Abstract
Objectives
High-density lipoprotein cholesterol (HDL-C) may not always be cardio-protective in postmenopausal women. HDL particles (HDL-P) via ion-mobility may better reflect the anti-atherogenicity of HDL. Objectives were 1) to evaluate associations of HDL-C and ion-mobility HDL-P with carotid intima-media thickness (cIMT) and plaque (cPlaque) separately and jointly in women; 2) to assess interactions by age at and time since menopause.
Approaches and Results
Analysis included 1,380 females from the Multi-Ethnic Study of Atherosclerosis (Age: 61.8±10.3; 61% natural-, 21% surgical-, and 18% peri-menopause). Women with unknown or early menopause (age at non-surgical menopause ≤45 years) were excluded. Adjusting for each other, higher HDL-P but not HDL-C was associated with lower cIMT (P=0.001), whereas higher HDL-C but not HDL-P was associated with greater risk of cPlaque presence (P=0.04). Time since menopause significantly modified the association of large but not small HDL-P with cIMT; higher large HDL-P was associated with higher cIMT close to menopause but with lower cIMT later in life. The pro-atherogenic association reported for HDL-C with cPlaque was most evident in women with later age at menopause who were >10 years postmenopausal.
Conclusions
Elevated HDL-C may not always be cardio-protective in postmenopausal women. The cardio-protective capacity of large HDL-P may adversely compromise close to menopause supporting the importance of assessing how the menopause transition might impact HDL quality and related CVD risk later in life.
Keywords: Intima-media thickness, carotid plaque, postmenopause, high-density lipoproteins, ion mobility
Subject codes: Lipids and Cholesterol, Aging, Epidemiology, Women, Atherosclerosis
Introduction
The well-known cardio-protective features of high-density lipoprotein (HDL) can be lost under certain conditions such as chronic inflammation (1). Women who transition through menopause are subjected to many adverse physiological changes including alterations in sex hormones, body fat deposition and lipid profile (2). The accrual of these changes could trigger a status of chronic inflammation over time (3) that could potentially impact the cardio-protective capacity of HDL. Several studies have showed that higher level of the conventional measure of HDL, HDL-cholesterol (HDL-C), in middle aged and older women is not always protective and are associated with greater risk of non-fatal stroke, cerebral infarction and carotid atherosclerosis (4–7). Recent findings from the Study of Women’s Health Across the Nation (SWAN) suggest a potential role of the menopause transition. A larger increase in HDL-C over time was significantly associated with a greater progression of carotid intima-media thickness (cIMT) independent of potential risk factors in women at midlife (8). It is unknown whether the cardio-protective capacity of HDL is compromised in postmenopausal women. The crude measure of total cholesterol carried by HDL particles may not fully characterize the HDL effect on cardiovascular disease (CVD) risk.
A novel promising method that physically quantifies HDL subclasses is the ion mobility analysis, a well-established method in the field of aerosol science. It is the latest method that provides accurate, reproducible, direct determination of size and concentration for a wide range of lipoproteins by separating lipoproteins by size based on movement of charged particles in a gas-phase under the impact of an electric field (9, 10). No previous study has evaluated the effect modification of menopause related factors including age at menopause and time since menopause on the associations between concentrations of ion-mobility HDL particles (HDL-P) and carotid atherosclerosis (level of carotid intima-media thickness and risk of carotid plaque presence) in midlife and older women.
The Multi-Ethnic Study of Atherosclerosis (MESA) is a large, well characterized, ethnically diverse cohort providing a unique opportunity to assess whether the cardio-protective associations of conventional and ion-mobility measures of HDL vary by menopause related parameters in midlife and older women. The proposed analyses will help in identifying other metrics that could better capture the cardio-protective capacity of HDL in women.
Materials and Methods
The authors declare that all supporting data are available within the article [and its online supplementary files].
Study population
MESA is a longitudinal multicenter cohort study of the prevalence and correlates of subclinical CVD and the factors that impact its development (11). At baseline (2000–2002), 6,814 community-dwelling multi-racial/ethnic men (n=3,213) and women (n=3,601) 45 to 84 years old were recruited from six US sites. Exclusion criteria at baseline included self-reported CVD, weight >136 kg, pregnancy, cancer, or cognitive impairment.
For the current analysis, 2,028 women had carotid artery scan and HDL ion mobility data available at MESA baseline visit (Exam 1). Women with triglycerides level >400 mg/dl (n=24) and those with unknown menopausal status (n=367) or early menopause (age at non-surgical menopause ≤45 years) (n=257) were excluded, leaving 1,380 women for the current analyses on HDL metrics, carotid measures and menopausal status. Analyses that assessed effect modifications by age at and time since menopause additionally excluded 242 women for whom age at and time since menopause were missing, leaving 1,138 women for these analyses.
Study protocols were approved by the institutional review board at each site and participants provided written informed consent.
Carotid Ultrasonography
At baseline, B-mode ultrasound images of the left and right common, bifurcation, and internal carotid artery segments were recorded using the M12L transducer (General Electric Medical Systems; common carotid artery frequency, 13 MHz) on Super-VHS videotape with a Logiq 700 ultrasound system. Video images were digitized at high resolution and frame rates using a Medical Digital Recording device (PACSGEAR, Pleasanton, CA) and converted into DICOM-compatible digital records. Ultrasound images were read at the University of Wisconsin Carotid Ultrasound Reading Center. Images were imported into syngo Ultrasound Workplace reading stations loaded with Arterial Health Package software (Siemens Medical, Malvern, PA) for cIMT and carotid plaque presence (cPlaque). As described previously (12), cIMT was defined as the mean of the mean left and right mean far wall distal common carotid artery wall thicknesses (the distal 10 mm of the common carotid artery, proximal to the carotid bifurcation point, where the diameter remains uniform). Carotid plaque was defined as a discrete, focal wall thickening ≥1.5 cm or focal thickening at least 50% greater than the surrounding IMT (12). The intraclass correlation coefficient for intra-reader and inter-reader reproducibility for mean cIMT were 0.99 and 0.95, respectively. For carotid plaque presence, intra-reader and inter-reader reproducibility were κ=0.83 (95% CI, 0.70–0.96) and 0.89 (95% CI, 0.72–1.00), respectively (12).
Ion Mobility and conventional lipids
Ion mobility lipoproteins were measured at the Quest Diagnostics Nichols Institute (San Juan Capistrano, CA). Compared to nuclear magnetic resonance (NMR) spectroscopy methodology which relies on the composition of particles as it measures the proton resonance associated with the methyl group on esterified cholesterol, ion mobility separates particles based on size and counts the number of particles that are separated at each size. The measurement is independent of the composition of the particles. Ion mobility can measure lipoprotein concentration of the entire size spectrum of lipoprotein particles ranging from 5nm-53nm at a high size resolution (<0.1nm diameter on average). Thus, it allows unbiased analysis of the entire lipoprotein spectrum without making any assumption about what specific size range should be analyzed (10). All specimens were collected as part of MESA exam 1 (2000–2002) and analyzed using ion mobility in 2015. The complete lipoprotein profiles from these samples were very much like the profiles obtained from fresh samples. The LDL and HDL regions were similar in size, number and shape. Prior to ion mobility fractionation, lipoproteins were isolated by dextran sulfate precipitation [plasma was treated with 17% ethanol which removed >97% of fibrinogen, and lipoproteins were then precipitated with dextran sulfate (2 mg/mL) and calcium (0.15 M)]. Precipitated lipoproteins were harvested on paramagnetic particles, washed to remove free salt and proteins and then re-suspended in 25 mM ammonium acetate for analysis by ion mobility. This method recovered all measureable apoB (105%), apoA-I (96%) and total cholesterol (103%). Removal of plasma proteins was assessed by the following proteins (final concentration remaining after extraction compared with original serum concentration): IgG (3%), albumin (<4%), transferrin (0%). This new isolation procedure has excellent recovery of the lipoprotein particles based on the apolipoprotein and total cholesterol recoveries (13). After isolation, the lipoproteins were fractionated and quantitated in a single scan using gas-phase electrophoresis (13). Based on the electrical potential applied, the dimensions of the differential mobility analyzer, and the airflow rate, the particle diameter and the number of particles in each size range can be determined. HDL-P were separated to large HDL-P (size range: 14.5–10.5nm), equivalent to HDL2b, or small particles (size range: 10.5–7.65nm), equivalent to HDL3+2a (14). Total HDL-P is the summation of the concentrations of the large and small HDL-Ps. Concentration of total LDL-P was available for the current analysis. The intra-assay and inter-assay variations were <10% and <13% for HDL-P and LDL-P concentrations, respectively. Since the original publication of the method (10), refinement to the technique have been made that address concerns about the method (13, 15, 16).
Total and HDL-C and triglycerides were measured at a central laboratory (Fairview-University Medical Center, Minneapolis, MN) after a 12-h fast. Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation (17).
Menopausal status
Menopausal status was determined by asking women at baseline if they have gone through “menopause” or “change of life”. Women were required to state the age at which they experienced menopause if they answered “Yes” to this question. Women who answered “No” or “Don’t know” were asked to provide the date of their last menstrual period and the number of periods they had experienced in the last 12 months. Women were additionally asked if they have had surgery to remove uterus or ovaries. Those who answered “Yes” to these questions were required to state the age at which they had their uterus or ovaries removed and how many ovaries removed if any. Responses to the above questions were cross-evaluated comprehensively to classify women into one of the following four categories: 1) perimenopause, 2) natural menopause, 3) surgical menopause, and 4) unknown menopausal status. Women were classified as perimenopause if they answered “No” or “Don’t know” to the question on “menopause” or “change of life” and reported at least 1 menstrual period in the last 12 months. Premenopausal women, who reported 12+ periods in the last 12 months, were combined with perimenopausal women due to small sample size (only 47 women were classified as premenopausal). Women were classified as natural menopause if they answered “Yes” to the question on “menopause” or “change of life” or reported no period in the last 12 months that was not due to a hysterectomy procedure. Women were classified as surgical menopause if they reported that they had bilateral oophorectomy. Women who had hysterectomy and/or bilateral oophorectomy at an age older than the reported age at menopause were classified as natural menopause. Women with inconsistent data regarding assessing menopausal status (e.g. reported having bleeding in the past 12 months and said that they are gone through menopause) and those who could not be classified into any of the above categories (e.g. women who did hysterectomy with/out unilateral oophorectomy, missing menopausal information or answering “unknown” to menopause and surgical procedure questions) were considered as unknown menopausal status. Women who classified as menopause (not due to bilateral oophorectomy) at an age ≤45 years were considered early menopausal. Women with early or unknown menopausal status were excluded from the current analysis.
Ages at natural or surgical menopause was self-reported by study participants. Time since menopause in years was calculated as the age at baseline minus the age at natural menopause or surgical menopause. Women reported hormone therapy use as either current or ever.
Study covariates
Race/ethnicity was self-reported. Smoking status was coded as never, former or current smoker. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Systolic blood pressure (SBP) was measured three times using an automated sphygmomanometer (Critikon, Tampa, FL) while seated, and the mean of the last two measurements was used. Physical activity was estimated as the total amount of intentional exercise performed in a usual week and measured in metabolic equivalent task (MET)–minutes per week. Hypertension was defined as having SBP ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or taking medications for high blood pressure. Diabetes was defined as the use of insulin or oral hypoglycemic medications or a fasting glucose level of ≥126 mg/dL. C-reactive protein (CRP) was measured using the BNII nephelometer (N High Sensitivity CRP; Dade Behring Inc., Deerfield, IL) and Interleukin-6 (IL-6) was measured by ultra-sensitive ELISA (Quantikine HS Human IL-6 Immunoassay; R&D Systems, Minneapolis, MN).
Statistical analysis
The distribution of cIMT was slightly skewed thus it was evaluated with and without a log transformation. As results were comparable, models using the original scale were presented for simplicity of interpretation. Descriptive statistics were used to characterize study participants in the total study sample and by menopausal status. ANOVA/Kruskal-Wallis or Chi-square tests were used as appropriate to assess differences by menopausal status categories. Pairwise comparisons across menopausal categories were performed on serum lipids and ion mobility HDL metrics adjusting for multiple testing. To assess correlates of HDL-C and HDL ion mobility metrics Spearman correlation coefficients were estimated.
To evaluate associations between each HDL metric and each of cIMT and cPlaque, linear and logistic regression were used, respectively. The model building strategy was based on a priori selection of common risk factors in the association between carotid measure and HDL metrics which was confirmed by univariate analysis (Supplemental Table I). The first model included age, race/ethnicity, study site, and menopausal status. In the second model, we further adjusted for BMI, SBP, smoking, physical activity, antihypertensive, lipid lowering medications, ever use hormone replacement therapy, IL-6 and CRP. The third model was built to determine the independent effect of HDL metrics, since each of HDL-C and HDL-P subclasses measure different aspects of HDL (total cholesterol contents carried by all particles vs. concentration of HDL particles of different size), adjusting for each other and for LDL metrics (LDL-C and LDL-P) on carotid measures such that: for 1) HDL-C models we additionally adjusted for total HDL-P and LDL-P; 2) total HDL-P, large HDL-P or small HDL-P models were additionally adjusted for HDL-C and LDL-P. We built a fourth model for large and small HDL-P, but not other HDL metrics, to determine their independent associations with carotid measures by additionally adjusting for large HDL-P in small HDL-P and vice versa. In model 5, we additionally adjusted for triglycerides and LDL-C for all HDL metrics.
Effect modifications of the associations between carotid measures and HDL metrics by time since and age at menopause were tested unadjusted and fully adjusted. We found significant effect modification of time since menopause on association between large HDL-P and cIMT. To visually present this effect modification, we estimated adjusted means of cIMT by percentiles of large HDL-P and time since menopause. Additionally, the effect of HDL-C on cPlaque presence was marginally modified by age at menopause. We performed additional analysis testing the interaction between HDL-C and age at menopause stratified by time since menopause given the potential possibility of recall bias of age at menopause among MESA participants.
Potential multi-collinearity was evaluated in all models using variance inflation factors and all values were within acceptable limit (≤5) indicative of no multicollinearity in any of the fitted models.
As a sensitivity analysis, we conducted all multivariable analyses while excluding women on lipid lowering medications and again by not adjusting for study site. All statistical analyses were conducted using SAS version 9.4; SAS Institute, Inc., Cary, North Carolina and significance was set at P < 0.05.
Results
Participants’ characteristics in total and by menopausal status are presented in Table 1. Correlates of HDL metrics are presented in Table 2. HDL-C was correlated weakly with total HDL-P, and this correlation was stronger with large than small HDL-P. HDL-C was inversely correlated with LDL-C and LDL-P. In contrast, total, small and large HDL-Ps were positively correlated with LDL-P.
Table 1.
Study Participants Characteristics in Total and by Menopausal Status
| Study Variable | Total N=1,380 |
Surgical menopause N=290 (21.0%) |
Natural menopause N=848 (61.4%) |
Perimenopause N=242 (17.5%) |
P value |
|---|---|---|---|---|---|
| Age, Year, Mean ± SD | 61.8 ± 10.3 | 63.3 ± 9.6 | 65.1 ± 8.8 | 48.4 ± 3.0 | <.0001 |
| Race / Ethnicity, N (%) | <.0001 | ||||
| White | 531 (38.5) | 101 (34.8) | 331 (39.0) | 99 (40.9) | |
| Chinese American | 195 (14.1) | 21 (7.2) | 148 (17.5) | 26 (10.7) | |
| African American | 360 (26.1) | 115 (39.7) | 180 (21.2) | 65 (26.9) | |
| Hispanic | 294 (21.3) | 53 (18.3) | 189 (22.3) | 52 (21.5) | |
| Age at menopause, Year, Median (Q1, Q3) * | 50.0 (48.0, 53.0) | 44.0 (38.0, 49.0) | 50.5 (50.0, 53.0) | ----- | <.0001 |
| Time since menopause, Median (Q1, Q3) * | 14.0 (7.0, 22.0) | 20.0 (9.0, 28.0) | 13.0 (6.0, 20.0) | ----- | <.0001 |
| BMI, Kg/m2, Mean ± SD | 28.6 ± 6.2 | 29.6 ± 6.4 | 28.2 ± 5.9 | 28.9 ± 6.8 | 0.004 |
| SBP, mmHg, Mean ± SD | 126.9 ± 23.0 | 130.6 ± 23.1 | 129.6 ± 22.9 | 112.9 ± 16.9 | <.0001 |
| Smoking status, N (%) | 0.0001 | ||||
| Never | 836 (60.8) | 161 (55.9) | 531 (62.8) | 144 (59.5) | |
| Former | 392 (28.5) | 88 (30.6) | 248 (29.3) | 56 (23.1) | |
| Current | 148 (10.8) | 39 (13.5) | 67 (7.9) | 42 (17.4) | |
| Intentional physical activity, metabolic equivalent task (MET) minutes/week, Median (Q1, Q3) | 720.0 (105.0, 1725.0) | 735.0 (105.0, 1710.0) | 720.0 (150.0, 1732.5) | 637.5 (75.0, 1710.0) | 0.98 |
| Hypertension, N (%) | 651 (47.2) | 171 (59.0) | 437 (51.5) | 43 (17.8) | <.0001 |
| Diabetes, N (%) | 148 (10.7) | 44 (15.2) | 90 (10.6) | 14 (5.8) | 0.002 |
| Antihypertensive medications, N (%) | 506 (36.7) | 145 (50.0) | 328 (38.7) | 33 (13.6) | <.0001 |
| Lipid lowering medications, N (%) | 220 (16.0) | 56 (19.3) | 157 (18.5) | 7 (2.9) | <.0001 |
| Ever use hormone replacement therapy, N (%) | 531 (38.5) | 227 (80.2) | 384 (45.4) | 29 (24.4) | <.0001 |
| CRP, mg/L, Median (Q1, Q3) | 2.5 (1.0, 5.4) | 3.5 (1.5, 7.8) | 2.4 (1.0, 4.7) | 1.7 (0.7, 4.7) | <.0001 |
| IL-6, pg/mL, Median (Q1, Q3) | 1.3 (0.8, 2.0) | 1.3 (0.9, 2.1) | 1.3 (0.8, 2.0) | 1.1 (0.6, 1.8) | <.0001 |
| Lipids, mg/dL, Mean ± SD † | |||||
| HDL-C | 56.3 ± 15.3 | 58.1 ± 15.7 ‡ | 56.5 ± 15.3 ‡ | 53.7 ± 14.8 | 0.004 |
| LDL-C | 118.0 ± 31.8 | 120.2 ± 34.5 | 118.4 ± 31.7 | 113.8 ± 28.5 | 0.06 |
| Total cholesterol | 199.2 ± 34.8 | 204.7 ± 36.6 ‡ | 200.2 ± 34.1‡ | 189.3 ± 33.0 | <.0001 |
| Triglycerides † | 109.0 (76.0, 157.0) | 116.0 (85.0, 163.0) ‡ | 112.0 (77.0, 160.5) ‡ | 93.5 (63.0, 134.0) | <.0001 |
| Ion mobility particle concentrations, umol/L, Mean ± SD | |||||
| Total HDL-P | 26.65 ± 5.48 | 27.47 ± 6.07 ‡ | 26.60 ± 5.43 | 25.81 ± 4.77 | 0.002 |
| Large HDL-P | 6.81 ± 1.88 | 7.08 ± 2.07 ‡ | 6.80 ± 1.89 | 6.51 ± 1.53 | 0.002 |
| Small HDL-P | 19.84 ± 4.04 | 20.39 ± 4.40 ‡ | 19.81 ± 4.01 | 19.30 ± 3.64 | 0.008 |
| Total LDL-P | 1.29 ± 0.36 | 1.31 ± 0.34 | 1.29 ± 0.36 | 1.26 ± 0.36 | 0.26 |
| Mean cIMT, um, Mean ± SD | 749.94 ± 183.23 | 775.86 ± 195.87 | 775.35 ± 182.72 | 630.22 ± 106.42 | <.0001 |
| cPlaque presence, N (%) | 664 (48.1) | 151 (52.1) | 464 (54.7) | 49 (20.2) | <.0001 |
Sample size for analysis on age at menopause and time since menopause =1,138 women
Except for triglycerides where median (Q1, Q3) were presented
Significantly different from perimenopausal women adjusting for multiple comparisons
BMI: body mass index; cIMT: carotid intima-media thickness; cPlaque: carotid plaque; CRP: C-reactive protein, HDL-C: high-density lipoprotein cholesterol; HDL-P: high-density lipoprotein particles; IL-6: interleukin-6; LDL-C: low-density lipoprotein cholesterol; LDL-P: low-density lipoprotein particles; Q1: first quartile; Q3: third quartile; SBP: systolic blood pressure; SD: standard deviation.
Table 2.
Spearman Correlation Coefficients of HDL-C and HDL-Ps with Lipids and Study Measures
| HDL-C | Total HDL-P | Large HDL-P | Small HDL-P | |
|---|---|---|---|---|
| HDL-C | 1 | 0.33* | 0.60* | 0.17* |
| LDL-C | −0.08† | 0.03 | −0.03 | 0.06‡ |
| Total Cholesterol | 0.20* | 0.21* | 0.19* | 0.19* |
| Triglycerides | −0.45* | 0.07‡ | −0.18* | 0.18* |
| Total HDL-P | 0.33* | 1 | 0.84* | 0.96* |
| Large HDL-P | 0.60* | 0.84* | 1 | 0.68* |
| Small HDL-P | 0.17* | 0.96* | 0.68* | 1 |
| Total LDL-P | −0.21* | 0.39* | 0.20* | 0.45* |
| CRP | −0.13* | 0.12* | 0.06‡ | 0.14* |
| IL-6 | −0.21* | 0.03 | −0.01 | 0.05 |
| BMI | −0.29‡ | 0.02 | −0.10† | 0.07‡ |
| SBP | −0.05‡ | 0.02 | −0.001 | 0.04 |
| Intentional physical activity | 0.13* | 0.01 | 0.06‡ | −0.02 |
P≤0.001;
0.001<P≤0.005;
0.005<P<0.05
BMI: body mass index; CRP: C-reactive protein; HDL-C: high-density lipoprotein cholesterol; HDL-P: high-density lipoprotein particles; IL-6: interleukin-6; LDL-C: low-density lipoprotein cholesterol; LDL-P: low-density lipoprotein particles; SBP: systolic blood pressure.
cIMT and HDL metrics
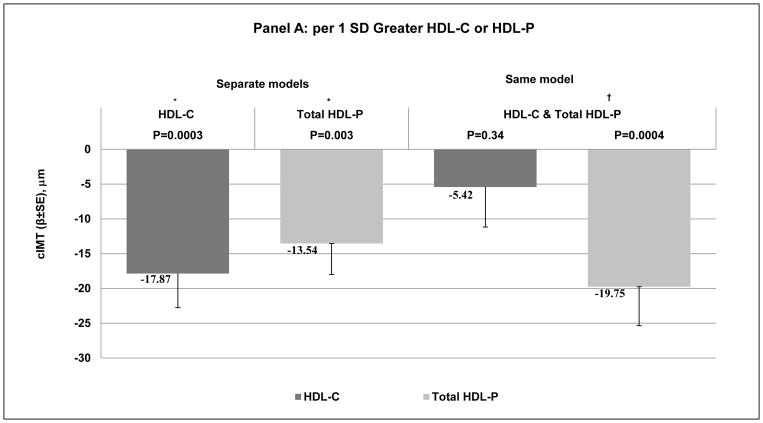
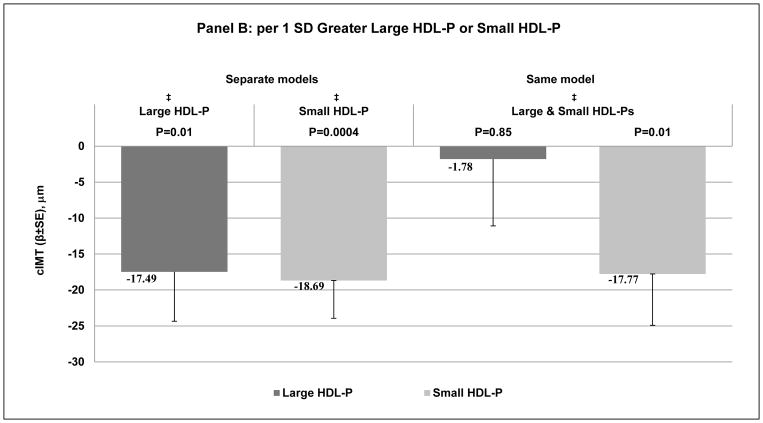
Each HDL metric was negatively associated with cIMT when modeled separately and after adjusting for age, race, study site, menopausal status, BMI, SBP, smoking, physical activity, anti-hypertensive, lipid lowering medications, ever use of HT, IL6 and CRP, Figure 1, A and B - left side and Supplemental Table II - Model 2. Adjusting for each other (HDL-C and total HDL-P in the same model), study covariates and LDL-P, total HDL-P but not HDL-C remained negatively associated with cIMT, Figure 1, A - right side and Supplemental Table II – Model 3. In models adjusted for study covariates, HDL-C, and LDL-P, small but not large HDL-P significantly associated with lower cIMT, Figure 1, B - right side, Supplemental Table II – Model 4. Same results were observed for each HDL metric with cIMT after further adjustment for LDL-C and triglycerides, Supplemental Table II – Model 5.
Figure 1. Difference in cIMT (μm) per 1 SD Greater A: HDL-C or HDL-P; B: Large HDL-P or Small HDL-P.
* Adjusted for age, race, study site, menopausal status, BMI, SBP, smoking, physical activity, anti-hypertensive, lipid lowering medications, ever use hormone replacement therapy, IL-6 and CRP.
† * + LDL-P.
‡ * + HDL-C and LDL-P
BMI: body mass index; cIMT: carotid intima-media thickness; CRP: C-reactive protein; HDL-C: high-density lipoprotein cholesterol; HDL-P: high-density lipoprotein particles; IL-6: interleukin-6; LDL-P: low-density lipoprotein particle particles; SBP: systolic blood pressure.
Effect modifications of menopausal factors on associations between HDL metrics and cIMT
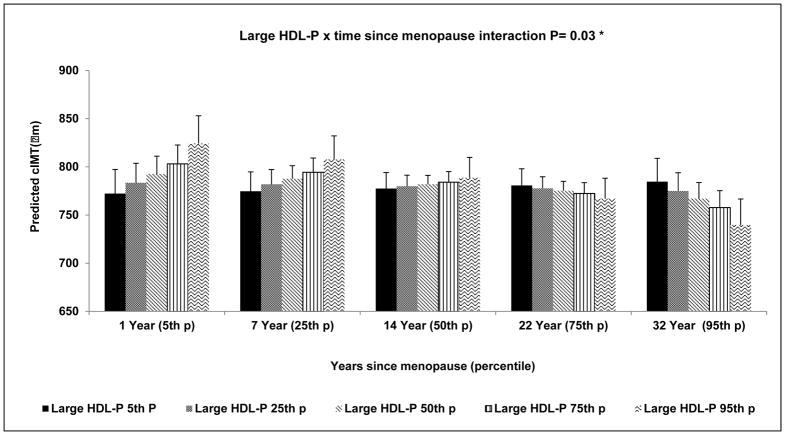
Significant interaction between large HDL-P and time since menopause (P=0.03) was found in relation to cIMT independent of study covariates, LDL-P, HDL-C and small HDL-P; such that a greater concentration of large HDL-P was significantly associated with lower cIMT as time since menopause increased, Figure 2. This effect modification of time since menopause remained significant even after further adjustment for triglycerides and LDL-C (P=0.03), data not shown. Association between small HDL-P and cIMT did not vary by time since menopause. Age at menopause did not modify associations between cIMT and HDL metrics.
Figure 2. Interaction of Large HDL-P with Time since Menopause in relation with cIMT.
* Adjusted for age, race, study site, menopausal status, BMI, SBP, smoking, physical activity, anti-hypertensive, lipid lowering medications, ever use hormone replacement therapy, IL-6, CRP, HDL-C, small HDL-P and LDL-P
BMI: body mass index; cIMT: carotid intima-media thickness; CRP: C-reactive protein; HDL-C: high-density lipoprotein cholesterol; HDL-P: high-density lipoprotein particles; IL-6: interleukin-6.; LDL-P: low-density lipoprotein particle particles; SBP: systolic blood pressure.
cPlaque and HDL metrics
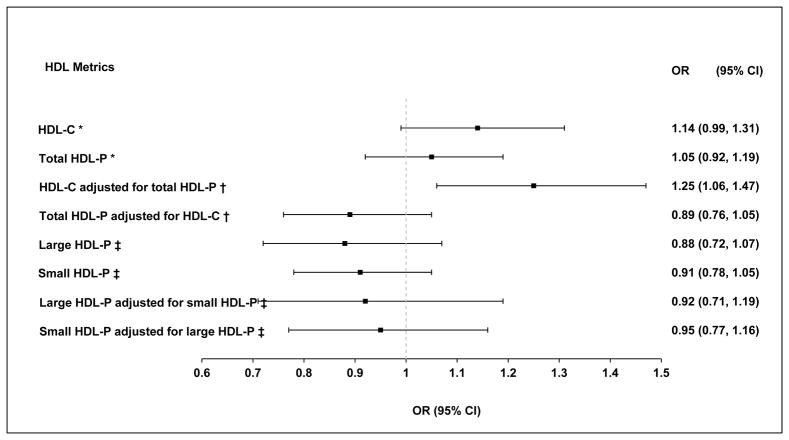
In midlife and older women, only higher HDL-C was marginally associated with greater risk of cPlaque presence (P=0.06) independent of age, race, study site, menopausal status, BMI, SBP, smoking, physical activity, anti-hypertensive, lipid lowering medications, ever use of HT, IL6 and CRP, Figure 3 and Supplemental Table III - Model 2. Adjusting for each other (HDL-C and total HDL-P), study covariates and LDL-P, higher HDL-C but not HDL-P became strongly associated with greater risk of cPlaque, Figure 3 and Supplemental Table III - Model 3. No significant association was observed between small or large HDL-P and cPlaque when either modeled separately or adjusted for each other and study covariates, Figure 3 and Supplemental Table III. Same results were observed for each HDL metric with cPlaque after further adjustment for LDL-C and triglycerides, Supplemental Table III – Model 5.
Figure 3. Adjusted Odds Ratio (95% CI) of cPlaque Presence per 1 SD Greater HDL-C, HDL-P, Large HDL-P or Small HDL-P.
* Adjusted for age, race, study site, menopausal status, BMI, SBP, smoking, physical activity, antihypertensive, lipid lowering medications, ever use hormone replacement therapy, IL-6 and CRP
† * + LDL-P
‡ * + HDL-C and LDL-P
BMI: body mass index; cPlaque: carotid plaque; CRP: C-reactive protein; HDL-C: high-density lipoprotein cholesterol; HDL-P: high-density lipoprotein particles; IL-6: interleukin-6; LDL-P: low-density lipoprotein particles; SBP: systolic blood pressure.
Effect modifications of menopausal factors on associations between HDL metrics and cPlaque
The positive association of HDL-C with higher risk of cPlaque was more evident at older age at menopause, P value for adjusted interaction from model 5 = 0.05). This marginal finding should be interpreted with caution, since this interaction was no longer significant after excluding age as a covariate. Additionally, further assessment of this interaction stratified by time since menopause (≤ 5 years since menopause, 6–10 years since menopause and >10 years since menopause) revealed that the positive interaction between age at menopause and HDL-C was only evident in women with >10 years since menopause (n=717), data not shown. Interestingly, the mean age of this group of women was 69.6 and the mean reported age at menopause was 48.3 years. Age at or time since menopause did not modify associations between ion mobility HDL metrics and cPlaque.
Additional analyses
Additional analyses were conducted; 1) excluding site variable from multivariable analysis, and 2) re-running models excluding women on lipid lowering medication. In general, results from these analyses were similar to findings from the main analysis, data not shown.
Discussion
We reported distinct associations of HDL-C and total HDL-P with carotid atherosclerotic measures among a large sample of midlife and older multi-racial/ethnic women. Adjusting for each other (total HDL-P and HDL-C) and CVD risk factors, we found that higher total HDL-P was associated with lower cIMT, whereas higher HDL-C was associated with higher risk of cPlaque presence. The pro-atherogenic association found for HDL-C was most evident in women who reported later age at menopause and were > 10 years postmenopausal at MESA Exam 1, suggesting that higher HDL-C in older women could be a marker of underlying HDL dysfunction. The current results further suggest that time elapsed since menopause may impact specific subclasses of HDL that could not be captured by HDL-C. Interestingly, small but not large HDL-P was negatively associated with cIMT and this association was not modified by time elapsed since menopause or age at menopause. On the other hand, greater concentration of large HDL-P was significantly associated with lower cIMT mainly after longer time since menopause, suggesting a potential adverse change in the quality of these particles close to menopause, which seems to be restored later in life. However, longitudinal studies will need to confirm these cross-sectional findings and test our proposed hypotheses by evaluating additional compositional and functional metrics of HDL in women transition through menopause.
Although specific HDL subclasses have different biological functions and roles in promoting the macrophage reverse cholesterol transport (18), the best recognized anti-atherogenic function of HDL particles (19), it is still unclear whether specific HDL subclasses are more cardio-protective or could be useful measures of benefits of HDL-therapeutic medications. Findings vary by methods used to quantify HDL subclasses, characteristics of the evaluated study population and whether independent associations of HDL subclasses were evaluated (18, 20). A few studies utilizing NMR to quantify HDL subclasses have been conducted in postmenopausal women, but they did not evaluate effect modifications by menopause-related factors or assess independent effect of HDL subclasses in the same models (21–23). In postmenopausal women, smaller NMR HDL size (21) and lower total and medium (8.3–9.3 nm) NMR HDL-Ps (22) were associated with incident CVD, while lower large NMR HDL-P (9.4–14.0 nm) was associated with extent of coronary calcification (23). Our findings of a protective association with small HDL-Ps are consistent with the above results in obese postmenopausal women showing medium NMR HDL-P to be protective independent of total HDL-P and LDL-P (22); since medium NMR HDL-P (8.3–9.3 nm) overlaps with small ion mobility HDL-P (7.7–10.5 nm). Irrespective of this agreement, it is important to emphasize that direct comparison between different methods of quantifying lipoprotein particles may not adequately address variabilities due to 1) different methods of separation, 2) different definitions of large, medium and small size and 3) different HDL component measured in each approach (24).
Interestingly, we found that associations between large, but not small, HDL-P and cIMT was modified by time elapsed since menopause; such that higher concentrations of large HDL-P were associated with higher cIMT close to menopause but with lower cIMT later in life. This finding suggests large HDL-P to be more prone to dysfunctionality over the menopause transition than small HDL-P. Interestingly, large HDL-P is more vulnerable to oxidative modification compared to small dense HDL-P (18). Recently, we assessed changes in concentrations of HDL subclasses, as measured by calibrated ion mobility (25), and HDL cholesterol efflux capacity among 46 women (mean baseline age = 47.1 years) before and after menopause as indexed by the final menstrual period from the SWAN study. Interestingly, small HDL-P did not correlate with macrophage cholesterol efflux capacity either before or after menopause, whereas higher large HDL-P concentration significantly correlated with greater HDL cholesterol efflux capacity and this correlation was stronger before than after menopause, suggesting that the menopause transition may impair large HDL-P’s ability to promote cholesterol efflux capacity. Our findings from MESA women’s cohort showing higher HDL-P to be positively associated with cIMT close to menopause are in line with our earlier findings from SWAN (25). The current analysis suggests large HDL-P to play a protective role later after menopause. Interestingly, individuals with exceptional longevity and their offspring have larger HDL-P sizes and a lower prevalence of CVD (26). Additionally, although no observed significant variation in large HDL particles was seen between young and elderly in a small study investigating the effect of aging on capacity of HDL particles to promote CEC, large HDL2 in elderly showed a trend of higher capacity to promote CEC than in young participants (27).
Several studies have showed that higher level of HDL-C in middle aged and older women is not always cardio-protective (28). Our findings of a positive association between higher level of HDL-C and risk of cPlaque are in line with these previous studies (4–8) and suggest HDL-C as a marker of underlying dysfunctionality rather than a true indicator of CVD risk. Interestingly, a recent study of P376L variant of the hepatic HDL scavenger receptor BI, encoded by the gene SCARB1, demonstrated that the gene increased both HDL-C and CVD risk (29). In-vivo studies in Scarb1 KO mice showed that scavenger receptor BI is an important positive regulator of macrophage reverse cholesterol transport, despite lowering HDL-C serum concentration (30). Thus, a decline in hepatic scavenger receptor BI function in humans may cause impaired reverse cholesterol transport, which leads to increased risk of CVD despite elevation in HDL-C levels. HDL-C is a crude measure of the total cholesterol contents carried by all HDL particles and thus may not correctly capture any changes impacting HDL particles composition or functions.
Our results of a consistent negative association of total HDL-P but not HDL-C with cIMT after including both metrics in the same model was in line with a previous work from MESA, which was not specifically focusing on menopause (31) and evaluated the independent associations of HDL-C and total HDL-P (measured via NMR spectroscopy) with cIMT among the full MESA cohort. The agreement between our findings and previous work from MESA (31) irrespective of using different methods to quantify total HDL-P, suggest that total HDL-P is a better indicator of HDL anti-atherogenic features than HDL-C in midlife and older women among whom the cardio-protective capacity of HDL-C has been challenged (28). Total HDL-P concentrations can provide consistent information on CVD risk that is independent of HDL-C (32–34).
The current study should be viewed in the context of several limitations. It was limited by the cross-sectional design and thus reversal causality is possible. Additionally, we could not assess the impact of the menopause transition on HDL quality changes since this would require repeated measures of HDL metrics over the menopause transition. In MESA, menopausal status as well as age at menopause were self-reported and could be subjected to recall bias given the age of the MESA women participants. By design, MESA did not include sufficient number of regularly menstruating women which limited our ability to compare associations between HDL subclasses and carotid atherosclerosis in regularly menstruating women and postmenopausal women. The lack of medical history data impacting menopausal status in early menopause women not due to bilateral oophorectomies resulted in excluding those women from the current analysis. Thus, our findings may not be generalizable to non-surgical early menopause women. The reported findings should be interpreted with cautions given the multiple testing performed, which increases the chance of a type I error. However, the tested lipoproteins are correlated, and we interpreted the results, emphasizing the consistency with prior studies. MESA did not include any measure of HDL functionality, such as HDL cholesterol efflux capacity. It would be interesting to assess capacity of HDL subclasses to promote cholesterol efflux by time elapsed since menopause. Finally, the lack of studies about long-term stability of HDL in frozen samples is always a concern with any retrospective analysis of clinical study samples where samples have been stored (all-be-it under ideal storage conditions) for long periods of time. However, lipoprotein profiles from MESA stored samples have been shown to be consistent with those obtained from fresh frozen specimens per Quest Diagnostics laboratory. Irrespective of these limitations, this is the first large cross-sectional analysis in midlife and older women comparing cardio-protective characteristics of HDL-C with HDL subclasses physically quantified based on size and charges of HDL-P using ion mobility method (9, 10). Given the large sample size we were able to control for many potential confounds and assess the joint effect of HDL subclasses, an important analytical methodology that should be evaluated in all studies given the dynamic remodeling between large and small HDL-Ps and the significant correlates (18).
In this large cross-sectional analysis among midlife and older women from the MESA cohort, adjusting for each other and traditional risk factors, higher HDL-P was associated with lower cIMT, whereas higher HDL-C was associated with higher risk of cPlaque presence. The protective association between small HDL-P and cIMT was not modified by menopausal characteristics, suggesting small particles to not affect by the menopause transition. On the other hand, large HDL-P may be compromiozed at menopause. We hypothesize that higher HDL-C in older women could be a marker of underlying HDL dysfunction. Findings from this study support the importance of assessing how the menopause transition might impact HDL quality and how that might impact women risk of CVD later in life. A longitudinal study over the menopause transition that includes comprehensive metrics of HDL composition and function will significantly enhance our understanding of the complex association of the menopause and HDL.
Supplementary Material
Highlights.
Higher HDL-C was associated with greater atherosclerosis risk, whereas higher HDL-P was protective in midlife and older women.
Time since menopause modified the association of large but not small HDL-P with atherosclerosis risk.
Higher large HDL-P was associated with higher atherosclerosis risk close to menopause but with lower risk thereafter.
Acknowledgments
The authors would like to thank Mr. Mohammed R. El Khoudary for designing the graphical abstract of this article.
Source of Funding:
This research was supported by R01 HL071739 and a grant from Quest Diagnostics, and MESA was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1 TR 001079, and UL1-RR-025005 from National Center for Research Resources. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org
Abbreviations list
| Abbreviations | Description |
|---|---|
| BMI | Body mass index |
| cIMT | Carotid intima-media thickness |
| cPlaque | Carotid plaque score |
| CRP | C-reactive protein |
| CVD | Cardiovascular disease |
| HDL | High-density lipoprotein |
| HDL-C | High-density lipoprotein cholesterol |
| HDL-P | HDL particles |
| IL-6 | Interleukin-6 |
| LDL-C | Low-density lipoprotein cholesterol |
| LDL-P | Low-density lipoprotein cholesterol particles |
| MESA | The Multi-Ethnic Study of Atherosclerosis |
| SBP | Systolic blood pressure |
Footnotes
Disclosures:
Drs. El Khoudary, Ceponiene, Tattersall, Li, and Budoff and Mr. Samargandy have nothing to disclose.
Dr. Stein receives royalties from the Wisconsin Alumni Research Foundation for a patent related to carotid wall thickness and arterial aging. The arterial age algorithms were not used in this research project.
References
- 1.Ansell BJ, Fonarow GC, Fogelman AM. High-density lipoprotein: is it always atheroprotective? Curr Atheroscler Rep. 2006;8:405–411. doi: 10.1007/s11883-006-0038-4. [DOI] [PubMed] [Google Scholar]
- 2.El Khoudary SR. Gaps, limitations and new insights on endogenous estrogen and follicle stimulating hormone as related to risk of cardiovascular disease in women traversing the menopause: A narrative review. Maturitas. 2017 Oct;104:44–53. doi: 10.1016/j.maturitas.2017.08.003. Epub 2017 Aug 7. [DOI] [PubMed] [Google Scholar]
- 3.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–1575. doi: 10.1016/j.jacc.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bots ML, Elwood PC, Nikitin Y, Salonen JT, Freire de Concalves A, Inzitari D, Sivenius J, Benetou V, Tuomilehto J, Koudstaal PJ, Grobbee DE. Total and HDL cholesterol and risk of stroke. EUROSTROKE: a collaborative study among research centres in Europe. J Epidemiol Community Health. 2002;56:i19–i24. doi: 10.1136/jech.56.suppl_1.i19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keidar S, Bogner I, Gamliel-Lazarovich A, Leiba R, Fuhrman B, Kouperberg E. High plasma high-density lipoprotein levels, very low cardiovascular risk profile, and subclinical carotid atherosclerosis in postmenopausal women. J Clin Lipidol. 2009;3:345–350. doi: 10.1016/j.jacl.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Woodard GA, Brooks MM, Barinas-Mitchell E, Mackey RH, Matthews KA, Sutton-Tyrrell K. Lipids, menopause, and early atherosclerosis in Study of Women’s Health Across the Nation Heart women. Menopause. 2011;18:376–384. doi: 10.1097/gme.0b013e3181f6480e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan AZ, Dwyer JH. Sex differences in the relation of HDL cholesterol to progression of carotid intima-media thickness: the Los Angeles Atherosclerosis Study. Atherosclerosis. 2007;195:e191–e196. doi: 10.1016/j.atherosclerosis.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 8.El Khoudary SR, Wang L, Brooks MM, Thurston RC, Derby CA, Matthews KA. Increase HDL-C level over the menopausal transition is associated with greater atherosclerotic progression. J Clin Lipidol. 2016;10:962–969. doi: 10.1016/j.jacl.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musunuru K, Orho-Melander M, Caulfield MP, Li S, Salameh WA, Reitz RE, Berglund G, Hedblad B, Engström G, Williams PT, Kathiresan S, Melander O, Krauss RM. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler Thromb Vasc Biol. 2009;29:1975–1980. doi: 10.1161/ATVBAHA.109.190405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caulfield MP, Li S, Lee G, Blanche PJ, Salameh WA, Benner WH, Reitz RE, Krauss RM. Direct determination of lipoprotein particle sizes and concentrations by ion mobility analysis. Clin Chem. 2008;54:1307–1316. doi: 10.1373/clinchem.2007.100586. [DOI] [PubMed] [Google Scholar]
- 11.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 12.Tattersall MC, Gassett A, Korcarz CE, Gepner AD, Kaufman JD, Liu KJ, Astor BC, Sheppard L, Kronmal RA, Stein JH. Predictors of carotid thickness and plaque progression during a decade: the Multi-Ethnic Study of Atherosclerosis. Stroke. 2014;45:3257–3262. doi: 10.1161/STROKEAHA.114.005669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mora S, Caulfield MP, Wohlgemuth J, Chen Z, Superko HR, Rowland CM, Glynn RJ, Ridker PM, Krauss RM. Atherogenic Lipoprotein Subfractions Determined by Ion Mobility and First Cardiovascular Events After Random Allocation to High-Intensity Statin or Placebo: The Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) Trial. Circulation. 2015;132:2220–2229. doi: 10.1161/CIRCULATIONAHA.115.016857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banche PJ, Gong EL, Forte TM, Nichols AV. Characterization of human high-density lipoproteins by gradient gel electrophoresis. Biochim Biophys Acta. 1981;665:408–419. doi: 10.1016/0005-2760(81)90253-8. [DOI] [PubMed] [Google Scholar]
- 15.Otvos JD, Rudel LL, McConnell JP. Concerns regarding lipoprotein particle measurement by ion mobility analysis. Clin Chem. 2008;54:2086–2087. doi: 10.1373/clinchem.2008.113795. author reply 2088–2089. [DOI] [PubMed] [Google Scholar]
- 16.Noori N, Caulfield MP, Salameh WA, Reitz RE, Nicholas SB, Molnar MZ, Nissenson AR, Kovesdy CP, Kalantar-Zadeh K. Novel lipoprotein subfraction and size measurements in prediction of mortality in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:2861–2870. doi: 10.2215/CJN.03650411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18.Camont L, Chapman MJ, Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med. 2011;17:594–603. doi: 10.1016/j.molmed.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Rosenson RS, Brewer HB, Jr, Chapman MJ, Fazio S, Hussain MM, Kontush A, Krauss RM, Otvos JD, Remaley AT, Schaefer EJ. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin Chem. 2011;57:392–410. doi: 10.1373/clinchem.2010.155333. [DOI] [PubMed] [Google Scholar]
- 20.Kontush A. HDL particle number and size as predictors of cardiovascular disease. Front Pharmacol. 2015;6:218. doi: 10.3389/fphar.2015.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119:931–939. doi: 10.1161/CIRCULATIONAHA.108.816181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackey RH, McTigue KM, Chang YF, Barinas-Mitchell E, Evans RW, Tinker LF, Lewis CE5, Manson JE, Stefanick ML, Howard BV, Phillips LS, Liu S, Kulick D, Kuller LH. Lipoprotein particles and size, total and high molecular weight adiponectin, and leptin in relation to incident coronary heart disease among severely obese postmenopausal women: The Women’s Health Initiative Observational Study. BBA Clin. 2015;3:243–250. doi: 10.1016/j.bbacli.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackey RH, Kuller LH, Sutton-Tyrrell K, Evans RW, Holubkov R, Matthews KA. Lipoprotein subclasses and coronary artery calcium in postmenopausal women from the healthy women study. Am J Cardiol. 2002;90:71i–76i. doi: 10.1016/s0002-9149(02)02636-x. [DOI] [PubMed] [Google Scholar]
- 24.Meeusen JW. Comparing Measures of HDL: On the Right Path with the Wrong Map. Clin Chem. 2018;64:424–426. doi: 10.1373/clinchem.2017.284208. [DOI] [PubMed] [Google Scholar]
- 25.El Khoudary SR, Hutchins PM, Matthews KA, Brooks MM, Orchard TJ, Ronsein GE, Heinecke JW. Cholesterol efflux capacity and subclasses of HDL particles in healthy women transitioning through menopause. J Clin Endocrinol Metab. 2016;101:3419–3428. doi: 10.1210/jc.2016-2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barzilai N, Atzmon G, Schechter C, Schaefer EJ, Cupples AL, Lipton R, Cheng S, Shuldiner AR. Unique lipoprotein phenotype and genotype associated with exceptional longevity. JAMA. 2003;290:2030–2040. doi: 10.1001/jama.290.15.2030. [DOI] [PubMed] [Google Scholar]
- 27.Berrougui H, Isabelle M, Cloutier M, Grenier G, Khalil A. Age-related impairment of HDL-mediated cholesterol efflux. J Lipid Res. 2007;48:328–336. doi: 10.1194/jlr.M600167-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.El Khoudary SR. HDL and the menopause. Curr Opin Lipidol. 2017;28:328–336. doi: 10.1097/MOL.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 29.Zanoni P, Khetarpal SA, Larach DB, et al. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016;351:1166–1171. doi: 10.1126/science.aad3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Da Silva JR, Reilly M, Billheimer JT, Rothblat GH, Rader DJ. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest. 2005;115:2870–2874. doi: 10.1172/JCI25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackey RH, Greenland P, Goff DC, Jr, Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2012;60:508–516. doi: 10.1016/j.jacc.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khera AV, Demler OV, Adelman SJ, Collins HL, Glynn RJ, Ridker PM, Rader DJ, Mora S. Cholesterol Efflux Capacity, High-Density Lipoprotein Particle Number, and Incident Cardiovascular Events: An Analysis From the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin) Circulation. 2017;135:2494–504. doi: 10.1161/CIRCULATIONAHA.116.025678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mora S, Glynn RJ, Ridker PM. HDL cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013;128:1189–1197. doi: 10.1161/CIRCULATIONAHA.113.002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Harchaoui K, Arsenault BJ, Franssen R, Després JP, Hovingh GK, Stroes ES, Otvos JD, Wareham NJ, Kastelein JJ, Khaw KT, Boekholdt SM. High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med. 2009;150:84–93. doi: 10.7326/0003-4819-150-2-200901200-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.