Abstract
Rationale
Blacks compared to Whites have a greater risk of adverse cardiovascular outcomes. Impaired regenerative capacity, measured as lower levels of circulating progenitor cells (CPCs), is a novel determinant of adverse outcomes, however, little is known about racial differences in CPCs.
Objectives
To investigate the number of CPCs, PC-mobilizing factors, PC mobilization during acute myocardial infarction (AMI) and the predictive value of CPC counts in Blacks compared with Whites.
Methods and Results
CPCs were enumerated by flow cytometry as CD45med+ blood mononuclear cells expressing CD34+,CD133+,VEGF2R+ and CXCR4+ epitopes in 1747 subjects, mean age 58.4±13, 55%male, 26%self-reported Black. Patients presenting with AMI (n=91) were analyzed separately. Models were adjusted for relevant clinical variables. Stromal cell-derived factor-1α(SDF-1α), vascular endothelial growth factor(VEGF), and matrix metallopeptidase-9(MMP-9) levels were measured (n=561) and 623 patients were followed for median of 2.2 years for survival analysis. Blacks were younger, more often female, with a higher burden of cardiovascular risk and lower CPC counts. Blacks had fewer CD34+ cells (−17.6%;95%CI[−23.5%,−11.3%];P<0.001), CD34+/CD133+ cells (−15.5%;95%CI[−22.4%,−8.1%];P<0.001), CD34+/CXCR4+ cells (−17.3%;95%CI[−23.9%,−10.2%];P<0.001) and CD34+/VEGF2R+ cells (−27.9%;95%CI[−46.9%,−2.0%];P=0.04) compared to Whites. The association between lower CPC counts and Black race was not affected by risk factors or cardiovascular disease. Results were validated in a separate cohort of 411 patients. Blacks with AMI had significantly fewer CPCs compared to Whites (P=0.02). Blacks had significantly lower plasma MMP-9 levels (P<0.001) which attenuated the association between low CD34+ and Black race by 19% (95%CI13-33%). However, VEGF and SDF-1α levels were not significantly different between the races. Lower CD34+ counts were similarly predictive of mortality in Blacks (HR=2.83[95%CI 1.12-7.20];P=0.03) and Whites (HR=1.79[95%CI 1.09-2.94];P=0.02) without significant interaction.
Conclusion
Black subjects have lower levels of CPCs compared to Whites which is partially dependent on lower circulating MMP-9 levels. Impaired regenerative capacity is predictive of adverse outcomes in Blacks and may partly account for their increased risk of cardiovascular events.
Keywords: Progenitor cells, Black, biomarker, regenerative capacity, CD34+, CD133+, VEGFR2+, CXCR4+, VEGF, SDF-1, MMP-9
INTRODUCTION
Across the U.S., Blacks compared to Whites suffer from a greater burden of cardiovascular disease (CVD) including incident myocardial infarction, heart failure, stroke, and other adverse cardiovascular events1, 2. This can only partly be explained by a higher prevalence of traditional risk factors such as obesity, hypertension, diabetes mellitus, or tobacco use and it has been suggested that socioeconomic factors account for the remaining disparity.
There is mounting experimental and clinical evidence to indicate that tissue injury is accompanied by a simultaneous reparative response, mediated by local and circulating stem and progenitor cells, that leads to tissue regeneration. Although these processes have been long established for conditions such as blood loss, in the last two decades it has become clear that even vascular and myocardial injury is followed by regeneration that is mediated by circulating and resident vascular progenitors. When the regenerative potential is insufficient or deficient, then vascular injury progresses. We and others have shown that circulating progenitor cells (CPCs) can be accurately phenotyped in humans. CPCs have been characterized as CD34-expressing mononuclear cells that possess the potential to differentiate into hematopoietic, endothelial, and non-hematopoietic (mesenchymal, lacking CD45 expression) lineages and contribute to vascular and myocardial regeneration3–7. CD133 is a 5-transmembrane antigen of primitive stem cells which is lost during maturation and dual expression of these markers (CD34+/CD133+) can detect a PC-enriched subpopulation8, 9. Co-expression of vascular endothelial growth factor receptor-2 (VEGFR2) appears to identify a scarcer subpopulation of CPCs further enriched for endothelial progenitors10, 11. Co-expression of Chemokine (C-X-C Motif) Receptor 4 (CXCR4) permits homing of CPCs to stromal-derived factor-rich hypoxic milieu and further characterizes the sub-population of CD34+ CPCs with capacity for homing and tissue repair12.
The bone marrow is a major reservoir for progenitor cells (PCs) where they are maintained in quiescent state. We and others have shown that CPC numbers and activity are modulated by age, sex, presence of CVD risk factors and subclinical and clinical CVD13–19. The hemostasis of the PCs in bone marrow niches is regulated by signaling pathways that include cytokines, chemokines and inflammatory factors. Under specific conditions such as acute myocardial infarction (AMI), PC-mobilizing factors such as stromal cell-derived factor-1 α (SDF-1α), vascular endothelial growth factor (VEGF), and matrix metallopeptidase-9 (MMP-9) permit PC release from their bone marrow niches, and their subsequent proliferation, differentiation, and mobilization into the circulation15, 20, 21. Furthermore, chemokines such as monocyte chemoattractant proteins (MCPs) regulate trafficking of leukocytes to sites of inflammation and injury. It remains unknown if there are racial differences in PC mobilization or PC-signaling pathways.
Finally, we and others have demonstrated that impaired regenerative capacity, estimated as reduced levels of CPCs independently predicts outcomes in patients with coronary artery disease (CAD), acute coronary syndromes, peripheral artery disease, heart failure, stroke and other conditions15–18. Whether differences in endogenous regenerative capacity contributes to the racial disparities of CVD remains unknown and is the focus of this investigation. Herein, we hypothesized that compared to Whites, Blacks will have (1) significantly lower level of CPCs; (2) diminished mobilization of PCs in the setting of AMI; (3) differences in circulating levels of PC-mobilizing factors; and (4) similar predictive value of low CPC levels for adverse outcomes.
METHODS
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study design and subjects
We identified adults from a database that included patients recruited into the Emory Cardiovascular Biobank, a prospective registry of patients undergoing cardiac catheterization, from the Mental Stress Ischemia Prognosis Study, a prospective study that recruited patients with stable coronary artery disease (CAD), and Emory Predictive Health Initiative, a study that recruited employees of Emory University and Georgia Institute of Technology without overt CVD, as previously described22–24. We excluded subjects who had cardiac transplantation, cancer or were on immunosuppressive agents. Race was self-reported, and other non-White racial groups were excluded for the purpose of this analysis. Demographic characteristics, medical history, medication use, and behavioral habits were documented as previously described22, 25. At the time of enrollment, CPCs were measured from peripheral blood samples.
Subjects presenting with acute myocardial infarction (AMI) were analyzed separately. AMI was diagnosed according to the ACC/AHA guidelines by the presence of self-reported chest pain and evidence of cardiac ischemia on 12-lead EKG or elevated cardiac enzymes greater than twice the upper limit of normal with angiographic findings consistent with AMI diagnosis. The study was approved at Emory University (Atlanta, GA) by the Institutional Review Board. All participants provided written informed consent.
Validation cohort
To validate the association between CPCs and Black race, we performed a separate analysis in an independent cohort of 411 subjects (16% Black) enrolled between 2006 and 2008 at Emory University affiliated hospitals. Both cohorts were enrolled under the same protocol, with identical sampling strategies and collection methods but separated in time and by a modified cell quantification methodology.
Progenitor cells assays
Peripheral blood was collected in EDTA tubes and incubated with fluorochrome-labeled monoclonal antihuman mouse antibodies within 4 hours. Cell populations enriched for circulating progenitor cells were enumerated using flow cytometry as CD45dim cells co-expressing CD34+, CD133+, VEGFR2+, or CXCR4+. CD34+/CD45med+ represent 95% of the total CD34+; CD34+/CD133+/CD45med+ represent 98% of CD34+/CD133+; CD34+/CXCR4+/CD45med+ represent 94% of CD34+/CXCR4+; and CD34+/VEGF+/CD45med represent 64% of CD34+/VEGF. This selection of CD45med helps eliminate a subset of other progenitors like mesenchymal or osteoprogenitor cells as these are typically CD45neg26. Moreover, eliminating the rare CD45bright CD34+ cells helps eliminate hematogones (early B-cell precursors). We incubated 300 μL of peripheral blood with 7 μL of FITC-CD34 (BD Biosciences), PerCP-CD45 (BD Biosciences), PE-VEGFR2 (R&D system) and 5 μL APC-CD133 (Miltenyi), and 3ul PE-Cy7-conjugated anti-CXCR4 (EBioscience, clone 12G5) in the dark for 15 minutes27. Then 1.5 mL ammonium chloride lysing buffer was added to lyse red blood cells. 1.5 mL staining medium (PBS with 3% heat-inactivated serum and 0.1% sodium azide) was added to stop the lysing reaction. Prior to flow cytometry, 100 μL of AccuCheck Counting Beads (Invitrogen, Cat#: PCB100) were added to act as an internal standard for direct estimation of the concentration of target cell subsets. At least 2.5 million events were acquired from the cytometer. Flow data were analyzed with Flowjo software (Treestar, Inc.). Absolute mononuclear cell count was estimated as the sum of lymphocytes and monocytes using a Coulter ACT/Diff cell counter (Beckman Coulter). Progenitor cells populations are reported as cell counts/mL. In 20 samples that were repeatedly analyzed on two occasions by the same technician, the coefficients of variation of the cell types were: CD34+ 2.9%; CD34+/CD133+ 4.8%; CD34+/CXCR4+ 6.5% and CD34+/CD133+/CXCR4+ 7.5%, CD34+/VEGFR2+ cells 21.6%. There were significant correlations between the progenitor cells subtypes, with moderate-strong correlations between CD34+, CD34+/CD133+, CD34+/CXCR4+ (r range 0.82-0.91, P<0.001), and weak correlations (r range 0.08-0.29, P<0.001) between CD34+/VEGFR2+ subtypes and the aforementioned progenitor cells. In the validation cohort, similar methods were used to enumerate CPCs (CD34+ and CD34+/CD133+) but without the addition AccuCheck Counting Beads or PE-Cy7-conjugated anti-CXCR4.
Biomarker measurements
Biomarkers were measured in 561 subjects enrolled in the Mental Stress Ischemia study including high-sensitivity C-reactive protein (hs-CRP), stromal cell-derived factor 1 (SDF-1α), vascular endothelial growth factor (VEGF), matrix metallopeptidase 9 (MMP-9), and monocyte chemoattractant protein-1 (MCP-1). We utilized high sensitivity assays using the MesoScale system (Meso Scale Diagnostics Rockville, Maryland) using the SECTOR Imager 2400 to quantitate hs-CRP, SDF-1α, VEGF, MMP-9 and MCP-1 according to the protocols supplied by the manufacturer. The Mesoscale multiplex assay system uses electrochemiluminescence for high sensitivity and broad dynamic range. Lower limits of detection for our experiment were: hsCRP: 1.33 × 10−6 mg/L; SDF‐1α, 27.8 pg/mL; VEGF, 1.12 pg/mL; MMP-9: 0.011 ng/mL; and MCP-1: 0.09 pg/mL. The inter assay coefficient of variations for midpoint standards were 3.79% for SDF‐1α, 4.26% for VEGF, 9.38% for MMP-9, and 4.99% for MCP-1. The intra-assay coefficients of variations were 2.33% for hsCRP; 3.45% for MCP-1 and 5.95% for MMP-9.
Follow-up and outcomes
Patients enrolled in the Emory Cardiovascular Biobank were followed for determination of the primary outcome of all-cause death obtained by phone contact, electronic medical record review, and data from the social security death index and state records. Adjudication was conducted by personnel blinded to the data. Baseline characteristics for follow-up cohort is shown in supplemental Table I.
Statistical analysis
Subject characteristics were reported as descriptive statistics with means, medians, standard deviations and ranges. Differences between groups were assessed using the t-test for continuous variables, and chi-square or Fischer exact tests for categorical variables where appropriate. Spearman rank correlation coefficients were used for examining associations between PCs and continuous variables. Two-sided P-value < 0.05 were considered statistically significant. For non-normally distributed variables such as CPC counts and biomarkers, Mann-Whitney U test was used to compare groups in unadjusted analyses. For multivariable analyses, PCs counts were examined as continuous variables after log-transformation to achieve normality. Characteristics incorporated in multivariable analyses included age, sex, race (White vs. Black), body mass index, hypertension, hyperlipidemia, diabetes, estimated glomerular filtration rate (GFR), history of CAD, history of heart failure. Analyses were repeated in the validation cohort after adjusting for the same variables. The association between CPC counts and outcomes was examined in Kaplan-Meier and Cox regression models. We analyzed CPC counts as a continuous variable after log-transformation and as binary variables using a pre-specified cutoffs that were previously shown to be predictive of worse prognosis (737 cells/mL for CD34+ subset)26. Analyses were performed using IBM SPSS Statistics Version 23, (Armonk, NY, USA).
RESULTS
Of the 1,747 subjects enrolled aged 59±13 years, 457 (26%) were Black. Compared to Whites, Black subjects were younger, less likely to be male, with a higher body mass index and were more likely to smoke, have hypertension, diabetes, and heart failure, Table 1A.
Table 1.
| A Baseline characteristics of White and Black subjects | |||
|---|---|---|---|
|
| |||
| White (n=1290) | Black (n=457) | P-value | |
| Age, years | 59.7 (13.8) | 54.6 (12) | <0.001 |
| Male | 758 (58.76) | 197 (43.11) | <0.001 |
| Body Mass Index kg/m2 | 28.4 (5.8) | 31 (7) | <0.001 |
| Smoking | 515 (40.02) | 211 (46.27) | 0.02 |
| Diabetes | 293 (22.71) | 173 (37.86) | <0.001 |
| Hypertension | 787 (61.01) | 351 (76.81) | <0.001 |
| Hypercholesterolemia | 689 (53.41) | 236 (51.64) | 0.51 |
| Estimated GFR mL/min/1.73 m2 | 86.5 (30.4) | 89.6 (43.5) | 0.05 |
| History of coronary artery disease | 638 (49.92) | 217 (48.76) | 0.67 |
| History of myocardial infarction | 191 (23.67) | 91 (30.03) | 0.03 |
| History of PCI | 381 (47.21) | 128 (42.24) | 0.14 |
| History of CABG | 234 (29) | 61 (20.13) | <0.001 |
| History of heart failure | 212 (16.43) | 113 (24.73) | <0.001 |
| Ejection fraction % | 54.3 (12.4) | 49.1 (15.9) | <0.001 |
|
| |||
| ACE/ARB use | 409 (50.68) | 166 (54.97) | 0.20 |
| Aspirin use | 644 (77.78) | 238 (76.53) | 0.65 |
| Clopidogrel use | 276 (33.33) | 88 (28.3) | 0.10 |
| Statin use | 677 (52.48) | 231 (50.66) | 0.50 |
| Beta blocker use | 563 (69.76) | 239 (79.14) | <0.001 |
|
| |||
| Mononuclear cells/μL | 470 (341-640) | 410 (300-620) | 0.15 |
| CD34+ cells/mL | 1874 (1208-2783) | 1644 (1006-2645) | 0.004 |
| CD34+/CD133+ cells/mL | 818 (494-1271) | 789 (459-1243) | 0.42 |
| CD34+/VEGF2R+ cells/mL | 34 (7-109) | 30 (6-112) | 0.33 |
| CD34+/CXCR4+ cells/mL | 827 (512-1316) | 722 (420-1267) | 0.01 |
|
| |||
| Biomarkers (n=561) | n=395 | n=166 | |
|
| |||
| SDF-1α pg/mL | 1244 (1002-1486) | 1304 (1045-1607) | 0.13 |
| VEGF pg/mL | 54 (39-77) | 53 (37-96) | 0.85 |
| MMP-9 ng/mL | 67 (46-102) | 50 (37-72) | <0.001 |
| MCP-1 ng/mL | 120 (101-142) | 136 (110-162) | <0.001 |
| HsCRP | 1.4 (0.6-2.9) | 2.2 (1-4.9) | <0.001 |
| B Baseline characteristics among White and Black subjects in the validation cohort. | |||
|---|---|---|---|
|
| |||
| White (n=344) | Black (n=67) | P-Value | |
| Age, years | 63.7 (10.9) | 59.7 (10.5) | 0.01 |
| Male | 225 (65.4) | 34 (50.7) | 0.02 |
| Body Mass Index kg/m2 | 30.1 (6.4) | 32.7 (7.2) | <0.001 |
| Estimated GFR mL/min/1.73 m2 | 71.6 (19.3) | 77.2 (24.3) | 0.03 |
| Smoking | 272 (79.1) | 43 (64.2) | 0.01 |
| Diabetes | 99 (28.8) | 23 (34.3) | 0.36 |
| Hypertension | 253 (73.6) | 55 (83.3) | 0.09 |
| Hypercholesterolemia | 265 (77.0) | 46 (68.7) | 0.14 |
| History of heart failure | 84 (24.4) | 19 (28.4) | 0.50 |
| Ejection fraction % | 54.8 (10.3) | 55.1 (11.9) | 0.30 |
| History of coronary artery disease (%) | 253 (73.5) | 39 (58.2) | 0.01 |
| History of myocardial infarction | 81 (23.5) | 11 (16.4) | 0.20 |
| History of PCI | 139 (40.4) | 29 (43.3) | 0.66 |
| History of CABG | 90 (26.2) | 8 (11.9) | 0.01 |
| ACE/ARB use | 209 (60.8) | 42 (62.7) | 0.77 |
| Aspirin use | 269 (78.2) | 45 (67.2) | 0.05 |
| Clopidogrel use | 172 (50.5) | 29 (43.3) | 0.31 |
| Statin use | 245 (71.2) | 40 (59.7) | 0.06 |
| Beta blocker use | 219 (63.7) | 40 (59.7) | 0.54 |
|
| |||
| CD34+ cell/mL | 1223 (737-1960) | 1025 (616-1597) | 0.02 |
| CD34+/CD133+ cell/mL | 572 (350-950) | 474 (260-732) | 0.05 |
Data presented as n (%) and mean ± SD.
Progenitor cells and biomarkers counts are presented as median (interquartile range).
GFR=Glomerular filtration rate; PCI=Percutaneous coronary intervention ; CABG=Coronary artery bypass surgery; ACE/ARB=Angiotensin converting enzyme inhibitors/Angiotensin receptor blockers
SDF-1 α = Stromal cell-derived factor-1 α; VEGF= Vascular endothelial growth factor; MMP-9= Matrix metallopeptidase-9; MCP-1= Monocyte chemoattractant protein-1; HsCRP=High-sensitivity C-reactive protein
Race and CPC counts.
GFR=Glomerular filtration rate
PCI=Percutaneous coronary intervention
CABG=Coronary artery bypass surgery
ACE/ARB=Angiotensin converting enzyme inhibitors/Angiotensin receptor blockers
Race and CPC counts
In unadjusted analyses, both CD34+ and CD34+/CXCR4+ cell counts were lower in Blacks compared to Whites. After multivariable adjustment for age, race, smoking history, BMI, hypertension, diabetes, dyslipidemia, GFR, heart failure, CAD, and mononuclear cell counts, Black race was an independent determinant of CPC counts and was associated with fewer CD34+ cells (−17.6%; 95%CI [−23.52%,−11.25%]; P<0.001), CD34+/CD133+ cells (−15.52%; 95%CI [−22.36%,−8.07%]; P<0.001), CD34+/CXCR4+ cells (−17.33%; 95%CI [−23.94%,−10.15%]; P<0.001) and CD34+/VEGF2R+ cells (−27.86%; 95%CI [−46.88%,−2.02%]; P=0.04) (Table 2).
Table 2.
Linear regression model for predictors of PC subsets
| CD34+ % difference (95%CI) | P-value | CD34+/CD133+ % difference (95%CI) | P-value | CD34+/CXCR4+ % difference (95%CI) | P-value | CD34+/VEGF2R+ % difference (95%CI) | P-value | |
|---|---|---|---|---|---|---|---|---|
| Male | 16.56% (9.15%,24.48%) | <0.001 | 13.34% (5.2%,22.11%) | 0.001 | 15.11% (6.91%,23.94%) | <0.001 | −36.5% (−52.33%,−15.41%) | 0.002 |
| Black | −16.95% (−22.92%,−10.53%) | <0.001 | −14.6% (−21.52%,−7.07%) | <0.001 | −16.77% (−23.46%,−9.49%) | <0.001 | −29.14% (−48.82%,−1.89%) | 0.04 |
| Smoking history | 5.49% (−1.59%,13.07%) | 0.13 | 8.37% (0.17%,17.25%) | 0.05 | 12.38% (3.94%,21.5%) | <0.001 | −31.83% (−49.65%,−7.69%) | 0.013 |
| Age (per 10 years higher) | −12.1% (−14.63%,−9.49%) | <0.001 | −14.44% (−17.23%,−11.56%) | <0.001 | −11.92% (−14.77%,−8.98%) | <0.001 | −7.95% (−18.98%,4.57%) | 0.20 |
| Body Mass Index (per 5 units higher) | 4.55% (1.78%,7.4%) | 0.001 | 7.02% (3.81%,10.34%) | <0.001 | 2.41% (−0.64%,5.55%) | 0.12 | 3.56% (−7.91%,16.45%) | 0.56 |
| CAD history | −3.78% (−10.72%,3.69%) | 0.31 | −1.02% (−9.07%,7.75%) | 0.81 | 1.19% (−6.98%,10.08%) | 0.78 | 54.75% (11.62%,114.54%) | 0.01 |
| Heart failure history | −16.53% (−23.22%,−9.27%) | <0.001 | −14.34% (−22.08%,−5.84%) | 0.001 | −18.47% (−25.77%,−10.44%) | <0.001 | 8.19% (−24.84%,55.75%) | 0.67 |
| Diabetes | 3.62% (−3.85%,11.68%) | 0.35 | 3.06% (−5.33%,12.2%) | 0.49 | 2.8% (−5.51%,11.83%) | 0.52 | −4.26% (−30.96%,32.77%) | 0.79 |
| Hypertension | 15.48% (6.91%,−24.74%) | <0.001 | 15.84% (6.14%,26.43%) | 0.001 | 21.17% (11.1%,32.15%) | <0.001 | 70.19% (21.54%,138.32%) | <0.001 |
| Hyperlipidemia | −3.58% (−10.63%,4.04%) | 0.35 | −3.24% (−11.23%,5.47%) | 0.45 | −1.88% (−9.92%,6.87%) | 0.66 | −47.07% (−62.01%,−26.25%) | <0.001 |
| Estimated Glomerular filtration rate (per 10 units higher) | 1.69% (0.54%,2.85%) | 0.004 | 1.16% (−0.14%,2.47%) | 0.08 | 0.84% (−0.44%,2.14%) | 0.20 | −3.82% (−8.48%,1.08%) | 0.12 |
| Mononuclear cells (log) | 5.31% (1.08%,9.71%) | 0.01 | 7.37% (2.5%,12.47%) | <0.001 | 3.96% (−0.72%,8.86%) | 0.099 | −17.43% (−30.94%,−1.26%) | 0.036 |
Validation Analysis
The racial differences in the CPC subsets between White and Black subjects was validated in an independent cohort of 411 subjects (mean age 63.0 ±11, 63% male, and 16% Black). Baseline characteristics are shown in Table 1B. The results validated a similar association between low CPC counts analyzed as continuous variables and Black race even after adjusting for aforementioned clinical variables. Thus, Black race was an independently associated with fewer CD34+ cells (−28.2%; 95%CI [−41.97%,−11.17%]; P=0.002) and CD34+/CD133+ cells (−29.84%; 95%CI [−43.64%,−12.65%]; P=0.002).
Racial differences in CPCs and interactions with demographic and risk factor characteristics
There was a decline in CPCs with age and men had higher CPC counts compared to women as previously reported25. Blacks had lower CPC levels at all ages (Figure 1) and Black women had lower CPC counts compared to Black men (15.26% fewer CD34+ cells (95%CI 0.2%,32.45%; P=0.04). Thus, CPC counts in Whites were similar to Blacks who were on average 14 years younger. There were no significant interactions between lower CPC counts in Blacks compared to Whites and either age, sex, or the individual risk factors including diabetes, hypertension, hyperlipidemia, chronic kidney disease, or presence of CVD (CAD, heart failure or peripheral arterial disease), (P for interaction >0.1 for all), Figure 2.
Figure 1.
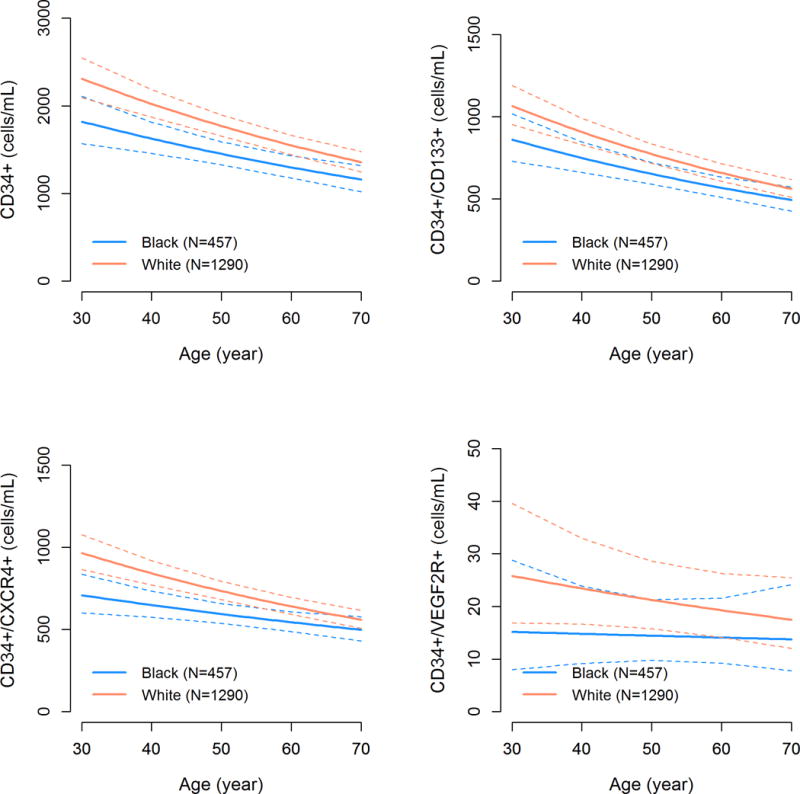
Age-related decline in CPC counts among Black and White subjects. P-values are derived from adjusted linear regression models.
Figure 2.
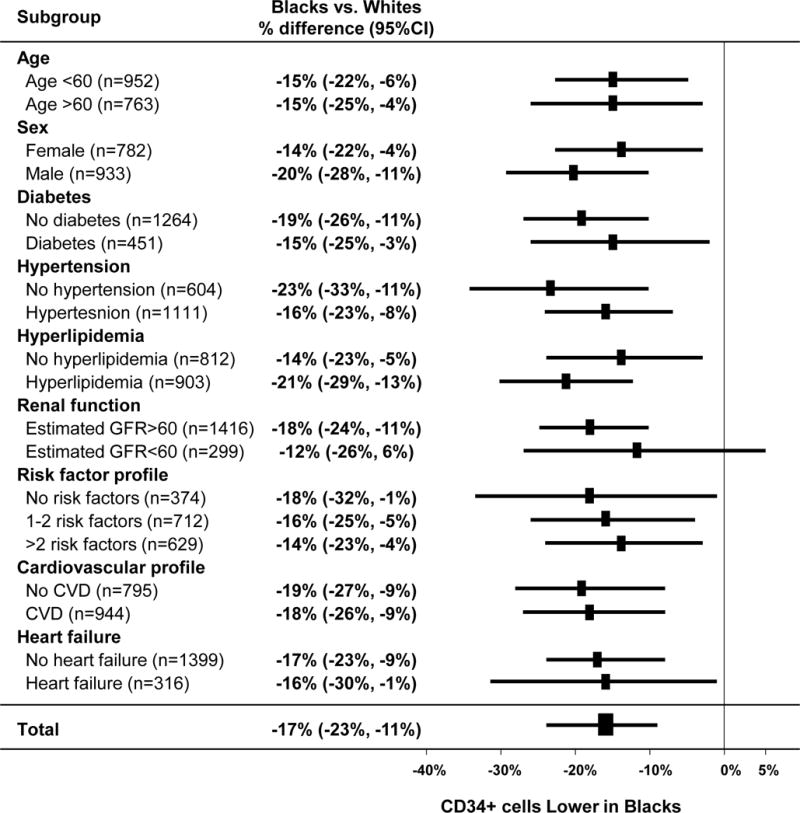
Forest plot of CPC counts in Blacks vs. Whites stratified by baseline characteristics (n=1715 subjects)
To ensure that the differences we observed in the entire population was not because of the overall increased risk factor burden in Blacks, we investigated racial differences in CPCs in a subset of patients (n=343) without any risk factors (hypertension, diabetes, hyperlipidemia, or smoking). Even in this risk factor-free subset, Black race remained significantly associated with fewer CPCs; CD34+ cells (−17.63% [95%CI −31.75%,−0.7%]; P=0.04), CD34+/CD133+ (−14.36% [95%CI −30.3%,5.23%]; P=0.13), CD34+/CXCR4+ cells (−20.15% [95%CI −35.21%,−1.59%]; P=0.03) but the lower numbers of CD34+/VEGF+ cells (−33.44% [95%CI −68.56%,41.06%]; P=0.28) did not reach statistical significance.
Racial differences in CPCs in patients with AMI
Baseline characteristics of 91 patients admitted with an AMI (30% Black, 45 with STEMI) are shown in Table 3. We measured CPCs at the time of the angiogram which is typically done within 24 after presentation with an acute myocardial infarction. CPC counts were higher in those with AMI compared to stable CAD patients even after adjustment for clinical risk factors, as previously shown.15 Black subjects had significantly lower CD34+ counts compared to Whites (median 1316 [IQR 938-2300] vs. 2231 [IQR 1409-3586] cell/mL, P=0.01), lower CD34+/CD133+ (median 749 [IQR 228-1431] vs. 998 [IQR 633-1795] cell/mL, P=0.05) and lower CD34+/CXCR4+ (621 [IQR 385-960] vs. 1011 [578-1630] cell/mL, P=0.01) but not CD34+/VEGF+ cells, (P>0.2). In multivariable analysis adjusted for age, sex, diabetes, hypertension, hyperlipidemia, renal function, heart failure history and troponin levels, Black race remained an independent predictor of lower CD34+ (−30.62%; 95%CI [−49.08%,−5.47%]; P=0.02), CD34+/CD133+ (−30.87%; 95%CI [−51.88%,−0.69%]; P=0.04) and CD34+/CXCR4+ (−34.08%; 95%CI [−53.24%,−7.07%]; P=0.02) cell counts, but not the CD34+/VEGF2R+ cell counts (P>0.1).
Table 3.
Baseline characteristics among White and Black subjects with AMI presentation
| White (n=64) | Black (n=27) | P-Value | |
|---|---|---|---|
| Age, years | 64.4 (12.8) | 61.8 (8.7) | 0.35 |
| Male | 47 (73.4) | 16 (59.3) | 0.18 |
| Body Mass Index kg/m2 | 29.2 (5.2) | 30.6 (5.9) | 0.39 |
| Estimated GFR mL/min/1.73 m2 | 77 (21) | 75 (27) | 0.92 |
| Smoking | 27 (54) | 11 (57.9) | 0.77 |
| Diabetes | 18 (28.1) | 10 (40) | 0.28 |
| Hypertension | 58 (90.6) | 22 (84.6) | 0.41 |
| Hypercholesterolemia | 53 (82.8) | 23 (88.5) | 0.50 |
| History of heart failure | 19 (29.7) | 12 (44.4) | 0.17 |
| Ejection fraction % | 46 (14.5) | 46 (16.1) | 0.87 |
| History of myocardial infarction | 36 (76.6) | 10 (66.7) | 0.44 |
| History of PCI | 35 (70) | 9 (47.4) | 0.08 |
| History of CABG | 17 (34) | 3 (15.8) | 0.14 |
| ST-elevation myocardial infarction at enrollment | 29 (45) | 16 (59) | 0.22 |
| Non-ST-elevation myocardial infarction at enrollment | 35 (56) | 11 (41) | 0.22 |
| Peak Troponin ng/mL | 18 (9-80) | 34 (9-89) | 0.46 |
|
| |||
| CD34+ cell/mL | 2231 (1409-3586) | 1316 (938-2300) | 0.01 |
| CD34+/CD133+ cell/mL | 998 (633-1795) | 749 (288-1431) | 0.05 |
| CD34+/VEGF2R+ cell/mL | 39 (7-155) | 25 (0-68) | 0.53 |
| CD34+/CXCR4+ cell/mL | 1011 (578-1630) | 621 (385-960) | 0.01 |
Data presented as n (%) and mean ± SD.
Progenitor cells and troponin levels are presented as median (interquartile range).
GFR=Glomerular filtration rate
Racial differences in circulating biomarkers and their relationship with CPCs
In 561 subjects, Blacks compared to Whites had higher levels of hs-CRP and MCP-1, and lower levels of MMP-9. However, circulating VEGF and SDF-1α levels were not significantly different between the races, Table 1. In adjusted analyses, MCP-1 correlated negatively with CPCs while MMP-9 levels correlated positively with CPC counts (Figure 3). SDF-1α, hs-CRP or VEGF levels were not independent predictors of CPCs (Table 4). Thus, MMP-9 levels (log) were independently associated with 30.25% higher CD34+ count (95%CI 18.5%, 43.17%; P<0.001).
Figure 3.
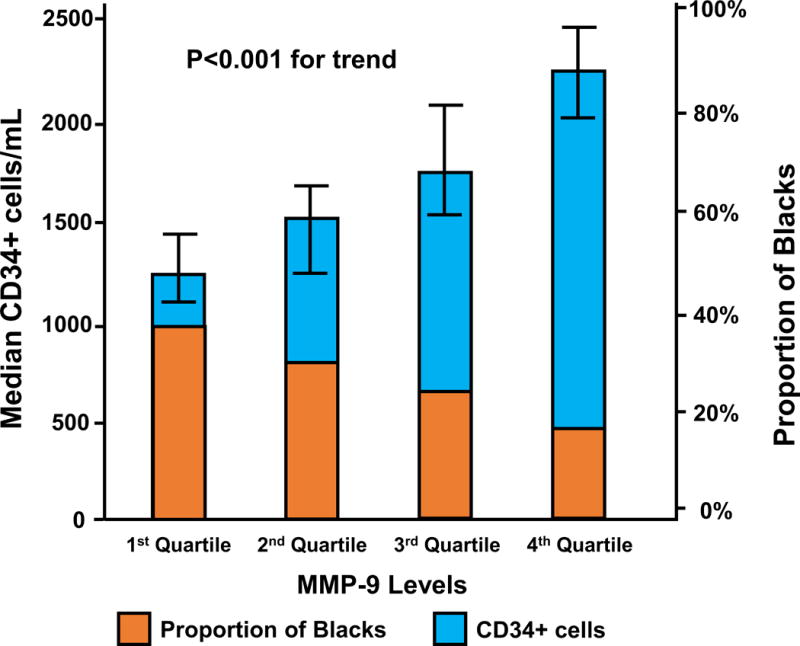
Correlation between matrix metallopeptidase 9 (MMP-9) quartiles and CPC counts.
Table 4.
Linear regression model for the association between CPCs and biomarkers in 561 subjects
| Biomarkers (log) | CD34+ % difference (95%CI) | P-value | CD34+/CD133+ % difference (95%CI) | P-value | CD34+/CXCR4+ % difference (95%CI) | P-value |
|---|---|---|---|---|---|---|
| Stromal cell-derived factor-1 α | −14.1% (−27.74%,2.12%) | 0.08 | −13.84% (−28.5%,3.82%) | 0.12 | −16.38% (−32.37%,3.38%) | 0.10 |
| Vascular endothelial growth factor | 2.5% (−7.27%,13.31%) | 0.63 | 1.42% (−8.97%,12.99%) | 0.80 | −0.79% (−12.26%,12.19%) | 0.90 |
| Matrix metallopeptidase-9 | 30.25% (18.5%,43.17%) | <0.0001 | 29.01% (16.51%,42.86%) | <0.0001 | 25.12% (11.42%,40.51%) | <0.0001 |
| Monocyte chemoattractant protein-1 | −22.81% (−37.93%,−4%) | 0.02 | −24.01% (−39.93%,−3.87%) | 0.02 | −19.71% (−38.55%,4.92%) | 0.11 |
| High-sensitivity C-reactive protein | −0.38% (−4.9%,4.36%) | 0.87 | −0.86% (−5.7%,4.23%) | 0.74 | −2.47% (−7.88%,3.25%) | 0.39 |
Adjusted for age, sex, race (White vs. Black), body mass index, hypertension, hyperlipidemia, diabetes, estimated glomerular filtration rate, history of heart failure and mononuclear cell counts.
In multivariable analysis, the association between CPCs and Black race was attenuated by adding MMP-9 levels to the model. Thus, there was 19% (95%CI 13-33%) attenuation for the association between Black race and CD34+ cells, 18% (95%CI 12-35%) for CD34+/CD133+ cells and 17% (95%CI 10-40%) for CD34+/CXCR4+ cells. However, Black race remained a significant predictor for lower numbers of CPCs without significant attenuation after additional adjustment for serum hs-CRP, VEGF, SDF-1α, or MCP-1 levels.
CPCs and prognosis
Among 623 patients with a median follow-up of 2.2 (IQR 1.3-4.5) years, there were 108 deaths (17%) from all causes. The outcome of death was not different based on race (P=0.9). A low CD34+ count, as a continuous variable, was predictive of all-cause mortality (lower CD34+ [log] HR=1.53 [95%CI 1.17-2.00]; P=0.002) independent of race. Thus, a lower CD34+ count, as continuous variable, was similarly predictive of mortality in Whites (HR=1.49 [95%CI 1.11-2.00]) and Blacks (HR=1.40 [95%CI 0.80-2.53]), without significant interaction (interaction P=0.8).
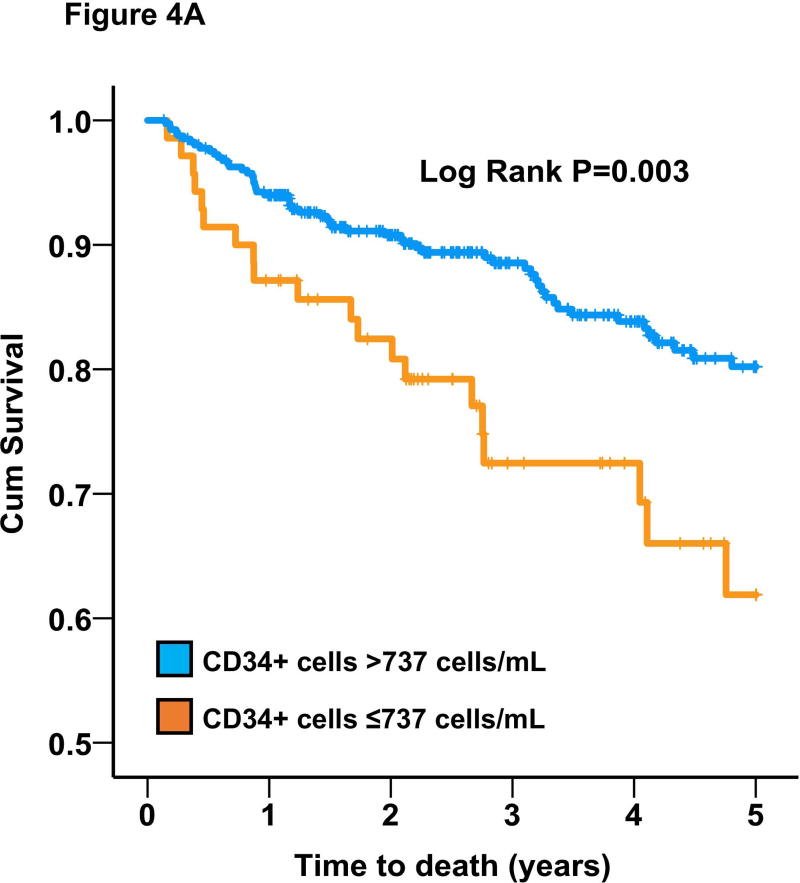
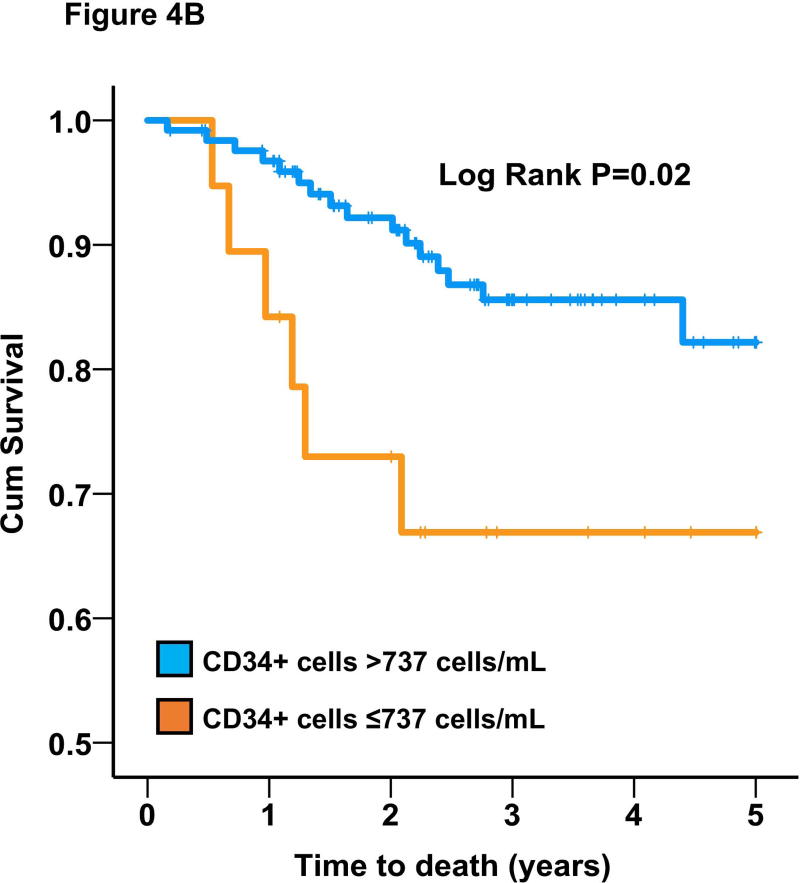
We also analyzed survival based on a pre-specified cutoffs that were previously shown to be predictive of worse prognosis (737 cells/mL for CD34+ subset)16, 25. In the entire cohort, CD34+ cells were also predictive of all-cause mortality (adjusted HR=1.91 [95%CI 1.21-3.00]; P=0.005) based on this cutoff value. Similarly, CD34+ levels below median was associated with HR=1.53 (95%CI 1.04-2.27; P=0.03). Black subjects had a 2-fold higher adjusted odds of having CD34+ cells below the pre-specified cutoff (737 cells/mL). Moreover, using stepwise backwards Cox regression model with P<0.1 as the threshold to retain a variable, a low CD34+ cell count (<737 cells/mL) was similarly predictive of survival in Blacks (adjusted HR=2.83 [95%CI 1.12-7.20]; P=0.03) and Whites (adjusted HR=1.79 [95%CI 1.09-2.94]; P=0.02) with non-significant interaction (P>0.2), Figure 4.
Figure 4.
Survival curves for the association between CD34+ cells and all-cause mortality in (A) Whites and (B) Blacks.
DISCUSSION
In a large well-characterized bi-racial cohort, we demonstrate that Black participants had significantly lower CPC counts compared to Whites, even after adjustment for differences in demographic factors and CVD risk factors. These results were validated in an independent cohort. Thus, on average, after adjustment for sex and other CVD risk factors, Blacks have CPC levels that are approximately 15 to 30% lower compared to Whites, even in subjects free of risk factors. CPC levels decline with age, reaching on average half the levels at age 80 compared to age 2025. We found that Blacks have CPC counts equivalent to those in Whites who are 14 years older. CPC levels are higher after AMI as a result of mobilization due to injury15. We show for first time that Blacks have 30-35% lower CPC mobilization in the setting of AMI.
We also investigated whether the racial differences in CPCs were driven by differences in inflammation, chemokine or cytokine signaling pathways previously associated with PC mobilization. Our results demonstrate that although levels of MMP-9 were lower and MCP-1 were higher in Blacks compared to Whites, only the lower levels of MMP-9 levels in Blacks, at least partly, contribute to the lower levels of CPCs. To explore whether there are any adverse consequences of low PC counts in Blacks compared to Whites, we examined the prognostic value of CPCs in patients with CAD. Our data shows that low CPC counts were similarly associated with a higher mortality in both racial groups. Because Blacks have lower CPC counts, this reduced endogenous regenerative capacity may be one additional reason for the observed disparities in CVD outcomes between Blacks and Whites.
Racial differences in CPCs have not been systematically investigated previously. One study reported similar CD34+/VEGF2R+ cell levels in 36 White and Black hypertensive subjects28, findings that may be explained by the small sample size and lack of structured multivariable adjustment for confounders.
In response to endogenous and exogenous stimuli, increased production of PC-mobilizing factors (such as hypoxia-inducible factor activation, VEGF and SDF-1α) increase levels of PCs21. Mechanisms contributing to cytokine-induced PC mobilization include cytokine gradients, cellular proliferation, modulation of adhesion molecules and modulation of the blood-bone marrow barrier29, 30. These factors also activate endothelial nitric oxide synthase, leading to an increased production of nitric oxide, which regulates the enzymatic activity of matrix metalloproteinases (MMPs)21. In particular, activated MMP-9 leads to the release of a soluble kit ligand from PCs in the bone marrow niches, resulting in PC release into the peripheral circulation20, 31. Availability of nitric oxide is important in PC mobilization and function that may also be impaired in Blacks32, 33. Both VEGF and SDF-1α stimulate PC mobilization from the bone marrow and regulate their angiogenic properties and their levels correlated with CPC levels in our study21. However, VEGF and SDF-1α levels did not differ between Black and White subjects and the association between lower CPCs and Black race was still significant after adding their levels to the model. In contrast, we found that MMP-9 levels correlated positively with CPC levels, its’ level was lower in Blacks, and accounted for 19% of the racial variability observed in CPC counts32. It is known that monocyte and PC mobilization from bone marrow niches requires stimulation of chemokine receptors by the activity of MMP-920, 33. Our findings demonstrate that lower MMP-9 activity in Blacks contributes, at least partly, to the reduced levels of CPCs in Blacks.
Studies using cord blood have shown that Blacks had significantly lower PC counts than other racial/ethnic groups, indicating that the racial differences in CPCs that we observed may be present even at birth and illustrate the importance of a potential genetic basis for the observed differences34. The Framingham Heart Study demonstrated that CD34+ PC frequency was highly heritable and polymorphisms in several loci, including OR4C12, ENO1/RERE, DGKB, and DSC3 correlated with CD34+ cell counts35. Additional variants in the MPL gene that regulates platelet and megakaryocyte receptors for thrombopoietin appear to regulate PCs in the bone marrow niche36. Further evaluation of race-associated genetic polymorphisms and CPC levels may help identify the potential genetic mechanisms underlying the impaired regenerative capacity in Blacks.
We also found that Blacks had about 30-35% lower hematopoietic-enriched CPC counts in the setting of large AMI compared to Whites. These findings suggest that not only do Blacks have lower CPC levels, but their ability to mobilize in response to injury is impaired. Previous studies have also reported that Blacks mobilize greater numbers of CD34+ PCs compared to Whites after granulocyte colony-stimulating factor (G-CSF) administration, suggesting that even though their response to endogenous stimuli is impaired, the response to exogenous mobilization agent (G-CSF) is in fact greater37,38. Our data didn’t show significant racial differences in hematopoietic-enriched CPCs in the setting of AMI which could be explained by their very low frequency and poor reproducibility compared to the more frequent hematopoietic-enriched CPC subsets.
In order to evaluate the long term consequences of lower CPC counts among Blacks, we assessed the prognostic value of CPCs in a population with CAD. Our study is the first to report the strong predictive value of CPCs in Blacks. We also found that the increased risk associated with lower CPC counts is similar among Blacks and Whites. Thus, the lower regenerative capacity puts Black subjects at added risk, over and above their risk factor burden, for worse CVD outcomes. These are novel and consequential findings that partly explain the increased CVD risk in Blacks and strategies to address regenerative capacity need to be further explored.
Strengths of our study include (1) a large study of more than 2300 subjects which allowed comparison of CPC counts across several groups with and without a variety of CVD phenotypes; (2) the use of state-of-the-art flow cytometry for quantification of CPCs by the same technical team27; (3) inclusion of subjects with and without risk factors, with and without CVD, and in the setting of acute MI; (4) exploration of a wide selection of CD34+ cell subpopulations encompassing hematopoietic and endothelial progenitors; (5) exploring the effect of inflammatory cytokines and chemokines on the association between race and CPCs and (6) long-term follow-up for mortality. Limitations of our study include (1) the determination of race by self-report that potentially introduces bias in individuals with mixed ancestry; (2) lack of comparison with other races; (3) lack of information on sickle cell trait that is more common in Blacks and could potentially have confounded our findings, although we and others have shown previously that patients with sickle cell disease have higher CPC counts39, 40 (4) absence of functional measurements of CPCs (5) single measurement at varying time points after the AMI and (6) our study recruited patients from Emory healthcare system and thus, the results might not be generalizable to other patient cohorts or other geographic regions.
In conclusion, this is the first study to demonstrate lower CPC counts in Blacks compared with Whites in a large cohort of subjects with and without cardiovascular risk factors and/or CVD which is partially dependent on lower MMP-9 levels in Blacks. Blacks also have reduced CPC mobilization in the setting of an AMI. These findings demonstrate that Blacks have impaired regenerative capacity compared with Whites, independent of their risk factor profile or biomarkers of chemotaxis. Because lower CPC counts are associated with worse long-term outcomes, these findings may explain the racial disparities in outcomes. Further studies are required to examine genetic and other mechanisms contributing to these disparities, and discuss potential targets for new interventions and research priorities.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Impaired regenerative capacity, measured as low circulating levels of progenitor cells, is associated with adverse clinical outcomes.
Progenitor cells are mobilized from the bone marrow into the circulation in response to ischemia and home to ischemic tissue, thereby contributing to repair and regeneration.
In comparison with Whites, Blacks have higher cardiovascular risk and worse outcomes, but little is known about racial differences in circulating numbers of progenitor cells.
What New Information Does This Article Contribute?
Compared to Whites, Blacks had lower numbers of circulating progenitor cells and diminished mobilization during acute myocardial injury.
Lower levels of progenitor cells in Blacks is partially dependent on lower circulating matrix metallopeptidase-9 levels.
Low circulating progenitor cell counts are predictive of higher mortality in Blacks. Thus, reduced regenerative capacity may partly explain the higher risk of adverse cardiovascular events in Blacks.
Progenitor cells originate from the bone marrow and contribute to tissue repair and regeneration. Compared to White subjects, Blacks with or without cardiovascular disease or risk factors have significantly lower circulating levels of hematopoietic and endothelial progenitor cells. Decreased regenerative capacity in Blacks, measured as lower levels of circulating progenitor cells can be partially explained by lower levels of circulating matrix metallopeptidase-9 levels in Blacks. Lower circulating progenitor cell levels are independently associated with a higher mortality rate in Blacks. Therapeutic interventions to enhance progenitor cell levels may represent a novel therapeutic target for reducing health disparities.
Acknowledgments
SOURCES OF FUNDING
AAQ is supported by 1R61HL138657-01, 1P30DK111024-01, AHA: 0000031288, 5P01HL101398-02, 1P20HL113451-01, 1RF1AG051633-01, R01 NS064162-01, R01 HL89650-01, 1U10HL110302-01, 2P01HL086773-06A1 and the Emory Predictive Health Institute. AST is supported by the Abraham J. & Phyllis Katz Foundation (Atlanta, GA) and NIH/NIA grant AG051633.
Nonstandard Abbreviations and Acronyms
- CVD
Cardiovascular Disease
- Cpcs
Circulating Progenitor Cells
- VEGFR2
Vascular Endothelial Growth Factor Receptor-2
- CXCR4
Chemokine (C-X-C Motif) Receptor 4
- Mcps
Monocyte Chemoattractant Proteins
- AMI
Acute Myocardial Infarction
- SDF-1α
Stromal Cell-Derived Factor-1 A
- VEGF
Vascular Endothelial Growth Factor
- MMP-9
Matrix Metallopeptidase-9
- CAD
Coronary Artery Disease
- GFR
Estimated Glomerular Filtration Rate
Footnotes
DISCLOSURES
None of the authors have conflicts of interest to disclose.
References
- 1.Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA, Jr, Willis M, Yancy CW, American Heart Association Council on E, Prevention, Council on Cardiovascular Disease in the Y, Council on C, Stroke N, Council on Clinical C, Council on Functional G, Translational B and Stroke C Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association. Circulation. 2017;136:e393–e423. doi: 10.1161/CIR.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond WD, Chambless LE, Heiss G, Mosley TH, Coresh J, Whitsel E, Wagenknecht L, Ni H, Folsom AR. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987-2008. Circulation. 2012;125:1848–57. doi: 10.1161/CIRCULATIONAHA.111.047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 4.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–7. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 6.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–53. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 7.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–9. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 8.Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schafer B, Hossfeld DK, Fiedler W. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–12. [PubMed] [Google Scholar]
- 9.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–12. [PubMed] [Google Scholar]
- 10.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110:624–37. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alaiti MA, Ishikawa M, Costa MA. Bone marrow and circulating stem/progenitor cells for regenerative cardiovascular therapy. Transl Res. 2010;156:112–29. doi: 10.1016/j.trsl.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Seeger FH, Rasper T, Koyanagi M, Fox H, Zeiher AM, Dimmeler S. CXCR4 expression determines functional activity of bone marrow-derived mononuclear cells for therapeutic neovascularization in acute ischemia. Arterioscler Thromb Vasc Biol. 2009;29:1802–9. doi: 10.1161/ATVBAHA.109.194688. [DOI] [PubMed] [Google Scholar]
- 13.Hammadah M, Samman Tahhan A, Mheid IA, Wilmot K, Ramadan R, Kindya BR, Kelli HM, O’Neal WT, Sandesara P, Sullivan S, Almuwaqqat Z, Obideen M, Abdelhadi N, Alkhoder A, Pimple PM, Levantsevych O, Mohammed KH, Weng L, Sperling LS, Shah AJ, Sun YV, Pearce BD, Kutner M, Ward L, Bremner JD, Kim J, Waller EK, Raggi P, Sheps D, Vaccarino V, Quyyumi AA. Myocardial Ischemia and Mobilization of Circulating Progenitor Cells. Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease. 2018;7:e007504. doi: 10.1161/JAHA.117.007504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topel ML, Hayek SS, Ko YA, Sandesara PB, Samman Tahhan A, Hesaroieh I, Mahar E, Martin GS, Waller EK, Quyyumi AA. Sex Differences in Circulating Progenitor Cells. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samman Tahhan AS, Hammadah M, Raad M, Almuwaqqat Z, Alkhoder A, Sandesara PB, Kelli H, Hayek SS, Kim JH, O’Neal WT, Topel ML, Grant AJ, Sabbak N, Heinl RE, Gafeer MM, Obideen M, Kaseer B, Abdelhadi N, Ko YA, Liu C, Ghaini I, Mahar EA, Vaccarino V, Waller EK, Quyyumi AA. Progenitor Cells and Clinical Outcomes in Patients with Acute Coronary Syndromes. Circ Res. 2018 doi: 10.1161/CIRCRESAHA.118.312821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samman Tahhan A, Hammadah M, Sandesara PB, Hayek SS, Kalogeropoulos AP, Alkhoder A, Mohamed Kelli H, Topel M, Ghasemzadeh N, Chivukula K, Ko YA, Aida H, Hesaroieh I, Mahar E, Kim JH, Wilson P, Shaw L, Vaccarino V, Waller EK, Quyyumi AA. Progenitor Cells and Clinical Outcomes in Patients With Heart Failure. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.117.004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayek SS, MacNamara J, Tahhan AS, Awad M, Yadalam A, Ko YA, Healy S, Hesaroieh I, Ahmed H, Gray B, Sher SS, Ghasemzadeh N, Patel R, Kim J, Waller EK, Quyyumi AA. Circulating Progenitor Cells Identify Peripheral Arterial Disease in Patients With Coronary Artery Disease. Circ Res. 2016;119:564–71. doi: 10.1161/CIRCRESAHA.116.308802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al Mheid I, Hayek SS, Ko YA, Akbik F, Li Q, Ghasemzadeh N, Martin GS, Long Q, Hammadah M, Maziar Zafari A, Vaccarino V, Waller EK, Quyyumi AA. Age and Human Regenerative Capacity Impact of Cardiovascular Risk Factors. Circ Res. 2016;119:801–9. doi: 10.1161/CIRCRESAHA.116.308461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Abdelhadi N, Alkhoder A, Obideen M, Pimple PM, Levantsevych O, Kelli HM, Shah A, Sun YV, Pearce B, Kutner M, Long Q, Ward L, Ko YA, Hosny Mohammed K, Lin J, Zhao J, Bremner JD, Kim J, Waller EK, Raggi P, Sheps D, Quyyumi AA, Vaccarino V. Telomere Shortening, Regenerative Capacity, and Cardiovascular Outcomes. Circulation research. 2017;120:1130–1138. doi: 10.1161/CIRCRESAHA.116.309421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein G, Schmal O, Aicher WK. Matrix metalloproteinases in stem cell mobilization. Matrix Biol. 2015;44–46:175–83. doi: 10.1016/j.matbio.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann BR, Wagner JR, Prisco AR, Janiak A, Greene AS. Vascular endothelial growth factor-A signaling in bone marrow-derived endothelial progenitor cells exposed to hypoxic stress. Physiol Genomics. 2013;45:1021–34. doi: 10.1152/physiolgenomics.00070.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko YA, Hayek S, Sandesara P, Samman Tahhan A, Quyyumi A. Cohort profile: the Emory Cardiovascular Biobank (EmCAB) BMJ Open. 2017;7:e018753. doi: 10.1136/bmjopen-2017-018753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Shah AJ, Sun Y, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Raggi P, Sheps DS, Vaccarino V, Quyyumi AA. The Mental Stress Ischemia Prognosis Study: Objectives, Study Design, and Prevalence of Inducible Ischemia. Psychosom Med. 2017;79:311–317. doi: 10.1097/PSY.0000000000000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al Mheid I, Patel R, Murrow J, Morris A, Rahman A, Fike L, Kavtaradze N, Uphoff I, Hooper C, Tangpricha V, Alexander RW, Brigham K, Quyyumi AA. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J Am Coll Cardiol. 2011;58:186–92. doi: 10.1016/j.jacc.2011.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel RS, Li Q, Ghasemzadeh N, Eapen DJ, Moss LD, Janjua AU, Manocha P, Al Kassem H, Veledar E, Samady H, Taylor WR, Zafari AM, Sperling L, Vaccarino V, Waller EK, Quyyumi AA. Circulating CD34+ progenitor cells and risk of mortality in a population with coronary artery disease. Circ Res. 2015;116:289–97. doi: 10.1161/CIRCRESAHA.116.304187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Modder UI, Roforth MM, Nicks KM, Peterson JM, McCready LK, Monroe DG, Khosla S. Characterization of mesenchymal progenitor cells isolated from human bone marrow by negative selection. Bone. 2012;50:804–10. doi: 10.1016/j.bone.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahar EA, Mou L, Hayek SS, Quyyumi AA, Waller EK. Flow cytometric data analysis of circulating progenitor cell stability. Data Brief. 2017;10:346–348. doi: 10.1016/j.dib.2016.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SR, Eirin A, Herrmann SM, Saad A, Juncos LA, Lerman A, Textor SC, Lerman LO. Preserved endothelial progenitor cell angiogenic activity in African American essential hypertensive patients. Nephrol Dial Transplant. 2017 doi: 10.1093/ndt/gfx032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nervi B, Link DC, DiPersio JF. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem. 2006;99:690–705. doi: 10.1002/jcb.21043. [DOI] [PubMed] [Google Scholar]
- 30.Rennert RC, Sorkin M, Garg RK, Gurtner GC. Stem cell recruitment after injury: lessons for regenerative medicine. Regen Med. 2012;7:833–50. doi: 10.2217/rme.12.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–37. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–26. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ballen KK, Kurtzberg J, Lane TA, Lindgren BR, Miller JP, Nagan D, Newman B, Rupp N, Haley NR. Racial diversity with high nucleated cell counts and CD34 counts achieved in a national network of cord blood banks. Biol Blood Marrow Transplant. 2004;10:269–75. doi: 10.1016/j.bbmt.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Cohen KS, Cheng S, Larson MG, Cupples LA, McCabe EL, Wang YA, Ngwa JS, Martin RP, Klein RJ, Hashmi B, Ge Y, O’Donnell CJ, Vasan RS, Shaw SY, Wang TJ. Circulating CD34(+) progenitor cell frequency is associated with clinical and genetic factors. Blood. 2013;121:e50–6. doi: 10.1182/blood-2012-05-424846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaushansky K. The molecular mechanisms that control thrombopoiesis. J Clin Invest. 2005;115:3339–47. doi: 10.1172/JCI26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsu JW, Wingard JR, Logan BR, Chitphakdithai P, Akpek G, Anderlini P, Artz AS, Bredeson C, Goldstein S, Hale G, Hematti P, Joshi S, Kamble RT, Lazarus HM, O’Donnell PV, Pulsipher MA, Savani BN, Schears RM, Shaw BE, Confer DL. Race and ethnicity influences collection of granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells from unrelated donors, a Center for International Blood and Marrow Transplant Research analysis. Biol Blood Marrow Transplant. 2015;21:165–71. doi: 10.1016/j.bbmt.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vasu S, Leitman SF, Tisdale JF, Hsieh MM, Childs RW, Barrett AJ, Fowler DH, Bishop MR, Kang EM, Malech HL, Dunbar CE, Khuu HM, Wesley R, Yau YY, Bolan CD. Donor demographic and laboratory predictors of allogeneic peripheral blood stem cell mobilization in an ethnically diverse population. Blood. 2008;112:2092–100. doi: 10.1182/blood-2008-03-143677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ofori-Acquah SF, Buchanan ID, Osunkwo I, Manlove-Simmons J, Lawal F, Quarshie A, Quyyumi AA, Gibbons GH, Gee BE. Elevated circulating angiogenic progenitors and white blood cells are associated with hypoxia-inducible angiogenic growth factors in children with sickle cell disease. Anemia. 2012;2012:156598. doi: 10.1155/2012/156598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panch SR, Yau YY, Fitzhugh CD, Hsieh MM, Tisdale JF, Leitman SF. Hematopoietic progenitor cell mobilization is more robust in healthy African American compared to Caucasian donors and is not affected by the presence of sickle cell trait. Transfusion. 2016;56:1058–65. doi: 10.1111/trf.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.