Abstract
Quantifying HIV-1 transmission risk per act of anal intercourse (AI) is important for HIV-1 prevention. We updated previous reviews by searching Medline and Embase to 02/2018. We derived pooled estimates of receptive AI (URAI) and insertive AI (UIAI) risk unprotected by condoms using random effects models. Subgroup analyses were conducted by gender, study design, and whether antiretroviral treatment (ART) had been introduced by the time of the study.
Two new relevant studies were identified, one of which met inclusion criteria, adding three new cohorts and increasing number of individuals/partnerships included from 1869 to 14,277. Four studies, all from high-income countries, were included. Pooled HIV-1 risk was higher for URAI (1.25%,95%CI 0.55–2.23%,N=5,I2=87%) than UIAI (0.17%,95%CI 0.09–0.26%,N=3,I2=0%). The sole heterosexual URAI estimate (3.38%,95%CI 1.85–4.91%), from a study of 72 women published in a peer-reviewed journal, was significantly higher than the MSM pooled estimate (0.75%,95%CI 0.56–0.98%,N=4,p<0.0001) and higher than the only other heterosexual estimate identified (0.4%,95%CI 0.08–2.0%, based on 59 women, excluded for being a pre-2013 abstract). Pooled per-act URAI risk varied by study design (retrospective-partner studies: 2.56%,95%CI 1.20–4.42%,N=2 (one MSM, one heterosexual); prospective studies: 0.71,95%CI 0.51–0.93%,N=3 MSM, p<0.0001). URAI risk was lower for studies conducted in the ART era (0.75%,95%CI 0.52–1.03%) than pre-ART (1.67%,95%CI 0.44–3.67%) but not significantly so (p=0.537).
Prevention messages must emphasise that HIV-1 infectiousness through AI remains high, even in the ART era. Further studies, particularly among heterosexual populations and in resource-limited settings, are required to elucidate whether AI risk differs by gender, region and following population-level ART scale-up.
Keywords: HIV, anal intercourse, transmission probability, infectivity, review, meta-analysis, heterosexual, MSM, antiretroviral therapy
Introduction
Anal intercourse (AI) drives HIV-1 epidemics among men-who-have-sex-with-men (MSM), and numerous studies have demonstrated that substantial proportions of heterosexual populations also practise AI1, 2, potentially making it an important source of heterosexual HIV-1 transmission3. Quantifying the role of AI in HIV-1 epidemics is important for effective targeting of safe sex messages, for developing and implementing HIV-1 prevention technologies, and to inform mathematical models. Two previously published systematic reviews and meta-analyses have only included four studies providing estimates of the probability of HIV-1 transmission per AI act unprotected by condoms 4, 5.
Baggaley et al derived the first pooled receptive AI unprotected by condoms (URAI) per-act estimates in 2010 (1.37%, 95% confidence interval[95%CI] 0.20–2.54%)5. Patel et al4 updated the review to February 2012, and derived a similar pooled estimate to Baggaley et al despite excluding a study included in Baggaley et al6 and incorporating one new study (1.38%, 95%CI 1.02–1.86%)5, 7. Patel also reported a pooled estimate for insertive AI unprotected by condoms (UIAI): 0.1% (95%CI 0.0–0.3%). However, since their search, additional per-act estimates derived from large HIV-1 cohort datasets have been published8, 9. Given the scarce data on per-act AI HIV risk, it is important to update pooled estimates in light of new data, to reduce uncertainty and provide more reliable estimates to address public health questions and for use in models.
Addition of further data may enable evaluation of how HIV-1 infectiousness through AI varies by gender of participants, by ART use in the general population, region and other study characteristics. For example, recent evidence from animal studies suggests increased susceptibility of male rhesus macaques to HIV-1 acquisition following intrarectal challenge, compared to females (Diane Bolton, personal communication).
Our aim was to revise pooled estimates of URAI and insertive AI unprotected by condoms (UIAI) per-act HIV-1 transmission risk through incorporation of new data. We aimed to assess whether the addition of new data leads to significantly different pooled estimates of AI per-act risk; to evaluate the robustness of pooled estimates through sensitivity analysis; and to conduct subgroup analysis to investigate the influence of: 1) ART use among study participants or their partners; 2) gender; 3) region; and 4) study design.
Methods
The systematic review and meta-analysis were conducted in accordance with the PRISMA statement10.
Search strategy
We conducted literature searches to identify new studies reporting data on per-act HIV-1 transmission risk through anal intercourse (AI) published since searches originally performed by Baggaley et al5 (searched to September 2008), and Patel et al4 (searched to February 2012). Our search was harmonised to ensure inclusion of terms employed previously4, 5. We used the following search string: (HIV OR HIV infections OR human immunodeficiency virus OR AIDS) AND (disease transmission OR infectious OR infectivity OR infectiousness OR transmissibility OR contact OR contacts OR per-contact OR per-act OR effectiveness) AND (sexual OR heterosexual OR homosexual OR coital OR intercourse OR anal). We searched Medline (Ovid), Embase (Ovid), CINAHL (EbscoHost), Web of Science, Global Health, and the Cochrane Library for studies published February 2012 to February 2018 inclusive. See Supplementary Material for further search details.
Unlike Baggaley et al5, which focused on transmission risk estimates in the absence of ART, we also included studies where ART was likely used by a proportion of study participant partners. This change of inclusion criterion necessitated searching the exclusion lists of Baggaley et al5 to ensure no studies were excluded based on ART use. We defined ART use to include therapeutic use by index (i.e. initially infected) partners, or pre-exposure prophylaxis (PrEP) or post-exposure prophylaxis (PEP) use by their (initially uninfected) partners.
Study selection
Inclusion criteria were randomised controlled trials, longitudinal studies (prospective or retrospective) or other empirical observational studies that directly reported estimates of per-act HIV-1 transmission risk through AI. We excluded studies that did not stratify AI risk, receptive versus insertive. Abstracts pre-2013, studies using sample sizes less than 10, and estimates derived from dynamic transmission modelling studies fitted to empirical HIV-1 prevalence curves, were excluded. While we included studies where study populations included individuals using ART, we aimed to include “real life studies” only, and so excluded studies where successful, suppressive ART of index partners was an inclusion criterion. Abstracts and other unpublished data older than five years were excluded because they were unlikely to result in peer-reviewed publication. There was no restriction by study year, region, or language of publication. AI per-act estimates included in previous systematic reviews4, 5, which we refer to as “original estimates”, were included if they fulfilled the current inclusion criteria.
Data extraction
Study review was conducted independently by two separate authors (RFB and BNO). Data were extracted on the following study and participant characteristics: region, study design, study dates, gender (MSM or heterosexual study population), sample size, statistical method of estimating per-act risk, information on current and history of sexually transmitted infections (STIs), proportion of the study partner population using therapeutic ART and stage of HIV-1 infection of infected partners, condom use, intravenous drug use and ART use (PrEP or PEP). Discrepancies were resolved by consensus.
Statistical methods
We performed random-effects inverse-variance meta-analysis11 on arcsin-transformed study estimates, which were back-transformed to the original scale to produce pooled estimates for per-act risk of HIV-1 transmission through URAI and UIAI. We presented available study estimates and pooled URAI and UIAI estimates in forest plots.
Meta-regression and subgroup analysis were used to explore potential sources of heterogeneity: gender; study design e.g. retrospective-partner study, prospective cohort of individuals; and ART use among partners. We assessed the robustness of pooled estimates and the influence of each individual study using leave-one-out sensitivity analysis (i.e., an influence analysis11). We also assessed the influence of relaxing our inclusion criteria to include Halperin et al (0.4%,95%CI 0.08–2.0%, excluded for being unpublished data pre-20136). Heterogeneity across study estimates was assessed using I2 statistics. Analysis was performed using R version 3.4.212 and the metafor package.
Results
Search results
Of 5336 unique studies published from February 2012 to February 2018 that we identified in our online searches, 4985 were excluded for non-relevance based on title, and 349 excluded based on abstract or full text. Two new articles directly reported per-act HIV-1 transmission probability estimates8, 9. No study had been excluded from our previous review based on ART use. Figure 1 illustrates the study selection procedure.
Figure 1.
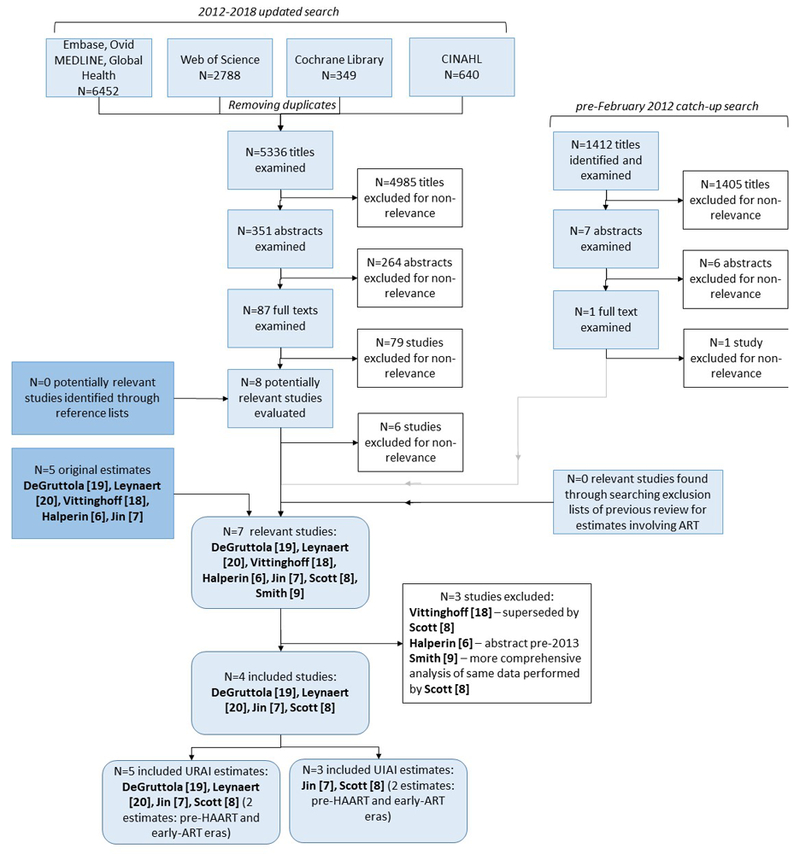
Flowchart summary of the literature search, comprising an update search from 2012 to February 2018 and a catch-up search to ensure the pre-2012 search included the same search terms as the updated search. “Original estimates” refers to studies included in either previous review4, 5. ART – antiretroviral therapy; CINAHL – Cumulative Index to Nursing and Allied Health Literature; UIAI – unprotected insertive anal intercourse; URAI – unprotected receptive anal intercourse.
Studies included in each systematic review
Table 1 summarises per-act URAI and UIAI transmission risk estimates and study characteristics for estimates included in Baggaley et al 20105, Patel et al4 and the current analysis. Detailed study characteristics are shown in Table S1, Supplementary Material. Data from 14,227 and 14,000 individuals/partnerships reported in the included studies were used to inform URAI and UIAI pooled estimates, respectively, compared to 1869 individuals/partnerships included in Baggaley et al5).
Table 1. Summary of per-act anal intercourse HIV-1 transmission probability studies included in meta-analyses reported by Baggaley et al 20105, Patel et al4, and the current analysis.
Reasons for study exclusion are provided, where applicable.
| Study | Population, sample size, setting |
Design. Study dates | Per-act estimate, % (95%CI) |
Included in: | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baggaley et al 2010 | Patel et al 2014 | Current analysis | |||||||
| URAI | |||||||||
| DeGruttola et al 198919 | 132 MSM (some infected, some uninfected) plus 155 sexual partners, US |
Retrospective-partner, study dates not stated |
0.5-3.0a | ✓ | ✓ | ✓ | |||
| Leynaert et al 199820 | 72heterosexual couples (male index) practising AI, Europe |
Retrospective-partner, 1987-1992 |
3.38 | (1.85-4.91) | ✓ | ✓ | ✓ | ||
| Vittinghoff et al 199918 | 1583 MSM, US | Prospective cohort of individuals, 1992-1994 |
0.82 | (0.24-2.76) | ✓ | ✓ |
× Supersededc |
||
| Halperin et al 2002 (abstract)6 plus S.C. Shiboski (personal communication, 2003) |
59 heterosexual couples (male index), US |
Retrospective-partner, participants recruited 1985-1986 |
0.4 | (0.08-2.0)b | ✓ |
× Estimate interpreted as a relative risk |
× Abstract pre-2013 |
||
| Jin et al 20107 | 1427 MSM, Australia | Prospective cohort of individuals, 2001-2007 |
0.91d | (0.41-2.07) |
× Data not yet published |
✓ |
✓ | ||
| Scott et al 20148 | MSM, US Pre-ART N=1813 c Early ART N=10,760f |
Four prospective cohorts of individuals: Jumpstart 1992-199515, EXPLORE 1999-200313, VAX 004 1998-200214, VPS 1995- 199916, 17 |
0.60e 0.73f |
(0.34-1.09) (0.45-0.98) |
× Data not yet published |
× Not includedg |
✓ | ||
| Smith et al 20159 | 3490 MSM, US |
Two prospective cohorts of individuals: EXPLORE 1999-200313, VAX 004 1998-200214 |
1.11h 0.41i |
(0.75-1.62) (0.30-0.55) |
× Data not yet published |
× Data not yet published |
× Study data reported by Scott et al 2014 8 |
||
| UIAI | |||||||||
| Vittinghoff et al 199918 | 1583 MSM, US | Prospective cohort of individuals, 1992-1994 |
0.06 | (0.02-0.19) |
× Estimate is per partner of HIV-1 positive or unknown serostatus |
✓ |
× Estimate is per partner of HIV-1 positive or unknown serostatus; supersededc |
||
| Jin et al 20107 | 1427 MSM, Australia | Prospective cohort of individuals, 2001-2007 |
0.16 | (0.05-0.31) |
× Data not yet published |
✓ | ✓ | ||
| Scott et al 20148 | MSM, US Pre-ART N=1813c Early ART N=10,760f |
Four prospective cohorts of individuals: Jumpstart 1992-199515, EXPLORE 1999-200313, VAX 004 1998-200214, VPS 1995- 199916, 17 |
0.14e 0.22f |
(0.04-0.29) (0.05-0.39) |
× Data not yet published |
× Not includedg |
✓ | ||
| Smith et al 20159 | 3490 MSM, US | Two prospective cohorts of individuals: EXPLORE 1999-200313, VAX 004 1998-200214 |
0.27h 0.20i |
(0.18-0.41) (0.15-0.27) |
× Data not yet published |
× Data not yet published |
× Study data reported by Scott et al 2014 8 |
||
NS – not stated.
Range rather than 95%CI reported by publication.
Range rather than 95%CI.
Estimate superseded by reanalysis of the dataset reported in Scott et al 20148.
Jin et al7 published per-act risk with ejaculation taking place inside the rectum (1.43%, 95%CI 0.48-2.85%) and with withdrawal prior to ejaculation (0.65%, 95%CI 0.15-1.53%). Per-act estimate regardless of when ejaculation occurred was reported in Patel et al4, obtained from study authors (James Jansson, personal communication).
Data taken from the pre-ART era (estimates use data from the Jumpstart study15).
Data taken from the early ART era (estimates use data from the EXPLORE13, VAX 00414, and VPS16, 17 studies).
Data mentioned in text but not included in meta-analysis
Data taken from the EXPLORE study13, restricted to study participants reporting never using condoms.
Data taken from the VAX 004 study14, restricted to study participants reporting never using condoms.
Of the two newly-identified studies8, 9, Scott et al8 was preferentially included. Smith et al9 used data from EXPLORE13 and VAX 00414 studies, while Scott et al8 additionally included Jumpstart15 and HIVNET Vaccine Preparedness Study (VPS)16, 17 data. Furthermore, Smith et al9 did not account for risk factors such as ethnicity and drug use, or for heterogeneity in per-act risk, as Scott did. Scott et al8 results also superseded and improved upon Vittinghoff et al18 estimates, which were conducted by the same research group and included the same Jumpstart study data. Vittinghoff et al18 data are therefore excluded. Halperin et al6, included by Baggaley et al5, was excluded for being a pre-2013 abstract. Further details of the advantages of Scott et al methodology, together with further information regarding excluded studies, are provided in Supplementary Material.
Study characteristics
Five URAI per-act study estimates reported by four studies7, 8, 19, 20 and three UIAI estimates reported by two studies7, 8 were included in the current analysis (Figure 1). Scott et al8 provided independent estimates for pre-highly active antiretroviral therapy (HAART, hereafter referred to as ART: study data from 1992–1995) and early ART (study data from 1995–2003) eras, for both URAI and UIAI, because they combined data from four cohorts13–17.
Data collection occurred between 1987 and 2007, although the earliest included publication did not state study dates19. URAI study estimates used data from Australia (N=17), the US (N=38, 19) and one multi-European country study20 (Table 1). UIAI study estimates used data from Australia (N=17) and the US (N=219). All but one included study estimate (Leynaert et al20, URAI) used data from MSM populations (Figure 2). Two URAI study estimates were from retrospective-partner studies19, 20; the remaining three used data from prospective cohorts of individuals7, 8.
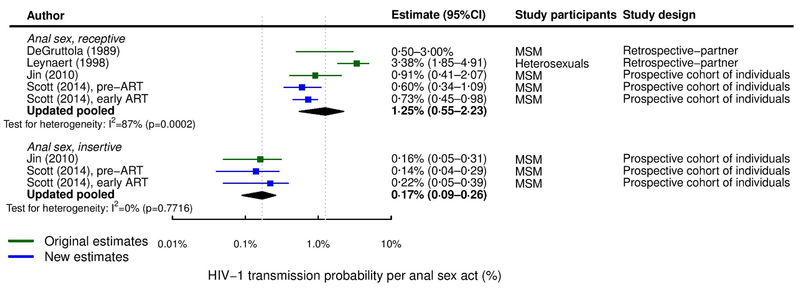
Figure 2.
Forest plot of studies estimating per-act HIV-1 transmission probability through anal intercourse. “Original estimates” refers to studies included in either previous review4, 5.
Three URAI study estimates used face-to-face interview (FTFI) data (8, 20 and pre-ART19), a third used FTFI combined with telephone interviewing7, and Scott et al’s8 early ART study estimate combined data gathered using FTFI (VAX00414 and VPS16, 17) and audio computer-assisted self-interview (ACASI) (Explore13). For UIAI, all three study estimates were from prospective studies and data were collected using FTFI (pre-ART19), FTFI plus telephone interview7 and FTFI plus ACASI combined (early ART8).
No studies reported on ART use of index partners. These data were not available from cohorts of individuals because they cannot be collected using this design7, 8. Authors discussed plausible ART coverage among infected partners but did not attempt to adjust estimates to account for ART use. Jin et al cited national data that 70% of Australian MSM used ART, and 75% of those had undetectable viral load7. For their early ART era estimates, Scott et al cited national data that only around 80% of those infected were aware of their status, and only 30% were virally suppressed, and that these levels were probably even lower during study periods. ART use was also not collected by retrospective-partner studies19, 20. Leynaert et al (retrospective-partner) reported that ART use data were not collected, but the study was conducted 1987–1992 and so use was minimal20. Similarly, DeGruttola et al (retrospective-partner) was published in 198919. Therefore ART use was minimal, likely 0%, in 3 of 5 (19, 20 and pre-ART8) and 1 (pre-ART8) of 3 URAI and UIAI study estimates, respectively. The remaining two studies were classed as having >0% ART use7, 8. Although no included studies reported any information on PEP or PrEP use by study participants, its use is expected to be very low, given the dates of data collection (all before 2007).
Study size varied considerably. Retrospective-partner studies enrolled 15519 and 7220 couples, while cohorts followed between 14277 and 4581 (EXPLORE13, included as part of Scott et al8) individuals. Number of AI acts with a partner appeared to vary considerably between individuals in the same study, with infectiousness similarly heterogeneous: Jin et al noted that 12 seroconversions in their cohort occurred as a result of <10 unprotected AI acts, while six men did not seroconvert despite reporting a total of 502 URAI acts with ejaculation7. Similarly, DeGruttola reported that 12 men reported >100 URAI acts with HIV-1-infected partners without seroconverting, while five men seroconverted after ≤10 such exposures to their infected partner and <3 partners outside the main relationship19.
Meta-analysis results
The updated pooled estimate of per-act URAI HIV-1 risk of 1.25% (95%CI 0.55–2.23%,N=5, I2=87%)7, 8, 19, 20) was considerably and statistically significantly higher (p=0.0026) and more heterogeneous than the UIAI risk (0.17%, 95%CI 0.09–0.26%, I2=0%,N=37, 8). Pooled and study estimates are shown in Figure 2.
Subgroup analysis
Table 2 shows the results of the subgroup analysis. The pooled per-act URAI HIV-1 risk was significantly lower for MSM (0.75% 95%CI 0.56–0.98%,N=4) than the sole heterosexual population estimate (3.38% 95%CI 1.85–4.91%,N=1) (p<0.0001). However, relaxing inclusion criteria to include Halperin et al6 (0.4% 95%CI 0.08–2.0%), one of just two identified estimates from heterosexual populations, excluded for being an abstract pre-2013, reduced the pooled heterosexual URAI estimate to 1.57% (95%CI 0.00–5.87%,N=2,I2=91%) which was no longer significantly different from the MSM estimate (p=0.370, Figure S1). MSM per-act estimates for both URAI and UIAI showed relatively little heterogeneity (I2<0.1%).
Table 2.
Subgroup analysis: meta-analytic pooled per-act HIV-1 transmission probability estimates for URAI and UIAI stratified by population subgroup (heterosexual and MSM), study design (retrospective-partner and prospective cohort of individuals) and plausible extent of ART use by sexual partners (0% versus >0%).
| Estimate type | Pooled estimate, % (95%CI) |
Pa | I2,b, (%) | N | References | p-valuea |
|---|---|---|---|---|---|---|
| URAI | ||||||
| Gender | ||||||
| Women | 3.38 (1.85-4.91) | 1.000 | 0.0% | 1 | 20 | |
| MSM | 0.75 (0.56-0.98) | 0.278 | <0.1% | 4 | 7, 8, 19c | p<0.0001 |
| Study design | ||||||
| Retrospective-partner | 2.56 (1.20-4.42) | 0.1296 | 56.5% | 2 | 19, 20 | |
| Prospective cohort of individuals | 0.71 (0.51-0.93) | 0.722 | 0.0% | 3 | 7, 8c | p<0.0001 |
| Plausible extent of ART use by sexual partners | ||||||
| 0% | 1.67 (0.44-3.67) | <0.0001 | 87.6% | 3 | 8, 19, 20d | |
| >0% | 0.75 (0.52–1.03) | 0.650 | 0.0% | 2 | 7, 8d | p=0.537 |
| Pooled estimate | 1.25 (0.55-2.23) | 0.0002 | 87.3% | 5 | 7, 8, 19, 20c | |
| UIAIe | ||||||
| Plausible extent of ART use by sexual partners | ||||||
| 0% | 0.14 (0.04-0.29) | 1.000 | 0.0% | 1 | 8 | |
| >0% | 0.18 (0.09-0.31) | 0.604 | 0.0% | 2 | 7, 8c | P=0.955 |
| Pooled estimate | 0.17 (0.09-0.26) | 0.7716 | 0.0% | 3 | 7, 8c | |
ART – antiretroviral treatment; N – number of study estimates; NA – not applicable; P – P-value; Q – heterogeneity statistic; UIAI – unprotected insertive anal intercourse; URAI – unprotected receptive anal intercourse.
“P” is the p-value for heterogeneity of the pooled estimate; “p-value” is the metaregression p-value defining the significance of the difference in pooled estimates between the two subgroups.
I2 is calculated as described in Higgins et al33. I2 lies between 0 and 100%; 0% indicates no observed heterogeneity and larger values show increasing heterogeneity.
Two URAI and UIAI estimates were provided by Scott et al8, using data from studies conducted in the pre-ART and early ART eras.
Scott et al’s8 pre-ART estimates are classed as likely 0% ART use; its early ART estimates are classed as >0% use.
All UIAI study estimates used data from prospective cohorts of individuals from MSM populations and so subgroup analysis could not be conducted gender or design.
Pooled per-act URAI risk from studies where ART was likely to have been used by >0% of sexual partners was lower than half (0.75%,95%CI 0.52–1.03%N=2) that without ART use (1.67%,95%CI 0.44–3.67%,N=3) but this difference was not significant (p=0.537). Per-act UIAI risks were similar by ART use (0.14%,95%CI 0.04–0.29% for 0% use vs. 0.18%,95%CI 0.09–0.31% for >0% use, p=0.955). When assessed in multivariate meta-regression analysis, only study design was (borderline) significantly associated with magnitude of URAI transmission risk (p=0.055), accounting for >99% of the heterogeneity across study estimates (R2=99.9%). Meta-regression analysis could not be undertaken for UIAI given the small number of estimates (N=3, all from MSM populations).
Sensitivity analysis
In the leave-one-out sensitivity analysis, only the omission of the heterosexual URAI estimate from Leynaert et al20 among heterosexual couples substantially reduced heterogeneity (I2 reduced from 87% to 0%), producing an all-MSM pooled URAI estimate (0.75%, 95%CI 0.56–0.98%) (Figure S1). Adding the Halperin et al6 study estimate did not substantially influence the URAI pooled estimate (1.10%,95%CI 0.50–1.94%,I2=85%, Figure S1). The pooled UIAI estimate was also not affected by any individual study estimate because study estimates were remarkably homogeneous (Figure 2, I2=0).
Discussion
Our updated review incorporates recently-published study estimates which strengthen the analysis and robustness of pooled per-act risk estimates by greatly increasing the number of included individuals (data from 14,227 individuals/partnerships, compared to 1869 individuals/partnerships in Baggaley et al5). Our results highlight that risk of HIV-1 transmission through AI remains high (1.25%,95%CI 0.55–2.23%,N=5 for URAI; 0.17%,95%CI 0.09–0.26%,N=3 for UIAI), and raises the question of whether HIV risk during URAI is higher for women than MSM, also highlighting the lack of data from resource-limited settings.
Our new pooled estimate is slightly lower than the previous pooled URAI estimates by Baggaley et al5 and Patel et al4, and a slight, nonsignificant increase on the previous pooled UIAI estimate reported by Patel et al4. We have explored sources of heterogeneity as far as possible, given the few included study estimates. In fact, URAI and UIAI estimates from MSM study populations were remarkably homogeneous (I2=0%). It is unclear whether gender or study design accounted for the heterogeneity across all URAI study estimates, but even after omitting the highest URAI estimate (i.e., the sole heterosexual estimate20, see Figure S1), the estimate of HIV-1 transmission risk through URAI remained high (0.75%,95%CI 0.56–0.98%). Even considering only study estimates which were conducted since the introduction of ART, risk remained nearly 10-fold riskier than unprotected receptive vaginal intercourse (VI): URAI 0.75%,95%CI 0.52–1.03% vs. unprotected receptive VI: 0.08%,95%CI 0.06–0.11%21. UIAI risk in the ART era is more than four-fold riskier than insertive VI (0.18%,95%CI 0.09–0.31% vs. 0.04%,95%CI 0.01–0.14%21).
It is unclear why the Leynaert et al URAI risk among females was so high (3.38%, 95%CI 1.85–4.91%20). All studies were conducted in industrialised countries, so difference by region is unlikely. Heterosexual study participants reported monogamy and no STIs. However, a large proportion of index cases (65% of the entire sample) were infected by intravenous drug use, so while their sexual partners reported no such use, it is possible that they underreported HIV-1 exposure and acquired HIV-1 via this route. Leynaert et al was a retrospective-partner study, and in multivariate meta-regression, study design explained a larger fraction of the variation across URAI estimates than gender, so the apparent difference by gender may be confounded by study design. HIV risk during URAI is especially uncertain because the only other identified URAI estimate among females, which was excluded for being a pre-2013 abstract, provided a markedly lower estimate than Leynaert et al (0.4% 95%CI 0.08–2.0%): it is in fact lower than all the five included URAI study estimates. This clouds the picture of potential differential risk by gender. The sample sizes of both Leynaert and Halperin were low (n<80), and given heterogeneity in infectiousness between individuals and by stage of HIV-1 infection25, the widely different estimates may be due to chance (95%CIs are wide and overlapping: 1.85–4.91%20 and 0.08–2.0%6). The lack of study design detail for the Halperin abstract makes it difficult to postulate reasons for the low estimate. However, our main results, based on the a priori exclusion of Halperin et al, mean we cannot exclude the possibility that women have an intrinsically higher URAI HIV-1 acquisition risk than men. This warrants further research, given its implication for HIV-1 prevention. There may exist underlying biological differences between the rectal compartments of males and females, rendering women more susceptible to infection. For example, there may be sex hormone differences, which alter rectal mucosal immunology and enhance susceptibility26. However, there has been little research conducted in this area to date, and recent evidence from animal studies suggested an opposite effect (Diane Bolton, person communication). Alternatively, variation in sexual practices by gender may play a role. MSM may be more likely to anticipate receptive AI and therefore prepare to reduce the likelihood of trauma (such as use of lubricants, cleansing the colon). Qualitative research has suggested that heterosexual AI often occurs without the explicit prior consent of women27, 28.
Our meta-regression found the pooled URAI risk among studies conducted in the ART era, when there was likely to be >0% ART use among sexual partners of study participants, was less than half that from pre-ART studies, but this difference failed to reach statistical significance, probably partly because of the small number of estimates and also the variability across estimates in the pre-ART era (from 0.60%8 to 3.38%20). For both URAI and UIAI, Scott et al pre-ART and early ART era per-act study estimates were very similar. Scott et al explained this lack of a significant association by suggesting that a relatively low proportion of infected MSM were on ART and had a suppressed viral load during the years in which data were collected. However, Jin et al7 URAI estimates were also high, and similar to Baggaley et al5 2010’s pooled estimate (without ART use), despite the likely high ART use in the Australian study population. 22, 23In fact, omitting the high heterosexual URAI estimate from Leynaert et al20 makes pre-ART and ART era URAI estimates more comparable: 1.00% (95%CI 0.22–2.33%) and 0.75% (95%CI 0.52–1.03%), respectively.
However, as Jin and Scott et al followed individuals rather than couples over time, information on infection status, current ART use and viral load of each sexual partner of each study participant was missing: data that are required to control for ART use adequately. 22, 23,29While evidence shows that HIV-infected individuals with ART-mediated viral suppression do not transmit HIV-122-24, our findings demonstrate that HIV-1 infectiousness through AI remains high, indicating that many HIV-infected individuals practising condomless AI are not on effective ART and remain infectious.
With ART coverage having continued to increase, now taken at earlier stages of HIV-1 infection and more tolerable regimens increasing levels of adherence, and with the advent of PrEP, it is expected that any future AI HIV-1 infectiousness studies would find further, significant reductions in infectiousness estimates. However, 22, 23,29HIV-infected MSM engaging in nondisclosing (not disclosing their HIV status to their partner), condomless AI have been found to be less ART-adherent and more likely to have unsuppressed HIV31 and so it is important to collect further data to monitor whether these population-level AI HIV-1 infectiousness estimates continue to decline over time.
There are some limitations to our findings, mainly due to scarcity of data. The few study estimates prevent us exploring the sources of heterogeneity in greater depth. Only one heterosexual study estimate was included, so it is difficult to know if differences in infectiousness by gender are real or confounded by study design. Included estimates were from only two study types: retrospective-partner and prospective studies of individuals. Both have advantages and disadvantages. For example, prospective studies are less likely to experience recall bias and therefore estimating numbers of sex acts may be more precise than retrospective studies. Recruiting individuals is easier than recruiting couples, providing larger sample sizes. Partner studies provide more reliable data on index cases, particularly regarding HIV-1 status, and in theory on their patterns of ART use. Studies of individuals rely on participants’ perceptions of the status of their sexual partners. However, couples may be more likely to underreport sexual partners outside the main relationship because of social desirability bias. Leynaert et al only reported from monogamous couples20, but all other study estimates included participants reporting multiple partners and multiple sexual behaviours. It can be challenging to estimate transmission risks using such data, especially where the HIV-1 infection and ART use status of sexual partners cannot be known with certainty: there are a lot of unknowns which must be accounted for. Different studies have used different statistical techniques to attempt this. All but one study used FTFI to gather sexual behaviour data, which may lead to social desirability bias32. These limitations may over- or underestimate per-act risk, and together with the small number of studies identified, and the variation in methods of data analysis, mean we recommend further data gathering using more confidential techniques such as ACASI, and analysis using standardised statistical methods, to increase comparability of studies and robustness of pooled estimates. Publication bias and selective reporting are likely to be low, because these studies are not assessing significance or effectiveness outcomes. This bias could be investigated using funnel plots if more study estimates became available.
In conclusion, current evidence suggests that practising unprotected AI continues to confer a high risk of HIV-1 transmission, particularly URAI, even in the ART era. More research is needed as important knowledge gaps regarding HIV-1 risk during AI remain. Given the high HIV-1 transmission risk associated with AI, it is remarkable that more research has not been conducted to evaluate if AI transmissibility differs by gender, high- and low-income countries and following ART scale-up at the population level. Standardised methods should be used to aid comparability between studies, and longitudinal studies reporting HIV transmission rates should be encouraged to use these methods to additionally report per-act estimates. Even today it continues to be important to design safe sex messaging that promotes the use of condoms in addition to interventions such as PrEP and other biotechnologies to prevent HIV-1 transmission through AI for both MSM and heterosexual populations.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health [Grant Number R01AI057020]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the US Army, or the US Department of Defense. We thank the HPTN Modelling Centre, which is funded by the U.S. National Institutes of Health (NIH UM1 AI068617) through HPTN, for partial funding of this work.
Footnotes
Conflict of interest statement
We do not have any commercial or other association that might pose a conflict of interest.
References
- 1.Owen BN, et al. , Prevalence and Frequency of Heterosexual Anal Intercourse Among Young People: A Systematic Review and Meta-analysis. AIDS Behav, 2015. 19(7): p. 1338–60. [DOI] [PubMed] [Google Scholar]
- 2.Owen BN, et al. , How common and frequent is heterosexual anal intercourse among South Africans? A systematic review and meta-analysis. J Int AIDS Soc, 2017. 19(1): p. 21162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maheu-Giroux M, et al. , Anal Intercourse Among Female Sex Workers in Cote d’Ivoire: Prevalence, Determinants, and Model-Based Estimates of the Population-Level Impact on HIV Transmission. Am J Epidemiol, 2018. 187(2): p. 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel P, et al. , Estimating per-act HIV transmission risk: a systematic review. AIDS, 2014. 28(10): p. 1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baggaley RF, White RG, and Boily MC, HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol, 2010. 39(4): p. 1048–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halperin DT, et al. , High level of HIV-1 infection from anal intercourse: a neglected risk factor in heterosexual AIDS prevention. Abstract ThPeC7438. International Conference on AIDS 2002, July 7–12. 2002. [Google Scholar]
- 7.Jin F, et al. , Per-contact probability of HIV transmission in homosexual men in Sydney in the era of HAART. AIDS, 2010. 24(6): p. 907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott HM, et al. , Age, race/ethnicity, and behavioral risk factors associated with per contact risk of HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr, 2014. 65(1): p. 115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith DK, et al. , Condom effectiveness for HIV prevention by consistency of use among men who have sex with men in the United States. J Acquir Immune Defic Syndr, 2015. 68(3): p. 337–44. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, et al. , Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg, 2010. 8(5): p. 336–41. [DOI] [PubMed] [Google Scholar]
- 11.Deeks JJ, Altman DG, and Bradburn MJ, Chapter 15: Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis In: Systematic review in health care: Meta-analysis in context. BMJ Books, London, U.K: 1995. [Google Scholar]
- 12.R Core Team (2012). R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria: ISBN 3–900051-07–0, URL http://http://www.R-project.org/. [Google Scholar]
- 13.Koblin B, et al. , Effects of a behavioural intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomised controlled study. Lancet, 2004. 364(9428): p. 41–50. [DOI] [PubMed] [Google Scholar]
- 14.Bartholow BN, et al. , Demographic and behavioral contextual risk groups among men who have sex with men participating in a phase 3 HIV vaccine efficacy trial: implications for HIV prevention and behavioral/biomedical intervention trials. J Acquir Immune Defic Syndr, 2006. 43(5): p. 594–602. [DOI] [PubMed] [Google Scholar]
- 15.Buchbinder SP, et al. , Feasibility of human immunodeficiency virus vaccine trials in homosexual men in the United States: risk behavior, seroincidence, and willingness to participate. J Infect Dis, 1996. 174(5): p. 954–61. [DOI] [PubMed] [Google Scholar]
- 16.Flynn NM, et al. , Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis, 2005. 191(5): p. 654–65. [DOI] [PubMed] [Google Scholar]
- 17.Seage GR 3rd, et al. , Are US populations appropriate for trials of human immunodeficiency virus vaccine? The HIVNET Vaccine Preparedness Study. Am J Epidemiol, 2001. 153(7): p. 619–27. [DOI] [PubMed] [Google Scholar]
- 18.Vittinghoff E, et al. , Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol, 1999. 150(3): p. 306–11. [DOI] [PubMed] [Google Scholar]
- 19.DeGruttola V, et al. , Infectiousness of HIV between male homosexual partners. J Clin Epidemiol, 1989. 42(9): p. 849–56. [DOI] [PubMed] [Google Scholar]
- 20.Leynaert B, Downs AM, and de Vincenzi I, Heterosexual transmission of human immunodeficiency virus: variability of infectivity throughout the course of infection. European Study Group on Heterosexual Transmission of HIV. Am J Epidemiol, 1998. 148(1): p. 88–96. [DOI] [PubMed] [Google Scholar]
- 21.Boily MC, et al. , Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis, 2009. 9(2): p. 118–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen MS, et al. , Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med, 2016. 375(9): p. 830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baggaley RF, et al. , Heterosexual HIV-1 infectiousness and antiretroviral use: systematic review of prospective studies of discordant couples. Epidemiology, 2013. 24(1): p. 110–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bavinton BR, et al. , Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet HIV, 2018. [DOI] [PubMed] [Google Scholar]
- 25.Boily MC, Baggaley RF, and Masse B, The role of heterosexual anal intercourse for HIV transmission in developing countries: are we ready to draw conclusions? Sex Transm Infect, 2009. 85(6): p. 408–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGowan I, et al. , A phase 1 randomized, double blind, placebo controlled rectal safety and acceptability study of tenofovir 1% gel (MTN-007). PLoS One, 2013. 8(4): p. e60147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marston C and Lewis R, Anal heterosex among young people and implications for health promotion: a qualitative study in the UK. BMJ Open, 2014. 4(8): p. e004996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds GL, Fisher DG, and Rogala B, Why women engage in anal intercourse: results from a qualitative study. Arch Sex Behav, 2015. 44(4): p. 983–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodger AJ, et al. , Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. JAMA, 2016. 316(2): p. 171–81. [DOI] [PubMed] [Google Scholar]
- 30.Powers KA, et al. , Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect Dis, 2008. 8(9): p. 553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalichman SC, et al. , HIV Disclosure and Transmission Risks to Sex Partners Among HIV-Positive Men. AIDS Patient Care STDS, 2016. 30(5): p. 221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langhaug LF, Sherr L, and Cowan FM, How to improve the validity of sexual behaviour reporting: systematic review of questionnaire delivery modes in developing countries. Trop Med Int Health, 2010. 15(3): p. 362–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins JP, et al. , Measuring inconsistency in meta-analyses. BMJ, 2003. 327(7414): p. 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.