Abstract
Cirrhosis is a morbid condition associated with frequent hospitalizations and high mortality. Management of cirrhosis requires complex medication regimens to treat underlying liver disease, complications of cirrhosis, and comorbid conditions. This review examines the complexities of medication management in cirrhosis, barriers to optimal medication use, and potential interventions to streamline medication regimens and avoid medication errors. A literature review was performed by searching PUBMED through December 2017 and article reference lists to identify articles relevant to medication management, complications, adherence, and interventions to improve medication use in cirrhosis. The structural barriers in cirrhosis include sheer medication complexity related to the number of medications and potential for cognitive impairment in this population, faulty medication reconciliation, and limited adherence. Tested interventions have included patient self-education, provider driven patient education, intensive case management including medication blister packs, and smartphone applications. Initiatives are needed to improve patient, caregiver, and provider education on appropriate use of medications in patients with cirrhosis. A multidisciplinary team should be established to coordinate care with close monitoring, address patient and caregiver concerns, and to provide timely access to outpatient evaluation of urgent/complex issues. Future studies evaluating the clinical outcomes and cost effectiveness of interventions are needed.
Keywords: End-Stage Liver Disease, Medication Adherence, Patient Education, Case Management
Introduction
Cirrhosis is the end result of chronic liver disease. It affects over 600,000 adults in the United States, with estimates that an additional five million have at least bridging fibrosis1,2. These numbers will rise as baby boomers with chronic hepatitis C age and the incidence of nonalcoholic fatty liver disease grows3. Indeed, patients with cirrhosis seeking medical attention has increased by 59% over the past decade4, and cirrhosis is now the 12th leading cause of death in the United States5. Compounding the problem of rising prevalence, cirrhosis is an expensive condition, with an estimated annual healthcare cost of $2 billion dollars in the United States6.
The primary drivers of morbidity and cost in cirrhosis are complications of decompensated disease: ascites, hepatic encephalopathy (HE), and variceal hemorrhage. Patients are frequently hospitalized for these conditions, with high 30-day readmission rates ranging from 25–52% 7,8. Providers prescribe a variety of medications with narrow therapeutic windows to treat these complications. Even for the most experienced provider, managing these medications is challenging. The risks of medication errors and serious side effects are magnified by difficulties with patient adherence, medication interactions, and the need for frequent dosage adjustments. Herein, we review the data on challenges and solutions for optimal medication management in patients with cirrhosis.
Methods
A literature review was performed by searching PUBMED for relevant full text articles through December 2017. The authors searched for articles using keywords: liver cirrhosis, end-stage liver disease, drug therapy, medication errors, medication adherence, medication reconciliation, patient education, disease management, case management. An additional search of article reference lists was performed to identify further studies. Only English language publications were considered.
Complications of Medical Therapy
This section reviews the special considerations that need to be made when using routine, even over-the-counter, medications in patients with cirrhosis (Table 1).
Table 1.
Commonly used Medications and Adverse Effects in Cirrhosis
| Medication Class | Indication | Take Home Point | Adverse Effects |
|---|---|---|---|
| NSAIDs | Acute and chronic pain | Readily available and often prescribed first line, but should not be used in the presence of ascites50 |
|
| Acetaminophen | Lower daily dose (≤ 2000 mg daily) is safe to use51 and preferred first line pain medication |
|
|
| Narcotics | Commonly prescribed (19–60% of patients)52,53 |
|
|
| Proton Pump Inhibitors | GERD, PUD, dyspepsia | Long term PPI use is often not indicated, up to 63% of patients are continued on a PPI indefinitely after a variceal bleed23 | |
| Statins | Cardiovascular risk reduction | Statins have been shown to be safe in compensated cirrhosis and should be continued as clinically indicated. Recent studies suggest they may be beneficial in patients with cirrhosis 54,55 |
|
NSAIDs: Non-Steroidal Anti Inflammatory Drugs
AKI: Acute Kidney Injury
HE: Hepatic Encephalopathy
PPI: Proton Pump Inhibitors
GERD: Gastroesophageal reflux disease
PUD: Peptic ulcer disease
Diuretics
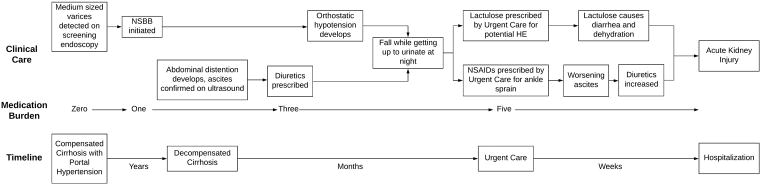
As many as 58% of patients with decompensated cirrhosis will develop ascites9. Few patients manage to adhere to the recommended two-gram sodium diet, and salt restriction alone is insufficient for many. Diuretic use requires vigilance to balance volume control versus the risks of over diuresis leading to electrolyte imbalance and acute kidney injury (AKI). First, patients need to have monitoring of their electrolytes and renal function. Active, anticipatory management demands patients getting regular labs, substantial non-reimbursed time to co-ordinate testing, and a medical team to follow up results. Up to 22% of readmissions after discharge for cirrhosis complications are attributable to AKI, hyponatremia, and hypo(or hyper)kalemia8,10. Second, diuretic therapy needs frequent adjustments. To accurately assess volume status, patients are asked to record their daily weights but many don’t and dose adjustments based on patient-reported swelling can be flawed. Whereas the complexities of warfarin adjustments are handled by anticoagulation clinics making dose adjustments based on standardized labs, systems of care for diuretics are lacking, and depend not only on labs but also on clinical assessment. An example of how AKI can occur in the course of seemingly routine care for patients with cirrhosis is laid out in Figure 1.
Figure 1.
Lactulose and Rifaximin
HE affects up to 40% of patients with decompensated cirrhosis11. Lactulose and rifaximin are the principal pharmacological therapies for HE. Lactulose must be taken several times a day, titrated to yield at least 2–3 bowel movements daily12. One missed dose may cause a downward spiral of progressive confusion, leading to further missed doses and worsening HE. Furthermore, lactulose is associated with abdominal cramping, and frequent loose stools (a desired effect) which contribute to poor adherence 13. Rifaximin, a poorly-absorbable antibiotic12, is well tolerated and has been shown to reduce hospital readmissions14,15. Still, only 60% of patients with overt HE are prescribed rifaximin16. Although rifaximin is generally cost saving in patients with a prior hospitalization for overt HE by reducing readmissions, coverage by insurers remains limited 17.
Beta Blockers
Non-selective beta-blockers (NSBBs) can prevent variceal hemorrhage in patients with large varices. Ideally, the dose should be titrated to decrease hepatic vein portal pressure gradient to < 12 mmHg or a decrease in 20% from baseline, but these measurements are not widely available18. In clinical practice, the dose of NSBB is titrated to decrease the resting heart rate to 55–60 beats per minute18, a target achieved by only 9.8% of patients19. Inadequate dosage accounts for treatment failure (variceal hemorrhage) and may be a result of lack of monitoring during clinical follow-up. In addition, NSBBs cause a myriad of side effects including fatigue, depression, sexual dysfunction, and orthostasis. Though controversial, NSBBs may increase the risk of AKI in patients with ascites, particularly those with baseline hypotension20,21.
Proton Pump Inhibitors (PPIs)
PPIs are one of the most widely prescribed medications22. They often are started for a specific indication, and continued without reviewing if they are still needed. Up to 63% of patients with end-stage liver disease (ESLD) on PPIs are prescribed them inappropriately, for example as long-term therapy after variceal bleeding (where reflexive in-hospital management of bleeding is continued on discharge)23,24. There is growing concern that chronic use is associated with adverse effects specific to patients with cirrhosis. PPI use and spontaneous bacterial peritonitis (SBP) have been associated in a meta-analysis, possibly because PPIs increase the risk of small intestinal bacterial overgrowth which, in turn, leads to bacterial translocation and SBP25. In addition, patients with cirrhosis on a PPI have an increased risk of C. difficile infection26. Through similar adverse changes in the gut microbiome, PPIs may increase the risk of HE and readmission to hospital27.
Structural Barriers to Optimal Medication Management
Medication Complexity
Patients with cirrhosis including some with compensated cirrhosis are on multiple medications with an average between 3 and 10 medications13,28,29. Volk et al. showed that in patients with cirrhosis, the number of medications at discharge can predict time to readmission29. In addition to the number of medications, dosing frequency and the need to actively titrate by symptom and effect make medication regimens for patients with cirrhosis complex. This is further complicated in patients with HE where impaired cognition limits the ability of patients to remember to take their medications and to adjust dosing according to their response. While caregivers can help assess responses to some medications such as body weight for diuretics, recording the number of bowel movements in response to lactulose is more difficult and reliant on patient reporting.
Medication List Discrepancies and Faulty Reconciliation
Patients often have medications prescribed by more than one doctor who do not have the complete medication list and are not necessarily aware of the changes made by other providers. When patients are hospitalized, medications are often started, adjusted, or stopped with limited teaching and reconciliation at the time of care transitions. The result is confusion which can lead to patient harm and readmissions. Pharmacy support for high-risk patients has been shown to decrease discrepancies, but it is unclear if decreased medication discrepancies leads to decreased health care utilization or improves patient outcomes30. Hayward et al. compared the dose, frequency and indications of each medication reported by a group of 50 patients with cirrhosis with a state-wide pharmacy record. Significant discrepancies were adjudicated by a panel of hepatologists (Table 2). Half of the patients had significant discrepancies with the potential for patient harm within seven days. Discrepancies were associated with older age, taking ≥5 medications each day, and lower medication adherence (according to the Morisky Medication Adherence Scale (MMAS-8 score))31. This study reinforces the importance of asking about over the counter or complementary medications, as only 31.8% of these medications were listed by patients without specific inquiry. It also highlights the need for structured patient education because only half of the patients reported being told how to take their medications and less than a third of the patients taking diuretics knew they should keep a record of their weight. This study was limited by patient recall as most patients did not bring their medication list in, and caregivers were not always present to help verify medications 32.
Table 2.
Medication Adherence in the Pre- and Post- Liver Transplant Patient Population
| Article | Patient population; n | Adherence measure | Significant findings | Affecting clinical outcomes? |
|---|---|---|---|---|
| Hayward 201632 | Cirrhosis n= 50; 40% decompensated | Self-reported medication list compared to medical record | 54% had ≥1 and 24% had ≥3 discrepancies between what patients were taking and the prescribed regimens | Not measured |
| Leevy 200735 | Hepatic encephalopathyn=145; 95% with cirrhosis, 12% on transplant list | Retrospective review of medical record | 92% took rifaximin for >75% of prescribed doses 30% took lactulose for > 75% of prescribed doses |
Fewer hospitalizations and hospital days when patients were taking rifaximin |
| Polis 201528 | Cirrhosis, n=29; mean MELD 11 | Patient response (MMAS-8) | 54% “sometimes forgot to take their medications” in the past 30 days 29% had missed 1 or more medication over the last two weeks |
Not measured |
| Kuo 201613 | Patients listed for liver transplant, n=181; mean MELD 13 | Patient response (MMAS-8) | 42% “sometimes forgot to take their medications” 28% missed 1 or more medications in the past 2 weeks 12% missed 1 or more medications in the day prior |
Not measured |
| Serper 201556 | Liver transplant recipients, n= 105; median 20 months from transplant | Structured interviews to determine patient knowledge and self-reported use compared with medical record abstraction and tacrolimus blood levels | 86% displayed correct medication treatment knowledge 78% could demonstrate simulated regimen use to researchers 14% self-reported as non-adherent 32% non-adherence based on tacrolimus levels |
Higher treatment knowledge scores and demonstrated regimen use associated with reduced readmissions after liver transplant |
MELD: Model for End-Stage Liver Disease
MMAS-8: Morisky Medication Adherence Scale
Medication Adherence
Broadly, adherence is related to the complexity of the regimen, side effects, costs, and patient understanding of the indications, regimen, and possible side effects. Measuring adherence is challenging in clinical and research settings as there are different criteria for what is considered “adherent” and no gold standard to measure it. With this in mind, adherence rates for patients in the general population with chronic conditions range between 43 and 78%33. Adherence rates are around 72% when patient reported measures are used34.
Polis et al. examined complete medication regimen adherence surveying 29 cognitively intact patients with Child A-B cirrhosis. These patients had been hospitalized at least once and were taking 3.2 medications on average. There are several key points from this study. First, 54% of the patients reported they missed at least one dose of their medications during the past 30 days. Reasons for missed doses included forgetfulness (42%), being away from home/change in routine (36%), sleeping through the dose time (32%), and running out of medications (25%). Conversely, adherence was associated with patients reporting less fatigue, less abdominal symptoms (such as pain, bloating), and higher emotional well-being. Second, only 62% of patients answered more than 75% of the questions correctly on a quiz focused on disease knowledge and treatments. Higher scores were not associated with adherence. Third, one in three patients stated they would adjust their medications if their symptoms improved without talking to their physicians 28.
Cirrhosis specifically has many medications that can be particularly noxious, further limiting adherence (Table 2). For instance, Lactulose adherence, as measured by patients’ report of taking more than 75% of their prescribed doses, can be as low as 31%35. The same study found that adherence to rifaximin use was 92%. The wide gap between taking more than 75% prescribed doses of lactulose and rifaximin argues that the adverse effects of lactulose may have a larger effect on adherence than HE itself.
Medication adherence in liver transplant candidates has been closely studied because poor adherence to anti-rejection medications is thought to be a leading cause of graft failure. In a study evaluating medication compliance in patients awaiting liver transplant, 70% of patients were “low adherers” to their medication regimens defined as not having a perfect score (<8) on the MMAS-8. The median number of medications taken in this cohort of patients was 7, not including supplements. Low-adherers were more likely to be diabetic, had a higher medication complexity judged by the MRCI score (Medication Regimen Complexity Index) and significantly lower self-reported health. The most common reasons for not taking medications were forgetting to do so (27%) and side effects (14%). Lactulose was the medication that these patients were least likely to take. High medication burden was associated with non-adherence, while high MELD (Model for End-Stage Liver Disease) or Child-Pugh scores were not associated with adherence in multivariate analysis13.
Interventions to Improve Medication Management
Congestive heart failure (CHF) is a similar disease model in that patients are medically complex and on multiple medications, which often include diuretics. Patients in this population have similar readmission rates as those with cirrhosis36. Research in CHF has shown that interventions to improve medication adherence, such as inpatient patient education, multidisciplinary care involvement, post discharge clinic follow-up and tele-monitoring, improves mortality and readmission rates37. The cost-effectiveness of these interventions combined or used individually is not well known38,39. Building on this model, a multi-faceted approach including patient and provider education, case management, and delivery system redesign is needed to improve medication management in patients with cirrhosis. Detailed below are examples of interventions aimed at improving medication use in patients with ESLD (Table 3).
Table 3.
Interventions in Medication Management
| Article | Domain | Intervention | Population; n | Process Measure | Process measures/Improved clinical outcomes? |
|---|---|---|---|---|---|
| Volk 201341 | Patient self-management | Educational booklet | Cirrhosis; 150;70% Child A cirrhosis |
Cirrhosis knowledge survey at baseline and at 3 months | Median knowledge increased/ not evaluated |
| Larrey 201140 | Nursing led education | Scheduled nurse clinic visits | CHC; 244; 29.4% F3–4 fibrosis | Adherence, SVR | 48-week adherence improved/ Higher SVR in treatment group |
| Asavakarn 201657 | Pharmacy led, multidisciplinary team educational approach | Education during hospitalization for LT and initial follow-up visit | LT recipients; 50; 64% ≤3 months post LT | Immunosuppressant knowledge questionnaire | Improved knowledge/ not evaluated |
| Promraj 201658 | Pharmacy led, multidisciplinary team educational approach | Education during hospitalization for LT and initial follow-up visit | LT recipients; 50;64% ≤ 3 months post LT | Immunosuppressive Therapy Adherence Scale | Higher medication knowledge scores correlated with higher ITAS/not evaluated |
| Russo 201659 | Multidisciplinary protocol to reduce readmissions | Outpatient service expansion, Pharmacist teaching |
LT recipients; 167; mean MELD at LT 21 | 30-day readmission Length of stay for index admission for liver transplant |
30-day readmission rate/30-day readmission rate decreased |
| Wigg 201342 | Chronic disease management program | Delivery system redesign Self-management support Protocol driven decision support Clinical information systems |
Cirrhosis with ascites; 40; mean MELD 11 | Days hospitalized for liver related reasons Attendance to outpatient clinic visits Quality of care measures |
Improved clinic attendance, Hepatocellular carcinoma screening rates, referral for LT, and Hepatitis A/B vaccination Did not reduce days hospitalized for liver related reasons or all cause admission rate |
| Ganapathy 2017 45 |
Smart phone App to reduce readmissions | Smart phone app to track medications, sodium intake, daily weight, signs of HE Patient and caregiver education Enhanced communication with health care team |
Decompensated cirrhosis; 40; mean MELD 19.5 | 30-day readmission Medication adherence Contact with study team |
No 30-day readmissions for HE App was tolerable for patients and caregivers |
CHC: Chronic Hepatitis C
HE: Hepatic Encephalopathy
ITAS: Immunosuppressive Therapy Adherence Scale
LT: Liver Transplant
MELD: Model for End-Stage Liver Disease
SVR: Sustained Virologic Response
Patient Education
The simplest intervention is patient self-education. Larrey et el. showed that frequent (5–6 sessions over 48 weeks) nursing-led education visits for patients undergoing interferon and ribavirin therapy for hepatitis C improved both adherence (69.7% v. 53.%, P<0.03) and sustained virologic response rates (38.2% vs. 24.8%)40. 35% of the included patients had extensive fibrosis, defined as F3-F4 fibrosis. Though these regimens are outdated, their results show the downstream benefits of investing in nursing visits for patient education.
In decompensated cirrhosis. Volk et al. gave patients with cirrhosis, 25% of whom were decompensated with HE, a booklet on prevention and management of complications of cirrhosis as well as health management topics such as surgery and hospitalizations. Patients took a quiz before and after receiving the booklet which focused on recommended salt intake and the safety of medications such as statins, acetaminophen, and NSAIDs (Non-Steroidal Anti Inflammatory Drugs). Only 53% of the 15 questions were answered correctly at baseline, but the correct response rate rose significantly to 67% after the intervention41. This is promising, but it is unknown whether this one-time intervention improved medication adherence or patient outcomes.
Case Management
Intensive outpatient case management and follow-up has also been evaluated to improve outcomes and to reduce hospital readmissions. Wigg et al. performed a randomized trial of a case management program in patients discharged from the hospital with cirrhosis and ascites. Their multimodal intervention included a booklet with nursing-led education on management of ascites and encephalopathy, medication blister packs, a post-discharge home-visit, weekly nurse phone calls, rapid access to care for patient concerns, and written and telephone reminders before appointments. This intervention did improve attendance at appointments and multiple process measures (hepatocellular carcinoma screening, transplant evaluation, and hepatitis A/B vaccination), but it did not reduce the number of days patients spent in the hospital42. While the authors did not examine medication use or adherence specifically, this study underscores how chronic disease management with patient education on medication use can improve patient centered outcomes. Preventable readmissions are an important target for interventions to optimize medication management in cirrhosis, but using this as the only target may be missing other key outcomes. Kanwal et al. found that improved contact with medical professionals was associated with increased hospital readmissions though mortality was decreased43, leading to the conclusion that readmissions to manage problems in earlier stages may be beneficial for patients8.
Smart Phone Applications
Smart phone based applications (“apps”), such as the “Patient Buddy” have been adapted to improve medication use, with an emphasis on reducing admissions for HE. A pilot study enrolled 40 patients admitted for decompensated cirrhosis (most with HE) and their caregivers to receive an iPhone loaded with a cirrhosis modified Patient Buddy app to track medications, sodium intake, weights, and weekly cognitive assessments (assessed via orientation questions and the EncephalApp Stroop test 44 performed by caregivers)45. Participants were educated on emergencies that should prompt them to reach out to their care team. Patients and caregivers were instructed to input the patient’s individual medication intake each day. If a critical medication entry was missing, an alert was delivered to the patient, caregiver, and study team. The study team followed up these alerts with a message via the application or a phone call. Caregiver identified changes in orientation questions or the EncephalApp results were classified as impending HE and expedited outpatient follow-up was arranged as needed. Overall, 42.5% of patients in this pilot study were readmitted within 30 days. However, no patients were admitted for HE.
Conclusions
Medication management for patients with cirrhosis is complex and is associated with multiple risks, including but not limited to orthostatic hypotension, falls, worsening ascites, and AKI (Figure 1). Optimal care requires coordinated follow-up with well-informed providers and close monitoring. In addition, efforts designed to promote patient self-management strategies and adherence while anticipating the pitfalls presented by cognitive dysfunction and frequent hospitalization, and to educate and empower caregivers to assist in the care of patients should be implemented. The existing literature suggests patients with cirrhosis and a high medication burden are at highest risk for poor outcomes through ineffective medication management, but further work needs to be done identifying at risk patients. There are also large gaps in the literature evaluating medication interventions for this population. Conclusions can be drawn from similarities in CHF, but clearly more research needs to be done in cirrhosis. Future interventions should be evaluated for clinical and cost effectiveness.
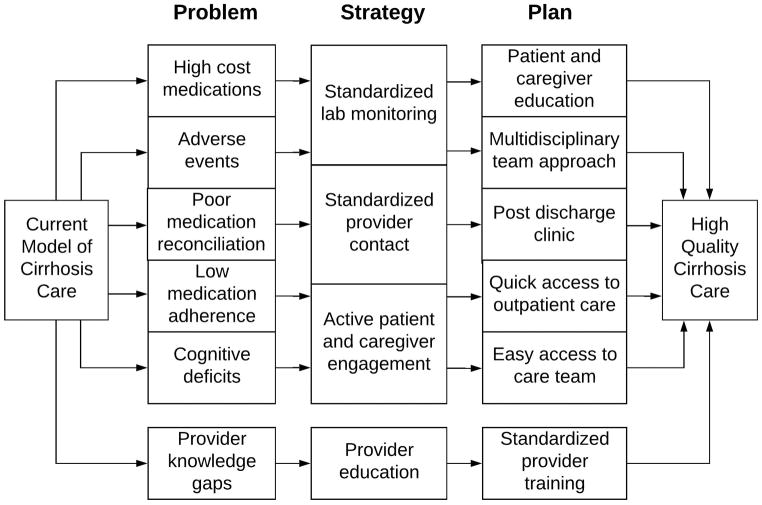
Based on the existing evidence, the optimal solution would create a system where there is multidisciplinary involvement (nurses, pharmacists, and physicians) in the inpatient and outpatient settings, standardized post hospitalization clinic visits, and easy access to providers (telephone or in-person) for patients concerns (Figure 2). Many aspects of this are included in the established chronic care model46, which focuses on active care between visits, mobilizing community support, enhancing patient self-management, and focusing on evidenced based care. This model has been proposed to be incorporated into cirrhosis care in the past47. Introducing a cirrhosis quality collaborative at each center may also help meet some of these goals by incorporating quality metrics into the electronic health record48. A clinical trial randomizing patients with decompensated cirrhosis to usual care v. pharmacist driven medication management and patient education is currently ongoing by Hayward et al. The primary outcome of this study is medication discrepancies, but they are also evaluating adherence, quality of life, medication beliefs, and clinical outcomes such as hospitalizations and mortality.49 All of these initiatives require systematic redesign of how patients with decompensated cirrhosis receive care. Despite the challenges of cost and provider buy-in, these changes are essential to improve the standard of medication management and clinical outcomes in cirrhosis.
Figure 2.
Key Points.
Providers should be aware of the known adverse effects of medications used to treat complications of cirrhosis, in addition to the adverse effects for routinely used medications in this population.
Complex medication regimens and medication list discrepancies are common in cirrhosis. Other factors that limit optimal and safe medication use include impaired cognition related to cirrhosis, medication adverse effects, suboptimal adherence, and provider knowledge gaps.
Patient education, case management, and novel technologies can be used to overcome these barriers. Restructuring delivery of care to include a multidisciplinary team approach and ensuring timely access to outpatient care is also needed.
Acknowledgments
Financial Support: This study was funded in part by an NIH Training Grant in Epidemiology and Health Services (T32 DK062708 - MJT) and an NIH Grant from the Michigan Institute for Clinical and Health Research (KL2 TR002241 - EBT).
Abbreviations
- HE
Hepatic Encephalopathy
- AKI
Acute Kidney Injury
- PPI
Proton Pump Inhibitor
- ESLD
End-Stage Liver Disease
- NSBB
Non-Selective Beta Blocker
- MMAS-8
Morisky Medication Adherence Scale
- MRCI
Medication Regimen Complexity Index
- MELD
Model for End-Stage Liver Disease
- NSAID
Non-Steroidal Anti-Inflammatory Drug
- GERD
Gastroesophageal Reflux Disease
- PUD
Peptic Ulcer Disease
- CHC
Chronic Hepatitis C
- ITAS
Immunosuppressive Therapy Adherence Scale
- SVR
Sustained Virologic Response
Footnotes
Conflict of Interest: No authors have any personal conflicts of interest to disclose.
References
- 1.Scaglione S, Kliethermes S, Cao G, et al. The Epidemiology of Cirrhosis in the United States: A Population-based Study. Journal of clinical gastroenterology. 2015;49(8):690–696. doi: 10.1097/MCG.0000000000000208. [DOI] [PubMed] [Google Scholar]
- 2.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2017 doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong R, Liu B, Bhuket T. Significant burden of nonalcoholic fatty liver disease with advanced fibrosis in the US: a cross-sectional analysis of 2011–2014 National Health and Nutrition Examination Survey. Alimentary Pharmacology & Therapeutics. 2017 doi: 10.1111/apt.14327. [DOI] [PubMed] [Google Scholar]
- 4.Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology. 2015;149(6):1471–1482. e1475. doi: 10.1053/j.gastro.2015.07.056. [DOI] [PubMed] [Google Scholar]
- 5.Murphy SL, Kochanek KD, Xu J. Deaths: final data for 2012. 2015 [PubMed] [Google Scholar]
- 6.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179–1187. e1173. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson B, Round J, Georgeson B, et al. Cirrhosis with ascites in the last year of life: a nationwide analysis of factors shaping costs, health-care use, and place of death in England. The lancet Gastroenterology & hepatology. 2017 doi: 10.1016/S2468-1253(17)30362-X. [DOI] [PubMed] [Google Scholar]
- 8.Tapper EB, Halbert B, Mellinger J. Rates of and reasons for hospital readmissions in patients with cirrhosis: a multistate population-based cohort study. Clinical Gastroenterology and Hepatology. 2016;14(8):1181–1188. e1182. doi: 10.1016/j.cgh.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Ginés P, Quintero E, Arroyo V, et al. Compensated cirrhosis: Natural history and prognostic factors. Hepatology. 1987;7(1):122–128. doi: 10.1002/hep.1840070124. [DOI] [PubMed] [Google Scholar]
- 10.Bajaj JS, Reddy KR, Tandon P, et al. The Three-Month Readmission Rate Remains Unacceptably High in a Large North American Cohort of Cirrhotic Patients. Hepatology. 2015 doi: 10.1002/hep.28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romero-Gomez M, Boza F, Garcia-Valdecasas MS, Garcia E, Aguilar-Reina J. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. The American journal of gastroenterology. 2001;96(9):2718–2723. doi: 10.1111/j.1572-0241.2001.04130.x. [DOI] [PubMed] [Google Scholar]
- 12.Wijdicks EF. Hepatic Encephalopathy. The New England journal of medicine. 2016;375(17):1660–1670. doi: 10.1056/NEJMra1600561. [DOI] [PubMed] [Google Scholar]
- 13.Kuo SZ, Haftek M, Lai JC. Factors Associated with Medication Non-adherence in Patients with End-Stage Liver Disease. Digestive diseases and sciences. 2017;62(2):543–549. doi: 10.1007/s10620-016-4391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Chang M, Lai M. A Quality Improvement Initiative Reduces 30-Day Rate of Readmission for Patients With Cirrhosis. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2016;14(5):753–759. doi: 10.1016/j.cgh.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. The American journal of gastroenterology. 2013;108(9):1458–1463. doi: 10.1038/ajg.2013.219. [DOI] [PubMed] [Google Scholar]
- 16.Landis CS, Ghabril M, Rustgi V, et al. Prospective Multicenter Observational Study of Overt Hepatic Encephalopathy. Digestive diseases and sciences. 2016;61(6):1728–1734. doi: 10.1007/s10620-016-4031-7. [DOI] [PubMed] [Google Scholar]
- 17.Orr JG, Currie CJ, Berni E, et al. The impact on hospital resource utilisation of treatment of hepatic encephalopathy with rifaximin-α. Liver International. 2016;36(9):1295–1303. doi: 10.1111/liv.13111. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65(1):310–335. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 19.Shukla R, Kramer J, Cao Y, et al. Risk and Predictors of Variceal Bleeding in Cirrhosis Patients Receiving Primary Prophylaxis With Non-Selective Beta-Blockers. The American journal of gastroenterology. 2016;111(12):1778–1787. doi: 10.1038/ajg.2016.440. [DOI] [PubMed] [Google Scholar]
- 20.Ge PS, Runyon BA. The changing role of beta-blocker therapy in patients with cirrhosis. Journal of hepatology. 2014;60(3):643–653. doi: 10.1016/j.jhep.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Krag A, Bendtsen F, Henriksen JH, Møller S. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut. 2010;59(01):105–110. doi: 10.1136/gut.2009.180570. [DOI] [PubMed] [Google Scholar]
- 22.Coté GA, Howden CW. Potential adverse effects of proton pump inhibitors. Current gastroenterology reports. 2008;10(3):208–214. doi: 10.1007/s11894-008-0045-4. [DOI] [PubMed] [Google Scholar]
- 23.Kalaitzakis E, Björnsson E. Inadequate use of proton-pump inhibitors in patients with liver cirrhosis. European journal of gastroenterology & hepatology. 2008;20(6):512–518. doi: 10.1097/MEG.0b013e3282f4aa01. [DOI] [PubMed] [Google Scholar]
- 24.Chavez-Tapia NC, Tellez-Avila FI, Garcia-Leiva J, Valdovinos MA. Use and overuse of proton pump inhibitors in cirrhotic patients. Medical Science Monitor. 2008;14(9):CR468–CR472. [PubMed] [Google Scholar]
- 25.Trikudanathan G, Israel J, Cappa J, O’sullivan D. Association between proton pump inhibitors and spontaneous bacterial peritonitis in cirrhotic patients–a systematic review and meta-analysis. International journal of clinical practice. 2011;65(6):674–678. doi: 10.1111/j.1742-1241.2011.02650.x. [DOI] [PubMed] [Google Scholar]
- 26.Bajaj JS, Ananthakrishnan AN, Hafeezullah M, et al. Clostridium difficile is associated with poor outcomes in patients with cirrhosis: a national and tertiary center perspective. The American journal of gastroenterology. 2010;105(1):106. doi: 10.1038/ajg.2009.615. [DOI] [PubMed] [Google Scholar]
- 27.Bajaj JS, Reddy KR, Tandon P, et al. The 3-month readmission rate remains unacceptably high in a large North American cohort of patients with cirrhosis. Hepatology. 2016;64:200–208. doi: 10.1002/hep.28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polis S, Zang L, Mainali B, et al. Factors associated with medication adherence in patients living with cirrhosis. Journal of clinical nursing. 2016;25(1–2):204–212. doi: 10.1111/jocn.13083. [DOI] [PubMed] [Google Scholar]
- 29.Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. The American journal of gastroenterology. 2012;107(2):247–252. doi: 10.1038/ajg.2011.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller SK, Sponsler KC, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: a systematic review. Archives of internal medicine. 2012;172(14):1057–1069. doi: 10.1001/archinternmed.2012.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. Journal of clinical hypertension (Greenwich, Conn) 2008;10(5):348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Hayward KL, Valery PC, Cottrell WN, et al. Prevalence of medication discrepancies in patients with cirrhosis: a pilot study. BMC gastroenterology. 2016;16:114. doi: 10.1186/s12876-016-0530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osterberg L, Blaschke T. Adherence to medication. The New England journal of medicine. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 34.DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Medical care. 2004;42(3):200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 35.Leevy CB, Phillips JA. Hospitalizations during the use of rifaximin versus lactulose for the treatment of hepatic encephalopathy. Digestive diseases and sciences. 2007;52(3):737–741. doi: 10.1007/s10620-006-9442-4. [DOI] [PubMed] [Google Scholar]
- 36.Ross JS, Chen J, Lin ZQ, et al. Recent national trends in readmission rates after heart failure hospitalization. Circulation: Heart Failure. 2009 doi: 10.1161/CIRCHEARTFAILURE.109.885210. CIRCHEARTFAILURE. 109.885210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruppar TM, Cooper PS, Mehr DR, Delgado JM, Dunbar-Jacob JM. Medication adherence interventions improve heart failure mortality and readmission rates: systematic review and meta-analysis of controlled trials. Journal of the American Heart Association. 2016;5(6):e002606. doi: 10.1161/JAHA.115.002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotb A, Cameron C, Hsieh S, Wells G. Comparative effectiveness of different forms of telemedicine for individuals with heart failure (HF): a systematic review and network meta-analysis. PloS one. 2015;10(2):e0118681. doi: 10.1371/journal.pone.0118681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hameed A, Sauermann S, Schreier G. The impact of adherence on costs and effectiveness of telemedical patient management in heart failure. Applied clinical informatics. 2014;5(03):612–620. doi: 10.4338/ACI-2014-04-RA-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larrey D, Salse A, Ribard D, et al. Education by a nurse increases response of patients with chronic hepatitis C to therapy with peginterferon-alpha2a and ribavirin. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2011;9(9):781–785. doi: 10.1016/j.cgh.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 41.Volk ML, Fisher N, Fontana RJ. Patient knowledge about disease self-management in cirrhosis. The American journal of gastroenterology. 2013;108(3):302–305. doi: 10.1038/ajg.2012.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wigg AJ, McCormick R, Wundke R, Woodman RJ. Efficacy of a chronic disease management model for patients with chronic liver failure. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2013;11(7):850–858. e851–854. doi: 10.1016/j.cgh.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Kanwal F, Asch SM, Kramer JR, Cao Y, Asrani S, El-Serag HB. Early outpatient follow-up and 30-day outcomes in patients hospitalized with cirrhosis. Hepatology. 2016;64(2):569–581. doi: 10.1002/hep.28558. [DOI] [PubMed] [Google Scholar]
- 44.Bajaj JS, Thacker LR, Heuman DM, et al. The Stroop smartphone application is a short and valid method to screen for minimal hepatic encephalopathy. Hepatology. 2013;58(3):1122–1132. doi: 10.1002/hep.26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ganapathy D, Acharya C, Lachar J, et al. The Patient Buddy App Can Potentially Prevent Hepatic Encephalopathy-Related Readmissions. Liver International. 2017 doi: 10.1111/liv.13494. [DOI] [PubMed] [Google Scholar]
- 46.Zwar N, Harris M, Griffiths R, et al. A systematic review of chronic disease management. 2017 [Google Scholar]
- 47.Mellinger JL, Volk ML. Multidisciplinary management of patients with cirrhosis: a need for care coordination. Clinical Gastroenterology and Hepatology. 2013;11(3):217–223. doi: 10.1016/j.cgh.2012.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanwal F, Volk M, Singal A, Angeli P, Talwalkar J. Improving quality of health care for patients with cirrhosis. Gastroenterology. 2014;147(6):1204–1207. doi: 10.1053/j.gastro.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 49.Hayward KL, Martin JH, Cottrell WN, et al. Patient-oriented education and medication management intervention for people with decompensated cirrhosis: study protocol for a randomized controlled trial. Trials. 2017;18(1):339. doi: 10.1186/s13063-017-2075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tapper EB, Bonder A, Cardenas A. Preventing and treating acute kidney injury among hospitalized patients with cirrhosis and ascites: a narrative review. The American journal of medicine. 2016;129(5):461–467. doi: 10.1016/j.amjmed.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 51.Chandok N, Watt KD. Pain management in the cirrhotic patient: the clinical challenge. Paper presented at: Mayo Clinic Proceedings; 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bajaj JS, Thacker LR, Heuman DM, et al. Cognitive performance as a predictor of hepatic encephalopathy in pretransplant patients with cirrhosis receiving psychoactive medications: a prospective study. Liver Transplantation. 2012;18(10):1179–1187. doi: 10.1002/lt.23484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tapper EB, Risech-Neyman Y, Sengupta N. Psychoactive medications increase the risk of falls and fall-related injuries in hospitalized patients with cirrhosis. Clinical gastroenterology and hepatology. 2015;13(9):1670–1675. doi: 10.1016/j.cgh.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 54.Cohen DE, Anania FA, Chalasani N. An assessment of statin safety by hepatologists. The American journal of cardiology. 2006;97(8):S77–S81. doi: 10.1016/j.amjcard.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 55.Kamal S, Khan MA, Seth A, et al. Beneficial Effects of Statins on the Rates of Hepatic Fibrosis, Hepatic Decompensation, and Mortality in Chronic Liver Disease: A Systematic Review and Meta-Analysis. The American journal of gastroenterology. 2017;112(10):1495–1505. doi: 10.1038/ajg.2017.170. [DOI] [PubMed] [Google Scholar]
- 56.Serper M, Patzer RE, Reese PP, et al. Medication misuse, nonadherence, and clinical outcomes among liver transplant recipients. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2015;21(1):22–28. doi: 10.1002/lt.24023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asavakarn S, Sirivatanauksorn Y, Promraj R, et al. Systematic Pharmaceutical Educational Approach to Enhance Drug Adherence in Liver Transplant Recipients. Transplantation proceedings. 2016;48(4):1202–1207. doi: 10.1016/j.transproceed.2015.12.100. [DOI] [PubMed] [Google Scholar]
- 58.Promraj R, Dumronggittigule W, Sirivatanauksorn Y, et al. Immunosuppressive Medication Adherence in Liver Transplant Recipients. Transplantation proceedings. 2016;48(4):1198–1201. doi: 10.1016/j.transproceed.2015.12.097. [DOI] [PubMed] [Google Scholar]
- 59.Russo MW, Levi DM, Pierce R, et al. A prospective study of a protocol that reduces readmission after liver transplantation. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2016;22(6):765–772. doi: 10.1002/lt.24424. [DOI] [PubMed] [Google Scholar]