Abstract
Objective
Macrophages play key roles in inflammation and diabetic vascular complications. Emerging evidence implicates long noncoding RNAs (lncRNAs) in inflammation, but their role in macrophage dysfunction associated with inflammatory diabetic complications is unclear and was therefore investigated in this study.
Approach and Results
RNA-sequencing and RT-qPCR demonstrated that a lncRNA Dynamin 3 opposite strand (Dnm3os) is upregulated in bone marrow derived macrophages from type 2 diabetic db/db mice, diet-induced insulin-resistant mice and diabetic ApoE−/− mice, as well as in monocytes from type 2 diabetic patients relative to controls. Diabetic conditions (High glucose and palmitic acid) induced Dnm3os in mouse and human macrophages. Promoter reporter analysis and chromatin-immunoprecipitation assays demonstrated that diabetic conditions induce Dnm3os via NF-κB activation. RNA-FISH and RT-qPCRs of sub-cellular fractions demonstrated nuclear localization and chromatin enrichment of Dnm3os in macrophages. Stable overexpression of Dnm3os in macrophages altered global histone modifications and upregulated inflammation and immune response genes, and phagocytosis. Conversely, RNAi-mediated knockdown of Dnm3os attenuated these responses. RNA-pull-down assays with macrophage nuclear lysates identified nucleolin and ILF-2 as protein binding partners of Dnm3os, which was further confirmed by RNA-IP and RNA-FISH-immunofluorescence. Furthermore, nucleolin levels were decreased in diabetic conditions, and its knockdown enhanced Dnm3os-induced inflammatory gene expression and histone H3K9-acetylation at their promoters.
Conclusions
These results demonstrate novel mechanisms involving upregulation of lncRNA Dnm3os, disruption of its interaction with nucleolin, and epigenetic modifications at target genes that promote macrophage inflammatory phenotype in diabetes. The data could lead to lncRNA-based therapies for inflammatory diabetes complications.
Keywords: Diabetes, atherosclerosis, macrophages, inflammation, long non coding RNA, Dnm3os, nucleolin, epigenetic mechanisms
INTRODUCTION
Metabolic syndrome and diabetes are associated with insulin resistance, hyperglycemia, and hyperlipidemia that contribute to chronic inflammation implicated in the pathogenesis of vascular complications such as atherosclerosis1–3. Monocyte recruitment into target tissues and differentiation into macrophages are key processes in inflammation associated with such complications. Diabetes accelerates these vascular complications by skewing monocyte/macrophage polarization, proliferation and dysfunction to enhance inflammation, foam cell formation, and promote defective apoptosis and efferocytosis2–5. These adverse pro-inflammatory effects of diabetes and insulin resistance on macrophages can be mediated by high glucose (HG), advanced glycation end products (AGEs), elevated free fatty acids such as palmitic acid (PA) and oxidized lipids2, 3, 6, 7. Several studies demonstrated the role of oxidative stress, activation of the receptor for AGEs (RAGE), signaling pathways including protein kinase C, and transcription factors (TF)s such as NF-κB8, 9. Epigenetic mechanisms, including promoter histone H3 lysine 9/14 acetylation (H3K9ac) and H3K4 methylation have also been implicated in inflammatory gene regulation associated with diabetic complications10. However, much less is known about the role of non-coding RNAs like long non-coding RNAs (lncRNAs) in regulating genes and processes associated with inflammation and macrophage dysfunction in diabetes.
Emerging evidence shows lncRNAs are involved in diverse biological processes such as cell proliferation and differentiation11, 12. LncRNAs are longer than 200 bp, lack coding potential and are expressed from intergenic regions or introns, as anti-sense to coding transcripts, pseudogenes, host genes of microRNAs, and from enhancers11–14. LncRNA actions include transcription regulation via directing epigenetic factors such as polycomb repressive complexes to chromatin, acting as scaffolds for chromatin remodeling proteins and TFs and regulation of mRNA stability/translation, and inhibition of microRNA functions11–13, 15. Changes in lncRNA expression can dysregulate key functions of cardiac, vascular and renal cells implicated in diabetic vascular complications and cardiovascular diseases14, 16–19. Recent studies have identified several lncRNAs expressed in macrophages and monocytes that mediate pro- and anti-inflammatory processes, cell differentiation, and survival20. These macrophage lncRNAs act through diverse mechanisms including regulation of pro-inflammatory TFs like NF-κB, interaction with hnRNPs and epigenetic regulators such as PRC complexes to promote chromatin remodeling at target gene promoters20–23. However, these aspects of lncRNA functions have not been studied in diabetes-induced macrophage dysfunction.
We recently demonstrated that bone marrow-derived macrophages (BMDMs) obtained from obese, type 2 diabetic (T2D) db/db mice exhibited enhanced inflammatory genes, and dysregulation of genes associated with macrophage alternate activation compared to BMDMs from control non-diabetic db/+ mice. Interestingly, these transcriptome changes were also associated with altered expression of several lncRNAs. One of the upregulated lncRNAs, E330013P06 was found to promote pro-inflammatory and pro-atherogenic phenotype in macrophages24. However, the regulation and function of other differentially expressed lncRNAs in diabetic macrophages are not known. Here, we demonstrate that a lncRNA Dynamin 3 opposite strand (Dnm3os) is upregulated in macrophages and monocytes under diabetic conditions via NF-κB activation. Furthermore, we found Dnm3os interacts with nucleolin, a nuclear protein, in macrophages, and that disruption of Dnm3os-nucleolin interactions under diabetic conditions can enhance inflammatory gene expression via epigenetic mechanisms. The results illustrate novel mechanisms underlying lncRNA dependent regulation of macrophage inflammation in diabetes.
Materials and Methods
Isolation of Bone marrow derived macrophages (BMDMs) and peritoneal macrophages (PMs) from mice
Animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee. BMDMs were prepared from 10–12 week old type 2 diabetic male and female db/db mice and control db/+ littermates, and male C57BL/6 mice (Jackson Laboratories, Maine) as described24. Bone marrow cells were obtained from femurs and tibia and differentiated into macrophages for 7–8 days in macrophage complete medium (MCM) containing DMEM (Cat. No. 12–707F, Lonza, Basel, Switzerland) with 5.5 mmol/L glucose, 10% heat inactivated fetal bovine serum (Cat. No. S11150, Atlanta Biologicals, Flowery Branch, GA), 2 mmol/L glutamine, penicillin/streptomycin (100 μmol/L), 50 μmol/L β-mercaptoethanol, and 10 mmol/L HEPES, pH 7.4 supplemented with 20 ng/mL of M-CSF (Cat. No. 416-ML-050, R&D Systems, Minneapolis MN). Thioglycollate elicited PMs were isolated from control C57BL/6 and STZ injected (50mg/kg per day for 5 days) T1D mice as well as C57BL/6 mice (8 weeks old) fed high fat diet (60% kcal, Research Diets Inc., D12492i) or normal chow diet (ND) for 8 weeks as described24. In addition, male Apoe−/− mice (Jackson Laboratory, stock# 002052) were injected with streptozotocin (STZ, 50mg/kg/d) or citrate buffer for 5 consecutive days. BMDMs were prepared at 20 weeks post diabetes (>500 mg/dL of glucose vs 160 mg/dL in Controls), when diabetic Apoe−/− mice exhibited accelerated atherosclerosis. Mouse macrophage cell line RAW264.7 (ATCC, Manassas, VA) was maintained in MCM. Where indicated, macrophages were treated with media containing 200 μmol/L palmitic acid (PA) (Nu-Check Prep, Inc. MN, USA) or control BSA for indicated periods. PA stock solutions (10 mmol/L) were freshly prepared in BSA, diluted to the indicated concentration in MCM, incubated for 30 min at 37°C, and filtered with 0.2 μm filter before treating macrophages and monocytes24.
Isolation of primary human monocytes and human macrophages
Human monocyte experiments were performed in accordance with approved protocols from the Baylor College of Medicine and the City of Hope Medical Center. Following informed consent CD14+ monocytes were obtained from T2D patients and healthy controls (age and sex matched) at the Baylor College of Medicine. Patient data and monocyte isolation methods have been described24. In some experiments, CD14+ monocytes from healthy donors (All Cells, Emeryville, CA) and THP1 monocytes (ATCC TIB-202) were differentiated into macrophages using MCSF (50 ng/mL) for one week and PMA (20 ng/mL, up to 48 hrs) respectively.
RNA isolation and gene expression
Total RNA was isolated using RNeasy mini kit (Cat. No. 74106, Qiagen, Valencia, CA), with on-column digestion with DNase I digestion (Cat. No. 79254, Qiagen). Total RNA (0.2–1 μg) was used to synthesize cDNA using the Prime ScriptTM RT Master Mix (Cat. No. RR036A, Takara, Mountain View, CA) for coding genes, QuantiTect Reverse Transcription Kit (Qiagen) or High-Capacity cDNA Reverse Transcription kit (Cat. No. 4368814, Thermo Fisher Scientific) for lncRNAs, and qScript microRNA cDNA Synthesis Kit (Quanta Bio, Beverly, MA) for miRNAs. Gene expression was analyzed by quantitative PCR (qPCR) using SYBR Green reagent and TaqMan assays (Cat. No. 4367659 and 7352042 respectively, Applied Biosystems, Foster City, CA) with gene specific primers (Online only Supplementary Table II), and PerfeCTa SYBR® Green SuperMix (Quantabio) for miRNAs in in triplicate on 7500 Fast Real-Time PCR system (Applied Biosystems). Relative gene expression between control and treated groups were determined using 2−ΔΔCt method24, 25 after normalization with Ppia, 18S, HPRT1 and Rplp0 (for coding genes and lncRNAs) and U6 (for miRNAs).
Transfection of macrophages
RAW macrophages were transfected with indicated siRNAs, expression vectors or promoter reporter plasmids using Nucleofection kit (Lonza, Gaitherberg, MD) for macrophages (program Y-001)24, 25 or RNAiMAX (Cat. No. 13778–150, Thermo Fisher Scientific) according to manufacturer’s protocols. THP1 macrophages were transfected with indicated GapmeRs (50 nmol/L) using RNAiMAX. Transfected cells were processed for RNA extraction, phagocytosis assays, luciferase assays and ChIP assays as indicated at 48–72 hrs post transfection.
RAW macrophage cell lines stably overexpressing Dnm3os
We generated Dnm3os expression vector (pDnm3os) by cloning Dnm3os (7.928 kb) into pcDNA 3.1 (+) (Thermo Fisher) and verified by DNA sequencing. RAW264.7 macrophages were transfected with pDnm3os or pcDNA3.1(+) empty vector (EV) using Nucleofection. Single cells clones stably expressing Dnm3os (RAW-Dnm3os) and EV (RAW-EV) were selected by Geneticin (Cat. No. 10131027, Thermo Fisher Scientific, 500 μg/mL).
Cloning of Dnm3os Promoter and Luciferase assays
Mouse genomic DNA fragments (1250 and 731 bp containing Dnm3os promoter (−1000 and −500 bp upstream from TSS) were cloned into SacI-XhoI sites of the pGL4.10[luc2] (Promega, Madison, WI) upstream of firefly luciferase and verified by DNA sequencing. RAW264.7 cells were co-transfected with Dnm3os reporters and pRL-TK expressing Renilla luciferase (internal control) using Nucleofection. Next day treated ± PA (6 hrs) or HG (24 hrs). Luciferase activity in cell lysates measured with Dual-Luciferase Reporter Assays (Cat. No. E1910, Promega). In some experiments, reporter plasmids were co-transfected with p65 (NF-κB) expression vector.
In vitro transcription/translation assay
We determined coding potential of Dnm3os using T7 TNT quick coupled transcription/translation system (Promega, Madison, WI, Cat. No. L1170) and transcend colorimetric non-radioactive translation detection system (Promega, Cat. No. L5070) following manufacturer’s instructions.
Dnm3os and Nucleolin Knockdown using siRNAs and GapmeRs
RAW264.7 cells and BMDMs from db/+ and db/db were transfected with siRNAs (20 nmol/L) targeting mouse Dnm3os (siDnm3os) (Cat. No. R-173881-00-0005, GE-Dharmacon, Lafayette, CO) or control siNTC oligos (Cat. No. D-001810-10-05, GE-Dharmacon) for 48 hrs. To knockdown nucleolin, RAW-EV and RAW-Dnm3os cell lines were transfected with Dicer-substrate siRNAs (20 nmol/L) targeting Nucleolin (Cat. No. DsiNA-NCL3 is mm.i.Ncl.13.3) and control NC1 oligos (from Integrated DNA Technologies, Skokie, Illinois) with RNAiMAX for 72 hrs. Then treated ± PA and processed for RNA isolation and ChIP assays. THP1 macrophages were transfected with antisense LNA-modified GapmeR targeting human DNM3OS (Cat No./ID: LG00201301-DDA, Qiagen) and a control antisense LNA GapmeR (Negative control-A, Cat# LG00000002, Qiagen) for 48 hrs to knockdown DNM3OS.
Phagocytosis assays
RAW Macrophages, BMDMs or THP-1 macrophages were transfected with siRNAs or indicated expression vectors and 48 hrs later plated on either cover slips in 24 well plates for microscopy or in black 96 well plates to measure fluorescence. Mouse macrophages were incubated with FITC-labeled E. coli BioParticles from Vybrant Phagocytosis Assay Kit (Thermo Fischer Scientific) for 2 hrs at 37 0C, washed twice with PBS, and incubated with Trypan blue to quench fluorescence from external E. coli bioparticles. Cells were fixed with paraformaldehyde (15 min) and washed with PBS. Cells on coverslips were mounted on slides using Vectashield containing DAPI and images collected using EVOS fluorescence microscope. Numbers of macrophages displaying phagocytosis were counted using Photoshop and results reported as % of Phagocytosed cells (FITC relative to total by DAPI stain). When cells were plated in 96 wells, fluorescence from phagocytosed E.coli bioparticles was read on Infinite 200 Pro 96 well fluorescent plate reader (Tecan) at 483/518 nm. Results were reported as arbitrary fluorescence units or fold over control cells. Phagocytosis assays in THP1 macrophages were performed in 96 wells by incubating with pHrodo Green E. coli BioParticles Conjugate for Phagocytosis (Cat. No. P35366, Thermo Fisher Scientific) for 2 hrs. Fluorescence from internalized particles was determined using Infinite 200 Pro (500/538 nm) and results expressed as % of Control GapmeR transfected cells.
Subcellular fractionation of macrophages
RNA from nuclear and cytoplasmic fractions of RAW macrophages and db/db mice BMDMs were prepared using Nuclear and cytoplasmic RNA purification kit (Cat. No. 21000, Norgen, ON, Canada) following manufacturer’s protocols. Chromatin from RAW macrophages was isolated following published methods26. RAW264.7 cells (80% confluent) were detached by Accutase and 2×107 cells were lysed with equal volumes of Buffer A (10 mmol/L HEPES pH 7.5, 10 mmol/L KCl, 10% glycerol, 340 mM sucrose, 4 mmol/L MgCl2, 1 mM DTT and 1× Complete protease inhibitor (Cat. No. 11697498001, Sigma) containing 0.2% Triton X-100 (12 min on ice). Lysates were centrifuged at 1,200×g, 5 min, 4° C and supernatants were saved as cytoplasmic fractions. The nuclear pellets were washed once (500×g for 5 min at 4° C) with 250 μl of NRB (20 mmol/L HEPES pH 7.5, 50% Glycerol, 75 mmol/L NaCl, 1mmol/L DTT, and 1× Complete protease inhibitor), resuspended in 250 μl NRB and mixed with an equal volume of NUN buffer (20 mmol/L HEPES, 300 mmol/L NaCl, 1M Urea, 1% NP-40, 10mmol/L MgCl2 and 1mmol/L DTT). After incubation for 5 min on ice, centrifuged (1,200×g, 5 min, 4° C) and soluble nuclear supernatant (SNE) was transferred to another tube. The depleted nuclear pellet was washed once with 1 ml Buffer A and chromatin enriched pellet was resuspended in 50 μl Buffer A. RNA was isolated from SNE and Chromatin enriched fractions using Trizol (Cat. No. 15596026, Thermo Fisher Scientific) and RNeasy mini kit.
Western Blotting
Whole cell protein lysates and western blotting were performed as described27. Western blots were probed with indicated primary antibodies for 24 hrs at 4°C, washed and incubated with HRP-conjugated anti-rabbit (Cat. No. AP307P, Millipore, Darmstadt, Germany, 0.33 μg/mL) or anti-mouse (Cat. No. 1706516, Biorad Hercules, CA, 0.07 μg/mL) secondary antibodies for 1 hr at room temperature. Blots were washed and protein bands visualized using chemiluminescence detection. Antibodies against Histone H3 (ab1791), H3K27ac (ab4729), H3K9ac (ab4441), and Nucleolin (ab-22758) were from Abcam, Cambridge, UK: Histone antibodies were used at 1 μg/mL and nucleolin at 0.5 μg/mL. As loading controls, blots were probed with actin (A5441, Sigma-Aldrich, 0.33 μg/mL) or tubulin (sc-5286, Santa Cruz Biotechnology Inc, Dallas, TX, 1 μg/mL) antibodies.
ELISA
Cell culture supernatants from mouse macrophages transfected with Dnm3os siRNAs (siDnm3os) or control siRNAs (siNTC) were centrifuged at 200 g for 10 min to remove cell debris and stored frozen at −80 °C until assayed using ELISA kits for the cytokines IL-6 (Cat. No. 550950, Lot no. 726959, BD Bioscience, La Jolla, CA) and TNF-α (Cat. No. MTA00B, Lot No. P161075, Minneapolis, MN) according to the manufacturer’s protocols. Results were expressed as pg/mL.
Chromatin immunoprecipitation (ChIP) assays
ChIP assays were performed as described28, 29. Briefly, RAW macrophages were crosslinked with 1% formaldehyde (Cat. No. F8775, Sigma Aldrich) for 10 min at room temperature, quenched with 0.125M glycine for 5 min, and washed with ice-cold PBS. Cells were lysed in ChIP lysis buffer and chromatin was sheared (upto 200–600 bp) by sonication (8 cycles, 30 sec on/30 sec off) using BioruptorR Pico (Diagenode, Denville, NJ). Immunoprecipitation was performed using 3 μg of antibodies specific to H3K9ac (Cat. Nos. ab10812, lot no. GR143846-2; Abcam, Cambridge, UK), or control rabbit IgG, overnight at 4°C. Immune complexes were collected onto 25 μl of magnetic protein G Dynabeads (Cat. No. 10003D, Novex, Waltham, MA) washed. ChIP DNA was purified and dissolved in MilliQ water. ChIP assays for transcription factor p65 (NF-κB) were performed using nuclear lysates prepared from cross-linked RAW macrophages30. Cells were lysed in cold lysis buffer 1 (140 mmol/L NaCl, 1 mmol/L EDTA, 50 mmol/L HEPES pH 7.5, 10% glycerol, 0.5% NP40, 0.25% Triton-x-100, 1× protease inhibitor) for 10 min at 4°C. Lysates were centrifuged at 2000 rpm for 5 min and the nuclear pellet was resuspended in lysis buffer 2 (10 mmol/L Tris pH 8.0, 200 mmol/L NaCl, 1 mmol/L EDTA, 0.5 mmol/L EDTA, 0.5 mmol/L EGTA and 1× protease inhibitor), incubated for 10 min at 4°C and centrifuged at 2000 rpm for 5 min. The chromatin pellet was resuspended in ChIP lysis buffer, sonicated and ChIP assays were performed using 5 μg of p65 specific antibody (F-6 antibody, Cat. Nos. Sc-8008x, lot no. A1817; Santa Cruz Biotechnology, Inc. Dallas, TX). ChIP DNA in technical triplicates were analyzed by qPCR using SYBR Green reagent on the 7500 Fast Real-Time PCR system using ChIP-qPCR primers (online only Supplementary Table II). ChIP enrichment relative to input (% input) was calculated using the formula 2−(CtChIP−Ct100%input).
RNA-FISH to determine subcellular localization of Dnm3os
RAW macrophages and indicated BMDMs were plated on coverslips and RNA-FISH was performed using 5′-end labeled (Cy5) LNA oligonucleotide probes targeting Dnm3os (Sequence-cagctaggccaagacaacaaaatg, Exiqon, Valencia, CA). Hybridization was carried out as described 31. Cells were blocked by incubating in pre-hybridization buffer (3% bovine serum albumin and 4× saline–sodium citrate buffer (SSC)) for 1 hr at room temperature. Hybridization was carried out with pre-warmed hybridization mix (10× dextran sulfate and 4× SSC) containing 25nM LNA-Cy5 probe, at 50°C (20–25°C below the predicted probe Tm) for 1 hr with gentle agitation. Then cells were washed with 4× SSC containing 0.1% Tween-20 three times and once each with 2× SSC, 1× SSC and PBS for 5 min each at room temperature. Cells were blocked with 1% BSA for 45 min at 4°C and incubated with primary anti-nucleolin antibody (Cat. Nos. ab22758, lot no. GR258417-1; Abcam, 1 μg/mL) or anti-ILF-2 (Cat. Nos. ab28772, lot no. GR178687-8; Abcam, diluted 10 μg/mL with 0.1% BSA) for 30 min at room temperature. Cells were washed three times with PBS, 5 min each, and incubated with appropriate Alexa Fluor conjugated secondary antibody (1:400 dilution in 0.1% BSA, Cat no. NEF710001EA, lot no.2031274; Perkin Elmer, Boston, MA) for 30 min at room temperature. After washing cells with PBS, nuclei were stained with 1 μg/mL 4′,6′-diamidino-2-phenylindole (DAPI) (Cat. No. D1306, Invitrogen by Thermo Fisher Scientific) and mounted in ProLong Gold antifade reagent (Cat. No. P36930, Thermo Fisher Scientific). The images were acquired in an LSM 10 Meta Confocal microscope (Carl Zeiss) and analyzed by the software provided by Carl Zeiss and Image J software.
Dnm3os RNA Pull-down and Mass spectrometry analysis
RNA Pull-down was performed as described before with some modifications32. Briefly, full-length Dnm3os sense and antisense strands were Biotin-labeled by in vitro transcription using Biotin RNA labeling Mix (Cat No. 11685597910, Roche) and T7 RNA Polymerase (Cat No. EP011, Stratagene). Biotinylated Dnm3os sense and antisense RNAs were treated with RNase-free DNase I and purified on G-50 Sephadex Quick Spin columns (Cat No.11274015001, Roche). Biotinylation efficiency for sense and anti-sense strands were determined by Biotin Chromogenic detection Kit (Cat No. K0661, Thermo Scientific). Biotinylated RNA (1 μg) was denatured by heating to 60° for 10 min and slow-cooled to 4° C. RNA was mixed with 1 mg of nuclear extract prepared from PMs (for mass spectrometry) and RAW macrophages (for western blot validations) in RNA-immunoprecipitation (RIP) buffer (150 mmol/L Kcl, 25 mmol/L Tris pH 7.4, 0.5 mmol/L DTT, 0.5% NP40, 100 mmlo/L PMSF and 1× protease inhibitor) and incubated at 4° C for 2 hrs. Then, 60 μl of Streptavidin agarose beads (Cat No. SA10004, Invitrogen) were added to each binding reaction and further incubated at 4° C for 2 hrs. Beads were quickly washed five times in Handee spin columns (Cat No. 69725 Pierce, Thermo Fisher Scientific). Proteins were eluted using SDS buffer, separated on a 4–15 % precast SDS gel (Cat No. 5671084, Criterion, Biorad, Hercules, CA) and stained with SimplyBlueTM SafeStain (Cat No. LC6065, Life Technologies). Bands were excised and subjected to protein identification by City of Hope’s Mass Spectrometry Core. In some experiments, eluted proteins were also analyzed by western blotting with indicated antibodies.
Gel separated proteins were reduced with DTT (Cat No. R0861, Thermo Fisher Scientific), alkylated with iodoacetamide (Cat No. A3221-10VL, Sigma-Aldrich), and digested with a mixture of trypsin and LysC (Promega, Madison, WI). Peptides were extracted from the gel, evaporated to dryness in a vacuum centrifuge, and resuspended in 0.1% formic acid. Digested peptides were analyzed by LC/MS using an Orbitrap Fusion Mass Spectrometer with an EasyNano1000 nanoflow UHPLC (both Thermo Fisher Scientific). Peptides were loaded onto a 75μm × 2cm PepMap trapping column packed with 3μm C18 silica particles, 100 Å pore size, then eluted through a 75μm × 25cm PepMap analytical column packed with 2μm C18 silica particles, 100 Å pore size (both Thermo Fisher Scientific) using a 85-minute linear buffer A/B gradient from 8% to 25% buffer B (buffer A: 0.1% aqueous formic acid, buffer B: 0.1% formic acid in acetonitrile). MS Spectra of the intact peptides were acquired in the Orbitrap, and CID MS/MS spectra were acquired in the ion trap. Data was searched using Sequest in Proteome Discoverer 2.1 (Thermo Fisher Scientific). The database used was a concatenation of the Mus musculus RefSeq proteome and a database of common laboratory contaminant proteins, and was searched separately in the forward (target) and reverse (decoy) direction. The search assumed tryptic specificity with a maximum of 2 missed cleavages, a precursor ion tolerance of 5ppm, and a fragment ion tolerance of 0.6 Da. It assumed quantitative carbamidomethylation of cysteine and potential oxidation of methionine and acetylation of the protein amino terminus. Search results were loaded into Scaffold version 4.8.4 for probability assignment.
RNA-seq and Ribosome profiling data analysis
RNA-seq was performed at City of Hope’s Integrative Genomics Core on HiSeq 2500 platform (Illumina, San Diego, CA). Raw sequences (online only Supplementary Table I) were aligned to the mouse reference genome mm9 using TopHat v2.033, and the expression levels of RefSeq genes were counted using customized R scripts. The counts were normalized by trimmed mean of M value (TMM) method and differentially expressed genes between groups were identified using Bioconductor package “edgeR” (fold change >1.5 and p<0.01)34. Additionally, DESeq2 package35 was used to normalize counts and identify differentially expressed genes (log2 fold change ≥ 0.5 and padj < 0.1). Differential genes identified using both methods were used to determine significantly modulated gene ontology and pathways using DAVID online annotation tools36 and Gene Set Enrichment Analysis (GSEA)37. Enriched gene sets were represented in a bubble plot using ‘plotly’ package in R. Briefly, expression values (transcripts per million, TPM) in RAW-EV and RAW-Dnm3os (overexpressed) datasets were calculated for genes in enriched gene sets and used to make the bubble plot with the size of the bubble representing the number of genes. Log2 fold change and –log10 p values of each gene were used to make a volcano plot using R scripts and differentially regulated genes are shown in green. Circos plot was generated using Circos software 38 with differentially regulated genes represented on mm9 chromosomes with links drawn from Dnm3os genomic location to the other genes. Only genes identified in the enriched gene sets from GSEA analysis are shown on the Circos plot. Motif analysis of differentially expressed gene promoters was performed using TRAP (http://trap.molgen.mpg.de/cgi-bin/home.cgi)39.
Ribosome profiling data (GSE100739) and corresponding RNA-seq data (GSE100873) were downloaded from Gene Expression Omnibus (GEO) database. Sequences were aligned to mm9 as above using TopHat, and expression levels were expressed as TPM. Ribosomal occupancy was calculated for selected RNA and expressed as the ratio of ribosomal profiling TPM to Input RNA-seq TPM as described40.
Data Deposition
RNAseq data is deposited in the GEO database (accession # GSE107557).
Statistical Analysis
Values are expressed as Mean+SEM. GraphPad Prism 7.02 software (GraphPad Prism Software Inc., San Diego, CA) was used for Statistical analyses. Normal distribution of each sample group was confirmed by Shapiro–Wilk normality test before comparing groups. For statistical comparison of two groupsunpaired two-tailed Student’s t-test was used. For the comparison of three or more groups with similar variances one-way ANOVA followed by Tukey’s or Dunnett’s post hoc tests were used. p-values < 0.05 were considered statistically significant for all tests used.
RESULTS
Macrophage Dnm3os is upregulated in Diabetes
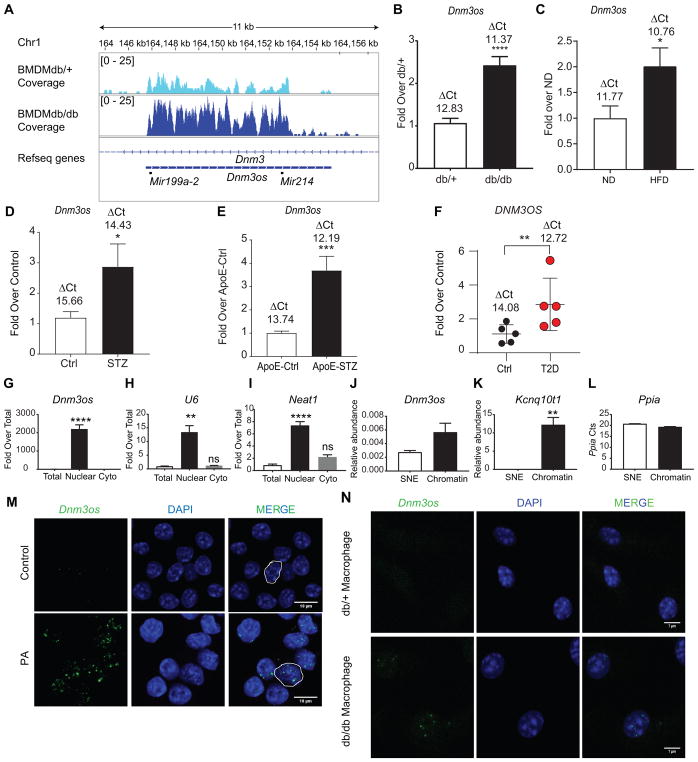
We previously reported that several lncRNAs were differentially expressed in BMDMs from T2D db/db mice compared with control non-diabetic db/+ mice, but the function of only one of them (E330013P06) was determined24. Here, we investigated the regulation and functional role of another lncRNA Dnm3os, which was highly upregulated in BMDMs from db/db mice compared to db/+ (Figure 1A). Dnm3os is expressed as a 7.928 kb long transcript containing a single exon on mouse chromosome 1 from the opposite strand of a coding gene Dynamin 3 (Dnm3), and its function in macrophages is unknown. To investigate Dnm3os functions, we first validated its upregulation using RT-qPCR in BMDMs from db/db macrophages compared to the control db/+ (Figure 1B). However, levels of the coding gene (Dnm3) on the opposite strand were not altered (results not shown). Next we examined Dnm3os expression in other mouse models of metabolic syndrome and diabetes. Results showed that Dnm3os expression is also increased in peritoneal macrophages (PMs) from high fat diet (HFD)-induced insulin-resistant mice versus normal chow diet (ND) fed mice (Figure 1C), PMs from streptozotocin (STZ)-induced type 1 diabetic mice (Figure 1D), and BMDMs from STZ-induced diabetic ApoE−/− mice (Figure 1E) that depict accelerated atherosclerosis41 versus respective controls. Thus, Dnm3os upregulation may play a role in macrophage dysfunction associated with diabetes, insulin resistance and macrovascular complications. A human ortholog, DNM3OS is also located on human chromosome 1 and shares 83% homology with mouse Dnm3os. We found that DNM3OS expression is significantly increased in CD14+ monocytes from T2D patients versus healthy controls (Figure 1F) demonstrating human disease relevance.
Fig. 1. Dnm3os is upregulated in macrophages in Diabetes and enriched in nucleus.
A. Normalized profiles of RNA-seq signals for lncRNA Dnm3os in macrophages. Each data track shown is on the same scale for BMDMs from control db/+ and T2D db/db mice. B. RT-qPCR validation of increased Dnm3os expression in BMDMs from db/db versus db/+ mice; C, PMs from HFD fed C57BL/6 mice versus normal diet (ND) mice; D, Control (Ctrl) and STZ-induced type 1 diabetic C57BL/6 mice (T1D); and E, BMDMs from Control ApoE−/− mice (ApoE-Ctrl) and STZ-injected ApoE−/− (ApoE-STZ) mice; and, F, CD14+ monocytes from control (Ctrl) and T2D patients. Gene expression normalized to reference gene is expressed as Fold over Control. Mean+SEM; n=12–14 (for B), n=6 (for C), n=8 (for D), n=5 (for E–F), *P<0.05; **P<0.01; *** P<0.001; **** P<0.0001, unpaired two tailed t-tests. The numbers above bar graphs represent ΔCt values (Dnm3os-reference gene). G–I. RT-qPCR analysis of indicated RNAs from total, nuclear and cytoplasmic (Cyto) fractions. U6 and Neat1 RNA were used as positive controls for nuclear RNAs. Gene expression normalized to Ppia is expressed as Fold over Total. Mean +SEM; n=3. *P<0.05; **P<0.01; **** P<0.0001, unpaired two tailed t-tests. J–K. Relative abundance of indicated RNAs in chromatin (Chr) and soluble-nuclear extracts (SNE) after normalization with Ppia transcript (L). Mean+SEM; n=3. **P<0.01, unpaired two tailed t-tests. M–N. RNA-FISH analysis of Dnm3os (green spots) in macrophages. M, control and PA (200 μmol/L) treated RAW macrophages; N, db/+ and db/db macrophages. Blue color- Nuclear staining with DAPI. Scale bars:10 μm (M) and 7 μm (N).
Nuclear localization and chromatin enrichment of Dnm3os
Since lncRNA functions are dependent on subcellular localization15, we first examined Dnm3os levels in nuclear and cytoplasmic fractions in mouse RAW 264.7 (RAW) macrophages. Results showed that Dnm3os is highly enriched in nuclear fractions compared with cytoplasmic (Figure 1G). Nuclear RNAs U6 snRNA and Neat1 served as controls (Figure 1H–I). As nuclear lncRNAs may interact with chromatin to regulate gene transcription, we examined Dnm3os levels in chromatin and soluble-nuclear extract (SNE)s from RAW macrophages. Results showed that Dnm3os is enriched by 2-fold in the chromatin fraction compared to SNE (Figure 1J). The known chromatin-associated lncRNA Kcnq10t1 26 served as positive control (Figure 1K). Ppia (loading control) showed similar Ct values in both chromatin and SNE fractions (Figure 1L). RNA fluorescence in-situ hybridization (RNA-FISH) further confirmed nuclear localization of Dnm3os and also showed that its levels increased in RAW cells treated with PA (200 μmol/L, 24 hrs), a free fatty acid elevated in diabetes (Figure 1M). Furthermore, Dnm3os was also localized in the nucleus in BMDMs from db/db and db/+ mice and its signal was clearly increased in db/db BMDMs versus db/+ cells (Figure 1N). Similar nuclear enrichment and PA mediated upregulation were also observed in NIH 3T3 fibroblasts (Figure I in the online-only Data Supplement). These results suggest that Dnm3os may have nuclear functions to modulate gene expression in macrophages.
Dnm3os is regulated by NF-κB under diabetic conditions
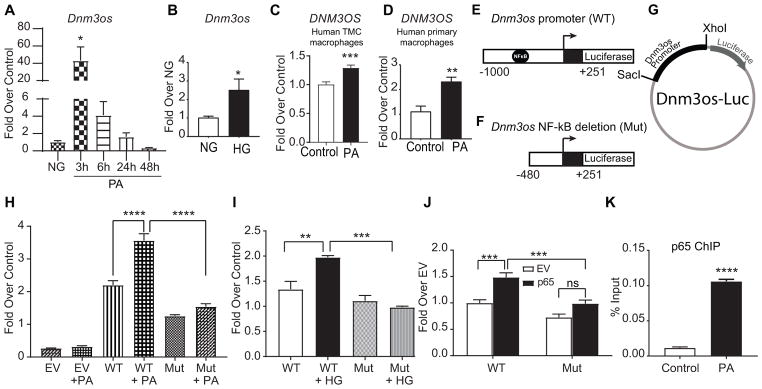
We next examined the mechanism of Dnm3os upregulation under diabetic conditions using BMDMs treated with PA, normal glucose (NG) and high glucose (HG). Dnm3os was significantly induced by PA with peak expression at 3 hrs compared with control (Figure 2A), and by HG at 24 hrs in BMDMs (Figure 2B). Moreover, PA also induced DNM3OS in human THP1 derived macrophages (TMC) as well as in human primary CD14+ monocyte-derived macrophages from multiple donors (Figure 2C–D), confirming similar regulation in human macrophages. To investigate TFs regulating Dnm3os expression, we used TRANSFAC and Consite databases42 to identify putative promoter TF binding sites. Results revealed a NF-κB binding site at −490 to −481 bp upstream of Dnm3os transcription start site. To verify the role of NF-κB, we generated two reporter constructs by cloning WT Dnm3os promoter (WT, −1000 to + 251 bp) and a mutant (Mut) promoter (−480 to + 251 bp) lacking the NF-κB site, upstream of firefly luciferase reporter gene (Figure 2E–G). Transient transfection of these constructs into RAW macrophages and luciferase assays showed that PA and HG increased the transcriptional activity of Dnm3os WT promoter, which was abrogated in NF-κB deletion mutant (Mut) (Figure 2H and I). In addition, co-transfection with p65 (NF-κB active subunit) expression vector transactivated Dnm3os WT but not Mut promoter in RAW macrophages (Figure 2J). Furthermore, ChIP assays with p65 antibody showed that PA treatment significantly increased p65 enrichment at the Dnm3os promoter compared to control in RAW macrophages (Figure 2K). These data clearly demonstrate that Dnm3os is upregulated by NF-κB binding to its promoter under diabetic conditions.
Fig. 2. Dnm3os is regulated by NF-κB under diabetic conditions.
A–D. PA and HG induce Dnm3os expression in macrophages. RT-qPCR analysis of Dnm3os expression in BMDMs treated with PA (200 umol/L) for indicated time points (A); with NG (5.5 mmol/L) or HG (25 mmol/L) for 24 hrs (B). C–D. PA induced DNM3OS expression in THP1 macrophages (TMC) and human primary macrophages. Mean+SEM; n=6 (for A–D), *P<0.05, **P<0.01, ***P<0.001, unpaired two tailed t-tests. E–G. Schematic of the Dnm3os wild type (WT) and NF-κB deletion (Mut) promoters, and luciferase reporter plasmid with Dnm3os promoters. H–I. PA and HG activate Dnm3os promoter via NF-κB. Activity of Dnm3os WT and Mut promoters in RAW macrophages co-transfected with indicated reporter plasmids and control Renilla luciferase (REN) plasmid after treatment ± PA (H) and HG or NG (I) for 24 hrs. Firefly luciferase activity normalized with REN expressed as fold over control cells. EV-empty luciferase vector pGL4. Mean +SEM; n=6 (for H), n=3 (for I), **P<0.01; ***P<0.001; ****P<0.0001, using one way ANOVA, Tukey’s multiple comparison test. J. NF-κB (p65) transactivates Dnm3os promoter. Relative luciferase activity in RAW macrophages co-transfected with indicated reporter plasmids along with NF-κB expression vector (p65) or empty vector (EV) and internal control REN plasmid. Mean+SEM; n=5, ***P<0.001, using one way ANOVA, Tukey’s multiple comparison test. K. ChIP assays showing p65 enrichment at NF-κB binding site in Dnm3os promoter in macrophages treated ± PA (200 μmol/L). Mean+SEM; n=3, ****P<0.0001 using unpaired two tailed t-tests.
Dnm3os overexpression promotes pro-inflammatory functions in macrophages
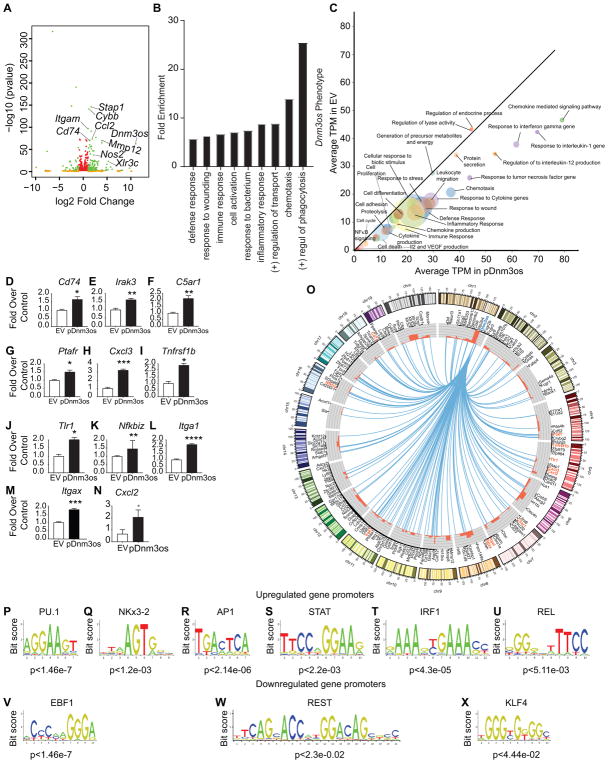
Next we examined the effect of Dnm3os overexpression in macrophages, mimicking its upregulation in diabetes. We cloned full length Dnm3os into pcDNA3.1 expression vector (Figure II in the online-only Data Supplement) and generated RAW cell lines stably transfected with Dnm3os expression vector (RAW-Dnm3os) and empty pcDNA3.1 vector (RAW-EV). We then performed RNA-seq analysis in biological replicates using RNA extracted from these two cell lines (Figure III in the online-only Data Supplement). Correlation and Principal component analysis (PCA) showed good correlation between the replicates (Figure IVA–B in the online-only Data Supplement). 203 genes were upregulated and 113 downregulated in Dnm3os overexpressing macrophages (RAW-Dnm3os) versus control RAW-EV (FDR <0.1, log2 fold change≥0.5) (Figure 3A and Figure IVC in the online-only Data Supplement). DAVID analysis of Dnm3os-induced genes showed enrichment of key macrophage phenotypes including phagocytosis, inflammation, immune response, cell activation, chemotaxis, and response to wounding (Figure 3B). Furthermore, GSEA37 showed enrichment of gene sets related to macrophage activation in genes upregulated by Dnm3os overexpression (Figure 3C). Using RT-qPCR we validated several key inflammatory genes upregulated by Dnm3os overexpression in RAW-Dnm3os versus RAW-EV (Figure 3D–N), although few upregulated genes tested could not be validated (Figure V in the online-only Data Supplement). Interestingly, Dnm3os overexpression increased the expression of genes not only on chr1 on which it is located, but also altered those present on other chromosomes (Figure 3O) suggesting both cis- and trans-actions. Furthermore, TF motif analysis showed enrichment of PU.1, NKx3-2, AP1, STAT, IRF1, REL binding sites in the promoters of upregulated genes (Figure 3P–U), whereas EBF1, REST and KLF4 sites were enriched in downregulated genes (Figure 3V–X). In addition, immunoblotting of cell lysates showed that Dnm3os overexpression increased global levels of permissive histone modifications such as H3K9ac and H3K27ac compared with RAW-EV (Figure VI in the online-only Data Supplement). Together, these data suggest that overexpression of Dnm3os augments pro-inflammatory genes possibly via changes in epigenetic histone modifications in macrophages.
Fig. 3. Dnm3os overexpression promotes pro-inflammatory functions in macrophages.
A. Volcano plot showing significantly upregulated and downregulated genes from RNA-seq data in Dnm3os stably overexpressing macrophage cell line RAW-Dnm3os (pDnm3os) versus control vector (RAW-EV). Genes depicted in green are those with padj < 0.05 and log2FC >0.6; Orange: padj>0.05 and log2FC >0.6; and red: padj <0.05 and log2FC<0.6. B. DAVID analysis of Dnm3os induced genes shows enrichment of key macrophage functions. C. GSEA analysis of genes upregulated in RAW-Dnm3os macrophages. Significant biological process gene sets (empirical P < 0.05) below the diagonal line are upregulated. The size of the circle is proportional to the number of significantly altered genes (0.5 < log2FC < −0.5) within each pathway. D–N. RT-qPCR validation of indicated Dnm3os induced genes identified by RNA-seq in RAW-Dnm3os macrophages relative to RAW-EV. Mean+SEM; n=3, *P<0.05; **P<0.01; *** P<0.001, ****P<0.0001 using unpaired two tailed t-tests. O. Circos plot depicting the genes upregulated by Dnm3os overexpression in RAW-Dnm3os macrophages. Upregulated genes (black and orange color fonts) are connected to the Dnm3os locus on chr1 (blue font) by blue lines. Orange fonts indicate upregulated genes validated by RT-qPCR in panels. Outer circle represents chromosome ideograms (chr Y is not shown). Inner circle shows histograms (red color) representing log2FC of each gene. P-X. Motif analysis of upregulated (P–U) and downregulated (V–X) gene promoters in RAW-Dnm3os macrophages.
Dnm3os enhances inflammation and phagocytosis
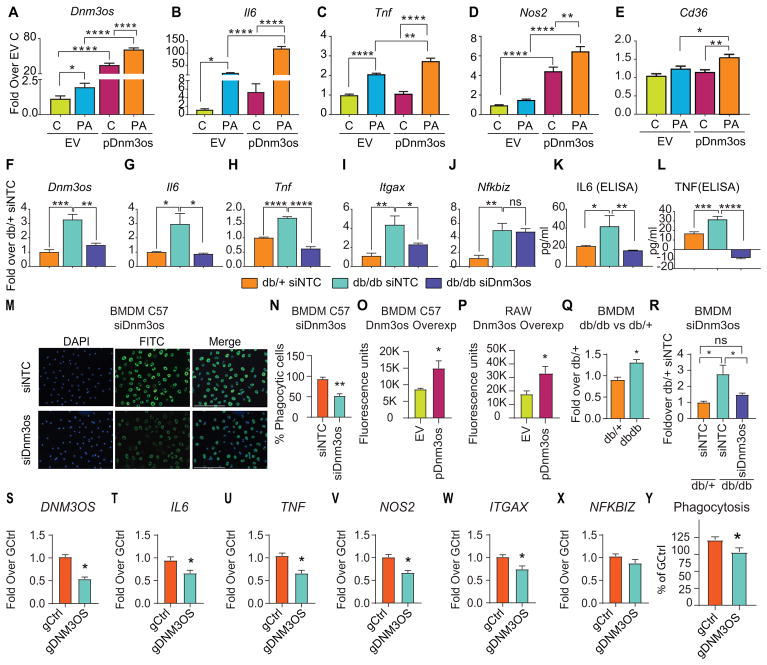
In order to further examine the function of Dnm3os and its target genes under diabetic conditions in vitro, we treated RAW-Dnm3os and RAW-EV macrophages with or without PA. Dnm3os was significantly increased by PA in both cell lines (Figure 4A) along with several inflammatory genes (Figure 4B–E). Furthermore, PA-induced expression of Il6, Tnf, Nos2, and Cd36 was significantly greater in RAW-Dnm3os macrophages (pDnm3os) relative to RAW-EV (Figure 4B–E), demonstrating that Dnm3os can further augment the pro-inflammatory effects of PA. Since lncRNAs can regulate nearby genes (cis- effects), we also tested if Dnm3os overexpression affects its nearby genes Mettl13 and Vamp4. However, both these genes were not affected (Figure VII in the online-only Data Supplement).
Fig. 4. Dnm3os promotes inflammation and phagocytosis.
A–E. Bar graphs represent the expression of indicated genes in RAW-EV (EV) and RAW-Dnm3os (pDnm3os) macrophages without (C) and with PA (PA) treatment (24 hrs). Results are expressed as fold over untreated RAW-EV (EV C). Mean+SEM; n=5–6, *P<0.05; **P<0.01; ****P<0.0001, one way ANOVA, Tukey’s multiple comparison test. F–L. Gene expression (RT-qPCR) and protein levels (ELISA) in BMDMs transfected with siDnm3os versus non-targeting control (siNTC) in db/db versus db/+ mice. Mean+SEM; n=4–6, * P<0.05, **P<0.01; ***P<0.001; ****P<0.0001, one way ANOVA, Tukey’s multiple comparison test. M–N. Effect of Dnm3os knockdown on phagocytosis. Representative images showing phagocytosis of FITC-labeled fluorescent E. coli bio-particles (green) in BMDMs (C57BL/6 mice) transfected with siDnm3os or control siNTC oligonucleotides (M) and quantification of phagocytosis (N). Mean+SEM; n=3, **P<0.01, unpaired two tailed t-tests. Blue color-DAPI. O–P: Effect of Dnm3os overexpression on phagocytosis in C57BL/6 BMDMs (O); transiently transfected with Dnm3os vector (pDnm3os) versus pcDNA3.1 (EV), and RAW-Dnm3os (pDnm3os) versus RAW-EV (EV) macrophages (P). Phagocytosis assays were performed in 96 wells and fluorescence of internalized particles measured on a plate reader. Mean+SEM *P<0.05; n=4, unpaired two tailed t-tests. Q–R: Phagocytosis assays were performed in BMDMs from db/+ and db/db mice without transfection (Q) or after transfection with siNTC (in db/+ and db/db) or siDnm3os (db/db) BMDMs (R) Mean+SEM; *P<0.05; n=5–6. S–X. Gene expression (S–X) and Phagocytosis (Y) in THP1 macrophages after DNM3OS knockdown using GapmeR (gDNM3OS) versus control GapmeR (gCtrl). Mean+SEM *P<0.05, unpaired t-test (n=6–9 for S-X and 9–10 for Y).
Next, we examined if Dnm3os knockdown can inhibit candidate genes that were found to be upregulated in Dnm3os overexpressing cells. Dnm3os knockdown was confirmed in RAW macrophages transfected with Dnm3os siRNA (siDnm3os) relative to non-targeting control siNTC (Figure VIIIA in the online-only Data Supplement). Furthermore, Dnm3os knockdown also significantly downregulated key inflammatory genes Il6 and Itgax (but not NFkbiz) (Figure VIIIB-D in the online-only Data Supplement) that were upregulated by Dnm3os. To examine direct relevance in diabetic macrophages, we next examined whether siRNA-mediated Dnm3os knockdown has similar effects in BMDMs from diabetic db/db mice, which exhibit enhanced expression of Dnm3os and pro-inflammatory genes in culture (ex vivo) relative to BMDMs from non-diabetic db/+ mice. We transfected db/db BMDMs with siNTC or siDnm3os and, as control, db/+BMDMs with siNTC oligonucleotides. siDnm3os significantly downregulated the increased expression of Dnm3os in BMDMs from db/db mice (Figure 4F) and, in parallel, also suppressed diabetes-induced enhanced inflammatory gene expression (Figure 4G–I) and cytokine secretion (Figure 4K–L) relative to siNTC transfected control db/+BMDMs. However, siDnm3os did not affect changes in Nfkbiz (Figure 4J) or the host gene Dnm3 (Figure IXA in the online-only Data Supplement). We also verified that the siRNA can target nuclear Dnm3os because siDnm3os significantly downregulated Dnm3os expression in nuclear fractions from db/db BMDMs versus siNTC transfected cells, but not in the cytoplasmic fractions where Dnm3os expression is much lower (Figure IXB–C in the online-only Data Supplement). Together, these results support a role for Dnm3os in diabetes-induced enhanced inflammatory phenotype of macrophages.
We next examined if Dnm3os regulates phagocytosis, an important function of macrophages, by incubating fluorescently labeled E. coli bioparticles with macrophages after Dnm3os knockdown or overexpression. siDnm3os significantly inhibited phagocytosis compared with siNTC in BMDMs from control C57BL6 mice (Figure 4M–N). In contrast, Dnm3os overexpression in BMDMs (via transient transfection), and RAW-Dnm3os macrophages significantly increased phagocytosis relative to EV (Figure 4O–P). Moreover, BMDMs from db/db mice exhibited increased phagocytosis relative to db/+ (Figure 4Q) that was attenuated by siDnm3os (Figure 4R). Interestingly, GapmeR-mediated human DNM3OS knockdown also significantly reduced inflammatory genes and phagocytosis in THP1 macrophages, suggesting similar mechanisms in human cells (Figure 4S–Y). These results demonstrate that Dnm3os promotes inflammation and phagocytosis in macrophages.
Dnm3os interacts with nuclear proteins in macrophages
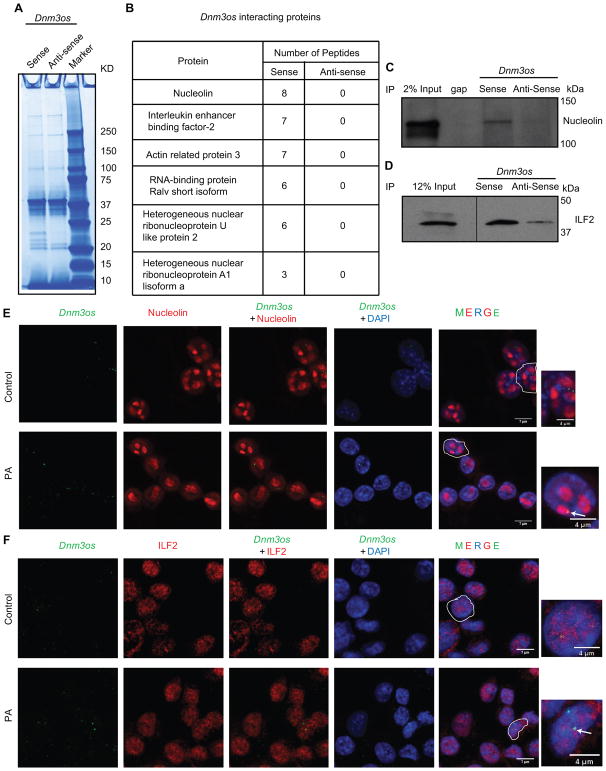
Interaction with key nuclear proteins is a major mechanism by which lncRNAs regulate gene expression11. Therefore, to gain additional insights into the mechanisms of Dnm3os actions, RNA-pulldown assays43 were performed to identify interacting protein partners of Dnm3os. Dnm3os sense and anti-sense (negative control) transcripts were biotinylated by in vitro transcription and RNA integrity as well as biotinylation efficiency of probes verified by denaturing gel electrophoresis and dot blot assays respectively (Figure X in the online-only Data Supplement). Nuclear extracts from normal mouse PMs were incubated with these two biotinylated probes and RNA-protein complexes resolved on an SDS-PAGE gel (Figure 5A). Parallel protein bands in different regions of the gel from sense and anti-sense lanes were subjected to mass spectrometry analysis. Results showed that several nuclear proteins were specifically associated with Dnm3os-sense strand, but not with anti-sense (Figure 5B). These included nucleolin, Interleukin enhancer binding factor-2 (ILF-2), actin related protein 3, RNA-binding protein Ralv short isoform, heterogeneous nuclear ribonucleoproteins U like protein 2 and heterogeneous ribonucleoprotein A1 isoform. To validate these interactions, RNA-protein complexes from RNA-pull-down assays were immunoblotted with antibodies to nucleolin and ILF-2. Results showed that both proteins interacted specifically with sense Dnm3os RNA but not anti-sense RNA (Figure 5C–D). Furthermore RNA-FISH with Dnm3os probe coupled with immunofluorescence using specific antibodies also showed the nuclear co-localization of Dnm3os with nucleolin and ILF-2 in both control and PA treated macrophages (Figure 5E–F). These results clearly demonstrate that Dnm3os interacts with nucleolin and ILF-2 in macrophages.
Fig. 5. Dnm3os interacts with key nuclear proteins in macrophages.
A. Gel image showing Simply Blue safe staining of proteins associated with biotinylated Dnm3os sense and control antisense RNAs using nuclear lysates from PMs. Proteins from both lanes were analyzed by mass spectrometry. B. List of top proteins associated only with Dnm3os sense RNA. C–D. Images of RNA pulldown followed by western blotting with nucleolin and ILF-2 antibodies. E–F. RNA FISH-coupled with immunofluorescence using Dnm3os probe (green color) and nucleolin or ILF-2 antibodies (red color) in Control and PA treated RAW macrophages. Scale bar 7 μm in all panels, except in enlarged images scale bars which are 4 μm. Arrows indicate location of Dnm3os.
Nucleolin modulates pro-inflammatory and epigenetic actions of Dnm3os in macrophages
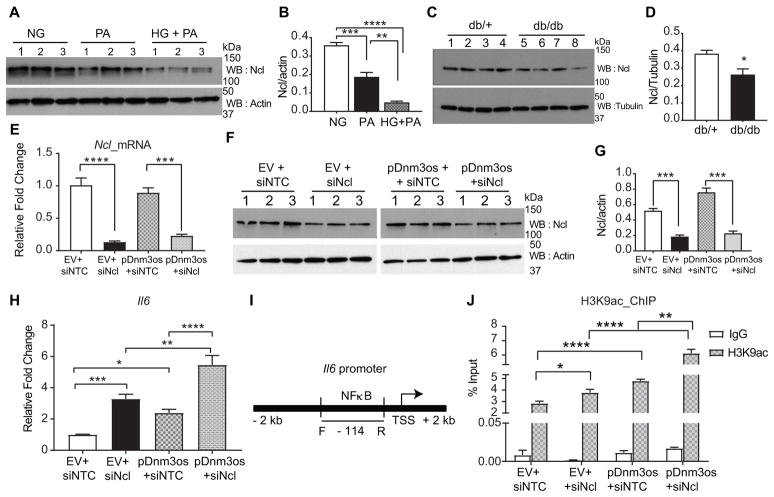
Nucleolin is a multifunctional nucleolar protein44 and one of its key functions includes regulation of chromatin structure. But its function in macrophages in diabetes is unknown. Recent studies suggested an atheroprotective function in macrophages45 and anti- inflammatory effects in HeLa cells46. Therefore, we hypothesized that nucleolin may negatively regulate pro-inflammatory functions of Dnm3os, and that inhibition of such interactions under diabetic conditions may promote inflammatory gene regulation. To test this, we checked nucleolin protein levels under diabetic conditions. PA and HG plus PA treatment significantly decreased nucleolin protein levels in RAW macrophages compared to the NG treated cells (Figure 6A–B). We also found that nucleolin protein levels were significantly reduced in BMDMs from diabetic db/db mice relative to control db/+ (Figure 6C–D). This supports the notion that inhibition of nucleolin under diabetic conditions in vivo might promote inflammatory gene expression.
Fig. 6. Nucleolin modulates pro-inflammatory and epigenetic actions of Dnm3os in macrophages.
A–B. Immunoblots showing nucleolin protein levels in macrophages after treatment with PA (for 24 hrs) and HG+PA (72 hrs for HG and 24 hrs for PA) compared to NG. B. Quantification of nucleolin protein in western blots. Mean+SEM; n=3, ** P<0.01, *** P<0.001, **** P<0.0001, one way ANOVA, Tukey’s multiple comparison test. C–D. Nucleolin protein levels and its quantification in BMDMs from db/db versus db/+ mice. Mean+SEM; n=4, * P<0.05, using unpaired two tailed t-tests. E–G. Downregulation of nucleolin mRNA (E) and protein (F–G) levels using nucleolin siRNA (siNcl) versus siNTC in RAW-EV (EV) and RAW-Dnm3os (pDnm3os) macrophages. Mean+SEM; n=3, *** P<0.001, **** P<0.0001, unpaired two tailed t-tests (for E and G). H. Bar graph showing Il6 expression in EV and RAW-Dnm3os macrophages after transfection with siNTC and siNcl. I. Schematic of Il6 promoter showing NF-κB binding sites and location of PCR primers. J. Bar graphs represent H3K9ac enrichment on the Il6 promoter (at NF-κB binding site) in RAW-EV and RAW-Dnm3os macrophages transfected with siNTC or siNcl. Data are shown as percent input. Mean+SEM; n=6 (for H) and 5–6 (for J), *P<0.05, **P<0.01; ***P<0.001; ****P<0.0001 one way ANOVA, Tukey’s multiple comparison tests (for H and J).
Next, we examined the effect of nucleolin knockdown on inflammatory gene expression in Dnm3os overexpressing macrophages. Transfection with nucleolin siRNA (siNcl) significantly inhibited nucleolin mRNA and protein versus siNTC in both RAW-Dnm3os and and RAW-EV macrophages (Figure 6E–G). Furthermore, nucleolin knockdown increased the expression of pro-inflammatory gene Il6 in RAW-EV macrophages and this effect was further significantly enhanced in RAW-Dnm3os macrophages (Figure 6H).
Next, we tested whether Dnm3os has trans-effects on inflammatory genes by augmenting permissive chromatin modifications like H3K9ac at their promoters. We examined the role of such epigenetic mechanisms in Il6 regulation by Dnm3os-nucleolin interactions using ChIP assays with H3K9ac antibody and qPCR with primers specific to Il6 promoter (Figure 6I). Interestingly, nucleolin depletion (siNcl) increased the enrichment of H3K9ac at Il6 promoter in control RAW- EV macrophages relative to siNTC (Figure 6J). Moreover, Dnm3os overexpression also increased H3K9ac at the Il6 promoter in RAW-Dnm3os macrophages and notably, this effect was further enhanced by nucleolin depletion (Figure 6J). However, nucleolin knockdown did not alter H3K9ac at Tnf and Nos2 promoters (Figure XI in the online-only Data Supplement). It is possible that other active chromatin marks besides H3K9ac may be involved in Dnm3os mediated increases in other target inflammatory genes besides Il6. Thus, Dnm3os-nucleolin interactions can regulate, in part, the macrophage inflammatory phenotype via epigenetic mechanisms such as increased permissive histone modifications and chromatin relaxation.
DISCUSSION
In this study we demonstrate a novel mechanism of enhanced macrophage inflammatory phenotype in diabetes by lncRNA Dnm3os. Macrophages are key players in promoting inflammation implicated in insulin resistance, lipid accumulation in the vessel wall and related vascular complications. Furthermore, increased oxidative stress in diabetes increases formation of oxidized LDL, which further enhances inflammation. Concerted actions of all these events play important roles in the initiation and progression of atherosclerosis3. Multiple signaling and epigenetic mechanisms have been implicated in augmenting these processes under diabetic states. Notably, lncRNAs have been recently discovered to play a role in macrophage inflammation under normal and diabetic conditions20, 24. Here, we showed that the expression of lncRNA Dnm3os is increased in macrophages derived from several mouse models of metabolic disorders including T1D, T2D, obesity and insulin resistance. Dnm3os expression was upregulated in BMDMs from both male (Figure 1) and female db/db mice (Figure XII in the online-only Data Supplement). Moreover, Dnm3os is also upregulated in STZ injected Apoe−/− mice, a model of diabetes-induced accelerated atherosclerosis41 indicating its association with diabetic vascular diseases. We confirmed that Dnm3os lacks coding potential using PhyloCSF, Cpat and macrophage ribosome profiling databases, and in vitro transcription/translation assays (Figure XIIIA–C in the online-only Data Supplement). Moreover, Dnm3os is conserved in humans and expressed in multiple tissues including adipose, aorta and whole blood (Figure XIV in the online-only Data Supplement). Interestingly, we found that Dnm3os is also upregulated in monocytes from T2D patients. These in vivo findings suggest that Dnm3os is regulated by hyperglycemia and hyperlipidemia in diabetes. Accordingly, our in vitro experiments clearly demonstrated that HG as well as PA, a free fatty acid abundant in obesity and T2D, increased Dnm3os expression in macrophages.
Reports show that Dnm3os is regulated by Twist and PPAR-α in cardiac and endothelial cells47, 48. Our studies show that HG and PA activate the Dnm3os promoter via NF-κB. PA can increase inflammatory gene expression via TLR4 activation, and HG can via activation of tyrosine kinases and protein kinase C. Both stimuli also activate NF-κB TF to induce inflammatory genes. Our results using macrophages derived from diabetic db/db mice support a role for Dnm3os in mediating diabetes-induced pro-inflammatory phenotype. Previous studies showed activation of NF-κB in this ex vivo model of diabetes-induced accelerated inflammation and macrophage activation4. Thus, activation of NF-κB by several factors in obesity and diabetes can increase Dnm3os in macrophages to further augment macrophage dysfunction. However, NF-κB activation in diabetes can clearly have effects on multiple inflammatory pathways and, whether Dnm3os mediates some or many of these effects in vivo has to be determined.
Mechanistically, lncRNAs regulate gene expression via distinct processes that promote or inhibit recruitment of transcription and epigenetic regulators to modulate chromatin structure15. In macrophages, lncRNAs THRIL, EPS and lncRNA-COX-2 interact with RNA binding proteins of hnRNP family to regulate NF-κB function and dysregulate chromatin structure around inflammatory genes20. We found that Dnm3os is highly enriched in the nucleus and associated with chromatin, supporting its role in gene regulation. We also observed Dnm3os interacts with nucleolin, ILF-2 and several RNA-binding proteins including hnRNPs. Interaction of hnRNPs with lncRNAs has been widely demonstrated in macrophages20. ILF-2 regulates IL2 expression in T-cells, but its function in other cell types is unclear49. Nucleolin, a 77 kDa protein highly enriched in the nucleolus, has multiple functions especially in chromatin structure and transcription44. However, nucleolin function in diabetes and lncRNA mediated gene regulation has not been previously examined. Our new results demonstrate that Dnm3os-nucleolin interaction could be a key mechanism regulating inflammatory phenotype of macrophages. We found that nucleolin inhibited Il6 gene expression which suggests anti-inflammatory functions. Moreover, Dnm3os-induced Il6 gene expression was further enhanced after nucleolin knockdown in macrophages. In support of our data, studies in HeLa cells showed nucleolin downregulation increased IL6 expression46, while in macrophages, nucleolin overexpression inhibited Ox-LDL induced foam cell formation through enhancing ABCA1 expression45. Further investigation is needed to determine whether Dnm3os can modulate macrophage foam cell formation through interactions with nucleolin. Our studies suggest that nucleolin-Dnm3os interaction inhibits inflammatory genes possibly via promoting repressive chromatin structure. Because diabetic conditions increase Dnm3os and decrease nucleolin levels, consequently, this can promote permissive histone modifications such as H3K9ac and open chromatin formation at inflammatory genes. Nucleolin functions are regulated by multiple post-translational modifications including phosphorylation and acetylation, which could affect its protein stability44, 50. Further studies are needed to examine how such complex regulatory mechanisms are involved in the downregulation of nucleolin by HG and PA.
We also observed Dnm3os increases phagocytosis in macrophages. It is possible Dnm3os enhances phagocytosis via upregulation of Cd36 (Figure 4E), a key regulator of phagocytosis and foam cell formation51. The role of phagocytosis in atherosclerosis is quite complex. Increased phagocytosis promotes excess uptake of oxidized lipids and foam cell formation, key steps in the initiation of lesions. Furthermore, in advanced complex lesions, non-specific increases in phagocytosis of apoptotic cells could affect plaque stability52. Thus, macrophage Dnm3os upregulation in diabetes may affect initiation and progression of vascular disease. However, further in vivo studies are needed to determine if Dnm3os also plays a role in clearance of pathogens and apoptotic cells, important events in infection and atherosclerosis respectively. Previous studies in cardiac and endothelial cells showed a role for Dnm3os during heart failure and hypertension47, 48, but they examined its function as a host gene for miR-199 and miR-214, and not as a lncRNA. In our study, overexpression of miR-199a and miR-214 did not affect most of the tested Dnm3os target genes (Figure XV in the online-only Data Supplement), suggesting mostly miRNA-independent effects for Dnm3os in diabetic macrophages. Other studies have also shown similar lncRNA functions of miRNA host genes in different cell types24, 53.
Taken together, our results demonstrate lncRNA Dnm3os-mediated novel mechanisms for increased macrophage inflammatory gene expression in diabetes. Under normal conditions, nucleolin interacts with and prevents the functional ability of Dnm3os to increase enrichment of permissive histone modifications like H3K9ac at promoters of inflammatory genes such as Il6. But, under diabetic conditions, increased Dnm3os and reduced nucleolin levels disrupt such interactions, allowing Dnm3os to enhance promoter H3K9ac, likely via recruitment of histone acetyl transferases (HATs), thereby leading to chromatin relaxation, upregulation of target inflammatory and macrophage dysfunction (Figure XVI in the online-only Data-Supplement). As certain human macrophage lncRNAs have also recently been associated with cardiometabolic disorders54, increased understanding of macrophage lncRNAs and their interacting proteins could aid the development of better therapies for inflammatory diabetic complications.
Supplementary Material
Highlights.
Diabetes upregulates lncRNA Dnm3os in mouse macrophages and human monocytes.
Diabetic conditions in vitro induce Dnm3os in macrophages via NF-κB activation.
Dnm3os increases inflammatory gene expression and phagocytosis in macrophages.
Human ortholog (DNM3OS) is also upregulated under diabetic conditions and exhibits pro-inflammatory phenotype in human macrophages.
Dnm3os interacts with nucleolin in macrophages and disruption of this interaction in diabetes upregulates inflammatory genes via epigenetic chromatin histone lysine acetylation.
Acknowledgments
Sources of Funding: This study was supported by grants from the National Institutes of Health (NIH), R01 DK065073, R01 HL106089 and R01 DK081705 (to RN). Research reported in this publication included work performed in the following Campus Cores: Integrative Genomics, DNA/RNA synthesis, and Mass Spectrometry and Proteomics (supported by the National Cancer Institute of the NIH under award number P30CA33572), and The Light Microscopy Core.
Abbreviations
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- BMDMs
Bone marrow derived macrophages
- lncRNA
long noncoding RNA
- FISH
Fluorescence in Situ hybridization
- RNA-IP
RNA immunoprecipitation
- ChIP
chromatin immunoprecipitation
Footnotes
Disclosures: None
References
- 1.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 2.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 3.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14:575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li SL, Reddy MA, Cai Q, et al. Enhanced proatherogenic responses in macrophages and vascular smooth muscle cells derived from diabetic db/db mice. Diabetes. 2006;55:2611–2619. doi: 10.2337/db06-0164. [DOI] [PubMed] [Google Scholar]
- 5.Nagareddy PR, Murphy AJ, Stirzaker RA, et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17:695–708. doi: 10.1016/j.cmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Natarajan R, Nadler JL. Lipid inflammatory mediators in diabetic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1542–1548. doi: 10.1161/01.ATV.0000133606.69732.4c. [DOI] [PubMed] [Google Scholar]
- 7.Yan SF, Ramasamy R, Schmidt AM. Mechanisms of disease: Advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Clin Pract Endocrinol Metab. 2008;4:285–293. doi: 10.1038/ncpendmet0786. [DOI] [PubMed] [Google Scholar]
- 8.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 9.Rask-Madsen C, King GL. Vascular complications of diabetes: Mechanisms of injury and protective factors. Cell Metab. 2013;17:20–33. doi: 10.1016/j.cmet.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy MA, Zhang E, Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia. 2015;58:443–455. doi: 10.1007/s00125-014-3462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mercer TR, Mattick JS. Structure and function of long noncoding rnas in epigenetic regulation. Nat Struct Mol Biol. 2013;20:300–307. doi: 10.1038/nsmb.2480. [DOI] [PubMed] [Google Scholar]
- 12.Quinn JJ, Chang HY. Unique features of long non-coding rna biogenesis and function. Nat Rev Genet. 2016;17:47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Notani D, Rosenfeld MG. Enhancers as non-coding rna transcription units: Recent insights and future perspectives. Nat Rev Genet. 2016;17:207–223. doi: 10.1038/nrg.2016.4. [DOI] [PubMed] [Google Scholar]
- 14.Kato M, Wang M, Chen Z, et al. An endoplasmic reticulum stress-regulated lncrna hosting a microrna megacluster induces early features of diabetic nephropathy. Nat Commun. 2016;7:12864. doi: 10.1038/ncomms12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rinn JL, Chang HY. Genome regulation by long noncoding rnas. Annu Rev Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uchida S, Dimmeler S. Long noncoding rnas in cardiovascular diseases. Circ Res. 2015;116:737–750. doi: 10.1161/CIRCRESAHA.116.302521. [DOI] [PubMed] [Google Scholar]
- 17.Long J, Badal SS, Ye Z, et al. Long noncoding rna tug1 regulates mitochondrial bioenergetics in diabetic nephropathy. J Clin Invest. 2016;126:4205–4218. doi: 10.1172/JCI87927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan B, Yao J, Liu JY, et al. Lncrna-miat regulates microvascular dysfunction by functioning as a competing endogenous rna. Circ Res. 2015;116:1143–1156. doi: 10.1161/CIRCRESAHA.116.305510. [DOI] [PubMed] [Google Scholar]
- 19.Leung A, Natarajan R. Long noncoding rnas in diabetes and diabetic complications. Antioxid Redox Signal. 2017 doi: 10.1089/ars.2017.7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen YG, Satpathy AT, Chang HY. Gene regulation in the immune system by long noncoding rnas. Nat Immunol. 2017;18:962–972. doi: 10.1038/ni.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Chao TC, Chang KY, et al. The long noncoding rna thril regulates tnfalpha expression through its interaction with hnrnpl. Proc Natl Acad Sci U S A. 2014;111:1002–1007. doi: 10.1073/pnas.1313768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carpenter S, Aiello D, Atianand MK, et al. A long noncoding rna mediates both activation and repression of immune response genes. Science. 2013;341:789–792. doi: 10.1126/science.1240925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atianand MK, Hu W, Satpathy AT, et al. A long noncoding rna lincrna-eps acts as a transcriptional brake to restrain inflammation. Cell. 2016;165:1672–1685. doi: 10.1016/j.cell.2016.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy MA, Chen Z, Park JT, et al. Regulation of inflammatory phenotype in macrophages by a diabetes-induced long noncoding rna. Diabetes. 2014;63:4249–4261. doi: 10.2337/db14-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin W, Reddy MA, Chen Z, et al. Small rna sequencing reveals micrornas that modulate angiotensin ii effects in vascular smooth muscle cells. J Biol Chem. 2012;287:15672–15683. doi: 10.1074/jbc.M111.322669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werner MS, Ruthenburg AJ. Nuclear fractionation reveals thousands of chromatin-tethered noncoding rnas adjacent to active genes. Cell Rep. 2015;12:1089–1098. doi: 10.1016/j.celrep.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahar S, Reddy MA, Wong C, et al. Cooperation of src-1 and p300 with nf-kappab and creb in angiotensin ii-induced il-6 expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:1528–1534. doi: 10.1161/ATVBAHA.107.145862. [DOI] [PubMed] [Google Scholar]
- 28.Das S, Senapati P, Chen Z, et al. Regulation of angiotensin ii actions by enhancers and super-enhancers in vascular smooth muscle cells. Nat Commun. 2017;8:1467. doi: 10.1038/s41467-017-01629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cong R, Das S, Ugrinova I, et al. Interaction of nucleolin with ribosomal rna genes and its role in rna polymerase i transcription. Nucleic Acids Res. 2012;40:9441–9454. doi: 10.1093/nar/gks720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orlando DA, Chen MW, Brown VE, et al. Quantitative chip-seq normalization reveals global modulation of the epigenome. Cell Rep. 2014;9:1163–1170. doi: 10.1016/j.celrep.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 31.de Planell-Saguer M, Rodicio MC, Mourelatos Z. Rapid in situ codetection of noncoding rnas and proteins in cells and formalin-fixed paraffin-embedded tissue sections without protease treatment. Nat Protoc. 2010;5:1061–1073. doi: 10.1038/nprot.2010.62. [DOI] [PubMed] [Google Scholar]
- 32.Rinn JL, Kertesz M, Wang JK, et al. Functional demarcation of active and silent chromatin domains in human hox loci by noncoding rnas. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trapnell C, Pachter L, Salzberg SL. Tophat: Discovering splice junctions with rna-seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson MD, McCarthy DJ, Smyth GK. Edger: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Love MI, Anders S, Kim V, et al. Rna-seq workflow: Gene-level exploratory analysis and differential expression. F1000Res. 2015;4:1070. doi: 10.12688/f1000research.7035.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using david bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 37.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krzywinski M, Schein J, Birol I, et al. Circos: An information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas-Chollier M, Hufton A, Heinig M, et al. Transcription factor binding predictions using trap for the analysis of chip-seq data and regulatory snps. Nat Protoc. 2011;6:1860–1869. doi: 10.1038/nprot.2011.409. [DOI] [PubMed] [Google Scholar]
- 40.Wang P, Xu J, Wang Y, et al. An interferon-independent lncrna promotes viral replication by modulating cellular metabolism. Science. 2017;358:1051–1055. doi: 10.1126/science.aao0409. [DOI] [PubMed] [Google Scholar]
- 41.Bucciarelli LG, Wendt T, Qu W, et al. Rage blockade stabilizes established atherosclerosis in diabetic apolipoprotein e-null mice. Circulation. 2002;106:2827–2835. doi: 10.1161/01.cir.0000039325.03698.36. [DOI] [PubMed] [Google Scholar]
- 42.Wasserman WW, Sandelin A. Applied bioinformatics for the identification of regulatory elements. Nat Rev Genet. 2004;5:276–287. doi: 10.1038/nrg1315. [DOI] [PubMed] [Google Scholar]
- 43.Huarte M, Guttman M, Feldser D, et al. A large intergenic noncoding rna induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rong C, Sadhan Das Bouvet P. The multiple properties and functions of nucleolin. In: Olson MOJ, editor. The nucleolus. Springer; 2011. pp. 185–212. [Google Scholar]
- 45.Li Y, Jiang B, Liang P, et al. Nucleolin protects macrophages from oxldl-induced foam cell formation through up-regulating abca1 expression. Biochem Biophys Res Commun. 2017;486:364–371. doi: 10.1016/j.bbrc.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 46.Kumar S, Gomez EC, Chalabi-Dchar M, et al. Integrated analysis of mrna and mirna expression in hela cells expressing low levels of nucleolin. Sci Rep. 2017;7:9017. doi: 10.1038/s41598-017-09353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li C, Mpollo MS, Gonsalves CS, et al. Peroxisome proliferator-activated receptor-alpha-mediated transcription of mir-199a2 attenuates endothelin-1 expression via hypoxia-inducible factor-1alpha. J Biol Chem. 2014;289:36031–36047. doi: 10.1074/jbc.M114.600775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.el Azzouzi H, Leptidis S, Dirkx E, et al. The hypoxia-inducible microrna cluster mir-199a approximately 214 targets myocardial ppardelta and impairs mitochondrial fatty acid oxidation. Cell Metab. 2013;18:341–354. doi: 10.1016/j.cmet.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Zhao G, Shi L, Qiu D, et al. Nf45/ilf2 tissue expression, promoter analysis, and interleukin-2 transactivating function. Exp Cell Res. 2005;305:312–323. doi: 10.1016/j.yexcr.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 50.Das S, Cong R, Shandilya J, et al. Characterization of nucleolin k88 acetylation defines a new pool of nucleolin colocalizing with pre-mrna splicing factors. FEBS Lett. 2013;587:417–424. doi: 10.1016/j.febslet.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 51.Rahaman SO, Lennon DJ, Febbraio M, et al. A cd36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schrijvers DM, De Meyer GR, Herman AG, et al. Phagocytosis in atherosclerosis: Molecular mechanisms and implications for plaque progression and stability. Cardiovasc Res. 2007;73:470–480. doi: 10.1016/j.cardiores.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 53.Niazi F, Valadkhan S. Computational analysis of functional long noncoding rnas reveals lack of peptide-coding capacity and parallels with 3′ utrs. RNA. 2012;18:825–843. doi: 10.1261/rna.029520.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H, Xue C, Wang Y, et al. Deep rna sequencing uncovers a repertoire of human macrophage long intergenic noncoding rnas modulated by macrophage activation and associated with cardiometabolic diseases. J Am Heart Assoc. 2017:6. doi: 10.1161/JAHA.117.007431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.