats after A-RDN (Figure 4A, 4B). Western blot data showed nNOS protein expression was decreased 51% in CHF that was abrogated by A-RDN (0.95 ± 0.20 CHF+CAP vs. 0.46 ± 0.11 CHF, P < 0.05) (Figure 4C). There was no difference in nNOS protein expression in the PVN after A-RDN between the sham and CHF groups.
Figure 4.
A. Representative photomicrograph of PVN with neuronal NOS (nNOS) staining (A) in four groups of rats, sham, CHF, sham+CAP and CHF+CAP. Bar = 100 μm. B. Summary data of nNOS immunostaining in the sham and CHF rats with/without capsaicin. C. Summary data of nNOS protein expression in the sham and CHF rats with/without capsaicin. Data are presented as mean ± SE. *P < 0.05 vs. sham; #P < 0.05 vs. without capsaicin.
L-NMMA microinjection into the PVN
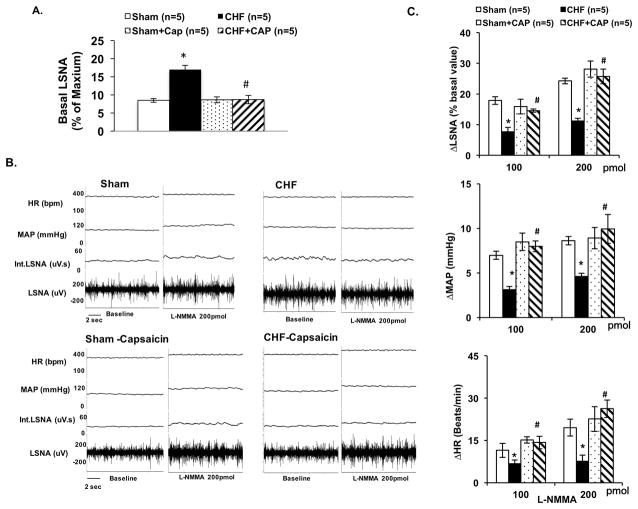
The basal lumbar sympathetic nerve activity (LSNA) was significantly increased in rats with CHF compared to the sham rats (17.0 ± 1.2 vs. 8.5 ± 0.5 % of Max, P < 0.05). A-RDN significantly reduced the basal LSNA in CHF group rats (8.7 ± 1.1 CHF+CAP vs. 17.0 ± 1.2 % of Max CHF, P < 0.05, Figure 5A). Baseline LSNA was normalized by A-RDN. Inhibiting NOS with L-NMMA in the PVN increased LSNA in both sham and CHF groups and shown previously while recording renal sympathetic nerve activity (RSNA) 7. The percentage change in LSNA to L-NMMA injection was blunted in CHF compared with sham (11 ± 2% vs. 24 ± 2% at 200 pmol L-NMMA, P < 0.05, Figures 5B, 5C). Mean arterial pressure (MAP) and heart rate (HR) responses to L-NMMA injection were also attenuated in the CHF condition. After A-RDN, RSNA, MAP and HR responses were all normalized in CHF rats (LSNA: 26 ± 2% CHF+CAP vs. 11 ± 2% CHF at 200 pmol L-NMMA, P < 0.05).
Figure 5.
A. Basal lumbar sympathetic nerve activity (LSNA) in four groups of rats, sham, CHF, sham+CAP, and CHF+CAP. B. Raw tracings of changes in LSNA, mean arterial pressure (MAP) and heart rate (HR) to administration of NOS inhibitor L-NMMA (200 pmol) in the PVN of four groups of rats. C. Summary data for the effect of L-NMMA in the PVN on changes in LSNA, MAP and HR in the four groups of rats. Data are presented as mean ± SE. *P < 0.05 vs. sham; #P < 0.05 vs. without capsaicin.
Resting afferent renal nerve discharge in CHF rats
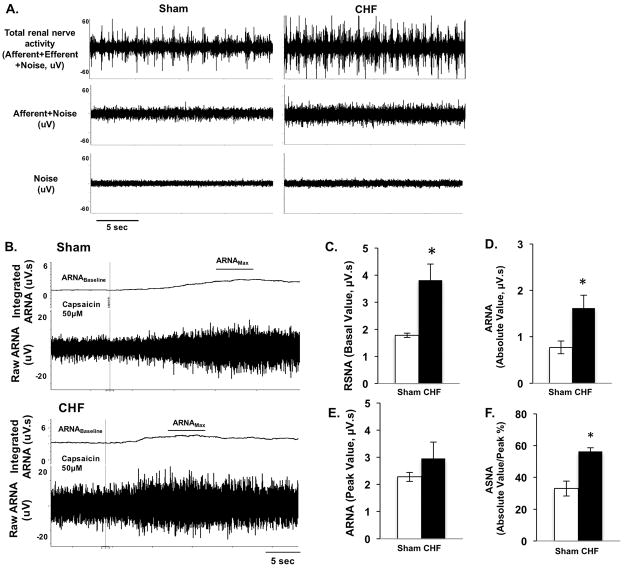
Examples of recordings showing tracing of RSNA, resting afferent renal nerve activity (ARNA) after sectioning of the proximal end of the nerve and background noise, from sham and CHF rats are shown in Figure 6A. The total RSNA was significantly increased in CHF rats compared to the sham rats (3.82 ± 0.59 vs. 1.78 ±0.08 μV.s, P < 0.05, Figure 6C). The resting ARNA was higher in CHF rats after section the proximal end of the renal nerve (1.62 ± 0.27 vs. 0.77 ±0.14 μV.s, P < 0.05, Figure 6D). The peak response to intrapelvic capsaicin was not significantly different between the sham and CHF groups (2.96 ± 0.60 vs. 2.28 ±0.16 μV.s, P > 0.05, Figure 6B, 6E). Final resting ARNA was expressed as percentage of peak ARNA response to intrapelvic capsaicin (%Amax). Resting ARNA was significantly higher in CHF rats compared to the sham rats (56.3 ± 2.4 vs 33.0 ± 4.7 %Amax, P < 0.05, Figure 6F).
Figure 6.
A. Raw tracings of nerve recordings showing, total renal sympathetic nerve activity (RSNA), afferent renal nerve activity (ARNA) and background noise from one sham and one CHF rat. B. Raw tracings of integrated and raw ARNA from one sham and one CHF rats. The ARNA was recorded before and after intrapelvic injection of capsaicin (50 μM) to establish the ARNA baseline and ARNAmax. C–F. Summary data for total RSNA (C), resting ARNA after section the proximal end of renal nerve (D), peak ARNA responses to intrapelvic capsaicin (E) and resting ARNA presented as a percent of ARNAmax (F) in sham and CHF rats, n=5. Data are presented as mean ± SE. *P < 0.05 vs. sham.
Discussion
The present study shows that A-RDN by capsaicin abrogates the increase in lumbar sympathetic outflow and elevated plasma NE in CHF. At the same time A-RDN restored the endogenous nNOS in the PVN that had been decreased in rats with CHF. Consistent with these observations RDN normalized the blunted LSNA response to inhibition of endogenous nNOS within the PVN observed in CHF rats. Furthermore, we found that the resting ARN activity was increased in CHF rats. These results demonstrate a critical role for the PVN in relaying neural signals from the kidney that may be tonically active in the CHF condition, and are abrogated by specific A-RDN. A possible mechanism for the therapeutic effects of A-RDN during CHF on sympathetic outflow may be through an NO-dependent mechanism within the PVN.
Selective ablation of afferent renal nerves
Recently, a novel method of selective ablation of ARN by periaxonal application of capsaicin has been reported 34–36. An adapted technique was performed by exposure of the renal nerves to capsaicin causing ablation of afferent but not efferent renal nerves. Capsaicin ablates small unmyelinated C-fibers and destroys afferent neurons in the kidney 37. Using similar technique, we applied capsaicin to the surface of renal artery and vein to ablate renal afferent nerve in both sham and CHF rats. Seven days after intervention, capsaicin treated rats had significantly depleted levels of an afferent nerve marker CGRP in the renal pelvis, as confirmed by immunohistology and Western blot. Capsaicin application had no effect on renal pelvic content of the efferent nerve markers TH and NE. These results demonstrate and confirm that capsaicin caused A-RDN 34–36 effectively ablating ARN but having no effect on intact efferent renal nerves in rats.
Renal afferent denervation normalizes the sympathoexcitation in CHF
Exaggerated sympathoexcitation is characteristic of both hypertension and CHF. RDN has been shown to reduce arterial pressure and sympathetic activity in experimental forms of hypertension as well as drug resistant hypertensive patients 20, 21, 38. In our previous studies, bilateral surgical RDN known to remove both afferent and efferent renal nerves was sufficient to reduce overall sympathetic outflow, as evident by a reduction in urinary excretion of NE as well as basal level of LSNA 32. The results from the present study show that A-RDN by capsaicin similarly reduced the basal LSNA and serum NE levels in CHF rats while having no significant effects on serum NE levels in the sham rats. A-RDN did not reduce the kidney pelvic NE content in both sham and CHF rats suggesting that the efferent component of the renal nerves was intact after capsaicin treatment. Our results suggest that A-RDN reduces sympathetic outflow by ablation of ARN that does not disrupt efferent renal nerves.
The present data shows that the body weight, whole heart weight and infarct size were significantly increased in the CHF group. A-RDN had no significant effects on these parameters in both sham and CHF groups. However, LVEDP was significantly reduced by A-RDN, while +dP/dt and –dP/dt were not significantly affected by A-RDN. The data confirm that the rats in the CHF groups were experiencing cardiac dysfunction and that A-RDN partially improved left ventricular function by restoring LVEDP. It is possible that the damage induced by coronary artery ligation is fairly severe and prevalent for four weeks, which are difficult to reverse with reduction in sympathetic outflow mediated by A-RDN. Nevertheless, overall these data are consistent with our previous study showing that total renal denervation (ablation of both afferent and efferent renal nerves) improved cardiac function in rats with CHF 39.
PVN and activated renal afferent nerve signal
A number of studies indicate that the renal afferent information is transmitted to sites within the spinal cord that relay information to the central nervous system associated with cardiovascular regulation, including nucleus tractus solitarius, RVLM, preoptic area, subfornical organ, lateral hypothalamus and the PVN in the hypothalamus 16, 18, 40. The PVN is an important site that integrates and responds to a variety of neural and humoral signals regulating sympathetic drive and extracellular fluid volume 3, 41, 42. Previous studies including ours have demonstrated that the discharge frequency of preautonomic neurons in the PVN was increased during stimulation of ARN 11, 32. We have found that electrical stimulation of the ARN activated RVLM projecting PVN neurons to a greater extent in rats with CHF than sham rats 32. These data suggest that afferent signal from the kidneys of CHF rats are more potent than those in sham rats in eliciting activation of preautonomic neurons within the PVN. We also found that basal activity of RVLM projecting PVN neurons is increased in CHF rats, 5 suggesting that perhaps there is a potential tonic activation by the ARN during CHF condition, which may contribute to an increased overall sympathoexcitation under basal conditions.
In order to examine if the renal afferents are activated tonically in the CHF condition, in the present study we directly recorded baseline ongoing renal afferent activity in both Sham and CHF rats. Resting ARN activity, expressed as a percent of peak ARN activity, was substantially increased in CHF compared to the sham rats. This is direct evidence to support the idea that there is enhanced afferent activity from the kidneys of CHF rats compared to the sham rats. There is other evidence to suggest that the diseased kidneys may exert an excitatory effect on sympathetic nerve activity in various pathological conditions involving renal injury, including hypertension, CHF, chronic renal failure, diabetes, and obesity 10, 16, 43–46. Since renal inflammation is prevalent in many of these pathological conditions, it is tempting to speculate that such a signal may contribute to the increased sympathetic outflow via activation of ARN in rats with CHF 16.
Renal afferent denervation improves nNOS expression and functional NOS activity in the PVN during CHF
Nitric oxide, well known to inhibit neuronal activity, is decreased in the PVN during CHF 47, 48. Sympathoexcitatory and hemodynamic responses to endogenous blockade of NO within the PVN were impaired in CHF 7, suggesting that the PVN contributes to the increased sympathoexcitation exhibited in CHF via an altered NO mechanism. Our results show that A-RDN by capsaicin restores the levels of nNOS within the PVN of rats with CHF suggesting that A-RDN reestablishes the endogenous inhibitory nNOS mechanisms in rats with CHF. Further, the levels of diaphorase positive cells are also restored in rats with CHF suggesting that the NOS activity index is normalized after A-RDN. The functional activity of NOS within the PVN of rats with CHF is also reestablished. The improved LSNA responses to microinjection of L-NMMA into the PVN are concomitant with enhanced nNOS levels in the PVN of rats with CHF after A-RDN. The amended central nNOS mechanism/s within the PVN in turn would alleviate the enhanced sympathoexcitation in rats with CHF. Taken together, the improvement in centrally mediated sympathoinhibition by A-RDN appears to be mediated via the nitroxidergic mechanism within the PVN. The source of this signal or its modality appears to be associated with ARN, but the salient characteristics of this signal remain to be identified.
Perspectives
Although RDN has been shown to lower arterial pressure in some humans with resistant hypertension, possibly by reducing overall sympathetic outflow, a similar effect in CHF is not conclusively established. In a safety trial, RDN in patients with CHF showed that there was no significant reduction in systolic pressure but improved 6-min walk distance 49. Further in a pilot study RDN showed a reduction in ventricular tachyarrhythmias in patients with CHF 50. Furthermore, RDN in hypertensive patients with cardiomyopathy have been shown to have reduced left ventricular mass 51, 52 and increased ejection fraction 52. Previously we have demonstrated that RDN in rats with CHF reduces centrally mediated sympathetic tone, specifically by the nNOS mechanism within the PVN 32. However, it is not known whether the inhibitory effect is due to ablation of afferent or efferent renal nerves. One well-known function of the renal nerves is to stimulate renin release from the kidney and renal nerve activity has been reported to be elevated in CHF 38, 53. It is conceivable that this activation of efferent renal nerves may be causally related to increased levels of angiotensin II in CHF. It has also been shown previously that angiotensin II causes an increased activation of the sympathetic activation via the PVN in CHF 54–56. Further, we have observed that angiotensin II may also cause a decrease in levels of nNOS in vitro as well as in the PVN of rats with CHF 57. Consequently, it is conceivable that the changes in the PVN are influenced by the elimination of this angiotensin II component by removal of renal efferent nerves.
Therefore, it is crucially important to address; 1) whether ARN are involved in the sympathoexcitatory state in CHF, 2) if they are involved, whether we can target ARN specifically to optimize the efficacy and reduce the possible side effects of RDN, and 3) whether we can identify specific central sites and/or molecular mechanisms that can be targeted therapeutically. As a first step toward addressing these points, we have selectively ablated the ARN in rats with CHF. This procedure, termed RDN by capsaicin, effectively ablated ARN, and restored nNOS levels within the PVN of rats with CHF and concomitantly reduced enhanced sympathetic tone. These results suggest that ARN may be critically involved in sympathoexcitation of CHF, making ARN both an important subject for future basic science research, as well as a possible target for human CHF. Further studies will be needed to determine the mechanisms underlying this anti-sympathoexcitatory effect, but one likely possibility is restoration of nNOS within the PVN. Understanding the inhibitory factors at the level of the kidney and central mechanisms underlying the reduction in central sympathetic drive may be critical to our understanding of the therapeutic effects of RDN and to the development of more targeted treatments.
Supplementary Material
Novelty and Significance.
What is New?
The present study shows that afferent renal denervation by capsaicin abrogates the increase in sympathetic outflow in chronic heart failure.
Afferent renal denervation restored the endogenous nNOS in the PVN that had been decreased in rats with chronic heart failure.
Renal denervation normalized the blunted lumbar sympathetic nerve activity response to inhibition of endogenous nNOS within the PVN observed in chronic heart failure rats.
Resting afferent renal nerve activity was increased in chronic heart failure rats.
What is Relevant?
Renal denervation has been shown to restore endogenous nNOS in the PVN, and at the same time reduce sympathetic drive during chronic heart failure, however the role of afferent versus efferent renal nerves in this response remains to be determined.
The present study was conducted to assess the role of afferent renal nerves in the nNOS within the PVN mediated sympathetic outflow in rats with chronic heart failure.
Summary
This study suggests that intact afferent renal nerve contribute to the reduction of nNOS in the PVN. Afferent renal denervation restores nNOS and thus attenuates the sympathoexcitation. Resting afferent renal nerve activity is elevated in chronic heart rats, which may highlight a crucial neural mechanism arising from the kidney in the maintenance of enhanced sympathetic drive in chronic heart failure.
Acknowledgments
Sources of funding
This work was supported by NIH grants R56 HL124104, P01 HL62222 and R01 DK114663.
Footnotes
Disclosures
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension. 2009;54:690–7. doi: 10.1161/HYPERTENSIONAHA.108.119883. [DOI] [PubMed] [Google Scholar]
- 2.Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J. 2012;33:1058–66. doi: 10.1093/eurheartj/ehs041. [DOI] [PubMed] [Google Scholar]
- 3.Patel KP. Role of paraventrivular nucleus in mediating sympathetic outflow in heart failure. Heart Fail Rev. 2000;5:73–86. doi: 10.1023/A:1009850224802. [DOI] [PubMed] [Google Scholar]
- 4.Coote JH. A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney. Exp Physiol. 2005;90:169–73. doi: 10.1113/expphysiol.2004.029041. [DOI] [PubMed] [Google Scholar]
- 5.Xu B, Zheng H, Patel KP. Enhanced Activation of RVLM Projecting PVN Neurons in Rats with Chronic Heart Failure. Am J Physiol Heart Circ Physiol. 2012;302:H1700–11. doi: 10.1152/ajpheart.00722.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res. 2003;93:990–7. doi: 10.1161/01.RES.0000102865.60437.55. [DOI] [PubMed] [Google Scholar]
- 7.Zhang K, Li YF, Patel KP. Blunted nitric oxide-mediated inhibition of renal nerve discharge within PVN of rats with heart failure. Am J Physiol Heart Circ Physiol. 2001;281:H995–H1004. doi: 10.1152/ajpheart.2001.281.3.H995. [DOI] [PubMed] [Google Scholar]
- 8.Zheng H, Liu X, Li Y, Sharma NM, Patel KP. Gene transfer of neuronal nitric oxide synthase to the paraventricular nucleus reduces the enhanced glutamatergic tone in rats with chronic heart failure. Hypertension. 2011;58:966–73. doi: 10.1161/HYPERTENSIONAHA.111.176222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng H, Sharma NM, Liu X, Patel KP. Exercise training normalizes enhanced sympathetic activation from the paraventricular nucleus in chronic heart failure: role of angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2012;303:R387–94. doi: 10.1152/ajpregu.00046.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlaich MP, Socratous F, Hennebry S, Eikelis N, Lambert EA, Straznicky N, Esler MD, Lambert GW. Sympathetic activation in chronic renal failure. J Am Soc Nephrol. 2009;20:933–9. doi: 10.1681/ASN.2008040402. [DOI] [PubMed] [Google Scholar]
- 11.Ciriello J. Afferent renal inputs to paraventricular nucleus vasopressin and oxytocin neurosecretory neurons. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1745–R1754. doi: 10.1152/ajpregu.1998.275.6.R1745. [DOI] [PubMed] [Google Scholar]
- 12.Xu B, Zheng H, Liu X, Patel KP. Activation of afferent renal nerves modulates RVLM-projecting PVN neurons. Am J Physiol Heart Circ Physiol. 2015;308:H1103–11. doi: 10.1152/ajpheart.00862.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel KP, Knuepfer M. Effect of afferent renal nerve stimulation on blood pressure and heart rate and noradrenergic activity in conscious rats. J Auton Nerv Syst. 1986;17:121–130. doi: 10.1016/0165-1838(86)90087-1. [DOI] [PubMed] [Google Scholar]
- 14.Solano-Flores LP, Rosas-Arellano MP, Ciriello J. Fos induction in central structures after afferent renal nerve stimulation. Brain Res. 1997;753:102–119. doi: 10.1016/s0006-8993(96)01497-7. [DOI] [PubMed] [Google Scholar]
- 15.Day TA, Ciriello J. Effects of renal receptor activation on neurosecretory vasopressin cells. Am J Physiol. 1987;253:R234–41. doi: 10.1152/ajpregu.1987.253.2.R234. [DOI] [PubMed] [Google Scholar]
- 16.Kopp UC. Role of renal sensory nerves in physiological and pathophysiological conditions. Am J Physiol Regul Integr Comp Physiol. 2015;308:R79–95. doi: 10.1152/ajpregu.00351.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caverson MM, Ciriello J. Contribution of paraventricular nucleus to afferent renal nerve pressor response. Am J Physiol. 1988;254:R531–43. doi: 10.1152/ajpregu.1988.254.3.R531. [DOI] [PubMed] [Google Scholar]
- 18.Ciriello J, Caverson MM. Central organization of afferent renal nerve pathways. Clinical and experimental hypertension Part A, Theory and practice. 1987;9(Suppl 1):33–46. doi: 10.3109/10641968709160162. [DOI] [PubMed] [Google Scholar]
- 19.Booth LC, May CN, Yao ST. The role of the renal afferent and efferent nerve fibers in heart failure. Front Physiol. 2015;6:270. doi: 10.3389/fphys.2015.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kline RL. Renal nerves and experimental hypertension: evidence and controversy. Can J Physiol Pharmacol. 1987;65:1540–1547. doi: 10.1139/y87-243. [DOI] [PubMed] [Google Scholar]
- 21.Krum H, Schlaich MP, Whitbourn R, Sobotka P, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler MD. Catheter-based renal sympathetic denervation for resistant hypertension. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 22.Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013;61:457–64. doi: 10.1161/HYPERTENSIONAHA.111.00194. [DOI] [PubMed] [Google Scholar]
- 23.Fink GD, Bryan WJ, Mann M, Osborn J, Werber A. Continuous blood pressure measurement in rats with aortic baroreceptor deafferentation. Am J Physiol. 1981;241:H268–72. doi: 10.1152/ajpheart.1981.241.2.H268. [DOI] [PubMed] [Google Scholar]
- 24.Schreihofer AM, Sved AF. Use of sinoaortic denervation to study the role of baroreceptors in cardiovascular regulation. Am J Physiol Regul Integr Comp Physiol. 1994;266:R1705–R1710. doi: 10.1152/ajpregu.1994.266.5.R1705. [DOI] [PubMed] [Google Scholar]
- 25.Patel KP, Ciriello J, Kline RL. Noradrenergic mechanisms in brain and peripheral organs after aortic nerve transection. Am J Physiol. 1981;240:H481–H486. doi: 10.1152/ajpheart.1981.240.4.H481. [DOI] [PubMed] [Google Scholar]
- 26.Ito CS, Scher AM. Regulation of arterial blood pressure by aortic baroreceptors in the unanesthetized dog. Circ Res. 1978;42:230–6. doi: 10.1161/01.res.42.2.230. [DOI] [PubMed] [Google Scholar]
- 27.Ito CS, Scher AM. Hypertension following denervation of aortic baroreceptors in unanesthetized dogs. Circ Res. 1979;45:26–34. doi: 10.1161/01.res.45.1.26. [DOI] [PubMed] [Google Scholar]
- 28.Krieger EM. Neurogenic Hypertension in the Rat. Circ Res. 1964;15:511–21. doi: 10.1161/01.res.15.6.511. [DOI] [PubMed] [Google Scholar]
- 29.Takeda K, Hayashi J, Itoh H, Hirata M, Nakata T, Oguro M, Kawasaki S, Sasaki S, Nakagawa M. Transection of aortic depressor nerve fails to raise blood pressure in spontaneously hypertensive rats. Cardiovasc Res. 1989;23:573–6. doi: 10.1093/cvr/23.7.573. [DOI] [PubMed] [Google Scholar]
- 30.Kline RL, Patel KP, Ciriello J, Mercer PF. Effect of renal denervation on arterial pressure in rats with aortic nerve transection. Hypertension. 1983;5:468–475. doi: 10.1161/01.hyp.5.4.468. [DOI] [PubMed] [Google Scholar]
- 31.Patel KP, Kline RL. Influence of renal nerves on noradrenergic responses to changes in arterial pressure. Am J Physiol. 1984;247:R615–R620. doi: 10.1152/ajpregu.1984.247.4.R615. [DOI] [PubMed] [Google Scholar]
- 32.Patel KP, Xu B, Liu X, Sharma NM, Zheng H. Renal Denervation Improves Exaggerated Sympathoexcitation in Rats With Heart Failure: A Role for Neuronal Nitric Oxide Synthase in the Paraventricular Nucleus. Hypertension. 2016;68:175–84. doi: 10.1161/HYPERTENSIONAHA.115.06794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kleiber AC, Zheng H, Schultz HD, Peuler JD, Patel KP. Exercise training normalizes enhanced glutamate-mediated sympathetic activation from the PVN in heart failure. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1863–72. doi: 10.1152/ajpregu.00757.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foss JD, Wainford RD, Engeland WC, Fink GD, Osborn JW. A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. Am J Physiol Regul Integr Comp Physiol. 2015;308:R112–22. doi: 10.1152/ajpregu.00427.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foss JD, Fink GD, Osborn JW. Differential role of afferent and efferent renal nerves in the maintenance of early- and late-phase Dahl S hypertension. Am J Physiol Regul Integr Comp Physiol. 2016;310:R262–7. doi: 10.1152/ajpregu.00408.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, Shimizu Y, Osborn JW. Resting Afferent Renal Nerve Discharge and Renal Inflammation: Elucidating the Role of Afferent and Efferent Renal Nerves in Deoxycorticosterone Acetate Salt Hypertension. Hypertension. 2016;68:1415–1423. doi: 10.1161/HYPERTENSIONAHA.116.07850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- 38.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 39.Zheng H, Liu X, Sharma NM, Patel KP. Renal denervation improves cardiac function in rats with heart failure: effects on expression of beta-adrenoceptors. Am J Physiol Heart Circ Physiol. 2016;311:H337–46. doi: 10.1152/ajpheart.00999.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuo DC, Nadelhaft I, Hisamitsu T, de Groat WC. Segmental distribution and central projections of renal afferent fibers in the cat studied by transganglionic transport of horseradish peroxidase. J Comp Neurol. 1983;216:162–74. doi: 10.1002/cne.902160205. [DOI] [PubMed] [Google Scholar]
- 41.Li YF, Mayhan WG, Patel KP. Role of the paraventricular nucleus in renal excretory responses to acute volume expansion: role of nitric oxide. Am J Physiol Heart Circ Physiol. 2003;285:H1738–46. doi: 10.1152/ajpheart.00727.2002. [DOI] [PubMed] [Google Scholar]
- 42.Patel KP, Zheng H. Central neural control of sympathetic nerve activity in heart failure following exercise training. Am J Physiol Heart Circ Physiol. 2012;302:H527–37. doi: 10.1152/ajpheart.00676.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giamouzis G, Butler J, Triposkiadis F. Renal function in advanced heart failure. Congest Heart Fail. 2011;17:180–8. doi: 10.1111/j.1751-7133.2011.00240.x. [DOI] [PubMed] [Google Scholar]
- 44.Henegar JR, Zhang Y, De Rama R, Hata C, Hall ME, Hall JE. Catheter-based radiorefrequency renal denervation lowers blood pressure in obese hypertensive dogs. Am J Hypertens. 2014;27:1285–92. doi: 10.1093/ajh/hpu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linz D, Hohl M, Schutze J, Mahfoud F, Speer T, Linz B, Hubschle T, Juretschke HP, Dechend R, Geisel J, Rutten H, Bohm M. Progression of kidney injury and cardiac remodeling in obese spontaneously hypertensive rats: the role of renal sympathetic innervation. Am J Hypertens. 2015;28:256–65. doi: 10.1093/ajh/hpu123. [DOI] [PubMed] [Google Scholar]
- 46.Ott C, Mahfoud F, Schmid A, Ditting T, Veelken R, Ewen S, Ukena C, Uder M, Bohm M, Schmieder RE. Improvement of albuminuria after renal denervation. Int J Cardiol. 2014;173:311–5. doi: 10.1016/j.ijcard.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 47.Zhang K, Zucker IH, Patel KP. Altered number of diaphorase (NOS) positive neurons in the hypothalamus of rats with heart failure. Brain Res. 1998;786:219–225. doi: 10.1016/s0006-8993(97)01449-2. [DOI] [PubMed] [Google Scholar]
- 48.Patel KP, Zhang K, Zucker IH, Krukoff TL. Decreased gene expression of neuronal nitric oxide synthase in hypothalamus and brainstem of rats in heart failure. Brain Res. 1996;734:109–115. [PubMed] [Google Scholar]
- 49.Davies JE, Manisty CH, Petraco R, Barron AJ, Unsworth B, Mayet J, Hamady M, Hughes AD, Sever PS, Sobotka PA, Francis DP. First-in-man safety evaluation of renal denervation for chronic systolic heart failure: primary outcome from REACH-Pilot study. Int J Cardiol. 2013;162:189–92. doi: 10.1016/j.ijcard.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 50.Ukena C, Bauer A, Mahfoud F, Schreieck J, Neuberger HR, Eick C, Sobotka PA, Gawaz M, Bohm M. Renal sympathetic denervation for treatment of electrical storm: first-in-man experience. Clin Res Cardiol. 2012;101:63–7. doi: 10.1007/s00392-011-0365-5. [DOI] [PubMed] [Google Scholar]
- 51.Doltra A, Messroghli D, Stawowy P, Hassel JH, Gebker R, Leppanen O, Grafe M, Schneeweis C, Schnackenburg B, Fleck E, Kelle S. Potential reduction of interstitial myocardial fibrosis with renal denervation. J Am Heart Assoc. 2014;3:e001353. doi: 10.1161/JAHA.114.001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahfoud F, Urban D, Teller D, Linz D, Stawowy P, Hassel JH, Fries P, Dreysse S, Wellnhofer E, Schneider G, Buecker A, Schneeweis C, Doltra A, Schlaich MP, Esler MD, Fleck E, Bohm M, Kelle S. Effect of renal denervation on left ventricular mass and function in patients with resistant hypertension: data from a multi-centre cardiovascular magnetic resonance imaging trial. Eur Heart J. 2014;35:2224–31b. doi: 10.1093/eurheartj/ehu093. [DOI] [PubMed] [Google Scholar]
- 53.Patel KP, Zhang K, Carmines PK. Norepinephrine turnover in peripheral tissues of rats with heart failure. Am J Physiol Regul Integr Comp Physiol. 2000;278:R556–R562. doi: 10.1152/ajpregu.2000.278.3.R556. [DOI] [PubMed] [Google Scholar]
- 54.Zheng H, Li YF, Wang W, Patel KP. Enhanced angiotensin-mediated excitation of renal sympathetic nerve activity within the paraventricular nucleus of anesthetized rats with heart failure. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1364–74. doi: 10.1152/ajpregu.00149.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang ZH, Francis J, Weiss RM, Felder RB. The renin-angiotensin-aldosterone system excites hypothalamic paraventricular nucleus neurons in heart failure. Am J Physiol Heart Circ Physiol. 2002;283:H423–33. doi: 10.1152/ajpheart.00685.2001. [DOI] [PubMed] [Google Scholar]
- 56.Huang BS, Zheng H, Tan J, Patel KP, Leenen FH. Regulation of hypothalamic renin-angiotensin system and oxidative stress by aldosterone. Exp Physiol. 2011;96:1028–38. doi: 10.1113/expphysiol.2011.059840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma NM, Zheng H, Mehtam PP, Li YF, Patel KP. Decreased nNOS in the PVN leads to increased sympathoexcitation in chronic heart failure: role for CAPON and Ang II. Cardivasc Res. 2011;92:348–57. doi: 10.1093/cvr217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.