Abstract
Oxytocin (OT) and arginine-vasopressin (AVP) are involved in the regulation of complex social behaviors across a wide range of taxa. Despite this, little is known about the neuroanatomy of the OT and AVP systems in most non-human primates, and less in humans. The effects of OT and AVP on social behavior, including aggression, mating, and parental behavior, may be mediated primarily by the extensive connections of OT- and AVP-producing neurons located in the hypothalamus with the basal forebrain and amygdala, as well as with the hypothalamus itself. However, OT and AVP also influence social cognition, including effects on social recognition, cooperation, communication, and in-group altruism, which suggests connectivity with cortical structures. While OT and AVP V1a receptors have been demonstrated in the cortex of rodents and primates, and intranasal administration of OT and AVP has been shown to modulate cortical activity, there is to date little evidence that OT-and AVP-containing neurons project into the cortex. Here, we demonstrate the existence of OT- and AVP-containing fibers in cortical regions relevant to social cognition using immunohistochemistry in humans, chimpanzees, and rhesus macaques. OT-immunoreactive fibers were found in the straight gyrus of the orbitofrontal cortex as well as the anterior cingulate gyrus in human and chimpanzee brains, while no OT-immunoreactive fibers were found in macaque cortex. AVP-immunoreactive fibers were observed in the anterior cingulate gyrus in all species, as well as in the insular cortex in humans, and in a more restricted distribution in chimpanzees. This is the first report of OT and AVP fibers in the cortex in human and non-human primates. Our findings provide a potential mechanism by which OT and AVP might exert effects on brain regions far from their production site in the hypothalamus, as well as potential species differences in the behavioral functions of these target regions.
Keywords: axons, cingulate, insula, neuropeptides, primates
1 INTRODUCTION
Research in a wide range of taxa has revealed a significant role for the neuropeptides oxytocin (OT) and arginine-vasopressin (AVP) in social cognition and behavior (Caldwell & Albers, 2015; Johnson & Young, 2017). Many of these behaviors are species-specific and reflect differences in the OT and AVP systems; the clearest example is the presence of OT and AVP receptors in the reward system of pair-bonding vole species, and their absence in these regions in non-pair-bonding but closely related species (Johnson & Young, 2017; Young, Murphy Young & Hammock, 2005). There is increasing evidence that OT and AVP modulate social cognition and behavior in humans and non-human primates (Crockford, Deschner, Ziegler & Wittig, 2014; Feldman, Monakhov, Freeman & Young, 2016) and that disruptions of the OT and AVP systems in humans may be associated with disorders such as autism, Williams syndrome, and schizophrenia (Dai et al., 2012; Guastella & Hickie, 2016; Meyer-Lindenberg, Domes, Kirsch & Heinrichs, 2011; Zhang, Zhang, Han & Han, 2017). However, there is limited knowledge of the neurobiology underlying the OT and AVP systems in humans, or how these systems may vary among primates. Here, we used immunohistochemistry to examine OT- and AVP-immunoreactive (ir) innervation of regions of the cerebral cortex relevant to social cognition in humans, chimpanzees, and rhesus macaques.
Intranasal administration of OT and AVP has been the primary source of information about the effects of these peptides in humans. Although effects on behavior using this procedure are varied and complex, several themes emerge. OT appears to facilitate social cognition and social approach, depending on the context (Preckel, Scheele, Kendrick, Maier & Hurlemann, 2014; Scheele et al., 2012) and may play a role in social attachment (King, Walum, Inoue, Eyrich & Young, 2016). OT also modulates amygdala reactivity to social stimuli, with potential for anxiety reduction (Chen et al., 2016; Petrovic, Kalisch, Singer & Dolan, 2008). The prosocial effects of OT may be context dependent and may depend on individual characteristics as well as the group status (ingroup vs. outgroup) of an interaction partner (De Dreu, 2012; Marsh et al., 2017). AVP effects are also context-dependent, as it can promote pair-bonding behavior and cooperation (Brunnlieb et al., 2016; Rilling et al., 2014), but can also increase reactivity to threat and enhance anxiety (Morales-Medina, Witchey & Caldwell, 2016).
Several of these behavioral and cognitive traits associated with OT and AVP—bonding, disposition toward outgroup individuals, and anxiety—show remarkable diversity among primate species. Humans differ from chimpanzees and rhesus macaques in their tendency to form pair-bonds. Chimpanzees and rhesus macaques live in multi-male, multi-female societies in which individual animals mate with multiple members of the opposite sex. Although the human mating system is characterized by flexibility and varies across cultures, stable mating relationships are widespread across human societies (Quinlan & Quinlan, 2008), suggesting humans possess the biological substrates to facilitate pair-bonding to a greater degree than chimpanzees or macaques. Chimpanzees are pronounced in their xenophobia and territoriality (Furuichi & Thompson, 2007), and they commonly engage in lethal aggression, while human societies show high variation in rates of intergroup and lethal aggression (Fry & Soderberg, 2013).
Despite this behavioral variation, the neurobiology of the OT and AVP systems in human and non-human primates is not well characterized. OT and AVP are produced in the paraventricular, supraoptic, and suprachiasmatic (AVP only) hypothalamic nuclei in all mammalian species studied, as well as a limited number of cell groups outside the hypothalamus, which vary according to species (Kelly & Goodson, 2014; Ragen & Bales, 2013; Sofroniew, 1980). OT- and AVP-producing cells in the hypothalamus send dense fiber projections through the median eminence into the posterior pituitary to be released into the bloodstream. These hypothalamic cells can also affect the brain by volume transmission, or by sending axonal fibers to distant regions for fast, targeted release (for reviews see Albers, 2015; Johnson & Young, 2017).
There is evidence that OT and AVP affect the activity of cortical regions in humans. For example, OT can modulate insula and inferior frontal gyrus responses to infant crying in women (Riem et al., 2011). Both OT and AVP have also been shown to modulate insula activation of human subjects during a dyadic social interaction task (Feng et al., 2015). Finally, OT can attenuate the effect of aversive conditioned responses to neutral faces, associated with activity in the anterior cingulate gyrus and right medial temporal lobe (Petrovic et al., 2008). It is unknown whether these effects are exerted through direct modulation of cortical regions by OT and AVP, or indirectly through potential connections within a larger brain network (Chini, Verhage & Grinevich 2017). To resolve this, it is important to know whether OT-and AVP-producing cell groups send fiber projections to cortical regions and whether receptors for the peptides are found there.
A few studies have demonstrated the presence of OT and AVP V1a receptors in non-human primate cortex. AVP V1a receptors are present in various cortical regions in rhesus macaques (Young, Toloczko, & Insel, 1999), titi monkeys (Freeman et al., 2014), and common marmosets (Freeman & Young, 2016; Schorscher-Petcu, Dupre, & Tribollet, 2009). OT receptors have been found in primary visual cortex in titi monkeys (Freeman et al., 2014). Two studies suggest that OT and AVP receptors may be present in cortical regions in humans: Loup, Tribollet, Dubois-Dauphin and Dreifuss (1991) reported AVP V1a receptors in entorhinal cortex, and Boccia, Petrusz, Suzuki, Marson and Pedersen (2013) reported OT receptors in anterior cingulate gyrus. Notably, these studies were conducted using disparate methodologies and examined very few cortical regions. The use of pharmacologically optimized methods to demonstrate OT and AVP V1a receptors in areas of the human brainstem (Freeman, Smith, Goodman & Bales, 2016) offers potential for the full elucidation of these receptors in the cortex and other forebrain regions.
The presence of OT and AVP V1a receptors in primate cortex suggests that there are fiber projections to the cortex from peptide-containing cell bodies. There is mixed evidence for fiber projections within the cortex across mammalian species. OT fibers have been found in the insula of rats (Knobloch et al., 2012) and AVP in the insula of mice (Rood & De Vries, 2011). However, no OT or AVP cortical fibers were found in the tree shrew, the closest living relative of primates (Ni et al., 2014). In non-human primates, AVP fibers have been found in the limbic cortical regions of crab-eating macaques, specifically the primary olfactory (piriform) and entorhinal cortex, but not in neocortex, and no OT or AVP fibers have been reported in the other species examined, which include rhesus macaques, Japanese macaques, marmosets, and squirrel monkeys (see Ragen & Bales, 2013 for review). Previous reports of OT and AVP fibers in humans have focused on a limited set of subcortical limbic (Fliers, Guldenaar, van de Wal & Swaab, 1986) and brainstem (Sofroniew, 1980) regions, so it is unknown whether any cortical regions are innervated by OT and AVP in humans. Moreover, no previous study has attempted to identify OT or AVP fibers in great apes. In the present study, we address these gaps by examining the distribution of OT- and AVP-immunoreactive fibers in select cortical (including neocortical) regions of human, chimpanzee, and rhesus macaque cortex.
2 METHODS
2.1 Specimens
All human tissue (three males, ages 28, 31, 54 years) was sourced from the Warren Alpert Medical School at Brown University and the Lifespan Consortium, and research with this material is approved under FAW00001230 by Lifespan’s IRB and 836–2005 by Emory’s IRB. Brains were collected post-mortem and fixed by immersion in 10% formalin. All chimpanzee (one male, age 43; 2 females, ages 23, 50 years) and rhesus macaque (three males, ages 4, 25, 25; two females, ages 4, 20 years) tissue came from animals housed at Yerkes National Primate Research Center (YNPRC), the brains being opportunistically collected after animals died of natural causes or after euthanasia per veterinary order. All procedures with macaques and chimpanzees were carried out in accordance with protocols approved by the YNPRC and the Emory University Institutional Animal Care and Use Committee (IACUC, approval # YER-2001206). Brain tissue was rinsed with phosphate-buffered saline. Chimpanzee brains were separated into hemispheres, with one hemisphere stored frozen at −80 °C, while the other was fixed; only the fixed tissue, prepared by immersion in 10% neutral-buffered formalin, was used in this study. The macaque brains were processed whole. Tissue was perfusion or immersion fixed in 10% neutral-buffered formalin. After fixation, the human, chimpanzee, and macaque tissue was stored in an ethylene-glycol-based cryopreserva-tive solution at −20 °C until use. Post-mortem intervals for macaques and chimpanzees were <3 hr, and <48 hr for humans. Previous studies using similarly prepared tissue have shown binding specificity using antibodies for nonphosphorylated neurofilament protein (SMI-32), calbindin, and VGLUT2 (Bryant et al., 2012; Preuss & Coleman, 2002).
2.2 Regions investigated
Figure 1 depicts a lateral view of the regions sampled in each species. In every macaque, both hemispheres were examined from the most rostral part of the striatum through the posterior hippocampus, corresponding to Paxinos, Huang, and Toga (2000) atlas levels 24 through 96. Due to the much larger size of chimpanzee and, especially, human brains, select cortical regions were examined in tissue blocks of one hemisphere spanning the most rostral part of the striatum through the anterior hippocampus. These blocks included posterior orbital, cingulate, and insular cortex, as well as portions of fronto-parietal and anterior temporal cortex, and correspond to level 15 through 56 in the Ding et al. (2016) human atlas. An additional, more anterior sample of human frontal cortex (level 10) was also examined. In all three species, sections from the hypothalamus were also used as positive controls.
FIGURE 1.
Mid-sagittal view of anatomical regions included in this analysis for (a), rhesus macaques, (b), chimpanzees, and (c), humans. Regions inside the rectangles represent the blocks examined in each species. Important target regions are labeled. See Figures 4–6 for a coronal view including insular regions not visible in a mid-sagittal view
2.3 Tissue sectioning, antibodies, and immunohistochemistry
Fixed tissue was sectioned in the coronal plane at 40 μm thickness using a freezing microtome (Leica Biosystems, Wetzlar, Germany); blockface images were collected for every second section using a camera mounted over the freezing stage. The sections were first washed with phosphate-buffered saline (PBS) and then incubated in citrate buffer at 37.5 °C for 30 min for antigen retrieval. Next, sections were washed with 3% peroxide in methanol for 10 min to inactivate endogenous peroxidase. Sections were incubated for 1 hr in blocking buffer containing PBS, 2% serum, and 0.2% Tween-20, then incubated overnight at 4 °C in primary antibody at a dilution of 1:20,000. The next morning, sections were washed, then incubated in secondary antibody for 1 hr followed by a solution of biotinylated peroxide plus avidin (Vector ABC reagent). Sections were then reacted with diaminobenzi-dine (DAB) solution using the Vector DAB peroxidase substrate kit (Vector Laboratories, Inc, Burlingame, CA).
The OT antibody (Millipore, Burlington, MA, MAB5296) was a mouse monoclonal antibody made against OT conjugated to thyro-globulin. Specificity was tested by competitive ELISA and no reactivity to AVP or vasotocin was found (Millipore technical information datasheet). The AVP antibody (Peninsula Laboratories International, San Carlos, CA, T-4563) was a rabbit polyclonal antibody made against a synthetic AVP peptide. Specificity was tested with a no-primary control. Based on dilution tests, a concentration of 1:20,000 was found to be optimal for both the OT and AVP antibodies. In all three species, a 1:12 series of sections was labeled for OT, and an adjacent 1:12 series of sections labeled for AVP. In addition, a 1:12 series of sections adjacent to AVP was stained for Nissl substance. Stained sections were mounted on gelatin-coated slides, air-dried, and coverslipped.
2.4 Digital image capture and examination
Digital images of the stained sections were captured using an Aperio Digital Pathology Slide Scanner (Leica Biosystems) and analyzed qualitatively for localization of cell bodies and fibers. Imagery for each stained section was comprehensively examined at high magnification on a computer screen by at least two investigators. Possible immunoreactive (ir) fibers were flagged using Aperio’s ImageScope software and each flagged site evaluated by multiple investigators. The images used in Figures 2–6 were extracted from the original scans using ImageScope software.
FIGURE 2.
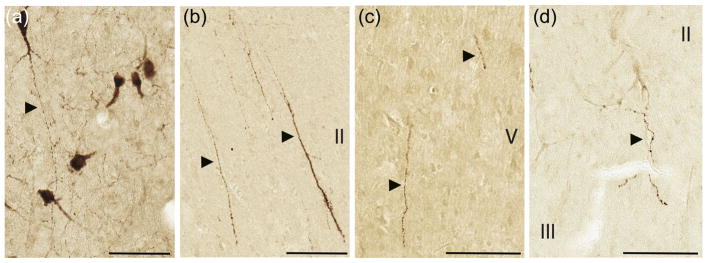
Morphology of AVP-ir fibers in the cortex closely resemble those in the hypothalamus. (a) Labeled hypothalamic cell bodies and fibers in the human supraoptic nucleus. (b) Cortical fibers in human insula. (c) Cortical fibers in chimpanzee insula. (d) Cortical fibers in macaque subgenual cingulate gyrus. OT and AVP fibers in all species showed the same morphological features. Roman numerals indicate cortical layers. Scale bar = 100 μm
FIGURE 6.
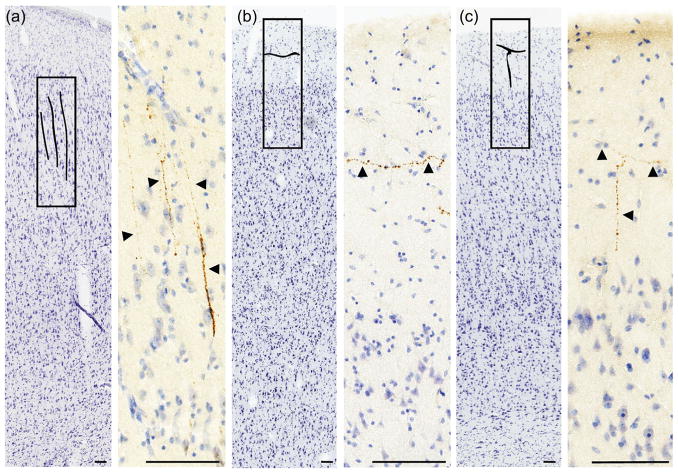
Nissl section illustrating a selection of regions where AVP fibers were found in macaque cortex. Letters within rectangles represent AVP fibers. a,b) High magnification view of AVP fibers. Letters on section view correspond to histology panels. Fiber images are from nearby sections (within 1 mm). AON, olfactory nucleus; Cd, caudate. Scale bar = 100 μm
3 RESULTS
3.1 Fiber morphology and orientation
OT and/or vasopressin fibers were found in a set of cortical regions in each species (see Table 1). In all three species, axonal fibers in the cortex showed morphology typical of those found in the hypothalamus and other subcortical structures, characterized by large varicosities that likely include en passant boutons (Figure 2). They are distinguishable from blood vessels by their thin and beaded appearance. OT and AVP fibers were observed in all cortical layers and showed no laminar preference. In deep cortical layers, a majority of fibers were oriented radially (parallel to the columns of cerebral cortex), with some oriented tangentially (perpendicular to the columns of cerebral cortex) or twisted in no obvious configuration. In layer I, fibers were mainly oriented tangentially (Figure 3). OT and AVP fibers were very sparse in every region in which they were observed.
TABLE 1.
Summary of results
| Rhesus macaque | Chimpanzee | Human | |
|---|---|---|---|
| OXYTOCIN | |||
| Paleocortex | |||
| Anterior olfactory nucleus | Ø | Ø | Ø |
| Primary olfactory cortex | Ø | Ø | Ø |
| Periallocortex | |||
| Agranular insula | Ø | Ø | Ø |
| Proisocortex | |||
| Dysgranular insula | Ø | Ø | Ø |
| Straight gyrus of orbitofrontal cortex | Ø | + | + |
| Ventral anterior cingulate gyrus (Area 24) | Ø | + | + |
| Subgenual anterior cingulate gyrus (Area 25) | Ø | + | + |
| Isocortex | |||
| Frontal operculum | Ø | Ø | Ø |
| Frontal pole | NE | NE | Ø |
| VASOPRESSIN | |||
| Paleocortex | |||
| Anterior olfactory nucleus | + | + | + |
| Primary olfactory cortex | + | + | + |
| Periallocortex | |||
| Agranular insula | Ø | + | + |
| Proisocortex | |||
| Dysgranular insula | Ø | Ø | + |
| Straight gyrus of orbitofrontal cortex | Ø | + | + |
| Ventral anterior cingulate gyrus (Area 24) | Ø | Ø | Ø |
| Subgenual anterior cingulate gyrus (Area 25) | + | + | + |
| Isocortex | |||
| Frontal operculum | Ø | Ø | + |
| Frontal pole | NE | NE | Ø |
A plus sign indicates fibers observed in that region. A Ø indicates no fibers were found in our sampling. NE indicates that the region was not examined in that species.
FIGURE 3.
AVP-ir fibers in the human insula. Nissl sections are shown at lower magnification. AVP fibers are shown at higher magnification, enhanced for contrast, and brightness with Aperio ImageScope software. (a) Radially oriented fibers in cortical layer II. (b) Fibers in the human insula oriented tangentially in cortical layer I. (c) A radial fiber that appears to end with tangential branches in cortical layer I. Scale bar = 100 μm
3.2 Fiber distribution
Table 1 provides a summary of results.
3.3 Humans
OT-immunoreactive (OT-ir) fibers were found in the straight gyrus (also known as gyrus rectus) and the ventral and subgenual anterior cingulate gyrus (Brodmann’s areas 24 and 25) in human cortex (Figure 4). Notably, due to the much larger size of the human brain, it was not feasible to examine the entire anterior cingulate gyrus, as we did in macaques and chimpanzees. OT-ir fibers were not observed in the insula or primary olfactory cortex. In all three human brains, immunoreactive AVP fibers were found in the insular cortex. These fibers were found in agranular insula (regions of insula lacking the granule cells of layer IV), including frontoinsular cortex, as well as dysgranular insula (transition area between agranular and more posterior granular insula). Fibers were observed in all layers of human insular cortex. Sparse AVP-ir fibers were also found in the frontal operculum, primary olfactory cortex, and the subgenual cingulate gyrus (Figure 4).
FIGURE 4.
Nissl section illustrating a selection of regions where OT and AVP fibers were found in human cortex. The midline is to the left; the dashed line at the dorsal edge indicates where the section was cut through the corpus callosum, internal capsule, and superior parts of frontal cortex. Tissue with OT fibers are marked by letters within circles; letters within rectangles represent AVP fibers. a–h) High magnification view of OT and AVP fibers. Letters on section view correspond to histology panels. Fiber images are from nearby sections (within 1 mm). FI, frontoinsular cortex; AON, olfactory nucleus; SG, straight gyrus; Cd, caudate. Scale bar = 100 μm
3.4 Chimpanzees
OT-ir cortical fibers were found in the straight gyrus in chimpanzee brains, as in human brains. OT-ir fibers were also found in the chimpanzee ventral anterior cingulate gyrus, subgenual cingulate gyrus, and superior frontal gyrus. There were no OT-ir fibers in the insula in chimpanzee brains. AVP-ir fibers were found in the primary olfactory cortex, as well as the subgenual cingulate gyrus, of chimpanzees. Additionally, solitary AVP-ir fibers were found in agranular insular cortex in the chimpanzee (Figure 5). AVP-ir fibers were not observed in granular insula, dysgranular insula, or the ventral anterior cingulate gyrus.
FIGURE 5.
Nissl section illustrating a selection of regions where OT and AVP fibers were found in chimpanzee cortex. Tissue with OT fibers are marked by letters within circles; letters within rectangles represent AVP fibers. a–e) High magnification view of OT and AVP fibers. Letters on section view correspond to histology panels. Fiber images are from nearby sections (within 1 mm). FI, frontoinsular cortex; AON, olfactory nucleus; SG, straight gyrus; Cd, caudate. Scale bar = 100 μm
3.5 Rhesus macaques
OT-ir fibers were not found in any cortical region in rhesus macaques. AVP-ir fibers were found in the subgenual cingulate gyrus and primary olfactory cortex of rhesus macaques (Figure 6). AVP-ir fibers were not observed in the insular cortex or ventral anterior cingulate gyrus in this species.
4 DISCUSSION
To our knowledge, this is the first report of OT- and AVP-ir fibers in the cortex in humans and the first report of OT-ir fibers in the cortex in non-human primates. Previous studies of human OT and AVP distribution of extra-hypothalamic projections have focused on non-cortical limbic and brainstem regions. It is not clear whether those studies examined the cortex and found no OT- or AVP-containing fibers or did not examine enough of the cortex to observe them. Both are plausible, given that the fibers are very sparse in all species examined here, consistent with the sparsity of OT innervation of rodent cortex (Knobloch et al., 2012). Here, OT and/or AVP axonal projections into the cortex were found in multiple non-isocortical regions in human, chimpanzee, and macaque brains. In addition, AVP fibers were observed in isocortical (neocortical) areas of human cortex (Table 1). Isocortical regions are comprised of six layers and are the most evolutionarily recent form of cortex.
OT- and AVP-ir cortical fibers in all three species were observed to have the same morphological features as hypothalamic fibers. These axonal fibers appear beaded due to varicosities (sites of neurotrans-mitter release). Release from varicosities of these fibers with en passant boutons, rather than classic axo-dendritic synapses, could be the mechanism by which OT and AVP modulate cortical activity (Grinevich & Charlet, 2017; Putnam, Young, & Gothard, 2018), similar to serotonin and catecholamine innervation of the cortex. Axonal release of a single vesicle of OT is estimated to be sufficient for 50% occupancy of OT receptors within a 55 μm radius (Chini et al., 2017). Thus even a limited number of projections may be able to influence cortical activity.
In our samples, OT and AVP fibers were most commonly oriented radially (parallel to the columns of cerebral cortex) in deep cortical layers and tangentially (perpendicular to cortical columns) in layer I, but both orientations as well as fibers twisted in no particular configuration were observed in all layers. While OT and AVP are produced in the hypothalamus, in several species, receptors with known functional effects have been found in brain regions that are likely too far from the hypothalamus to receive peptide through passive diffusion (Chini et al., 2017; Freeman & Young, 2016). Our results suggest that OT- and AVP-producing cells may be able to modulate cortical activity through targeted axonal release. Due to the columnar organization of the cerebral cortex, the tangential orientation of fibers traveling across these columns, particularly in layer I, could make peptide available to the apical dendrites of pyramidal cells to an extent out of proportion to the relatively small number of these peptidergic fibers. Further studies can be done tracing projections through adjacent sections to clarify their exact path. Additionally, OT and AVP fibers could modulate cortical activity via receptors not detectable in studies using autoradiography, such as those expressed in presynaptic terminals, distal to their site of synthesis in the cell body (Dölen, Darvishzadeh, Huang & Malenka, 2013). For example, OT receptors are abundantly expressed in the nucleus basalis of Meynert of nonhuman primates and humans, and this region is known to send cholinergic projections to many cortical structures (Freeman & Young, 2016). It is possible that OT receptors are transported to these distal projections and expressed in axon terminals. Further research is needed to determine if this is a mechanism by which OT and AVP innervation can modulate cortical activity. Regardless, the presence of axonal fibers may suggest the presence of receptors that are difficult to localize in postmortem samples.
With respect to localization, OT-ir fibers were found in the straight gyrus (also called gyrus rectus) within the medial orbitofrontal cortex in human and chimpanzee brains. The medial orbitofrontal cortex generally is associated with decision-making and emotional regulation (Bechara, Damasio & Damasio, 2000; Hsu & Price, 2007). The function of the straight gyrus is not well understood, but it has been proposed as a site of sensory integration (Passingham & Wise, 2012), and is known to have extensive connections to areas important for motivation, reward, and emotion, including the amygdala, hippocampus, cingulate gyrus, and insular cortex (Morecraft, Geula & Mesulam, 1992). Chimpanzees and rhesus macaques show some differences in the reward value of social stimuli; for example, rhesus macaques avoid direct eye gaze (Mendelson, Haith & Goldman-Rakic, 1982) while chimpanzees do not (Leavens & Hopkins, 1998). It is possible that OT acts in this region to affect social reward, though this interpretation is at the moment speculative.
OT-ir fibers were also found in regions of the anterior cingulate gyrus of chimpanzees and humans. In both species, OT-ir fibers were present in the subgenual cingulate gyrus as well as the ventral anterior cingulate. The ventral anterior cingulate gyrus is involved in assessing emotional salience (Apps, Rushworth & Chang, 2016). Interestingly, OT receptors in the anterior cingulate gyrus have been shown to mediate empathy-related consoling behavior in prairie voles (Burkett et al., 2016). It may be that OT has a conserved role in this region in detecting and responding to the emotional state of others.
AVP-ir fibers were observed in the paleocortical primary olfactory cortex and the subgenual cingulate gyrus in all three species. While primates have evolved to depend highly on their visual system for social communication, olfaction is the main social sense for most mammals (Barton, Purvis, & Harvey, 1995). Nevertheless, within primates, aspects of olfactory-related neuroanatomy correlate with social group size (Barton, 2006). The presence of vasopressin fibers in the primary olfactory cortex of all three species is possibly a conserved mammalian trait. AVP-ir fibers were also found in the subgenual cingulate gyrus (Brodmann’s area 25), a non-isocortical region with high connectivity with the hypothalamus, insula, and amygdala, all of which are involved in emotion processing (Phan, Wager, Taylor & Liberzon, 2002).
The most numerous AVP-containing fibers were observed in the agranular and dysgranular insula (including frontoinsular cortex) in humans. AVP fibers were also present in agranular insula in chimpanzees. In humans, the insula has been implicated in empathy and interoception (Craig, 2002; Singer & Lamm, 2009), and it has been proposed that the agranular cortex of the anterior insula is uniquely enlarged in humans (Bauernfeind et al., 2013; Nieuwenhuys, 2012). In one study, intranasal AVP increased empathic concern among individuals who had experienced parental warmth (Tabak et al., 2015). It may be that targeted release of AVP in the human insula contributes to the role of AVP in empathy. Additionally, the frontoinsular cortex, as well as the anterior cingulate gyrus, contains Von Economo neurons, large bipolar neurons present in humans, anthropoid primates, and other large-brained mammals, including cetaceans and farm animals (Allman et al., 2011; Raghanti et al., 2015). Though the functional role of this class of neurons is not well understood, they are known to be altered in some individuals with neuropsychiatric conditions affecting social skills and emotional function (Butti, Santos, Uppal & Hof, 2013). Although we did not observe direct contact between OT or AVP fibers and Von Economo neurons, further research is needed to determine whether local OT or AVP release modulates the activity of these neurons.
Among primates, there is evidence for human-specific modifications to the localization of cortical axonal fibers in several neuromodulatory systems (Sousa, Meyer, Santpere, Gulden, & Sestan, 2017); the OT and AVP systems appear to be no exception. However, while our sample included individuals of both sexes in chimpanzees and rhesus macaques, our human brain tissue was from males only. Sex differences in neurobiology and behavior have been demonstrated for OT and AVP in rodents (Albers, 2015; Dumais & Veenema, 2016) and in behavior after intranasal administration in humans (Feng et al., 2015; Rilling et al., 2014). Further study is needed to determine whether target regions of OT and AVP projections in humans may be different in females. Finally, due to the large size of the human brain, our study focused on selected regions of cortex and did not include the entirety of sizable regions such as the anterior cingulate gyrus. Future studies should investigate a greater number of areas, such as the fusiform gyrus, superior temporal sulcus, and right somatosensory cortex, which are involved in processing social information (Adolphs, 2001), as well as more posterior and dorsal regions of anterior cingulate gyrus in humans. Our results suggest that in primates, OT and AVP effects on cortical function and social cognition may be mediated by direct cortical projections from the hypothalamus, and the targets of these projections may be species-specific.
Acknowledgments
Funding information
The Leakey Foundation, Grant number: 38217; NIH OD, Grant number: P51OD11132; The Templeton Foundation, Grant number: 40463; NIH, Grant number: P50MH100023
This research was supported by funding from The Leakey Foundation (#38217) to CR and The Templeton Foundation (#40463) to HEA, LJY, JKR, and TMP. Additional support was provided by NIH grants P50MH100023 to LJY and by NIH OD P51OD11132 to YNPRC.
References
- Adolphs R. The neurobiology of social cognition. Current Opinion in Neurobiology. 2001;11(2):231–239. doi: 10.1016/S0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Albers HE. Species, sex and individual differences in the vasotocin/vasopressin system: Relationship to neurochemical signaling in the social behavior neural network. Frontiers in Neuroendocrinology. 2015;36:49–71. doi: 10.1016/j.yfrne.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, … Hof PR. The von Economo neurons in the frontoinsular and anterior cingulate cortex. Annals of the New York Academy of Sciences. 2011;1225:59–71. doi: 10.1111/j.1749-6632.2011.06011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps MA, Rushworth MF, Chang SW. The anterior cingulate gyrus and social cognition: Tracking the motivation of others. Neuron. 2016;90(4):692–707. doi: 10.1016/j.neuron.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton RA. Olfactory evolution and behavioral ecology in primates. American Journal of Primatology. 2006;68(6):545–558. doi: 10.1002/ajp.20251. [DOI] [PubMed] [Google Scholar]
- Barton RA, Purvis A, Harvey PH. Evolutionary radiation of visual and olfactory brain systems in primates, bats and insectivores. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 1995;348(1326):381–392. doi: 10.1098/rstb.1995.0076. [DOI] [PubMed] [Google Scholar]
- Bauernfeind AL, de Sousa AA, Avasthi T, Dobson SD, Raghanti MA, Lewandowski AH, … Sherwood CC. A volumetric comparison of the insular cortex and its subregions in primates. Journal of Human Evolution. 2013;64(4):263–279. doi: 10.1016/j.jhevol.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10(3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Petrusz P, Suzuki K, Marson L, Pedersen CA. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience. 2013;253:155–164. doi: 10.1016/j.neuroscience.2013.08.048. [DOI] [PubMed] [Google Scholar]
- Brunnlieb C, Nave G, Camerer CF, Schosser S, Vogt B, Munte TF, Heldmann M. Vasopressin increases human risky cooperative behavior. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(8):2051–2056. doi: 10.1073/pnas.1518825113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant KL, Suwyn C, Reding KM, Smiley JF, Hackett TA, Preuss TM. Evidence for ape and human specializations in geniculostriate projections from VGLUT2 immunohistochemistry. Brain, Behavior, and Evolution. 2012;80(3):210–221. doi: 10.1159/000341135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FB, Young LJ. Oxytocin-dependent consolation behavior in rodents. Science. 2016;351(6271):375–378. doi: 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butti C, Santos M, Uppal N, Hof PR. Von Economo neurons: Clinical and evolutionary perspectives. Cortex. 2013;49(1):312–326. doi: 10.1016/j.cortex.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Albers HE. Oxytocin, vasopressin, and the motivational forces that drive social behaviors. In: Simpson EH, Balsam PD, editors. Behavioral neuroscience of motivation. Cham: Springer; 2015. pp. 51–103. [DOI] [PubMed] [Google Scholar]
- Chen X, Hackett PD, DeMarco AC, Feng C, Stair S, Haroon E, … Rilling JK. Effects of oxytocin and vasopressin on the neural response to unreciprocated cooperation within brain regions involved in stress and anxiety in men and women. Brain Imaging and Behavior. 2016;10(2):581–593. doi: 10.1007/s11682-015-9411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini B, Verhage M, Grinevich V. The action radius of oxytocin release in the mammalian CNS: From single vesicles to behavior. Trends in Pharmacological Sciences. 2017;38(11):982–991. doi: 10.1016/j.tips.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Crockford C, Deschner T, Ziegler TE, Wittig RM. Endogenous peripheral oxytocin measures can give insight into the dynamics of social relationships: A review. Frontiers in Behavioral Neuroscience. 2014;8:68. doi: 10.3389/fnbeh.2014.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Carter CS, Ying J, Bellugi U, Pournajafi-Nazarloo H, Korenberg JR. Oxytocin and vasopressin are dysregulated in Williams Syndrome, a genetic disorder affecting social behavior. PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0038513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu CK. Oxytocin modulates cooperation within and competition between groups: An integrative review and research agenda. Hormones and Behavior. 2012;61(3):419–428. doi: 10.1016/j.yhbeh.2011.12.00909. [DOI] [PubMed] [Google Scholar]
- Ding SL, Royall JJ, Sunkin SM, Ng L, Facer BA, Lesnar P, … Lein ES. Comprehensive cellular-resolution atlas of the adult human brain. Journal of Comparative Neurology. 2016;525(2):407. doi: 10.1002/cne.24080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501(7466):179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Frontiers in Neuroendocrinology. 2016;40:1–23. doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Monakhov M, Pratt M, Ebstein RP. Oxytocin pathway genes: Evolutionary ancient system impacting on human affiliation, sociality, and psychopathology. Biological Psychiatry. 2016;79(3):174–184. doi: 10.1016/j.biopsych.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Feng C, Hackett PD, DeMarco AC, Chen X, Stair S, Haroon E, … Rilling JK. Oxytocin and vasopressin effects on the neural response to social cooperation are modulated by sex in humans. Brain Imaging and Behavior. 2015;9(4):754–764. doi: 10.1007/s11682-014-9333-9. [DOI] [PubMed] [Google Scholar]
- Fliers E, Guldenaar SE, van de Wal N, Swaab DF. Extrahypothalamic vasopressin and oxytocin in the human brain; presence of vasopressin cells in the bed nucleus of the stria terminalis. Brain Research. 1986;375(2):363–367. doi: 10.1016/0006-8993(86)90759-6. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Smith AL, Goodman MM, Bales KL. Selective localization of oxytocin receptors and vasopressin 1a receptors in the human brainstem. Social Neuroscience. 2016;12(2):113–123. doi: 10.1080/17470919.2016.1156570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Young LJ. Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: Translational implications. Journal of Neuroendocrinology. 2016;28(4) doi: 10.1111/jne.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Walum H, Inoue K, Smith AL, Goodman MM, Bales KL, Young LJ. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus) Neuroscience. 2014;273:12–23. doi: 10.1016/j.neuroscience.2014.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry DP, Soderberg P. Lethal aggression in mobile forager bands and implications for the origins of war. Science. 2013;341(6143):270–273. doi: 10.1126/science.1235675. [DOI] [PubMed] [Google Scholar]
- Furuichi T, Thompson J. The bonobos: Behavior, ecology, and conservation. New York: Springer Sciences & Business Media; 2007. [Google Scholar]
- Grinevich V, Charlet A. Oxytocin: Pain relief in skin. Pain. 2017;158(11):2061–2063. doi: 10.1097/j.pain.0000000000001006. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Hickie IB. Oxytocin treatment, circuitry, and autism: A critical review of the literature placing oxytocin into the autism context. Biological Psychiatry. 2016;79(3):234–242. doi: 10.1016/j.biopsych.2015.06.028. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Price JL. Midline and intralaminar thalamic connections with the orbital and medial prefrontal networks in macaque monkeys. Journal of Comparative Neurology. 2007;504(2):89–111. doi: 10.1002/cne.21440. [DOI] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ. Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neuroscience & Biobehavioral Reviews. 2017;76(Pt A):87–98. doi: 10.1016/j.neubiorev.2017.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL. Social functions of individual vasopressin-oxytocin cell groups in vertebrates: What do we really know? Frontiers in Neuroendocrinology. 2014;35(4):512–529. doi: 10.1016/j.yfrne.2014.04.005. [DOI] [PubMed] [Google Scholar]
- King LB, Walum H, Inoue K, Eyrich NW, Young LJ. Variation in the oxytocin receptor gene predicts brain region-specific expression and social attachment. Biological Psychiatry. 2016;80(2):160–169. doi: 10.1016/j.biopsych.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, … Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73(3):553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD. Intentional communication by chimpanzees: A cross-sectional study of the use of referential gestures. Developmental Psychology. 1998;34(5):813. doi: 10.1037//0012-1649.34.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high- affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Research. 1991;555(2):220–232. doi: 10.1016/0006-8993(91)90345-V. [DOI] [PubMed] [Google Scholar]
- Marsh N, Scheele D, Feinstein JS, Gerhardt H, Strang S, Maier W, Hurlemann R. Oxytocin-enforced norm compliance reduces xenophobic outgroup rejection. Proceedings of the National Academy of Sciences in the United States of America. 2017;114(35):9314–9319. doi: 10.1073/pnas.1705853114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson MJ, Haith MM, Goldman-Rakic PS. Face scanning and responsiveness to social cues in infant rhesus monkeys. Developmental Psychology. 1982;18(2):222. doi: 10.1037/0012-1649.18.2.222. [DOI] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nature Reviews Neuroscience. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Morales-Medina JC, Witchey SK, Caldwell HK. Melatonin, Neuroprotective Agents and Antidepressant Therapy. India: Springer; 2016. The role of vasopressin in anxiety and depression; pp. 667–685. [Google Scholar]
- Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. Journal of Comparative Neurology. 1992;323(3):341–358. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- Ni RJ, Shu YM, Wang J, Yin JC, Xu L, Zhou JN. Distribution of vasopressin, oxytocin and vasoactive intestinal poly-peptide in the hypothalamus and extrahypothalamic regions of tree shrews. Neuroscience. 2014;265:124–136. doi: 10.1016/j.neuroscience.2014.01.034. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R. The insular cortex: A review. Progress in Brain Research. 2012;195:123–163. doi: 10.1016/B978-0-444-53860-4.00007-6. [DOI] [PubMed] [Google Scholar]
- Passingham RE, Wise SP. The neurobiology of the prefrontal cortex: anatomy, evolution, and the origin of insight (No. 50) Oxford, UK: Oxford University Press; 2012. [Google Scholar]
- Paxinos G, Huang XF, Toga AW. The rhesus monkey brain in stereotaxic coordinates. San Diego, USA: Academic Press; 2000. [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. Journal of Neuroscience. 2008;28(26):6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Preckel K, Scheele D, Kendrick KM, Maier W, Hurlemann R. Oxytocin facilitates social approach behavior in women. Frontiers in Behavioral Neuroscience. 2014;8:191. doi: 10.3389/fnbeh.2014.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss TM, Coleman GQ. Human-specific organization of primary visual cortex: Alternating compartments of dense Cat-301 and calbindin immunoreactivity in layer 4A. Cerebral Cortex. 2002;12(7):671–691. doi: 10.1093/cercor/12.7.671. [DOI] [PubMed] [Google Scholar]
- Putnam PT, Young LJ, Gothard KM. Bridging the gap between rodents and humans: The role of non-human primates in oxytocin research. American Journal of Primatology. 2018:e22756. doi: 10.1002/ajp.22756. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan RJ, Quinlan MB. Human lactation, pair-bonds, and alloparents: A cross-cultural analysis. Human Nature. 2008;19(1):87–102. doi: 10.1007/s12110-007-9026-9. [DOI] [PubMed] [Google Scholar]
- Ragen BJ, Bales KL. Oxytocin and vasopressin in non-human primates. Oxytocin, vasopressin and related peptides in the regulation of behavior. Cambridge, UK: Cambridge University Press; 2013. pp. 288–306. [Google Scholar]
- Raghanti MA, Spurlock LB, Treichler FR, Weigel SE, Stimmelmayr R, Butti C, … Hof PR. An analysis of von Economo neurons in the cerebral cortex of cetaceans, artiodactyls, and perissodactyls. Brain Structure and Function. 2015;220(4):2303–2314. doi: 10.1007/s00429-014-0792-y. [DOI] [PubMed] [Google Scholar]
- Riem MM, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MA, Vermeiren RR, … Rombouts SA. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: A randomized controlled trial. Biological Psychiatry. 2011;70(3):291–297. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Demarco AC, Hackett PD, Chen X, Gautam P, Stair S, … Pagnoni G. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology. 2014;39:237–248. doi: 10.1016/j.psyneuen.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, De Vries GJ. Vasopressin innervation of the mouse (Mus musculus) brain and spinal cord. Journal of Comparative Neurology. 2011;519(12):2434. doi: 10.1002/cne.22635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D, Striepens N, Gunturkun O, Deutschlander S, Maier W, Kendrick KM, Hurlemann R. Oxytocin modulates social distance between males and females. Journal of Neuroscience. 2012;32(46):16074–16079. doi: 10.1523/JNEUROSCI.2755-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Dupre A, Tribollet E. Distribution of vasopressin and oxytocin binding sites in the brain and upper spinal cord of the common marmoset. Neuroscience Letters. 2009;461(3):217–222. doi: 10.1016/j.neulet.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Singer T, Lamm C. The social neuroscience of empathy. Annals of the New York Academy of Sciences. 2009;1156:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Projections from vasopressin, oxytocin, and neurophysin neurons to neural targets in the rat and human. Journal of Histochemistry & Cytochemistry. 1980;28(5):475–478. doi: 10.1177/28.5.7381192. [DOI] [PubMed] [Google Scholar]
- Sousa AM, Meyer KA, Santpere G, Gulden FO, Sestan N. Evolution of the human nervous system function, structure, and development. Cell. 2017;170(2):226–247. doi: 10.1016/j.cell.2017.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak BA, Meyer ML, Castle E, Dutcher JM, Irwin MR, Han JH, … Eisenberger NI. Vasopressin, but not oxytocin, increases empathic concern among individuals who received higher levels of paternal warmth: A randomized controlled trial. Psychoneuroendocrinology. 2015;51:253–261. doi: 10.1016/j.psyneuen.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Murphy Young AZ, Hammock EA. Anatomy and neurochemistry of the pair bond. Journal of Comparative Neurology. 2005;493(1):51–57. doi: 10.1002/cne.20771. [DOI] [PubMed] [Google Scholar]
- Young LJ, Toloczko D, Insel TR. Localization of vasopressin (V1a) receptor binding and mRNA in the rhesus monkey brain. Journal of Neuroendocrinology. 1999;11(4):291–297. doi: 10.1046/j.1365-2826.1999.00332.x. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang HF, Han JS, Han SP. Genes related to oxytocin and arginine-vasopressin pathways: Associations with autism spectrum disorders. Neuroscience Bulletin. 2017;33(2):238–246. doi: 10.1007/s12264-017-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]