Abstract
Depression and chronic inflammation are common in persons infected with the Human immunodeficiency virus (HIV+). Although depression and response to peripheral inflammation are represented in common neural networks little is known regarding the functional integrity of these networks in HIV+ persons. The relationship between resting-state functional connectivity (rsFC) of the subgenual anterior cingulate cortex (sgACC) and Beck Depression Inventory-1 (BDI) scores were compared between 23 HIV+ and 23 HIV-negative comparison adults. In HIV+ individuals, greater rsFC between the sgACC and the right and left amygdala was associated with higher BDI scores. Total BDI scores and plasma levels of IL-6, IL-8, TNF-α and IL-10, made available from 10 HIV+ patients, were regressed on an index of spontaneous whole-brain activity at rest, i.e., amplitude of low-frequency fluctuations (ALFF). Elevated levels of depression and IL-6 were associated with increased ALFF in the orbitomedial frontal cortex (OFC). Although global reductions in rsFC and task-based activity have been reported in HIV, within this sample, greater burden of depressive symptomology and peripheral inflammation was reflected in stronger spontaneous activity and functional connectivity of ventromedial and subcortical limbic regions.
Keywords: cytokines, perigenual cingulate, seed-based connectivity, salience network, immunodeficiency
1. Introduction
Depression is one of the most common neuropsychiatric presentations in persons chronically infected with the Human immunodeficiency virus (HIV), with lifetime prevalence estimated as high as 40% (Rabkin, Ferrando et al. 2000, Rabkin 2008). Resting-state functional connectivity (rsFC) is a popular technique in functional magnetic resonance neuroimaging (fMRI) that allows for characterization of patterns of inter- and intra-network brain connectivity in order to distinguish healthy individuals from those with depression and other neuropsychiatric disorders (Greicius, Krasnow et al. 2003, Fox and Greicius 2010). Although the use of rsFC continues to provide a clearer picture of the neural etiology of depression in healthy individuals comparatively less is known regarding the state of functional brain connectivity in depressed persons living with HIV.
Altered rsFC of the default mode network (DMN) is present in nearly every known stage of depression including first episode, current-treatment, recurrent, late-life, and remission (Alexopoulos, Hoptman et al. 2012, Marchetti, Koster et al. 2012, Zhu, Wang et al. 2012). Although connectivity of this network has not been exclusively studied with relation to depression in persons with HIV altered rsFC of the DMN has been noted. For example, lower rsFC within the DMN was noted in addition to lower inter-network connectivity with hubs of other major neural networks, (e.g., salience and dorsal attention network) in a study comparing HIV+ to healthy HIV-negative individuals (Thomas, Brier et al. 2013). In addition to the DMN rsFC research has uncovered substantial contributions from the subgenual anterior cingulate (sgACC) in the presentation of major depression (Greicius, Flores et al. 2007; Fransson and Marrelec 2008). In a mixed cohort of a treatment-naïve and recurrent depressive adults increased rsFC was observed between the sgACC and medial prefrontal cortex (Sheline, Price et al. 2010). A similar pattern of greater rsFC between sgACC and medial prefrontal cortex was observed in treatment-naïve adults (Zhu, Wang et al. 2012). A more recent study of treatment-naïve adolescents diagnosed with first-episode depression uncovered greater rsFC between sgACC and the insula when compared to well-matched control subjects (Connolly, Wu et al. 2013). This rsFC work has not been replicated in an HIV+ cohort, however, low-resolution electromagnetic tomography was recently used to show elevations in depressive symptomology were associated with greater resting activity in the rostral and sgACC (Kremer, Lutz et al. 2016). Rather, the emphasis of neuroimaging findings with respect to the presentation of cognitive-affective symptoms in persons living with HIV/AIDS (PLWH) has been structural and functional alterations of the bilateral amygdala (Ances, Ortega et al. 2012, Spies, Ahmed-Leitao et al. 2016, Clark, Sweet et al. 2017, Thames, Kuhn et al. 2018). What most, but not all (Behrman-Lay, Paul et al. 2016), of these studies suggest is that HIV-related stress and trauma history have negative effects on morphology and activity of the bilateral amygdala as well as other limbic regions such as ACC and hippocampus.
There is evidence to suggest that the burden of depression is heavier in HIV patients, moreover, chronic immunosuppression may further contribute to the severity of depressive symptomology in this group. As the virus replicates, spread of gene products (e.g., gp120) activate lymphocytes, monocytes, and microglial cells that enhance pro-inflammatory cytokine and chemokine expression (Appay and Sauce 2008). Furthermore, increased burden of depressive symptomology may contribute to alterations in hypothalamic-pituitary-adrenal (HPA) axis activity which in turn can lead to further T-helper cell suppression and viral proliferation (Leserman 2003, Cole 2008). Despite suppression of viral transcription by antiretroviral therapy (ART), chronic monocyte activation and expression of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and IL-1β remain a growing concern for HIV disease management because of their ability to predict all-cause mortality (Kuller, Tracy et al. 2008, Sandler, Wand et al. 2011). It should also be noted that this pro-inflammatory profile is linked to decreases in monoamines and brain-derived neurotrophic factor, both of which are implicated in the etiology of depression in HIV (Del Guerra, Fonseca et al. 2013). Accordingly, elevated levels of IL-6 and peripheral monocyte are linked to severity of fatigue and depressive symptomology in HIV-infected adults (Pala, Steca et al. 2016).
Recent years have seen increased use of fMRI to study the neural representation of peripheral inflammation, and in doing has uncovered significant overlap with brain regions involved in the presentation of depression, most notably the sgACC. A double-blind, randomized, crossover-study comparing brain activation during an emotional face-processing task revealed increased sgACC activity paralleled a rise in peripheral levels of IL-6 2–3 hours following typhoid vaccination (Harrison, Brydon et al. 2009). Moreover, reduced connectivity of the sgACC with the amygdala, basal ganglia, and medial prefrontal cortex buffered the inflammation-related increase in mood disturbance observed post-vaccination. In another double-blind randomized study increases in salivary IL-1β and soluble tumor necrosis factor receptor were associated with greater activity in the sgACC and orbitofrontal cortex (OFC) during a grief-elicitation task (O’Connor, Irwin et al. 2009). These studies suggest functional activation of the sgACC and inferior medial frontal cortex correspond to acute inflammatory responses in otherwise healthy humans. Whether elevated levels of inflammation persons living with chronic HIV-infection is reflected in heightened activity of the sgACC/OFC and does this activity overlap with depressive symptomology has not been formally tested, to date.
The primary aim of the current study is to determine whether in prediction of depressive symptomology there exists an interactive effect for HIV serostatus and functional connectivity between a seed region in the sgACC and bilateral amygdala. Yet another application for the resting-state blood oxygen level dependence (BOLD) signal is quantification of spontaneous brain activity, in the absence of explicit cognitive tasks or stimulation, to perhaps reflect a cumulative effect of specific experience over time (Lewis, Baldassarre et al. 2009). An exploratory aim, carried out in a subset of the HIV+ individuals, was to determine whether severity of depressive symptomology and burden of peripheral levels of pro-inflammatory cytokines predict the amplitude of low-frequency fluctuation (ALFFs) in a subset of HIV+ individuals from which this data was collected. ALFF is a measure of spontaneous activity at rest that has been used to compare with MDD and depressive symptomology in non-clinical samples (Guo, Liu et al. 2012, Liu, Ma et al. 2013, Liu, Guo et al. 2013, Tadayonnejad, Yang et al. 2015).
2. Methods
2.1. Participants
Participants included HIV+ individuals enrolled in the Hawaii Aging with HIV–Cardiovascular Disease (HAHC-CVD) cohort study, and HIV− comparison subjects, who underwent resting-state functional magnetic resonance imaging. HIV+ individuals were also drawn from another study with the same inclusion/exclusion criteria and neuroimaging protocol as the HAHC-CVD. HIV+ participants were recruited through referrals from the clinic at the Hawaii Center for AIDS, community physicians, community advisory board members, and AIDS service organizations. Individuals were eligible for the study if they were ≥ 40 years old, had a documented history of HIV infection, had been on stable ART ≥ 3 months, spoke English as their primary language, and could understand and provide informed consent. Exclusion criteria were uncontrolled major affective disorder, active psychosis, loss of consciousness > 5 minutes, factors precluding MRI (e.g., claustrophobia), and any past or present condition (e.g., central nervous system infection, stroke, substance use) considered by the evaluating physician to introduce confounding variables. HIV− comparison subjects were recruited from the same region (Hawaii) as the HIV+ participants. The HIV− individuals were over 40 years old, spoke English as their primary language, were seronegative on enzyme- linked immunosorbent assay (ELISA), fulfilled the same exclusion criteria as the HIV+ participants, and underwent MRI and similar clinical testing (non-HIV-related variables) although cytokine data were not obtained for this group.
All study participants underwent a physical exam and neuroimaging, and provided details of their medical history with a focus on HIV disease and CVD events and risk factors. Nadir CD4 count, years since HIV diagnosis, and date of ART initiation were provided by subject self-report. Blood specimens were obtained, and plasma HIV RNA and CD4 cell counts performed by a local commercial Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory. Basic laboratory evaluations included a complete metabolic profile, complete blood count (CBC), high sensitivity (hs)-CRP, fasting glucose, insulin and lipids. HIV-specific labs included assessment of plasma HIV-RNA and CD4 and CD8 counts. IRB approval was obtained from the University of Hawaii and all participants provided written informed consent prior to study entry.
2.2. Inflammatory biomarkers
Blood was separated within 30 minutes of the blood draw and plasma aliquots were cryopreserved until used for cytokine/chemokine assessments in a subset of the HIV+ individuals. Testing was conducted using Milliplex Human Cardiovascular Disease panels (EMD Millipore, USA) as outlined in the manufacturer’s protocols. The soluble biomarkers assessed were IL-6, IL-8, IL-10, and TNF-α.
2.3. Depressive symptomology
Depressive symptomology was assessed in all study participants by the Beck Depression Inventory I (BDI) (Beck, Ward et al. 1961). Component (somatic and cognitive-affective) and total BDI scores were obtained.
2.4. fMRI Preprocessing
Magnetic resonance imaging was performed on a 3.0-Tesla Philips Medical Systems Achieva scanner equipped with an 8-channel head coil (InVision Imaging, Honolulu). For each subject, a high-resolution anatomical volume was acquired with a sagittal T1-weighted 3D turbo field echo (T1W 3D TFE) sequence (echo time TE/repetition time TR = 3.2 ms/6.9 ms; flip angle 8°; slice thickness 1.2 mm with no gap; in-plane resolution 1.0 mm2; field of view 256 × 256 mm2; scan time=10.2 min). Rs-fMRI echo-planar imaging (EPI) BOLD data were acquired with subjects’ eyes closed and with whole-brain coverage repetition time/echo time (TR/TE= 1600ms/22ms with 262 time points; flip angle 70°; 3.5 mm isotropic voxels; 37 sagittal slices with no gap; scan time = 7.5 min).
Standard methods for preprocessing of the blood oxygen level dependent (BOLD) signal were implemented for the 4D time series using a modified version of a shell script generated by afni_proc.py (http://afni.nimh.nih.gov/pub/dist/doc/program_help/afni_proc.py.html) from AFNI (Cox 1996). Images were skull-stripped and co-registered to the fourth time point of the time series data. The initial 3 time points were discarded to allow T1 saturation. We then applied standard preprocessing methods which included despiking the time series data; slice time correction; motion correction; smoothing with a 4 mm full-width half-maximum Gaussian kernel; and head motion correction (Power, Mitra et al. 2014). Data were filtered using a bandpass filter (0.008 < f < 0.08 Hz). During motion correction, 6 demeaned rigid body motion profiles and their derivatives were calculated by the 3dvolreg command and used to censor the data. Participants were excluded if they had head movement > 0.3 mm, in any direction, at more than 25% of the time points in the rsfMRI scan. Nuisance covariates (signals associated with deep white matter and with cerebrospinal fluid averaged over the ventricles) were also computed. To remove confounding effects of physiological noise and participant movement, the time courses for white matter, cerebrospinal fluid, and six motion parameters were regressed out in a whole-brain linear model for rsFC in order to balance Type I and Type II error rates (Lieberman and Cunningham 2009).
2.5. Region-of-interest selection
Montreal Neurological Institute (MNI) coordinates (MNI: x, y, z: 0, 22, −10) was selected to correspond with the subgenual anterior cingulate (i.e., subcallosal anterior cingulate gyrus or sgACC). This region corresponding with Brodmann’s area (BA) 25, is bordered dorsal-anteriorly by BA 24 or the rostral anterior cingulate. A 10 mm3 sphere was created around this point using the AFNI program 3dmaskave. This seed region coincides with meta-analytic fMRI findings of increased metabolism within this region for individuals diagnosed with MDD (Alexopoulos, Hoptman et al. 2012).
2.6. Seed-based resting-state functional connectivity
Each participant’s residual 4D volumes were spatially normalized by applying the previously computed transformation to MNI-152 template at 2 mm standard space. Within each subject’s native space, we then performed a correlation analysis for each ROI using the AFNI program 3dfim+. The analyses produced participant- level correlation maps of all voxels in the brain that were positively or negatively correlated with the sgACC seed time series. The correlation maps were converted to Z-value maps using Fisher’s r-to-z transformation to improve normality, and were corrected for degrees of freedom (time points after censoring) using the equation: z = log [(1 + r)/(1 − r)] ×√((n − 3))/2, where n= degrees of freedom. Z-score maps were entered into second-level statistical analyses. These z-scores represent the magnitude of positive rsFC between the sgACC seed regions and the whole brain in a voxel-wise fashion. Statistical maps were obtained by segmenting the mean normalized high resolution T1-weighted images of all subjects to include only areas within gray matter. Prior to the seed-to-seed extraction of bilateral amygdala ROI, whole brain sgACC connectivity maps were averaged together and then compared between groups. Gaussian random field (GRF) theory (voxel significance p < 0.001 and cluster significance p < 0.01) was used to balance for type I and II error (Lieberman and Cunningham 2009).
2.7. ALFF Analysis
Following the pre-processing of the time series data, an ALFF analysis (Yang, Long et al. 2007, Zou, Zhu et al. 2008) was conducted using AFNI software (AFNI_proc.py) on a subset of 10 HIV+ individuals for whom plasma cytokine assays were performed. Briefly, ALFF maps were acquired for each individual wherein each brain voxel a fast Fourier transform was used to convert the pre-processed filtered time series to a frequency domain and power spectrum. The square-root of the power spectrum was then averaged across 0.01–0.08 Hz at each voxel and standardized resulting in a spatial map representing mean ALFF (Zou, Zhu et al. 2008). The resulting Z-map was spatially smoothed with an isotropic Gaussian kernel of 6 mm of full-width at half-maximum and registered to a Montreal Neurological Institute (MNI) mask (Zou, Zhu et al. 2008). At the second-level analysis stage, regression of whole-brain ALFF upon plasma IL-6 levels and total BDI scores was carried out using the SPM12 software package http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). A GRF theory multiple comparison correction was also implemented fort the ALFF results (voxel significance p < 0.001 and cluster significance p < 0.01).
2.8. Model for rsFC Predicting Depressive Symptomology
A region of interest (ROI) seed-to-whole brain and seed-to-seed analysis was used to create subject-specific maps in order to test the hypotheses that functional connectivity between the sgACC and bilateral central amygdala will be related to depressive symptomology as a function of HIV serostatus. The sgACC seed-to-whole-brain map results are reported when significant at the voxel (p < 0.001) as well as cluster level (p <.01) (Chumbley, Worsley et al. 2010). All coordinates reported below refer to peak activations in anatomical MNI space.
First, two ROIs were located in bilateral amygdala based upon a match between the MNI-152 atlas and group seed-to-whole-brain connectivity map. This yielded two regions: left amygdala (AMG) (−22, −6, −14) and right AMG (22, −6 −14). A 5-mm sphere was placed on these two points. This procedure was applied separately to the HIV+ and HIV− groups. Fisher’s Z-transformed correlations were computed to quantify functional connectivity between the sgACC seed and target ROIs and extracted from each individual subject map of resting brain activity.
Next, we tested several regression models in order to assess a possible interaction effect between HIV+ status and sgACC-amygdala connectivity on depressive symptomology. Separate models were created for BDI total, somatic, and cognitive-affective scores with the interaction term controlling for age, education level, gender, HIV serostatus and seed-to-seed connectivity. Demographic variables were controlled in the rst stage of the model; biomarker data was entered into the second stage of the model; and the interaction term entered last. A signi cance test for the change in variance (ΔR2) at each step of the model was reported in addition to beta coef cients and overall F-tests for model signi cance. This allowed us to explore whether HIV-related patterns in sgACC connectivity with bilateral amygdala differentially predict subtypes of depressive symptomology while controlling for sociodemographic factors associated with endorsement of greater depressive symptoms in this population (Scarinci, Beech et al. 2002, Olley, Gxamza et al. 2003, Gupta, Dandu et al. 2010).
3. Results
3.1. Behavioral Data
Table 1 shows the mean and standard deviation for demographic, clinical and BDI data between the HIV+ (N=23) and HIV-negative (N=23) groups. The groups did not differ in age, gender or ethnicity. Fourteen HIV+ individuals had undetectable plasma viral load (< 50 copies/mL). As predicted, the HIV+ group had significantly higher total BDI scores than HIV-negative controls t(41) = 2.039, p = 0.048, η2 = 0.09.
Table 1.
Median demographic and clinical characteristics for HIV+ and HIV-negative study participants.
| Characteristics | HIV+ (N=23) | SD | HIV− (N=23) | SD | p - value | * HIV+ (n =10) | SD |
|---|---|---|---|---|---|---|---|
| Age (years) | 53.8 | 7.4 | 56.6 | 8.63 | NS | 49.6 | 7.5 |
| Male gender (% n) | 87.0% | 69.6% | NS | 90% | |||
| Caucasian (% n) | 52.0% | 65.2% | NS | 60% | |||
| Education (years) | 13.8 | 1.9 | 15.2 | 2.19 | 0.030 | ||
| Beck Depression Inventory | 9.0 | 5.9 | 5.77 | 4.37 | 0.048 | 8.2 | 3.4 |
| Undetectable HIV RNA (% n) | 60.8% | 80% | |||||
| Current CD4+ count (cells/μl) | 500.9 | 222.5 | 475.0 | 228.3 | |||
| Nadir CD4+ count (cells/μl) | 127.1 | 147.3 | 122.3 | 125.5 | |||
| CD8_absolute | 1083.7 | 397.6 | 981.7 | 397.7 | |||
| Time of infection (years) | 13.3 | 9.8 | 14.2 | 7.2 | |||
| Time on Antiretroviral therapy | 13.0 | 7.1 | 13.0 | 7.1 | |||
| Interlukin-6* | 1.18 | 0.8 | |||||
| Interlukin-8* | 5.21 | 2.68 | |||||
| Interlukin-10* | 2.93 | 2.99 | |||||
| TNFa* | 3.43 | 3.09 |
NS = non-significant.
Sub-sample of 10 HIV+ individuals for whom soluble cytokine markers were available.
3.2. Movement Parameters
Table 2 shows that the values for movement in the planes of yaw, pitch, roll, displacement in the left-right and anterior-posterior axes did not differ between groups. Furthermore, the total number and average number of TRs removed that exceeded the 0.3 mm threshold did not differ significantly between the HIV+ and HIV-negative groups. However, results from the afni_restproc.py program indicate that HIV-negative individuals demonstrated more movement on the superior-inferior plane than their HIV+ counterparts t(44) = 7.78, p = 0.008, η2 = 0.15.
Table 2.
Comparison of motion parameters between groups.
| Motion Parameter | HIV+ (N=23) | SD | HIV-negative (N=23) | SD | p - value |
|---|---|---|---|---|---|
| Roll | −0.038 | 0.077 | −0.045 | 0.077 | NS |
| Pitch | −0.036 | 0.080 | −0.056 | 0.080 | NS |
| Yaw | −0.036 | 0.071 | −0.027 | 0.071 | NS |
| Superior-inferior displacement | −0.029 | 0.072 | −0.090 | 0.072 | p = 0.008 |
| Left-right displacement | −0.037 | 0.085 | −0.058 | 0.085 | NS |
| Anterior-posterior displacement | −0.033 | 0.067 | −0.059 | 0.067 | NS |
| Number TRs above motion limit | 17.35 | 22.42 | 27.435 | 22.42 | NS |
| Average TRs above motion limit (%) | 14.1 | 15.9 | 0.04 | NS |
NS = non-significant
3.3. Group Differences in rsFC
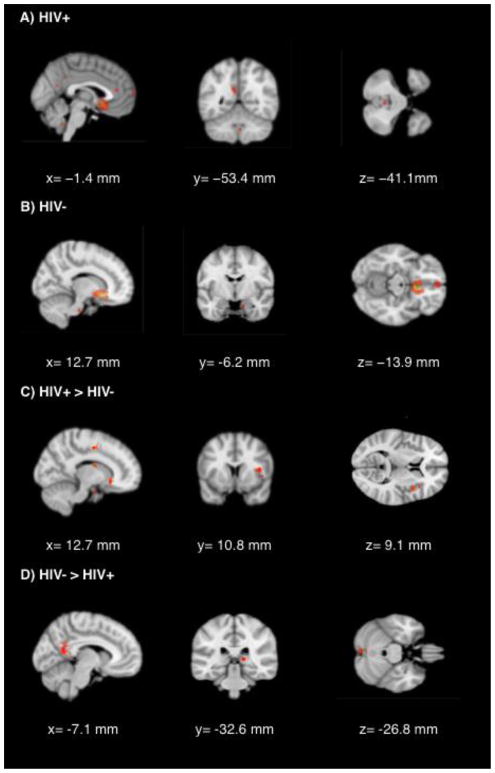
Figure 2 depicts rsFC of the sgACC as a function of HIV status. Table 3 lists the MNI coordinates, cluster sizes, z-scores and p-values (cluster-wise corrected at p < .01) for brain regions showing above threshold rsFC with the sgACC seed region, separately, for each group. Notably, HIV+ participants showed lower sgACC connectivity with large clusters in the medial prefrontal cortex and brainstem, but evidenced greater rsFC with clusters in the anterior and posterior cingulate (Table 3).
Figure 2.
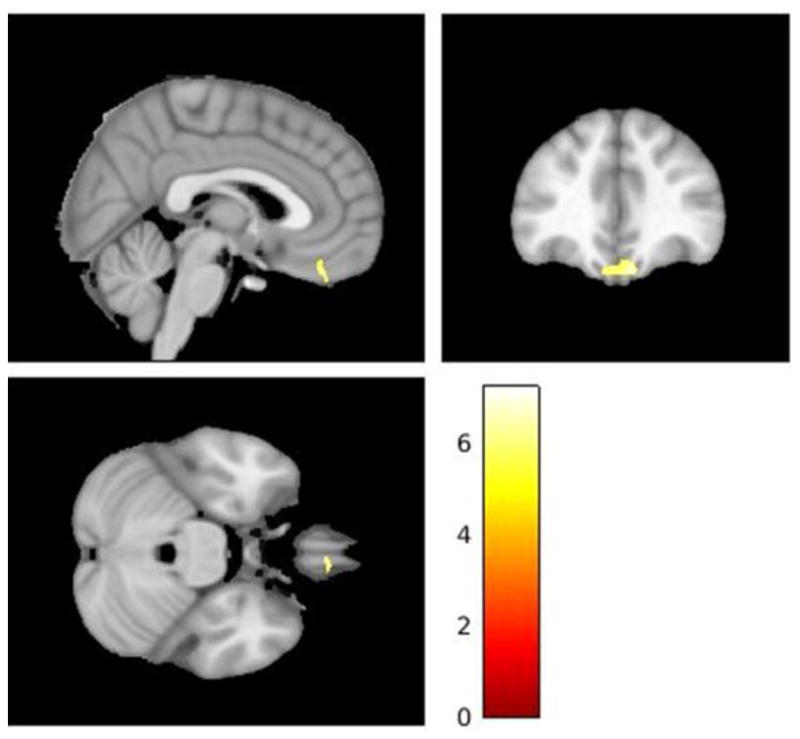
Significant (p < 0.001, uncorrected) spontaneous resting-state activity in a cluster of voxels (k=716) within the ventromedial prefrontal cortex associated with greater levels of IL-6 and depressive symptomology in 10 HIV+ individuals.
Table 3.
Brain regions showing significant resting-state functional connectivity with the sgACC.
| HIV+ | ||||
|---|---|---|---|---|
|
| ||||
| Structure | Brodmann Area | MNI Coordinates | Cluster Size | T-value |
| Left Posterior Cingulate | 23 | −6, −54, 22 | 275 | 8.45 |
| Left Medial Frontal Gyrus | 10 | −4, 70, 12 | 55 | 7.06 |
| Right Posterior Cingulate | 10, −52, 20 | 27 | 6.98 | |
| Left Anterior Cingulate | 32 | −4, 36, 14 | 74 | 6.68 |
| Right Parahippocampal Gyrus | 30 | 16, −34, −10 | 42 | 6.34 |
| HIV− | ||||
|---|---|---|---|---|
|
| ||||
| Structure | Brodmann Area | MNI Coordinates | Cluster Size | T-value |
| Right Medial Frontal Gyrus | 11 | 2, 52, −12 | 235 | 7.33 |
| Right Brain Stem | 10, −22, −36 | 72 | 6.74 | |
| Right Anterior Cingulate | 24 | 8, 26, 18 | 18 | 6.67 |
| Right Uncus (rPG, rAMG) | 34 | 16, −6, −24 | 27 | 6.62 |
| HIV− > HIV+ | ||||
|---|---|---|---|---|
|
| ||||
| Structure | Brodmann Area | MNI Coordinates | Cluster Size | T-value |
| Right Anterior Cingulate | 32 | 8, 20, −8 | 154 | 4.67 |
| Right Cingulate Gyrus | 16, −12, 48 | 144 | 4.61 | |
| Right Parahippocampal Gyrus | 34 | 16, −6, −22 | 119 | 4.52 |
| Right Insula | 13 | 38, 12, 16 | 130 | 3.85 |
| Left Postcentral Gyrus | 0, −46, 72 | 45 | 3.84 | |
| Right Caudate | 12, −2, 18 | 52 | 3.82 | |
| Right Insula | 40, 6, 18 | 33 | 3.72 | |
| Left Middle Frontal Gyrus | −22, 70, 16 | 33 | 3.49 | |
| HIV+ > HIV− | ||||
|---|---|---|---|---|
|
| ||||
| Structure | Brodmann Area | MNI Coordinates | Cluster Size | T-value |
| Left Cingulate Gyrus (lPG) | −10, −52, 26 | 202 | 5.13 | |
| Left Declive | −4, −84, −28 | 57 | 4.16 | |
| Right Cerebellar Tonsil | 4, −52, −42 | 64 | 4.01 | |
| Left Insula | 13 | −36, 0, −12 | 22 | 3.71 |
| Right Thalamus (Right Pulvinar) | 16, −32, 6 | 20 | 3.59 | |
For the contrast reflecting greater rsFC of the sgACC with whole brain between HIV+ and HIV− individuals several regions survived cluster-wise correction. Figure 1 depicts lower rsFC between the sgACC and clusters throughout the right anterior cingulate, right insula, and right amygdala in HIV+ individuals compared to HIV− controls. The HIV+ group also showed greater rsFC between the sgACC and a large cluster within the posterior cingulate gyrus (see Figure 1).
Figure 1.
Significant (p < 0.001) regions of resting-state activity with the sgACC as a seed. A) FWE corrected activity in HIV+ individuals B) FWE corrected activity in HIV− individuals C) Activity significantly greater in HIV+ than HIV− individuals D) Activity significantly lower in HIV+ than HIV− individuals.
3.4. Interaction HIV and rsFC on Depressive Symptomology
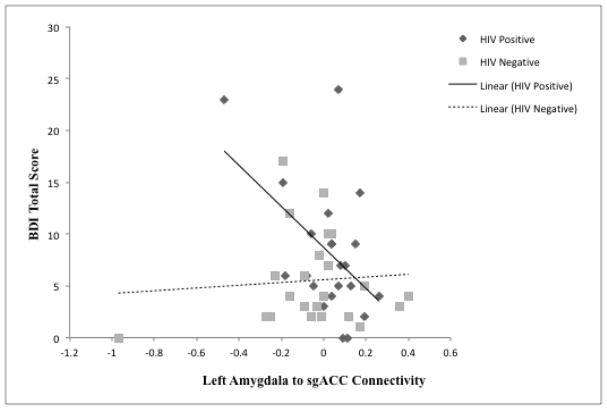
The regression model for rsFC of sgACC and left amygdala seed predicted 25.8% of the variance in total depressive symptomology F(6,38) = 2.20, p = .064 after accounting for the effects of age, gender, and education level. The interaction for HIV status and connectivity of the sgACC to left amygdala showed a trend towards significance (β = −0.35, p = .06). This right-lateralized model also predicted 27.4% of the variance in the cognitive-affective subtype of depressive symptomology on the BDI F(6,38) = 2.39, p = .047. Whereas a trending positive association arose between HIV+ serostatus and cognitive-affective depression (β = 0.25, p = .10), the interaction with sgACC to left amygdala rsFC was significant (β = −0.37, p = .047) with this term explaining an additional 8.1% of the variance in the model ΔF(1, 38) = 4.20, p = .047. The model for somatic complaints was not significant.
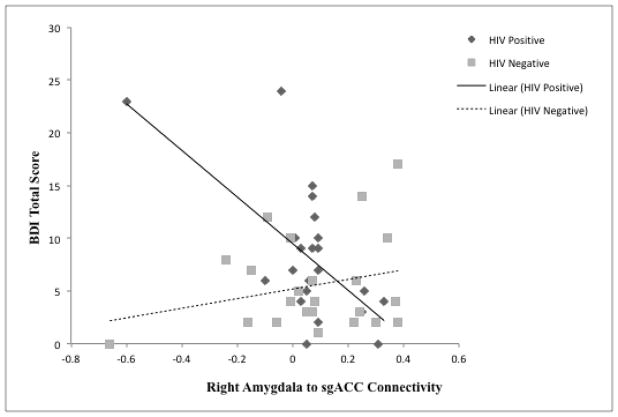
The regression model for rsFC of sgACC and right amygdala seed predicted 60.3% of the variance in total depressive symptomology F(6,38) = 3.61, p = .006 after accounting for the effects of age, gender, and education level. The final stage of the model revealed a positive association between HIV+ serostatus and total depressive symptomology (β = 0.32, p = .032). The interaction of HIV with sgACC to right amygdala rsFC was also significant (β = −0.56, p = .002) with this term explaining an additional 19.1% of the variance in the model ΔF(1, 38) = 11.55, p = .002. The right-lateralized model also predicted 44.1% of the variance in the cognitive-affective subtype of depressive symptomology on the BDI F(6,38) = 4.99, p < .001. There was a positive association between HIV+ serostatus and the cognitive-affective subtype of depressive symptomology (β = 0.35, p = .015). The interaction of HIV with sgACC to left amygdala rsFC was also significant (β = −0.66, p < .001) with this term explaining an additional 26.4% of the variance in the model ΔF(1, 38) = 17.89, p < .001. The model for somatic complaints was also not significant.
The overall model results indicate an effect for HIV+ serostatus on total as well as cognitive-affective depressive symptomology. The interaction of HIV status and rsFC of sgACC and bilateral amygdala suggests that lower positive connectivity of these regions of interest predicts greater depressive symptomology for HIV+ but not HIV− individuals (see Figures 3 & 4).
Figure 3.
Interaction between resting-state connectivity between the sgACC and the Right Amygdala and HIV status.
Figure 4.
Interaction between resting-state connectivity between the sgACC and the Left Amygdala and HIV status.
3.5. Comparison of ALFF with BDI and IL-6 in HIV+ subset
Demographic and HIV disease-related data for the subsample of HIV+ individuals (N=10) is presented in Table 1. A cluster of 714 voxels in the VMPFC centered around (x, y, z = 10, 38, −26, t = 7.64, p = 0.02) with a clusterwise correction was associated with higher BDI-1 scores and peripheral levels of IL-6 (see Figure 4). There was no significant activation associated with IL-8, IL-10, or TNF-α in the HIV+ subset.
4. Discussion
Previous work has shown elevated activity within the sgACC to be a signature of depressive symptomology in HIV-infected individuals (Kremer, Lutz et al. 2016). We expected that altered rsFC between the sgACC and regions of interest within the bilateral amygdala would correspond to the greater depressive symptomology in HIV+ participants. At rest, greater rsFC was observed between sgACC and several structures located throughout salience and default mode networks including right amygdala. Our model for total report of depressive symptomology revealed an interaction effect for HIV+ status and right as well as left amygdala connectivity with sgACC such that lower positive connectivity predicts greater depressive symptomology in HIV+ but not HIV− individuals. These effects also present for the cognitive-affective subtype although the effects of HIV on this subtype only trended towards significance. Also identified in a subset of 10 HIV+ individuals for whom cytokine assays were available were brain regions where greater spontaneous neural activity in the resting state correlated with higher peripheral levels of peripheral inflammation and depressive symptomology. Greater ALFF within a cluster of over 700 voxels in the ventromedial pre-frontal cortex, adjacent to the orbitofrontal cortex (OFC) and most rostral portions of the sgACC, was associated with higher total BDI-1 scores and plasma IL-6. What follows is a discussion of the implications of these preliminary findings as they relate to self-reported depressive symptoms in HIV-infected individuals.
Evidence of altered rsFC of the ACC and amygdala go back at least 15 years. Low frequency bold fluctuations compared between depressed patients and healthy controls revealed lower positive, i.e., greater negative connectivity of the ACC and left amygdala at rest as well as during presentation of negative picture stimuli (Anand, Li et al. 2005). Another study comparing depressed patients to healthy controls reported greater negative connectivity between a seed in the basolateral amygdala and both subgenual and rostral anterior cingulate (Tahmasian, Knight et al. 2013). In contrast, healthy controls show a pattern of positive connectivity between the basolateral amygdala and sgACC (Roy, Shehzad et al. 2009), effects that have been confirmed in task studies involving regulation of negative emotions (Ochsner, Ray et al. 2004). More recently adolescents with MDD showed significantly weaker bottom-up connectivity from amygdala to sgACC compared to healthy controls (Musgrove, Eberly et al. 2015). These studies suggest a regulatory effect for the ACC over limbic areas, i.e., amygdala, may contribute to the depressed state.
Despite considerable examination into structural abnormalities in subcortical regions such as the amygdala, with most but not all showing HIV-related atrophy over time (Ances, Ortega et al. 2012, Clark, Cohen et al. 2012, Towgood, Pitkanen et al. 2013, Janssen, Meulenbroek et al. 2015, Wade, Valcour et al. 2015), there is little known regarding connectivity with medial frontal structures. Assessing the extent of aberrant rsFC in persons with HIV is difficult because of the myriad of analytical approaches to conceptualizing brain connectivity. However, there is some indirect evidence that through the course of infection virally suppressed HIV+ individuals may be more prone rsFC alterations to the salience network. Prominent cortical and subcortical structures of the salience networks include anterior insula, cingulate, amygdala and thalamus state (Gusnard and Raichle 2001). For example, in a study comparing HIV-negative controls to a heterogenous group of HIV+ patients both on and off ART revealed patterns of decreased within- and between-network connectivity of the salience and default mode network as a function of HIV+ status and increasing age (Thomas, Brier et al. 2013). Coinciding, a study of HIV+ adults over the age of 60 used a ROI approach to assess connectivity within attention, control, sensorimotor, salience and default mode networks, and found no differences as a function of seropositivity but instead lower salience network connectivity in those with detectable viral load (Guha, Wang et al. 2016). More recently, a seed-based connectivity analysis of virally-suppressed patients with HIV-associated neurocognitive disorder was used to show lower rsFC within central executive and salience networks compared to demographically matched controls (Chaganti, Heinecke et al. 2017). Collectively, these findings beg the question of whether the virus or inflammatory pathways may preferentially alter rsfc within the salience network of HIV-positive individuals. Indeed, pre-clinical and clinical studies suggest acute and chronic inflammatory conditions and the resulting sickness-behavior may develop into depression and the catalyst for the neural injury which leads to this transition may mediated by pro-inflammatory activation of neurotoxic kynurenine metabolites including the NMDA-receptot agonist quinolinic acid (Dantzer 2016). Compared to other neurological diseases, activity within quinolinic acid and kynuerenine pathways are disproportionately higher in HIV-1 infection and has been attributed to neuropsychiatric symptoms in this population (Heyes, Saito et al. 1992, Gostner, Becker et al. 2015, Anderson, Croteau et al. 2018). Evidence has emerged which now implicates increasing levels of quinolinic acid on aberrant rsFC of the pgACC in adolescents with MDD (DeWitt, Bradley et al. 2018). With the emphasis in inflammatory- immune regulation shifting to pro-inflammatory peripheral blood mononuclear cells in chronic HIV-infection specific attention must be paid to the propensity of kynurenine pathway expression in these cells and their impact on functional connectivity of networks contributing salience and emotional processing (Jones, Franco et al. 2015).
The correlation between greater spontaneous activity in the VMPFC-OFC region and elevated levels of IL-6 and depressive symptomology observed in our HIV+ sample is supported by several fMRI studies. Studies linking peripheral levels of cytokines to limbic activity, albeit in response to affective visual stimuli, do implicate activity within the VMPFC area. Bereaved women with higher salivary concentrations of pro-inflammatory IL-1β and TNF-α receptors showed greater activity in the sgACC and OFC following presentation of grief-eliciting stimuli (O’Connor, Irwin et al. 2009). In another study, acute endotoxemia was found in response to bolus injection of bacterial lipopolysaccharide (Kullmann et al., 2013). The resulting increase of plasma TNF-α corresponded to enhanced activity in the right inferior OFC in response to emotional visual stimuli. Moreover, this effect was mediated by ACC and anterior insula activity (Kullmann, Grigoleit et al. 2013).
The role of the sgACC in depression is concomitant with the signaling of peripheral inflammation. Increased activity within the ACC and insula of individuals with asthma was observed during presentation of asthma-relevant emotional stimuli compared to neutral stimuli. Moreover, this activation accounted for over 40% of the increase in peripheral TNF-α within these asthmatic individuals (Rosenkranz et al., 2005). A recent positron emission tomography (PET) study showed that increased glutaminergic metabolism in the dorsal ACC, sgACC, and basal ganglia correlated with depressive symptomology following interferon therapy in individuals with Hepatitis C infection (Taylor et al., 2014). Increases in inflammatory cytokines associated with endotoxin administration have been correlated not only with elevations in depressive mood but also with higher normalized glucose metabolism in the right anterior insula (Hannestad et al., 2012). Another PET investigation of persons with MDD showed that plasma IL-18 levels were associated with higher baseline and sadness-evoked activity in the sgACC during an emotion regulation paradigm (Prossin et al., 2011). Further, results from PET reveal elevated translocator density protein levels, a marker of microglial activation, in the medial frontal cortex, anterior cingulate, and insula during major depressive episodes (Setiawan et al., 2015). This microglial activation may reflect pro-inflammatory cytokine infiltration through transporters at the blood-brain barrier (Vitkovic et al., 2000), although alternate mechanisms may involve peripheral neural (i.e., vagal or humoral) pathways involving Toll-like receptors (TLRs) on cells resembling macrophages circumventricular organs and choroid plexus (Quan et al., 1998, Dantzer et al., 2008). These findings converge on the unique role of the sgACC and right anterior insula in the representation of both peripheral inflammation and depression.
4.1. Limitations
The results presented here are not without limitations. Most evident are the small sample size and the non FWE-corrected results involving the BDI and cytokine biomarker data. To compensate for this we used a threshold correction that strikes a balance between type I and II errors (Lieberman and Cunningham 2009). Another potential limitation is the disproportionate number of males among the HIV+ participants. It is possible that the low number of females in this sample may have obscured some of the HIV-related findings, as gender effects have been observed for resting-state perigenual cingulate activity associated with depressive symptomology in HIV (Kremer et al., 2016).
4.2. Conclusions
Our data suggest that elevated levels of depression in HIV+ individuals are associated with less positive rsFC between the sgACC and bilateral amygdala. This aberrant pattern of connectivity may reflect lower top-down control of amygdala activity or reductions in neural integrity of the amygdala in response to cortical neurotransmission. Although the role of sgACC connectivity with subcortical limbic structures in mood disturbance has been previously documented these findings should be corroborated in a larger sample of clinically depressed HIV+ individuals. The finding of greater spontaneous brain activity in a region of the ventromedial prefrontal cortex adjacent to the sgACC may reflect the involvement of this structure in the signaling of peripheral inflammation to brain regions known to influence motivational state. It should be noted that neural, humoral, and cellular pathways participate in the communication of the peripheral inflammatory state to the brain. Future studies should take into account the effects of HIV-1 viral proteins such as Tat and gp120, which activate pro-inflammatory monocytes that may contribute to neurobehavioral disturbances upon transmigration across the blood-brain barrier (Barak, Goshen et al. 2002, Lawson, Kelley et al. 2011). Nonetheless, our findings add to a larger body of literature implicating the neural representation of inflammation and depression in chronic disease populations (Frodl and Amico 2014) and illustrate the utility of resting-state functional connectivity in helping to elucidate psychoneuroimmunological mechanisms of chronic inflammation and depression in HIV infection.
Table 4.
Hierarchical regression model for rsFC of right amygdala in prediction of depression
| Predictor | BDI Total Score | BDI Cognitive/Affect | BDI Somatic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| R2 | ΔR2 | ΔF2 | β | R2 | ΔR2 | ΔF2 | β | R2 | ΔR2 | ΔF2 | β | |
| Step 1: Demographic | .13 | .13 | 2.05 | .12 | .12 | 1.93 | .09 | .09 | 1.51 | |||
| Education | −.27 | −.20 | −.32* | |||||||||
| Sex | .14 | .16 | .04 | |||||||||
| Age | −.09` | −.14 | .06 | |||||||||
| Step 2 | .17 | .04 | .93 | .18 | .05 | 1.23 | .11 | .01 | .29 | |||
| R. Amygdala.-sgACC rsFC | −.11 | −.17 | .04 | |||||||||
| HIV status | .32* | .35* | .12 | |||||||||
| Step 3 | .36 | .19 | 11.55* | .44 | .26 | 17.9** | .13 | .02 | .80 | |||
| Interaction of HIV & R. Amygdala-sgACC rsFC | −.56* | −.66** | −.17 | |||||||||
p < 0.10.
p<0.05.
p< 0.01 and 0.0001
Table 5.
Hierarchical regression model for rsFC of left amygdala in prediction of depression
| Predictor | BDI Total Score | BDI Cognitive/Affect | BDI Somatic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| R2 | ΔR2 | ΔF2 | β | R2 | ΔR2 | ΔF2 | β | R2 | ΔR2 | ΔF2 | β | |
| Step 1: Demographic | .13 | .13 | 2.05 | .12 | .12 | 1.93 | .09 | .09 | 1.51 | |||
| Education | −.27` | −.20` | −.32* | |||||||||
| Sex | .14 | .16 | .04 | |||||||||
| Age | −.09 | −.14 | .06 | |||||||||
| Step 2 | .18 | .05 | 1.29 | .19 | .07 | 1.67 | .11 | .01 | .27 | |||
| L. Amyg.-sgACC rsFC | −.20 | −.25 | −.02 | |||||||||
| HIV status | .12 | −.11 | .12 | |||||||||
| Step 3 | .26 | .07 | 3.77` | .27 | .08 | 4.20* | .14 | .02 | 1.03 | |||
| Interaction of HIV & L. Amygdala-sgACC rsFC | −.35~ | −.37* | −.19 | |||||||||
p < 0.10.
p<0.05.
p< 0.01 and 0.0001
Acknowledgments
The authors thank the study participants, clinical and laboratory staff of the Hawaii Center for AIDS, and the staff at InVision Imaging. This study was supported by NIH grants U54RR026136, U54MD007584, R01HL095135 and R21 N5080656.
References
- Alexopoulos GS, et al. Functional connectivity in the cognitive control network and the default mode network in late-life depression. Journal of affective disorders. 2012;139(1):56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biological psychiatry. 2005;57(10):1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Ances BM, et al. Independent effects of HIV, aging, and HAART on brain volumetric measures. Journal of acquired immune deficiency syndromes (1999) 2012;59(5):469. doi: 10.1097/QAI.0b013e318249db17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AM, et al. HIV, prospective memory, and cerebrospinal fluid concentrations of quinolinic acid and phosphorylated Tau. Journal of neuroimmunology. 2018;319:13–18. doi: 10.1016/j.jneuroim.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. The Journal of pathology. 2008;214(2):231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- Barak O, et al. Involvement of brain cytokines in the neurobehavioral disturbances induced by HIV-1 glycoprotein120. Brain research. 2002;933(2):98–108. doi: 10.1016/s0006-8993(02)02280-1. [DOI] [PubMed] [Google Scholar]
- Beck AT, et al. An inventory for measuring depression. Archives of general psychiatry. 1961;4(6):561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Behrman-Lay AM, et al. Human immunodeficiency virus has similar effects on brain volumetrics and cognition in males and females. Journal of neurovirology. 2016;22(1):93–103. doi: 10.1007/s13365-015-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaganti J, et al. Functional Connectivity in Virally Suppressed Patients with HIV-Associated Neurocognitive Disorder: A Resting-State Analysis. American Journal of Neuroradiology. 2017;38(8):1623–1629. doi: 10.3174/ajnr.A5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumbley J, et al. Topological FDR for neuroimaging. Neuroimage. 2010;49(4):3057–3064. doi: 10.1016/j.neuroimage.2009.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, et al. Effects of HIV and early life stress on amygdala morphometry and neurocognitive function. Journal of the International Neuropsychological Society. 2012;18(4):657–668. doi: 10.1017/S1355617712000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, et al. High early life stress and aberrant amygdala activity: risk factors for elevated neuropsychiatric symptoms in HIV+ adults. Brain imaging and behavior. 2017;11(3):649–665. doi: 10.1007/s11682-016-9542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW. Psychosocial influences on HIV-1 disease progression: Neural, endocrine, and virologic mechanisms. Psychosomatic medicine. 2008;70(5):562–568. doi: 10.1097/PSY.0b013e3181773bbd. [DOI] [PubMed] [Google Scholar]
- Connolly CG, et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biological psychiatry. 2013;74(12):898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Inflammation-Associated Depression: Evidence, Mechanisms and Implications. Springer; 2016. Role of the Kynurenine metabolism pathway in inflammation- induced depression: preclinical approaches; pp. 117–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Guerra F, et al. Human immunodeficiency virus-associated depression: contributions of immuno- inflammatory, monoaminergic, neurodegenerative, and neurotrophic pathways. Journal of neurovirology. 2013;19(4):314–327. doi: 10.1007/s13365-013-0177-7. [DOI] [PubMed] [Google Scholar]
- DeWitt SJ, et al. A pilot resting-state functional connectivity study of the kynurenine pathway in adolescents with depression and healthy controls. Journal of affective disorders. 2018;227:752–758. doi: 10.1016/j.jad.2017.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Frontiers in systems neuroscience. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42(3):1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Frodl T, Amico F. Is there an association between peripheral immune markers and structural/functional neuroimaging findings? Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2014;48:295–303. doi: 10.1016/j.pnpbp.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Gostner JM, et al. Disturbed amino acid metabolism in HIV: association with neuropsychiatric symptoms. Frontiers in psychiatry. 2015;6:97. doi: 10.3389/fpsyt.2015.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, et al. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha A, et al. Intrinsic network connectivity abnormalities in HIV-infected individuals over age 60. Journal of neurovirology. 2016;22(1):80–87. doi: 10.1007/s13365-015-0370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W-b, et al. Alterations of the amplitude of low-frequency fluctuations in treatment-resistant and treatment-response depression: a resting-state fMRI study. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2012;37(1):153–160. doi: 10.1016/j.pnpbp.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Gupta R, et al. Depression and HIV in Botswana: a population-based study on gender-specific socioeconomic and behavioral correlates. PloS one. 2010;5(12):e14252. doi: 10.1371/journal.pone.0014252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2(10):685. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Harrison NA, et al. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological psychiatry. 2009;66(5):407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes M, et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992;115(5):1249–1273. doi: 10.1093/brain/115.5.1249. [DOI] [PubMed] [Google Scholar]
- Janssen MA, et al. Cognitive functioning, wellbeing and brain correlates in HIV-1 infected patients on long-term combination antiretroviral therapy. AIDS. 2015;29(16):2139–2148. doi: 10.1097/QAD.0000000000000824. [DOI] [PubMed] [Google Scholar]
- Jones SP, et al. Expression of the kynurenine pathway in human peripheral blood mononuclear cells: implications for inflammatory and neurodegenerative disease. PloS one. 2015;10(6):e0131389. doi: 10.1371/journal.pone.0131389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer H, et al. Interhemispheric asymmetries and theta activity in the rostral anterior cingulate cortex as EEG signature of HIV-related depression gender matters. Clinical EEG and neuroscience. 2016;47(2):96–104. doi: 10.1177/1550059414563306. [DOI] [PubMed] [Google Scholar]
- Kuller LH, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann JS, et al. Neural response to emotional stimuli during experimental human endotoxemia. Human brain mapping. 2013;34(9):2217–2227. doi: 10.1002/hbm.22063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson MA, et al. Intracerebroventricular administration of HIV-1 Tat induces brain cytokine and indoleamine 2, 3-dioxygenase expression: a possible mechanism for AIDS comorbid depression. Brain, behavior, and immunity. 2011;25(8):1569–1575. doi: 10.1016/j.bbi.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leserman J. HIV disease progression: depression, stress, and possible mechanisms. Biological psychiatry. 2003;54(3):295–306. doi: 10.1016/s0006-3223(03)00323-8. [DOI] [PubMed] [Google Scholar]
- Lewis CM, et al. Learning sculpts the spontaneous activity of the resting human brain. Proceedings of the national academy of sciences. 2009;106(41):17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social cognitive and affective neuroscience. 2009:nsp052. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, et al. Resting-state brain activity in major depressive disorder patients and their siblings. Journal of affective disorders. 2013;149(1):299–306. doi: 10.1016/j.jad.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Liu F, et al. Abnormal amplitude low-frequency oscillations in medication- naive, first-episode patients with major depressive disorder: a resting-state fMRI study. Journal of affective disorders. 2013;146(3):401–406. doi: 10.1016/j.jad.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Marchetti I, et al. The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychology review. 2012;22(3):229–251. doi: 10.1007/s11065-012-9199-9. [DOI] [PubMed] [Google Scholar]
- Musgrove DR, et al. Impaired bottom-up effective connectivity between amygdala and subgenual anterior cingulate cortex in unmedicated adolescents with major depression: results from a dynamic causal modeling analysis. Brain connectivity. 2015;5(10):608–619. doi: 10.1089/brain.2014.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MF, et al. When grief heats up: pro-inflammatory cytokines predict regional brain activation. Neuroimage. 2009;47(3):891–896. doi: 10.1016/j.neuroimage.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, et al. For better or for worse: neural systems supporting the cognitive down-and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Olley BO, et al. Psychopathology and coping in recently diagnosed HIV/AIDS patients-the role of gender. South African Medical Journal. 2003;93(12):928–931. [PubMed] [Google Scholar]
- Pala AN, et al. Subtypes of depressive symptoms and inflammatory biomarkers: An exploratory study on a sample of HIV-positive patients. Brain, behavior, and immunity. 2016 doi: 10.1016/j.bbi.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, et al. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin JG. HIV and depression: 2008 review and update. Current HIV/AIDS Reports. 2008;5(4):163–171. doi: 10.1007/s11904-008-0025-1. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, et al. Relationships among apathy, depression, and cognitive impairment in HIV/AIDS. The Journal of neuropsychiatry and clinical neurosciences. 2000;12(4):451–457. doi: 10.1176/jnp.12.4.451. [DOI] [PubMed] [Google Scholar]
- Roy AK, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45(2):614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler NG, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. Journal of Infectious Diseases. 2011;203(6):780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarinci IC, et al. Depression, socioeconomic status, age, and marital status in black women: a national study. Ethnicity & disease. 2002;12(3):421–428. [PubMed] [Google Scholar]
- Sheline YI, et al. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences. 2010;107(24):11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies G, et al. Effects of HIV and childhood trauma on brain morphometry and neurocognitive function. Journal of neurovirology. 2016;22(2):149–158. doi: 10.1007/s13365-015-0379-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadayonnejad R, et al. Clinical, cognitive, and functional connectivity correlations of resting-state intrinsic brain activity alterations in unmedicated depression. Journal of affective disorders. 2015;172:241–250. doi: 10.1016/j.jad.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasian M, et al. Aberrant intrinsic connectivity of hippocampus and amygdala overlap in the fronto-insular and dorsomedial-prefrontal cortex in major depressive disorder. Frontiers in human neuroscience. 2013;7:639. doi: 10.3389/fnhum.2013.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, et al. Effects of social adversity and HIV on subcortical shape and neurocognitive function. Brain imaging and behavior. 2018;12(1):96–108. doi: 10.1007/s11682-017-9676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JB, et al. Pathways to neurodegeneration Effects of HIV and aging on resting-state functional connectivity. Neurology. 2013;80(13):1186–1193. doi: 10.1212/WNL.0b013e318288792b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towgood KJ, et al. Regional cerebral blood flow and FDG uptake in asymptomatic HIV-1 men. Human brain mapping. 2013;34(10):2484–2493. doi: 10.1002/hbm.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade BS, et al. Mapping abnormal subcortical brain morphometry in an elderly HIV+ cohort. NeuroImage: Clinical. 2015;9:564–573. doi: 10.1016/j.nicl.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, et al. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage. 2007;36(1):144–152. doi: 10.1016/j.neuroimage.2007.01.054. [DOI] [PubMed] [Google Scholar]
- Zhu X, et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biological psychiatry. 2012;71(7):611–617. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Zou QH, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. Journal of neuroscience methods. 2008;172(1):137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]