Abstract
Aim
To determine the efficacy of human relaxin-2 (hRLX2) in reversing radiation-induced bladder fibrosis and lower urinary tract dysfunction (LUTD). Radiation cystitis is a consequence of radiotherapy for pelvic malignancies. Acutely, irradiation leads to reactive oxygen/nitrogen species in urothelial cells, apoptosis, barrier disruption, and inflammation. Chronically, this results in collagen deposition, bladder fibrosis, and attenuated storage and voiding functions. In severe cases, cystectomies are performed as current therapies do not reverse fibrosis.
Methods
We developed a mouse model for selective bladder irradiation (10 Gray; 1 Gy = 100 rads) resulting in chronic fibrosis within 6 weeks, with decreased bladder compliance, contractility, and overflow incontinence. Seven weeks post-irradiation, female C57Bl/6 mice were continuously infused with hRLX2 (400 μg/kg/day/14 days) or vehicle (saline) via subcutaneous osmotic pumps. Mice were evaluated in vivo using urine spot analysis, cystometrograms and external urethral sphincter electromyograms; and in vitro using length-tension measurements, Western blots, histology, and immunohistochemistry.
Results
hRLX2 reversed fibrosis, decreased collagen content, improved bladder wall architecture, and increased bladder compliance, detrusor smooth muscle Cav1.2 expression and detrusor contractility in mice with chronic radiation cystitis. hRLX2 treatment outcomes were likely caused by the activation of RXFP1/2 receptors which are expressed on the detrusor.
Conclusion
hRLX2 may be a new therapeutic option for rescuing bladders with chronic radiation cystitis.
Keywords: Cav1.2, fibrosis, human relaxin-2 (hRLX2), matrix metalloproteases (MMPs), radiotherapy
1 | INTRODUCTION
1.1 | Radiation cystitis
Radiation is a major intervention in treating pelvic organ malignancies. However, the risk for developing complications such as radiation cystitis limits the radiation dose.1 The consequences of radiotherapy include a dose-dependent detrim ental effect on normal organ function within the irradiated field.2
Chronic radiation cystitis can develop 6–12 months following radiotherapy with a prevalence of ~7%.3 Consequences include vascular endothelial cell damage, inflammation, ischemia, collagen deposition, and decreased bladder compliance.3 A major feature of the chronic phase is mild to life-threatening hematuria.4 Severely decreased bladder compliance due to collagen deposition can impair ureteric emptying causing renal dysfunction. Voiding failure can develop as the detrusor becomes progressively underactive and eventually acontractile. Ultimately the patient may require a cystectomy, to prevent renal failure and preserve some quality of life. Current therapies include anticholinergic agents and β3-adrenergic receptor agonists for frequency and urgency, pain relief medications, cranberry juice capsules, or instillation of hyaluronic acid and/or chondroitin sulfate which are symptomatic, invasive, and often ineffective. Crucially, they do not reverse fibrosis to improve bladder compliance, and in theory could worsen it. There is currently no effective treatment to reverse bladder fibrosis, so it remains an unmet public health problem.
1.2 | Contributing mechanisms of bladder fibrosis
The urinary bladder is composed of mucosa and muscular detrusor layers. The mucosa includes the urothelium and lamina propria; with the latter containing an extracellular matrix (ECM), composed of elastin, collagen-I and -III fibers. The ECM provides strength during contractions, high compliance during relaxation, and low pressure storage of urine.5 Collagen is continuously synthesized and degraded, but locally produced proinflammatory cytokines and other activators can impair this homeostatic balance by transforming fibroblasts to myofibroblasts to drive fibrosis.6 Transforming growth factor beta-1 (TGF-β1) is implicated in the stimulation of membrane-cytoskeletal structural protein formation and in the synthesis of ECM through multiple signaling pathways.7 TGF-β1 exerts ECM-preserving actions by suppressing matrix metalloproteinases (MMPs) activity and by inducing synthesis of protease inhibitors, such as tissue inhibitor of metalloproteinase (TIMP).
1.3 | Relaxin hormone
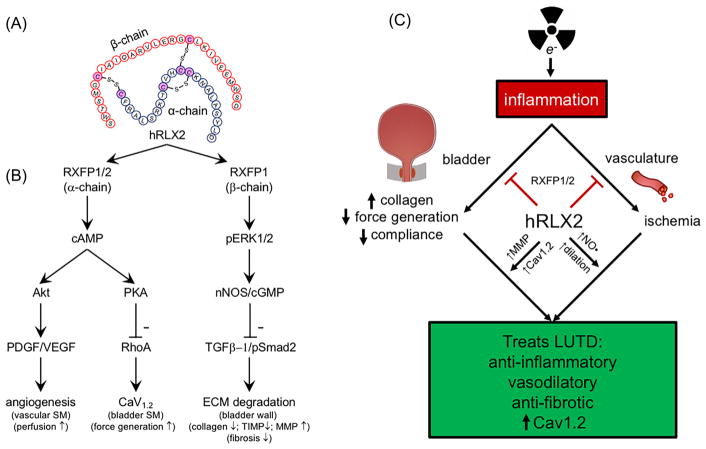
Relaxin is a 6 kilodalton hormone first described in 1926 for inducing relaxation of uterine smooth muscle and softening of the pubic symphysis during pregnancy. However, this direct relaxation effect has only been observed on the uterine tissue of humans, pigs, and rodents during pregnancy.8 Non-pregnant pigs9 and rats10–12 must be pre-treated with high-dose estrogen for at least 3 days to induce direct relaxation of uterine smooth muscle. Direct relaxing effects of the hormone have not been reported for other smooth muscle including bladder. This 6 kilodalton hormone is also produced in the prostate and testes to enhance sperm motility.13 It belongs to the insulin superfamily that includes relaxin-1 to -4 and insulin-like peptide-3 to -6. Peptides signal through four G-protein coupled receptors (RXFP1-4), with RXFP1 having the highest affinity for hRLX2.14
The differential effects of hRLX2 are hypothesized to be mediated by at least three pathways where two are activated by the α-chain acting through RXFP1/2 and a third by the β-chain acting on RXFP1 (Figure 1). The α-chain pathway elicits elevations of cAMP that enhance smooth muscle contractility through protein kinase A (PKA) inhibition of RhoA, which may also increase the expression of voltage-gated Ca2+ channel currents.15 The cAMP pathway is also involved in enhancement of proangiogenic signaling. The β-chain is mediated through pERK1/2 and the cGMP pathway.16
FIGURE 1.
Structure of hRLX2, hypothetical pathway intermediates of RXFP1/2 and the benefits of hRLX2 therapy in radiation cystitis. A and B, The α-chain of hRLX2 can bind to RXFP1/2 receptors located on detrusor smooth muscle to increase cAMP levels and the expression of Cav1.2 (potentially via inhibition of RhoA activity) resulting in enhancement of force generation. hRLX2-mediated cAMP generation in the bladder vasculature may also increase Akt phosphorylation, platelet derived growth factor (PDGF), and vascular endothelial growth factor (VEGF) expression to promote angiogenesis. The β-chain of hRLX2 can interact with RXFP1 to selectively stimulate pERK1/2 pathways upregulating nNOS and cGMP levels. This leads to decreased collagen synthesis and tissue inhibitors of matrix metalloproteases (TIMP), and increased matrix metalloprotease (MMP) expression to reverse fibrosis in the ECM. C, One of the initial responses following radiation exposure is inflammation due to urothelial apoptosis and urine infiltration. Concurrently, there is damage to the vascular endothelium leading to ischemia. These processes cause increased collagen deposition, and decreased bladder compliance and force generation. Treatment with hRLX2 reverses fibrosis through inhibition of collagen synthesis and enhancement of its degradation by MMPs. It also enhances contractile function through increased Cav1.2 (ie, L-type Ca2+ channel) expression and improved tissue perfusion via NO• induced vasodilation. hRLX2 is also anti-inflammatory, inhibiting recurrent damage to the bladder wall
RXFP1 activation by the β-chain increases phosphorylation of extracellular signal-regulated protein kinase 1 and 2 (pERK1/2) that enhances neuronal nitric oxide synthase (NOS) activity and cyclic guanosine monophosphate (cGMP) generation.14 Activation of this pathway disrupts profibrotic TGF-β/Smad2 phosphorylation (pSmad2) signaling17 to inhibit collagen synthesis, promote expression of MMPs,18 and decrease expression of TIMPs.7 RXFP1 may potentially prevent further inflammatory responses by directly inhibiting immune cell activation.19
The present study on the potential efficacy of relaxin in irradiation-induced fibroses in LUT organs was motivated by the published clinical findings on the antifibrotic properties of hRLX2 in acute heart failure.20 Furthermore, neither the expression of RXFP receptors nor the effect of relaxin peptides in the LUT have been described. In this study, we utilized a mouse model of selective bladder irradiation to demonstrate the efficacy of hRLX2 in reversing the fibrotic consequences of chronic radiation cystitis.
2 | METHODS
2.1 | Selective bladder irradiation
Adult female C57Bl/6 mice (6–18 weeks, Envigo Labs, Fredrick, MD) were anesthetized with 2,2-tribromoethanol (300 mg/kg, intraperitoneal) and a small incision was made into the lower abdominal wall to expose the bladder. A suture was tied to the urachus and mice placed sideways on a Lexan platform, allowing the organ to be externalized during irradiation (Figure 2A). Bladders were catheterized with a FEP shield from a 24 Ga BD angiocath, emptied and filled with 75 μL of saline for standardization. Mice were placed in an X-RAD 320 biological irradiator (Precision X-Ray, North Branford, CT) and the collimator and table height adjusted to focus the irradiation beam to ensure that only the bladder was irradiated. After delivery of a 10 Gy irradiation dose, the bladder was returned to the abdominal cavity, the incision sutured, and mice allowed to recover for up to 10 weeks. ALZET© osmotic pumps (model 1002, Cupertino, CA) filled with saline (control) or recombinant hRLX2 (50–400 μg/kg/day) were implanted subcutaneously at the lower mid-region of the animal’s back 7 weeks post-irradiation. Animals were used for experiments 15 days following implantation.
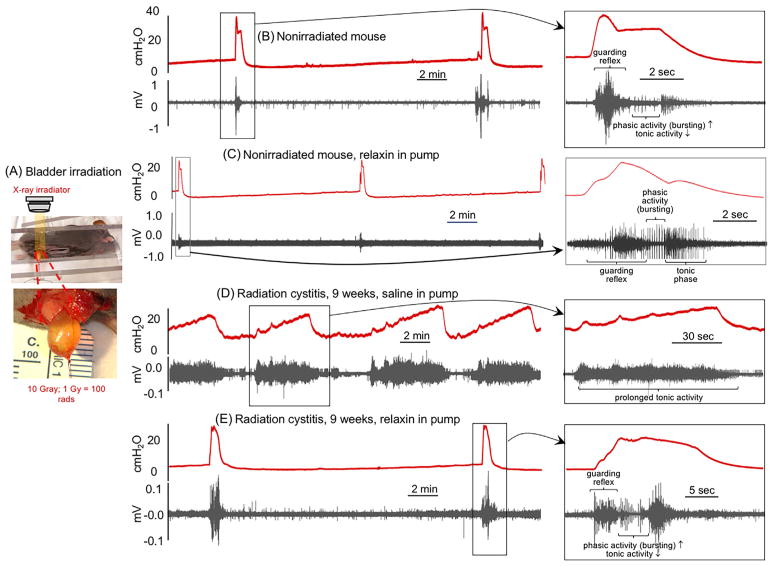
FIGURE 2.
Bladder cystometrograms (CMGs) and external urethral sphincter (EUS) electromyograms (EMGs) from irradiated mice with and without hRLX2 treatment. A, Method for selective irradiation of the urinary bladder. B–E, CMGs/EUS-EMGs in decerebrated mice. B, Control, nonirradiated mouse (N = 6). C, Nonirradiated mouse treated with hRLX2 (400 μg/kg/day) for 2 weeks (N = 2). D, Irradiated mouse with saline infusion via a subcutaneous osmotic pump for 2 weeks (N = 6). E, Irradiated mouse with hRLX2 infusion (400 μg/kg/day) via a subcutaneous osmotic pump for 2 weeks (N = 4). Treatment in D and E commenced 7 weeks after irradiation. hRLX2 treated mice exhibited more efficient voiding, longer intercontractile intervals, higher bladder compliances, and a normalized EUS activity
2.2 | Urine spot assay
Mice were placed individually for 2 h, between 11:00 am and 2:00 pm, in identical clean metabolic cages lined with Whatman filter paper. Food and water were withheld during this period. At the end, filter papers were collected for illumination with UV light and images retained as TIFF files for analysis with ImageJ software as reported previously.21 Briefly, images were grayscaled, inverted, auto-thresholded using “Max entropy” method, converted to binary and an “analyze particles” function used to exclude particles smaller than 135 pixel2 (<0.5 μL). A calibration curve corresponding spot sizes to volumes was built based on known volumes of urine pipetted on the filter paper. Linear relation was confirmed with R2 = 0.9975, where volume [μL] = 0.0037 × spot size [pixel2] (Supplemental Figure S9).
2.3 | Cystometrogram/electromyogram (CMG/ EMG) recordings from decerebrate mice
Mice were anesthetized using isoflurane (5% induction/2% maintenance in O2) and an incision made into the neck to expose the carotid arteries and the trachea. Ligatures were placed around the carotid arteries to decrease cerebral blood flow and a tracheotomy was performed using PE-60 tubing connected to the anesthesia delivery system. A craniotomy was performed and the brain rostral to the supracollicular level sectioned away. Decerebration permits bladder cystometry without the use of anesthetics, which can modify reflex bladder contractions. A PE-50 catheter was inserted through the bladder dome, secured using a suture and connected to a pressure transducer and syringe pump. Two epoxy-coated, stainless steel 50 μm EMG electrodes were inserted transperineally 1 mm lateral to the mid urethra to record from the EUS. To perform voiding cystometry, the bladder was manually emptied and then filled with saline at 0.01 mL/min until reflex contractions were elicited. Bladder compliance was calculated as the volume infused into the bladder between two consequent contractions divided by the difference between pressure threshold and baseline pressure [μL/cmH2O]. Voided and residual volumes were estimated knowing the volume of saline infused.
2.4 | Blood collection and assays of exogenously administered hRLX2 or endogenous mRLX1
Micro-hematocrit capillary tubes were used to collect 100–150 μL of blood from mouse tails. Tubes were centrifuged at 20 000g for 15 min to separate plasma from the cellular components. Plasma samples were rapidly frozen and stored at −80°C until used for ELISA measurements of hRLX2 (R&D Systems, Minneapolis, MN) or mRLX1 (LifeSpan BioScience, Seattle, WA).
2.5 | In vitro measurement of passive and active contractile properties
Strips of bladder (8 mm by 1–2 mm) were obtained by cutting the bladder along the ventral midline. These were mounted in a temperature-controlled recording chamber22 and connected to an isometric tension transducer and an anchor connected to a computer-controlled stepper motor to implement stretch protocols. Strips were superfused with a modified Tyrode’s solution23 and maintained at 36 ± 0.5°C. The baseline was stabilized and electrical field stimulation (EFS) with platinum electrodes (20 Hz, 3 s train, 0.1 ms pulse width) was performed. Preparations were stretched incrementally to their optimal length (LO) at which peak EFS contractions are elicited24 and subsequent stretches resulted in decreased contractions. EFS stimulation was then switched off and responses to muscarinic (oxotremorine-M, 0.1–10 μM) and KCl-induced depolarization (120 mM) were examined. All forces were normalized to cross-sectional area and expressed as milli-Newtons per milli-meter squared (mN/mm2).
2.6 | Histology
Mice were treated with 200 units of heparin (intraperitoneal injection), anesthetized using 2,2-tribromoethanol and transcardially perfused with oxygenated Krebs solution before removal of the bladders. Bladders were cut open along the ventral aspect from urethra to dome and flattened between glass plates in 10% buffered formalin for 1 h; then fixed overnight without the plates, embedded in paraffin and 2 μm sections cut. Sections were stained with Van Gieson Solution (Sigma, St. Louis, MO) and visualized using bright field microscopy. The percentage of collagen per total tissue area was calculated using ImageJ software from three TIFF images per section.
2.7 | Immunofluorescence
Bladders were isolated, cut open into sheets, placed into cryo-molds, covered in optimal cutting temperature medium, and frozen on dry ice. Slide mounted sections (10 μm) were post-fixed in 4% paraformaldehyde and blocked with 10% donkey serum in 1× tris buffered saline + 0.1% Triton X-100. Sections were incubated overnight with antibodies against RXFP1, RXFP2 (Santa Cruz Biotechnology, Dallas, TX), α-smooth muscle actin (Abcam, Cambridge, MA) or Cav1.2 (Alomone Labs, Jerusalem, Israel) followed by incubation in Alexa Fluor (488 and 594 nm) anti-rabbit or goat IgG conjugates and DAPI for nuclear staining and examined using widefield or confocal fluorescence microscopy. For negative control, the primary antibody was omitted in the incubation solution. Refer to Supplemental Figure S11 for full details of antibodies.
2.8 | Western blotting
Tissue samples were homogenized in Hank’s balanced salt solution containing complete protease inhibitor cocktail (1 tablet/10 mL, Roche, Indianapolis, IN) and phosphatase inhibitor cocktail (1:100; Sigma). After centrifugation (10 000g; 15 min at 4°C), the supernatant was collected, and the membrane protein fraction prepared by suspending pellets in lysis buffer (0.3M NaCl, 50 mM Tris-HCl pH7.6, and 0.5% Triton X-100) with protease/phosphatase inhibitors as above. Supernatants were pooled for whole cell lysates and protein concentrations determined using a BCA protein assay (Pierce, Rockford, IL). After denaturation (100°C for 5 min) in Laemmli sample buffer, each lysate was separated on a 4–15% TGX Stain-Free SDS-PAGE gel (Bio-Rad, Hercules, CA). Proteins were transferred to PVDF membranes and incubated overnight at 4°C with primary antibodies against RXFP1, RXFP2 and smooth muscle actin (details of antibodies in Supplemental Figure S11) diluted in Tris-buffered saline with 0.1% Tween-20 (TBS-T) containing 5% (w/v) milk. Membranes were incubated with appropriate horseradish peroxidase conjugated secondary antibodies in 5% (w/v) Milk TBS-T, washed, and incubated in WesternBright Quantum (Advansta, Menlo Park, CA) for chemiluminescent imaging (ChemiDoc MP, Bio-Rad). Optical density of each protein species was normalized to total protein levels using Image Lab software (Bio-Rad).
2.9 | RT-qPCR
Tissues were harvested from four female mice and lysed using a bead homogenizer (MP FastPrep-24). Total RNA was extracted using an RNeasy mini kit (Qiagen, Germantown, MD) and used to generate cDNA using the iScript cDNA Synthesis Kit (Bio-Rad). RT-qPCR was performed on a CFX Connect (Bio-Rad). Each PCR reaction was completed with 1.5 μL of cDNA using the TaqMan Fast Advanced Master Mix (Life Technologies, Carlsbad, CA). TaqMan probes were RXFP1 (Mm01220214_m1), RXFP2 (Mm01218503_m1), and reference gene HPRT (Mm00446968_m1). Expression levels were quantified using the 2−ΔCt method.
2.10 | Data and statistical analysis
Data from tension recordings were expressed as mean ± standard deviation from “N” experiments. Wilcoxon ranked sum test was used to determine differences between control versus irradiated or vehicle versus relaxin treated data sets. The null hypothesis was rejected at P < 0.05.
2.11 | Study approval
All animal procedures were in accordance to the National Institutes of Health “Guide for the Care and Use of Laboratory Animals and received ethical approval from the Institutional Animal Care and Use Committee of the authors” University.
3 | RESULTS
3.1 | hRLX2 treatment restores normal bladder function in mice with chronic radiation cystitis
As abdominal irradiation at the selected dose could be lethal (LD50 ≃ 8 Gy)25 we developed a mouse model of chronic radiation cystitis by performing a laparotomy where the bladder is briefly exteriorized for selective high dose (10 Gy) irradiation (Figure 2A). hRLX2 did not significantly affect the voiding function of nonirradiated mice (Figure 2C). Cystometry performed 9 weeks post-irradiation (N = 6) demonstrated a loss of the micturition response and exhibited overflow incontinence as shown in Figure 2D. Respective external urethral sphincter electromyogram (EUS-EMG, black traces) demonstrated that animals had prolonged guarding reflexes and that normal phasic bursting activity as seen in Figure 2B did not occur. However, when hRLX2 was administered (400 μg/kg/day) for 2 weeks in 7-week post-irradiated animals (N = 4), the CMGs and EUS-EMGs (Figure 2E) became similar to those seen in nonirradiated mice (N = 6; Figure 2B) with the return of a normalized guarding reflex and bursting (Figure 2E, right panel) permitting voiding. It is important to note that while human and rodent sphincters exhibit a guarding reflex as bladder pressures approach threshold, the sphincter in humans completely relaxes, whereas rodents normally exhibit a pattern of intermittent phasic activity (“bursting”)26 during which decreased tonic activity permits pulsatile voiding to occur (Figures 2B, 2C, and 2E). Detailed CMG and EUS-EMG parameters are listed in the tables in Supplemental Figures S6 and S7, respectively.
3.2 | Chronic radiation cystitis results in a time-dependent development of voiding dysfunction which is reversed by hRLX2 treatment
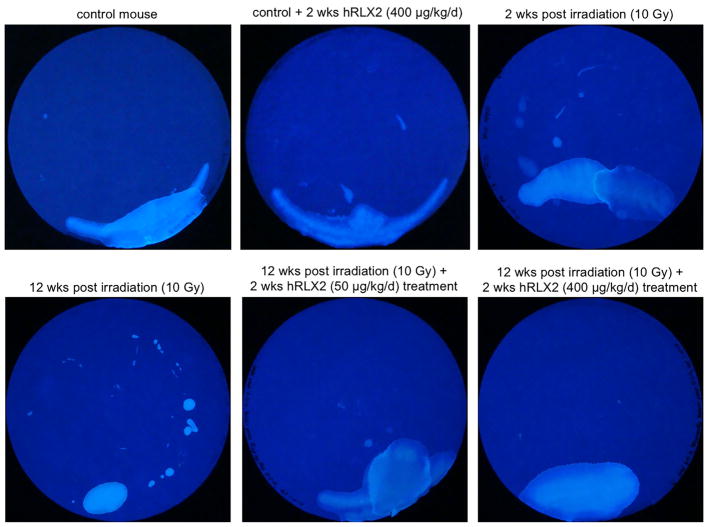
The voiding patterns of mice following bladder irradiation were evaluated at different time points by noninvasive void spot assay (Figure 3). Nonirradiated control animals (N = 6) display continence by generally voiding in one area of the cage. hRLX2 treatment does not affect this behavior (N = 2). In contrast, voiding spot analysis performed 2 weeks following irradiation (N = 6), revealed urine leakage suggestive of incontinence. At 12 weeks post-irradiation (N = 6), there were random patterns of urine spots with smaller voided volumes (Supplemental Figure S8). hRLX2 treatment (50 and 400 μg/kg/day for 14 days; N = 4 at each dosage) normalized the chronic radiation cystitis induced voiding pattern, as indicated by effective bladder emptying and larger voided volumes. The increased voided volumes in irradiated mice treated at the lower dose of 50 μg/kg/day were marred by indications of unresolved incontinence (ie, multiple small urine spots). At 400 μg/kg/day, mice showed a voiding pattern like that of control mice, with only one to two large urine spots present at the end of the assay comparable to those of nonirradiated controls.
FIGURE 3.
Urine spot test samples of irradiated mice with and without hRLX2 treatment. hRLX2 did not have significant effect on mouse voiding behavior (N = 2). Chronic irradiated mice (12 weeks post-irradiation) were incontinent and exhibited urine leakage (multiple small spots) with decreased voided volumes (N = 6). hRLX2 increased voided volumes and decreased the number of spots, restoring continence and normal bladder function (N = 4; see also supplemental Figure S8)
3.3 | Decrease in tissue compliance and contractility secondary to bladder irradiation-mediated fibrosis is reversed by hRLX2 treatment
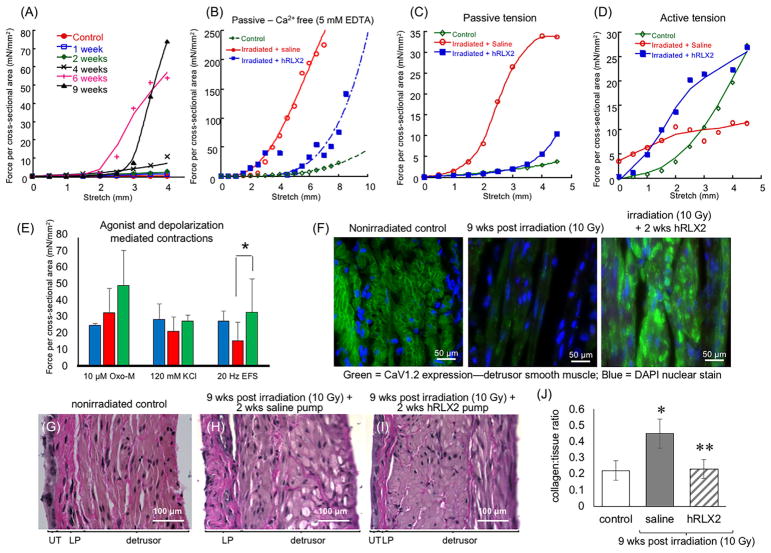
The effect of ionizing radiation on bladder tissue contractility and compliance was evaluated by organ bath experiments with isolated bladder strips. In length-tension studies, there was a marked increase in passive tension of irradiated mouse bladders (ie, decrease in tissue compliance) which was evident by 4 weeks post-irradiation and peaked by 6–9 weeks (N = 3 at each time point; Figure 4A). There was no significant difference at time points up to 16 weeks post-exposure (not shown). Moreover, the increased passive tension in irradiated bladders was also revealed by measurements performed with 5 mM EDTA-Tyrode’s solution (Ca2+ free; N = 5 for each group) (Figure 4B), further supporting that increased ECM deposition alone alters relaxation properties of the bladder wall. In chronic radiation cystitis, the decreased contractility and compliance were reversed by a 2 weeks treatment with hRLX2 (400 μg/kg/day) with experimental recordings becoming comparable to those of nonirradiated mouse bladders (Figure 4C). Furthermore, hRLX2 treatment increased active force generation even beyond that of nonirradiated controls (Figure 4D). There was a significant increase in the force generation evoked by electrical field stimulation (20 Hz) in hRLX2 treated preparations (Figure 4E; Wilcoxon ranked sum test, P = 0.029 versus vehicle treated irradiated mice). Contractions evoked by muscarinic agonist, oxotremorine-M, and high KCl were not significantly different between groups. Additionally, Cav1.2, the α-subunit of the L-type Ca2+ channel which is responsible for detrusor contraction, was found to decrease in the detrusor layer in chronic radiation cystitis compared to non-irradiated controls (Figure 4F) and the expression was restored following a 2-week hRLX2 treatment (N = 3 each group).
FIGURE 4.
Passive properties, bladder wall compliance, detrusor contractility and collagen content changes in chronic radiation cystitis and its reversal by hRLX2 treatment. A, The bladders were isolated at 1, 2, 4, 6, and 9 weeks post-irradiation and contractile function was measured in organ bath experiments (N = 3 each time point). Passive tension profiles (an indicator of tissue stiffness) showed significant increases at 6–9 weeks post-irradiation. B, Passive tension recorded in Ca2+-free Tyrode’s solution demonstrated that hRLX2 decreased tension generation, compared to saline treated irradiated mice, suggesting that this effect was due to changes in the elastic properties of the bladder and not smooth muscle relaxation, (N = 5 each). C and D, At 9 weeks post-irradiation, mouse bladders showed increased passive tension and decreased active force generation (red traces) compared to nonirradiated mice (green traces). Two weeks treatment with hRLX2 (subcutaneous, 400 μg/kg/day) commenced 7-week post-irradiation resulted in a passive tension profile similar to nonirradiated controls and increased contractile responses to EFS (blue traces and bars, N = 4 for control and N = 5 each for irradiated + vehicle or hRLX2). E, Force generation in response to EFS were enhanced in hRLX2-treated compared to vehicle treated irradiated mouse bladders (*P < 0.05, Wilcoxon ranked sum test). Responses to muscarinic agonist, oxotremorine-M and 120 mM KCl were not significantly different between the groups (N = 4 for control and N = 5 each for irradiated + vehicle or hRLX2). F, The expression of L-type Ca2+ channels (Cav1.2) was decreased in the detrusor layer of mice with chronic radiation cystitis and was increased following hRLX2 treatment (N = 3 each). G, Van Gieson staining of control mouse bladder sections. H, Sections from irradiated bladders showed denuding of the UT and significant collagen staining in the lamina propria (LP) and throughout the detrusor. I, Mice treated with hRLX2 showed a decrease in bladder collagen content that was comparable to nonirradiated mice and an intact urothelial layer. J, Collagen: tissue ratio was analyzed using ImageJ (N = 4 each group, Wilcoxon ranked sum test, * indicate P < 0.01 vs non-irradiated control and **P < 0.01 vs irradiated + vehicle)
3.4 | Urothelial loss and bladder collagen deposition in chronic radiation cystitis contribute to LUT dysfunction
Bladder sections stained with Van Gieson solution showed urothelial layer disruption, increased collagen content (intense pink staining) and significant muscle damage 9 weeks post-injury (Figure 4H) compared to age matched controls (Figure 4G). In contrast, mice receiving hRLX2 treatment showed a return of the urothelial layer and normal collagen and smooth muscle architecture (Figure 4I) that is indifferent from nonirradiated controls. Quantification of collagen to total tissue area ratio showed a significant increase in collagen content of irradiated mouse bladders which was reversed by hRLX2 treatment to a level comparable to nonirradiated controls (N = 4 each group; Figure 4J).
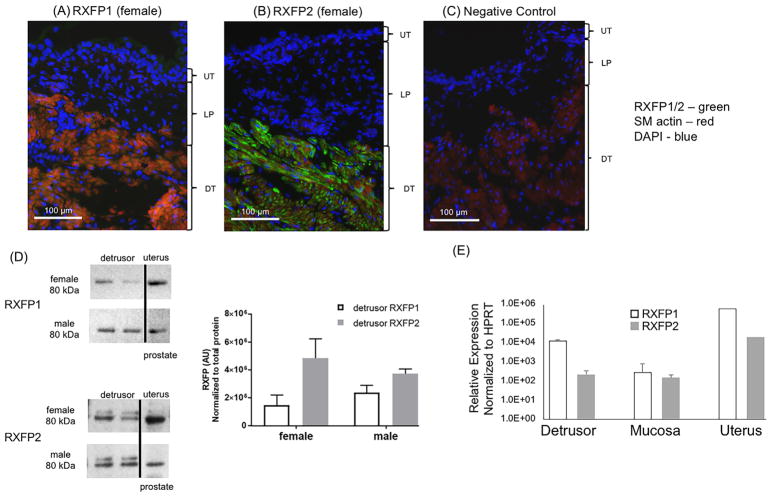
3.5 | The hRLX2 receptors, RXFP1 and RXFP2 are expressed in mouse bladders
Immunofluorescence analysis of normal femalemouse bladder sections demonstrates that the receptors for hRLX2, RXFP1/2 are expressed in the detrusor layer, with RXFP2 being the dominant subtype (N = 3; Figures 5A and 5B, green—RXFP, red—smooth muscle actin, blue—DAPI nuclear stain; Figure 5C—negative control without the primary antibody). Confirmation of immunofluorescence findings by Western blot analysis (N = 3 for each sex; Figure 5D) supports that there is direct action of hRLX2 on bladder smooth muscle and, possibly, myofibroblasts in the mouse bladder—positive controls for RXFP1 and RXFP2 in uterus and prostrate are also shown. Expression of RXFP1 and RXFP2 in the bladder was also demonstrated by RT-qPCR, with expression in detrusor higher than in mucosa (N = 4; Figure 5E).
FIGURE 5.
Expression of hRLX2 receptors, RXFP1 and RXFP2, in mouse bladders. Immunofluorescence analysis of RXFP1/2 in the female C57Bl/6 mouse bladders (N = 3) showed that these receptors are expressed on the detrusor smooth muscle (RXFP1/2, 1:1000 dilution—green, smooth muscle actin—red, DAPI nuclear stain—blue), with surprisingly little expression in the lamina propria (LP) and urothelium (UT). A, The expression of RXFP1 was less robust than RXFP2 (B) in histological sections. C, Negative control omitting the primary antibody did not show any fluorescence. D, Bladder western blot analysis (N = 3 for each sex) and positive controls for uterus and prostate. E, RT-qPCR analysis confirmed the expression of RXFP1 and RXFP2 in the female mouse bladder (N = 4), with expression in detrusor higher than in mucosa
3.6 | Measurements of endogenous and exogenously administered relaxin in mouse plasma
Supplemental Figure S10 shows our data on the (1) measurements of endogenous mouse relaxin-1 (mRLX1; the homologue to hRLX2) plasma levels in control male, female, and pregnant mice and (2) continuous subcutaneous infusion of hRLX2 produced a dose-dependent increase in plasma levels of hRLX2 (N = 4 each group). The plasma levels of hRLX2 measured in the mouse group receiving 50 μg/kg/day were equivalent to the levels of hRLX2 in human pregnancy27 and mRLX1 detected at day 10 of gestation. Chronic infusion at 400 μg/kg/day by day 7 raised the plasma levels to 17.5 ng/mL in non-pregnant female mice, which is approximately 10 times higher than E10 pregnant mice27. These levels were stable for the rest of the treatment period (up to 14 days).
4 | DISCUSSION
Fibrosis has been implicated as a central mechanism in a wide variety of pathologies including LUT dysfunction secondary to chronic inflammation that leads to urinary retention.2,3 This has a substantial effect on the quality of life and has severe health implications including the potential for development of progressive renal dysfunction. As such, patients may need to use intermittent self-catheterization and, in severe cases, undergo a cystectomy, as there are presently no effective therapies that reverse fibrosis. Anti-fibrotic therapy would have clear benefits not only in radiation cystitis, but also in other fibrosis-driven bladder dysfunctions, including neurogenic bladder and outlet obstruction.
The present study leveraged the findings of earlier reports to investigate the therapeutic benefits from sustained infusion of exogenous hRLX2 in a diseased mouse model without deleting endogenous relaxin. We found that subcutaneous infusion of hRLX2 (400 μg/kg/day for 14 days), reversed the fibrosis (Figure 4G–J), increased bladder compliance (Figure 2) and force generation (Figure 4) to restore bladder function in our mouse model of chronic radiation cystitis. Sustained plasma levels are necessary for inducing genomic changes in the LUT organs, just as maternal physiological adaptions in pregnancy are mediated by a sustained plasma elevation in the level of endogenous relaxin.28 Our findings demonstrate that a sustained rise in plasma levels of relaxin is a key determinant for deriving therapeutic benefits in reversing the fibrosis in non-pregnant female mice because direct application of hRLX2 was devoid of any discernible effect on contractility of bladder strips (data not shown). Therefore, we choose the regimen of chronic administration for the present study. From previous reports, 400 μg/kg/day was the minimum dose needed to prevent and reduce fibrosis in various models.29 hRLX2 may also prevent recurrent inflammation potentially via inhibition of immune cell activation.30 We propose that hRLX2 acts via G-protein coupled RXFP1/2 in the bladder (Figure 5), causing transient elevations of cAMP and cGMP, via a NOS dependent pathway, activation of kinases and transcription factors that lead to anti-inflammatory, vasodilatory, anti-oxidative, and antifibrotic properties to reverse fibrosis (Figure 1C).
The high active tension in hRLX2 treated irradiated mice compared to control suggests that hRLX2 likely shifts the increased nitric oxide (NO•) signaling in irradiated mice away from the proapoptotic/profibrotic pathway towards NO•-dependent/cGMP signaling, which promotes collagen degrading gelatinase activity. The contractile strength of detrusor smooth muscles is further increased as indicated by the increased EFS responses that may result from genomic changes in the expression of Cav1.2.31
5 | CONCLUSION
These studies, although pre-clinical with transferability to the human system yet to be determined, demonstrate the therapeutic potential of hRLX2 in treating LUT pathologies due to radiation cystitis. Relaxin is a natural hormone which has passed human safety tests in clinical trials, increases Cav1.2 expression to improve detrusor contractility, arrests collagen deposition, and reverses fibrosis to increase bladder compliance.
Supplementary Material
Supplemental Figure 10. Endogenous mRLX and exogenous hRLX2 plasma levels from mice.
Supplemental Figure 11. Antibodies used for western blot and immunofluorescence.
Supplemental Figure 6. CMG parameters from nonirradiated and irradiated mice with and without hRLX2 treatment. Data sets were analyzed with Wilcoxon ranked sum test and significant differences indicated with * p< 0.01 and ** p<0.05.
Supplemental Figure 7. EUS-EMG parameters from nonirradiated, and irradiated mice with and without hRLX2 treatment. Data sets were analyzed with Wilcoxon ranked sum test and significant differences indicated with * p< 0.01.
Supplemental Figure 8. Spot test data. Number of spots and total volume voided during the test of control, nonirradiated mice, irradiated mice 2 and 12 weeks following irradiation and 12 weeks following irradiation with hRLX2 treatment.
Supplemental Figure 9. Calibration of urine spot sizes to volumes of urine voided. Spots of 10, 40, 50, 60 and 70 μl were pipetted onto the Whatman paper, spot size analyzed, and a calibration curve built where linear relation was confirmed with R2 = 0.9975.
Acknowledgments
The authors would like to thank Dr. Guy Salama, University of Pittsburgh, for kindly providing the hRLX2 used in this study.
Footnotes
Karl-Erik Andersson led the peer-review process as the Associate Editor responsible for the paper.
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- 1.Kibrom AZ, Knight KA. Adaptive radiation therapy for bladder cancer: a review of adaptive techniques used in clinical practice. J Med Radiat Sci. 2015;62:277–285. doi: 10.1002/jmrs.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cetinel B. Chemotherapy and pelvic radiotherapy-induced bladder injury. Urologia. 2015;82:S2–S5. doi: 10.5301/uro.5000144. [DOI] [PubMed] [Google Scholar]
- 3.Smit SG, Heyns CF. Management of radiation cystitis. Nat Rev Urol. 2010;7:206–214. doi: 10.1038/nrurol.2010.23. [DOI] [PubMed] [Google Scholar]
- 4.Browne C, Davis NF, Mac Craith E, et al. A narrative review on the pathophysiology and management for radiation cystitis. Adv Urol. 2015;2015:346812. doi: 10.1155/2015/346812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aitken KJ, Bagli DJ. The bladder extracellular matrix. Part I: architecture, development and disease. Nat Rev Urol. 2009;6:596–611. doi: 10.1038/nrurol.2009.201. [DOI] [PubMed] [Google Scholar]
- 6.Nogueira A, Pires MJ, Oliveira PA. Pathophysiological mechanisms of renal fibrosis: a review of animal models and therapeutic strategies. In Vivo. 2017;31:1–22. doi: 10.21873/invivo.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mauviel A. Transforming growth factor-beta: a key mediator of fibrosis. Methods Mol Med. 2005;117:69–80. doi: 10.1385/1-59259-940-0:069. [DOI] [PubMed] [Google Scholar]
- 8.Downing SJ, Hollingsworth M. Action of relaxin on uterine contractions-a review. J Reprod Fertil. 1993;99:275–282. doi: 10.1530/jrf.0.0990275. [DOI] [PubMed] [Google Scholar]
- 9.Sarosi P, Schmidt CL, Essig M, Steinetz BG, Weiss G. The effect of relaxin and progesterone on rat uterine contractions. Am J Obstet Gynecol. 1983;145:402–405. doi: 10.1016/0002-9378(83)90307-1. [DOI] [PubMed] [Google Scholar]
- 10.McGovern PG, Goldsmith LT, Schmidt CL, Von Hagen S, Linden M, Weiss G. Effects of endothelin and relaxin on rat uterine segment contractility. Biol Reprod. 1992;46:680–685. doi: 10.1095/biolreprod46.4.680. [DOI] [PubMed] [Google Scholar]
- 11.Grazi RV, Goldsmith LT, Schmidt CL, Von Hagen S, Weiss G. Synergistic effect of relaxin and progesterone on cyclic adenosine 3′,5′-monophosphate levels in the rat uterus. Am J Obstet Gynecol. 1988;159:1402–1406. doi: 10.1016/0002-9378(88)90564-9. [DOI] [PubMed] [Google Scholar]
- 12.Goldsmith LT, Skurnick JH, Wojtczuk AS, Linden M, Kuhar MJ, Weiss G. The antagonistic effect of oxytocin and relaxin on rat uterine segment contractility. Am J Obstet Gynecol. 1989;161:1644–1649. doi: 10.1016/0002-9378(89)90942-3. [DOI] [PubMed] [Google Scholar]
- 13.Lessing JB, Brenner SH, Colon JM, et al. Effect of relaxin on human spermatozoa. J Reprod Med. 1986;31:304–309. [PubMed] [Google Scholar]
- 14.Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ. Relaxin family peptides and their receptors. Physiol Rev. 2013;93:405–480. doi: 10.1152/physrev.00001.2012. [DOI] [PubMed] [Google Scholar]
- 15.Morelli A, Squecco R, Failli P, et al. The vitamin D receptor agonist elocalcitol upregulates L-type calcium channel activity in human and rat bladder. Am J Physiol Cell Physiol. 2008;294:C1206–C1214. doi: 10.1152/ajpcell.90634.2007. [DOI] [PubMed] [Google Scholar]
- 16.Hossain MA, Kocan M, Yao ST, et al. A single-chain derivative of the relaxin hormone is a functionally selective agonist of the G protein-coupled receptor, RXFP1. Chem Sci. 2016;7:3805–3819. doi: 10.1039/c5sc04754d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuel CS. Relaxin: antifibrotic properties and effects in models of disease. Clin Med Res. 2005;3:241–249. doi: 10.3121/cmr.3.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C, Kemp-Harper BK, Kocan M, Ang SY, Hewitson TD, Samuel CS. The anti-fibrotic actions of relaxin are mediated through a NO-sGC-cGMP-dependent pathway in renal myofibroblasts in vitro and enhanced by the NO donor, diethylamine NONOate. Front Pharmacol. 2016;7:91. doi: 10.3389/fphar.2016.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masini E, Nistri S, Vannacci A, Bani Sacchi T, Novelli A, Bani D. Relaxin inhibits the activation of human neutrophils: involvement of the nitric oxide pathway. Endocrinology. 2004;145:1106–1112. doi: 10.1210/en.2003-0833. [DOI] [PubMed] [Google Scholar]
- 20.Metra M, Cotter G, Davison BA, et al. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol. 2013;61:196–206. doi: 10.1016/j.jacc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Yu W, Ackert-Bicknell C, Larigakis JD, et al. Spontaneous voiding by mice reveals strain-specific lower urinary tract function to be a quantitative genetic trait. Am J Physiol Renal Physiol. 2014;306:F1296–F1307. doi: 10.1152/ajprenal.00074.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikeda Y, Zabbarova IV, Birder LA, et al. Botulinum neurotoxin serotype A suppresses neurotransmitter release from afferent as well as efferent nerves in the urinary bladder. Eur Urol. 2012;62:1157–1164. doi: 10.1016/j.eururo.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pakzad M, Ikeda Y, McCarthy C, Kitney DG, Jabr RI, Fry CH. Contractile effects and receptor analysis of adenosine-receptors in human detrusor muscle from stable and neuropathic bladders. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:921–929. doi: 10.1007/s00210-016-1255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev. 2004;84:935–986. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- 25.Nunamaker EA, Artwohl JE, Anderson RJ, Fortman JD. Endpoint refinement for total body irradiation of C57BL/6 mice. Comp Med. 2013;63:22–28. [PMC free article] [PubMed] [Google Scholar]
- 26.Ito H, Pickering AE, Igawa Y, Kanai AJ, Fry CH, Drake MJ. Muroneuro-urodynamics; a review of the functional assessment of mouse lower urinary tract function. Front Physiol. 2017;8:49. doi: 10.3389/fphys.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unemori EN, Erikson ME, Rocco SE, et al. Relaxin stimulates expression of vascular endothelial growth factor in normal human endometrial cells in vitro and is associated with menometrorrhagia in women. Hum Reprod. 1999;14:800–806. doi: 10.1093/humrep/14.3.800. [DOI] [PubMed] [Google Scholar]
- 28.Conrad KP. Emerging role of relaxin in the maternal adaptations to normal pregnancy: implications for preeclampsia. Semin Nephrol. 2011;31:15–32. doi: 10.1016/j.semnephrol.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samuel CS, Bodaragama H, Chew JY, Widdop RE, Royce SG, Hewitson TD. Serelaxin is a more efficacious antifibrotic than enalapril in an experimental model of heart disease. Hypertension. 2014;64:315–322. doi: 10.1161/HYPERTENSIONAHA.114.03594. [DOI] [PubMed] [Google Scholar]
- 30.Nistri S, Cinci L, Perna AM, Masini E, Mastroianni R, Bani D. Relaxin induces mast cell inhibition and reduces ventricular arrhythmias in a swine model of acute myocardial infarction. Pharmacol Res. 2008;57:43–48. doi: 10.1016/j.phrs.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Han X, Habuchi Y, Giles WR. Relaxin increases heart rate by modulating calcium current in cardiac pacemaker cells. Circ Res. 1994;74:537–541. doi: 10.1161/01.res.74.3.537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 10. Endogenous mRLX and exogenous hRLX2 plasma levels from mice.
Supplemental Figure 11. Antibodies used for western blot and immunofluorescence.
Supplemental Figure 6. CMG parameters from nonirradiated and irradiated mice with and without hRLX2 treatment. Data sets were analyzed with Wilcoxon ranked sum test and significant differences indicated with * p< 0.01 and ** p<0.05.
Supplemental Figure 7. EUS-EMG parameters from nonirradiated, and irradiated mice with and without hRLX2 treatment. Data sets were analyzed with Wilcoxon ranked sum test and significant differences indicated with * p< 0.01.
Supplemental Figure 8. Spot test data. Number of spots and total volume voided during the test of control, nonirradiated mice, irradiated mice 2 and 12 weeks following irradiation and 12 weeks following irradiation with hRLX2 treatment.
Supplemental Figure 9. Calibration of urine spot sizes to volumes of urine voided. Spots of 10, 40, 50, 60 and 70 μl were pipetted onto the Whatman paper, spot size analyzed, and a calibration curve built where linear relation was confirmed with R2 = 0.9975.