Abstract
Rationale
A primary goal of therapy for patients with peripheral arterial disease and intermittent claudication (PAD+IC) is increased ambulatory function. Supervised exercise rehabilitation was recently shown to confer superior walking benefits to pharmacological or surgical interventions. Increases in plasma inorganic nitrite, via oral nitrate, have been shown to increase exercise performance in both human and animal models, especially in hypoxic conditions.
Objective
To determine whether a 36-session exercise rehabilitation program while consuming oral inorganic nitrate (4.2mmol concentrated beetroot juice- EX+BR) would produce superior benefits over exercise plus placebo (EX+PL) in pain free walking and markers of increased skeletal muscle perfusion in patients with PAD+IC.
Methods and Results
This was a randomized, double-blind, per-protocol study design. Following the 12-week protocol claudication onset time (COT) on a maximal treadmill test increased by 59.2±57.3 sec for the EX+PL group (n=13), and by 180.3±46.6 sec for the EX+BR group (n=11) (p≤0.05). This produced a between treatment medium to large standardized effect size (Cohen’s d) of 0.62 (95%CI = −0.23 to +1.44). The data for six minute walk (6MW) distance showed a similar pattern with increases of 24.6±12.1 m and 53.3±19.6 m (p≤0.05) in the EX+PL and EX+BR groups respectively. Measures of gastrocnemius perfusion including ABI, peak reactive hyperemic blood flow and tissue deoxygenation characteristics during exercise (assessed my near infra-red spectroscopy) all changed significantly for the EX+BR group with moderate to large effect sizes over EX+PL changes.
Conclusions
While it is premature to speculate on overall clinical utility of a nitrate based therapy for PAD, this early pilot study evidence is encouraging. Specifically, our data suggests that increasing plasma nitrite prior to exercise may allow PAD subjects to train with less pain, at higher workloads for longer durations at each training session thereby maximizing the beneficial peripheral vascular and skeletal muscle adaptations.
Clinical Trial Registration
Keywords: Nitrate, nitrite, nitric oxide, exercise, peripheral arterial disease, intermittent claudication, vasculature
Subject Terms: Clinical Studies, Endothelium/Vascular Type/Nitric Oxide, Peripheral Artery Disease, Physiology, Rehabilitation
INTRODUCTION
Peripheral arterial disease (PAD) is a highly prevalent1 and costly2 condition. Intermittent claudication (IC), defined as ischemic leg pain that occurs with walking and resolves with rest, affects 90% of symptomatic PAD patients. PAD+IC results in functional impairment3, reduced daily physical activity4, a lower quality of life5 and an accelerated loss of mobility3. Accordingly, a primary goal of therapy for PAD+IC is increased ambulatory function.
Although the pathophysiologic mechanisms contributing to the functional impairment and decline in PAD are not fully delineated, current evidence suggests that a range of tissue maladaptation’s in response to chronic under perfusion are involved. These include vascular endothelial dysfunction, reduced nitric oxide (NO) bioavailability6, capillary density rarefaction7, 8, increased reactive oxygen species9, mitochondrial dysfunction9, and a preferential loss of type I oxidative fibers10. Overall this results in the uncoupling of skeletal muscle cellular metabolism from tissue perfusion11 and the exhibition of a glycolytic phenotype, which promotes the early onset of fatigue and exercise intolerance.
NO is an important signalling molecule, which is essential for vascular function/health. In healthy human exercise studies, increased vascular NO bioavailability has been shown to provide ergogenic benefits, especially in low oxygen conditions or in high-intensity activities that rely predominantly on fast–twitch muscle fibres12. Interestingly, in vascular pathologies, including PAD, peripheral tissue maladaptation’s to chronic under perfusion and disuse (coupled with endothelial dysfunction and the inability to endogenously upregulate NO) may provide an ideal target for exogenous supplementation therapies. To date, the use of NO-donor compounds has been limited in clinical applications, primarily due to systemic vascular effects often resulting in hypotension.
Plasma nitrite was once considered a biologically inert byproduct and marker of endothelial NO production13, 14. However, several studies have demonstrated an endocrine-like role for NO equivalents15, 16, including nitrite17, 18, which function to conserve and transport NO, and “release” it under hypoxic and acidic conditions17. This suggests increasing plasma nitrite may be an innovative way to create a “delivery pool” for NO which would target the peripheral tissues that are under-perfused due to occlusive vascular disease and maybe especially beneficial during exercise. It would also avoid systemic pressor effects.
In patients with PAD+IC, we have observed; a) a net consumption of plasma nitrite following maximal exercise6, b) a change in plasma nitrite which predicted an increase in exercise performance19, 20 and, c) a single oral dose of a inorganic nitrate (9mmol) in the form of beetroot juice, produced an 18% increase in pain free walking (COT) on a graded treadmill test21. These findings suggest that elevated plasma nitrite allows patients with PAD+IC to exercise with less pain, at higher workloads for longer durations.
Currently, treatment options to increase function in PAD are limited. Supervised exercise rehabilitation has a Class IA recommendation and confers superior ambulatory benefits than pharmacological or surgical interventions22, 23, with lower costs, morbidity and mortality24. Supervised exercise was recently approved for reimbursement coverage by the Centres for Medicare and Medicaid Services, so will likely be utilized on a much wider basis in the future. It is unknown if the concurrent addition of an inorganic nitrate supplementation to exercise rehabilitation would “beet the best” available therapeutic approach for PAD+IC.
Accordingly, the primary hypothesis of this pilot trial was, in patients with PAD+IC, regular consumption of a high inorganic-nitrate containing supplement (beetroot juice), in conjunction with supervised exercise (EX+BR) over a 12-week period, would produce greater clinical benefit via increases in pain free exercise tolerance (COT, 6MW), relative to exercise plus placebo (EX+PL). A secondary hypothesis was that these differences would be mediated by increased NO bioavailability/signalling, skeletal muscle microvascular tissue perfusion and tissue structural adaptations.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The NO-PAD trial was a randomized, double-blind, per-protocol design, details of which have been published25 and registered on www.clinicaltrials.gov (NCT01684930 and NCT01785524). Briefly, we recruited patients aged between 40 and 80 years with diagnosed PAD (Ankle Brachial Index (ABI) <0.90) (Table 1). All subjects were required to have stable, IC pain as the limiting factor in their exercise ability. The study was approved by the Duke University Medical Centre Institutional Review Board and all subjects signed an informed consent prior to participation.
Table 1.
Subject Demographics
| All n=24 | EX+PL n=13 | EX+BR n=11 | |
|---|---|---|---|
|
| |||
| Age (years) | 69.7 ±8.1 | 71.5 ± 7.3 | 67.5 ± 8.6 |
| Male, n (%) | 15 (62.5) | 6 (46.2) | 9 (82) |
| Height (cm) | 171.9 ± 9.8 | 170.3 ± 8.5 | 173.7 ± 11.3 |
| Mass (kg) | 80.4 ±15.8 | 79.7 ± 12.6 | 81.3 ±19.6 |
| BMI | 27.1 ± 4.4 | 27.5 ± 4 | 26.6 ± 5.1 |
| Diabetes, n (%) | 8 (33.3) | 2 (15.4) | 6 (54.5) |
| Blood Glucose (mg/dL) | 128.3 ± 29.7 | 139.5 ± 39.2 | 118.9 ± 14.2 |
| Statin status, n (%) | 18 (75) | 10 (77) | 8 (73) |
| Smoking Status, n (%) | |||
| Current | 9 (37.5) | 5 (38.5) | 4(36.4) |
| Former | 9 (37.5) | 5 (38.5) | 4(36.4) |
| Never | 6 (25) | 3 (23.0) | 3 (27.2) |
| Myocardial Infarct, n (%) | 4 (16.7) | 2 (15.4) | 2 (18.2) |
| Stroke, n (%) | 4 (16.7) | 1 (7.6) | 3 (27.3) |
| Periph Intervention, n (%) | 4 (16.7) | 1 (7.6) | 3 (27.3) |
| Race, n (%) | |||
| White | 15 (62.5) | 7 (53.8) | 8 (72.7) |
| African-American | 7 (29.2) | 5 (38.5) | 2 (18.2) |
| Asian | 1 (4.2) | 0 (0) | 1 (9.1) |
| Hispanic | 1 (4.2) | 1 (7.7) | 0 (0) |
Mean ± SD except where otherwise note. There were no significant differences between any of the metrics.
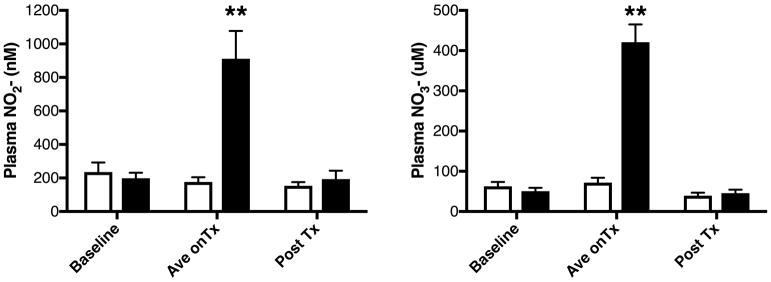
Following baseline exercise and vascular testing, subjects were randomly assigned to consume either 70ml (4.2mmol NO3-) beetroot juice (EX+BR) or an identical nitrate-depleted placebo (EX+PL), 3 hours prior to each exercise training visit (3 times per week for 12 weeks). In order to check for beverage compliance and nitrate to nitrite conversion, several scheduled and unannounced blood draws were obtained. Based on previous studies and known variabilities in nitrate to nitrite conversion26, an a priori acceptable range for resting plasma NO2- concentrations (prior to exercise) was set at ≥400nM nitrite for EX+BR and ≤400nM for EX+PL (fig 1)25. Additionally, all subjects included in the per-protocol analysis completed at least 34 of the 36 supervised exercise training sessions. Each session included at least 30 minutes of actual walking, with the intensity tailored to each subjects’ initial baseline maximal graded exercise test results (GXT). When claudication pain became moderately severe (3–4/5 on a 5-point claudication pain scale) they would step off the treadmill and rest until the pain subsided (rest periods were not included in the 30 minutes exercise time). Typically, after a patient was able to walk 8–10 minutes at the initial work load, the grade was increased by 0.5%, or the speed increased by 0.1 mph as tolerated.
Figure 1.
Venous Plasma (left) NO2- and (right) NO3- concentrations at baseline, during the 12 weeks of training (mean of several tests-Ave on Tx) and at end of study testing (Post Tx). EX+PL (clear bars/circles) or EX+BR (black bars/circles). **=p<0.01 versus other values.
Statistics
This was a repeated-measures design, with the purpose of assessing change over time (12 weeks) for the two intervention groups. Since this was a pilot study, the overarching goal of the analyses is to derive effect sizes (ie, effectiveness) for this intervention for a future larger, confirmatory study.
Comparisons were made using a General Linear Model analysis of variance adjusted for baseline values. P<0.05 (two-tailed) was employed as the criterion to declare statistical significance. Changes in individual and treatment group near-infra-red spectroscopy (NIRS) spectra, generated from the incident leg gastrocnemius muscle tissue monitoring during the pre and post treatment GXT was examined for statistical significance by χ2 test between groups. Deoxy-hemoglobin spectra were fit to a 3 parameter exponential decay curve (f= Yo+a*e(b*x)), while oxy-hemoglobin spectra were fit to a single rectangular 2 parameter hyperbola(f=a*x/(b+x)). Individual parameters were directly compared by z score using the Ho of no significant difference between treatment groups.
RESULTS
Thirty-five patients were enrolled in the study and following baseline testing were randomly allocated to EX+BR (n=17) or EX+PL (n=18). During the trial 3 participants from each group withdrew participation. A further 3 subjects from EX+BR and 2 from EX+PL were excluded due to plasma nitrite concentrations outside the a-priori specified ranges.
There were no differences plasma nitrate and nitrite concentrations at the initial baseline testing visit or at the end of the 12-week study testing (approximately 1 week after the final BR dosage -figure 1).
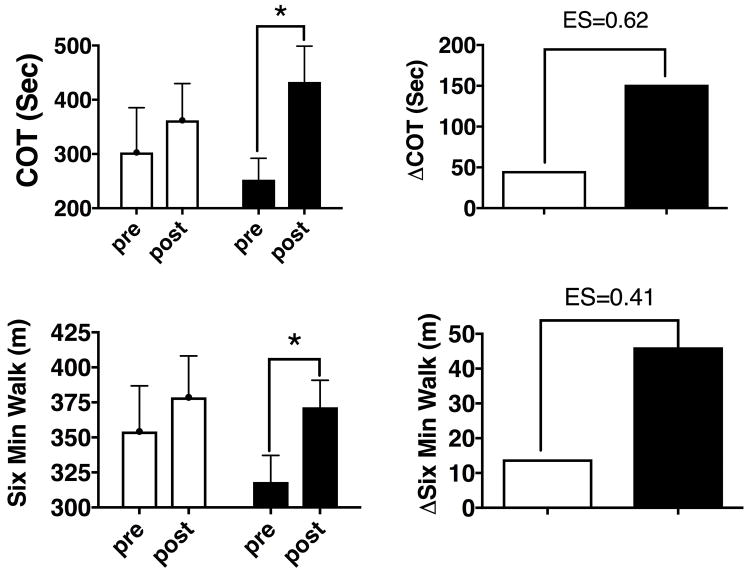
Measures of clinical function (figure 2), or tissue perfusion (figure 3) were also not different at baseline (pre). Following the 12-week protocol (post) COT increased by 59.2±57.3 sec for the EX+PL group, and by 180.3±46.6 sec for the EX+BR group (p≤0.05). This produced a between treatment medium to large standardized effect size (Cohen’s d) of 0.62 (95%CI = −0.23 to +1.44) (figure 2). The data for six minute walk (6MW) showed a similar pattern with increases of 24.6±12.1 m and 53.4±19.6 m (p≤0.05) in the EX+PL and EX+BR groups respectively with a standardized effect size of 0.43 (95%, CI = −0.44 to +1.21, figure 2). Peak walk time (PWT) increased significantly for both groups 238.7±207.0 sec and 269.9±195.3 sec respectively (both p≤0.01, data not shown).
Figure 2.
(left) raw data and (right) group changes for (upper panels) Claudication Onset Time (COT) and (lower panels) Six Minute Walk Test following 12 weeks of EX+PL (clear bars/circles) or EX+BR (black bars/circles). **=p<0.01 within treatment.
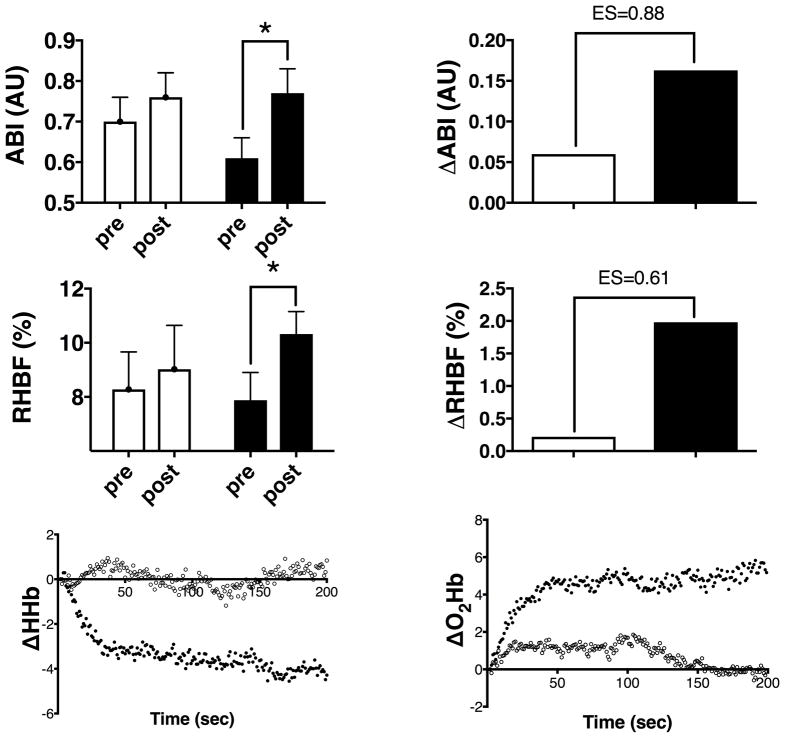
Figure 3.
(left) raw data and (right) group changes for (upper panels) Ankle Brachial Index (ABI-arbitrary units); (middle panels) Reactive Hyperemic Blood Flow (RHBF-ml/min/100mltissue or %). Lower panels show the group average changes in NIRS spectra from pre to post treatment (left) deoxy-haemoglobin and (right) oxy-haemoglobin.
EX+PL (clear bars/circles) or EX+BR (black bars/circles). *=p<0.05, **=p<0.01 within treatment
Indices of gastrocnemius perfusion showed similar responses to treatments. Resting ABI measures showed no changes following EX+PL but increased by 0.16±0.11 after EX+BR (p≤0.05) with a standardized effect size of 0.88 (95%, CI = −0.01 to +1.70, figure 3). Measures of limb blood flow at rest (using strain-gauge plethysmography) were not different between groups and did not change following either treatment. Responses following a 5-minute arterial occlusion of the thigh (peak reactive hyperemic blood flow) did not change (0.74+2.67ml blood/100mltissue/min) for EX+PL but increased by 2.57±2.65ml blood/100mltissue/min (p≤0.05) for EX+BR, with a between treatment effect size of 0.65 (95%, CI = −0.21 to +1.46) (figure 3).
Similarly, measures of gastrocnemius tissue perfusion during the GXT revealed differences in the NIRS spectra characteristics between treatments (figure 3e, f and online figure I). There was no difference in the deoxygenation characteristics of the EX+PL group over time, however, the change in initial desaturation rate within the EX+BR group from pre to post was significantly reduced as reflected in an increased parameter ‘a’, (4.07 ± 0.111, p≤0.05) (figure 3e). Conversely, the change in the Oxyhaemoglobin (oxyHb) spectra over time indicated a significant increase in the maintenance of oxygenation in both the EX+PL and EX+BR groups following the 12 week treatment period. This improvement was again significantly greater in the EX+BR treatment group, both in the initial rate (parameter a) and in plateau rate (parameter b)(figure 3f and online Table I).
DISCUSSION
This study shows that oral inorganic nitrate supplementation prior to exercise rehabilitation three times per week for 12 weeks produced increases in pain free exercise tolerance. However, the overall hypothesis that these changes would be superior to exercise rehabilitation alone could not be confirmed; although moderate to strong effect sizes were observed. COT was chosen as the major endpoint of this trial because it may best represent changes following treatment in gastrocnemius tissue perfusion and mitochondrial metabolism. Interestingly, the increase in COT for exercise alone was similar to our previous exercise-only PAD trial19 confirming that the current pilot study was likely effective but underpowered.
In a practical context, the combination of exercise and beet juice improved COT by 121.1sec (95% CI: −23.7, 265.9sec), which was a 200% increase over exercise alone. This is slightly more than one complete stage on the treadmill graded exercise test. For the 6-minute walk test, often regarded as more representative of activities of daily living27, a clinically meaningful change in patients with chronic heart failure, who have a similar functional level to PAD, is 30m28. In the current study, the EX+BR group walked 28.8 m (95% CI: −16.3, 73.9) further than the EX+PL group. These data are suggestive that the addition of beet juice to the intervention was clinically beneficial beyond the currently optimal treatment of exercise alone.
The secondary hypothesis that the differences were mediated by increased NO bioavailability/signaling is supported by the significantly greater plasma nitrite levels during the 12-week training intervention. Importantly, the ABI and peak blood flow measurements were made following the training period but without acute nitrate/nitrite/NO supplementation. Therefore, the increases in ABI and peak limb blood flow for the EX+BR group at the post intervention testing suggest greater tissue structural changes may have occurred. One possible mechanism for such a structural change is an increase in collateralization and downstream angiogenesis via NO mediated mechanisms, which result in reduced tissue desaturation. In this regard, it is interesting that exercise alone produced no significant decrease in tissue deoxygenation within the gastrocnemius upon exercise, as determined by NIRS. However, the EX+BR combination resulted in reduced tissue deoxygenation upon exercise initiation (figure 3e). These data suggest that oxygen extraction within the working tissue is improved with BR supplementation. Although we have no direct evidence from tissue samples in this study, a murine hind limb permanent femoral artery ligation model demonstrated a dose dependant relationship between nitrite dose (via intraperitoneal injection twice daily for 7 days) and improved tissue perfusion via angiogenesis29. Co-administration of the NO scavenger carboxy-PTIO with the nitrite completely abrogated the increase in perfusion suggesting the mechanism of effect is NO mediated.
Despite exercise training alone delivering substantial improvements in functional capacity for patients with PAD+IC, limb ischemia and claudication pain are still major limiting factors30, 31 in the intensity, duration and progression that can be achieved in therapeutic exercise programs. These factors likely contribute to the poor adherence rates often experienced in PAD+IC rehabilitation programs. It is clear that new therapeutic approaches which can raise the ischemic threshold and reduce the burden of exercise in PAD+IC are needed.
The limitations of the current study include the small sample size, 17% drop out rate (not unusual in this population) and lack of an intent to treat design make it premature to speculate on overall clinical utility of a nitrate based therapy for PAD. However, the early evidence is encouraging. Specifically, our data suggests that increasing plasma nitrite prior to exercise may allow PAD subjects to train with less pain, at higher workloads for longer durations at each training session21 thereby maximizing the beneficial peripheral vascular and skeletal muscle adaptations.
The data from this study suggests that a sample size of 58 subjects per group will have 80% power for detecting a 90 second or greater difference in mean COT improvement, at the two-sided significance level of 0.05. To plan for a 20% loss to follow-up, 73 individuals (58/(1–0.20) ~ 73)) would need to be recruited in each treatment arm.
If our hypotheses are confirmed by such additional clinical trial and basic science research, this novel, relatively simple, and inexpensive to implement, therapeutic approach would provide clinically meaningful changes in exercise capacity beyond the current best practice option. This outcome could significantly reduce the burden of exercise participation and may lead a paradigm shift in the treatment and rehabilitation recommendations for PAD+IC.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Peripheral artery disease (PAD) with intermittent claudication (IC) is associated with a markedly impaired quality of life and a high perception of disability.
Increased walking capacity is a primary goal of therapy for PAD+IC.
Supervised exercise training confers walking benefits superior to pharmacological or surgical interventions
What New Information Does this Article Contribute?
Regular oral inorganic nitrate supplementation prior to exercise training produced superior functional benefits in comparison with supervised exercise training plus placebo.
Increasing plasma nitrite prior to exercise may allow PAD subjects to train with less pain, at higher workloads for longer durations at each training session; thereby, maximizing the beneficial peripheral vascular and skeletal muscle adaptations.
This relatively simple and inexpensive therapeutic approach could provide clinically meaningful changes in exercise capacity beyond current best practice.
Peripheral arterial disease (PAD) with intermittent claudication (IC) is defined as lower limb ischemic pain which occurs with walking and is relieved by rest. It results in significant reductions in walking ability and quality of life. Here we report that oral inorganic nitrate supplementation in conjunction with a 3-month supervised exercise rehabilitation program produces superior functional benefits than supervised exercise rehabilitation training plus placebo. This approach, if confirmed, could have the ability to provide clinically meaningful changes in exercise capacity beyond the current best practice option. This could significantly reduce the burden of exercise participation, and would be a significant step forward in the available therapeutic options for patients with PAD+IC.
Acknowledgments
SOURCES OF FUNDING
This work was supported by grants to J.D. Allen from the National Heart, Lung, and Blood Institute R21HL111972 and R21HL113717
Nonstandard Abbreviations and Acronyms
- ABI
Ankle Brachial Index
- BR
Beetroot juice
- COT
Claudication Onset Time
- EX
Exercise
- IC
Intermittent claudication
- GXT
Maximal Graded Exercise Test
- NIRS
Near Infra-red Spectroscopy
- NO
Nitric oxide
- NO2−
Nitrite
- OxyHb
Oxyhaemoglobin
- PWT
Peak walk Time
- PAD
Peripheral Arterial Disease
- PL
Placebo
- 6MW
Six Minute Walk
Footnotes
DISCLOSURES
None.
References
- 1.Fowkes FGR, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UKA, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. The Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 2.Mahoney EM, Wang K, Keo HH, Duval S, Smolderen KG, Cohen DJ, Steg G, Bhatt DL, Hirsch AT. Vascular Hospitalization Rates and Costs in Patients With Peripheral Artery Disease in the United States. Circulation: Cardiovascular Quality and Outcomes. 2010;3:642–651. doi: 10.1161/CIRCOUTCOMES.109.930735. [DOI] [PubMed] [Google Scholar]
- 3.McDermott MM. Lower Extremity Manifestations of Peripheral Artery Disease. The Pathophysiologic and Functional Implications of Leg Ischemia. 2015;116:1540–1550. doi: 10.1161/CIRCRESAHA.114.303517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sieminski DJ, Gardner AW. The Relationship between Free-Living Daily Physical Activity and the Severity of Peripheral Arterial Occlusive Disease. Vascular Medicine. 1997;2:286–291. doi: 10.1177/1358863X9700200402. [DOI] [PubMed] [Google Scholar]
- 5.Olsen P, Gustafsen J, Rasmussen L, Lorentzen J. Long-term results after arterial surgery for arteriosclerosis of the lower limbs in young adults. Eur J Vasc Surg. 1988;2:15–18. doi: 10.1016/s0950-821x(88)80101-4. [DOI] [PubMed] [Google Scholar]
- 6.Allen J, Miller E, Schwark E, Robbins J, Duscha B, Annex B. Plasma Nitrite Response and Arterial Reactivity Differentiate Cardiovascular Health Status and Performance. Nitric Oxide. 2009;20:231–237. doi: 10.1016/j.niox.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Askew CD, Green S, Walker PJ, Kerr GK, Green AA, Williams AD, Febbraio MA. Skeletal muscle phenotype is associated with exercise tolerance in patients with peripheral arterial disease. J Vasc Surg. 2005;41:802–7. doi: 10.1016/j.jvs.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 8.Robbins J, Jones W, Duscha B, Allen J, Kraus W, Regensteiner J, Hiatt W, Annex B. Relationship between Leg Muscle Capillary Density and Peak Hyperemic Blood Flow with Endurance Capacity in Peripheral Artery Disease. Journal of Applied Physiology. 2011 doi: 10.1152/japplphysiol.00141.2011. online before print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pipinos II, Judge AR, Zhu Z, Selsby JT, Swanson SA, Johanning JM, Baxter BT, Lynch TG, Dodd SL. Mitochondrial defects and oxidative damage in patients with peripheral arterial disease. Free Radical Biology and Medicine. 2006;41:262–269. doi: 10.1016/j.freeradbiomed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, Haykowsky M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. American journal of physiology Heart and circulatory physiology. 2014;306:H1364–70. doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson JD, Epstein FH, Meyer CH, Hagspiel KD, Wang H, Berr SS, Harthun NL, Weltman A, DiMaria JM, West AM, Kramer CM. Multifactorial Determinants of Functional Capacity in Peripheral Arterial Disease: Uncoupling of Calf Muscle Perfusion and Metabolism. Journal of the American College of Cardiology. 2009;54:628–635. doi: 10.1016/j.jacc.2009.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woessner MN, McIlvenna LC, Zevallos JOd, Neil CJ, Allen JD. Dietary nitrate supplementation in cardiovascular health: an ergogenic aid or exercise therapeutic? American Journal of Physiology-Heart and Circulatory Physiology. 2018;314:H195–H212. doi: 10.1152/ajpheart.00414.2017. [DOI] [PubMed] [Google Scholar]
- 13.Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. PNAS. 2001;98:12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen JD, Cobb FR, Gow AJ. Regional and whole-body markers of nitric oxide production following hyperemic stimuli. Free Radical Biology and Medicine. 2005;38:1164–1169. doi: 10.1016/j.freeradbiomed.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Cannon RO, III, Schechter AN, Panza JA, Ognibene FP, Pease-Fye ME, Waclawiw MA, Shelhamer JH, Gladwin MT. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. JClinInvest. 2001;108:279–287. doi: 10.1172/JCI12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 17.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nature reviews Drug discovery. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 18.Pinder A, Pittaway E, Morris K, James P. Nitrite directly vasodilates hypoxic vasculature via nitric oxide dependent and independent pathways. British Journal of Pharmacology. 2009;158:1523–1530. doi: 10.1111/j.1476-5381.2009.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen JD, Stabler T, Kenjale A, Ham KL, Robbins JL, Duscha BD, Dobrosielski DA, Annex BH. Plasma nitrite flux predicts exercise performance in peripheral arterial disease following 3 months of exercise training. Free Radical Biology and Medicine. 2010;49:1138–1144. doi: 10.1016/j.freeradbiomed.2010.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen JD, Stabler T, Kenjale AA, Ham KL, Robbins JL, Duscha BD, Kraus WE, Annex BH. Diabetes status differentiates endothelial function and plasma nitrite response to exercise stress in peripheral arterial disease following supervised training. Journal of diabetes and its complications. 2014;28:219–225. doi: 10.1016/j.jdiacomp.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenjale AA, Ham KL, Stabler T, Robbins JL, Johnson JL, VanBruggen M, Privette G, Yim E, Kraus WE, Allen JD. Dietary Nitrate Supplementation Enhances Exercise Performance in Peripheral Arterial Disease. Journal of Applied Physiology. 2011;110:1582–1591. doi: 10.1152/japplphysiol.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patterson RB, Pinto B, Marcus B, Colucci A, Braun T, Roberts M. Value of a supervised exercise program for the therapy of arterial claudication. Journal of Vascular Surgery. 1997;25:312–319. doi: 10.1016/s0741-5214(97)70352-5. [DOI] [PubMed] [Google Scholar]
- 23.Regensteiner JG, Hiatt WR. Current medical therapies for patients with peripheral arterial disease: a critical review. The American Journal of Medicine. 2002;112:49–57. doi: 10.1016/s0002-9343(01)01034-8. [DOI] [PubMed] [Google Scholar]
- 24.Treesak C, Kasemsup V, Treat-Jacobson D, Nyman JA, Hirsch AT. Cost-effectiveness of exercise training to improve claudication symptoms in patients with peripheral arterial disease. Vascular Medicine. 2004;9:279–285. doi: 10.1191/1358863x04vm570oa. [DOI] [PubMed] [Google Scholar]
- 25.Woessner MN, VanBruggen MD, Pieper CF, O’Reilly EK, Kraus WE, Allen JD. Combined Dietary Nitrate and Exercise Intervention in Peripheral Artery Disease: Protocol Rationale and Design. JMIR Res Protoc. 2017;6:e139. doi: 10.2196/resprot.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James PE, Willis GR, Allen JD, Winyard PG, Jones AM. Nitrate pharmacokinetics: Taking note of the difference. Nitric Oxide. 2015 doi: 10.1016/j.niox.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. Journal of the American Geriatrics Society. 1998;46:706–11. doi: 10.1111/j.1532-5415.1998.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 28.Shoemaker MJ, Curtis AB, Vangsnes E, Dickinson MG. Triangulating Clinically Meaningful Change in the Six-minute Walk Test in Individuals with Chronic Heart Failure: A Systematic Review. Cardiopulmonary Physical Therapy Journal. 2012;23:5–15. [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar D, Branch BG, Pattillo CB, Hood J, Thoma S, Simpson S, Illum S, Arora N, Chidlow JH, Langston W, Teng X, Lefer DJ, Patel RP, Kevil CG. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proceedings of the National Academy of Sciences. 2008;105:7540–7545. doi: 10.1073/pnas.0711480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibson JME, Kenrick M. Pain and powerlessness: the experience of living with peripheral vascular disease. Journal of Advanced Nursing. 1998;27:737–745. doi: 10.1046/j.1365-2648.1998.00599.x. [DOI] [PubMed] [Google Scholar]
- 31.Treat-Jacobson D, Halverson SL, Ratchford A, Regensteiner JG, Lindquist R, Hirsch AT. A Patient-Derived Perspective of Health-Related Quality of Life With Peripheral Arterial Disease. Journal of Nursing Scholarship. 2002;34:55–60. doi: 10.1111/j.1547-5069.2002.00055.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.