Abstract
Background and Purpose
Increased systolic blood pressure variability (BPV) is associated with worse outcome after acute ischemic stroke and may also have a negative impact after intracerebral hemorrhage. We sought to determine if increased BPV was detrimental in the Antihypertensive Treatment of Acute Cerebral Hemorrhage II (ATACH-2) trial.
Methods
The primary outcome of our study was a 3 month follow-up modified Rankin Scale of 3–6 and the secondary outcome was a utility-weighted modified Rankin Scale. We calculated blood pressure mean and variability using systolic blood pressure from the acute period (2–24 hours post-randomization) and subacute period (days 2, 3, and 7).
Results
The acute period included 913 patients and the subacute included 877. For five different statistical measures of systolic BPV there was a consistent association between increased BPV and worse neurologic outcome in both the acute and subacute periods. This association was not found for systolic blood pressure mean.
Conclusions
In this secondary analysis of ATACH-2, we show that increased systolic BPV is associated with worse long-term neurological outcome. Additional research is needed to find techniques that allow early identification of patients with an expected elevation of BPV and to study pharmacologic or protocol-based approaches to minimize BPV.
Keywords: Intracranial hemorrhage, blood pressure, hypertension
Subject terms: Intracranial hemorrhage, blood pressure, hypertension
Introduction
Acute intracerebral hemorrhage (ICH) is a major cause of death and disability with few effective treatment options. Hypertension is both a risk factor for ICH and predicts worse outcome.1 However, large clinical trials have not shown that aggressive blood pressure reduction following ICH lowers the rate of death or disability.1,2 Increased systolic blood pressure variability (BPV) is associated with a higher risk of incident stroke, coronary artery disease, and poor outcome after ischemic stroke.3,4 Post-hoc analyses of the Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial (INTERACT2) and Field Administration of Stroke Therapy-Magnesium (FAST-MAG) indicated that increased BPV was harmful after ICH.5,6 We sought to determine whether increased BPV was detrimental in the Antihypertensive Treatment of Acute Cerebral Hemorrhage II (ATACH-2) trial.1
Methods
This is a secondary analysis of ATACH-2, a randomized trial of patients with non-traumatic ICH to determine if an intensive antihypertensive protocol (goal SBP of 110 to 139 mm Hg) was superior to a standard approach (goal SBP of 140 to 179 mm Hg). The anonymized ATACH-2 dataset, received with a local IRB waiver, is available through NINDS at https://www.ninds.nih.gov. We created an acute (2–24 hours post-randomization) and subacute period (days 2–7). In the acute period, the ATACH-2 trial recorded the highest and lowest SBP for every hour and, in the subacute period, the two highest and lowest SBP readings separated by 1 hour for day 2, 3 and 7. We censored blood pressure readings for hours 0–2 after randomization, when the study intervention caused a rapid decrease in blood pressure.
Our primary outcome was “unfavorable neurological outcome,” defined as a modified Rankin scale (mRS) of 3 to 6, measured 3 months after randomization. The secondary outcome was a utility-weighted mRS (UW-mRS).7 Additional outcomes included change in ICH volume (ΔICH), midline shift (Δshift), and cerebral edema (Δedema). We calculated systolic mean and five measures of systolic BPV: standard deviation (SD), coefficient of variation (CV), average real variability (ARV), successive variation (SV), and residual SD (RSD). Blood pressure variables were analyzed both per 10 mm Hg shift and in quintiles.4 We performed separate analyses for the acute and subacute time periods using logistic regression and used Spearman’s rank correlation to test for association between BPV and ΔICH, Δshift, and Δedema.
Results
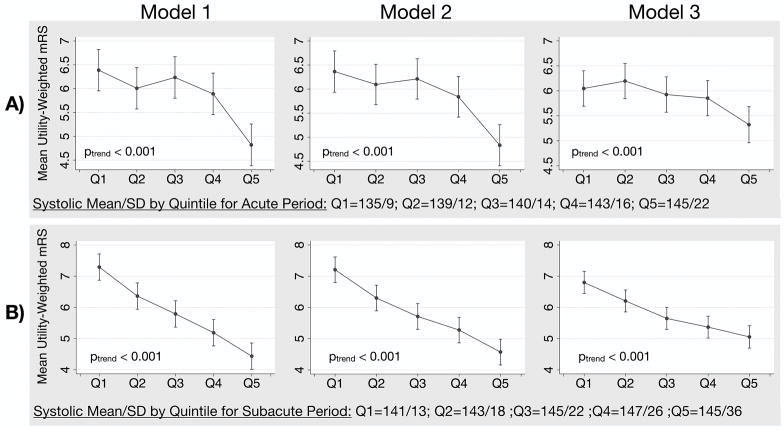
Of the 1,000 patients enrolled in ATACH-2, our acute period included 913 and the subacute included 877 (Table 1). The censored patients had either no data on the primary outcome (n=39), incomplete blood pressure data (n=16 in the acute and n=58 in the subacute period), or incomplete clinical information (n=32 in the acute and n=26 in the subacute period). The five measures of systolic BPV, but not systolic mean, were consistently associated with the primary outcome in both time periods (Table 2). The SD was significantly higher for patients with the primary outcome (acute period = 15.1±5.8 versus 13.7±4.4 mm Hg, p<0.001; subacute period = 25.4±8.6 versus 21.1±7.8 mm Hg, p<0.001). This association was stronger in the subacute than the acute period, with tighter confidence intervals and larger effect sizes. Increased BPV was also associated with worse UW-mRS in both time periods (Figure 1, ptrend < 0.001). Our findings relating BPV to the primary and secondary outcomes remained significant after adding systolic mean as a covariable or after stratifying the cohort by intensive versus standard treatment arm and running regression models separately (data not shown). In the acute period, there were 901 patients with a CT prior to randomization and 24 hours later. In this group of patients, there were no significant associations or trends between the acute period BPV or mean and ΔICH, Δedema, or Δshift. For SD, the Spearman’s rho was r=0 for ΔICH (p=0.944), r=0.02 for Δedema (p=0.560), and r=0.06 for Δshift (p=0.09).
Table 1.
Patient demographics in the two time periods.
| Variable* | Acute Period (hours 2–24, n=913) | Subacute Period (days 2–7, n=877) |
|---|---|---|
| Age | 62.1±13.1 | 61.9±12.9 |
| Male | 563 (61.7) | 541 (61.7) |
| Asian | 534 (58.4) | 539 (61.5) |
| Glasgow Coma Score | 13.7±2.1 | 13.7±2.1 |
| NIH Stroke Scale | 11.5±6.7 | 11.6±6.7 |
| Intensive SBP control randomization arm | 452 (49.5) | 442 (50.4) |
| Prior stroke | 147 (16.1) | 140 (16.0) |
| Coronary artery disease | 40 (4.4) | 37 (4.2) |
| Antihypertensive medication* | 448 (49.1) | 420 (47.9) |
| Diabetic medication* | 132 (14.5) | 123 (14.0) |
| Minutes from ICH onset to randomization | 184.0±56.8 | 182.5±56.2 |
| Right-sided ICH** | 475 (52.0) | 455 (51.9) |
| Lobar ICH** | 105 (11.5) | 95 (10.8) |
| Intraventricular extension of ICH** | 244 (26.7) | 233 (26.6) |
| ICH volume (mL)** | 15.1±14.8 | 15.2±14.3 |
| Cerebral edema volume (mL)** | 2.8±3.7 | 2.8±3.6 |
| Septum pellucidum shift (mm)** | 1.4±1.9 | 1.4±1.9 |
| Number of blood pressure readings | 46±1 | 12±0 |
| Systolic mean (mm Hg) | 140.2±13.5 | 144.0±10.8 |
| Systolic SD (mm Hg) | 14.5±5.3 | 22.7±8.3 |
| Systolic CV (mm Hg) | 10.3±3.6 | 15.8±5.8 |
| Systolic ARV (mm Hg) | 13.9±6.7 | 26.0±11.4 |
| Systolic SV (mm Hg) | 18.0±8.2 | 31.6±13.2 |
| Systolic RSD (mm Hg) | 13.9±5.3 | 22.3±8.9 |
Continuous variables: mean±SD; categorical data: n(%).
Pre-morbid
Pre-randomization CT
Table 2.
Logistic regression models showing the effect of 10 mm Hg shift in systolic mean or BPV predictors on the odds of an unfavorable neurological outcome.
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Acute Period | ||||||
| Mean | 1.11 (1.01, 1.22) | 0.035 | 1.49 (1.25, 1.78) | <0.001 | 1.14 (1.01, 1.28) | 0.036 |
| SD | 1.72 (1.30, 2.26) | <0.001 | 1.74 (1.30, 2.32) | <0.001 | 1.47 (1.06, 2.03) | 0.021 |
| CV | 2.03 (1.37, 3.02) | <0.001 | 1.91 (1.28, 2.85) | 0.001 | 1.57 (0.98, 2.52) | 0.061 |
| ARV | 1.37 (1.11, 1.69) | 0.004 | 1.33 (1.07, 1.65) | 0.011 | 1.26 (0.98, 1.62) | 0.077 |
| SV | 1.38 (1.15, 1.65) | 0.003 | 1.34 (1.11, 1.61) | 0.002 | 1.25 (1.01, 1.54) | 0.039 |
| RSD | 1.65 (1.24, 2.19) | 0.001 | 1.65 (1.24, 2.19) | 0.001 | 1.45 (1.05, 2.01) | 0.024 |
| Subacute Period | ||||||
| Mean | 1.05 (0.93, 1.19) | 0.402 | 1.11 (0.98, 1.27) | 0.116 | 1.00 (0.86, 1.17) | 0.997 |
| SD | 1.92 (1.60, 2.30) | <0.001 | 1.83 (1.52, 2.20) | <0.001 | 1.56 (1.26, 1.93) | <0.001 |
| CV | 2.49 (1.91, 3.24) | <0.001 | 2.28 (1.75, 2.99) | <0.001 | 1.88 (1.38, 2.56) | <0.001 |
| ARV | 1.58 (1.39, 1.81) | <0.001 | 1.54 (1.35, 1.76) | <0.001 | 1.39 (1.19, 1.63) | <0.001 |
| SV | 1.48 (1.32, 1.66) | <0.001 | 1.45 (1.29, 1.62) | <0.001 | 1.32 (1.16, 1.51) | <0.001 |
| RSD | 1.86 (1.57, 2.21) | <0.001 | 1.78 (1.49, 2.12) | <0.001 | 1.54 (1.26, 1.89) | <0.001 |
Model 1 = unadjusted, Model 2 = adjusted for patient age, sex, and treatment arm, Model 3 = adjusted for variables in Table 1 chosen with a stepwise backwards selection with model inclusion set at p<0.01: age, baseline NIH stroke scale, premorbid antihypertensive medication, intraventricular hemorrhage, laterality of ICH.
Figure 1.
Mean utility-weighted mRS with 95% CI by quintiles of increasing SD for patients in the A) acute and B) subacute period. Lower utility-weighted mRS values represent a worse neurologic outcome.
*Model 1 = unadjusted, Model 2 = adjusted for patient age, sex, and treatment arm, Model 3 = adjusted for age, ICH volume (mL), intraventricular hemorrhage, location of ICH, NIH stroke scale, intraventricular catheter, premorbid antihypertensive medication.
Conclusions
In ATACH-2 patients, we find a consistent association between unfavorable neurologic outcome and increased systolic BPV, which is also associated with worse UW-mRS, an outcome measure with better weighting from the patient or caregiver perspective. Our findings are similar to the secondary analyses of INTERACT2 and FAST-MAG,5,6 with an important difference – we find a stronger association between increased BPV and worse outcome in the subacute period. The FAST-MAG analysis did not look at subacute blood pressure. The discrepancy between our findings and the INTERACT2 analysis may be because the trial interventions can reduce BPV and ATACH-2’s only lasted 24 hours whereas INTERACT2’s continued for a full 7 days.
This study has several strengths compared to prior analyses, including considerably more blood pressure readings, which increase the precision of BPV, and a single first-line antihypertensive treatment (nicardipine). The main limitation is the methodology of blood pressure collection in ATACH-2, which introduced bias by recording the highest and lowest values. This increases BPV for all patients, but our results remain relevant because outliers are what is targeted in protocols to treat blood pressure. ATACH-2 also only enrolled patients with a SBP >180 after ICH onset and excluded patients with very severe ICH.
This study, and the similar results of the INTERACT2 and FAST-MAG analyses, should prompt clinicians to consider monitoring BPV after ICH and reinforce the need for additional research into reducing BPV after stroke. Potential therapeutic interventions include transitioning from the nicardipine used in ATACH-2 to an ultrashort-acting antihypertensive, which could be titrated rapidly to achieve steady blood pressure levels, or a more intensive monitoring and titration protocol of blood pressure management for an extended period of time. Research is also needed to find techniques that allow early identification of patients with an expected elevation of BPV.
Acknowledgments
NIH-NINDS and the ATACH-2 Trial Investigators for making the entire ATACH-2 dataset publicly available.
Sources of Funding: Dr. Rost is in part supported by the NIH-NINDS R01NS082285 and R01NS086905. The remaining authors have no disclosures.
Footnotes
Disclosures: None
Contributor Information
Adam de Havenon, University of Utah, Department of Neurology.
Jennifer J. Majersik, University of Utah, Department of Neurology.
Gregory Stoddard, University of Utah, Department of Medicine.
Ka-Ho Wong, University of Utah, Department of Neurology.
J. Scott McNally, University of Utah, Department of Radiology.
A. Gordon Smith, Virginia Commonwealth University, Department of Neurology.
Natalia S. Rost, Harvard Medical School.
David L. Tirschwell, University of Washington, Department of Neurology.
References
- 1.Qureshi AI, Palesch YY, Barsan WG, Hanley DF, Hsu CY, Martin RL, et al. Intensive Blood-Pressure Lowering in Patients with Acute Cerebral Hemorrhage. N Engl J Med. 2016;375:1033–1043. doi: 10.1056/NEJMoa1603460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355–2365. doi: 10.1056/NEJMoa1214609. [DOI] [PubMed] [Google Scholar]
- 3.Muntner P, Whittle J, Lynch AI, Colantonio LD, Simpson LM, Einhorn PT, et al. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure and mortality: A cohort study. Ann Intern Med. 2015;163:329–338. doi: 10.7326/M14-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manning LS, Rothwell PM, Potter JF, Robinson TG. Prognostic Significance of Short-Term Blood Pressure Variability in Acute Stroke: Systematic Review. Stroke. 2015;46:2482–2490. doi: 10.1161/STROKEAHA.115.010075. [DOI] [PubMed] [Google Scholar]
- 5.Manning L, Hirakawa Y, Arima H, Wang X, Chalmers J, Wang J, et al. Blood pressure variability and outcome after acute intracerebral haemorrhage: a post-hoc analysis of INTERACT2, a randomised controlled trial. Lancet Neurol. 2014;13:364–373. doi: 10.1016/S1474-4422(14)70018-3. [DOI] [PubMed] [Google Scholar]
- 6.Chung P-W, Kim J-T, Sanossian N, Starkmann S, Hamilton S, Gornbein J, et al. Association Between Hyperacute Stage Blood Pressure Variability and Outcome in Patients With Spontaneous Intracerebral Hemorrhage. Stroke. 2018;49:348–354. doi: 10.1161/STROKEAHA.117.017701. [DOI] [PubMed] [Google Scholar]
- 7.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]