Abstract
Objective
Coronary artery thrombosis can occur in the absence of plaque rupture due to superficial erosion. Erosion-prone atheromata associate with more neutrophil extracellular traps (NETs) than lesions with stable or rupture-prone characteristics. The effects of NETs on endothelial cell (EC) inflammatory and thrombogenic properties remain unknown. We hypothesized that NETs alter EC functions related to erosion-associated thrombosis.
Approach and Results
Exposure of human ECs to NETs increased VCAM-1 and ICAM-1 mRNA and protein expression in a time- and concentration-dependent manner. THP-1 monocytoid cells and primary human monocytes bound more avidly to NET-treated human umbilical vein ECs than to unstimulated cells under flow. Treatment of human ECs with NETs augmented the expression of tissue factor (TF) mRNA, increased EC TF activity, and hastened clotting of re-calcified plasma. Anti-TF neutralizing antibody blocked NET-induced acceleration of clotting by ECs. NETs alone did not exhibit TF activity or acceleration of clotting in cell-free assays. Pre-treatment of NETs with anti-IL-1α-neutralizing antibody or IL-1 receptor antagonist (IL1Ra)—but not with anti-IL-1β-neutralizing antibody or control IgG—blocked NET- induced VCAM-1, ICAM-1, and TF expression. Inhibition of cathepsin G, a serine protease abundant in NETs, also limited the effect of NETs on EC activation. Cathepsin G potentiated the effect of IL-1α on ECs by cleaving the pro-IL-1α precursor and releasing the more potent mature IL-1α form.
Conclusions
NETs promote EC activation and increased thrombogenicity through concerted action of IL-1α and cathepsin G. Thus, NETs may amplify and propagate EC dysfunction related to thrombosis due to superficial erosion.
Keywords: neutrophil extracellular traps, endothelial cells, interleukin-1, cathepsin G, vascular cell adhesion molecules, tissue factor
Subject codes: Cell Biology/Structural Biology, Growth Factors/Cytokines, Inflammation, Mechanisms, Pathophysiology, Vascular Biology, Cardiovascular Disease, Atherosclerosis, Thrombosis
INTRODUCTION
Superficial erosion of atherosclerotic plaques, a distinct pathway to coronary thrombosis of likely rising prevalence in the statin era, currently accounts for about one-third of acute coronary syndromes.1–3 Eroded plaques provoke thrombosis at focal areas of intimal endothelial cell (EC) loss without plaque fibrous cap rupture.4 The composition of erosion-prone plaques differs markedly from that of rupture-prone plaques: those with erosion contain more proteoglycans, glycosaminoglycans, and smooth muscle cells but fewer macrophages and less lipid and necrotic cores than ruptured plaques.5 While the mechanisms that drive erosion remain poorly understood, the characteristic morphology and composition of eroded plaques suggest the operation of distinct pathophysiologic mechanisms.
Recent data from our laboratory support a role for neutrophils in erosion-associated thrombosis.2, 6–8 Disturbed blood flow distal to stenoses and engagement of Toll-like receptor 2 induce EC activation and neutrophil recruitment to plaques. Neutrophils promote EC apoptosis and desquamation, favoring platelet recruitment, thrombin generation, and thrombus formation.2, 6, 7, 9–13 Neutrophil activation can trigger the release of neutrophil extracellular traps (NETs)— macromolecular aggregates that contain DNA, histones, and granular enzymes—through a programmed cell death pathway known as NETosis.14, 15 We recently reported that NETs participate causally in acute thrombotic complications of mouse intimal lesions that recapitulate features of superficial erosion in humans.8 Erosion-prone human carotid artery plaques exhibit a higher content of NETs than plaques with rupture-prone or stable characteristics.7 NETs can promote thrombosis directly by several mechanisms including acting as a “solid-state reactor” by entrapping circulating platelets and coagulation factors, and activating platelets via neutrophil proteases and histones.16–21 Furthermore, NET-associated effectors including neutrophil elastase (NE), proteinase 3 (PR3), and histones as well as HOCl—produced locally by the abundant NET component myeloperoxidase (MPO)—can induce EC expression of tissue factor (TF), the initiator of the extrinsic coagulation cascade.22–25 These studies suggest that NETs may augment plaque thrombogenicity in several ways.
This study tested the hypothesis that NETs evoke activation and accentuate thrombogenicity of human vascular ECs. Our findings unveil a novel mechanism by which NETs can propagate and amplify endothelial dysfunction and induce thrombogenicity, in a process that depends on interleukin (IL)-1α and cathepsin G (CatG). The results provide new insight into the mechanisms of superficial erosion, and have substantial translational potential given the availability of therapeutic anti-human IL-1α antibodies.26
MATERIALS AND METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Reagents
Glutathione S-transferase (GST)-pro-IL1α and GST-pro-IL1β fusion proteins purified from a wheat germ expression system were purchased from Abnova (Taipei, Taiwan), recombinant human IL-1α and IL-1β from Peprotech (Rocky Hill, New Jersey), thapsigargin from Abcam (Cambridge, MA), and N-formyl-methionyl-leucyl-phenylalanine (fMLP) from Sigma-Aldrich (St. Louis, MO). Anti-IL-1α-neutralizing antibody (MABp1) and IgG isotype control were obtained from Dr. John Simard at XBiotech (Austin, TX) and anti-IL-1β-neutralizing antibody (canakinumab) from Dr. Hermann Gram at Novartis (Basel, Switzerland). Anti-TF-neutralizing antibody (TF8-5G9) was provided by Drs. James Morrisey and Kevin Croce. CatG from human neutrophils was purchased from Sigma-Aldrich, CatG inhibitor I from Cayman Chemical Co. (Ann Arbor, MI), CatG substrate from Bachem (Torrance, CA), and NE substrate from EMD Millipore (Billerica, MA).
Cell culture
Human saphenous vein ECs (HSVECs) or umbilical vein ECs (HUVECs) were harvested respectively from patients undergoing coronary artery bypass grafting or from umbilical cord segments as described.27, 28 Human aortic endothelial cells (HAECs) were purchased from Lonza Biologics (Portsmouth, NH). Cells were cultured on plates coated with 0.1% gelatin (Sigma-Aldrich) in Medium 199 (Lonza) supplemented with fetal bovine serum (FBS, 20% for HSVECs and HAECs or 10% for HUVECs), penicillin-streptomycin, L-glutamine, heparin, and bovine hypothalamic endothelial cell growth supplement (Alfa Aesar, Haverhill, MA). After reaching confluence, HSVECs or HAECs were incubated in OptiMEM (Lonza Biologics Inc., Portsmouth, NH) supplemented with 3% FBS for 16 hours prior to the addition of the indicated reagents for various time periods as described in each experiment. Treatment of HUVECs with NETs was carried out in HUVEC growth medium. Primary human monocytes were isolated by RosetteSep™ Human Monocyte Enrichment Cocktail (Stem Cell Technologies, Vancouver, Canada) following the manufacturer’s instructions.
Preparation of NETs
Human neutrophils were isolated from buffy coats obtained from Massachusetts General Hospital. NETs were generated by stimulating neutrophils with 50 nM phorbol myristate acetate (PMA) for 4 hours, followed by extensive washing with PBS and NET isolation as described.29 Some experiments used NETs prepared from neutrophils stimulated with 500 nM thapsigargin for 4 hours. Characterization of NETs included DNA quantification using Sytox green nucleic acid labeling (Thermo Fisher Scientific Inc., Waltham, MA, USA); colorimetric assays that measured the enzymatic activity of NE + PR3 (0.142 ± 0.022 units/ng DNA, n = 7) and myeloperoxidase (0.497 ± 0.074 units/ng DNA, n = 6); fluorometric assays that determined the enzymatic activity of CatG (0.127 ± 0.030 units/ng DNA, n = 6); immunoblotting showing the presence of neutrophil elastase (NE), proteinase 3 (PR3), CatG, and citrullinated histone H3 (R26) (H3Cit); and immunofluorescence microscopy that co-localized DNA with H3Cit and NE (Supplemental Fig. I).
RNA Isolation and Reverse Transcription-Quantitative Polymerase Chain Reaction
Total RNA was isolated using an Ambion PureLink mini kit (Thermo Fisher Scientific Inc., Waltham, MA) and reverse-transcribed by Superscript II (Invitrogen, Carlsbad, CA). Quantitative PCR was performed in a MyiQ Single-Color Real-Time PCR system using SYBR Green I (Bio-Rad, Hercules, CA). The mRNA levels of the genes tested were normalized to 18S or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as internal controls as indicated in each figure. The primer sequences were: 18S, 5′-ATGGCCGTTCTTAGTTGGTG-3′ and 5′-GAACGCCACTTGTCCCTCTA-3; GAPDH, 5’-GAAGGTGAAGGTCGGAGTCA-3’ and 5’-AATGAAGGGGTCATTGATGG-3’; vascular cell adhesion molecule 1 (VCAM-1), 5’-GTCAATGTTGCCCCCAGAGA-3’ and 5’-TGCCTGCTCCACAGGATTTT-3’; intercellular adhesion molecule 1 (ICAM-1), 5’-AGCTTCGTGTCCTGTATGGC-3’ and 5’-TTT TCTGGCCACGTCCAGTT-3’; TF, 5’-GTCTTCGCCCAGGTGGC-3’ and 5’-TGACTTAGTGCTTATTTGAACAGTG-3’.
Immunoblot and Cell Surface Biotinylation
Whole-cell lysates from 25,000 ECs were fractionated on 4% to 12% gradient SDS-PAGE gels (Life Technologies, Grand Island, NY) and transferred to polyvinylidene difluoride membranes. After blocking with 5% defatted milk and incubating with the appropriate antibodies, membranes were developed using a chemiluminescence reagent (Thermo Scientific, Waltham, MA). Anti-IL-1α (Cat.# ab9614), anti-PR3 (Cat.# 133613), anti-H3Cit R2-8-17 (Cat.# ab5103), anti-NE (Cat.# 131260), and anti-CatG (Cat.# 50845) were from Abcam; anti-IL-1β (Cat. #7884) from Santa Cruz (Santa Cruz, CA); anti-ICAM-1 (Cat.# 611704) from BD Biosciences (San José, CA); and anti-VCAM-1 (Cat.# 13662), anti-β-actin (Cat.# 4967), anti-GAPDH (Cat.# 2118), anti-calnexin (Cat.# 2433), anti-nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) inhibitor α (IκBα) (Cat# 9242), and anti-phospho-IκBα (Ser32) (Cat.# 2859) were from Cell Signaling (Danvers, MA). Biotinylation of cell surface glycoproteins and precipitation with streptavidin-agarose were performed as described previously.30
Interaction of HUVECs with THP-1 Cells under Flow
Confluent HUVEC monolayers were cultured on 25-mm glass coverslips coated with 5 µg/ml fibronectin (Corning, NY). After cell treatment with NETs for 6 hours, the coverslips were transferred to a parallel-plate flow chamber mounted on an inverted microscope as described.31 THP-1 cells or primary human monocytes were drawn through the chamber at an estimated shear stress of 0.75 dynes/cm2 for 5 min. Interactions were recorded using a 10× objective and a video microscope connected to Videolab software (Ed Marcus Laboratories, Boston, MA) and quantified by counting the number of firmly adhered THP-1 cells in 4 different fields.
Protease, TF, and MPO Activity Assays
NE + PR3 activity was determined by incubating NETs with Succinyl-Ala-Ala-Pro-Val-p-nitroanilide in 50 mM Tris-HCl buffer pH 7.5 at 37°C, following the release of p-nitroaniline by measuring optical density (OD) at 410 nm every 2 minutes on a SpectraMax i3× multimode detection platform (Molecular Devices LLC, Sunnyvale, CA). CatG activity was determined by incubating NETs with Succinyl-Ala-Ala-Pro-Phe-7-amido-4-methyl-coumarin in 50 mM Tris-HCl buffer pH 7.5 at 37°C, following the release of 7-amino-4-methylcoumarin by measuring fluorescence (excitation, 370 nm; emission, 450 nm) every 2 minutes. Myeloperoxidase activity was assessed by incubating NETs with 3,3′,5,5′-tetramethylbenzidine, following substrate oxidation by measuring OD at 650 nm every 2 minutes. One activity unit of NE or MPO is defined as the amount of activity corresponding to a ΔOD = 1 per hour under standard assay conditions. One activity unit of CatG is defined as the amount of activity corresponding to a ΔRFU = 1 per hour under standard assay conditions (RFU = relative fluorescence units).
To assess TF pro-coagulant activity, HSVECs were incubated with NETs for the time intervals indicated in each experiment and lysed by incubating with 15 mM octyl-β-D-glucopyranoside (Sigma-Aldrich) at 37°C for 15 minutes. Activity was determined using a chromogenic assay (Abcam, Cambridge, UK) that measures levels of Factor X activation by the complex of cell lysate TF and exogenous Factor VII, following the manufacturer’s instructions. One activity unit is defined as the activity corresponding to 1 pM recombinant TF under the standard assay conditions.
Re-Calcification Clotting Assay
The effect of NETs on HSVEC’s plasma clotting activity was assessed in 96-well plates by a modified re-calcification assay previously described32 using either intact monolayers or lysates from control or NET-treated HSVECs. One hundred microliters of cell lysates were incubated with 50 µL of pooled platelet-poor human plasma (George King Biomedical Inc., Overland Park, KS) at 37°C for 2 minutes, followed by the addition of 50 µL of pre-warmed 25 mM CaCl2. The rate of fibrin formation was quantified by measuring OD at 405 nm every 10 seconds for 30 minutes. Clotting time is defined as the time elapsed between the first read and 50% of the maximal OD405 reading. To assess clotting in the setting of an intact EC monolayer, HSVECs were stimulated with NETs for 8 hours, followed by two washes with Hanks’ balanced salt solution and the addition of 150 µL of plasma. The clotting assay was then completed as described above.
Immunofluorescence
To visualize translocation of NFκB p65 to the nucleus, HSVECs were cultured on Permanox slides (Thermo Fisher Scientific Inc., Waltham, MA, USA) coated with Cultrex basement membrane extract (Trevigen, Gaithersburg, MD, USA), treated with NETs for 90 minutes in OptiMEM containing 3% FBS, and then fixed with 4% paraformaldehyde. Slides were stained overnight with 4’6-diamidino-2-phenylindole (DAPI) and with anti-NFκB p65 antibody (Abcam, Cat.# ab16502), followed by staining with goat anti-rabbit secondary antibody conjugated to Alexa Fluor 555 (Thermo Fisher Scientific Inc.). For NET staining, isolated NETs were fixed and stained as above with DAPI and anti-H3cit or anti-NE (Abcam), followed by goat anti-rabbit secondary antibody conjugated to Alexa Fluor 555. Slides were visualized using a BX61WI microscope with an Olympus FV1000 confocal unit, and photographed using an attached Olympus DP72 camera and Fluoview 10-ASW software.
Statistical Analysis
Results are presented as mean values ± SEM. Data were analyzed to assess distribution normality. For normally distributed variables, statistical significance was assessed by Student’s unpaired t-test (2 groups) or by ordinary one-way ANOVA (> 2 groups). For non-normally-distributed variables, statistical significance was assessed by Mann-Whitney test (2 groups) or Kruskal Wallis test for multiple comparisons (p<0.05 considered significant). Graphpad Prism software was used to run statistical tests.
RESULTS
NETs induce EC expression of leukocyte adhesion molecules
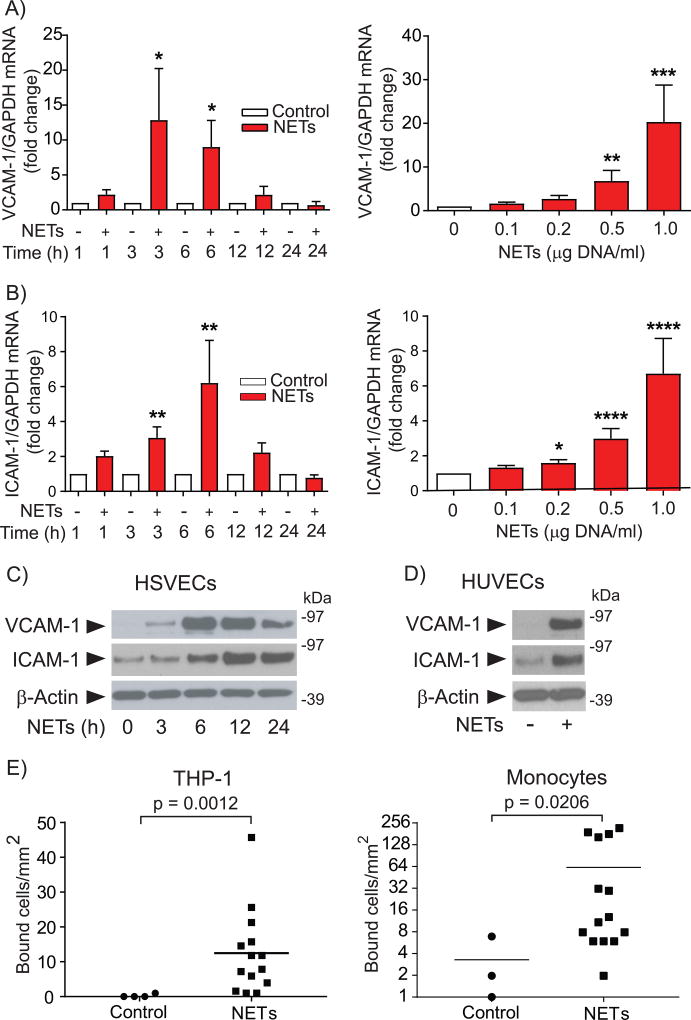
To test the hypothesis that NETs activate EC functions related to superficial erosion, this study evaluated the expression of VCAM-1 and ICAM-1 in HSVECs exposed to various concentrations of NETs for different time intervals. Compared to unstimulated controls, cells treated with NETs exhibited higher mRNA and protein concentrations corresponding to these adhesion molecules. The mRNA expression of VCAM-1 and ICAM-1, which depended on NET concentration, reached maximal levels at 3–6 hours of stimulation and then decayed (Fig. 1, A & B). The expression of VCAM-1 protein reached maximum at 6–12 hours, whereas the levels of ICAM-1 peaked at 12 hours and remained elevated up to 24 hours (Fig. 1C). Treatment with NETs increased the expression of adhesion molecules on the surface of HSVECs, assessed by biotinylation of cell surface glycoproteins and precipitation with streptavidin-agarose (Supplemental Fig. II). NETs also induced robust expression of VCAM-1 and ICAM-1 in HUVECs (Fig. 1D) and HAECs (Supplemental Fig. III) and augmented the adhesion of THP-1 monocytoid cells and primary human monocytes to NET-treated HUVEC monolayers (Fig. 1E).
Figure 1. NETs increase the endothelial expression of leukocyte adhesion molecules and augment leukocyte adhesion to ECs under flow.
(A–B) HSVECs were incubated with NETs (0.5 µg DNA/ml) for the indicated periods of time (left panels) or for 3 hours with various concentrations of NETs (right panels), followed by RNA extraction and determination of VCAM-1 and ICAM-1 mRNA levels by RT-qPCR. Levels of GAPDH mRNA served as an internal control for adjustment between samples. Left panels, n = 7; right panels, n = 9. P values: *<0.05, **<0.01, ***<0.001, ****<0.0001 vs. the respective controls. (C) HSVECs were incubated with NETs (0.5 µg DNA/ml) for the indicated periods of time. Whole-cell extracts were fractionated by SDS-PAGE and immunoblotted with antibodies to VCAM-1, ICAM-1, or β-actin. (D) HUVECs were treated with NETs (0.5 µg DNA/ml) for 6 hours and analyzed by immunoblot as in (C). (E) HUVECs were treated with NETs (0.5 µg DNA/ml) for 6 hours, followed by analysis of the adhesion of THP-1 cells (left panel, n = 4) or primary human monocytes (right panel, n = 3) under flow as described in the Materials and Methods section.
The experiments throughout this study used NETs produced by neutrophils stimulated with PMA, a well-established method to elicit NETosis.33, 34 NETs prepared in an independent manner from neutrophils stimulated with thapsigargin—a treatment that increases cytoplasmic Ca2+ concentration and thus activates peptidylarginine deiminase 4 (PAD4), a Ca2+-dependent enzyme that causes DNA decondensation and NETosis—likewise induced expression of VCAM-1 and ICAM-1 in HSVECs (Supplemental Fig. IV). Conversely, the addition of medium conditioned by neutrophils treated with fMLP—a potent neutrophil activator that does not induce NETosis34—did not augment the expression of these adhesion molecules.
IL-1α and CatG mediate NET-induced EC activation
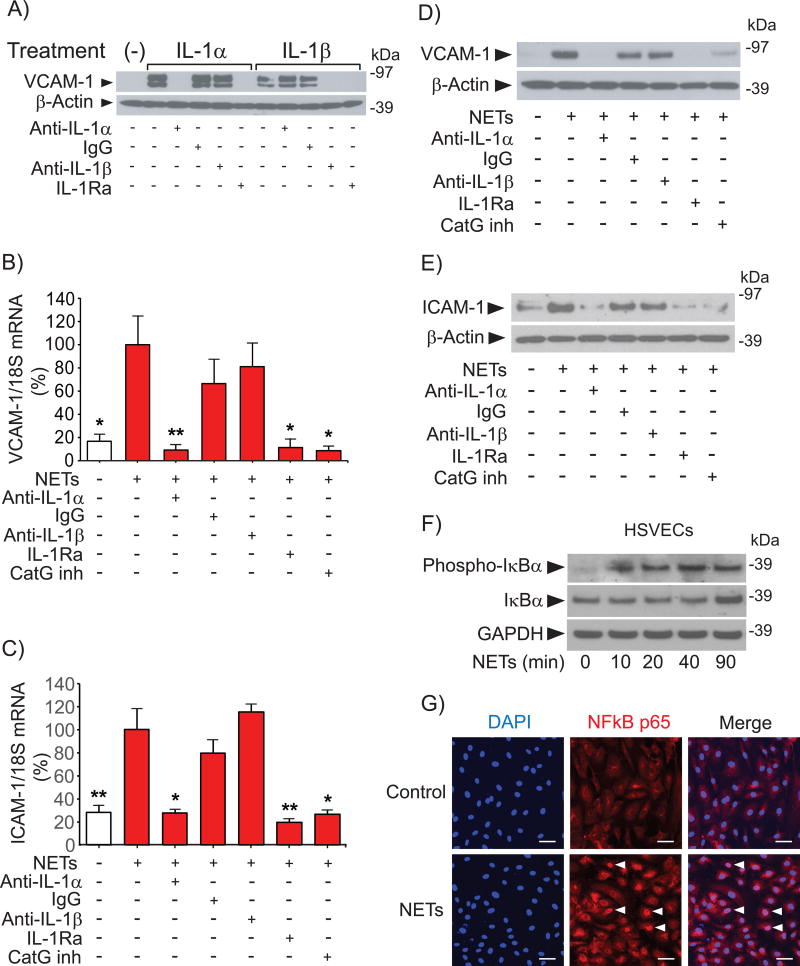
To explore the mechanism by which NETs activate ECs, we examined the content of various cytokines in NET preparations by ELISA. NETs contained IL-1α (2.1 ± 1.1 pg/µg DNA, n = 5) and IL-1β (2.4 ± 1.4 pg/µg DNA, n = 5). Human monoclonal antibodies to IL-1α (MABp1) or IL-1β (canakinumab), both used in clinical trials,26, 35 enabled testing the effect of selective neutralization of IL-1 isoforms on NET-induced expression of VCAM-1 and ICAM-1 in ECs. Assessing the effect of these antibodies on VCAM-1 expression in HSVECs stimulated with recombinant IL-1α or IL-1β validated their specificity. Anti-IL-1α abrogated VCAM-1 expression induced by IL-1α but not by IL-1β, whereas anti-IL-1β inhibited VCAM-1 expression elicited by IL-1β but not by IL-1α. IL-1Ra, which blocks the IL-1 receptor 1 that mediates the effect of both IL-1 isoforms, inhibited VCAM-1 expression induced by either IL-1α or IL-1β as expected (Fig. 2A).
Figure 2. IL-1α and CatG mediate NET-induced expression of leukocyte adhesion molecules in HSVECs.
(A) Cells were incubated with 50 pg/ml IL-1α or IL-1β for 3 hours in the presence of the indicated antibodies (20 ng/ml) or IL-1Ra (1 µg/ml). Whole-cell extracts were fractionated by SDS-PAGE and immunoblotted with antibodies to VCAM-1 or β-actin. (B–C) Cells were incubated with NETs (0.5 µg DNA/ml) for 3 hours in the presence of the indicated antibodies (20 ng/ml), IL-1Ra (1 µg/ml), or CatG inhibitor I (50 µM), followed by RNA extraction and determination of VCAM-1 and ICAM-1 mRNA levels by RT-qPCR. N = 3–7. P values: *<0.05, **<0.01 vs. NETs alone, defined as 100%. (D–E) Cells were incubated as in (B-C) for 6 hours, followed by fractionation of whole-cell extracts by SDS-PAGE and immunoblotting with antibodies to VCAM-1, ICAM-1, or β-actin. (F) Cells were incubated with NETs (0.5 µg DNA/ml) for the indicated periods of time, followed by fractionation of whole-cell extracts by SDS-PAGE and immunoblotting with antibodies to phospho-IκBa, IκBα, or GAPDH. (G) Cells were incubated with NETs (0.5 µg DNA/ml) for 90 minutes, fixed, and stained with anti-NFκB p65 (1/1000 dilution) and DAPI as described in the Materials and Methods section. The arrowheads indicate NFκB localized to the nucleus. Scale bar = 50 µM. Omission of the primary antibody yielded no signal.
Anti-IL-1α or IL-1Ra—but not anti-IL-1β or control IgG—abolished NET-induced expression of VCAM-1 and ICAM-1 in HSVECs (Fig. 2, B–E) and in HUVECs (Suppl. Fig. V), indicating that IL-1α mediates the effect of NETs on the expression of these adhesion molecules in ECs. Concordant with the participation of IL-1α in NET-induced EC activation, NETs elicited NFκB signaling, assessed by phosphorylation of IκB (Fig. 2F) and by translocation of NFκB p65 to the nucleus of HSVECs (Fig. 2G).
NETs contain an array of molecules that participate in neutrophils’ host defense functions. This arsenal includes the serine proteases NE, PR3, and CatG, enzymes implicated in the pathogenesis of several human diseases.36–38 Further experiments investigated whether these proteases participate in the induction of adhesion molecules elicited by NETs in ECs. HSVEC stimulation experiments included 3% FBS, a condition that suppressed the activity of NE and PR3 present in NETs as assessed by determination of the rate of hydrolysis of Succinyl-Ala-Ala-Pro-Val-p-NA, a common substrate for both proteases. This inhibition likely reflects the action of blood anti-proteinases. In contrast, NETs retained 30–40% of their CatG activity in the presence of 3% FBS, evaluated by measuring the hydrolysis of Succinyl-Ala-Ala-Pro-Phe-MCA. Indeed, either fetal bovine or human sera inhibited the activity of these serine proteases differentially (Suppl. Fig. VI). This finding prompted the examination of the effect of CatG inhibition on EC activation induced by NETs. Addition of the reversible competitive CatG inhibitor I limited VCAM-1 and ICAM-1 expression induced by NETs in HSVECs (Fig. 2, B–E) and HUVECs (Suppl. Fig. V), demonstrating that CatG participates in NET-induced EC activation.
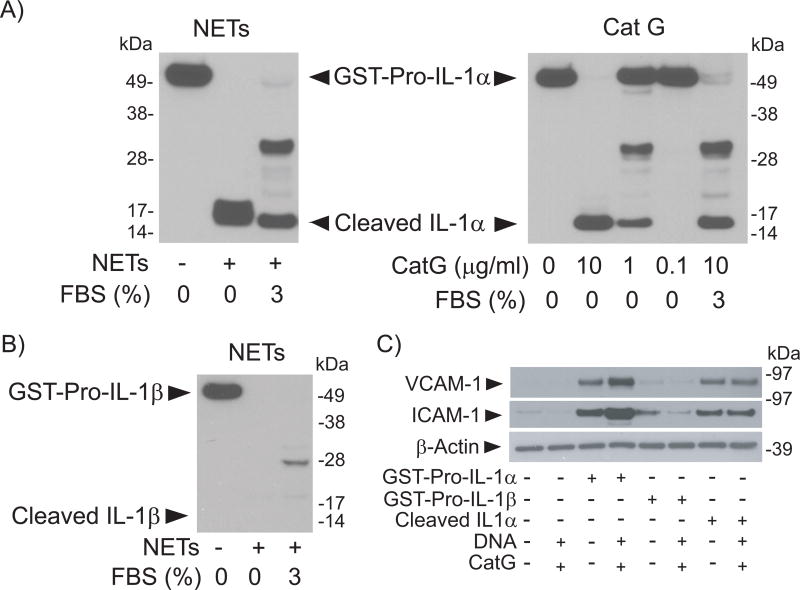
Proteolytic processing enhances the biological activity of the IL-1α precursor, but unprocessed pro-IL-1α can still bind to the IL-1 receptor and activate target cells.39, 40 Conversely, IL-1β requires proteolytic processing of its precursor to attain biological activity. The observation that IL-1α but not IL-1β mediates NET-induced EC activation prompted the examination of the effect of NETs on IL-1 isoforms in vitro. Incubation of NETs with recombinant pro-IL-1α fused to the C-terminus of GST resulted in cleavage of the precursor yielding 16–18 kDa polypeptides corresponding in molecular weight to mature IL-1α (Fig. 3A, left panel). The addition of 3% FBS, a condition that inhibits elastase and PR3 but not CatG, resulted in the elaboration of only the faster-migrating polypeptide, which has a mobility identical to the polypeptide generated by incubation of GST-pro-IL-1α with recombinant CatG (Fig. 3A, right panel). In striking contrast, incubation of GST-pro-IL-1β with NETs in the presence or absence of FBS resulted in the degradation of the fusion protein without accumulation of intermediate polypeptides including the mature form of this cytokine (Fig. 3B).
Figure 3. NETs act differentially on Pro-IL-1α or Pro-IL-1β.
(A) GST-Pro-IL-1α (3.6 µg/ml) was incubated with NETs (left panel, 4 µg DNA/ml), or with various concentrations of recombinant CatG (right panel) for 1 hour at 37°C in the presence or absence of 3% FBS. Samples were fractionated by SDS-PAGE and immunoblotted with antibodies to IL-1α. (B) GST-Pro-IL-1β (3.8 µg/ml) was incubated with NETs (4 µg DNA/ml) for 1 hour at 37°C in the presence or absence of 3% FBS. Samples were fractionated by SDS-PAGE and immunoblotted with antibodies to IL-1β. (C) HSVECs were incubated for 6 hours with GST-Pro-IL-1α (20 pg/ml), GST-Pro-IL-1β (20 pg/ml), or cleaved IL-1α (100 pg/ml) in the presence or absence of DNA (0.16 µg/ml) and CatG (0.032 units/ml). Whole-cell extracts were fractionated by SDS-PAGE and immunoblotted with antibodies to VCAM-1, ICAM-1, or β-actin.
Treatment of HSVECs with pro-IL-1α or with cleaved IL-1α induced the expression of VCAM-1 and ICAM-1 (Fig. 3C). The addition of CatG and DNA in similar quantities to those found in NETs increased the effect of pro-IL1α but not of cleaved IL-1α, indicating that the effect of CatG stems primarily from its ability to process the IL-1α precursor and yield a more active cytokine. CatG—added together with DNA (Fig. 3C) or alone (Suppl. Fig. VII)—did not induce the expression of leukocyte adhesion molecules. In contrast to the action of pro-IL-1α, exposure of HSVECs to Pro-IL-1β in the presence or absence of CatG induced minimal amounts of VCAM-1 and ICAM-1 (Fig. 3C). Collectively, the results indicate that NETs induce EC activation in a process that depends on IL-1α and that involves processing of the IL-1α precursor by CatG.
NETs augment HSVEC thrombogenicity through induction of TF expression
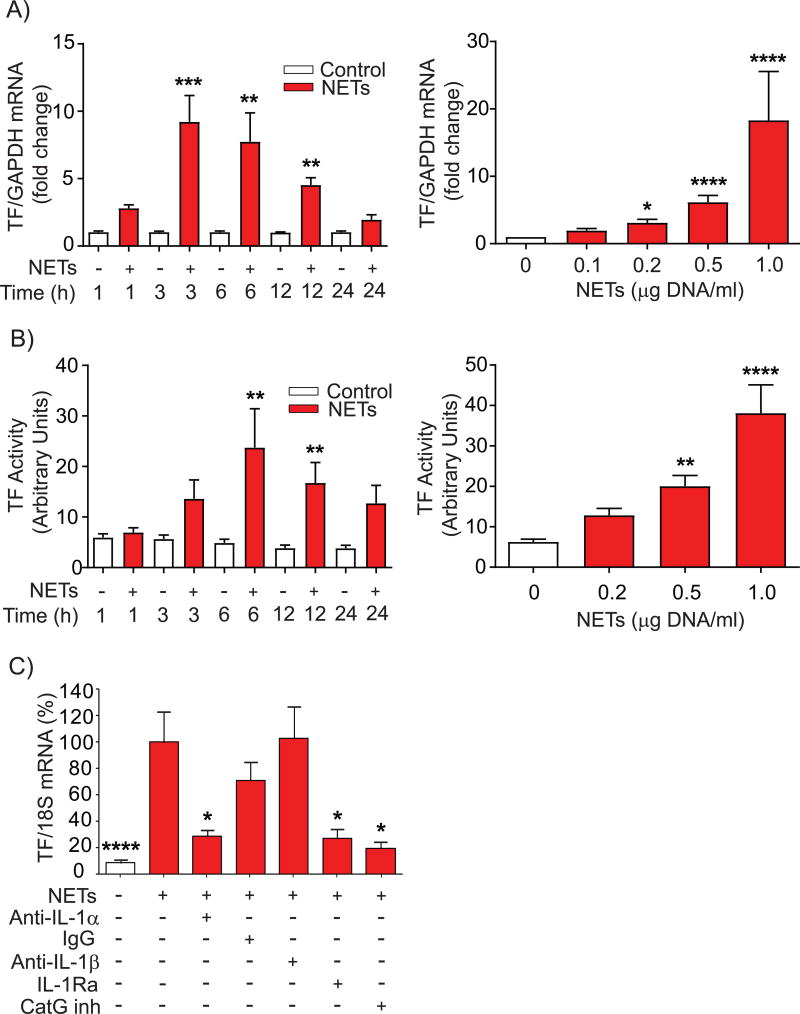
Evaluation of the effect of NETs on EC thrombogenicity involved exposure of HSVECs to NETs at various concentrations and times and examination of the expression and activity of TF. NETs increased TF mRNA expression with a peak after 3 hours (Fig. 4A, left panel), accompanied by augmented TF activity that peaked at 6 hours and remained elevated after 24 hours of treatment (Fig. 4B, left panel). These effects depended on NET concentration (Fig.4, A & B, right panels). Inhibition of the action of IL-1α—either with neutralizing antibodies or by blocking the IL-1 receptor with IL-1Ra—or the addition of CatG inhibitor I limited the effect of NETs on TF mRNA expression (Fig. 4C). Treatment of HSVECs with NETs in the presence of the MPO inhibitors 4-aminobenzoic acid hydrazide (ABAH) or PF-1355 did not reduce NET-induced TF mRNA expression (Suppl. Fig. VIII). Taken together, these results indicate that HSVECs regulate the expression of adhesion molecules and TF by a shared mechanism.
Figure 4. NETs induce endothelial TF expression and pro-coagulant activity.
(A) HSVECs were incubated with NETs (0.5 µg DNA/ml) for the indicated periods of time (left panel, n = 6) or for 3 hours with various concentrations of NETs (right panel, n = 9), followed by RNA extraction and determination of TF mRNA levels by RT-qPCR. Levels of GAPDH mRNA served as an internal control for adjustment between samples. (B) HSVECs were incubated with NETs (0.5 µg DNA/ml) for the indicated periods of time (left panel, n = 8) or for 6 hours with various concentrations of NETs (right panel, n = 6), followed by cell lysis and determination of TF activity as described in the Materials and Methods section. (C) HSVECs were incubated with NETs (0.5 µg DNA/ml) for 3 hours in the presence of the indicated antibodies (20 ng/ml), IL-1Ra (1 µg/ml), or CatG inhibitor I (50 µM), followed by RNA extraction and determination TF mRNA levels by RT-qPCR. N = 4–9. P values: *<0.05, **<0.01, ***<0.001, ****<0.0001 vs. the respective controls (A–D) or vs. NETs alone, defined as 100% (E).
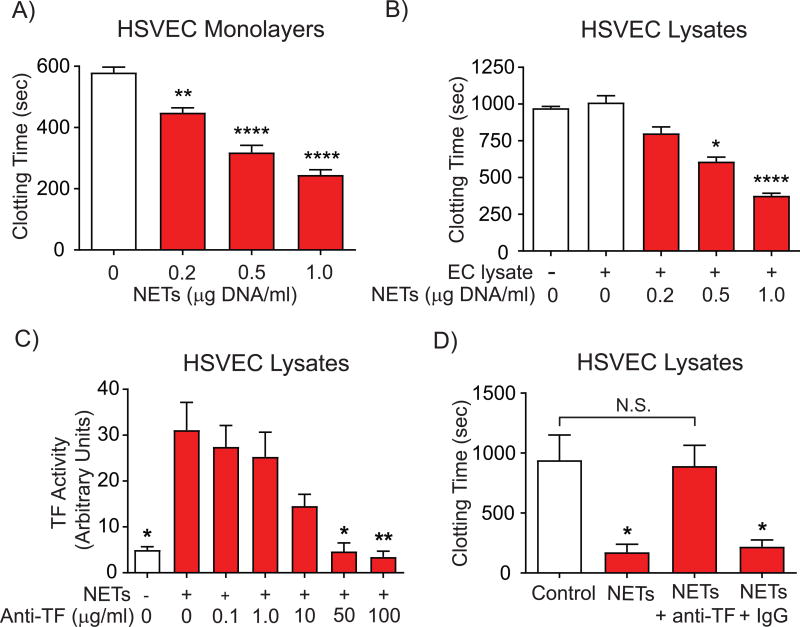
Exposure of HSVECs to NETs accelerated plasma clotting by cell monolayers (Fig. 5A) or lysates (Fig. 5B), achieving a 60–70% decrease in clotting time at the highest NET concentration. NETs alone did not accelerate plasma clotting in cell-free assays (Suppl. Fig. IX-A) nor did they exhibit detectable TF activity (Suppl. Fig. IX-B). TF–neutralizing antibodies inhibited NET-induced TF activity in a concentration-dependent manner (Fig. 5C) and abrogated NET-stimulated acceleration of plasma clotting by HSVEC lysates (Fig. 5D). Cell treatment with NETs did not significantly change mRNA expression of other proteins involved in blood coagulation such as tissue factor pathway inhibitor, thrombomodulin, or Von Willebrand factor (not shown). Together, these data demonstrate that increased TF production after exposure of HSVECs to NETs augments their thrombogenicity.
Figure 5. NETs accelerate EC-mediated plasma clotting in a TF-dependent manner.
(A) HSVECs were incubated with various concentrations of NETs for 8 hours, followed by washing and determination of plasma clotting activity of the intact monolayers as described in Materials and Methods. N = 4. (B) HSVECs were incubated with NETs (0.5 µg DNA/ml) as in (A) for 6 hours, followed by cell lysis and determination of plasma clotting activity. N = 7. (C–D) HSVECs were incubated with NETs (0.5 µg DNA/ml) or left unstimulated for 6 hours. TF activity of cell lysates was measured in the presence of various concentrations of anti-TF neutralizing antibody (C). The plasma clotting activity of cell lysates was determined in the presence or absence of 50 µg/ml anti-TF neutralizing antibody or control IgG (D). N = 5. P values: *<0.05, **<0.01, ****<0.0001 vs. the respective controls (A, B, and D) or vs. NETs alone (C). N.S., not significant.
DISCUSSION
Superficial erosion of human coronary arterial plaques often causes thrombosis and acute coronary syndromes. Yet no contemporary therapies target superficial erosion specifically, in part because the mechanisms that underlie this condition remain poorly understood. Endothelial dysfunction appears to participate critically in the pathophysiology of superficial erosion. Activated ECs promote neutrophil recruitment to plaques and release of NETs, which localize to the surface of human plaques with morphologic characteristics associated with superficial erosion.2, 6, 7, 41 This study demonstrated that NETs can amplify and propagate local processes that lead to endothelial injury by eliciting EC activation and increased adhesivity, which in turn could promote further leukocyte recruitment to lesion sites and thus potentiate the regional inflammatory response. Additionally, NETs induced TF expression and accelerated plasma clotting by ECs. Collectively, these results illustrate a novel mechanism by which NETs can aggravate thrombosis at sites of superficial erosion of atherosclerotic plaques (Fig. 6).
Figure 6. Interleukin-1α mediates endothelial cell activation by neutrophil extracellular traps.
When neutrophils undergo NETosis, the extruded DNA strands associate with numerous proteins including the precursor forms of IL-1α and β and serine proteinases produced by the granulocytes. The results presented here show that the neutrophil-derived proteinase cathepsin G processes pro-IL-1α to the more active mature form by limited proteolysis, but degrades pro-IL-1b to inactive fragments. This pathway likely operates in vivo, as cathepsin G retains activity even in the presence of plasma proteinase inhibitors. NETs bearing mature IL-1α can activate endothelial cells to express adhesion molecules that can recruit further leukocytes, and elicit the local production of the potent procoagulant tissue factor. Thus, activation of endothelial cells by this NET-associated cytokine can amplify, sustain, and propagate local vascular inflammation and also promote thrombosis. These results have particular importance for postulated mechanisms of thrombosis due to superficial erosion. We have proposed a "multi-hit" pathway for the pathogenesis of this mode of arterial thrombosis: an initial endothelial desquamation with inadequate local repair, followed by an amplification phase. NET-associated IL-1α could participate in the amplification phase by aggravating the consequences of local endothelial erosion and promotion of the formation and persistence of arterial thrombi that lead to clinical events.
NETs are multimolecular structures composed of chromatin decorated with granular and cytoplasmic proteins released by neutrophils undergoing a specialized form of cell death.14, 42 Examination of the content and function of various cytokines present in NETs revealed that these structures contain both IL-1 isoforms. Two decades before the discovery of NETs, Tiku et al. reported that neutrophils stimulated with soluble (PMA) or insoluble (zymosan) agonists released IL-1, a cytokine not expressed by resting neutrophils.43 Subsequent studies demonstrated IL-1 expression by isolated neutrophils treated with various pro-inflammatory stimuli and in those in the synovial fluid of patients with rheumatoid arthritis.44–46 Association with NETs should retain IL-1 isoforms and other potent effector molecules at sites of intimal injury, and furnish a solid-state scaffold for their concerted actions to amplify and propagate the local response.
The present study demonstrated that IL-1α, but not IL-1β, mediates the action of NETs on the aspects of EC activation examined here. This finding was somewhat surprising given that both IL-1 isoforms share a common cell surface receptor, elicit the same signaling pathways, and exhibit considerable redundancy in their action on target cells.47, 48 The observation, however, agrees with the known role of IL-1α as a danger signal when released by dead and dying cells, where it initiates inflammatory responses characterized by neutrophil recruitment to injury sites.49, 50
IL-1α and IL-1β have distinct biochemical, cell biological, functional, and pathophysiologic aspects,40, 48 including exhibiting different requirements for proteolytic processing to exert biological activity. IL-1α possesses activity without processing, but cleavage of its precursor with generation of a 16–18–kDa C-terminal fragment can enhance its activity. IL-1β, in contrast, requires proteolytic processing to attain biological activity through highly regulated intracellular pathways that involve the inflammasomes and multiple triggering stimuli and co-stimuli that result in caspase-1 activation.39, 40, 48 IL-1 isoforms can also undergo proteolytic processing extracellularly after being released by damaged or dying cells. We previously reported differential processing of IL-1β by various matrix metalloproteinases implicated in atherogenesis.51 Although several purified proteases can process either IL-1 isoform yielding the corresponding mature, more active polypeptides,39, 40, 52–57 the present data indicate that NETs exert differential effects on IL-1 isoforms, processing the IL-1α precursor to a more active form via limited proteolysis but degrading the IL-1β precursor more completely. These results explain the present study’s finding that IL-1α, but not IL-1β, mediates the effects of NETs on ECs and suggest that the lack of redundancy of IL-1 in various pathophysiologic scenarios may stem in part from their differential susceptibility to proteolysis. CatG appears to be the most relevant NET protease participating in pro-IL-1α processing under the experimental conditions of this study. Incubation of pro-IL-1α with NETs yields a product of identical electrophoretic mobility to that generated by purified CatG. Moreover, unlike NE or PR3, CatG retains enzymatic activity in the presence of endogenous proteinase inhibitors in blood. Also, a recent study showed that NE associated with NETs exhibits very low activity.58 Our results demonstrate a novel pathway by which local IL-1α processing by NET-associated CatG at the intimal surface acts analogously to activation of pro-IL-1β by caspase 1 in inflammasomes in other circumstances.
The work reported here documents that NETs can induce the EC expression of TF—a critical modulator of the thrombogenicity of human atheromata—,59 which results in the acceleration of plasma clotting by EC lysates or monolayers. NETs did not exhibit detectable TF activity in cell-free assays in the present study, while NETs isolated from the blood withdrawn from culprit arteries of patients with acute coronary syndrome contain TF.60 This result, which concurs with a recent report by Noubouosie et al.,61 suggests that neutrophils do not act as the primary source of TF found in NETs detected in ACS patients. Rather, in addition to their prothrombotic action on ECs, NETs may aggravate thrombosis by providing a surface that can bind TF released from dying cells or circulating in the blood, as well as trapping platelets, further leukocytes, and fibrin.
Data from our recent study that categorized human atherosclerotic plaques based on morphologic features revealed an association of neutrophils and NETs with eroded plaques but not with rupture-prone plaques.7 The present work supports a growing body of evidence indicating that NETs contribute pivotally to coronary thrombosis in the setting of superficial erosion and may therefore comprise therapeutic targets. Other recent data from our group affirms this concept by demonstrating that NETs participate causally in acute thrombotic complications of intimal lesions tailored in mice to recapitulate features of superficial erosion in humans.8 This experimental approach demonstrated that abrogation of NETosis by genetic deficiency of peptidyl arginine deiminase 4 or promoting disassembly of NETs by administration of soluble DNAse I reduced intimal permeability, endothelial injury and death, endothelial expression of TF, and thrombosis.
Imaging of culprit lesions with intravascular optical coherence tomography can now differentiate erosion from rupture in patients with ACS. Patients with ruptured or intact fibrous caps present distinct expression profiles of intracoronary cytokines.62 This differential cytokine expression can help understand the mechanisms underlying ACS but may not suffice as a diagnostic tool to discriminate between plaque erosion and rupture. Blood biomarkers of NETosis such as free DNA or citrullinated histones might provide a means to distinguish erosion from rupture non-invasively at point of care upon presentation. Such advances might guide more precision treatment of ACS based on pathobiologic mechanisms rather than the surface ECG,63 as erosion may respond best to management strategies distinct from those appropriate for rupture.10, 64
The availability of selective anti-IL-1α antibodies already in use in clinical investigation points to the ready translatability of the results presented here. Administration of such agents acutely to patients with ACS due to superficial erosion might mitigate the amplification and propagation of local thrombosis. Moreover, as reperfusion injury or “no-reflow” may involve EC interaction with neutrophils and possibly NETosis, anti-IL-1α therapy merits consideration as adjunctive therapy in these conditions as well. The recent demonstration that long-term administration of an anti-IL-1β antibody can reduce recurrent cardiovascular events in patients in the stable phase post ACS who have persistent inflammation validates the feasibility and concept of targeting of IL-1 therapeutically with a monoclonal antibody in coronary artery disease.35 The present data provide a mechanistic basis for a strategy of short term acute treatment of patients with ACS due to erosion with an anti-IL-1α inhibitor. Such an acute intervention could precede a more prolonged chronic treatment with an anti-IL-1β inhibitor or broader spectrum anti-inflammatory therapy in those with residual inflammatory risk, given the evidence for distinct drivers of inflammation in the acute and chronic phases of atherothrombosis.
Supplementary Material
HIGHLIGHTS.
Neutrophil extracellular traps induce the expression of adhesion molecules in human endothelial cells and increase leukocyte adhesion to cell monolayers.
Neutrophil extracellular traps augment endothelial cell thrombogenicity by eliciting expression of tissue factor and consequently accelerating blood clotting by cell lysates or monolayers.
Interleukin-1α and cathepsin G mediate the effects of neutrophil extracellular traps on endothelial cell activation.
Acknowledgments
We thank Kay Case for HUVEC preparation, Dr. Hermann Gram for providing canakinumab, Dr. John Simard for providing anti-IL-1α-neutralizing antibody (MABp1), James Morrisey and Kevin Croce for supplying the anti-TF antibody, and Chelsea Swallom for editorial assistance.
SOURCES OF FUNDING
This work was in part supported by grants from the National Heart, Lung, and Blood Institute (R01HL080472 to P.Libby and R01HL125780 to F Luscinskas), and support from the RRM Charitable Fund. A. Vromman received the Harold M. English Fellowship Fund from Harvard Medical School (Boston, USA).
ABBREVIATIONS
- EC
endothelial cell
- HSVEC
human saphenous vein endothelial cell
- HUVEC
human umbilical cord endothelial cell
- HAEC
human aortic endothelial cell
- NET
neutrophil extracellular trap
- NE
neutrophil elastase
- PR3
proteinase 3
- MPO
myeloperoxidase
- TF
tissue factor
- IL
interleukin
- IL-1Ra
interleukin-1 receptor antagonist
- CatG
cathepsin G
- GST
glutathione-S-transferase
- VCAM-1
vascular cell adhesion molecule 1
- ICAM-1
intercellular adhesion molecule 1
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- IκBα
NFκB inhibitor α
- pNA
para-nitroanilide
- MCA
7-amido-4-methylcoumarin
- DAPI
4’6-diamidino-2-phenylindole
- OD
optical density
- kDa
kiloDaltons
- ABAH
4-aminobenzoic acid hydrazide
- fMLP
formyl-methionyl-leucyl-phenylalanine
Footnotes
DISCLOSURES
Dr. Libby is an unpaid consultant to, or involved in clinical trials for XBiotech, Inc.
References
- 1.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 2.Quillard T, Franck G, Mawson T, Folco E, Libby P. Mechanisms of erosion of atherosclerotic plaques. Curr Opin Lipidol. 2017 doi: 10.1097/MOL.0000000000000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Partida RA, Libby P, Crea F, Jang IK. Plaque erosion: a new in vivo diagnosis and a potential major shift in the management of patients with acute coronary syndromes. Eur Heart J. 2018 doi: 10.1093/eurheartj/ehx786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farb A, Burke AP, Tang AL, Liang TY, Mannan P, Smialek J, Virmani R. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93:1354–1363. doi: 10.1161/01.cir.93.7.1354. [DOI] [PubMed] [Google Scholar]
- 5.Kolodgie FD, Burke AP, Farb A, Weber DK, Kutys R, Wight TN, Virmani R. Differential accumulation of proteoglycans and hyaluronan in culprit lesions: insights into plaque erosion. Arterioscler Thromb Vasc Biol. 2002;22:1642–1648. doi: 10.1161/01.atv.0000034021.92658.4c. [DOI] [PubMed] [Google Scholar]
- 6.Franck G, Mawson T, Sausen G, et al. Flow Perturbation Mediates Neutrophil Recruitment and Potentiates Endothelial Injury via TLR2 in Mice: Implications for Superficial Erosion. Circ Res. 2017;121:31–42. doi: 10.1161/CIRCRESAHA.117.310694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quillard T, Araujo HA, Franck G, Shvartz E, Sukhova G, Libby P. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur Heart J. 2015;36:1394–1404. doi: 10.1093/eurheartj/ehv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franck G, Mawson TL, Folco EJ, et al. Roles of PAD4 and NETosis in Experimental Atherosclerosis and Arterial Injury: Implications for Superficial Erosion. Circ Res. 2018 doi: 10.1161/CIRCRESAHA.117.312494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–1161. [PubMed] [Google Scholar]
- 10.Jia H, Abtahian F, Aguirre AD, et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62:1748–1758. doi: 10.1016/j.jacc.2013.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mullick AE, Soldau K, Kiosses WB, Bell TA, 3rd, Tobias PS, Curtiss LK. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J Exp Med. 2008;205:373–383. doi: 10.1084/jem.20071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tricot O, Mallat Z, Heymes C, Belmin J, Leseche G, Tedgui A. Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques. Circulation. 2000;101:2450–2453. doi: 10.1161/01.cir.101.21.2450. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Gao H, Kessinger CW, Schmaier A, Jaffer FA, Simon DI. Myeloid-related protein-14 regulates deep vein thrombosis. JCI Insight. 2017;2 doi: 10.1172/jci.insight.91356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 15.Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, Resink TJ. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 2010;584:3193–3197. doi: 10.1016/j.febslet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gould TJ, Vu TT, Swystun LL, Dwivedi DJ, Mai SH, Weitz JI, Liaw PC. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol. 2014;34:1977–1984. doi: 10.1161/ATVBAHA.114.304114. [DOI] [PubMed] [Google Scholar]
- 18.Martinod K, Wagner DD. Thrombosis: tangled up in NETs. Blood. 2014;123:2768–2776. doi: 10.1182/blood-2013-10-463646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massberg S, Brand K, Gruner S, Page S, Muller E, Muller I, Bergmeier W, Richter T, Lorenz M, Konrad I, Nieswandt B, Gawaz M. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. 2002;196:887–896. doi: 10.1084/jem.20012044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonald B, Davis RP, Kim SJ, Tse M, Esmon CT, Kolaczkowska E, Jenne CN. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood. 2017;129:1357–1367. doi: 10.1182/blood-2016-09-741298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Bruhl ML, Stark K, Steinhart A, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haubitz M, Gerlach M, Kruse HJ, Brunkhorst R. Endothelial tissue factor stimulation by proteinase 3 and elastase. Clin Exp Immunol. 2001;126:584–588. doi: 10.1046/j.1365-2249.2001.01587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JE, Yoo HJ, Gu JY, Kim HK. Histones Induce the Procoagulant Phenotype of Endothelial Cells through Tissue Factor Up-Regulation and Thrombomodulin Down-Regulation. PLoS One. 2016;11:e0156763. doi: 10.1371/journal.pone.0156763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugiyama S, Kugiyama K, Aikawa M, Nakamura S, Ogawa H, Libby P. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arterioscler Thromb Vasc Biol. 2004;24:1309–1314. doi: 10.1161/01.ATV.0000131784.50633.4f. [DOI] [PubMed] [Google Scholar]
- 25.Yang X, Li L, Liu J, Lv B, Chen F. Extracellular histones induce tissue factor expression in vascular endothelial cells via TLR and activation of NF-kappaB and AP-1. Thromb Res. 2016;137:211–218. doi: 10.1016/j.thromres.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Reichert JM. Antibodies to watch in 2013: Mid-year update. MAbs. 2013;5:513–517. doi: 10.4161/mabs.24990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bevilacqua MP, Pober JS, Wheeler ME, Cotran RS, Gimbrone MA., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985;76:2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Libby P, Alroy J, Pereira ME. A neuraminidase from Trypanosoma cruzi removes sialic acid from the surface of mammalian myocardial and endothelial cells. J Clin Invest. 1986;77:127–135. doi: 10.1172/JCI112266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Najmeh S, Cools-Lartigue J, Giannias B, Spicer J, Ferri LE. Simplified Human Neutrophil Extracellular Traps (NETs) Isolation and Handling. J Vis Exp. 2015 doi: 10.3791/52687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jindal HK, Folco EJ, Liu GX, Koren G. Posttranslational modification of voltage-dependent potassium channel Kv1.5: COOH-terminal palmitoylation modulates its biological properties. Am J Physiol Heart Circ Physiol. 2008;294:H2012–2021. doi: 10.1152/ajpheart.01374.2007. [DOI] [PubMed] [Google Scholar]
- 31.Alcaide P, Lim YC, Luscinskas FW, Fresno M. Mucin AgC10 from Trypanosoma cruzi Interferes with L-selectin-mediated monocyte adhesion. Infect Immun. 2010;78:1260–1268. doi: 10.1128/IAI.00794-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okamoto Y, Ishii S, Croce K, Katsumata H, Fukushima M, Kihara S, Libby P, Minami S. Adiponectin inhibits macrophage tissue factor, a key trigger of thrombosis in disrupted atherosclerotic plaques. Atherosclerosis. 2013;226:373–377. doi: 10.1016/j.atherosclerosis.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoppenbrouwers T, Autar ASA, Sultan AR, Abraham TE, van Cappellen WA, Houtsmuller AB, van Wamel WJB, van Beusekom HMM, van Neck JW, de Maat MPM. In vitro induction of NETosis: Comprehensive live imaging comparison and systematic review. PLoS One. 2017;12:e0176472. doi: 10.1371/journal.pone.0176472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011;18:581–588. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 36.Chistiakov DA, Bobryshev YV, Orekhov AN. Neutrophil's weapons in atherosclerosis. Exp Mol Pathol. 2015;99:663–671. doi: 10.1016/j.yexmp.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 37.Korkmaz B, Horwitz MS, Jenne DE, Gauthier F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol Rev. 2010;62:726–759. doi: 10.1124/pr.110.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 39.Afonina IS, Muller C, Martin SJ, Beyaert R. Proteolytic Processing of Interleukin-1 Family Cytokines: Variations on a Common Theme. Immunity. 2015;42:991–1004. doi: 10.1016/j.immuni.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durand E, Scoazec A, Lafont A, Boddaert J, Al Hajzen A, Addad F, Mirshahi M, Desnos M, Tedgui A, Mallat Z. In vivo induction of endothelial apoptosis leads to vessel thrombosis and endothelial denudation: a clue to the understanding of the mechanisms of thrombotic plaque erosion. Circulation. 2004;109:2503–2506. doi: 10.1161/01.CIR.0000130172.62481.90. [DOI] [PubMed] [Google Scholar]
- 42.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tiku K, Tiku ML, Skosey JL. Interleukin 1 production by human polymorphonuclear neutrophils. J Immunol. 1986;136:3677–3685. [PubMed] [Google Scholar]
- 44.Goh K, Furusawa S, Kawa Y, Negishi-Okitsu S, Mizoguchi M. Production of interleukin-1-alpha and -beta by human peripheral polymorphonuclear neutrophils. Int Arch Allergy Appl Immunol. 1989;88:297–303. doi: 10.1159/000234815. [DOI] [PubMed] [Google Scholar]
- 45.Marucha PT, Zeff RA, Kreutzer DL. Cytokine regulation of IL-1 beta gene expression in the human polymorphonuclear leukocyte. J Immunol. 1990;145:2932–2937. [PubMed] [Google Scholar]
- 46.Quayle JA, Adams S, Bucknall RC, Edwards SW. Interleukin-1 expression by neutrophils in rheumatoid arthritis. Ann Rheum Dis. 1995;54:930–933. doi: 10.1136/ard.54.11.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beltrami-Moreira M, Vromman A, Sukhova GK, Folco EJ, Libby P. Redundancy of IL-1 Isoform Signaling and Its Implications for Arterial Remodeling. PLoS One. 2016;11:e0152474. doi: 10.1371/journal.pone.0152474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 49.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 50.Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E, White MR, Dinarello CA, Apte RN. IL-1alpha and IL-1beta recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187:4835–4843. doi: 10.4049/jimmunol.1102048. [DOI] [PubMed] [Google Scholar]
- 51.Schonbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol. 1998;161:3340–3346. [PubMed] [Google Scholar]
- 52.Afonina IS, Tynan GA, Logue SE, Cullen SP, Bots M, Luthi AU, Reeves EP, McElvaney NG, Medema JP, Lavelle EC, Martin SJ. Granzyme B-dependent proteolysis acts as a switch to enhance the proinflammatory activity of IL-1alpha. Mol Cell. 2011;44:265–278. doi: 10.1016/j.molcel.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Black RA, Kronheim SR, Cantrell M, Deeley MC, March CJ, Prickett KS, Wignall J, Conlon PJ, Cosman D, Hopp TP, Mochizuki DY. Generation of biologically active interleukin-1 beta by proteolytic cleavage of the inactive precursor. J Biol Chem. 1988;263:9437–9442. [PubMed] [Google Scholar]
- 54.Clancy DM, Henry CM, Sullivan GP, Martin SJ. Neutrophil extracellular traps can serve as platforms for processing and activation of IL-1 family cytokines. FEBS J. 2017;284:1712–1725. doi: 10.1111/febs.14075. [DOI] [PubMed] [Google Scholar]
- 55.Coeshott C, Ohnemus C, Pilyavskaya A, Ross S, Wieczorek M, Kroona H, Leimer AH, Cheronis J. Converting enzyme-independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc Natl Acad Sci U S A. 1999;96:6261–6266. doi: 10.1073/pnas.96.11.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hazuda DJ, Strickler J, Kueppers F, Simon PL, Young PR. Processing of precursor interleukin 1 beta and inflammatory disease. J Biol Chem. 1990;265:6318–6322. [PubMed] [Google Scholar]
- 57.Li P, Allen H, Banerjee S, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 58.Kasperkiewicz P, Poreba M, Snipas SJ, Parker H, Winterbourn CC, Salvesen GS, Drag M. Design of ultrasensitive probes for human neutrophil elastase through hybrid combinatorial substrate library profiling. Proc Natl Acad Sci U S A. 2014;111:2518–2523. doi: 10.1073/pnas.1318548111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toschi V, Gallo R, Lettino M, Fallon JT, Gertz SD, Fernandez-Ortiz A, Chesebro JH, Badimon L, Nemerson Y, Fuster V, Badimon JJ. Tissue factor modulates the thrombogenicity of human atherosclerotic plaques. Circulation. 1997;95:594–599. doi: 10.1161/01.cir.95.3.594. [DOI] [PubMed] [Google Scholar]
- 60.Stakos DA, Kambas K, Konstantinidis T, Mitroulis I, Apostolidou E, Arelaki S, Tsironidou V, Giatromanolaki A, Skendros P, Konstantinides S, Ritis K. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur Heart J. 2015;36:1405–1414. doi: 10.1093/eurheartj/ehv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noubouossie DF, Whelihan MF, Yu YB, Sparkenbaugh E, Pawlinski R, Monroe DM, Key NS. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood. 2017;129:1021–1029. doi: 10.1182/blood-2016-06-722298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chandran S, Watkins J, Abdul-Aziz A, Shafat M, Calvert PA, Bowles KM, Flather MD, Rushworth SA, Ryding AD. Inflammatory Differences in Plaque Erosion and Rupture in Patients With ST-Segment Elevation Myocardial Infarction. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crea F, Libby P. Acute Coronary Syndromes: The Way Forward From Mechanisms to Precision Treatment. Circulation. 2017;136:1155–1166. doi: 10.1161/CIRCULATIONAHA.117.029870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Libby P. Superficial erosion and the precision management of acute coronary syndromes: not one-size-fits-all. Eur Heart J. 2017;38:801–803. doi: 10.1093/eurheartj/ehw599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.