Abstract
Background and Purpose
Endovascular therapy in an extended time window has been shown to be beneficial in selected patients. This study correlated angiographic outcomes of patients randomized to endovascular therapy with clinical and imaging outcomes in the DEFUSE 3 study.
Methods
Angiograms were assessed for the primary arterial occlusive lesion and the modified Thrombolysis in Cerebral Infarction (TICI) Score at baseline and the final modified TICI Score. Clinical outcomes were assessed using an ordinal analysis of 90 day modified Rankin Scale (mRS) and a dichotomous analysis for functional independence (mRS 0–2). TICI scores were correlated with outcome, types of device used for thrombectomy and 24-hour follow-up imaging.
Results
TICI 2B-3 reperfusion was achieved in 70 of 92 patients (76%). TICI 2B-3 reperfusion showed a more favorable distribution of Rankin scores compared to TICI 0–2A, OR=2.77 (95% CI 1.17–6.56), p=0.019. Good functional outcome (90-day mRS 0–2) increased with better TICI scores (p=0.0028). There was less disability comparing TICI 3 patients to TICI 2B patients (p=0.037). Successful reperfusion (TICI 2B-3) was independent of the device used, the site of occlusion (ICA or M1) or adjunctive use of carotid angioplasty and stenting. Significantly less infarct growth at 24 hours was seen in TICI 3 patients compared to TICI 0–2A (p=0.0015) and TICI 2B (p= 0.0002) patients.
Conclusions
Thrombectomy in an extended time window demonstrates similar rates of TICI 2B-3 reperfusion to earlier time window studies. Successful reperfusion was independent of the device used, the site of occlusion or adjunctive use of carotid angioplasty and stenting. TICI 3 reperfusion was more likely to result in low rates of infarct growth at 24 hours and good functional outcome at 90 days.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02586415
Keywords: stroke, thrombectomy, reperfusion
Subject Terms: ischemic stroke, angiography, revascularization
INTRODUCTION
Endovascular thrombectomy improves outcome for acute ischemic stroke patients with large artery anterior circulation strokes, when performed within 6 hours of stroke onset1. Recently two trials demonstrated benefit extended to 16–24 hours after last seen well in selected patients2, 3. The DEFUSE 3 trial was a randomized study comparing medical therapy to endovascular treatment plus medical therapy 6–16-hours after last known well3.
DEFUSE 3 utilized FDA cleared thrombectomy devices for use in an 8-hour time window. Data from randomized studies prior to DAWN and DEFUSE 3 provides limited information about the effectiveness of these devices beyond 6 hours4. In addition, data from DEFUSE 3 offers an opportunity to correlate angiographic findings before and after endovascular treatment with baseline and follow-up non-invasive imaging. This study correlates the angiographic findings at endovascular treatment with clinical outcome, device choice and non-invasive imaging.
METHODS
The study protocol was previously described5. DEFUSE 3 was a multicenter, prospective, randomized, trial enrolling patients for endovascular therapy 6–16 hours after last known well. Subjects were randomized 1:1 to endovascular therapy plus medical therapy versus medical therapy alone. Ethics approval was obtained from the local institutional review board at each center and written informed consent was obtained from patients. The study was halted in May 2017, when an early interim analysis demonstrated efficacy according to the pre-specified efficacy boundary with 182 patients randomized, 92 to endovascular treatment and 90 to medical treatment. Anonymized data from the DEFUSE 3 study will be made publicly available at the NIH repository for open data.
Imaging eligibility was determined by either CT perfusion or MR perfusion-diffusion studies showing a Target Mismatch Profile, previously described5. Mismatch between core and salvageable ischemic tissue (penumbra) were determined from CT or MR using a previously described automated processing system (RAPID, iSchemaView, Menlo Park, CA- versions 4.51 and 4.6)6, 7. Patients also had baseline MRA (Magnetic Resonance Angiography) or CTA (Computed Tomographic Angiography) to confirm an intracranial ICA (Internal Carotid Artery) or MCA (Middle Cerebral Artery) M1 occlusion.
Endovascular Therapy
Interventionalists were pre-approved based on training and post-training experience as previously described3. Interventionalists could use any FDA approved device for thrombectomy (approved for use within an 8-hour window following stroke onset and used in the DEFUSE 3 study with an FDA investigational device exemption). The devices used were the Trevo Retriever (Stryker Neurovascular, Fremont CA), the Solitaire Revascularization Device (Medtronic, Irvine, CA), Covidien MindFrame Capture Revascularization Device (Medtronic), and the Penumbra Suction Thrombectomy system (Penumbra, Alameda, CA).
The interventionalist could utilize any device or combination of devices in the ICA or MCA M1 segment. They could also use the devices to remove thrombus from MCA M2 segments. If the interventionalists encountered severe common or proximal ICA stenosis or occlusion, they could also perform angioplasty or stenting with FDA approved devices. Adjuvant intraarterial thrombolytic agents were prohibited in DEFUSE 3.
Outcome Measures
The modified Rankin Scale was determined at day 90. An ordinal analysis of Rankin Scores was performed and functional independence at day 90, defined as a modified Rankin Scale score 0–2, was also determined.
Follow-up imaging was performed at 24 (±6) hours with MRI (CT was allowed if MR was not possible). This was utilized to determine (1) Lesion growth between baseline and 24 hours; (2) Successful reperfusion, (>90% Tmax>6sec lesion volume reduction), and (3) recanalization on CTA/MRA. Baseline and follow-up MRI and CT images were assessed by core lab readers blinded to randomization assignment.
Angiographic Assessment
Sites were asked to provide a baseline pre-treatment angiogram and a final post-treatment angiogram of the involved carotid circulation. Angiographic studies were assessed at baseline for the primary arterial occlusive lesion (AOL) and the modified Thrombolysis in Cerebral Infarction (TICI) Score8. The final angiogram was assessed for the modified TICI score. A lateral projection was available for the final angiogram in 90 cases. In the two cases where this was not available the primary AOL had not been reperfused and the TICI score was 0. The endovascular procedure was deemed successful if the final angiogram demonstrated a modified TICI score of 2b (50 to 99% reperfusion) or 3 (complete reperfusion). Angiographic studies were evaluated by 2 independent raters at the core laboratory at Stanford and, in cases of disagreement, a consensus reading was used.
Statistical Analysis
Recanalization categories were compared with respect to the 90-day mRS distribution via Wilcoxon rank-sum test, and the effect of reperfusion is estimated via the proportional odds model. Univariate association between categorical variables were assessed using Chi-square test. Gradual change in the good functional outcome rates, recanalization and reperfusion across levels of TICI reperfusion were assessed by the Cochran-Armitage test. Exact tests were used when cell counts were <5 for categorical variables. Infarct growth between levels of TICI reperfusion were compared by Wilcoxon rank-sum test.
All statistical tests were two-sided and conducted using level of significance 0.05. Statistical analysis was done with SAS version 9.4.
RESULTS
Baseline clinical and non-invasive imaging data for the 92 patients randomized to endovascular therapy are presented in Table I (Data Supplement). Baseline angiograms showed 35 patients (38%) had ICA-CCA (Common Carotid Artery) occlusions, 54 (59%) had MCA M1 occlusions, 2 (2%) had MCA M2 occlusions, and one patient (1%) had no occlusion. Baseline TICI scores of 0–1 were present in 90 patients, one patient was TICI 2A and one TICI 3. The final angiogram showed TICI 2B-3 reperfusion in 70 patients (76%), with 52 (57%) TICI 2B and 18 (20%) TICI 3. In addition, 10 patients (11%) were TICI 0 and 12 (13%) were TICI 2A. The TICI 2B-3 rates were similar when the AOL was the carotid artery (74%) versus the MCA M1 (76%). The two readers TICI scoring agreed in 85/92=92% (95% CI 85–96), with a Kappa of 0.87 (95% CI 0.78–0.97).
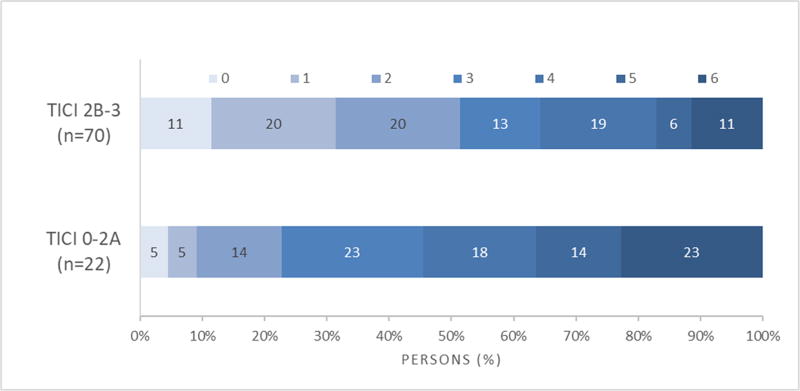
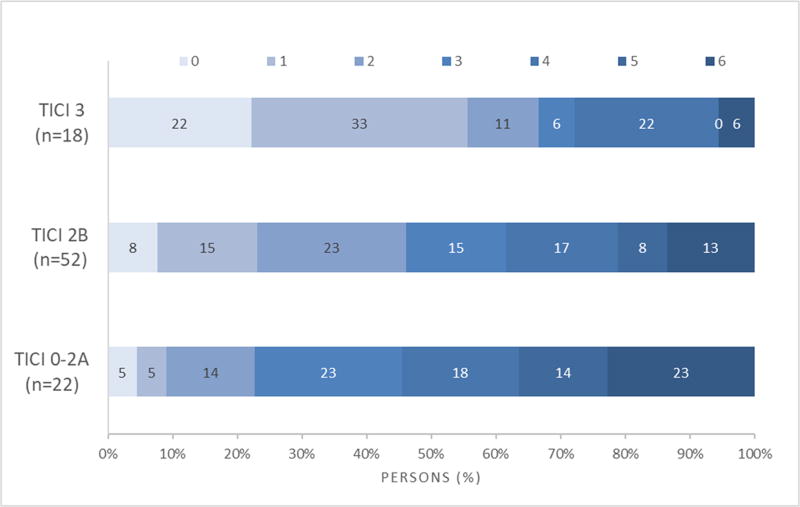
Figure 1 shows the 90-day Rankin score distribution stratified by TICI scores. There was a more favorable distribution of scores (less disability) comparing TICI 2B-3 patients to TICI 0–2A patients, OR=2.77 (95% CI 1.17–6.56), p=0.019 (Figure 1A). There was less disability comparing TICI 3 patients to TICI 2B patients (p=0.037). However, there was not a significant difference in disability comparing TICI 2B patients to TICI 0–2A patients (p=0.07) (Figure 1B). Table 1 shows the rates of good functional outcome (90-day mRS 0–2) by TICI score, which consistently increased with better TICI scores (p=0.0028). TICI score was an independent predictor of good functional outcome (p=0.006, for TICI 0–2A, 2B, 3) and there was no interaction found between TICI score and age (p=0.956) or baseline NIHSS (p=0.384).
Figure 1.
A 90-day mRS distribution stratified by TICI 0–2A, 2B-3
B 90-day mRS distribution stratified by TICI 0–2A, 2B and 3
Table 1.
TICI Score versus Good Functional Outcome
| TICI Score | mRS 0–2 N (%) |
|---|---|
| 0(n=10) | 1 (10%) |
| 2A (n=12) | 4 (33%) |
| 2B (n=52) | 24 (46%) |
| 3 (n=18) | 12 (67%) |
Table II (Supplementary Material) shows the recanalization, reperfusion and infarct growth rates at 24-hour follow-up imaging stratified by TICI score. Rates of recanalization significantly increased across higher TICI scores (p=0.0004) as did rates of successful reperfusion (p<0.0001). The decline in infarct growth however was not found to be gradual across TICI scores. While there was no difference between TICI 0–2A and 2B (p=0.887), infarct growth was attenuated with TICI 3 reperfusion compared to TICI 0–2A (p=0.0015) and TICI 2B (p= 0.0002).
Ninety of 92 patients (98%) randomized to endovascular therapy had an intervention. Eighty-eight had attempted thrombectomy, 87 with approved devices and one with placement of a self-expanding stent. Two patients had ICA stenting alone without subsequent thrombectomy. Eleven of 88 patients with attempted thrombectomy also had extracranial carotid angioplasty or stenting. Two patients with cervical carotid occlusions (CCA and ICA) had no intervention because the endovascular therapist felt that treatment was not feasible. Table III (Supplementary Material) displays the angiographic and clinical results when aspiration or stent retrievers were used as first-line therapy. There were no differences in successful reperfusion (TICI 2B-3) or good functional outcome between the two groups, however, treatment with the alternative therapy was used more frequently when aspiration was used first. There were 5 protocol violations and 2 device related complications (described in Supplementary Material).
Eleven patients with carotid angioplasty or stenting and attempted thrombectomy did not have significantly different TICI 2B-3 rates (82%, 9/11) or rates of good functional outcome (45%, 5/11) when compared to the 76 patients that were treated solely with approved thrombectomy devices (TICI 2B-3 76%, 58/76, p=0.73; GFO 45%, 34/76, p=1.0). Two other patients who underwent carotid stenting without thrombectomy had mRS scores of 2 and 5 at 90 days.
Interventionalists performed 2.3 ± 1.5 (mean ± SD) passes in the thrombectomy patients. The rates of good functional outcome at 90 days (mRS 0–2) were similar for patients treated with one pass (50%, 16/32) or two passes (56%, 14/25), and were significantly higher than those patients treated with ≥ 3 passes (27%, 8/30) [p=0.024].
DISCUSSION
Successful reperfusion (TICI 2B-3) was achieved in 76% of the patients treated in this extended time window using FDA approved thrombectomy devices. This rate is similar to the HERMES meta-analysis of earlier time window trials (71%) and the recently reported DAWN trial (84%)1,2. There was no difference in the TICI 2B-3 rates achieved if the first-line device was a suction thrombectomy catheter or a stent retriever, consistent with the results of an earlier time window trial, which also showed comparable TICI 2B-3 rates for the two techniques.9 In our study, interventionalists did switch to the alternative device more frequently when they started with a suction thrombectomy catheter. DEFUSE 3 allowed interventionalists to perform angioplasty and stenting of the cervical carotid artery. This added procedure did not adversely affect TICI 2B-3 rates or outcomes when compared to patients treated solely with thrombectomy devices.
TICI 2–3 reperfusion has been used as a standard for FDA thrombectomy device approval studies evaluating devices in an earlier time window10, 11, and TICI 2B-3 reperfusion has been considered a benchmark for successful reperfusion in other randomized trials1. TICI 2B-3 reperfusion has also been identified in a consensus statement as successful reperfusion8. Our results show the degree of TICI reperfusion was an independent predictor of good outcome and TICI 3 reperfusion results in better clinical outcomes than TICI 2B. Similarly, TICI 3 reperfusion has been shown in an earlier time window retrospective study to have significantly better outcomes when compared to TICI 2B reperfusion12. These differences in clinical outcome between TICI 2B and TICI 3 are supported by our finding that TICI 3 reperfusers had significantly less infarct growth on follow-up imaging (median 5 ml, IQR 1–15) when compared to TICI 2B patients (median 32 ml, IQR 14–103). The infarct growth measurement at 24 hours may not represent the full size of the eventual infarct as there is evidence that final infarct volume is not realized for several days in tissue that is not reperfused13. However, the values we measured suggest that TICI 3 reperfusion freezes the infarct size when compared to TICI 2B. These results suggest the potential importance of striving for full TICI 3 reperfusion.
These results also suggest the ability to reperfuse large arteries with current devices is not adversely affected by treatment in an extended time window. Several factors have been postulated to influence the ability to reperfuse large artery occlusions in acute ischemic stroke, including collateral status14, 15. Patents with good collaterals have been shown to have higher rates of reperfusion14, 15. DEFUSE 3 selected patients with a good collateral status by using a mismatch profile yielding relatively small cores in an extended time window. An important limitation of the present study is the limited sample size for analysis.
In conclusion, endovascular treatment with FDA approved devices in an extended time window yielded similar rates of TICI 2B-3 reperfusion to earlier time window studies. Rates of good reperfusion were independent of the device used, the site of occlusion or adjunctive use of carotid angioplasty and stenting. TICI 3 reperfusion was associated with less infarct growth at 24 hours and a higher rate of good functional outcome at 90 days.
Supplementary Material
Acknowledgments
FUNDING
NIH NINDS U10NS086487 and U01NS092076
Footnotes
DISCLOSURES
MP Marks- Thrombx Medical- stock
JJ Heit-Medtronic- consultant
S Christensen- IschemiaView- Stock and consultant
CP Derdeyn- Pulse Therapeutics- scientific advisory board, stock options; Bayer- honorarium
PA Rasmussen - Modest: Stryker Neurovascular, Medtronic Neurovascular, Neurvive Medical, Neurvana Medical, Boston Scientific, Perflow; Significant, Mehana Medical
OO Zaidat- Stryker, Penumbra, Neuravi, and Medtronic-consultant
SD Yeatts- Genentech-consultant
GW Albers- iSchemaView- stock; iSchemaView and Medtronic-consultant
Contributor Information
Michael P Marks, Stanford Stroke Center, Stanford, CA.
Jeremy J Heit, Stanford Stroke Center, Stanford, CA.
Maarten G Lansberg, Stanford Stroke Center, Stanford, CA.
Stephanie Kemp, Stanford Stroke Center, Stanford, CA.
Soren Christensen, Stanford Stroke Center, Stanford, CA.
Colin P Derdeyn, Department of Radiology, University of Iowa, Iowa City, IA.
Peter A Rasmussen, Department of Neurological Surgery and the Cerebrovascular Center, Cleveland Clinic, Cleveland, OH.
Osama O. Zaidat, Mercy Health, Toledo, OH.
Joseph P Broderick, Department of Neurology, University of Cincinnati, Cincinnati, OH.
Sharon D Yeatts, Medical University of South Carolina, Charleston SC.
Scott Hamilton, Stanford Stroke Center, Stanford, CA.
Michael Mlynash, Stanford Stroke Center, Stanford, CA.
Gregory W Albers, Stanford Stroke Center, Stanford, CA.
References
- 1.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–31. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 2.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 3.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018;378:708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, Lugt AVD, Menon BK, Majoie CB, Dippel DW, et al. Time to Treatment with Endovascular Thrombectomy and Outcomes from Ischemic Stroke: A Meta-analysis. JAMA. 2016;316(12):1279–88. doi: 10.1001/jama.2016.13647. [DOI] [PubMed] [Google Scholar]
- 5.Albers GW, Lansberg MG, Kemp S, Tsai JP, Lavori P, Christensen S, et al. A multicenter randomized controlled trial of endovascular therapy following imaging evaluation for ischemic stroke (DEFUSE 3) Int J Stroke. 2017;12:896–905. doi: 10.1177/1747493017701147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurology. 2012;11:860–7. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lansberg MG, Christensen S, Kemp S, Mlynash M, Mishra N, Federau C, et al. Computed tomographic perfusion to Predict Response to Recanalization in ischemic stroke. Ann Neurol. 2017;81:849–56. doi: 10.1002/ana.24953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–63. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lapergue B, Blanc R, Gory B, Labreuche J, Duhamel A, Marnat G, et al. Effect of Endovascular Contact Aspiration vs Stent Retriever on Revascularization in Patients With Acute Ischemic Stroke and Large Vessel Occlusion: The ASTER Randomized Clinical Trial. JAMA. 2017;318:443–452. doi: 10.1001/jama.2017.9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nogueira RG, Frei D, Kirmani JF, Zaidat O, Lopes D, Turk AS, 3rd, et al. Safety and Efficacy of a 3-Dimensional Stent Retriever With Aspiration-Based Thrombectomy vs Aspiration-Based Thrombectomy Alone in Acute Ischemic Stroke Intervention, A Randomized Clinical Trial. JAMA Neurology. 2018;75:304–311. doi: 10.1001/jamaneurol.2017.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380:1231–40. doi: 10.1016/S0140-6736(12)61299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dargazanli C, Consoli A, Barral M, Labreuche J, Redjem H, Ciccio G, et al. Impact of Modified TICI 3 versus Modified TICI 2b Reperfusion Score to Predict Good Outcome following Endovascular Therapy. AJNR. 2017;38:90–96. doi: 10.3174/ajnr.A4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Federau C, Mlynash M, Christensen S, Zaharchuk G, Cha B, Lansberg MG, et al. Evolution of Volume and Signal Intensity on Fluid-attenuated Inversion Recovery MR Images after Endovascular Stroke Therapy. Radiology. 2016;280:184–92. doi: 10.1148/radiol.2015151586. [DOI] [PubMed] [Google Scholar]
- 14.Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011;42:693–9. doi: 10.1161/STROKEAHA.110.595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liebeskind DS, Tomsick TA, Foster LD, Yeatts SD, Carrozzella J, Demchuk AM, et al. Collaterals at angiography and outcomes in the interventional management of stroke (IMS) III Trial. Stroke. 2014;45:759–64. doi: 10.1161/STROKEAHA.113.004072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.