Abstract
Background
Diagnosis of heart failure with preserved ejection fraction (HFpEF) is challenging in euvolemic patients with dyspnea, and no evidence-based criteria are available. We sought to develop and then validate non-invasive diagnostic criteria that could be used to estimate the likelihood that HFpEF is present among patients with unexplained dyspnea in order to guide further testing.
Methods
Consecutive patients with unexplained dyspnea referred for invasive hemodynamic exercise testing were retrospectively evaluated. Diagnosis of HFpEF (case) or non-cardiac dyspnea (control) was ascertained by invasive hemodynamic exercise testing. Logistic regression was performed to evaluate the ability of clinical findings to discriminate cases from controls. A scoring system was developed and then validated in a separate test cohort.
Results
The derivation cohort included 414 consecutive patients (267 HFpEF and 147 controls, HFpEF prevalence 64%). The test cohort included 100 consecutive patients (61 HFpEF, prevalence 61%). Obesity, atrial fibrillation, age>60 years, treatment with 2 or more antihypertensives, echocardiographic E/e′ ratio>9 and echocardiographic pulmonary artery systolic pressure>35 mmHg were selected as the final set of predictive variables. A weighted score based on these six variables was used to create a composite score (H2FPEF score) ranging from 0–9. The odds of HFpEF doubled for each 1 unit score increase [OR 1.98 [1.74–2.30], p<0.0001], with an AUC of 0.841 (p<0.0001). The H2FPEF score was superior to a currently-used algorithm based upon expert consensus (increase in AUC of +0.169 [+0.120 to +0.217], p<0.0001). Performance in the independent test cohort was maintained [AUC 0.886, p<0.0001].
Conclusions
The H2FPEF score, which relies upon simple clinical characteristics and echocardiography, enables discrimination of HFpEF from non-cardiac causes of dyspnea, and can assist in determination of the need for further diagnostic testing in the evaluation of patients with unexplained exertional dyspnea.
Keywords: HFpEF, exercise testing, exercise catheterization
Introduction
Exertional dyspnea may be caused by cardiac and noncardiac disorders. Among the cardiovascular causes, heart failure with preserved ejection fraction (HFpEF) is an increasingly common etiology characterized by pathologic increases in cardiac filling pressures at rest or with exertion.1–6 Decompensated patients with HFpEF typically display overt congestion on physical examination and chest radiography, and in this setting the diagnosis is straightforward. However, compensated, euvolemic patients presenting with exertional dyspnea in the absence of overt clinical, radiographic or biomarker evidence of congestion present a greater diagnostic challenge.
The reference standard to diagnose HFpEF in these patients is by right heart catheterization followed by invasive exercise testing if resting intracardiac pressures are normal.7–10 Because of its invasive nature, technical complexity and cost, this test is impractical for routine evaluation, but is more logically reserved for situations where diagnosis remains uncertain after less invasive test results are equivocal.7 In order to make this determination, the probability of disease must first be estimated, allowing clinicians to decide whether disease is likely present or absent, or intermediate, where more definitive testing is required. Currently, there are no data available to guide this sort of Bayesian approach to the evaluation of unexplained dyspnea.
To fill this gap, we evaluated clinical data from consecutive patients where the diagnosis of HFpEF or a non-cardiac etiology of dyspnea was ascertained conclusively by invasive exercise testing in order to develop a scoring system that could be used in the diagnostic evaluation of HFpEF. We then validated this new scoring system in a separate cohort.
Methods
This was a retrospective analysis of all consecutive patients undergoing invasive exercise testing for the evaluation of unexplained dyspnea between 2006 and 2016 at the Mayo Clinic in Rochester, MN. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. Exclusion criteria included ejection fraction<50% (current or prior), significant valvular heart disease (>mild stenosis, >moderate regurgitation), pulmonary arterial hypertension, constrictive pericarditis, primary cardiomyopathies, or heart transplant. All patients referred for hemodynamic catheterization were evaluated by Mayo staff cardiologists and concluded to have dyspnea not explainable by pulmonary disease based upon evaluations performed at the discretion of the referring physicians.
HFpEF patients were identified by elevated pulmonary capillary wedge pressure at rest (≥15 mmHg) or during exercise (≥25 mmHg).7, 8 Non-cardiac dyspnea was defined as patients with no evidence of a cardiac etiology for dyspnea after exhaustive clinical evaluation, including normal rest and exercise hemodynamics. Data included in the study were authorized by the patient for use in research with informed consent, and the study was approved by the Mayo Clinic Institutional Review Board.
Clinical Evaluation
All patients were evaluated by a board certified cardiologist. Medical history was determined from detailed manual chart review by 2 independent observers (YR and MO, discrepancies arbitrated by BAB). Hypertension was defined by treatment with antihypertensive medications, and the number of antihypertensive medications was quantified for each patient. Atrial fibrillation was determined from clinical history and electrocardiogram. Diabetes was defined by treatment with anti-diabetic medications, fasting plasma glucose ≥126 mg/dl, or a hemoglobin A1c ≥6.5 mg/dl. Prediabetes was defined as fasting plasma glucose between 100–126 mg/dl or a hemoglobin A1c between 5.7–6.5 mg/dl. Laboratories including hemoglobin and creatinine were obtained on the day of catheterization. NT-proBNP levels were obtained from samples drawn within 3 months of the assessment. Echocardiography was performed according to American Society of Echocardiography guidelines and interpreted by Mayo Clinic staff cardiologists.11 Details of the echocardiographic measurements performed are included in the online supplement.
Ascertainment of Diagnosis
Subjects were studied on chronic medications in the fasted state and supine position using high fidelity micromanometer catheters and directly measured O2 consumption at rest and during supine cycle ergometry exercise to exhaustion as previously described.7, 8 Pressures in the right atrium, pulmonary artery, and pulmonary artery wedge positions were measured at end-expiration from electronically stored continuous recordings of pressure tracings digitized at 240 Hz. Systemic and mixed venous O2 content was determined by blood sampling. Cardiac output was determined by the Fick method.
Statistical analysis
Data are reported as mean and standard deviation or median (25th–75th interquartile range). Chi square, Wilcoxon rank sum test or T test were used as appropriate to examine differences between HFpEF and controls. Prior to development of the final models, data in the development cohort were imputed using random forest imputation (missForest package version 1.4).12 Two modeling strategies were considered. The primary analysis plans were to develop a multivariable logistic regression model that could be summarized using a simple additive score based upon prior knowledge of HFpEF pathophysiology while allowing for categorization of variables to be considered in the modeling process.13 As an alternative to address the limitations of variable categorization, a model consisting of only continuous variables was also estimated. Second, two agnostic supplemental models were built as sensitivity analyses: (1) a classification and regression tree (CART) model to enable easier graphical representation with inclusion of higher order interaction terms that would be complex to represent using the additive score, and (2) a fully agnostic multivariable logistic model.
Predictors for HFpEF were first analyzed with simple logistic regression to identify candidate variables that were significantly associated with disease status. For ease of clinical use, continuous variables that were significant were dichotomized using Receiver Operating Characteristic curves to identify optimal cut points for discrimination, which were rounded to the nearest clinically significant integer when applicable. Next, significant variables (p<0.05) were entered into multivariable logistic regression models to determine a final model.
Obesity14, 15 and atrial fibrillation16, 17 are known to be important in HFpEF pathogenesis, and these variables were a priori forced into the model. Additional variables that were significant on univariable analysis were added, with care taken to avoid clinically relevant collinearity. Once the full multivariable model was created, stepwise backward elimination was performed with the least significant variable removed one variable at a time, until all included model variables were statistically significant. A non-invasive prediction score was created using the variables and strength of association by beta coefficients, as previously described.13 In addition to the points-based score a continuous model was built using the same variables.
Using this prediction score on a continuous scale, we then evaluated its diagnostic performance by the Area Under the Receiver Operating Charactersitic Curve (AUC), or c statistic. To evaluate the model’s likelihood to generalize to a new sample, Harrell’s optimism was calculated using 1000 bootstrap replicates18, and to evaluate for incremental discrimination beyond existing criteria, we compared the AUCs from our derived scoring system to the current and prior consensus algorithms endorsed by the European Society of Cardiology guidelines (Supplemental Figure 1) using the DeLong test.5 Calibration of the predicted probabilities with the empirical probabilities for HFpEF was assessed using the Hosmer-Lemeshow goodness of fit test.
Two completely agnostic models were built as a sensitivity analysis. First, an agnostic multivariable logistic model included all significant predictors of HFpEF on univariable analysis, with stepwise backward regression using a probability to leave of 0.05. Second, random forest classifiers were constructed using the full list of candidate predictors in the development data to develop a CART model. Variable importance plots were used to begin sub-setting the number of variables. The subset of variables was then used to train a CART model using the rpart package in R under the default tuning configuration. Both the resulting CART model and prediction score were validated on an independent test cohort from patients. All tests were 2-sided, with a P value <0.05 considered significant. Analyses were performed using JMP 13.0.0 (SAS Institute, Cary, NC) and R 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients with HFpEF (n=267) were older, had higher body mass index (BMI), and more hypertension, glucose intolerance, atrial fibrillation, NTproBNP elevation and renal dysfunction as compared to patients with non-cardiac causes of dyspnea (n=147, Table 1). They were more likely to have a pacemaker, QRS, QTc and PR interval prolongation on electrocardiogram, and cardiomegaly on chest radiography. Two-thirds of patients came from local practices served by the Mayo Clinic (n=273, 66%) with the remainder referred from tertiary academic centers. Of patients found to have HFpEF, 45% (n=121) displayed elevation in filling pressures only during exercise (“early stage HFpEF”).
Table 1.
Baseline Characteristics:
| Non-Cardiac Dyspnea (n=147) | HFpEF (n=267) | p value | |
|---|---|---|---|
| Age, years | 56±15 | 68±11 | <0.0001 |
| Female, % | 59 | 61 | 0.4 |
| Body mass index, kg/m2 | 28.2±5.4 | 33.0±7.4 | <0.0001 |
|
| |||
| Comorbidities | |||
| Hypertension, % | 53 | 86 | <0.0001 |
| Number of anti-hypertensive drugs, n | 1.2±1.3 | 2.2±1.3 | <0.0001 |
| Impaired glucose tolerance any, % | 29.9 | 54.7 | <0.0001 |
| Prediabetes, % | 17.7 | 26.6 | |
| Diabetes, % | 12.2 | 28.1 | |
| Atrial Fibrillation any, % | 4.1 | 34.4 | <0.0001 |
| Paroxysmal Atrial Fibrillation, % | 3.4 | 17.2 | |
| Permanent Atrial Fibrillation, % | 0.7 | 17.2 | |
| Hemoglobin, g/dl | 12.9±1.3 | 12.2±1.5 | <0.0001 |
| Diuretic, % | 23 | 48 | <0.0001 |
| NT-proBNP, pg/ml | 122 [52–259] | 384 [131–1111] | <0.0001 |
| Creatinine, mg/dl | 0.96±0.22 | 1.13±0.40 | <0.0001 |
| Glomerular Filtration Rate, ml/min/1.73m2 | 93±31 | 83±37 | 0.006 |
| Kidney Disease grade 3 or higher, % | 10 | 26 | <0.0001 |
|
| |||
| Electrocardiography | |||
| Pacemaker, % | 0.7 | 12.7 | <0.0001 |
| QRS duration, ms | 94±19 | 99±27 | 0.04 |
| Left Bundle Branch Block,% | 2 | 2 | 0.7 |
| Left atrial enlargement, % | 1 | 4 | 0.2 |
| PR interval, ms | 159±26 | 175±39 | <0.0001 |
| LV hypertrophy, % | 2 | 3 | 0.7 |
| QTc interval, ms | 435±26 | 445±33 | 0.0009 |
|
| |||
| Chest Radiography | |||
| Cardiomegaly, % | 4 | 24 | <0.0001 |
| Pleural effusion, % | 2 | 4 | 0.4 |
|
| |||
| Echocardiography | |||
| Left ventricle | |||
| LV end diastolic dimension, mm | 48±5 | 48±5 | 0.1 |
| LV mass index, g/m2 | 84±19 | 92±23 | <0.0001 |
| LV hypertrophy, % | 12 | 26 | 0.0009 |
| LV ejection fraction, % | 63±5 | 63±6 | 0.7 |
| LV global longitudinal strain, % | 16.3±2.6 | 15.2±3.0 | 0.0001 |
| LA volume index, ml/m2 | 29±9 | 38±14 | <0.0001 |
| E/e′ ratio | 10±4 | 14±7 | <0.0001 |
| Septal e′, cm/s | 8±3 | 7±2 | <0.0001 |
|
| |||
| Right ventricle | |||
| Pulmonary artery systolic pressure, mmHg | 30±5 | 38±12 | <0.0001 |
| TAPSE, mm | 23±3 | 21±4 | <0.0001 |
| TV lateral s′, cm/s | 14±3 | 13±3 | 0.002 |
| RV Fractional Area Change, % | 53±5 | 50±9 | 0.0003 |
| RV basal diameter, mm | 31±5 | 33±6 | 0.0003 |
| RV mid diameter, mm | 24±4 | 25±6 | 0.004 |
| Visual RV dysfunction,% | 6 | 22 | <0.0001 |
| Visual RV dilation, % | 12 | 31 | <0.0001 |
LV-Left Ventricle; LA-Left Atrium; RV-Right Ventricle, TV-Tricuspid valve, TAPSE-Tricuspid Annular Plane Systolic Excursion; E/e′-ratio of early diastolic mitral inflow velocity to septal mitral annulus tissue relaxation velocity , e′-septal mitral annulus tissue relaxation velocity in early diastole
Transthoracic echocardiography revealed that HFpEF patients were more likely to have diastolic dysfunction, with higher non-invasive estimates of filling pressure (higher E/e′ ratio). Although ejection fraction was similar, HFpEF patients had subtle impairment in systolic function as evidenced by lower global longitudinal strain (Table 1). Estimated pulmonary artery pressure was higher and right ventricular dysfunction and dilatation were more common in HFpEF. While group differences for many variables were highly significant, there was a substantial degree of overlap between the two groups (Table 1).
At cardiac catheterization, HFpEF patients displayed higher ventricular filling pressures and pulmonary artery pressures and lower cardiac output compared to non-cardiac dyspnea patients, as expected (Supplemental Table 1).
Univariable Predictors of HFpEF
Clinical, demographic, and echocardiographic criteria were evaluated as predictors of HFpEF in isolation (Table 2, Supplemental Table 2). Certain variables were highly specific for the presence of HFpEF, including grade II obesity (BMI>35 kg/m2, specificity 88%), chronic kidney disease (≥stage 3, 90%), atrial fibrillation (96%), diabetes (88%), the presence of a pacemaker (99%), cardiomegaly on chest film (96%), mildly depressed EF of 50–54% (96%), E/e′>14 (89%), pulmonary artery systolic pressure>35 mmHg (86%), NTproBNP>450 pg/ml (85%) and the presence of right ventricular dysfunction.
Table 2.
Univariate predictors of HFpEF
| OR [95% CI] | Beta estimate | AUC | Sensitivity | Specificity | p value | |
|---|---|---|---|---|---|---|
| Clinical | ||||||
| Age>60 years | 6.20 [3.96–9.69] | 1.82 | 0.704 | 80 | 60 | <0.0001 |
| Body Mass Index>30 kg/m2 | 3.46 [2.27–5.29] | 1.24 | 0.651 | 65 | 65 | <0.0001 |
| Body Mass Index>35 kg/m2 | 4.02 [2.23–7.07] | 1.39 | 0.615 | 35 | 88 | <0.0001 |
| NTproBNP> 275pg/ml | 4.82 [3.06–7.59] | 1.57 | 0.680 | 59 | 77 | <0.0001 |
| NTproBNP> 450pg/ml | 4.93 [3.00–8.40] | 1.60 | 0.657 | 46 | 85 | <0.0001 |
| Chronic Kidney Disease grade 3 or higher | 3.38 [1.88–6.47] | 1.22 | 0.584 | 26 | 90 | <0.0001 |
| Any Hypertension | 5.33 [3.35–8.61] | 1.67 | 0.664 | 86 | 47 | <0.0001 |
| Treatment with 2 or more antihypertensives | 4.49 [2.94–6.94] | 1.50 | 0.678 | 72 | 63 | <0.0001 |
| Atrial Fibrillation, any | 12.35 [5.69–302.41] | 2.51 | 0.652 | 35 | 96 | <0.0001 |
| Paroxysmal Atrial Fibrillation | 5.91 [2.51–17.36] | 1.78 | 0.569 | 17 | 97 | <0.0001 |
| Permanent Atrial Fibrillation | 30.39 [6.53–540.93] | 3.41 | 0.583 | 17 | 99 | <0.0001 |
| Diabetes | 2.80 [1.60–4.90] | 1.03 | 0.579 | 28 | 88 | 0.0003 |
| Prediabetes or Diabetes | 2.82 [1.84–4.33] | 1.04 | 0.624 | 55 | 70 | <0.0001 |
| Pacemaker | 21.30 [2.89–157.31] | 3.06 | 0.560 | 13 | 99 | <0.0001 |
|
| ||||||
| Chest Radiography | ||||||
| Cardiomegaly | 7.56 [3.45–19.97] | 2.02 | 0.601 | 24 | 96 | <0.0001 |
| Pleural effusion | 4.96 [1.70–21.08] | 1.60 | 0.537 | 9 | 98 | 0.002 |
| Cardiomegaly or Pleural effusion | 5.99 [3.05–13.21] | 1.79 | 0.610 | 28 | 94 | <0.0001 |
|
| ||||||
| Echocardiogram | ||||||
| Ejection Fraction<55% | 2.39 [0.88–6.47] | 0.87 | 0.522 | 8 | 96 | 0.09 |
| Global longitudinal strain<16% | 2.10 [1.39–3.16] | 0.74 | 0.591 | 62 | 56 | 0.0004 |
| Left Ventricular Hypertrophy | 2.55 [1.48–4.59] | 0.94 | 0.570 | 26 | 88 | 0.0006 |
| Left Atrial Volume Index>30 ml/m2 | 5.65 [3.64–8.79] | 1.73 | 0.704 | 70 | 71 | <0.0001 |
| E/e′ ratio>9 | 5.23 [3.37–8.11] | 1.65 | 0.687 | 78 | 59 | <0.0001 |
| E/e′ ratio>13 | 5.20 [3.09–8.76] | 1.65 | 0.661 | 46 | 86 | <0.0001 |
| Septal e′ velocity <7, cm/s | 2.90 [1.87–4.59] | 1.07 | 0.619 | 48 | 76 | <0.0001 |
| Right atrial pressure>10 mmHg | 6.80 [2.05–22.58] | 1.91 | 0.564 | 16 | 97 | <0.0001 |
| Pulmonary Artery Systolic Pressure >35mmHg | 5.05 [3.05–8.69] | 1.61 | 0.657 | 46 | 86 | <0.0001 |
| RV Fractional Area Change<48% | 4.88 [2.78–8.59] | 1.59 | 0.637 | 39 | 88 | <0.0001 |
| Tricuspid Annular Plane Systolic Excursion<21 mm | 3.69 [2.29–5.94] | 1.30 | 0.637 | 46 | 81 | <0.0001 |
| Visual RV dysfunction | 4.26 [2.04–8.87] | 1.45 | 0.578 | 22 | 94 | <0.0001 |
| Visual RV dilation | 3.45 [1.96–6.09] | 1.24 | 0.598 | 32 | 88 | <0.0001 |
Cut points derived from Receiver operating curve analysis as shown in Supplementary Table 2. NTproBNP-N Terminal pro Brain Natriuretic Peptide; RV-Right Ventricle; E/e′-ratio of early diastolic mitral inflow velocity to septal mitral annulus tissue relaxation velocity; e′-septal mitral annulus tissue relaxation velocity in early diastole
Derivation of the H2FPEF score
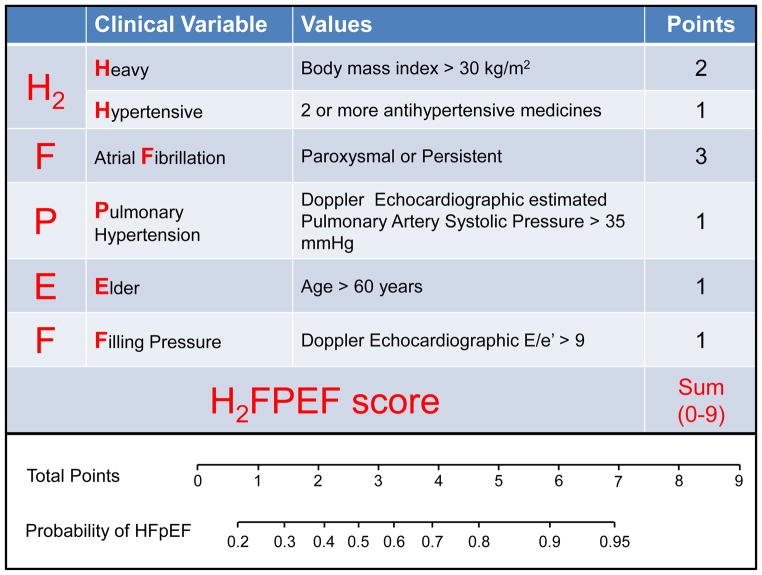
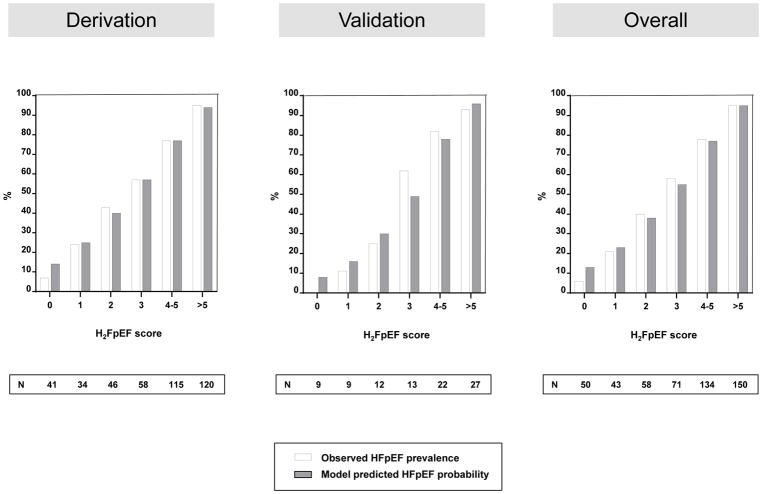
The variables identified through univariable screening were entered into a multivariable model (Table 3). Obesity (BMI>30 kg/m2), atrial fibrillation, age>60 years, treatment with 2 or more antihypertensive drugs, E/e′>9 and pulmonary artery systolic pressure>35 mmHg were associated with HFpEF in combination (all p<0.05). A score was assigned to these six variables based on strength of association in logistic regression with HFpEF [atrial fibrillation-3 points, obesity-2 points, others-1 each], creating a H2FPEF score ranging from 0–9 (Figure 1, upper box). The probability of HFpEF increased with increasing H2FPEF score (Figure 1, lower box). Model-based probabilities closely matched the observed prevalence for each given score value, indicating good calibration (Figure 2).
Table 3.
Multivariable predictors of HFpEF
| Multivariable model | OR [95% CI] | Beta estimate | p value |
|---|---|---|---|
| (AICc=393.72) AUC-0.854, <0.0001 | |||
| Atrial Fibrillation | 4.59 [1.84–13.22] | 1.52 | 0.0007 |
| Body Mass Index>30 kg/m2 | 2.90 [1.68–5.09] | 1.07 | 0.0001 |
| Age>60 years | 2.12 [1.12–3.82] | 0.75 | 0.01 |
| Treatment with 2 or more antihypertensives | 1.78 [1.04–3.02] | 0.58 | 0.03 |
| E/e′ ratio>9 | 1.87 [1.07–3.26] | 0.63 | 0.03 |
| Pulmonary Artery Systolic Pressure>35 mmHg | 1.74 [0.92–3.35] | 0.55 | 0.09 |
| Diabetes or prediabetes | 1.67 [0.97–2.87] | 0.51 | 0.06 |
| Left atrial volume index>30 ml/m2 | 1.59 [0.88–2.88] | 0.47 | 0.1 |
| Chronic kidney disease stage 3 or greater | 1.46 [0.66–3.30] | 0.37 | 0.4 |
| NT proBNP>275 pg/ml | 1.26 [0.66–2.41] | 0.23 | 0.5 |
|
| |||
| H2FPEF score | |||
| (AICc=393.36), AUC-0.841, <0.0001 | |||
| Body Mass Index>30 kg/m2 | 3.10 [1.85–5.18] | 1.13 (Score2) | <0.0001 |
| Atrial Fibrillation | 5.78 [2.28–14.62] | 1.75 (Score3) | <0.0001 |
| Age>60 years | 2.83 [1.65–4.84] | 1.04 (Score1) | 0.0001 |
| 2 or more antihypertensives | 1.99 [1.18–3.33] | 0.69 (Score1) | 0.01 |
| E/e′>9 | 2.15 [1.27–3.67] | 0.77 (Score1) | 0.005 |
| Pulmonary Artery Systolic Pressure>35 mmHg | 2.05 [1.11–3.78] | 0.72 (score1) | 0.02 |
AICc-Akaike information criterion corrected; AUC-Area Under the Curve; OR-Odds ratio; CI-Confidence Interval; NT-proBNP–N terminal pro Brain Natriuretic Peptide; E/e′-ratio of early diastolic mitral inflow velocity to septal mitral annulus tissue relaxation velocity
Figure 1.
Description of the H2FPEF score and point allocations for each clinical characteristic (top box), with associated probability of having HFpEF based upon the total score as estimated from the model (lower box).
Figure 2.
Calibration of H2FPEF score. The Hosmer-Lemeshow goodness of fit test results using deciles of predicted probabilities were p=0.14, 0.53, and 0.18 for the derivation, validation and pooled overall sample, respectively, indicating support for a properly calibrated model.
The odds of having HFpEF increased by a factor of 2 for every 1 unit increase in the score [OR 1.98, 95% CI: 1.73 to 2.30]. The H2FPEF score provided strong discrimination of HFpEF from controls [AUC 0.841, 95% CI:0.802 – 0.881]. The H2FPEF score better discriminated HFpEF from non-cardiac causes of dyspnea compared to widely used diagnostic algorithms based upon expert consensus4, 5 (AUC comparison +0.169 [95% CI +0.120 to +0.217] vs 2016 ESC guidelines and +0.173 [95% CI +0.132 to +0.215] vs 2007 ESC guidelines, both p<0.0001, Supplemental Table 3). The use of NTproBNP levels did not incrementally add diagnostic ability to the H2FPEF score (Table 3).
Because the points-based score can result in loss of information due to dichotomization, we also evaluated the H2FPEF score on a continual scale (Supplemental Figure 2). This resulted in a slightly better performing model (AUC comparison +0.022 [+0.002 to +0.042], p=0.03; Supplemental Table 3). In contrast to the points-based H2FPEF model, the number of hypertension medicines did not remain predictive in the continuous model, so this variable was not included. Calibration remained robust using the continuous model with a goodness of fit p-values >0.1 (Supplemental Figure 3). Findings for the models were upheld in the bootstrap (internal) validation, with an optimism-corrected AUC of 0.838 for the categorical model and 0.857 for the continuous model.
Sensitivity Analyses
The agnostic CART model used a more nuanced diagnostic scheme as it allows for empirically determined thresholds and interactions based on patterns in the data. As a result, the CART was slightly more predictive than the logistic regression derived H2FPEF score, with an AUC of 0.8831, an increase of 0.044 (p=0.002) (Supplemental Figure 4, Supplemental Table 3). The agnostic logistic model based upon automated stepwise backward selection of all predictors also verified similar discrimination to the H2FPEF score (AUC 0.857) and included the same variables, except that RV Fractional Area Change supplanted pulmonary artery systolic pressure as being predictive in the final agnostic logistic model (Supplemental Table 4).
Sensitivity analyses applying the H2FPEF model restricted to local patients from the regional practice (AUC 0.841) or patients with early stage HFpEF (AUC 0.814) demonstrated similar performance as the overall cohort (Supplemental Figure 5).
Validation in the test cohort
The test cohort included 100 consecutive patients (61 HFpEF and 39 controls) whose baseline characteristics were similar to the derivation cohort (Supplemental Table 5). Performance of the points-based H2FPEF score [AUC 0.886] and continuous variable based score [AUC 0.910] remained robust in this cohort (Supplemental table 3).
Discussion
Heart failure with preserved ejection fraction accounts for half of HF hospitalizations, and in hospitalized patients, overt congestion is typically obvious from physical examination, chest radiography and natriuretic peptide assays.1 However, in outpatients with exertional dyspnea, overt congestion is often absent at rest and the diagnosis may be challenging.7, 8 Right heart catheterization, with exercise if resting filling pressures are normal, is the gold standard for HFpEF diagnosis, but is not universally available, and non-invasive estimates of cardiac filling pressures lack sensitivity.1–8 In this study we derived and then validated a new score using clinical and echocardiographic variables that are widely available in clinical practice. In the derivation and test cohorts, and in sensitivity analyses restricted to community-based patients and those with early stage HFpEF, the H2FPEF score effectively discriminated patients with HFpEF from a comparator population of patients with exertional dyspnea that was not caused by heart failure, ascertained using the gold standard of invasive hemodynamic exercise testing. Inclusion of this control group was crucial to our study design, since it would not have otherwise been possible to judge the ability of clinical characteristics to estimate the likelihood of HFpEF without the ability to definitively identify or exclude disease based upon invasive criteria.
Diagnostic algorithms for HFpEF used in practice and for entry to clinical trials are based upon expert consensus opinion.4, 5 When these criteria have been prospectively evaluated, specificity was robust but sensitivity was poor.7 As such, HFpEF remains underdiagnosed in the community. In recent years, there has been increased utilization of invasive cardiopulmonary exercise testing to evaluate patients with exertional dyspnea, which is the gold standard to establish or refute the diagnosis of HFpEF.7–10 While this definitive approach has been shown to be cost-effective and safe,19 its uniform application is not practical for all diagnostic evaluations, given the enormous number of patients in the community presenting with exertional dyspnea.
By establishing the probability of disease, the H2FPEF score may be used to effectively rule out disease among patients with low scores (e.g. 0 or 1), establish the diagnosis with reasonably high confidence at higher scores (e.g. 6–9), and identify patients where additional testing is needed with intermediate scores (e.g. 2–5). Rather than forcing a probabilistic diagnosis (HFpEF) into binary categories (present or absent), this Bayesian approach provides a framework that can be used to determine whether there is sufficient confidence in the working diagnosis, or whether further evaluation is necessary based upon the identified probability of disease. This system could be readily applied for diagnostic purposes in clinical care as well as research settings to help refine enrollment criteria for clinical trials. Although the categorical H2HPEF score is easily calculated even at the bedside to rapidly estimate low or high probability of HFpEF, the more complex continual HFPEF calculator (Online Supplement) can also be used to provide a more precise estimate of the probability of HFpEF in an individual when required for clinical use, screening or research settings.
Selection of Final Model
In this analysis, we examined complementary modeling strategies that strove to balance parsimony, ease of calculation, and discriminatory capabilities. While we also considered more complex machine learning approaches, we finalized our models using multiple logistic regression analysis and the agnostic classification and regression tree analysis (CART). Many of the candidate variables for the models were highly collinear, so multiple sets of variables were often found to be equally discriminatory. The final model reflected a combination of variables selected a priori because of their central role in HFpEF pathogenesis (such as obesity and atrial fibrillation), as well as stepwise multivariable regression with systematic backwards elimination to only include variables that were independently predictive of HFpEF in combination. This yielded the components of our final H2FPEF score.
Sensitivity analyses using purely agnostic methods including an unbiased logistic model yielded nearly identical results, apart from the inclusion of right ventricular fractional area change in place of pulmonary artery systolic pressure (Supplemental Table 4). Because right ventricular fractional area change (a measure of right ventricular function) varies inversely with pulmonary artery pressure,20 it is not surprising that both measures can discriminate HFpEF from non-cardiac dyspnea. Since estimated pulmonary artery systolic pressure is a well-established marker of HFpEF21 and is more commonly measured in practice, we chose to include this in the final model rather than right ventricular fractional area change, which is not part of the routine clinical echocardiogram in many centers.
The lack of a particular variable in the final model (such as NT-proBNP) should not be interpreted as revealing a lack of association with HFpEF. Rather, what our data does suggest is that NT-proBNP may not add incremental information to clinical variables and echocardiography in diagnosing HFpEF among patients with unexplained dyspnea. This is in contrast to patients presenting with acute dyspnea that is present at rest, where the diagnostic performance of the natriuretic peptides are well established.22, 23 While discrimination of cases and controls was slightly improved using the classification and regression tree model and the continuous HFPEF score model, the differences were minor, and we propose that the simplicity of the H2FPEF score system outweighs this difference because it improves the feasibility of applying this approach in everyday practice. However, if precise estimation of an individual patient’s probability of underlying HFpEF is to be calculated, the use of the more complex continuous variable version of the HFPEF score from our online calculator can be applied.
Association of comorbidities with HFpEF
HFpEF is currently believed to be a systemic disorder driven in large part by comorbidities.2, 3 We observed that two comorbidities, obesity and atrial fibrillation, independently increase the probability that HFpEF is present. Severe hypertension identified by treatment with 2 or more antihypertensive drugs was another independent predictor. Diabetes is common in HFpEF, seen in 30–40%,24 but the presence of abnormal glucose tolerance did not add incremental diagnostic value beyond obesity alone, supporting the emerging evidence of the importance of obesity as an important cause of HFpEF.14
Limitations
NTproBNP data was missing at random in 24% of patients, due to the fact that some cardiologists did not obtain this laboratory during their evaluation. Therefore imputation was performed to account for the missing data, which may have affected the inclusion of NTproBNP in the final model. However, a sensitivity analysis yielded similar results in the 76% of patients that did have directly measured NTproBNP, increasing our confidence in the imputation derived values. This study was single center, limiting generalizability. There is referral bias in that all patients were referred for invasive testing, which may have inflated the prevalence of HFpEF. However, this analysis would not have been possible without the use of a gold standard assessment. Although this study was performed in a tertiary referral center, our practice also serves the local population and sensitivity analysis restricted to local patients revealed that the H2FPEF score performed similarly well in this subset (AUC 0.841), increasing confidence in generalizability of our results. While discrimination was maintained in our separate validation cohort, external validation was not performed and replication in other centers is necessary. Physical examination findings were not included in the models because there may be variability in examination skill and interpretation,25 and because overt congestion was absent in the patients included in this study, who were deemed to have indeterminate dyspnea after thorough evaluation by board certified cardiologists based upon history, physical examination and echocardiography. As such, the current results may not apply to patients with more frank evidence of tissue congestion, where testing beyond the history and physical examination may not be necessary to diagnose HFpEF. Assessment for lung disease was performed at the discretion of referring physicians and was not obtained in all patients. However, this reflects practice in the community, and the presence or absence of pulmonary disease is not relevant to the primary study goal of discriminating cardiac dyspnea (HFpEF) and non-cardiac dyspnea.
Conclusion
The H2FPEF score, which utilizes six clinical and echocardiographic characteristics that are universally obtained in the evaluation of patients with unexplained exertional dyspnea, enables robust discrimination of HFpEF from non-cardiac etiologies of dyspnea at low and high scores, while identifying patients at intermediate probability where additional testing is needed to refine diagnosis.
Supplementary Material
Clinical Perspectives.
1) What is new?
We show that using simple, universally-available clinical and echocardiographic characteristics the probability that HFpEF is present can be accurately estimated in the patient presenting with unexplained exertional dyspnea.
2) What are the clinical implications?
The H2FPEF score enables providers and patients to estimate the probability of underlying HFpEF.
This allows for more informed decision making about the likelihood of disease and thus the yield of additional testing to confirm or refute the diagnosis in a Bayesian approach.
Acknowledgments
Funding Sources
BAB is supported by R01 HL128526, R01 HL 126638, U01 HL125205 and U10 HL110262, from the NIH. YNR is supported by T32 HL007111 from the NIH. REC is supported by R01 HL128526 and UL1 TR02377 from the NIH. MO is supported by a research fellowship from the Uehara Memorial Foundation, Japan.
Footnotes
Disclosures
None.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2016;375:1868–1877. doi: 10.1056/NEJMcp1511175. [DOI] [PubMed] [Google Scholar]
- 3.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-specific treatment of heart failure with preserved ejection fraction: A multiorgan roadmap. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: A consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the heart failure and echocardiography associations of the european society of cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 5.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 esc guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the european society of cardiology (esc) developed with the special contribution of the heart failure association (hfa) of the esc. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 accf/aha guideline for the management of heart failure: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 7.Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: A simultaneous invasive-echocardiographic study. Circulation. 2017;135:825–838. doi: 10.1161/CIRCULATIONAHA.116.024822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maron BA, Cockrill BA, Waxman AB, Systrom DM. The invasive cardiopulmonary exercise test. Circulation. 2013;127:1157–1164. doi: 10.1161/CIRCULATIONAHA.112.104463. [DOI] [PubMed] [Google Scholar]
- 10.Givertz MM, Fang JC, Sorajja P, Dimas V, Forfia PR, Kapur NK, Kern MJ, Naidu SS, Borlaug BA. Executive summary of the scai/hfsa clinical expert consensus document on the use of invasive hemodynamics for the diagnosis and management of cardiovascular disease. J Card Fail. 2017;23:487–491. doi: 10.1016/j.cardfail.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the american society of echocardiography and the european association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 12.Stekhoven DJ, Buhlmann P. Missforest--non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28:112–118. doi: 10.1093/bioinformatics/btr597. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan LM, Massaro JM, D’Agostino RB., Sr Presentation of multivariate data for clinical use: The framingham study risk score functions. Stat Med. 2004;23:1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 14.Obokata M, Reddy YN, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. doi: 10.1161/CIRCULATIONAHA.116.026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitzman DW, Lam CSP. Obese heart failure with preserved ejection fraction phenotype: From pariah to central player. Circulation. 2017;136:20–23. doi: 10.1161/CIRCULATIONAHA.117.028365. [DOI] [PubMed] [Google Scholar]
- 16.Reddy YNV, Obokata M, Gersh BJ, Borlaug BA. High prevalence of occult heart failure with preserved ejection fraction among patients with atrial fibrillation and dyspnea. Circulation. 2018;137:534–535. doi: 10.1161/CIRCULATIONAHA.117.030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam CS, Rienstra M, Tay WT, Liu LC, Hummel YM, van der Meer P, de Boer RA, Van Gelder IC, van Veldhuisen DJ, Voors AA, Hoendermis ES. Atrial fibrillation in heart failure with preserved ejection fraction: Association with exercise capacity, left ventricular filling pressures, natriuretic peptides, and left atrial volume. JACC Heart Fail. 2017;5:92–98. doi: 10.1016/j.jchf.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 19.Huang W, Resch S, Oliveira RK, Cockrill BA, Systrom DM, Waxman AB. Invasive cardiopulmonary exercise testing in the evaluation of unexplained dyspnea: Insights from a multidisciplinary dyspnea center. Eur J Prev Cardiol. 2017;24:1190–1199. doi: 10.1177/2047487317709605. [DOI] [PubMed] [Google Scholar]
- 20.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–3462. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: A community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinhart B, Thorpe KE, Bayoumi AM, Moe G, Januzzi JL, Jr, Mazer CD. Improving the diagnosis of acute heart failure using a validated prediction model. J Am Coll Cardiol. 2009;54:1515–1521. doi: 10.1016/j.jacc.2009.05.065. [DOI] [PubMed] [Google Scholar]
- 23.Januzzi JL, Jr, Chen-Tournoux AA, Christenson RH, Doros G, Hollander JE, Levy PD, Nagurney JT, Nowak RM, Pang PS, Patel D, Peacock WF, Rivers EJ, Walters EL, Gaggin HK. N-terminal pro-b-type natriuretic peptide in the emergency department: The icon-reloaded study. J Am Coll Cardiol. 2018;71:1191–1200. doi: 10.1016/j.jacc.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Lindman BR, Davila-Roman VG, Mann DL, McNulty S, Semigran MJ, Lewis GD, de las Fuentes L, Joseph SM, Vader J, Hernandez AF, Redfield MM. Cardiovascular phenotype in hfpef patients with or without diabetes: A relax trial ancillary study. J Am Coll Cardiol. 2014;64:541–549. doi: 10.1016/j.jacc.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.From AM, Lam CS, Pitta SR, Kumar PV, Balbissi KA, Booker JD, Singh IM, Sorajja P, Reeder GS, Borlaug BA. Bedside assessment of cardiac hemodynamics: The impact of noninvasive testing and examiner experience. Am J Med. 2011;124:1051–1057. doi: 10.1016/j.amjmed.2011.05.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.