Summary
Elbasvir/grazoprevir (EBR/GZR) is an all-oral direct-acting antiviral agent (DAA) with high sustained virologic response (SVR) in clinical trials. This study’s primary objective was to evaluate effectiveness of EBR/GZR among HCV-infected patients in a real-world clinical setting. We conducted a nationwide retrospective observational cohort study of HCV-infected patients in the US Department of Veterans Affairs (VA) using the VA Corporate Data Warehouse. The study population included patients with positive HCV RNA who initiated EBR/GZR from February 1 to August 1, 2016. We calculated the 95% confidence interval for binomial proportions for SVR overall and by demographic subgroups. Clinical and demographic characteristics were also evaluated. We included 2,436 patients in the study cohort. Most were male (96.5%), African-American (57.5%), with mean age of 63.5 (SD=5.9), and 95.4% infected with genotype (GT) 1 [GT1a (34.7%), GT1b (58.6%)]. Other comorbidities included diabetes (53.2%), depression (57.2%), and HIV (3.0%). More than 50% had history of drug or alcohol abuse (53.9% and 60.5%, respectively). 31.4% of the cohort had cirrhosis. A total of 95.6% (2,328/2,436; 95% CI: 94.7%−96.4%) achieved SVR. The SVR rates by subgroups were: male, 95.5% (2245/2350); female, 96.5% (83/86); GT1a, 93.4%, GT1b, 96.6%, GT4, 96.9%, African-American, 95.9% (1,342/1,400); treatment-experienced, 96.3% (310/322); cirrhosis, 95.6% (732/766); stage 4–5 CKD, 96.3% (392/407); and HIV, 98.6% (73/74). SVR rates were high overall and across patient subgroups regardless of gender, race/ethnicity, cirrhosis, renal impairment, or HIV. This study provided important data regarding the effectiveness of EBR/GZR in a large clinical setting.
Keywords: Direct-acting antiviral agents, hepatitis C virus, Veterans Affairs, treatment effectiveness
INTRODUCTION
Chronic hepatitis C virus (HCV) infection affects 1.3% of the U.S. population 1, 2 and is the leading cause of cirrhosis, hepatocellular cancer, and death from liver disease in the U.S. 3. The new all-oral direct-acting antivirals (DAAs) result in SVR in an unprecedented >90% of patients in clinical trials 4–13. In early 2016, the US Food and Drug Administration (FDA) approved the fixed dose combination of elbasvir/grazoprevir (EBR/GZR) for the treatment of chronic HCV genotypes (GT) 1 and 4 infections. EBR/GZR demonstrated high sustained virologic response (SVR) overall as well as in special populations of patients including those with advanced chronic kidney disease (CKD), persons who inject drugs, and those with inherited blood disorders with low adverse-event rates 13–15.
Randomized controlled trials of EBR/GZR reported high efficacy for the treatment-naïve and treatment-experienced patients including special populations. In integrated analyses of EBR/GZR trials among GT 1 patients with and without cirrhosis, SVR rates were greater than 95% in all patient subgroups 16, 17. Among patients infected with HCV GT 1 and stage 4–5 CKD, the phase 3 clinical trial of EBR/GZR showed that the SVR rates were 99% with low adverse-event rates 18. However, the chasm between efficacy (in clinical trials) and effectiveness (in clinical practice) persists in the DAA era. The few data that are available from real-world experience suggest that SVR rates with all-oral DAAs may be lower than those reported in clinical trials (~75–80% vs. >90%, respectively) 19–22. In contrast, other data suggest that the effectiveness of DAAs may be comparable to efficacy observed in clinical trials 23, 24. Previous real-world studies and clinical trials have also reported factors associated with lower response rates including non-adherence, African American race 4, 25, 26, having cirrhosis 22, 24, 26, and male gender 22.
To our knowledge there have been no published reports examining the effectiveness of EBR/GZR regimens in real-world clinical practice. Therefore, the objective of the study was to evaluate effectiveness among HCV-infected patients treated with EBR/GZR in a real-world clinical setting in the US using data from the US Department of Veterans Affairs (VA) healthcare system.
MATERIALS AND METHODS
This was a retrospective analysis of administrative and clinical data using the VA Corporate Data Warehouse (CDW) which is a national data repository of VA electronic medical records including those of almost 9 million veterans. The CDW includes data from pharmacy, laboratory, inpatient and outpatient encounters by ICD-9/10 diagnosis codes, as well as vital status from October 1, 1999 through present day. The VA is the largest single provider of HCV care in the US due to the fact that veterans are 3 times more likely to be infected with HCV 27, 28.
Study populations and definitions
The study cohort included subjects who initiated EBR/GZR treatment after the FDA approval beginning on February 1, 2016 to August 1, 2016 from 128 VA Medical Centers nationwide. To allow for adequate follow-up time to determine SVR rates, SVR outcomes were captured through February 15, 2017.
We included subjects who initiated EBR/GZR regimens and were at least 18 years of age. Patients were required to have at least one positive HCV RNA test to confirm chronic HCV and had at least one inpatient or outpatient visit within a one-year period prior to treatment initiation. We excluded patients with undetermined regimen such as having ribavirin (RBV) added ≥30 days after treatment initiation or those treated with EBR/GZR for greater than seventeen weeks because these regimens were not consistent with those approved by the FDA. The index date was defined as the first EBR/GZR initiation date during the study period.
The primary study was conducted among the evaluable population (EP), which included all patients who had HCV RNA test data available at least 4 weeks after the end of treatment. The EP cohort included patients who did not complete treatment regimen. As a sensitivity analysis, we examined the per protocol (PP) population, which included all the patients in the EP who completed treatment regimen of EBR/GZR. Treatment completion was defined as those who were prescribed EBR/GZR for at least 11 weeks (77 days) of treatment. The EBR/GZR regimens consisted of EBR/GZR without RBV, EBR/GZR with RBV, and EBR/GZR + sofosbuvir (SOF) +/− RBV.
Outcomes measures and demographic and clinical variables
SVR was defined as HCV RNA below the limit of quantification performed at least 12 weeks after the end of treatment. If HCV RNA data at ≥12 weeks were not available, SVR was defined based on HCV RNA testing available from week 4 to 12 weeks after the end of treatment to account for variability of clinic visits and availability of laboratory data in clinical practice. This definition of SVR has been used in other similar database studies 25, 29 and SVR4 has been shown to be highly concordant with SVR12 30. The end of treatment was calculated from the last day covered by the medication dispensed by totaling the number of days supply. Treatment duration was calculated until there was a gap of at least 45 days without EBR/GZR. HCV patients were considered to have completed 12 weeks and 16 weeks of EBR/GZR regimens if they had received between 77–91 days and 105–119 days of medication, respectively.
Demographic variables included age, gender, race/ethnicity (White, African American, Hispanic, Asian, others). HCV treatment experience was defined as previous exposure to any interferon-based regimen (Peg-IFN) with RBV, first generation (i.e. boceprevir: BOC, telaprevir: TEL), and all-oral DAAs regimens (i.e. simeprevir: SIM, sofosbuvir: SOF, ledipasvir/sofosbuvir: LDV/SOF, ombitasvir/paritaprevir/ritonavir/dasabuvir: PrOD) prior to EBR/GZR treatment any time after October 1, 1999. If patients received more than one type of previous treatment, they were classified based on the most recent type received prior to EBR/GZR. HCV GT was also determined by using laboratory results for GT tests any time prior to treatment index date. High baseline viral load was defined as those with HCV RNA greater than 6M IU/ml.
Comorbidities such as cirrhosis, diabetes, hypertension, depression, anxiety, history of alcohol and/or drug abuse, and HIV were identified by the presence of at least one ICD-9 or 10 diagnosis codes any time prior to the index date, many of which have been used in previously published studies 31, 32. Decompensated cirrhosis (end-stage liver disease) was defined as the presence of inpatient or outpatient claims for ascites, variceal bleeding, hepatic encephalopathy, and hepatorenal syndrome based on inpatient or outpatient ICD-9/10 codes. Hepatocellular carcinoma was defined from its diagnosis codes at any time prior to index date. Kidney and liver transplant were defined by presence of CPT or ICD procedure codes any time prior to index date. The Deyo modification of the Charlson comorbidity index was also calculated to determine comorbidity status in the 1 year prior to index date 33.
We used estimated glomerular filtration rate (eGFR) to categorize patients by severity of CKD if they had at least two eGFR values ≥3 months apart within 2 years prior to the index date as follows: eGFR >60, Stage 3 CKD (eGFR of 30–59), and Stages 4–5 CKD (eGFR <30). We ascertained eGFR from automated reporting of VA laboratory tests that used the Modification of Diet in Renal Disease (MDRD) equation 34. If the two tests classified a patient in discrepant stages, the patient was classified using the most recent stage. Stage of CKD was assigned based on the criteria published by the National Kidney Foundation 35.
Data analysis
Descriptive statistics were used to describe demographic and clinical characteristics such as HCV GT, baseline HCV viral load, HIV co-infection, alcohol or substance use, depression, and comorbid physical health conditions by treatment regimen type. Mean or median values were compared using chi-squared test, Fisher Exact test, or the Wilcoxon signed-rank test for continuous variables, as appropriate. We conducted primary data analysis in the EP cohort. The SVR rates were calculated overall and by demographic and clinical variables defined above. We calculated the 95% confidence interval for binomial proportions for SVR overall and by demographic and clinical subgroups. SVR rates by treatment regimen and treatment experience were also analyzed. We further stratified by GT 1 and GT 4 and calculated SVR by prior treatment status, treatment regimen, and duration while excluding patients with history of decompensated cirrhosis or who had prior use of all-oral DAAs. We calculated 95% confidence intervals (CIs) for SVR rates using the binomial distribution. Overall SVR was calculated in the PP cohort as a sensitivity analysis. We used SAS® (version 9.4, SAS Institute Inc., Cary, NC, USA) for analyses. The study was approved by the VA Central Institutional Review Board.
RESULTS
During the study period, we identified 2,985 chronic HCV-infected patients who initiated EBR/GZR regimens. After applying study inclusion and exclusion criteria, 2,436 patients were included in the EP cohort as a primary study population. When excluding those who did not complete treatment regimen (7.3%, n=179), the PP cohort included 2,257 patients (Figure 1).
Fig. 1.
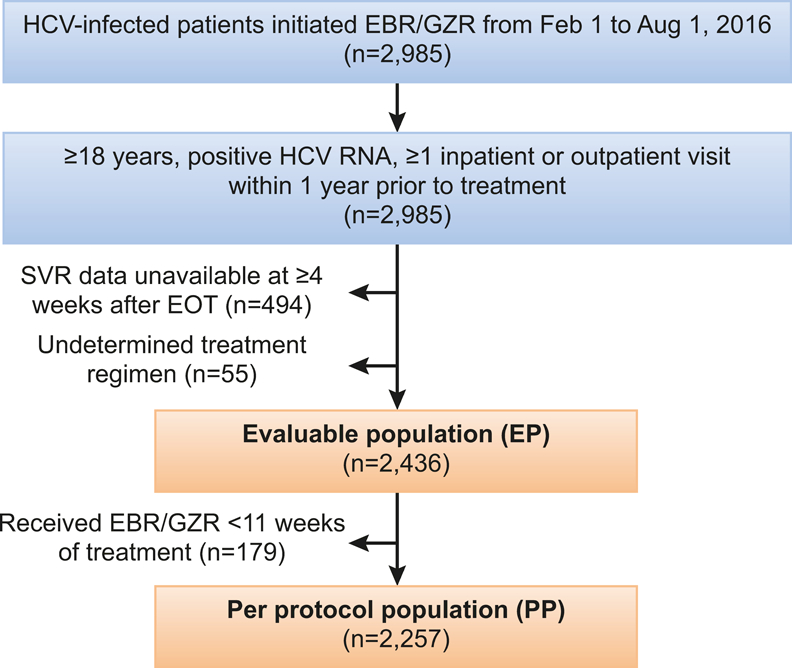
Study cohort diagram
Cohort characteristics overall and by treatment regimen
Baseline demographic and clinical characteristics of EP by EBR/GZR treatment regimen are shown in Table 1. Of 2,436 patients in the evaluable population, 2,246 (92.2%) were prescribed EBR/GZR without RBV, followed by EBR/GZR+RBV (6.4%, n = 156) and EBR/GZR +SOFs+/−RBV (1.4%, n = 34). The mean age was 63.5 years old (SD = 5.9); and the majority were African American (57.5%) and male (96.5%). There were 2,324 patients (95.4%) infected with GT 1 [GT1a (34.7%, n = 844), GT 1b (58.6%, n = 1428)]. The prevalence of co-morbidities was as follows: diabetes (53.2%), depression (57.2%), CKD stage 3–5 (32.8%), and HIV co-infection (3%). A third (31.4%) of the cohort had a diagnosis of cirrhosis; 13.6% overall had decompensated cirrhosis. Additionally, more than half of the patients had a history of drug (53.9%) or alcohol (60.5%) abuse. The population included 448 treatment-experienced patients (18.4%). We observed that EBR/GZR were given to some patient populations that are not indicated by the US FDA labelling such as the 117 patients (4.8%) who had prior NS5A-exposure of LDV/SOF or PrOD and the 332 patients (13.6%) who had decompensated cirrhosis. We also observed 4 GT 3 patients and 2 GT 2 patients.
Table 1.
Baseline and clinical characteristics among chronic HCV-infected patients treated with EBR/GZR in the evaluable population
| Characteristics | EBR/GZR N=2246 | EBR/GZR+ RBV N=156 |
EBR/GZR+ SOF+/−RBV n = 34 |
All patients N=2436 |
P-value+ |
|---|---|---|---|---|---|
| Age, mean (S.D.) | 63.7 (5.9) | 61.9 (6.6) | 63.6 (5.5) | 63.5 (5.9) | 0.003 |
| Race/ethnicity, N (%) | |||||
| White (non-Hispanic) | 726 (32.3) | 80 (51.3) | 18 (53.0) | 824 (33.8) | N/A* |
| African American | 1321 (58.8) | 65 (41.7) | 14 (41.2) | 1400 (57.5) | |
| Hispanic | 77 (3.4) | 3 (1.9) | 1 (2.9) | 81 (3.3) | |
| Others | 33 (1.5) | 1 (0.6) | 1 (2.9) | 35 (1.4) | |
| Missing | 89 (4.0) | 7 (4.5) | 0 (0) | 96 (4.0) | |
| Male, N (%) | 2169 (96.6) | 148 (94.9) | 33 (97.1) | 2350 (96.5) | 0.529 |
| Genotype, N (%) | |||||
| GT1a | 699 (31.1) | 122 (78.2) | 23 (67.6) | 844 (34.7) | N/A* |
| GT1b | 1399 (62.3) | 25 (16.0) | 4 (11.8) | 1428 (58.6) | |
| GT1 unknown subtype | 42 (1.9) | 5 (3.2) | 5 (14.7) | 52 (2.1) | |
| GT2 | 2 (0.1) | 0 (0) | 0 (0) | 2 (0.1) | |
| GT3 | 1 (0.04) | 1 (0.6) | 2 (5.9) | 4 (0.2) | |
| GT4 | 62 (2.7) | 2 (1.3) | 0 (0) | 64 (2.6) | |
| Missing GT | 41 (1.8) | 1 (0.7) | 0 (0) | 42 (1.7) | |
| Treatment-naïve, N (%) | 1853 (82.5) | 133 (85.3) | 2 (5.9) | 1988 (81.6) | <0.001 |
| Treatment-experienced, N (%) | 393 (17.5) | 23 (14.7) | 33 (94.1) | 448 (18.4) | |
| Prior interferon | 314 (14.0) | 1 (0.6) | 1 (2.9) | 316 (13.0) | |
| Prior BOC/TEL | 6 (0.3) | 0 (0) | 0 (0) | 6 (0.2) | |
| Prior SIM+SOF, SOF | 5(0.2) | 4 (2.6) | 0 (0) | 9 (0.4) | |
| Prior LVD/SOF and PrOD | 68 (3.0) | 18 (11.5) | 31 (91.2) | 117 (4.8) | |
| Deyo Charlson Comorbidity Index - mean (S.D.) | 2.3 (2.6) | 1.9 (2.2) | 1.2 (1.3) | 2.3 (2.5) | 0.042 |
| Anxiety, N (%) | 640 (28.5) | 47 (30.1) | 11 (32.4) | 698 (28.7) | 0.810 |
| Cirrhosis, N (%) | 699 (31.1) | 47 (30.1) | 20 (58.8) | 766 (31.4) | 0.002 |
| Compensated cirrhosis, N (%) | 400 (17.8) | 23 (14.7) | 11 (32.4) | 434 (17.8) | 0.052 |
| Decompensated cirrhosis, N (%) | 299 (13.3) | 24 (15.4) | 9 (26.5) | 332 (13.6) | 0.069 |
| Ascites, N (%) | 83 (3.7) | 10 (6.4) | 2 (5.9) | 95 (3.9) | 0.199 |
| Hepatic encephalopathy, N (%) | 129 (5.7) | 6 (3.9) | 1 (2.9) | 136 (5.6) | 0.484 |
| Depression, N (%) | 1279 (57.0) | 92 (59.0) | 23 (67.7) | 1394 (57.2) | 0.412 |
| Hepatocellular carcinoma, N,(%) | 45 (2.0) | 4 (2.6) | 3 (8.8) | 52 (2.1) | 0.039 |
| Diabetes, N (%) | 1208 (53.8) | 73 (46.8) | 14 (41.2) | 1295 (53.2) | 0.088 |
| History of drug abuse, N (%) | 1202 (53.5) | 91 (58.3) | 20 (58.8) | 1313 (53.9) | 0.428 |
| History of alcohol abuse, N (%) | 1361 (60.6) | 93 (59.6) | 19 (55.9) | 1473 (60.5) | 0.834 |
| HIV, N (%) | 68 (3.0) | 6 (3.9) | 0 (0) | 74 (3.0) | 0.566 |
| History of kidney transplant, N (%) | 33 (1.5) | 1 (0.6) | 1 (3.0) | 35 (1.4) | 0.478 |
| History of liver transplant, N (%) | 13 (0.6) | 1 (0.6) | 0 (0) | 14 (0.6) | 0.680 |
| eGFR, Mean (S.D.) | 68.9 (35.8) | 75.6 (36.9) | 96.9 (25.8) | 69.7 (35.9) | <0.001 |
| CKD stage 3–5, N (%) | 759 (33.8) | 37 (23.7) | 4 (11.8) | 800 (32.8) | 0.014 |
| Baseline HCV RNA ≥6M IU/ml*, N (%) (missing n = 110) | 373 (16.6) | 23 (14.7) | 4 (11.8) | 400 (16.4) | 0.522 |
Fisher’s exact test calculated for variables with sample size less than 5.
Insufficient memory space to process Fisher exact test.
Comparing baseline characteristics across regimens, patients receiving EBR/GZR+RBV were slightly younger than patients receiving other regimen types. Patients receiving EBR/GZR alone were more likely to be Black (58.8% vs. 41.7% and 41.2% for EBR/GZR+RBV and EBR/GZR+SOF+/−RBV, respectively) and infected with GT1b (62.3% vs. 16% and 11.8% for EBR/GZR+RBV and EBR/GZR+SOF+/−RBV, respectively) than other regimens. There were 91.2% (31/35) of patients treated with EBR/GZR+SOF+/−RBV who had prior LDV/SOF or PrOD treatment compared with only 11.5% for EBR/GZR+RBV and 3% for EBR/GZR. Comorbidities were similar across regimen types except for cirrhosis, which was higher among patients who received EBR/GZR+SOF+/−RBV (58.8% any cirrhosis vs. 31.1% and 30.1% in EBR/GZR and EBR/GZR+RBV, respectively). EBR/GZR+SOF+/−RBV patients were also less likely to have CKD stage 3–5 than the other regimens (11.8% vs. 33.8% and 23.7% in EBR/GZR and EBR/GZR+RBV, respectively).
SVR rates overall and by subgroups
In the EP cohort (n=2,436), 95.6% (95%CI: 94.7%−96.4%) of veterans prescribed EBR/GZR regimens achieved SVR (Table 2). When including only patients who completed at least 11 weeks of treatment, the SVR rate in the PP cohort was 97.0% (2,190/2,257; 95%CI: 96.3%−97.7%).
Table 2.
SVR in patients treated with EBR/GZR+/− RBV (all genotypes) by baseline characteristics in the evaluable population (N=2,436)
| Characteristics | N | N | SVR (%) | Confidence Interval |
|---|---|---|---|---|
| All patients (SVR) | 2328 | 2436 | 95.6% | 94.7%−96.4% |
| Gender | ||||
| Male | 2245 | 2350 | 95.5% | 94.6%−96.3% |
| Female | 83 | 86 | 96.5% | 90.1%−99.3% |
| Age | ||||
| >65 years | 828 | 866 | 95.6% | 94.0%−96.9% |
| ≤65 years | 1500 | 1570 | 95.5% | 94.4%−96.5% |
| Cirrhosis | ||||
| No | 1556 | 1628 | 95.6% | 94.5%−96.5% |
| Yes | 732 | 766 | 95.6% | 93.9%−96.9% |
| Compensated | 420 | 434 | 96.8% | 94.6%−98.2% |
| Decompensated | 312 | 332 | 94.0% | 90.9%−96.3% |
| Race | ||||
| African American | 1342 | 1400 | 95.9% | 94.7%−96.8% |
| Hispanic | 77 | 81 | 95.1% | 87.8%−98.6% |
| White | 783 | 824 | 95.0% | 93.3%−96.4% |
| Other | 33 | 35 | 94.3% | 80.8%−99.3% |
| Missing race | 93 | 96 | 96.9% | 91.1%−99.4% |
| Genotype | ||||
| Genotype 1 (all) | 2218 | 2324 | 95.4% | 94.5%−96.3% |
| Genotype 1a | 788 | 844 | 93.4% | 91.5%−94.9% |
| Genotype 1b | 1379 | 1428 | 96.6% | 95.5%−97.5% |
| Genotype 2 or 3 | 6 | 6 | 100.0% | 54.1%−100% |
| Genotype 4 | 62 | 64 | 96.9% | 89.2%−99.6% |
| Unknown genotype | 42 | 42 | 100.0% | 91.6%−100% |
| Treatment history | ||||
| Treatment-naïve | 1910 | 1988 | 96.1% | 95.1%−96.9% |
| Treatment-experienced (IFN or BOC/TEL) | 310 | 322 | 96.3% | 93.6%−98.1% |
| Treatment-experienced (including all-oral DAAs) | 418 | 448 | 93.3% | 90.6%−95.4% |
| LDV/SOF | 68 | 82 | 82.9% | 73.0%−90.3% |
| PrOD | 31 | 35 | 88.6% | 73.3%−96.8% |
| SIM+SOF, SOF+RBV | 9 | 9 | 100.0% | 66.3%−100% |
| BOV/TEL | 5 | 6 | 83.3% | 35.9%−99.6% |
| IFN+/−RBV | 305 | 316 | 96.5% | 93.9%−98.2% |
| CKD* | ||||
| eGRF ≥60ml | 1533 | 1611 | 95.2% | 94.0%−96.2% |
| Stage 3 CKD | 380 | 393 | 96.7% | 94.4%−98.2% |
| Stage 4–5 CKD | 392 | 407 | 96.3% | 94.0%−97.9% |
| HIV | ||||
| Positive | 73 | 74 | 98.6% | 92.7%−99.9% |
| Negative | 2255 | 2362 | 95.5% | 94.6%−96.3% |
| History of drug abuse | ||||
| Yes | 1251 | 1313 | 95.3% | 94.0%−96.4% |
| No | 1077 | 1123 | 95.9% | 94.6%−97.0% |
| History of alcohol abuse | ||||
| Yes | 1412 | 1473 | 95.9% | 94.7%−96.8% |
| No | 916 | 963 | 95.1% | 93.6%−96.4% |
| Baseline HCV RNA | ||||
| ≥6M IU/ml | 370 | 400 | 92.5% | 89.5%−94.9% |
| <6M IU/ml | 1853 | 1926 | 96.2% | 95.3%−97.0% |
Patients without 2 or more eGFR values are not included in this subgroup.
Table 2 showed that SVR rates by baseline characteristics ranged from 93% to 97%. SVR by these subgroups were reported as follows: male, 95.5% (2,245/2,350); female, 96.5% (83/86); African American, 95.9% (1,342/1,400); Hispanic, 95.1% (77/81); White, 95.0% (783/824); previously untreated, 96.1% (1,910/1,988); cirrhosis, 95.5% (772/808); without cirrhosis, 95.6% (1556/1628); stage 3 CKD, 96.7% (380/393); stage 4–5 CKD, 96.3% (392/407); HIV positive, 98.6% (73/74); HIV negative, 95.5% (2,255/2,362); history of alcohol abuse, 95.9% (1,412/1,473); no history of alcohol abuse, 95.1% (916/963); history of drug abuse, 95.3% (1,251/1,313); and no history of drug abuse, 95.9% (1,077/1,123). The SVR rate for treatment-experienced patients was 93.3% (418/448); of those, 117 patients (26.1%) had prior NS5A exposure of LDV/SOF or PrOD with SVR rates of 82.9% and 88.6%, respectively.
SVR in the 34 patients treated with EBR/GZR +SOFs+/−RBV was 97.1% (33/34) (data not shown). The one treatment failure was a >65 year old black male, with GT1a, treated with EBR/GZR+SOF without RBV who did not complete the treatment course (i.e. had less than 11 weeks of treatment). He was previously treated by LDV/SOF with baseline HCV RNA ≥ 6 million copies, and had HIV infection, stage 4/5 CKD, and prior exposure of drug abuse.
SVR rates among GT1- and GT4 HCV-infected patients by treatment regimen
In the subgroup analysis that excluded patients with prior treatment experience with all-oral DAAs or those who had decompensated cirrhosis which were not indicated in the US FDA labeling for EBR/GZR, the majority of patients were treated with EBR/GZR without RBV (94% in GT1 and 96.6% in GT4) (Table 3). Overall SVR (EP) rates among GT1 patients were 96.2% (1842/1915); . SVR was 96.4% (1737/1801) for those treated with EBR/GZR without RBV; 92% (104/113) for EBR/GZR+RBV; and 100% (1/1) for EBR/GZR+SOF+/−RBV. Of those who had GT1a (N = 647), 546 patients (84.4%) received 12 weeks of treatment, 49 patients (7.6%) received 16 weeks of treatment, and 52 (8%) patients did not receive at least 11 weeks of treatment. SVR rates of EBR/GZR-based regimens for 12 weeks was 97% (531/546) and 90% (44/49) among those treated for 16 weeks. Specifically, SVR for EBR/GZR without RBV for 12 weeks was 98% (471/481) and SVR for EBR/GZR+RBV for 16 weeks was 83.3% (20/24).
Table 3.
SVR by treatment regimen and prior HCV treatment among GT1- and GT4 HCV-infected patients in the evaluable population
| Treatment-naïve | Treatment-experienced (Peg-IFN+RBV, BOC, TEL) |
Total | |||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Genotype 1 (n=1915) | |||||||
| Overall | 1605/1668 | 96.2 | 237/247 | 96.0 | 1842/1915 | 96.2 | |
| By regimen | |||||||
| EBR/GZR | 1501/1555 | 96.5 | 236/246 | 95.9 | 1737/1801 | 96.4 | |
| EBR/GZR+RBV | 104/113 | 92.0 | -- | -- | 104/113 | 92.0 | |
| EBR/GZR+SOF+/−RBV | -- | -- | 1/1 | 100 | 1/1 | 100 | |
| By treatment regimen | |||||||
| EBR/GZR 12 wk | 1387/1416 | 98.0 | 208/213 | 97.7 | 1595/1629 | 97.9 | |
| EBR/GZR 16 wk | 28/28 | 100 | 16/18 | 88.9 | 44/46 | 95.7 | |
| EBR/GZR+RBV 12 wk | 74/79 | 93.7 | -- | -- | 74/79 | 93.7 | |
| EBR/GZR+RBV 16 wk | 27/31 | 87.1 | -- | -- | 27/31 | 87.1 | |
| EBR/GZR+SOF+/−RBV 12 wk | -- | -- | 1/1 | 100 | 1/1 | 100 | |
| Genotype 4 (n=59) | |||||||
| Overall | 53/54 | 98.2 | 5/5 | 100 | 58/59 | 98.3 | |
| By regimen | |||||||
| EBR/GZR | 51/52 | 98.1 | 5/5 | 100 | 56/57 | 98.2 | |
| EBR/GZR+RBV | 2/2 | 100 | -- | -- | 2/2 | 100 | |
| By treatment regimen | |||||||
| EBR/GZR 12 wk | 49/49 | 100 | 1/1 | 100 | 50/50 | 100 | |
| EBR/GZR 16 wk | -- | -- | 2/2 | 100 | 2/2 | 100 | |
| EBR/GZR+RBV 12 wk | -- | -- | -- | -- | |||
| EBR/GZR+RBV 16 wk | 2/2 | 100 | -- | -- | 2/2 | 100 | |
Note: excluding decompensated cirrhosis.
Of 1,668 GT1 treatment-naïve patients, majority (93%) were treated with EBR/GZR without RBV. SVR rates were 96.2% overall [EBR/GZR: 96.5% (1501/1555), EBR/GZR+RBV: 92% (104/113)]. In 247 GT1 treatment-experienced (i.e. Peg-IFN or BOC or TEL) patients, SVR was 95.9%. Finally, among 59 patients with GT4, 98.3% achieved SVR overall ranging from 98.2%−100% by regimen and treatment duration.
DISCUSSION
This study evaluated the real-world effectiveness of EBR/GZR in the first 6 months after approval in the large VA national health care system. Our study showed that EBR/GZR was highly effective, with an SVR of 95.6% overall and 97% in patients who completed a full course of treatment. SVR rates were high across patient subgroups regardless of gender, race/ethnicity, presence of cirrhosis, renal impairment, or HIV co-infection. To our knowledge, this is the largest real-world effectiveness study of EBR/GZR of 2,436 patients. Randomized controlled trials demonstrated high SVR rates for EBR/GZR ranging from 90% to100% 13–15. Compared to the integrated analysis of 6 clinical trials among patients with HCV GT 1, 4, and 6 infection 36, HCV patients in this study were older, had a higher prevalence of comorbidities, and had a higher percent of treatment experience including those with prior use of all-oral DAAs. Our findings show that the real-world effectiveness of EBR/GZR among HCV-infected GT1 or GT4 patients in the VA population is comparable to efficacy rates reported in clinical trials.
The effectiveness of EBR/GZR reported in this study is slightly higher than other previous VA studies of all-oral DAAs (not including EBR/GZR). Backus et al. reported an overall SVR of 88%−91% in 21,242 VA patients with GT 1 who received LDV/SOF or PrOD 26, and Fox et al. reported SVR rates of 83–92% for 11,464 VA patients on all-oral DAAs through July 1, 2015 22. Ioannou et al. evaluated 17,487 VA patients treated with SOF, LDV/SOF and PrOD regimens from January 2014 to June 2015. The SVR12 was 92.8% for those with HCV GT 1 and 89.6% for those with GT 4 29. The study population in this study was comparable to other studies in using the intent-to-treat definition that included those who discontinued treatment early. Compared to other VA studies, some differences in baseline characteristics were observed that could be important for the interpretation of SVR rates. First, the percentage of African Americans in this cohort was higher than in other VA studies (i.e. 59% in this study vs. 39%, Backus et al.; 34%, Fox et al.; and 29%, Ioannou et al.) 22, 26, 29. Second, this study cohort had higher percentages of patients with depression and diabetes, but lower percentages of patients with HIV-co infection compared with the above studies. Third, about 30–35% of patients with cirrhosis were observed among all these findings. While the other three studies reported negative impact on response rates in patients with cirrhosis, patients with cirrhosis in our study did not have an attributably lower SVR than patients without cirrhosis as the SVR rates were 95.6% in both groups.
Table 2 showed that SVR rates of EBR/GZR were 95% or higher across many patient subgroups including GTs, those with HIV-coinfection, cirrhosis, history of drug/alcohol abuse, and moderate to severe CKD. Although several studies of other DAAs demonstrated negative impact of high viral load, African American race, cirrhosis, decompensated liver disease, and those with concomitant proton pump inhibitors (PPI) upon treatment response among those treated with LDV/SOF in the VA 25, 26. In contrast, we observed slightly higher SVR rates among African Americans compared to the overall cohort. Our findings reported a slightly lower SVR rate of 92.5% in patients with high viral load compared with SVR overall. In addition, high SVR was achieved in HCV-infected patients with CKD across all CKD stages. One-third of patients treated had CKD stage 3–5, which was higher than those treated with other all-oral DAAs observed in literature (i.e. 6.9%−27.3%) 37, 38. Our results suggest that HCV can be effectively treated among these highly vulnerable patients with CKD or in the high-risk population. Finally, although the US FDA labelling of EBR/GZR is not indicated for those with prior exposure to NS5A DAAs or those with decompensated cirrhosis, about 5% had prior use of NS5A and 14% had decompensated cirrhosis. Our finding reported slightly lower SVR rates than overall in patients with prior exposure to LDV/SOF or PrOD (i.e. 82.9%−88.6%) during the first six months after the approval.
Our study has several strengths. First, we used data from the nationwide VA database, which is the largest integrated healthcare data source for patients with HCV, including those who are racially and clinically diverse. Second, missing data is relatively low because the VA healthcare system has an established electronic medical record system as well as a robust pharmacy benefits management system including prescriptions data across all VA facilities. However, this study is subject to certain limitations. The VA population may not be generalizable to the entire U.S. population due to the potential for differing demographic risk factors. Misclassification bias may exist, as diagnoses and co-morbidities were identified through ICD-9/10 codes. Sample sizes were low for some subgroups (i.e. EBR/GZR+RBV for 16 weeks) and larger studies are needed to determine more robust results. Additionally, some laboratory data, including data on the presence of baseline NS5A resistance-associated substitutions (RAS), was not available at the time of this analysis. Adverse events data was also not available in this study. Future studies are needed to examine the impact of RAS and RBV on real-world effectiveness of EBR/GZR.
In conclusion, EBR/GZR regimens were highly effective, with an overall SVR of 96% and 97% in the EP and PP cohorts, respectively. SVR rates were high across patient subgroups regardless of gender, race/ethnicity, presence of cirrhosis, history of drug or alcohol abuse, renal impairment, or HIV co-infection. This studied provides important data regarding the effectiveness of EBR/GZR in a large clinical setting.
Acknowledgments
Michele McColgan of Merck & Co., Inc. provided editorial support.
Financial Support: The research reported here was supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development Service (VA IIR 13–059). Drs. Kramer, Erickson, El-Serag, and Kanwal are Research Health Scientists at the Center for Innovations in Quality, Effectiveness and Safety (#CIN 13–413), Michael E. DeBakey VA Medical Center, Houston, TX. This work is also partly funded by NIH grant T32 DK083266–01A1, NIH/National Institute of Diabetes and Digestive and Kidney Disease to Hashem El-Serag and Center Grant P30 DK56338. This study also was funded by Merck & Co., Inc., Kenilworth, NJ.
Abbreviations
- BOC
boceprevir
- CI
confidence interval
- CKD
chronic kidney disease
- DAA
direct-acting antiviral agent
- EBR/GZR
elbasvir/grazoprevir
- eGFR
estimated glomerular filtration rate
- EP
evaluable population
- FDA
Food and Drug Administration
- GT
genotype
- HCV
hepatitis C virus
- LDV
ledipasvir
- MDRD
Modification of Diet in Renal Disease
- Peg-IFN
pegylated interferon
- PP
per protocol
- PPI
proton pump inhibitor
- PrOD
ombitasvir/paritaprevir/ritonavir/dasabuvir
- RAS
resistance-associated substitution
- RBV
ribavirin
- SIM
simeprevir
- SOF
sofosbuvir
- SVR
sustained virologic response
- TEL
telaprevir
- VA
Veterans Affairs
- VA CDW
Veterans Affairs Corporate Data Warehouse
Footnotes
Conflicts of Interest: Dr. Kanwal received research grant funding to complete this work. Drs. Kramer, Kanwal, and El-Serag have received research funding from Merck & Co. and Gilead Sciences for studies outside this work.
Authors Contributions: Conceptualization of study and study design: Kramer, Kanwal, Puenpatom; Acquisition of data: Smith and Cao; Data construction and statistical analysis: Kramer, Cao, Puenpatom, Smith, Erickson; Interpretation of the data: Kramer, Kanwal, Puenpatom, Erickson, El-Serag; Drafting of the manuscript: Kramer, Kanwal, Puenpatom; Critical revision of manuscript: Kramer, Kanwal, Puenpatom, Erickson, Cao, Smith, El-Serag; Final manuscript approval: Kramer, Kanwal, Puenpatom, Erickson, Cao, Smith, El-Serag; Obtained funding: Kanwal, Kramer, Smith
Disclaimer: The opinions and assertions contained herein are the sole views of the authors and are not to be construed as official or as reflecting the views of the Department of Veterans Affairs or the United States.
Ethical Approval: This study was approved by the VA Central Institutional Review Board and the Michael E. DeBakey VA Medical Center Research & Development subcommittee.
References
- 1.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Annals of internal medicine. 2006;144(10):705–14. [DOI] [PubMed] [Google Scholar]
- 2.Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology (Baltimore, Md: ). 2015;62(5):1353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim WR, Brown RS Jr., Terrault NA, El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology (Baltimore, Md: ). 2002;36(1):227–42. [DOI] [PubMed] [Google Scholar]
- 4.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. The New England journal of medicine. 2014;370(20):1889–98. [DOI] [PubMed] [Google Scholar]
- 5.Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. The New England journal of medicine. 2014;370(17):1594–603. [DOI] [PubMed] [Google Scholar]
- 6.Ferenci P, Bernstein D, Lalezari J, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. The New England journal of medicine. 2014;370(21):1983–92. [DOI] [PubMed] [Google Scholar]
- 7.Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. The New England journal of medicine. 2014;370(20):1879–88. [DOI] [PubMed] [Google Scholar]
- 8.Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet (London, England: ). 2014;384(9956):1756–65. [DOI] [PubMed] [Google Scholar]
- 9.Pearlman BL, Ehleben C, Perrys M. The combination of simeprevir and sofosbuvir is more effective than that of peginterferon, ribavirin, and sofosbuvir for patients with hepatitis C-related Child’s class A cirrhosis. Gastroenterology. 2015;148(4):762–70.e2; quiz e11–2. [DOI] [PubMed] [Google Scholar]
- 10.Pol S, Sulkowski MS, Hassanein T, et al. Sofosbuvir plus pegylated interferon and ribavirin in patients with genotype 1 hepatitis C virus in whom previous therapy with direct-acting antivirals has failed. Hepatology (Baltimore, Md: ). 2015;62(1):129–34. [DOI] [PubMed] [Google Scholar]
- 11.Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. The New England journal of medicine. 2014;370(21):1973–82. [DOI] [PubMed] [Google Scholar]
- 12.Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. The New England journal of medicine. 2014;370(21):1993–2001. [DOI] [PubMed] [Google Scholar]
- 13.Zeuzem S, Ghalib R, Reddy KR, et al. Grazoprevir-Elbasvir Combination Therapy for Treatment-Naive Cirrhotic and Noncirrhotic Patients With Chronic Hepatitis C Virus Genotype 1, 4, or 6 Infection: A Randomized Trial. Annals of internal medicine. 2015;163(1):1–13. [DOI] [PubMed] [Google Scholar]
- 14.Lawitz E, Gane E, Pearlman B, et al. Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C-WORTHY): a randomised, open-label phase 2 trial. Lancet (London, England: ). 2015;385(9973):1075–86. [DOI] [PubMed] [Google Scholar]
- 15.Roth D, Nelson DR, Bruchfeld A, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4–5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet (London, England: ). 2015;386(10003):1537–45. [DOI] [PubMed] [Google Scholar]
- 16.Serfaty L, Zeuzem S, Vierling JM, et al. High Efficacy of the Combination Hcv Regimen Grazoprevir and Elbasvir for 8 or 12 Weeks With or Without Ribavirin in Treatment-naive, Noncirrhotic Hcv Gt1b-infected Patients: An Integrated Analysis. Hepatology (Baltimore, Md: ). 2015;62:555A–6A. [Google Scholar]
- 17.Thompson A, Zeuzem S, Rockstroh J, Kwo P. The combination of elbasvir and grazoprevir±RBV is highly effective for the treatment of GT1a-infected patients. Proceedings of the American Association for the Study of Liver Diseases Liver Meeting, San Francisco, CA: of Conference; 2015. [Google Scholar]
- 18.Hezode C, Colombo M, Bourliere M, et al. Elbasvir/Grazoprevir for Patients With Hepatitis C Virus Infection and Inherited Blood Disorders: A Phase III Study. Hepatology (Baltimore, Md: ). 2017;66(3):736–45. [DOI] [PubMed] [Google Scholar]
- 19.Kanwal F, Hoang T, Kramer JR, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140(4):1182–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saeed S, Strumpf EC, Walmsley SL, et al. How Generalizable Are the Results From Trials of Direct Antiviral Agents to People Coinfected With HIV/HCV in the Real World? Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;62(7):919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu CJ, Roytman MM, Hong LK, et al. Real-world Experience with Sofosbuvir-based Regimens for Chronic Hepatitis C, Including Patients with Factors Previously Associated with Inferior Treatment Response. Hawai’i journal of medicine & public health : a journal of Asia Pacific Medicine & Public Health. 2015;74(9 Suppl 2):3–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Fox DS, McGinnis JJ, Tonnu-Mihara IQ, McCombs JS. Comparative treatment effectiveness of direct acting antiviral regimens for hepatitis C: Data from the Veterans administration. J Gastroenterol Hepatol. 2017;32(6):1136–42. [DOI] [PubMed] [Google Scholar]
- 23.Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology (Baltimore, Md: ). 2016;64(2):405–14. [DOI] [PubMed] [Google Scholar]
- 24.Terrault NA, Zeuzem S, Di Bisceglie AM, et al. Effectiveness of Ledipasvir-Sofosbuvir Combination in Patients With Hepatitis C Virus Infection and Factors Associated With Sustained Virologic Response. Gastroenterology. 2016;151(6):1131–40 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su F, Green PK, Berry K, Ioannou GN. The association between race/ethnicity and the effectiveness of direct antiviral agents for hepatitis C virus infection. Hepatology (Baltimore, Md). 2017;65(2):426–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness and predictors of sustained virological response with all-oral therapy in 21,242 hepatitis C genotype-1 patients. Antiviral therapy. 2016. [DOI] [PubMed] [Google Scholar]
- 27.Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology (Baltimore, Md: ). 2005;41(1):88–96. [DOI] [PubMed] [Google Scholar]
- 28.Maier MM, Ross DB, Chartier M, Belperio PS, Backus LI. Cascade of Care for Hepatitis C Virus Infection Within the US Veterans Health Administration. American journal of public health. 2016;106(2):353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of Sofosbuvir, Ledipasvir/Sofosbuvir, or Paritaprevir/Ritonavir/Ombitasvir and Dasabuvir Regimens for Treatment of Patients With Hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology. 2016;151(3):457–71 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida EM, Sulkowski MS, Gane EJ, et al. Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology (Baltimore, Md: ). 2015;61(1):41–5. [DOI] [PubMed] [Google Scholar]
- 31.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27(3):274–82. [DOI] [PubMed] [Google Scholar]
- 32.Solberg LI, Engebretson KI, Sperl-Hillen JM, Hroscikoski MC, O’Connor PJ. Are claims data accurate enough to identify patients for performance measures or quality improvement? The case of diabetes, heart disease, and depression. Am J Med Qual. 2006;21(4):238–45. [DOI] [PubMed] [Google Scholar]
- 33.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. [DOI] [PubMed] [Google Scholar]
- 34.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Annals of internal medicine. 2006;145(4):247–54. [DOI] [PubMed] [Google Scholar]
- 35.National Kidney Foundation. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International. 2013;3(1):S1–150. [DOI] [PubMed] [Google Scholar]
- 36.Jacobson IM, Lawitz E, Kwo PY, et al. Safety and Efficacy of Elbasvir/Grazoprevir in Patients With Hepatitis C Virus Infection and Compensated Cirrhosis: An Integrated Analysis. Gastroenterology. 2017;152(6):1372–82 e2. [DOI] [PubMed] [Google Scholar]
- 37.Butt AA, Yan P, Simon TG, Abou-Samra AB. Effect of Paritaprevir/Ritonavir/Ombitasvir/Dasabuvir and Ledipasvir/Sofosbuvir Regimens on Survival Compared With Untreated Hepatitis C Virus-Infected Persons: Results From ERCHIVES. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017;65(6):1006–11. [DOI] [PubMed] [Google Scholar]
- 38.Puenpatom A, Hull M, McPheeters J, Schwebke K. Disease Burden, Early Discontinuation, and Healthcare Costs in Hepatitis C Patients with and without Chronic Kidney Disease Treated with Interferon-Free Direct-Acting Antiviral Regimens. Clin Drug Investig. 2017;37(7):687–97. [DOI] [PMC free article] [PubMed] [Google Scholar]


