Abstract
Background
Spontaneous intracerebral hemorrhage (ICH) leaves most survivors dependent at follow-up. The importance of promoting M2-like microglial responses is increasingly recognized as a key element to ameliorate brain injury following ICH. The osmotherapeutic agents, mannitol and hypertonic saline (HTS), which are routinely used to reduce intracranial pressure, have been shown to reduce neuroinflammation in experimental ischemic and traumatic brain injury, but anti-inflammatory effects of osmotherapies have not been investigated in ICH.
Methods
We studied the effects of iso-osmotic mannitol and HTS in rat models of ICH utilizing high-dose and moderate-dose collagenase injections into the basal ganglia, associated with high and low mortality, respectively. We studied the effects of osmotherapies, first given 5 hours after ICH induction, then administered every 12 hours thereafter (4 doses total). Immunohistochemistry was used to quantify microglial activation and polarization.
Results
Compared to controls, mannitol and HTS increased plasma osmolarity 1 hour after infusion (301±1.5, 315±4.2 and 310±2.0 mOsm/kg, respectively), reduced mortality at 48 hours (82, 36 and 53%, respectively), and reduced hemispheric swelling at 48 hours (32, 21 and 17%, respectively). In both perihematomal and contralateral tissues, mannitol and HTS reduced activation of microglia/macrophages (abundance and morphology of Iba1+ cells), and in perihematomal tissues, reduced markers of the microglia/macrophage M1-like phenotype (nuclear p65, TNF and NOS2), increased markers of the microglia/macrophage M2-like phenotype (arginase, YM1 and pSTAT3), and reduced infiltration of CD45+ cells.
Conclusions
Repeated dosing of osmotherapeutics at regular intervals may be a useful adjunct to reduce neuroinflammation following ICH.
Keywords: intracerebral hemorrhage, mannitol, hypertonic saline, microglia, brain swelling
INTRODUCTION
Spontaneous intracerebral hemorrhage (ICH) accounts for 15% of all strokes, and leaves most survivors dependent at follow-up. Numerous mechanisms of secondary injury adversely affect outcomes in this type of stroke (1).
Osmotic agents such as mannitol and hypertonic saline (HTS) are mainstays of treatment to mitigate brain swelling and decrease intracranial pressure (ICP) following ICH (2). Apart from brain swelling, outcomes after ICH also depend on the inflammatory response of the brain to blood-breakdown products (3,4). An overexuberant inflammatory response in viable perihematomal or more remote tissues can cause “bystander” or secondary injury, which may worsen outcome.
Microglial activation is an important determinant of the neuroinflammatory response in ICH (5). Microglia respond to ICH by becoming activated and exhibiting classic M1-like or alternatively activated M2-like phenotypes, which in broad terms are characterized by the production of proinflammatory or anti-inflammatory cytokines and chemokines, respectively. In addition, M2-like microglia contribute to hematoma clearance by phagocytizing erythrocytes and tissue debris (6). The importance of promoting M2-like microglial responses, especially during the recovery phase of ICH, is increasingly recognized as a key element to ameliorate brain injury following ICH (5).
Apart from their salutary effects on brain swelling, osmotherapeutic agents also have been shown to attenuate inflammation. An increase of 10–20 mOsm/kg in plasma osmolality can affect certain functions of immune cells, including degranulation (7,8), reactive oxygen species (ROS) production (7,8), adhesion molecule expression (8), phagocytic ability and other functions (8–10). In a middle cerebral artery model of stroke, HTS alleviates cerebral edema by inhibiting tumor necrosis factor (TNF) and interleukin 1β (IL-1β) secretion by M1-like polarized microglia (11), and in rodent models of traumatic brain injury, both HTS and mannitol reduce neutrophil tissue invasion (12,13). To our knowledge, the potential effects of osmotherapies on inflammation in ICH have not been evaluated.
Here, we studied the effects of mannitol and HTS on perihematomal and contralateral neuroinflammation in a rat model of ICH. Of the two ICH models most often utilized in preclinical work – autologous blood injection and collagenase injection – we chose to study the collagenase model because it is considered to be more severe and it reportedly induces a more robust inflammatory response (3,14,15), a desirable feature when assessing an anti-inflammatory strategy. We used a high-dose (high-mortality) collagenase model to examine the effects of osmotherapies on mortality, and we used a moderate-dose (low mortality) collagenase model to study hemispheric swelling and the inflammatory response in brain tissues. We studied the effects of osmotherapies administered repeatedly at regular intervals, rather than in a “reactionary” manner to reduce ICP, as typically used in the ICU setting.
As expected, based on reported effects of these agents on ICP (16–18), both mannitol and HTS reduced mortality and brain swelling. In addition, mannitol and HTS suppressed microglial activation and promoted the M2-like phenotype in perihematomal and contralateral tissues. Our findings reveal a heretofore unrecognized, salutary effect of osmotherapies in experimental ICH that may merit further evaluation for possible translation.
METHODS
Ethics statement
We certify that all applicable institutional and governmental regulations concerning the ethical use of animals were followed during the course of this research. Animal experiments were performed under a protocol approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Maryland, Baltimore and in accordance with the relevant guidelines and regulations as stipulated in the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
Subjects and surgical procedure
Male Wistar rats, aged 12–16 weeks (325–400 gm) (Harlan, Indianapolis, IN, USA), were anesthetized (60 mg/kg ketamine plus 7.5 mg/kg xylazine, intraperitoneal) and allowed to breathe room air spontaneously. Core temperature was maintained at 37 °C using a heating pad regulated by a rectal temperature probe (Harvard Apparatus, Holliston, MA). Oxygen saturation and heart rate were monitored using a pulse oximeter (Mouse Ox™; STARR Life Sciences Corp., Oakmont, PA). Surgical incision sites were prepared using iodine and alcohol, and a sterile environment was maintained throughout the procedure. Rats were mounted in a stereotactic apparatus (Stoelting Co., Wood Dale, IL, USA). Lidocaine solution (2%) was injected prior to making an incision. A midline scalp incision was made to expose the skull. A 1-mm burr hole was made over the right basal ganglia (x,y: +0.2, – 3.0 mm relative to bregma), and the dura was opened sharply. The tip of the Hamilton syringe needle was advanced to z: –7.0 mm. Collagenase IV (cat #1889; Sigma; sterile-filtered), either 0.035 U or 0.055 U in 3.0 μL of sterile saline (“moderate-dose” vs. “high-dose”, respectively, for “low mortality” vs. “high mortality” models), or an equivalent volume of saline, was infused into the striatum over 10 min. After a 10-minute delay to prevent backflow, the needle was slowly removed. The hole in the skull was sealed with bone wax and the incision was sutured (15).
Following induction of ICH, the external jugular vein was exposed and catheterized using a rodent vascular access port with pre-attached 2 Fr silastic catheter (volume, 130 μL; #72-4375; Harvard Apparatus), with the port filled with normal saline (NS). The port itself was positioned within a subcutaneous pocket on the dorsal thorax and was used for intravenous (IV) administration of osmotherapeutic agents. After the surgical procedures, rats were given 10 mL of glucose-free NS subcutaneously and were nursed on a heating pad to maintain rectal temperature ~37° C until they emerged from anesthesia.
Exclusions
All animals receiving moderate-dose collagenase (series 2 and 3, see below) were evaluated at 24 hours using the vibrissae-elicited forelimb placing test, as described (19). Animals not exhibiting abnormal forelimb placing (5/64 rats) were considered to have insufficient ICH and were eliminated from further study. Necropsy generally was performed and invariably confirmed an insufficient or misplaced ICH. Excluded animals were replaced with new ICH animals of the proper treatment group.
Treatments
Animals were randomized by coin toss to receive either no treatment, or an equi-osmolar IV infusion of mannitol [20%, w/v; 5 mL/kg (1 gm/kg)] or HTS (23.4%; 0.7 mL/kg) over 3 minutes (20). After treatment, the port was flushed with NS (250 μL). The first dose of osmotherapeutic was administered 5 hours after ICH induction (hour 5), and then every 12 hours thereafter (hours 17, 29, and 41) for 4 doses total. Animals were euthanized either 7 hours (hour 48) or 7 days after the 4th treatment.
Sample size calculation
We calculated sample size based on the effect size of mannitol (1 gm/kg) on ICP reduction in a model of ICH reported by Qureshi et al. (16), with the values they reported yielding an effect size (Cohen’s d) of 1.37. We calculated sample size using an apriori sample size calculator with the following assumptions: α, 0.05; desired power, 80%; anticipated effect size (Cohen’s d), 1.37. Calculations indicated that a minimum of 8 rats/group (one tailed hypothesis) would be required for the experiments on brain swelling (series 2, see below).
Experimental series
We studied three series of rats. In series 1, 37 rats underwent high-dose collagenase injection; they were randomly assigned to receive no treatment (n=11), IV mannitol (n=11) or IV HTS (n=15). Animals in this series were used to evaluate the effect of treatment on mortality at 48 hours.
In series 2, 32 rats underwent saline (sham; n=5) or moderate-dose collagenase injection into the striatum (n=27); those with collagenase injection were randomly assigned to receive no treatment (n=8), IV mannitol (n=11) or IV HTS (n=8); ICH animals in this series were used to evaluate the effects of treatment on hemispheric swelling at 48 hours (7 hours after the 4th dose of mannitol or HTS). In addition, 5 sham brains and 5 brains from each ICH group, carefully matched for hemorrhage size within and between groups, were used to evaluate various immunohistochemical markers of inflammation at 48 hours.
In series 3, 27 rats underwent moderate-dose collagenase injection into the striatum and were randomly assigned to receive no treatment (n=11), mannitol (n=8) or HTS (n=8); animals in this series were used to evaluate the effects of treatment on neurobehavior at 48 hours and 7 days, compared to baseline pre-ICH neurobehavior.
Serum osmolarity
Using normal rats (no ICH), we measured plasma osmolarity using a vapor-pressure osmometer (VAPRO5520, Wescor) in 9 untreated control rats, and in 6 rats per group, 1 hour after IV infusion of a single dose of either mannitol or HTS.
Blinded outcome evaluations
Mortality, brain swelling, and neurological evaluations were conducted by investigators blinded to treatment group.
Mortality
Rats in series 1 were nursed post-operatively to maintain euvolemia and euthermia, and were closely monitored for neurological deterioration. Animals judged to be terminal by an experienced investigator blinded to treatment group were euthanized. Some rats were found to have died overnight. All remaining rats in this series were euthanized at 48 hours.
Swelling
Rats in series 2 were euthanized at 48 hours, exsanguinated and perfused with 10% neutral buffered formalin. Brains were sectioned coronally through the epicenter of the ICH and were imaged on a flatbed scanner. Images were segmented manually using software (Photoshop) to quantify the areas of the ipsilateral and contralateral hemisphere, Ai and Ac, respectively. Swelling was computed as Ai/Ac–1.
Neurodeficit Score
Rats in series 3 underwent neurofunctional testing at 48 hours and 7 days after ICH induction. Neurologic deficits were quantified using the Neurologic Deficit Scale, as described (21). Tests included: (i) spontaneous circling, graded from 0 for no circling to 3 for continuous circling; (ii) hind limb retraction, graded from 0 for immediate replacement to 3 for no retraction after the limb was displaced laterally; (iii) bilateral forepaw grasp, graded from 0 for normal grasping to 3 for a rat unable to grasp the bar at all; (iv) contralateral forelimb flexion, graded from 0 for uniform extension of forelimbs to 2 for full wrist flexion and shoulder adduction when the rat was lifted by the base of the tail; and (v) beam walking ability, graded from 0 for a rats that readily crossed the beam to 3 for a rat unable to stay on the beam for more than 10 seconds. Scores for each component were added for a maximum of 14 (greatest impairment). The score decreases with recovery.
Vibrissae-elicited forelimb placing test
This test was performed as described (19). A normal rat scores correctly 100% of the time, whereas an acutely injured ICH rat with a profound unilateral deficit scores 50%. The score increases with recovery.
Corner Turn Test
This test was performed as described (19).
Immunohistochemistry and quantification of specific labelling
Flat-bed images of coronal sections of rats from series 2 were screened to identify 5 from each treatment group that were best matched for hematoma size and location; tissues from these animals were subsequently processed for immunohistochemical evaluation. These brains, which had been perfused with 10% neutral buffered formalin, were post-fixed 2–3 days in paraformaldehyde, cryoprotected with 30% sucrose, frozen in OCT and cryosectioned (10 μm).
Immunohistochemistry was performed as described (22). Sections were incubated at 4 °C overnight with primary antibodies, including: rabbit anti-Iba1 (1:200; cat#019-19741; Wako, Osaka, Japan); goat anti-Iba1 (1:100; cat#5076; Abcam, Cambridge UK); rabbit anti-p65 (1:200; cat#sc372; Santa Cruz Biotechnology, Santa Cruz, CA); goat anti-TNF (1:200; cat#sc1350; Santa Cruz Biotechnology); rabbit anti-NOS2 (1:500; cat#482728; Millipore, Burlington, MA); goat anti-arginase (1:100; cat#sc18354; Santa Cruz); rabbit anti-YM1 (1:50; cat#60130; STEMCELL Technologies, Cambridge, MA); rabbit anti-pSTAT3 (Y705; 1:200; cat#9145; Cell Signaling, Danvers, MA); mouse anti-CD45 (1:100; cat#202201; BioLegend, San Diego, CA). After several rinses in phosphate buffered saline (PBS), sections were incubated with species-appropriate fluorescent secondary antibodies (Alexa Fluor 488 and 555, Molecular Probes, Invitrogen, Carlsbad, CA) for 1 hour at room temperature. Controls included the omission of primary antibodies.
Unbiased measurements of specific labeling within regions of interest (ROI) were obtained using NIS-Elements AR software (Nikon Instruments, Melville, NY) from sections immunolabeled in a single batch. All images for a given signal were captured using uniform parameters of magnification, area, exposure, and gain. Segmentation analysis was performed by computing a histogram of pixel intensity for a particular ROI, and pixels were classified as having specific labeling, based on signal intensity greater than 2x that of background. The area occupied by pixels with specific labeling was used to determine the percent area in the ROI with specific labeling (% ROI). For Iba1, the ROI was a rectangle, 2500 × 1000 μm, positioned at the lateral edge of the ICH; for p65, TNF, NOS2, arginine, YM1, pSTAT3, the ROIs were 3 rectangles, 400 × 300 μm, positioned randomly on the border of the ICH; for CD45, similar ROIs were positioned at the border inside the ICH. For sham animals, ROIs were placed in the same anatomical regions. To quantify M2-like markers (Arg, YM1 and pSTAT3), the integrated intensity relative to Iba1 was quantified as the sum of pixel fluorescence intensity for a specific label divided by the area of Iba1 positivity (23).
Immunoblot
Proteins were extracted from formalin-fixed frozen brains (series 2). Rat brain tissue lysates were prepared from the dorsal half of a 2-mm thick coronal slab at the epicenter, adjacent to the sections used for immunohistochemistry. Protein extraction was performed using a Qproteome FFPE tissue kit (Qiagen, Valencia, CA), following the manufacturer’s instructions. Briefly, tissues were homogenized in 100 μL of FFPE solution, incubated on ice for 5 minutes, following by incubation in a heating block incubator for 20 minutes at 100 °C, and 120 minutes at 80 °C, with vortexing every 5 minutes. Insoluble aggregates were removed by centrifugation, and the resultant supernatant was collected. The protein concentration was determined using a commercial protein assay solution (Bio-Rad, Hercules, CA). Protein lysates were used for immunoblot analysis of Iba1 (cat#019-19741; Wako Chemicals, Richmond VA).
Statistics
Nominal data are presented as mean±SE. Binary variables are represented as percentage. Mortality was analyzed using Fisher’s exact test (one sided). Nominal data were analyzed using an ANOVA with post-hoc Fisher’s comparisons. Statistical tests were performed using Origin Pro (V8; OriginLab, North Hampton, MA). Significance was assumed if P < 0.05.
RESULTS
Mortality, swelling and neurofunction
We first evaluated the effect on plasma osmolarity of equi-osmolar mannitol [20%, w/v; 5 mL/kg (1 gm/kg)] and HTS (23.4%; 0.7 mL/kg), infused IV over 3 minutes (20). Compared to controls, mannitol and HTS increased serum osmolarity 1 hour after infusion from 301±1.5 to 315±4.2 and 310±2.0 mOsm/kg, respectively (P<0.05).
We studied the effect of the osmotherapies on mortality using the high-dose collagenase model, which induced large hemorrhages with mass effect and midline shift (Fig. 1A, left). In untreated ICH rats, mortality at 48 hours was 82% (9/11 rats) (Fig. 1A, right). Mannitol and HTS reduced mortality to 36% (4/11 rats; P=0.04) and 53% (8/15 rats; P=0.14), respectively.
Figure 1. Mannitol and hypertonic saline reduce mortality and brain swelling in ICH models.
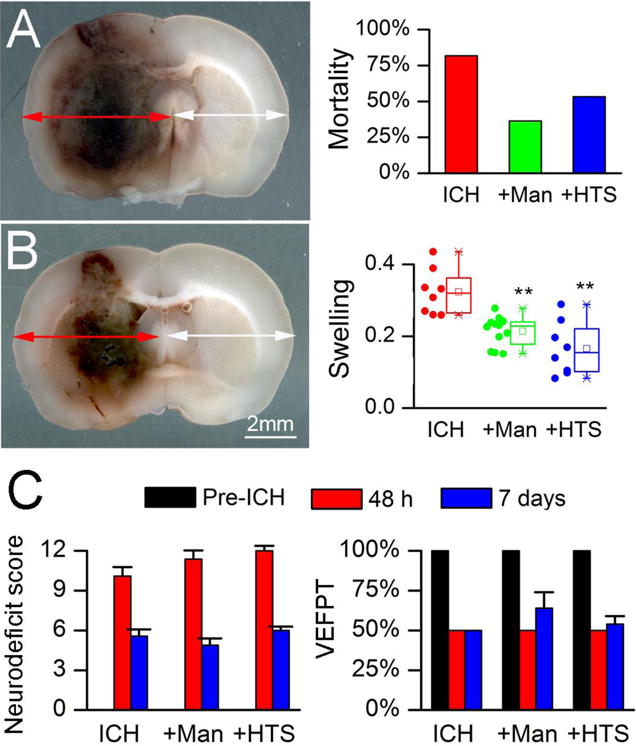
A: Image of coronal section of rat brain 48 hours after high-dose collagenase injection (left); note the marked mass effect and midline shift; percent mortality at 48 hours (right) in the high-dose collagenase injection model, in untreated animals (ICH; n=11), and ICH animals treated with mannitol (+Man; n=11) or hypertonic saline (+HTS; n=15); P=0.09. B: Image of coronal section of brain 48 hours after moderate-dose collagenase injection (left); note the presence of less mass effect and less midline shift compared to the high-dose model; swelling at 48 hours of the ipsilateral hemisphere (right), computed as Ai/Ac–1 (see Methods), in the moderate-dose collagenase injection model, in untreated animals (ICH; n=8), and ICH animals treated with IV mannitol (+Man; n=11) or IV hypertonic saline (+HTS; n=8); data are presented as scatter plots, and as box plots showing the mean, median, 1st and 3rd quartiles, and the minimum and maximum; **, P<0.01 with respect to untreated ICH. C: Neurodeficit score and vibrissae-elicited forelimb placing test (VEEPT) at baseline before ICH, and 48 hours and 7 days after ICH, in the moderate-dose collagenase injection model, in untreated animals (ICH; n=11), and ICH animals treated with mannitol (+Man; n=8) or hypertonic saline (+HTS; n=8); in all cases, significant neurological deficits were observed with ICH, and no significant effects of treatment were observed.
All subsequent experiments were carried out using the moderate-dose collagenase model, which induced more moderate hemorrhages with less pronounced with mass effect (Fig. 1B, left), and was associated with essentially no mortality. We used this model to examine the effect of the osmotherapies on hemispheric swelling, which is due to both extravasated blood and edema. In untreated ICH rats, mean swelling of the ipsilateral hemisphere at 48 hours was 32% (Fig. 1B, right). Mannitol and HTS reduced hemispheric swelling to 21% (P=0.001) and 17% (P=0.0004), respectively.
Neurological function was evaluated 48 hours and 7 days after induction of moderate-dose collagenase ICH. Scores on the neurologic deficit scale (Fig. 1C, left), the vibrissae-elicited forelimb placing test (Fig. 1C, right), and the corner turn test (not shown) were significantly abnormal, compared to baseline, consistent with all included animals having sustained a symptomatic ICH and equivalent brain injuries. No differences in neurological function were observed between treatment groups at 48 hours or 7 days.
Activation of microglia/macrophages
The effects of the osmotherapies on the activation of microglia/macrophages were evaluated 48 hours after induction of ICH using immunolabeling for Iba1. In sham animals, Iba1+ microglia were sparsely distributed and exhibited a ramified, non-reactive morphology (Fig. 2A,B, Sham), consistent with a quiescent phenotype. By contrast, in perihematomal tissues from untreated ICH rats, numerous Iba1+ cells with a plump, activated morphology were evident (Fig. 2A,B, ICH) (5). In perihematomal tissues, treatment with either mannitol or HTS was associated with reduced prominence of Iba1+ cells that exhibited less-plump morphologies (Fig. 2A, ICH+Man, ICH+HTS; Fig. 2B, ICH+Man). Quantification of Iba1 immunoreactivity confirmed a significant increase in untreated ICH compared to sham controls, and significant decreases in ICH rats treated with either mannitol or HTS, compared to untreated ICH (Fig. 2C). Immunoblots for Iba1 confirmed the findings obtained with immunohistochemistry (Fig. 2C).
Figure 2. Mannitol and hypertonic saline attenuate microglial activation ipsilateral and contralateral to the ICH.
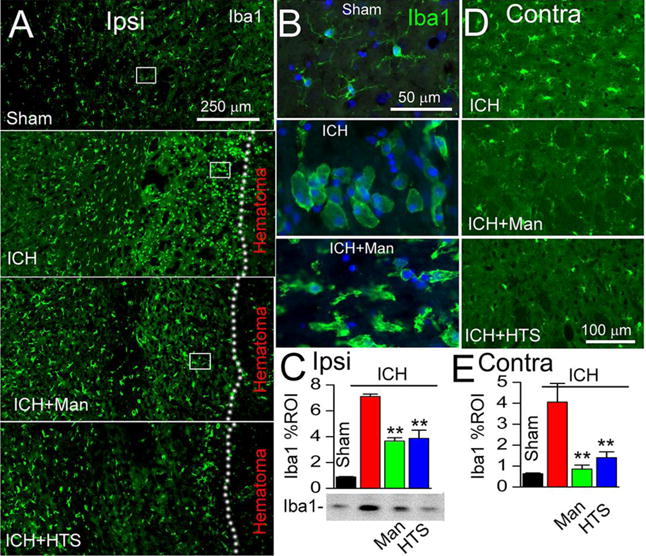
A–C: Immunolabeling at 48 hours for Iba1 ipsilateral to the ICH (moderate-dose collagenase model), shown at low magnification (A) and high magnification (B; corresponding to rectangles in A), for sham injury, ICH without treatment (ICH), ICH with mannitol treatment (ICH+Man), and ICH with hypertonic saline treatment (ICH+HTS), as indicated; the dotted line demarcates the hematoma from perihematomal tissues; the bar graphs show immunohistochemistry data quantified as percent region of interest (Iba1 % ROI) in the four conditions (C); below the bar graph is shown an immunoblot for Iba1 in the 4 conditions (one animal per lane, representative of 3 animals per condition), corroborating the immunohistochemistry data; **, P<0.01 for treatments compared to no treatment; n=5/group. D,E: Immunolabeling at 48 hours for Iba1 contralateral to the ICH (moderate-dose collagenase model), for ICH without treatment (ICH), ICH with mannitol treatment (ICH+Man), and ICH with hypertonic saline treatment (ICH+HTS), as indicated; the bar graphs show data quantified as percent region of interest in the four conditions (E); **, P<0.01 for treatments compared to no treatment; n=5/group.
ICH can be accompanied by abnormalities in the contralateral hemisphere (24). In contralateral tissues from untreated ICH rats, Iba1+ cells with a plump, activated morphology were evident (Fig. 2D, ICH), whereas treatment with either mannitol or HTS was associated with reduced prominence of Iba1+ cells contralaterally (Fig. 2D, ICH+Man, ICH+HTS). Quantification of Iba1 immunoreactivity in the contralateral hemisphere confirmed a significant increase in untreated ICH compared to sham controls, and significant decreases in ICH rats treated with either mannitol or HTS, compared to untreated ICH (Fig. 2E).
Microglial activation in ICH is characterized by NF-κB activation and TNF upregulation. NF-κB signaling was assessed by quantifying nuclear translocation of p65 (RelA). In shams, nuclear p65 was undetectable (Fig. 3A, Sham). In perihematomal tissues from untreated ICH rats, nuclear p65 was prominent, especially in Iba1+ microglia/macrophages (Fig. 3A, ICH). In perihematomal tissues, both mannitol (Fig. 3A, ICH+Man) and HTS (not shown) were associated with reduced nuclear p65. Quantification of nuclear p65 in Iba1+ cells confirmed a significant increase in untreated ICH, and significant decreases with both mannitol and HTS, relative to untreated ICH (Fig. 3B).
Figure 3. Mannitol and hypertonic saline attenuate p65 and TNF.
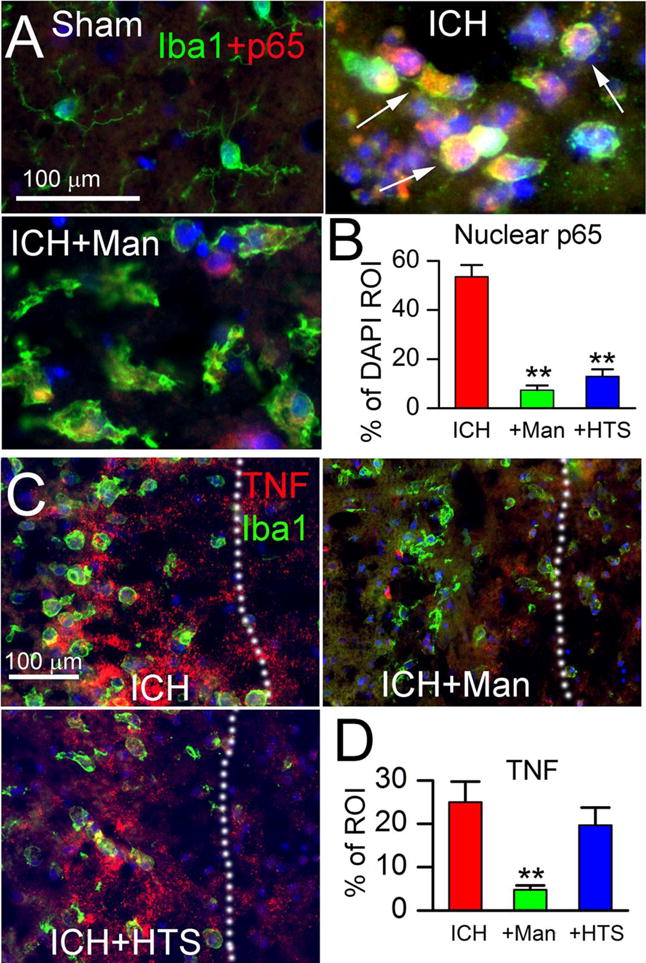
A,B: Double immunolabeling (merged images) at 48 hours for Iba1 (green) and p65 (red), with nuclear staining by DAPI, ipsilateral to the ICH (moderate-dose collagenase model), for sham injury, ICH without treatment (ICH), and ICH with mannitol treatment (ICH+Man), as indicated; arrows point to p65+ (red) and DAPI+ (blue) nuclei that appear pink; the bar graphs show quantification of p65 within DAPI+ nuclei (% of DAPI ROI) in the three conditions indicated (B); **, P<0.01 for treatments compared to no treatment; n=5/group. C,D: Double immunolabeling (merged images) at 48 hours for Iba1 (green) and TNF (red), ipsilateral to the ICH (moderate-dose collagenase model), for ICH without treatment (ICH), ICH with mannitol treatment (ICH+Man), and ICH with hypertonic saline treatment (ICH+HTS), as indicated; the dotted line demarcates the hematoma from perihematomal tissues; the bar graphs show quantification of TNF (% of ROI) in the three conditions indicated (D); **, P<0.01 for treatments compared to no treatment; n=5/group.
Tumor necrosis factor (TNF) is a critical downstream target of NF-κB signaling (5). In untreated ICH rats, TNF was prominent near Iba1+ microglia/macrophages (Fig. 3C, ICH) (5). By contrast, in animals treated with mannitol, TNF was markedly reduced (Fig. 3C, ICH+Man), an effect that was not evident with HTS (Fig. 3C, ICH+HTS). Quantification of TNF confirmed a significant increase in untreated ICH, and significant decrease with mannitol but not HTS (Fig. 3D).
Activated microglial phenotype
In ICH, activated microglia can exhibit classic M1-like (proinflammatory) or alternative M2-like (anti-inflammatory) phenotypes (5). A classic M1-like signature gene is inducible nitric oxide synthase 2 (NOS2) (5,25). In shams, NOS2 was undetectable (not shown). In perihematomal tissues, NOS2 was prominently expressed by Iba1+ cells from untreated ICH rats (Fig. 4A) (5), whereas both mannitol (Fig. 4B) and HTS (Fig. 4C) were associated with reduced NOS2 expression. Quantification of NOS2 in Iba+ cells confirmed a significant increase in untreated ICH, and significant decreases with both mannitol and HTS (Fig. 4D).
Figure 4. Mannitol and hypertonic saline attenuate nitric oxide synthase (NOS2) expression.
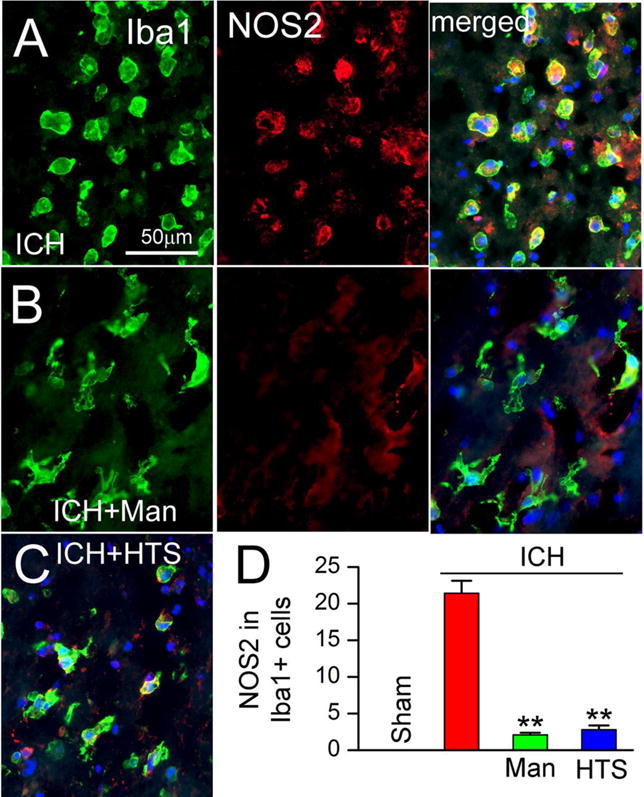
A–D: Double immunolabeling at 48 hours for Iba1 (green) and NOS2 (red), ipsilateral to the ICH (moderate-dose collagenase model), for ICH without treatment (ICH) (A), ICH with mannitol treatment (ICH+Man) (B), and ICH with hypertonic saline treatment (ICH+HTS) (C); merged images are shown in A, right panel, in B, right panel, and in C; the merged images also show nuclear staining with DAPI; the bar graphs show quantification of NOS2 in Iba1+ cells in the four conditions indicated (D); **, P<0.01 for treatments compared to no treatment; n=5/group.
To determine whether the attenuated microglial activation observed with mannitol and HTS was associated with increases in M2-like features, we assessed the expression of arginase, YM1 and pSTAT3, all of which are characteristic markers of the M2b/c phenotype (5). In perihematomal tissues, expression of arginase, YM1 and pSTAT3 was modest in untreated ICH rats (Fig. 5A–C, ICH), whereas in both mannitol and HTS, expression of arginase, YM1 and pSTAT3 was significantly increased in Iba+ cells (Fig. 5A–C).
Figure 5. Mannitol and hypertonic saline promote the expression of M2-like markers.
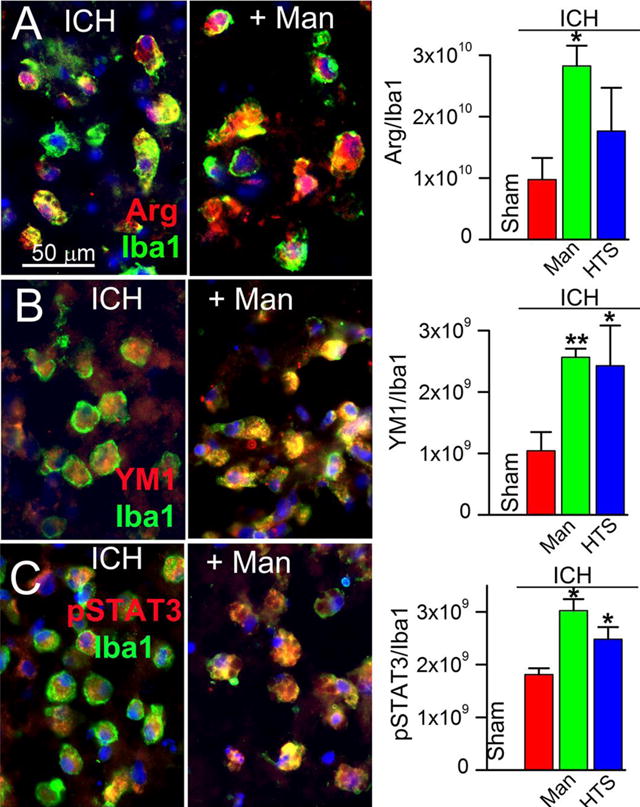
A–C: Double immunolabeling (merged images in left and middle panels) at 48 hours for Iba1 (green) plus arginase (Arg) (A), or YM1 (B) or pSTAT3 (C), with nuclear staining by DAPI, ipsilateral to the ICH (moderate-dose collagenase model), for ICH without treatment (ICH), and ICH with mannitol treatment (+Man), as indicated; the bar graphs show quantification of Arg, YM1 and pSTAT3 relative to Iba1 labeling in the four conditions indicated (right panels); *, P<0.05 and **, P<0.01 for treatments compared to no treatment; n=5/group.
Infiltrating immune cells
CD45 is a receptor-linked protein tyrosine phosphatase that is expressed on all leucocytes (26) and is often used to quantify tissue infiltration of peripheral immune cells. In shams, CD45+ cells were undetectable (not shown). In perihematomal tissues from untreated ICH rats, CD45+ cells were abundant (Fig. 6A, ICH). In perihematomal tissues, both mannitol (Fig. 6B) and HTS (Fig. 6C) were associated with reduced CD45+ cells. Quantification of CD45 confirmed a significant increase in untreated ICH, and significant decreases with both mannitol and HTS, relative to untreated ICH (Fig. 6D).
Figure 6. Mannitol and hypertonic saline reduce the infiltration of CD45+ cells.
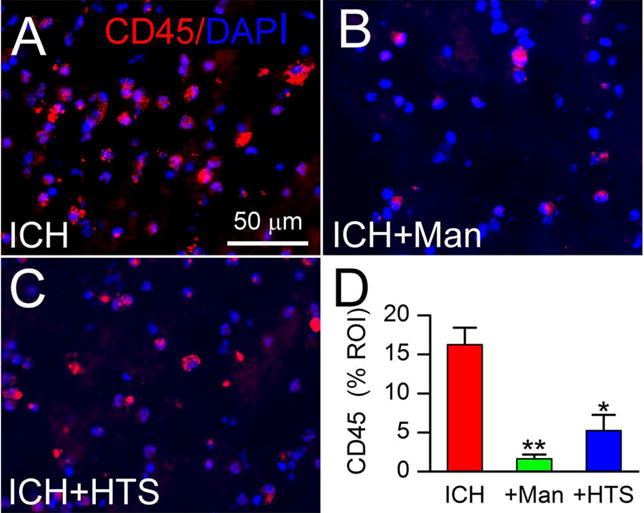
A–D: Immunolabeling at 48 hours for CD45 ipsilateral to the ICH (moderate-dose collagenase model), for ICH without treatment (ICH) (A), ICH with mannitol treatment (ICH+Man) (B), and ICH with hypertonic saline treatment (ICH+HTS) (C); nuclei stained with DAPI (blue); the bar graphs show CD45+ cells as percent region of interest (% ROI) in the three conditions (D); *, P<0.05 and **, P<0.01 for treatments compared to no treatment; n=5/group.
DISCUSSION
The principal findings of the present study are that, in collagenase models of ICH of two severities: (i) mannitol and HTS reduced death and brain swelling, commensurate with reports that osmotherapies reduce ICP (16–18); (ii) repeated dosing of mannitol and HTS over the course of 48 hours after ICH reduced the pro-inflammatory profile in viable perihematomal and contralateral tissues. The effects of osmotherapies on ICP were reported previously in a canine model of ICH (16), a rodent model with cold injury to the brain (17), and a rodent model of subarachnoid hemorrhage (18), but effects on mortality, swelling and inflammation in models of ICH have not been reported previously.
Mannitol and HTS were highly effective in quelling the inflammatory response in perihematomal and contralateral tissues in a rat model of ICH. Both mannitol and HTS reduced microglial activation, attenuated M1-like features, and promoted the anti-inflammatory, phagocytic M2-like microglial phenotype. The effects of mannitol and HTS on microglia that we identified in ICH tissues is in keeping with reports in rodent models of stroke (11) and traumatic brain injury (12,13).
On some outcome measures, specifically mortality and immunoreactivity for TNF and CD45, mannitol appeared to be more efficacious than equi-osmolar HTS, suggesting possible involvement of non-osmotic mechanisms. Prior studies comparing the two osmotherapies found mannitol to be superior to HTS regarding reduced leukocyte recruitment into the injured brain (13), free radical scavenging (27), and reduced malondialdehyde levels indicative of cellular oxidative damage (28). Anti-inflammatory effects of mannitol also have been linked to inhibition of lipid peroxidation and inhibition of NF-κB complex formation (29).
Tissues contralateral to the ICH exhibited microglial activation, and this response also was attenuated by osmotherapy. It was previously reported that the collagenase ICH model is associated with contralateral edema (excess water) (24), but to our knowledge, the present study is the first report to show an effect on microglial activation contralateral to the ICH. In patients with ICH, brain atrophy occurs in both the ipsilateral and contralateral hemispheres (30). It is tempting to think that atrophy in ICH may be related to neuroinflammation, as in other conditions, and that this potentially could be targeted by osmotherapy.
Guidelines from the American Heart Association/American Stroke Association recommend using mannitol in the context of ICH with increased ICP (31). Despite a variety of observational studies (32,33), clinical trials (34,35), and one systematic review (36), no clear long-term benefit of mannitol has been demonstrated. However, in these studies, mannitol invariably was administered in a “reactionary” manner to treat ICP elevations, with variable doses and durations of treatment. By contrast, the approach taken in the present study emphasized repeated bolus dosing at 12-hour intervals over 48 hours beginning 5 hours after ICH onset, with no regard to ICP. It remains an open question whether targeting inflammation using repeated dosing of osmotherapeutics would benefit humans with acute ICH.
The effects found here with mannitol and HTS resemble effects reported for minocycline, a recognized inhibitor of microglial activation. In rat models of ICH, minocycline protects the blood-brain barrier and reduces edema (24,37), reduces inflammation (38), and selectively inhibits M1 polarization of microglia (39), with these effects of minocycline associated with reduced neurological impairment and reduced brain atrophy (37).
The importance of promoting M2-like microglial responses, especially during the recovery phase of ICH, is increasingly recognized as a key element to ameliorate brain injury following ICH (5). Currently, several promising drugs are in early-phase clinical trials for ICH, including minocycline, fingolimod, rosuvastatin and erythropoietin [reviewed by Lan et al. (5)]. All exert neuroprotective effects in part by targeting M1-like microglial activation and promoting M2-like microglial anti-inflammatory pro-phagocytic responses. The data presented here suggest that osmotherapeutic agents that are already approved for use in humans with ICH could potentially serve as alternatives to these drugs by simply changing the dosing regimens currently used.
This study has shortcomings. We studied only the collagenase model of ICH, and it is possible that different findings would be obtained using the autologous blood injection model, which reportedly is associated with a less robust inflammatory response (3,15). The mortality experiment with the high-dose collagenase model was apparently underpowered. Our experiments were of short duration, 48 hours, and so cannot address important longer term outcomes such as the rate of clot resorption, long-term neurofunctional outcomes or brain atrophy. Our experiments did not address the molecular mechanism for the effects on microglia, although we speculate that a transcription factor sensitive to osmotic pressure, such as TonEBP (40), may be involved in the salutary effects observed with the osmotherapeutic agents.
In summary, we found that commonly used osmotherapeutic agents exert potent effects on microglial activation, attenuating the M1-like phenotype and promoting the M2-like phenotype in a manner that resembles the effects of bona fide inhibitors of microglial activation such as minocycline. Further exploration of the anti-inflammatory effects of these agents, especially mannitol, may be warranted in both preclinical and clinical settings.
Acknowledgments
This work was supported in part by a grant to DLS from the Department of Anesthesia. JMS is supported by a grant from the National Heart, Lung, and Blood Institute (HL082517).
Footnotes
CONFLICT OF INTEREST
All authors declare no conflict of interest.
References
- 1.Urday S, Kimberly WT, Beslow LA, et al. Targeting secondary injury in intracerebral haemorrhage–perihaematomal oedema. Nature reviews Neurology. 2015;11:111–22. doi: 10.1038/nrneurol.2014.264. [DOI] [PubMed] [Google Scholar]
- 2.Torre-Healy A, Marko NF, Weil RJ. Hyperosmolar therapy for intracranial hypertension. Neurocritical care. 2012;17:117–30. doi: 10.1007/s12028-011-9649-x. [DOI] [PubMed] [Google Scholar]
- 3.Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Progress in neurobiology. 2010;92:463–77. doi: 10.1016/j.pneurobio.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mracsko E, Veltkamp R. Neuroinflammation after intracerebral hemorrhage. Frontiers in cellular neuroscience. 2014;8:388. doi: 10.3389/fncel.2014.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lan X, Han X, Li Q, Yang QW, Wang J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nature reviews Neurology. 2017;13:420–33. doi: 10.1038/nrneurol.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durafourt BA, Moore CS, Zammit DA, et al. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60:717–27. doi: 10.1002/glia.22298. [DOI] [PubMed] [Google Scholar]
- 7.Junger WG, Hoyt DB, Davis RE, et al. Hypertonicity regulates the function of human neutrophils by modulating chemoattractant receptor signaling and activating mitogen-activated protein kinase p38. The Journal of clinical investigation. 1998;101:2768–79. doi: 10.1172/JCI1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Junger WG, Rhind SG, Rizoli SB, et al. Resuscitation of traumatic hemorrhagic shock patients with hypertonic saline-without dextran-inhibits neutrophil and endothelial cell activation. Shock. 2012;38:341–50. doi: 10.1097/SHK.0b013e3182635aca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shields CJ, O’Sullivan AW, Wang JH, Winter DC, Kirwan WO, Redmond HP. Hypertonic saline enhances host response to bacterial challenge by augmenting receptor-independent neutrophil intracellular superoxide formation. Annals of surgery. 2003;238:249–57. doi: 10.1097/01.sla.0000080827.77985.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizoli SB, Rhind SG, Shek PN, et al. The immunomodulatory effects of hypertonic saline resuscitation in patients sustaining traumatic hemorrhagic shock: a randomized, controlled, double-blinded trial. Annals of surgery. 2006;243:47–57. doi: 10.1097/01.sla.0000193608.93127.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang LQ, Zhu GF, Deng YY, et al. Hypertonic saline alleviates cerebral edema by inhibiting microglia-derived TNF-alpha and IL-1beta-induced Na-K-Cl Cotransporter up-regulation. Journal of neuroinflammation. 2014;11:102. doi: 10.1186/1742-2094-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soustiel JF, Vlodavsky E, Zaaroor M. Relative effects of mannitol and hypertonic saline on calpain activity, apoptosis and polymorphonuclear infiltration in traumatic focal brain injury. Brain research. 2006;1101:136–44. doi: 10.1016/j.brainres.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 13.Kumasaka K, Marks JA, Eisenstadt R, et al. In vivo leukocyte-mediated brain microcirculatory inflammation: a comparison of osmotherapies and progesterone in severe traumatic brain injury. American journal of surgery. 2014;208:961–8. doi: 10.1016/j.amjsurg.2014.08.004. discussion 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue M, Del Bigio MR. Intracerebral injection of autologous whole blood in rats: time course of inflammation and cell death. Neuroscience letters. 2000;283:230–2. doi: 10.1016/s0304-3940(00)00971-x. [DOI] [PubMed] [Google Scholar]
- 15.MacLellan CL, Silasi G, Poon CC, et al. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008;28:516–25. doi: 10.1038/sj.jcbfm.9600548. [DOI] [PubMed] [Google Scholar]
- 16.Qureshi AI, Wilson DA, Traystman RJ. Treatment of elevated intracranial pressure in experimental intracerebral hemorrhage: comparison between mannitol and hypertonic saline. Neurosurgery. 1999;44:1055–63. doi: 10.1097/00006123-199905000-00064. discussion 63–4. [DOI] [PubMed] [Google Scholar]
- 17.Mirski AM, Denchev ID, Schnitzer SM, Hanley FD. Comparison between hypertonic saline and mannitol in the reduction of elevated intracranial pressure in a rodent model of acute cerebral injury. Journal of neurosurgical anesthesiology. 2000;12:334–44. doi: 10.1097/00008506-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Bermueller C, Thal SC, Plesnila N, Schmid-Elsaesser R, Kreimeier U, Zausinger S. Hypertonic fluid resuscitation from subarachnoid hemorrhage in rats: a comparison between small volume resuscitation and mannitol. Journal of the neurological sciences. 2006;241:73–82. doi: 10.1016/j.jns.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–84. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- 20.Marks JA, Li S, Gong W, et al. Similar effects of hypertonic saline and mannitol on the inflammation of the blood-brain barrier microcirculation after brain injury in a mouse model. The journal of trauma and acute care surgery. 2012;73:351–7. doi: 10.1097/TA.0b013e3182592f76. [DOI] [PubMed] [Google Scholar]
- 21.MacLellan CL, Auriat AM, McGie SC, et al. Gauging recovery after hemorrhagic stroke in rats: implications for cytoprotection studies. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26:1031–42. doi: 10.1038/sj.jcbfm.9600255. [DOI] [PubMed] [Google Scholar]
- 22.Patel AD, Gerzanich V, Geng Z, Simard JM. Glibenclamide reduces hippocampal injury and preserves rapid spatial learning in a model of traumatic brain injury. Journal of neuropathology and experimental neurology. 2010;69:1177–90. doi: 10.1097/NEN.0b013e3181fbf6d6. [DOI] [PubMed] [Google Scholar]
- 23.Verdaasdonk JS, Lawrimore J, Bloom K. Determining absolute protein numbers by quantitative fluorescence microscopy. Methods in cell biology. 2014;123:347–65. doi: 10.1016/B978-0-12-420138-5.00019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wasserman JK, Schlichter LC. Minocycline protects the blood-brain barrier and reduces edema following intracerebral hemorrhage in the rat. Experimental neurology. 2007;207:227–37. doi: 10.1016/j.expneurol.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 25.Kurland DB, Gerzanich V, Karimy JK, et al. The Sur1-Trpm4 channel regulates NOS2 transcription in TLR4-activated microglia. Journal of neuroinflammation. 2016;13:130. doi: 10.1186/s12974-016-0599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altin JG, Sloan EK. The role of CD45 and CD45-associated molecules in T cell activation. Immunology and cell biology. 1997;75:430–45. doi: 10.1038/icb.1997.68. [DOI] [PubMed] [Google Scholar]
- 27.Ouriel K, Ginsburg ME, Patti CS, Pearce FJ, Hicks GL. Preservation of myocardial function with mannitol reperfusate. Circulation. 1985;72:II254–8. [PubMed] [Google Scholar]
- 28.Yilmaz N, Dulger H, Kiymaz N, Yilmaz C, Gudu BO, Demir I. Activity of mannitol and hypertonic saline therapy on the oxidant and antioxidant system during the acute term after traumatic brain injury in the rats. Brain research. 2007;1164:132–5. doi: 10.1016/j.brainres.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 29.Kim H, Seo JY, Kim KH. Effects of mannitol and dimethylthiourea on helicobacter pylori-induced IL-8 production in gastric epithelial cells. Pharmacology. 1999;59:201–11. doi: 10.1159/000028321. [DOI] [PubMed] [Google Scholar]
- 30.Kim JH, Kim YS, Kim SH, et al. Contralateral Hemispheric Brain Atrophy After Primary Intracerebral Hemorrhage. World neurosurgery. 2017;102:56–64. doi: 10.1016/j.wneu.2017.02.105. [DOI] [PubMed] [Google Scholar]
- 31.Hemphill JC, 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–60. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 32.Gigliuto CM, Stone KE, Algus M. The use of mannitol in intracerebral bleeds in the medical ICU. New Jersey medicine: the journal of the Medical Society of New Jersey. 1991;88:48–51. [PubMed] [Google Scholar]
- 33.Bereczki D, Mihalka L, Szatmari S, et al. Mannitol use in acute stroke: case fatality at 30 days and 1 year. Stroke. 2003;34:1730–5. doi: 10.1161/01.STR.0000078658.52316.E8. [DOI] [PubMed] [Google Scholar]
- 34.Kalita J, Misra UK, Ranjan P, Pradhan PK, Das BK. Effect of mannitol on regional cerebral blood flow in patients with intracerebral hemorrhage. Journal of the neurological sciences. 2004;224:19–22. doi: 10.1016/j.jns.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 35.Misra UK, Kalita J, Ranjan P, Mandal SK. Mannitol in intracerebral hemorrhage: a randomized controlled study. Journal of the neurological sciences. 2005;234:41–5. doi: 10.1016/j.jns.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 36.Bereczki D, Fekete I, Prado GF, Liu M. Mannitol for acute stroke. The Cochrane database of systematic reviews. 2007:CD001153. doi: 10.1002/14651858.CD001153.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu J, Yang S, Hua Y, Liu W, Keep RF, Xi G. Minocycline attenuates brain edema, brain atrophy and neurological deficits after intracerebral hemorrhage. Acta neurochirurgica Supplement. 2010;106:147–50. doi: 10.1007/978-3-211-98811-4_26. [DOI] [PubMed] [Google Scholar]
- 38.Wasserman JK, Schlichter LC. Neuron death and inflammation in a rat model of intracerebral hemorrhage: effects of delayed minocycline treatment. Brain research. 2007;1136:208–18. doi: 10.1016/j.brainres.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi K, Imagama S, Ohgomori T, et al. Minocycline selectively inhibits M1 polarization of microglia. Cell death & disease. 2013;4:e525. doi: 10.1038/cddis.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeong GR, Im SK, Bae YH, et al. Inflammatory signals induce the expression of tonicity-responsive enhancer binding protein (TonEBP) in microglia. Journal of neuroimmunology. 2016;295–296:21–9. doi: 10.1016/j.jneuroim.2016.04.009. [DOI] [PubMed] [Google Scholar]


