Abstract
Background:
Black race compared to white race is associated with more advanced stage and biologically aggressive breast cancer. Consequently, black patients are more frequently treated with neoadjuvant chemotherapy (NAC) than white patients. However, it is unclear how distant recurrence-free survival (DRFS) of black patients treated with NAC, compares to DRFS of black patients treated with adjuvant chemotherapy (AC). We evaluated the association between race, distant recurrence, and type of chemotherapy (AC or NAC) in localized or locally advanced breast cancer.
Patients and Methods:
We evaluated DRFS in 807 patients, including 473 black, 252 white, 56 Hispanic, and 26 women of other or mixed race. The association between AC or NAC and DRFS was examined using multivariate Cox proportional hazard models that included race, age, stage, estrogen receptor (ER) and triple negative (TN) status.
Results:
When the black and white subjects were pooled for the analysis the features associated with worse DRFS included stage III disease and age<50 years, but not ER-negative disease, TN disease, the use of NAC, or black race. However, in the analysis stratified by race NAC was associated with worse DRFS compared to AC in black (HR=2.70; 95% CI=1.73–4.22; p<0.0001), but not in white women (HR=1.29, 95% CI=0.56–2.95; p=0.36).
Conclusion:
Black patients treated with NAC had worse DRFS than black patients treated with AC, or white patients treated with either NAC or AC. These findings need to be validated in a large-scale observational study and the effect of NAC on the breast cancer microenvironment in black women needs to be further evaluated.
Keywords: breast cancer, distant recurrence, black patients, neoadjuvant chemotherapy, adjuvant chemotherapy
Introduction
Black women with operable breast cancer have higher recurrence and mortality rates than white women [1]. This has been attributed to more advanced stage at diagnosis [2], higher rates of ER-negative (ER-) and/or triple-negative (TN) disease [3–5], lower socioeconomic status [6], more comorbidities [7], and higher rates of toxicity due to therapy [8]. However, black women have lower overall survival and cancer-specific survival compared to white women when treated with systemic and local therapy, even after controlling for demographic and prognostic tumor variables [9]. Some reports have also indicated that black women treated with neoadjuvant chemotherapy (NAC) have higher recurrence rates and breast cancer mortality than white women treated with NAC [10], while others did not find a difference [11].
Since black women typically present with more advanced stage and more aggressive ER- disease than white women [10], they are treated with NAC more frequently than white women [12] because NAC decreases tumor burden and improves surgical outcome [13]. Large prospective randomized studies of distant recurrence free survival (DRFS) and overall survival (OS) in predominantly white patients with localized breast cancer have not shown differences between those treated with NAC compared to those treated with adjuvant chemotherapy (AC) [14,15]. However, data comparing DRFS in black women treated with AC versus black women treated with NAC are currently not available. Since breast cancer behaves more aggressively in blacks than in whites, and blacks are more commonly treated with NAC, it is important to investigate how DRFS in black patients treated with NAC compares to DRFS of black patients treated with AC and patients of other racial background treated with and AC or NAC. To address this question we performed a retrospective study to evaluate the association between DRFS and type of chemotherapy (AC versus NAC) in a multiracial cohort treated at an academic medical center in which patients received multidisciplinary care.
Methods
Data collection
The research protocol was approved by the Einstein/Montefiore institutional review board. Patient data were obtained from Clinical Looking Glass (CLG, http://exploreclg.montefiore.org/), an interactive software application developed at Montefiore Medical Center that integrates demographic, clinical, and administrative data sets, and which additionally allows for data extraction in a programmable format for statistical assessment [16].
We manually evaluated charts from all 214 patients treated with NAC to obtain clinical stage. Use of clinical stage, as opposed to pathological stage, was essential for multivariate analysis to avoid chemotherapy-induced down-staging that might have occurred in NAC treated patients if pathological stage was used.
Patient selection
The study was conducted in a cohort of 807 women (473 black, 252 white, 56 Hispanic and 26 others) with a first diagnosis of invasive breast cancer made between 2000 and 2016 at Montefiore Medical Center in the Bronx, NY who received either NAC or AC for non-metastatic breast cancer. The cohort only included patients with stage IIA to IIIC at presentation.
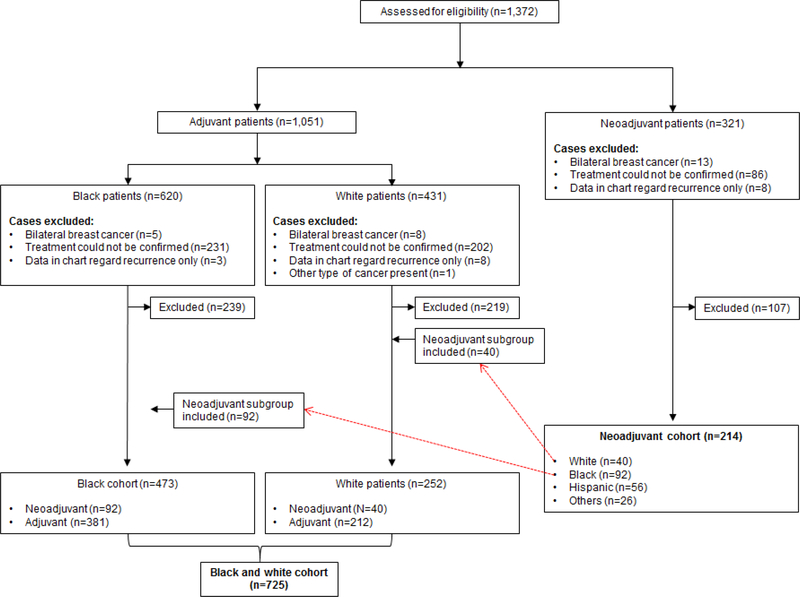
The exclusion criteria included: bilateral breast cancer, unclear record of the chemotherapy schedule in relationship to surgery, and insufficient data regarding their initial breast cancer diagnosis and treatment, and multiple cancers (Figure 1). The patients were grouped in 2 cohorts: 1) Black and white cohort (N=725), consisting of only black and white patients treated with either NAC or AC, and 2) Neoadjuvant cohort (N=214), consisting of only patients treated with NAC, as indicated in the consort diagram (Figure 1).
Figure 1.
Consort diagram.
Statistical Analysis
Bivariate comparisons in age, stage, ER, PR, HER2, and TN status were made between NAC and AC groups within white and black patients separately using a chi-square test, except for the continuous age where a two-sampled t-test was used. Distant recurrence-free survival (defined as a metastasis at a distant organ) was the end-point measurement used in this analysis. Kaplan-Meier (KM) survival curves and log-rank tests were used to compare DRFS between NAC and AC combined, as well as separate, for black and white patients. A multivariate Cox model was used to examine the effect of NAC vs AC on DRFS separately for blacks and whites, while adjusting for patient age, stage of tumor, and hormonal receptor status (including ER, PR, HER2, and TN). PR and HER2 were later removed from the model because of non-statistical significance. A formal comparison of the effect of NAC vs AC between black and white was made by combining the whites and blacks together and examining the interaction between NAC and race.
We used a propensity score approach to further examine if our results were subject to bias due to potential confounding that led to imbalanced groups between the NAC and AC groups. Specifically, separately for blacks and whites, a logistic regression model was used to model on the use of NAC versus AC treatment with patients’ age and tumor characteristics including tumor size, lymph node status, stage, ER, PR, and Her2 status as variables in the model. Then an inverse probability weighting method (IPW) was used to incorporate the propensity score into the multivariate Cox models and the robust variance was used to account for the weighting [17,18]. We did not use propensity score matching here because of the limited sample size. The IPW method to adjust for propensity has been widely adopted to control for potential bias induced by self-selected exposure in observational studies.
All the p-values were reported as two-sided. All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC 2014).
Results
Black and White Cohort
Demographic and tumor characteristics for the “Black and white” cohort (black, n=473; white, n=252) treated with either NAC or AC are shown in the Table 1. Compared to patients treated with AC, white patients treated with NAC were significantly more likely to have ER- (p=0.01), PR- (p<0.0001), and TN disease (p=0.02), whereas black patients treated with NAC were significantly more likely to be <50 years old (p=0.007) and have stage III disease (p<0.0001), as well as ER- (p=0.003), PR- (p=0.005), and TN disease (p=0.0002). Six out of 92 (6.2%) black and 3 out of 40 (7.5%) white patients treated with NAC in this cohort achieved pathologic complete response evidenced by absence of tumor cells in the breast and lymph nodes. Pooled data for the entire cohort are shown in the Table 2.
Table 1.
Black and white patient cohort: patient and tumor characteristics
| White patients |
Black patients |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Neoadjuvant N (%) | Adjuvant N (%) | Total | Chi-Square (p-value) | Neoadjuvant N (%) | Adjuvant N (%) | Total | Chi-Square (p-value) |
| All | 40 (15.87%) | 212 (84.13%) | 252 | 92 (19.45%) | 381 (80.55%) | 473 | ||
| Age | 0.92 | 0.007 | ||||||
| <50 | 11 (27.50%) | 60 (28.30%) | 71 | 45 (48.91%) | 129 (33.86%) | 174 | ||
| >50 | 29 (72.50%) | 152 (71.70%) | 181 | 47 (51.09%) | 252 (66.14%) | 299 | ||
| Stage* | 0.68 | <0.0001 | ||||||
| II | 27 (67.50%) | 150 (70.75%) | 177 | 50 (54.35%) | 287 (75.33%) | 337 | ||
| III | 13 (32.50%) | 62 (29.25%) | 75 | 42 (45.65%) | 94 (24.67%) | 136 | ||
| ER status | 0.01 | 0.0003 | ||||||
| Negative | 15 (37.50%) | 41 (19.34%) | 56 | 53 (57.61%) | 140 (36.75%) | 193 | ||
| Positive | 25 (62.50%) | 171 (80.66%) | 196 | 39 (42.39%) | 241 (63.25%) | 280 | ||
| PR status1 | <0.0001 | 0.005 | ||||||
| Negative | 25 (62.50%) | 68 (32.08%) | 93 | 60 (66.67%) | 190 (50.26%) | 250 | ||
| Positive | 15 (37.50%) | 144 (67.92%) | 159 | 30 (33.33%) | 188 (49.74%) | 218 | ||
| HER2 status2 | <0.0001 | 0.09 | ||||||
| Equivocal | 4 (10.00%) | 0 (0.00%) | 4 | 1 (1.14%) | 0 (0.00%) | 1 | ||
| Negative | 28 (70.00%) | 170 (80.19%) | 198 | 71 (80.86%) | 281 (77.41%) | 352 | ||
| Positive | 8 (20.00%) | 42 (19.81%) | 50 | 16 (18.18%) | 82 (22.59%) | 98 | ||
| Triple-negative status3 | 0.02 | 0.0002 | ||||||
| No | 28 (70.00%) | 181 (85.38%) | 209 | 46 (57.27%) | 262 (72.58%) | 308 | ||
| Yes | 12 (30.00%) | 31 (14.62%) | 43 | 42 (47.73%) | 99 (27.42%) | 141 | ||
Clinical stage was used for neoadjuvant and pathological stage for adjuvant cohort.
PR status missing from 8 patients: Blacks/Neoadjuvant, N=90; Blacks/Adjuvant, N=378.
HER2 status missing from 25 patients: Blacks/Neoadjuvant, N=88; Blacks/Adjuvant, N=363.
Triple-negative status missing from 27 patients: Blacks/Neoadjuvant, N=88; Black/Adjuvant 361.
ER = estrogen receptor; PR = progesterone receptor; HER2 = Human Epidermal Growth Factor Receptor 2
Table 2.
Black and white patient cohort: patient and tumor characteristics
| Black and white cohort pooled
analysis | ||||
|---|---|---|---|---|
| Characteristic | Neoadjuvant N (%) | Adjuvant N (%) | Total | Chi-Square (p-value) |
| All | 132 (18.21%) | 593 (81.79%) | 725 | |
| Age | ||||
| <50 | 56 (42.42%) | 189 (31.87%) | 245 | 0.02 |
| >50 | 76 (57.58%) | 404 (68.13%) | 480 | |
| Stage* | ||||
| II | 77 (58.33%) | 437 (73.69%) | 514 | 0.0004 |
| III | 55 (41.67%) | 156 (26.31%) | 211 | |
| ER status | ||||
| Negative | 68 (51.52%) | 181 (30.52%) | 249 | <0.0001 |
| Positive | 64 (48.48%) | 412 (69.48%) | 476 | |
| PR status1 | ||||
| Negative | 85 (65.38%) | 258 (43.73%) | 343 | <0.0001 |
| Positive | 45 (34.62%) | 332 (56.27%) | 377 | |
| HER2 status2 | ||||
| Equivocal | 5 (3.91%) | 0 (0.00%) | 5 | <0.0001 |
| Negative | 99 (77.34%) | 451 (78.43%) | 550 | |
| Positive | 24 (18.75%) | 124 (21.57%) | 186 | |
| Triple-negative status3 | ||||
| No | 74 (57.81%) | 443 (77.31%) | 517 | <0.0001 |
| Yes | 54 (42.19%) | 130 (22.69%) | 184 | |
Clinical stage was used for neoadjuvant and pathological stage for adjuvant cohort.
PR status missing from 4 patients: Neoadjuvant, N=131; Adjuvant, N=590.
HER2 status missing from 22 patients: Neoadjuvant, N=128; Adjuvant, N=575.
Triple-negative status missing from 24 patients: Neoadjuvant, N=128; Adjuvant, N=573
ER = estrogen receptor; PR = progesterone receptor; HER2 = Human Epidermal Growth Factor Receptor 2
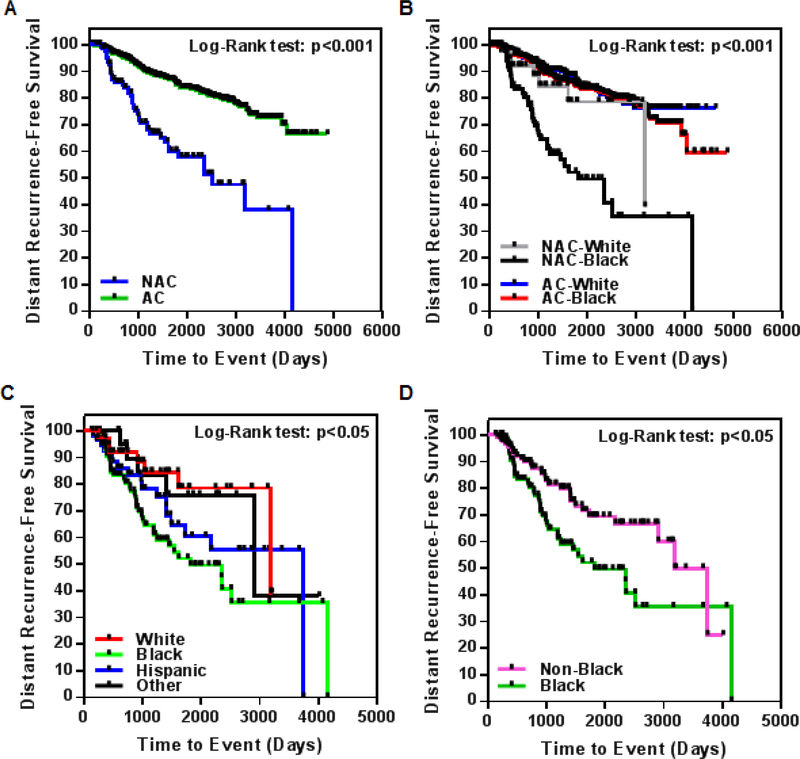
The Kaplan-Meier curve shows that in this cohort, composed of predominantly back patients (65% blacks, 35% whites), NAC-treated patients (70% blacks, 30% whites) have lower DRFS than AC-treated patients (64% blacks, 36% whites) (Figure 2A; p<0.001). When the stratification included not only treatment scheme (AC/NAC), but also race (Figure 2B), the Kaplan-Meier curve for DRFS showed that black patients receiving NAC have significantly lower DRFS rates compared to white patients receiving either AC or NAC, or black patients receiving AC (p<0.001 for all groups).
Figure 2.
(A-B) Kaplan-Meier curves demonstrating distant recurrence-free survival (DRFS) in black and white patients treated with either neoadjuvant (NAC) or adjuvant (AC) chemotherapy (A, non-stratified analysis; B, analysis stratified according to race and treatment). (C-D) KaplanMeier curves demonstrating DRFS in patients of various racial ethnicities, all treated with NAC. (C, race and ethnicity separated; D, DRFS for black vs. non-black races and ethnicities pooled).
Multivariate analysis of DRFS adjusted for race, age, stage, tumor size, ER, and triplenegative status for black and white patients combined is shown in Table 3 (top panel). Factors associated with significantly worse DRFS included stage III disease (p<0.0001; HR=4.43, 95% CI=3.12–6.37) and age <50 years (p<0.001; HR=1.78, 95% CI=1.26–2.53), but not black race (p=0.76), the use of NAC (p=0.36), ER-negative disease (p=0.17), or TN disease (p=0.23). However, NAC was associated with worse DRFS in black (HR=2.54, 95% CI=1.64–3.93) but not white (HR=1.46, 95% CI=0.63–3.33) patients. We used the inverse probability weighting (IPW), as an additional statistical method to control for baseline imbalance [17,18] and confirmed that NAC was associated with worse DRFS in black (HR = 3.61, 95%CI = 1.59, 8.20, p = 0.002 ), but not in white (HR = 0.72, 95CI = 0.04, 13.5, p = 0.82) women with breast cancer (Supplementary Tables 1a and 1b). Although we identified a much larger treatment effect of NAC in blacks as compared to whites, we failed to detect a significant interaction between race and treatment, mostly likely due to the limited sample size (p=0.24).
Table 3.
Multivariate analysis of DRFS in the Black and white patient cohort
| Pooled (non-stratified) analysis
(n=725, 100%) | ||
|---|---|---|
| Characteristic | p-value | Hazard ratio (95% CI) |
| Black1 | 0.76 | NAC:1.86 (0.82–4.22) AC: 1.07 (0.70–1.65) |
| Neoadjuvant | 0.36 | Black: 2.54
(1.64–3.93) White: 1.46 (0.64–3.33) |
| Race* Treatment interaction | 0.24 | - |
| Stage III* | <0.0001 | 4.46 (3.12–6.37) |
| Age <50 | 0.001 | 1.78 (1.26–2.53) |
| ER- | 0.17 | 1.50 (0.84–2.69) |
| Triple-negative | 0.23 | 1.44 (0.79–2.63) |
| Stratified-by-race
analysis | ||||
|---|---|---|---|---|
| White patients
(n=252, 35%) |
Black patients
(n=473, 65%) |
|||
| Characteristic | p-value | Hazard ratio | p-value | Hazard ratio |
| Neoadjuvant | 0.36 | 1.29 (0.56–2.95) | <0.0001 | 2.7 (1.73–4.22) |
| Stage III* | <0.0001 | 7.60(3.77–15.31) | <0.0001 | 3.66 (2.38–5.55) |
| Age <50 | 0.03 | 2.04 (1.10–3.92) | 0.02 | 1.63 (1.08–2.47) |
| ER− | 0.05 | 2.95 (1.02–8.56) | 0.70 | 1.14 (0.58–2.28) |
| Triple-negative | 0.68 | 1.28 (0.40–4.11) | 0.24 | 1.53 (0.76–3.09) |
Clinical stage was used for neoadjuvant and pathological stage for adjuvant cohort.
The reference race is white.
AC = adjuvant chemotherapy; NAC = neoadjuvant chemotherapy; DRFS = distant recurrence-free survival; ER = estrogen receptor; CI = confidence interval
In a stratified analysis (Table 3; bottom panel), stage and age showed the same trends as in the combined analysis for white and black patients independently. However, NAC was an independent indicator of poor prognosis in blacks (p<0.0001; HR=2.7, 95% CI 1.73–4.22), but not in whites (p=0.36; HR=1.29, 95% CI=0.56–2.95).
Neoadjuvant cohort
We also evaluated the characteristics and DRFS in a multiracial and multiethnic cohort of 214 patients consisting of 132 patients treated with NAC described above (40 white, 92 black), and an additional 82 patients (56 Hispanics, and 26 races other than black or white; Table 4). In this cohort, black race was associated with significantly higher rates of ER- (p=0.008) and TN disease (p=0.003) for the four race categories, but no significant differences (p>0.05) were found in patient age, stage, PR, HER2, status at the time of diagnosis.
Table 4.
Neoadjuvant cohort: patient and tumor characteristics
| Characteristic | Black N (%) | White N (%) | Hispanic N (%) | Other N (%) | Total | Chi-square (p-value) |
|---|---|---|---|---|---|---|
| All | 92 (42.99%) | 40(18.69%) | 56 (26.17%) | 26(12.15%) | 214 | |
| Age | ||||||
| <50 | 45 (48.91%) | 11 (27.50%) | 25 (44.64%) | 11 (42.31%) | 92 | 0.15 |
| >50 | 47(51.09%) | 29 (72.50%) | 31 (55.36%) | 15(57.69%) | 122 | |
| Clinical stage | ||||||
| I, II | 50 (54.35%) | 27 (67.50%) | 28 (50.00%) | 15(57.69%) | 120 | 0.38 |
| III | 42 (45.65%) | 13 (32.50%) | 28 (50.00%) | 11 (42.31%) | 94 | |
| ER status1 | ||||||
| Negative | 53 (57.61%) | 15 (37.50%) | 16(30.19%) | 10(40.00%) | 94 | 0.008 |
| Positive | 39 (42.39%) | 25 (62.50%) | 37 (69.81%) | 15(60.00%) | 116 | |
| PR status2 | ||||||
| Negative | 60 (66.67%) | 25 (62.50%) | 24 (48.00%) | 15(60.00%) | 124 | 0.19 |
| Positive | 30 (33.33%) | 15 (37.50%) | 26 (52.00%) | 10(40.00%) | 81 | |
| HER2 status3 | ||||||
| Equivocal | 1 (1.14%) | 4(10.00%) | 2 (3.85%) | 1 (4.00%) | 8 | 0.20 |
| Negative | 71 (80.68%) | 28 (70.00%) | 37 (71.15%) | 16(64.00%) | 152 | |
| Positive | 16(18.18%) | 8 (20.00%) | 13(25.00%) | 8 (32.00%) | 45 | |
| Triple-negative status4 | 0.003 | |||||
| No | 46 (52.27%) | 28 (70.00%) | 40 (80.00%) | 20 (80.00%) | 134 | |
| Yes | 42 (47.73%) | 12 (30.00%) | 10(20.00%) | 5 (20.00%) | 69 |
ER status missing from 4 patients: Hispanics, N=53; Others, N=25.
PR status missing from 9 patients: Blacks, N=90; Hispanics, N=50; Others, N=25.
HER2 status missing from 9 patients: Blacks, N=88; Hispanics, N=52; Others, N=25.
Triple-negative status missing from 11 patients: Blacks, N=88; Hispanics, N=50; Others, N=25
ER = estrogen receptor; PR = progesterone receptor; HER2 = Human Epidermal Growth Factor Recept
The Kaplan-Meier curve for DRFS stratified by race for patients treated with NAC is presented in Figure 2C. As shown, black patients have lower DRFS than Hispanic, white, and other races (p<0.05 for all groups).
Multivariate analysis of DRFS adjusted for race, age, stage, ER status, and TN status indicates that stage III disease is an independent indicator of worse DRFS in patients receiving NAC (Table 5. top panel; p<0.0001; HR=3.09, 95% CI=1.77–5.40). Also, black patients receiving NAC have worse DRFS rates when compared to white patients (p=0.08; HR=2.09, 95% CI=0.91–4.79), but no such difference is observed for Hispanics (p=0.33; HR=1.57, 95% CI=0.63–3.94) or patients grouped as other races (p=0.95; HR=0.99, CI=0.29–3.16).
Table 5.
Multivariate analysis of DRFS in the Neoadjuvant cohort (non-black patients separated)
| Characteristic | p-value | Hazard ratio |
|---|---|---|
| Black1 | 0.08 | 2.09 (0.91–4.79) |
| Hispanic | 0.33 | 1.57 (0.63–3.94) |
| Other | 0.95 | 0.99 (0.29–3.16) |
| Clinical stage III | <0.0001 | 3.09 (1.77–5.40) |
| Age <50 | 0.78 | 1.08 (0.65–1.79) |
| ER− | 0.32 | 1.51 (0.67–3.41) |
| Triple-negative | 0.27 | 1.60 (0.70–3.68) |
| Multivariate analysis of DRFS
in the Neoadjuvant cohort (non-black patients pooled together) | ||
|---|---|---|
| Characteristic | p-value | Hazard ratio |
| Black2 | 0.05 | 1.68 (1.00–2.85) |
| Clinical stage III | <0.0001 | 3.18 (1.83–5.54) |
| Age <50 | 0.68 | 1.11 (0.67–1.84) |
| ER− | 0.36 | 1.45 (0.65–2.23) |
| Triple-negative | 0.23 | 1.65 (0.73–3.73) |
The reference race is white.
The reference race is non-black.
DRFS = distant recurrence-free survival; ER = estrogen receptor
All the aforementioned analyses were additionally performed after pooling all patients other than black into a single category, designated as “non-black” (Table 6). Black patients receiving NAC have lower DRFS than non-black patients, as shown through multivariate analysis (Table 5, bottom panel; p=0.05, HR=1.68, 95% CI=1.00–2.85) and the corresponding Kaplan-Meier curve (Figure 2D; p<0.01; median survival: black, 1820 days; non-black, 3748 days).
Table 6.
Neoadjuvant cohort: patient and tumor characteristics (all non-black patients pooled as one group)
| Characteristic | Black N (%) | Non-Black N (%) | Total | Chi-square test (p-value) |
|---|---|---|---|---|
| All | 92 (42.99%) | 122 (57.01%) | 214 | |
| Age | 0.13 | |||
| <50 | 45 (48.91%) | 47 (38.52%) | 92 | |
| >50 | 47 (51.09%) | 75 (61.48%) | 122 | |
| Clinical stage | 0.66 | |||
| I,II | 50 (54.35%) | 70 (57.38%) | 120 | |
| III | 42 (45.65%) | 52 (42.62%) | 94 | |
| ER status | 0.0009 | |||
| Negative | 53 (57.61%) | 41 (34.75%) | 94 | |
| Positive | 39 (42.39%) | 77 (65.25%) | 116 | |
| PR status1 | 0.11 | |||
| Negative | 60 (66.67%) | 64 (55.65%) | 124 | |
| Positive | 30 (33.33%) | 51 (44.35%) | 81 | |
| HER2 status2 | 0.09 | |||
| Equivocal | 1 (1.14%) | 7 (5.98%) | 8 | |
| Negative | 71 (80.68%) | 81 (69.23%) | 152 | |
| Positive | 16 (18.18%) | 29 (24.79%) | 45 | |
| Triple-negative status3 | 0.003 | |||
| No | 46 (52.27%) | 88 (76.52%) | 134 | |
| Yes | 42 (47.73%) | 27 (23.48%) | 69 |
PR status missing from 9 patients: Blacks, N=90; Non-blacks, N=115.
HER2 status missing from 9 patients: Blacks, N=88; Non-blacks, N=117.
Triple-negative status missing from 11 patients: Blacks, N=88; Non-blacks, N=115.
ER = estrogen receptor; PR = progesterone receptor; HER2 = Human Epidermal Growth Factor Receptor 2
Treatment considerations
There was no difference between black and white subjects in regards to taxanecontaining versus non-taxane chemotherapy (p=0.4). However, chemotherapy was more often combined with endocrine therapy in white than in black patients (p<0.001) (Table 7).
Table 7.
Chemotherapy regimen in black versus white patients.
| Treatment | Black N (%) | White N (%) | Total | Chi-square (p-value) |
|---|---|---|---|---|
| All | 473 | 252 | 725 | |
| Chemotherapy1 | 0.40 | |||
| Taxane-containing | 227 (87.98%) | 110 (90.91%) | 337 | |
| No taxane | 31 (12.02%) | 11 (9.09%) | 42 | |
| All Treatments | <0.01 | |||
| Chemo | 278 (58.77%) | 107 (42.46%) | 385 | |
| Chemo + Endocrine | 143 (30.23%) | 118 (46.83%) | 261 | |
| Chemo + Trastuzumab | 21 (4.44%) | 8 (3.17%) | 29 | |
| Chemo + Endocrine + Trastuzumab | 31 (6.55%) | 19 (7.54%) | 50 |
Detailed chemotherapy information missing from 428 patients: Black N=258; Adjuvant, N=121
Although patients receiving NAC were more often treated with taxane-containing chemotherapy compared to patient receiving AC (p=0.01), there was no difference in overall treatment combinations between the NAC and AC groups (p=0.7) (Table 8).
Table 8.
Chemotherapy regimen in patients receiving neoadjuvant versus adjuvant chemotherapy. Treatment.
| Treatment | Neoadjuvant N (%) | Adjuvant N (%) | Total | Chi-square (p-value) |
|---|---|---|---|---|
| All | 214 (26.52%) | 593 (73.48%) | 807 | |
| Chemotherapy1 | 0.01 | |||
| Taxane-containing | 136 (95.10%) | 253 (87.24%) | 389 | |
| No taxane | 7 (4.90%) | 37 (12.76%) | 44 | |
| All Treatments | 0.70 | |||
| Chemo | 113 (52.80%) | 310 (52.28%) | 423 | |
| Chemo + Endocrine | 73 (34.11%) | 221 (37.27%) | 294 | |
| Chemo + Trastuzumab | 10 (4.67%) | 21 (3.54%) | 31 | |
| Chemo + Endocrine + Trastuzumab | 18 (8.41%) | 41 (6.91%) | 59 |
Detailed chemotherapy information missing from 374 patients: Neoadjuvant N=143; Adjuvant, N=290.
Discussion
Black race has been associated with higher recurrence rates and breast cancer mortality [10]. Previous randomized trials showed similar outcomes for patients with localized breast cancer treated with NAC or AC, however these studies included predominately white women [15,14]. The main objective of this retrospective study was to evaluate the association between black race and distant recurrence free survival (DRFS) in patients with stage II-III breast cancer receiving neoadjuvant chemotherapy (NAC) or adjuvant chemotherapy (AC). In the first part of the study we compared DRFS in a cohort of black and white women treated with AC and NAC, whereas in the second part we investigated the DRFS in multi-racial and multi-ethnic cohort treated with NAC. Using Cox proportional hazard models adjusted for age, stage, ER expression, and triple negative status, we found that blacks treated with NAC have significantly worse DRFS compared to blacks treated with AC, while whites treated with either NAC or AC have similar DRFS. Although the race-treatment interaction was not statistically significant, our findings suggest that despite receiving NAC black women have worse DRFS than white women receiving the same therapy. In addition to tumor characteristics, other potential confounders for which we could not control for may explain this difference in outcome following NAC in black patients. In particular, our database has limited information regarding other patient characteristics such as education, socioeconomic status, comorbidities and others which could have been potential cofounders.
Population-based studies indicate that black women are treated significantly more often with NAC compared to other racial groups [12], which is attributed to more advanced stage, and higher rates of ER- and TN disease [19], which is more common in blacks [20]. Large prospective clinical trials, performed predominantly in white patient populations, showed similar OS and DRFS in patients treated with either NAC or AC [15,14]. Our analysis, stratified according to treatment plan (AC, NAC) and race (black, white) is consistent with prior reports indicating that whites have similar DRFS when treated with either NAC or AC, but indicates a discordance in outcomes for black women receiving NAC compared to AC, an observation that requires further evaluation using larger scale observational study that can control for additional patient related potential confounders and ultimately in a randomized controlled trial. Although we identified much larger treatment effect of NAC in blacks as compared to whites, we failed to detect a significant interaction between race and treatment, mostly likely due to the limited sample size.
Some evidence indicates racial differences in the breast tumor microenvironment (TME) that could explain our findings [21,22]. Although it is known that NAC induces pro-metastatic changes in breast cancers, racial differences in these changes have not been thoroughly evaluated [23,24]. NAC promotes the assembly of structures called tumor microenvironment of metastasis (TMEM) that serve as doorways for intravasation of tumor cells [24,23,25,26] and it increases the proportion of the highly invasive MenaINV-hi/Mena11alo (MenaCalc-Hi) tumor cells which utilize the TMEM sites for hematogenous dissemination [25]. Functional TMEM sites are composed of a proangiogenic Tie2 expressing macrophage in contact with an endothelial cell and Mena-expressing cancer cell [25,26]. Interestingly, it has been reported that the density of Tie2 expressing pro-angiogenic macrophages is higher in blacks than in whites [27], raising the possibility that worse DRFS post-NAC in blacks versus whites reported here may be a consequence of higher TMEM activity in blacks compared to whites. Furthermore, Martin et al. reported increased macrophage and microvascular density in the breast TME of blacks compared to whites [21], again pointing to the function of TMEM as a possible cause for observed differences in DRFS between black and white patients.
Another possible explanation for the difference in response to NAC between black and white patients may be due to body-mass index (BMI), which we did not control for due to limited information regarding the BMI in our data set. It has been reported that blacks have significantly higher BMI than whites, which is associated with higher circulating levels of cytokines and proinvasive changes in cancer cells and TME [22].
Although we showed worse DRFS in blacks treated with NAC compared to blacks treated with AC using rigorous statistical methods, our study has limitations inherent to all retrospective studies such as lack of randomization and lack of reliable information regarding BMI, as well as lack of information regarding other parameters such as education, socioeconomic status, and comorbidities which could have been potential cofounders. Our cohort showed a lower rate of pathologic complete response (pCR) than reported in other studies, but this was present in both black and white patients, and therefore unlikely to affect the observed difference in DRFS. However, using this retrospective cohort we recapitulated the findings from large randomized prospective clinical trials which found equal DRFS in white patients treated with NAC and AC. Likewise, our results support the findings of Woodward et al. (12), whose report indicated a tendency for worse DRFS and statistically worse overall survival (OS) in blacks compared to whites treated with doxorubicin-based NAC [10].
In summary, although black breast cancer patients are frequently treated with NAC, this approach does not seem to result in better, or even equivalent, DRFS as compared to black patients treated with AC. Although this may be a result of confounders which our study could not control for, the biologic factors contributing to our findings warrant further evaluation. Likewise, prospective randomized trials need to be initiated to determine which treatment approach would result in the most improved long-term survival in black breast cancer patients who have biologically more aggressive disease.
Supplementary Material
Acknowledgments
Funding sources: This work was supported by the National Cancer Institute at the National Institutes of Health [grants number CA216248 to Condeelis, Entenberg, Oktay; CA100324 to Condeelis, Oktay, 1T32CA200561–01 to Pastoriza]; and the Integrated Imaging Program at the Albert Einstein College of Medicine.
List of Abbreviations:
- AC
adjuvant chemotherapy
- BMI
body mass index
- DRFS
distant recurrence-free survival
- ER
estrogen receptor
- IPW
inverse probability weighting
- KM
Kaplan-Meier
- NAC
neoadjuvant chemotherapy
- OS
overall survival
- pCR
pathologic complete response
- PR
progesterone receptor
- TME
tumor microenvironment
- TMEM
tumor microenvironment of metastasis
- TN
triple negative
Footnotes
Competing Interests: The authors have declared no conflicts of interest.
Institutional Review Board Approval: This investigation was approved by the Albert Einstein Institutional Review Board (IRB# 2016–6065).
References
- 1.N H, AM N, M K, D M, K B, CL K, M Y, J R, Z T, A M, DR L, HS C, EJ F, KA C (2017) SEER Cancer Statistics Review, 1975–2014, National Cancer Institute; Bethesda, MD. [Google Scholar]
- 2.Eley JW, Hill HA, Chen VW, Austin DF, Wesley MN, Muss HB, Greenberg RS, Coates RJ, Correa P, Redmond CK, et al. (1994) Racial differences in survival from breast cancer. Results of the National Cancer Institute Black/White Cancer Survival Study. JAMA 272 (12):947–954 [DOI] [PubMed] [Google Scholar]
- 3.Chu KC, Lamar CA, Freeman HP (2003) Racial disparities in breast carcinoma survival rates: seperating factors that affect diagnosis from factors that affect treatment. Cancer 97 (11):2853–2860. doi: 10.1002/cncr.11411 [DOI] [PubMed] [Google Scholar]
- 4.Li CI, Malone KE, Daling JR (2002) Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer Epidemiol Biomarkers Prev 11 (7):601–607 [PubMed] [Google Scholar]
- 5.Elledge RM, Clark GM, Chamness GC, Osborne CK (1994) Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. Journal of the National Cancer Institute 86 (9):705–712 [DOI] [PubMed] [Google Scholar]
- 6.Newman LA, Mason J, Cote D, Vin Y, Carolin K, Bouwman D, Colditz GA (2002) African-American ethnicity, socioeconomic status, and breast cancer survival: a meta-analysis of 14 studies involving over 10,000 African-American and 40,000 White American patients with carcinoma of the breast. Cancer 94 (11):2844–2854. doi: 10.1002/cncr.10575 [DOI] [PubMed] [Google Scholar]
- 7.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D (2005) Comorbidity and survival disparities among black and white patients with breast cancer. JAMA 294 (14):1765–1772. doi: 10.1001/jama.294.14.1765 [DOI] [PubMed] [Google Scholar]
- 8.Griggs JJ, Sorbero ME, Stark AT, Heininger SE, Dick AW (2003) Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res Treat 81 (1):21–31. doi: 10.1023/A:1025481505537 [DOI] [PubMed] [Google Scholar]
- 9.KS A, JM U, LF H (December 3–6, 2003) Outcome of African Americans on Southwest Oncology Group (SWOG) breast cancer adjuvant therapy tria ls Presented at the 26th Annual San Antonio Breast Cancer Symposium, San Antonio, TX [Google Scholar]
- 10.Woodward WA, Huang EH, McNeese MD, Perkins GH, Tucker SL, Strom EA, Middleton L, Hahn K, Hortobagyi GN, Buchholz TA (2006) African-American race is associated with a poorer overall survival rate for breast cancer patients treated with mastectomy and doxorubicin-based chemotherapy. Cancer 107 (11):2662–2668. doi: 10.1002/cncr.22281 [DOI] [PubMed] [Google Scholar]
- 11.Tichy JR, Deal AM, Anders CK, Reeder-Hayes K, Carey LA (2015) Race, response to chemotherapy, and outcome within clinical breast cancer subtypes. Breast Cancer Res Treat 150 (3):667–674. doi: 10.1007/s10549-015-3350-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Killelea BK, Yang VQ, Wang SY, Hayse B, Mougalian S, Horowitz NR, Chagpar AB, Pusztai L, Lannin DR (2015) Racial Differences in the Use and Outcome of Neoadjuvant Chemotherapy for Breast Cancer: Results From the National Cancer Data Base. J Clin Oncol 33 (36):4267–4276. doi: 10.1200/JCO.2015.63.7801 [DOI] [PubMed] [Google Scholar]
- 13.Mieog JS, van der Hage JA, van de Velde CJ (2007) Neoadjuvant chemotherapy for operable breast cancer. Br J Surg 94 (10):1189–1200. doi: 10.1002/bjs.5894 [DOI] [PubMed] [Google Scholar]
- 14.Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil SR, Tamkus D, King KM, Pajon ER, Wright MJ, Robert J, Paik S, Mamounas EP, Wolmark N (2008) Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 26 (5):778–785. doi: 10.1200/JCO.2007.15.0235 [DOI] [PubMed] [Google Scholar]
- 15.Mauri D, Pavlidis N, Ioannidis JP (2005) Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. Journal of the National Cancer Institute 97 (3):188–194. doi: 10.1093/jnci/dji021 [DOI] [PubMed] [Google Scholar]
- 16.Bellin E, Fletcher DD, Geberer N, Islam S, Srivastava N (2010) Democratizing information creation from health care data for quality improvement, research, and education-the Montefiore Medical Center Experience. Acad Med 85 (8):1362–1368. doi: 10.1097/ACM.0b013e3181df0f3b [DOI] [PubMed] [Google Scholar]
- 17.Austin PC (2011) An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 46 (3):399–424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin PC, Stuart EA (2015) Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Statistics in Medicine 34 (28):3661–3679. doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Konecny GE, Denkert C, Nekljudova V, Mehta K, Loibl S (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30 (15):1796–1804. doi: 10.1200/JCO.2011.38.8595 [DOI] [PubMed] [Google Scholar]
- 20.Lund MJ, Trivers KF, Porter PL, Coates RJ, Leyland-Jones B, Brawley OW, Flagg EW, O'Regan RM, Gabram SG, Eley JW (2009) Race and triple negative threats to breast cancer survival: a populationbased study in Atlanta, GA. Breast Cancer Res Treat 113 (2):357–370. doi: 10.1007/s10549-008-9926-3 [DOI] [PubMed] [Google Scholar]
- 21.Martin DN, Boersma BJ, Yi M, Reimers M, Howe TM, Yfantis HG, Tsai YC, Williams EH, Lee DH, Stephens RM, Weissman AM, Ambs S (2009) Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS One 4 (2):e4531. doi: 10.1371/journal.pone.0004531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deshmukh SK, Srivastava SK, Bhardwaj A, Singh AP, Tyagi N, Marimuthu S, Dyess DL, Dal Zotto V, Carter JE, Singh S (2015) Resistin and interleukin-6 exhibit racially-disparate expression in breast cancer patients, display molecular association and promote growth and aggressiveness of tumor cells through STAT3 activation. Oncotarget 6 (13):11231–11241. doi: 10.18632/oncotarget.3591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karagiannis GS, Pastoriza JM, Wang Y, Harney AS, Entenberg D, Pignatelli J, Sharma VP, Xue EA, Cheng E, D'Alfonso TM, Jones JG, Anampa J, Rohan TE, Sparano JA, Condeelis JS, Oktay MH (2017) Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Science translational medicine 9 (397). doi: 10.1126/scitranslmed.aan0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang YS, Jalgaonkar SP, Middleton JD, Hai T (2017) Stress-inducible gene Atf3 in the noncancer host cells contributes to chemotherapy-exacerbated breast cancer metastasis. Proc Natl Acad Sci U S A 114 (34):E7159–E7168. doi: 10.1073/pnas.1700455114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian BZ, Oktay MH, Pollard JW, Jones JG, Condeelis JS (2015) Real-Time Imaging Reveals Local, Transient Vascular Permeability, and Tumor Cell Intravasation Stimulated by TIE2hi Macrophage-Derived VEGFA. Cancer discovery 5 (9):932–943. doi: 10.1158/2159-8290.CD-15-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harney AS, Karagiannis GS, Pignatelli J, Smith BD, Kadioglu E, Wise SC, Hood MM, Kaufman MD, Leary CB, Lu WP, Al-Ani G, Chen X, Entenberg D, Oktay MH, Wang Y, Chun L, De Palma M, Jones JG, Flynn DL, Condeelis JS (2017) The Selective Tie2 Inhibitor Rebastinib Blocks Recruitment and Function of Tie2(Hi) Macrophages in Breast Cancer and Pancreatic Neuroendocrine Tumors. Mol Cancer Ther 16 (11):2486–2501. doi: 10.1158/1535-7163.MCT-17-0241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koru-Sengul T, Santander AM, Miao F, Sanchez LG, Jorda M, Gluck S, Ince TA, Nadji M, Chen Z, Penichet ML, Cleary MP, Torroella-Kouri M (2016) Breast cancers from black women exhibit higher numbers of immunosuppressive macrophages with proliferative activity and of crown-like structures associated with lower survival compared to non-black Latinas and Caucasians. Breast Cancer Res Treat 158 (1):113–126. doi: 10.1007/s10549-016-3847-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.