Abstract
Originally considered to be part of a cellular waste pathway, expansive research into exosomes has shown that these vesicles possess a vast array of functional utilities. As vital transporters of materials for communications between cells, particular interest has been generated in the ability of cancer cells to use exosomes to induce immune suppression, and to establish a thriving microenvironment, ideal for disease progression. Exosomes carry and transfer many types of cargo, including microRNAs (miRNAs; miRs), which are important modulators of messenger RNA (mRNA) expression. These miRNAs have been shown to be noteworthy components of the mechanisms used by tumor derived exosomes to carry out their functions. Alternatively, research has been expanding into using exosomes and miRNAs as both bio-markers for detecting cancer and disease progression, and as potential treatment tools. Here, we discuss some of the progress that researchers have made related to cancer exosomes, their suppression of the immune system, the importance of the miRNAs they shuttle, along with some of the shortcomings, obstacles, and challenges that lie ahead.
Introduction
Exosomes are a subset of extracellular vesicles (EVs), recognized to have diameters of 40–150 nm. Exosomes are produced and secreted by all cell types (1), and are found in all bodily fluids (2). They originate intracellularly during endosomal maturation, where intralumenal invaginations or “reverse budding” push intralumenal vesicles or intravesicular bodies inside multivesicular endosomes (also called multivesicular bodies). Canonically, these multivesicular endosomes were generally thought to be dismantled by lysosomes, with the contents being recycled. However, we now know that these endosomes may also fuse with the plasma membrane, releasing or secreting the intralumenal vesicles into the extracellular space, where they are now called exosomes. Other forms of extracellular vesicles include microvesicles and apoptotic bodies (2). Both these vesicle types are generally considered to be larger than exosomes, ranging from 100 nm to 1000 nm in diameter (for microvesicles) and 50–5000 nm for apoptotic bodies (3). Microvesicles are formed as “shed vesicles” from the cell plasma membrane by outward budding, while apoptotic bodies form as cells undergoing programmed cell death dismantle themselves with intracellular breakdown that leads to extracellular, often amorphous blobs. In the literature used in this review, most of the authors refer to the vesicles they isolated and studied as “exosomes”; we will continue to use that terminology in the rest of this work.
EVs were first observed by researchers as early as the 1960s (4, 5), and later, in the 1980s, were better characterized with the use of electron microcopy, where the EVs/exosomes were described as being part of a waste disposal pathway during reticulocyte maturation (6, 7). Those vesicles were generated in the endosomal system, as noted above, in multivesicular endosomes / multivesicular bodies, rather than protruding and releasing directly from the plasma membrane (and hence, were called exosomes – Rose Johnstone is frequently credited with popularizing that term). The study was certainly relevant in that it explained how transferrin receptor is lost as reticulocytes mature to erythrocytes at a point where the cells no longer have detectable lysosomes (8). This established the initial “garbage bag” nature of exosomes.
While waste disposal is certainly an important function, subsequent research has shown considerably more robust and exciting roles are played by exosomes, the mysteries of which we are still uncovering today (9). Particularly noteworthy was the discovery that B lymphocytes produced antigen presenting exosomes (10). This discovery demonstrated the exosome’s role as a functional and important piece of adaptive immunity. Further investigations showed that dendritic cells secrete exosomes (dexosomes, DEX) capable of producing antitumor immune responses in mice (11). These studies were eventually extended to include several clinical trials in humans using dendritic cell exosomes as anti-cancer vaccines (reviewed in (12)). Unmodified exosomes derived from cancer cells (tumor exosomes, TEX) have also been used as anti-cancer vaccines in murine models. However, there has only been one reported clinical trial using tumor-derived exosomes as a form of cancer immunotherapy (13) (also reviewed in (12)), suggesting that the immune stimulating properties of such anti-cancer vaccines are as yet insufficient to overcome the profound state of immune suppression found in most patients with cancer.
This interaction with the immune system is also of note; once considered as agents of immune stimulation, TEX soon became notorious for their implications in immune suppression (14, 15). Tumor cells evidently gain overall benefit from their exosome interactions with the immune system, as tumors produce exosomes which promote a favorable microenvironment that supports tumor growth, cell proliferation, and suppression of the immune response through the use of various exosome-mediated mechanisms (16). Exosome levels measured in the body fluids of cancer patients are typically elevated compared to healthy controls, including blood (17), where TEX interactions with immune cells could obviously occur. Another noteworthy discovery was that exosomes could bind and/or be absorbed by recipient cells neighboring their cells of exosome origin, with observable and function-altering effects. TEX interactions with cells in the tumor microenvironment include the co-opting of stromal cells (18), the “differentiation” of fibroblasts into cancer-associated fibroblasts (19) to support the tumor, to the full conversion of neighboring normal cells to cancer-like entities that aid and abet the tumor (20). This includes a willingness to undergo apoptosis and die for the sake of the tumor (21). Coupled with the discovery of mRNA and miRNA inside of exosomes (22), along with the observations of numerous nucleic acid binding proteins found in exosomes (23), it became clear that exosomes were being used to transfer both signaling proteins (24) and genetic information, which contributed to the production of proteins. Thus, the vesicles previously thought to be little more than garbage sacks had become immensely more interesting.
TEX-Induced Overall Immune Suppression
Exosomes are a vital part of the communication system between tumor cells, necessary for promoting proliferation, angiogenesis, and thrombosis as well as establishing tumor educated stromal cells (2). TEX are also involved in host matrix modulation and directing cell movement through tissues, influencing the migration speed of tumor cells (25, 26). Successful tumor growth requires establishment of a favorable microenvironment surrounding the tumor, including overcoming attacks by the immune system. The immunosuppressive abilities of TEX go beyond just creating a favorable microenvironment for the tumor, but also include a comprehensive altering of the immune system as a whole, to be more permissive of tumor growth, and allowing the tumor to spread more aggressively. As such, the mechanisms and targets of immune suppression by TEX are numerous.
TEX and Natural Killer (NK) Cells
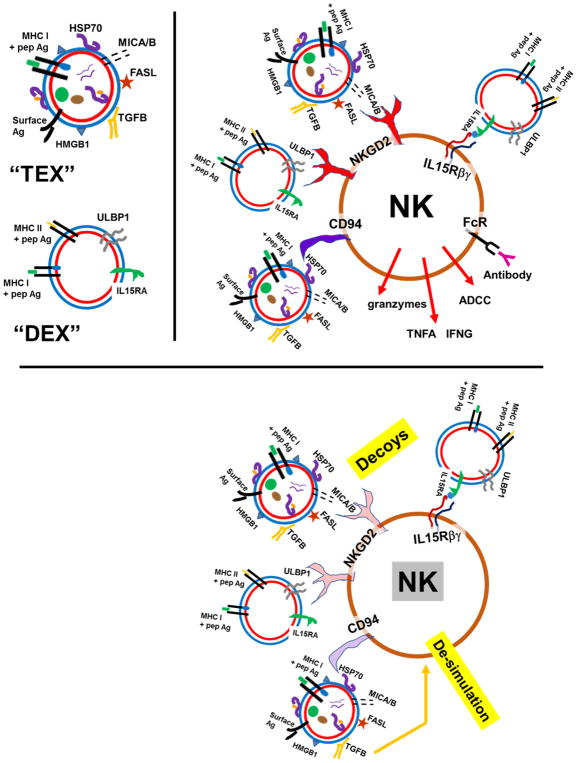
TEX, and also DEX, can stimulate natural killer (NK) cells via triggering of the NKG2D (activating natural-killer group 2 member D) receptor with NKG2D ligands on exosome surfaces, such as MICA and MICB (MHC class I polypeptide-related sequence A/B) on TEX, or ULBP1 (UL16 binding protein 1) on DEX (27, 28). Heat shock protein 70 (HSP70; HSPA1A) on TEX surfaces is a known ligand for the NK cell receptor CD94, and is also immune stimulatory in that context (29). However, the MICA/B and ULBP1/2 ligands may also promote down-regulation of NKG2D on NK cells (30), and tumors could use TEX in order to prevent apoptosis in modulating the expression of the NKG2D receptor (31). It is conceivable that TEX surface HSP70 could also lead to downregulation of NK CD94; in these instances, TEX could act as circulating decoys for NK cells (32). Transforming growth factor β (TGFB), known to be a component of TEX (33), has also been shown to be an immune suppressive moiety of NK cells (34). These stimulation/de-stimulation scenarios are shown in Figure 1.
Figure 1.
TEX and DEX interactions with NK cells, stimulatory and suppressive
Tumor cell exosomes (“TEX”) and dendritic cell exosomes (“DEX”) are depicted in the upper left portion of the figure. The upper right portion shows NK cell activation properties of TEX, where NKG2D ligands MICA and MICB provide stimulation, as does TEX surface HSP70 interacting with CD94. DEX ULBP1/2 can also trigger NKG2D, and IL15 can be delivered by DEX IL15RA (IL15 receptor alpha) to NK IL15 receptor beta (and gamma). However, the prolonged NKG2D stimulation from MICA/B or ULBPs can downregulate the NK receptor, as can HSP70 interacting with CD94 (bottom panel). Also, TGFB from TEX can de-activate NK cells, leading to overall suppression of NK cell anti-tumor activity.
TEX and T cells
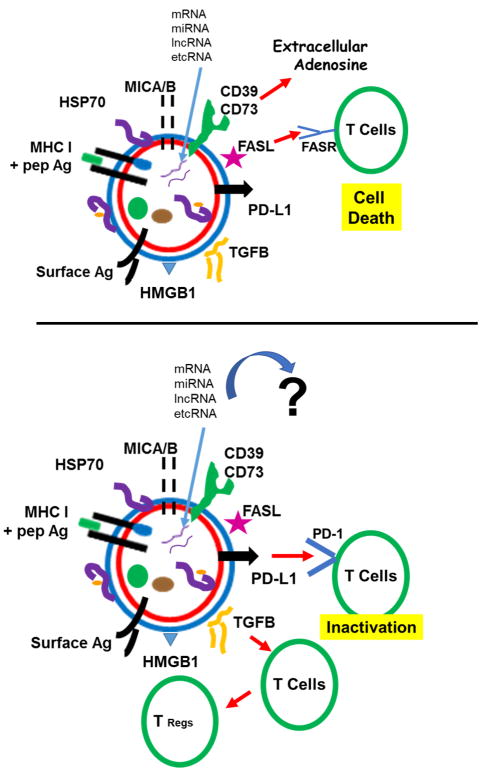
Induction of effector T cell apoptosis is another immunosuppression technique employed by TEX, through the activation of the Fas receptor (FasR; CD95) by Fas ligand (FasL; CD95L) (21). Another mechanism involving deactivation of T cells is via the PD-L1 / PD-1 (programmed cell death ligand 1 / programmed cell death 1) checkpoint, where TEX displaying PD-L1 can de-stimulate activated T cells expressing the PD-1 receptor (35). TEX also display active ectonucleotidases CD73 and CD39 (36), ecto-enzymes that sequentially convert extracellular ATP eventually to adenosine (37). Adenosine has numerous tumor-promoting activities as well as suppressive effects on T cells and other immune cells (38). TEX have been sho.wn to induce, expand, and up-regulate the generation of immunosuppressing regulatory T-cells (Treg), with possible influences of TGFB and interleukin 10 (IL10) (39). These T cell suppressive tendencies of TEX are shown in Figure 2.
Figure 2.
T cells and TEX-induced immune suppression
Different mechanisms of TEX-driven T cell immune suppression are shown. In the top panel, generation of adenosine from ATP to ADP to AMP (by ectonucleotidase CD39) followed by AMP to adenosine by CD73 leads to inactivation of T cells. Fas ligand (FASL) on TEX can directly promote T cell apoptosis by binding Fas receptor (FASR) on activated T cells. In the bottom panel, PD1 ligand (PD-L1) on TEX can inactivate T cells expressing the PD-1 receptor. TEX TGFB can promote the generation of regulatory T cells (Tregs), leading to further immune suppression by the potent regulatory/suppressive capacities of Tregs.
TEX and Monocytes
Disruption of dendritic cell differentiation and maturation is another example of TEX suppressing the immune system (12, 40). In particular, TEX reduced DC T cell stimulatory capabilities by promoting DC expression of CD73, the ectonucleotidase that hydrolyzes AMP to adenosine, which suppresses T cell function. TEX prostaglandin E2 (PGE2) was a trigger for DC expression of CD73 (41). However, TEX have been used as effective antigen-delivery systems to DCs (12), suggesting that TEX content, or DC responses to TEX, may be deciding factors in the abilities of DC to stimulate or suppress effector T cells. Myeloid-derived suppressor cells (MDSCs) are monocytic cells of myeloid lineage that regulate functions of DCs, macrophage, T and NK cells (42), and TEX can influence this lack of differentiation even in bone marrow, where TGFB and PGE2 play roles (43, 44). TEX also promote M2 macrophage polarization, which is associated with tumor-promoting activities (45), although there may be mixed macrophage phenotypes (46).
There has been a great deal of research conducted regarding TEX immune suppressive activities (12, 47). There have also been extensive efforts in characterizing TEX-encased microRNAs (miRNAs; miRs) and unraveling their roles in the pathology of tumors, and as potential biomarkers (48). Curiously, there is little overlap in these areas; the roles of TEX miRNAs (and other vesicular RNAs) in immune suppression is a relatively understudied area (as noted in Figure 2), and we will devote the remainder of this review to that area.
microRNAs in Brief
microRNAs are small (19 to 22 nucleotide length) noncoding RNAs, that participate in regulation of cellular development, differentiation, proliferation, apoptosis, and cancer cell metabolism. These are encoded within the genome both intergenically and intragenically; following transcription, miRNAs are cropped in the nucleus by the DROSHA complex, exported into the cytosol through Exportin 5 (XPO5)/RAN-GTP transporter, with further cytosolic processing by Dicer. The miRNA joins in the RISC (RNA-induced silencing complex) composed of Dicer, TRBP (TAR RNA binding protein), and AGO (Argonaute) proteins which the “guide strand” miRNA guides to the 3′-untranslated region (UTR) of mRNAs using a typically imperfect complementarity (the miRNA “passenger strand” is thought to be degraded, but this may not be the full account—see below). This complex in place on the mRNA 3′-UTR leads to reduced or inhibited mRNA translation and/or degradation of the message. Thus, miRNAs are mechanistically similar to RNA interference (RNAi) and silencing RNA (siRNA) (reviewed in(49)).
TEX and microRNAs
It has been shown that while exosomes can contain all major classes of small RNAs (50), and the microRNAs are certainly represented (2). TEX clearly serve as vehicles for miRNAs between different cells and cell types. How miRNAs become exosomal cargo is not entirely clear (as is true for most exosome cargo), but there are some published and proposed mechanisms. One involves the binding of sequence motifs in RNAs (EXOmotifs) by sumoylated hnRNPA2B1 (heterogenous nuclear ribonucleoprotein A2/B1) (51). Other concepts involve the interactions of appropriate or modified RNA sequences with the lipid raft domains of endosomes that will undergo invagination (“budding in” process) to form the intralumenal vesicles of multivesicular bodies, eventually leading to extracellular release as exosomes (52). Ceramide at the endosome cytoplasmic face appears important in this process (53). The role of RNA binding proteins (RBPs) was regarded as significant in getting RNAs in the vicinity of the endosomes (52), and we have noted the extensive variety of RBPs found in the EV proteome (23). Curiously, AGO2 (Argonaute 2) is relatively under-represented in exosome proteomes (54), which suggests that other RBPs may play roles in RNA loading into exosomes, including the passenger strands of microRNAs (55). For example, SYNCRIP (synaptotagmin-binding cytoplasmic RNA-interacting protein; hnRNPQ / NSAP1) is an RNA-binding protein that recognizes hEXO motifs for loading miRNAs into exosomes (56). Thus, there appear to be mechanisms for miRNA entry into the forming endosomal intralumenal vesicles that eventually become exosomes.
TEX and their miRNA cargoes are deposited within the tumor microenvironment, taken up by the various neighboring cells where they support communication, promote tumor growth, and drive immunosuppression (57, 58). Several studies have shown miRNAs present in exosomes released from tumor cells are able to induce angiogenesis in different cancer types, which is vital for tumor growth and survival. One example is miR-92a, found in exosomes derived from leukemia cells; it has been shown to improve endothelial cell migration and tube formation (59). Exosomes containing miR-210 can help promote the angiogenic activity of endothelial cells in tumors (60). Another study showed that exosomes containing miR-135b could directly suppress FIH1 (factor-inhibiting hypoxia-inducible factor 1), to induce angiogenesis in multiple myeloma (61). Some studies have also shown that exosomes can be utilized by tumors to remove miRNA that inhibit tumor metastasis. Tumor suppressor miRNAs such as miR-23b, miR-92 and, miR-224 could be found in exosomes released from bladder carcinoma cells (62) (thus revitalizing the idea of exosomes as garbage bags, but with a nefarious purpose from the perspective of the patient). Another important study (63) showed that breast cancer exosomes contained pre-miRNAs (ie, prior to Dicer cleavage) along with other RISC components, including AGO2 (which were not found in exosomes from normal cells). Passage of these TEX to mammary gland epithelial cells (MCF10A cells) resulted in the processing of active miRNAs that altered the transcriptome of the recipient cells (including reduced expression of tumor suppressors PTEN (phosphatase and tensin homolog) and HOXD10 (homeobox protein D10), whose mRNAs are targeted by miR-21 and miR-10b, respectively. TEX stimulated the normal cell recipients to become tumorigenic in immune-compromised mice; this was found to be Dicer-dependent. All of these instances show clear impacts of TEX-delivered microRNAs impacting tumor cells themselves, or cells of the microenvironment, to promote tumor growth, metastasis, and even a full co-opting of normal cells to become cancerous.
TEX, microRNAs, and Immune Suppression
The effects of miRNA transfer from TEX to immune cells may form a basis for immune suppression, assuming the miRNAs target key players in the immune cell activities. A major point to keep in mind is that the immune system indeed functions as a system; impacting mRNA expression/translation in one cell type may affect the downstream activities of another cell type. For instance, reduction of key members of the antigen presentation machinery in professional antigen presenting cells (APCs) such as B cells, macrophage, or DCs, could result in reduced or ineffective stimulation of T lymphocytes without the TEX-derived miRNA directly affecting the T cells. Reducing cytokine output in one cell type (eg, DCs, T cells, B cells, macrophage) could adversely affect NK cell activity. Thus, as we break down our focus into various immune cell types, our discussion of TEX miRNAs that target specific gene products in one class of immune cells may have profound effects on other immune cells.
TEX miRNAs and NK Cells
TEX (microvesicle-like)-derived miR-23a (lung cancer, leukemia) was shown to reduce expression of CD107a/LAMP1 (lysosomal associated membrane protein 1) (64), an NK cell activation marker with damage-mitigating properties (65). As the tumor vesicles also deliver TGFB that downregulates NKG2D expression, tumor MVs/TEX were seen as general NK cell suppressors. miR-362-5p has different impacts on different tumor cells themselves (66–68), but appears to be critical in promoting NK cell activation status (via downregulation of CYLD; ubiquitin carboxyl-terminal hydrolase CYLD) (69). While one would thus suspect that TEX transfer of miR-362-5p might benefit the immune response, there is a possibility that over-abundance of the miRNA could lead to over-stimulation resulting in hypo-responsiveness (70). Other TEX miRNA effects on NK cells may be due to expression of NK ligands on other cell types (see below).
TEX miRNA and T Cells
TEX from nasopharyngeal carcinoma were shown to contain over-expressed miRs-24-3p; -891a; -106a-5p; -20a-5p; and -1908. Bioinformatics analysis yielded almost 20 targets linked to the MAPK1 pathway for potential downregulation. The net effect on T cells (via downregulated ERK/STAT1/STAT3 phosphorylation) was a shift from TH1 and TH17 phenotypes towards TH2 and Treg phenotypes (71). Following assessment of upregulated tumor miRNAs in tissues from patients with breast cancer, hepatocellular carcinoma, non-small-cell lung cancer, or pancreatic cancer, the ubiquitous miR-21 and miR-214 were found to be consistently upregulated in tissue and in plasma exosomes/microvesicles from patients. This was extended to murine lung cancer and sarcoma models, where it was shown that miR-214 drove downregulation of PTEN in T cells, promoting a Treg phenotype. This is likely to involve varying levels of downstream signaling (72), but runs somewhat counter to other reports involving the role of PTEN in Treg maintenance (73, 74). It is conceivable that tumor-induced generation of Tregs may have different signaling and regulatory modes and outcomes than naturally-generated Tregs. While TEX miRNAs generally appear to promote Treg or immune restrictive phenotypes, T cells may also be affected by antigen presenting cells such as macrophage and DCs.
TEX miRNA and Monocytes
miR-212-3p found in TEX from pancreatic cancer cells was predicted and validated to downregulate the transcription factor RFXAP (regulatory factor X-associated protein) when those TEX were pulsed onto immature DCs. This led to reduced expression of MHC (major histocompatibility complex) Class II molecules HLA (human leukocyte antigen) DR/DP/DQ (75). While not tested, one would predict those DCs would be relatively ineffective at antigen presentation to CD4+ T cells as well as B cells. As noted above, TEX-pulsed DCs have been shown to promote effector T cells from the stimulation and supplied antigens from the TEX. As noted elsewhere (12), the context in which the immune system “sees” TEX is likely a critical factor in the distinction between immune stimulation vs immune suppression; thus, if the DCs were matured during this process, would the outcome have been different? The same group revealed a downregulation of stimulatory capacity in immature DCs when exposed to the same PANC-1 TEX (76); in this case, miR-203 was associated with reduced TLR4 (toll-like receptor 4) expression at the protein level (curiously, the mRNA levels were insignificantly affected). There was also reduced secretion of TNFA (tumor necrosis factor alpha) and IL12. It was not clear exactly how miR-203 promulgated the reduced expression of TLR4. It is possible that miR-203 in the RISC binds TLR4 mRNA, inhibiting its translation, but without mRNA degradation. This was not explored. Again, the alteration of the cytokine environment could impact the T cells (or B cells) receiving DC stimulation, so the impact of the miRNAs may not be directly on the effector cell population, but from an immune stimulatory standpoint, those cells would suffer from that impact on the DCs. Once again, this may depend on the context of the DCs, as immature DCs were used in this study.
Tumor-associated macrophages (TAMs) are closely tied to the tumor microenvironment in the tempest of constant inflammation (but with minimal anti-tumor activity) in a wound-healing milieu (77). TAM derivation to a polarized M2 state, and TAM maintenance are part-and-parcel with this pro-inflammatory environment -- but one that seldom deletes tumors, and instead, promotes them (78). Several years ago Fabbri et al (79) showed that TEX from non-small cell lung carcinoma (NSCLC) could deliver miRs-21 and -29a to macrophage entering the tumor microenvironment. These RNAs bound and activated TLR8 (murine TLR7), which activated the NFKB (nuclear factor kappa-light-chain-enhancer of activated B cells) pathway and led to secretion of IL6 and TFNA, leading to a pro-tumor inflammatory environment. TLR7/8 is one of the intracellular TLRs, in endosomal and other vesicular membranes, and is responsible for innate reactions against various pathogens (80). Fabbri’s group has also demonstrated cross-talk between TAMs and tumor cells via the miRNAs in their exosomes (81). miR-21 from neuroblastoma TEX could again trigger TLR8 in monocytes, which led to upregulation of miR-155 in those cells. miR-155 could then be passaged back to the tumor cells via exosomes, where the miR-155 target TERF1 (telomeric repeat binding factor 1) was downregulated. TERF1 is a telomerase inhibitor, and upregulation of telomerase promotes cisplatin resistance in cancer cells (82). Thus, TERF1 downregulation increases neuroblastoma drug resistance, and shows how cross-talk in the microenvironment generally benefits the tumor.
However, TEX miRNAs may have detrimental effects on the tumor when transferred to macrophage, as in the case where murine breast cancer cells (and in vivo tumors) were treated with EGCG (epigallocatechin gallate), a component of green tea extract with known anti-cancer properties (83). Tumor cells treated with EGCG upregulated miR-16 in cells and their TEX. Delivery of these TEX to macrophage altered expression of a number of cytokines, generally towards an M1 phenotype as opposed to M2. One target of miR-16 is CHUK/IKK α (inhibitor of nuclear factor kappa-B kinase subunit alpha). As this component of IKK complex is reduced, phosphorylation of IκB is reduced, maintaining its stability and preventing activated NFKB from translocating to the nucleus. As that NFKB pathway is known to promote differentiation of macrophage towards an M2 phenotype (84), miR-16 may be important in regulating that pathway via CHUK/IKK α (although the NFKB pathway is quite complicated and is likely not the whole story (85)).
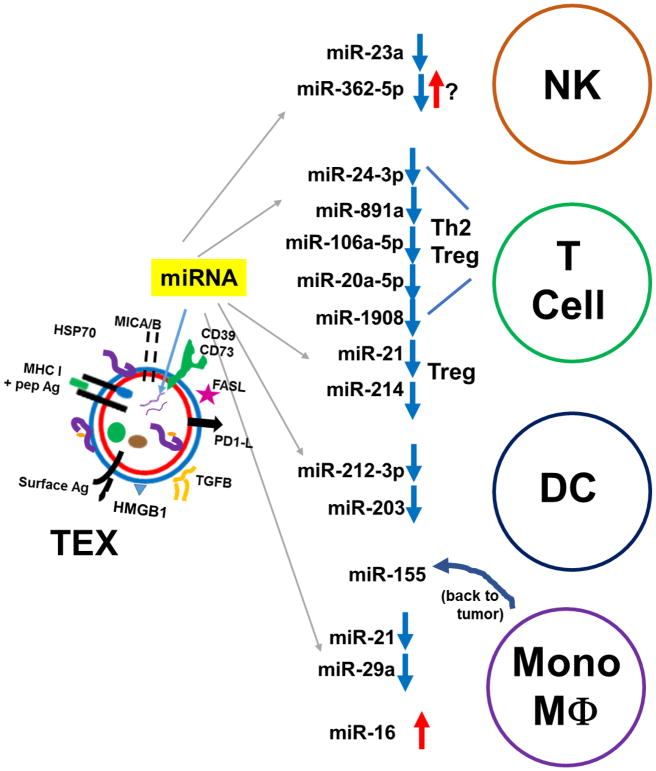
Overall, the general pattern seen is that TEX deliver miRNAs to immune system cells that ultimately lead to situations that benefit the tumor. These are shown in Figure 3. Three excellent reviews related to this topic are here (86–88).
Figure 3.
TEX microRNAs that downregulate immune activity in NK cells (NK), T cells, dendritic cells (DC), and monocytes/macrophage (Mono/MΦ). Downward blue arrows indicated suppression or reduction in activity, while upward red arrows indicate stimulation or activation. Note that miR-155 from TEX-influenced monocytes is passaged back to tumor cells in exosomes, resulting in tumor chemotherapy resistance. Details are given in the text.
TEX and the Development of Anti-Tumor Therapies and Biomarkers
Elucidating these TEX mechanisms in the context of immune suppression is important for the development of novel therapies. For example, the previously mentioned suppression of NK cells by modulating the expression of the NKG2D receptor has been targeted for therapeutic advancement, by using DEX equipped with NK cell-activating NKG2D ligands to restore NK cell function and induce tumor cell apoptosis (89). Additional modifications of TEX (and DEX) for utilization as anti-cancer vaccines have been reviewed elsewhere by us (12). The mechanisms cancer cells have developed to grow, suppress and evade the immune system, and to modify the existing biological processes of the host for their benefit, are vast. While extensive research has already been done (2), it is important to continue elucidating the exosome-mediated mechanisms of cancer, and to continue to develop novel treatments. For example, employing the tumor’s own mechanisms for transfer of miRNAs could be exploited, as noted above for EGCG-driven increase in miR-16 packaging and transfer to alter macrophage.
Screening for exosomal miRNAs obtained from biofluids allows them to be used as biomarkers for detecting cancer, which can be an invaluable diagnostic tool in a clinical setting. This is particularly desirable as blood and urine are biofluids obtained in minimally- or non-invasive settings. One screen of plasma exosomes from pancreatic cancer patients showed elevated levels of miR-1246, miR-3976, miR-4306, and miR-4644 (90). Another study of sera from colon cancer patients found increases in exosomal miRNAs including miR-21, miR-23a, miR-150, miR-223, miR-1229, and miR-1246, and that those levels were decreased after surgical resection of the tumor (91). Increases or decreases of miRNA levels can also be used to determine the aggressiveness of tumors, as in the case of miR-122, which in breast cancer has been shown to alter glucose metabolism and promote metastasis (92). Exosomes containing miR-126a have been identified as promoting lung cancer metastasis (93). TEX miRNAs are of high interest in the biomarker field, with many studies performed, and many more ongoing (48, 94, 95).
Exosomes can also be engineered to deliver specific mRNAs, miRNAs, siRNAs, in order to repress target genes in tumor cells, and thus used as a treatment method (96, 97). Anti-miR-9 has been used to sensitize GBM cells to chemotherapy drug temozolomide (98). miR-143 has been shown to inhibit tumor cell growth (99) [46]. The use of miRNAs both by TEX and as methods of treatment has been an exponentially increasing field of study in recent years. Conceptually, exosomes may be a biologically relevant means on delivering silencing RNAs with ongoing repackaging and further delivery (100). Expanding the knowledge pool of miRNAs and their associations and roles in cancer, and as potential treatments, is vital to understanding the challenges presented by cancer and moving to overcome those challenges.
Conclusion
Research on exosomes, has come a long way since their initial discovery. Once thought to be part of a cellular waste disposal pathway, the last few decades have seen incredible growth in the amount of research interest (and to some extent, funding) in exosomes and their miRNA cargo (57, 101). Exosomes have proven to be a key component of a tumor’s ability to create a viable microenvironment, orchestrate communication between cells, promote growth, and suppress the immune system (102). The field of cancer immunotherapy has also made substantial progress recent years. However, the exosome-mediated mechanisms of immune suppression utilized by cancer go well beyond the upregulation of immune checkpoints, which despite being a significant portion of current research, account for only a sampling of the complex communication system established by cancer cells throughout the disease (102). Regardless of the interest that has been generated in the field, there are still numerous shortcomings that prevent more robust and expansive research. Exosome labeling techniques are significantly lacking, particularly in the context of nanoparticles formed by the dyes themselves (103). These can lead to very misleading conclusions concerning exosome quantity, cellular uptake, and subcellular localization. We understand little about the nature of the exosomes being released by cancer cells in terms of numbers and fates of those exosomes in the microenvironment, especially the release and uptake dynamics. The variations between cell sources, purification methods, delivery methods, variations in exosome concentration determinations, and the use of immunodeficient animals for many tumor models, highlight the difficulties with deriving accurate and reliable conclusions (104). Developing new tools to expand and solidify our understanding will be important steps in translating our recent discoveries into practical clinical therapies. Despite the potential usefulness of miRNA’s as bio-markers, the results of previous screens have not been robust enough to account for a myriad of potential, alternative explanations for the data being generated, hampering the validity of their conclusiveness and their clinical utility (105). The recent discovery of miRNA’s ability to function similar to hormones and bind to and subsequently activate receptors, represents an exciting new molecular mechanism to be elucidated, but with it comes an ever-heightening tidal wave of unanswered questions and mysteries (106). Still, the expansive research that has been done, and continues to be done provide encouraging, exciting new prospects for the field of cancer immunotherapy in particular, and cancer biology in general. The recent studies showing the potential use of exosomes as a drug delivery system highlight the significance of the extensive and progress that has already been made, and that researchers continue to make (107). While the critical roles of TEX and their miRNAs in immune suppression continues to be illuminated, so too does the development of novel treatment strategies, that continue to be increasingly effective, and potentially life saving.
References
- 1.Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126:1208–15. doi: 10.1172/JCI81135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Kramer-Albers EM, Laitinen S, Lasser C, Lener T, Ligeti E, Line A, Lipps G, Llorente A, Lotvall J, Mancek-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen EN, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pallinger E, Del Portillo HA, Reventos J, Rigau M, Rohde E, Sammar M, Sanchez-Madrid F, Santarem N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szatanek R, Baran J, Siedlar M, Baj-Krzyworzeka M. Isolation of extracellular vesicles: Determining the correct approach (Review) Int J Mol Med. 2015;36:11–7. doi: 10.3892/ijmm.2015.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–88. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 5.Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969;41:59–72. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–39. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101:942–8. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–20. [PubMed] [Google Scholar]
- 9.De Toro J, Herschlik L, Waldner C, Mongini C. Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front Immunol. 2015;6:203. doi: 10.3389/fimmu.2015.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–72. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 12.Kunigelis KE, Graner MW. The Dichotomy of Tumor Exosomes (TEX) in Cancer Immunity: Is It All in the ConTEXt? Vaccines (Basel) 2015;3:1019–51. doi: 10.3390/vaccines3041019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. 2008;16:782–90. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. 2015;40:72–81. doi: 10.1016/j.semcdb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Meehan K, Vella LJ. The contribution of tumour-derived exosomes to the hallmarks of cancer. Crit Rev Clin Lab Sci. 2016;53:121–31. doi: 10.3109/10408363.2015.1092496. [DOI] [PubMed] [Google Scholar]
- 17.Hellwinkel JE, Redzic JS, Harland TA, Gunaydin D, Anchordoquy TJ, Graner MW. Glioma-derived extracellular vesicles selectively suppress immune responses. Neuro Oncol. 2015 doi: 10.1093/neuonc/nov170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wendler F, Stamp GW, Giamas G. Tumor-Stromal Cell Communication: Small Vesicles Signal Big Changes. Trends Cancer. 2016;2:326–9. doi: 10.1016/j.trecan.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 19.CRG, Bernard G, Tremblay S, Chabaud S, Bolduc S, Pouliot F. Exosomes Induce Fibroblast Differentiation into Cancer-associated Fibroblasts through TGFbeta Signaling. Mol Cancer Res. 2018 doi: 10.1158/1541-7786.MCR-17-0784. [DOI] [PubMed] [Google Scholar]
- 20.Oushy S, Hellwinkel JE, Wang M, Nguyen GJ, Gunaydin D, Harland TA, Anchordoquy TJ, Graner MW. Glioblastoma multiforme-derived extracellular vesicles drive normal astrocytes towards a tumour-enhancing phenotype. Philos Trans R Soc Lond B Biol Sci. 2018:373. doi: 10.1098/rstb.2016.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, Squarcina P, Accornero P, Lozupone F, Lugini L, Stringaro A, Molinari A, Arancia G, Gentile M, Parmiani G, Fais S. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195:1303–16. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 23.Ung TH, Madsen HJ, Hellwinkel JE, Lencioni AM, Graner MW. Exosome proteomics reveals transcriptional regulator proteins with potential to mediate downstream pathways. Cancer Sci. 2014;105:1384–92. doi: 10.1111/cas.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–24. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 25.Mu W, Rana S, Zoller M. Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia. 2013;15:875–87. doi: 10.1593/neo.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sung BH, Ketova T, Hoshino D, Zijlstra A, Weaver AM. Directional cell movement through tissues is controlled by exosome secretion. Nat Commun. 2015;6:7164. doi: 10.1038/ncomms8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vivier E, Tomasello E, Paul P. Lymphocyte activation via NKG2D: towards a new paradigm in immune recognition? Curr Opin Immunol. 2002;14:306–11. doi: 10.1016/s0952-7915(02)00337-0. [DOI] [PubMed] [Google Scholar]
- 28.Chitadze G, Bhat J, Lettau M, Janssen O, Kabelitz D. Generation of soluble NKG2D ligands: proteolytic cleavage, exosome secretion and functional implications. Scand J Immunol. 2013;78:120–9. doi: 10.1111/sji.12072. [DOI] [PubMed] [Google Scholar]
- 29.Gross C, Hansch D, Gastpar R, Multhoff G. Interaction of heat shock protein 70 peptide with NK cells involves the NK receptor CD94. Biol Chem. 2003;384:267–79. doi: 10.1515/BC.2003.030. [DOI] [PubMed] [Google Scholar]
- 30.Mincheva-Nilsson L, Baranov V. Cancer exosomes and NKG2D receptor-ligand interactions: impairing NKG2D-mediated cytotoxicity and anti-tumour immune surveillance. Semin Cancer Biol. 2014;28:24–30. doi: 10.1016/j.semcancer.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Clayton A, Tabi Z. Exosomes and the MICA-NKG2D system in cancer. Blood Cells Mol Dis. 2005;34:206–13. doi: 10.1016/j.bcmd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Hedlund M, Nagaeva O, Kargl D, Baranov V, Mincheva-Nilsson L. Thermal- and oxidative stress causes enhanced release of NKG2D ligand-bearing immunosuppressive exosomes in leukemia/lymphoma T and B cells. PLoS One. 2011;6:e16899. doi: 10.1371/journal.pone.0016899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graner MW, Alzate O, Dechkovskaia AM, Keene JD, Sampson JH, Mitchell DA, Bigner DD. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2009;23:1541–57. doi: 10.1096/fj.08-122184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica. 2011;96:1302–9. doi: 10.3324/haematol.2010.039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ichim TE, Zhong Z, Kaushal S, Zheng X, Ren X, Hao X, Joyce JA, Hanley HH, Riordan NH, Koropatnick J, Bogin V, Minev BR, Min WP, Tullis RH. Exosomes as a tumor immune escape mechanism: possible therapeutic implications. J Transl Med. 2008;6:37. doi: 10.1186/1479-5876-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187:676–83. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 37.Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol Rev. 2017;276:121–44. doi: 10.1111/imr.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spychala J. Tumor-promoting functions of adenosine. Pharmacol Ther. 2000;87:161–73. doi: 10.1016/s0163-7258(00)00053-x. [DOI] [PubMed] [Google Scholar]
- 39.Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg) PLoS One. 2010;5:e11469. doi: 10.1371/journal.pone.0011469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clayton A, Mason MD. Exosomes in tumour immunity. Curr Oncol. 2009;16:46–9. doi: 10.3747/co.v16i3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salimu J, Webber J, Gurney M, Al-Taei S, Clayton A, Tabi Z. Dominant immunosuppression of dendritic cell function by prostate-cancer-derived exosomes. J Extracell Vesicles. 2017;6:1368823. doi: 10.1080/20013078.2017.1368823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valenti R, Huber V, Iero M, Filipazzi P, Parmiani G, Rivoltini L. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 2007;67:2912–5. doi: 10.1158/0008-5472.CAN-07-0520. [DOI] [PubMed] [Google Scholar]
- 43.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–91. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rutkowski MR, Svoronos N, Perales-Puchalt A, Conejo-Garcia JR. The Tumor Macroenvironment: Cancer-Promoting Networks Beyond Tumor Beds. Adv Cancer Res. 2015;128:235–62. doi: 10.1016/bs.acr.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Vrij J, Maas SL, Kwappenberg KM, Schnoor R, Kleijn A, Dekker L, Luider TM, de Witte LD, Litjens M, van Strien ME, Hol EM, Kroonen J, Robe PA, Lamfers ML, Schilham MW, Broekman ML. Glioblastoma-derived extracellular vesicles modify the phenotype of monocytic cells. Int J Cancer. 2015;137:1630–42. doi: 10.1002/ijc.29521. [DOI] [PubMed] [Google Scholar]
- 46.Bardi GT, Smith MA, Hood JL. Melanoma exosomes promote mixed M1 and M2 macrophage polarization. Cytokine. 2018;105:63–72. doi: 10.1016/j.cyto.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whiteside TL. Exosomes carrying immunoinhibitory proteins and their role in cancer. Clin Exp Immunol. 2017;189:259–67. doi: 10.1111/cei.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salehi M, Sharifi M. Exosomal miRNAs as novel cancer biomarkers: Challenges and opportunities. J Cell Physiol. 2018 doi: 10.1002/jcp.26481. [DOI] [PubMed] [Google Scholar]
- 49.Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ragusa M, Barbagallo C, Cirnigliaro M, Battaglia R, Brex D, Caponnetto A, Barbagallo D, Di Pietro C, Purrello M. Asymmetric RNA Distribution among Cells and Their Secreted Exosomes: Biomedical Meaning and Considerations on Diagnostic Applications. Front Mol Biosci. 2017;4:66. doi: 10.3389/fmolb.2017.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, Perez-Hernandez D, Vazquez J, Martin-Cofreces N, Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M, Sanchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janas T, Janas MM, Sapon K, Janas T. Mechanisms of RNA loading into exosomes. FEBS Lett. 2015;589:1391–8. doi: 10.1016/j.febslet.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 53.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–52. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koppers-Lalic D, Hackenberg M, Bijnsdorp IV, van Eijndhoven MAJ, Sadek P, Sie D, Zini N, Middeldorp JM, Ylstra B, de Menezes RX, Wurdinger T, Meijer GA, Pegtel DM. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep. 2014;8:1649–58. doi: 10.1016/j.celrep.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 55.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, Fischer A, Engelhardt S, Wei Y, Schober A, Fiedler J, Thum T. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124:2136–46. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Santangelo L, Giurato G, Cicchini C, Montaldo C, Mancone C, Tarallo R, Battistelli C, Alonzi T, Weisz A, Tripodi M. The RNA-Binding Protein SYNCRIP Is a Component of the Hepatocyte Exosomal Machinery Controlling MicroRNA Sorting. Cell Rep. 2016;17:799–808. doi: 10.1016/j.celrep.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 57.Tkach M, Thery C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164:1226–32. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 58.Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell. 2016;30:836–48. doi: 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Umezu T, Ohyashiki K, Kuroda M, Ohyashiki JH. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013;32:2747–55. doi: 10.1038/onc.2012.295. [DOI] [PubMed] [Google Scholar]
- 60.Tadokoro H, Umezu T, Ohyashiki K, Hirano T, Ohyashiki JH. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J Biol Chem. 2013;288:34343–51. doi: 10.1074/jbc.M113.480822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124:3748–57. doi: 10.1182/blood-2014-05-576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ostenfeld MS, Jeppesen DK, Laurberg JR, Boysen AT, Bramsen JB, Primdal-Bengtson B, Hendrix A, Lamy P, Dagnaes-Hansen F, Rasmussen MH, Bui KH, Fristrup N, Christensen EI, Nordentoft I, Morth JP, Jensen JB, Pedersen JS, Beck M, Theodorescu D, Borre M, Howard KA, Dyrskjot L, Orntoft TF. Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res. 2014;74:5758–71. doi: 10.1158/0008-5472.CAN-13-3512. [DOI] [PubMed] [Google Scholar]
- 63.Melo SA, Sugimoto H, O’Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, Lucci A, Ivan C, Calin GA, Kalluri R. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–21. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berchem G, Noman MZ, Bosseler M, Paggetti J, Baconnais S, Le Cam E, Nanbakhsh A, Moussay E, Mami-Chouaib F, Janji B, Chouaib S. Hypoxic tumor-derived microvesicles negatively regulate NK cell function by a mechanism involving TGF-beta and miR23a transfer. Oncoimmunology. 2016;5:e1062968. doi: 10.1080/2162402X.2015.1062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohnen A, Chiang SC, Stojanovic A, Schmidt H, Claus M, Saftig P, Janssen O, Cerwenka A, Bryceson YT, Watzl C. Surface CD107a/LAMP-1 protects natural killer cells from degranulation-associated damage. Blood. 2013;122:1411–8. doi: 10.1182/blood-2012-07-441832. [DOI] [PubMed] [Google Scholar]
- 66.Ni F, Gui Z, Guo Q, Hu Z, Wang X, Chen D, Wang S. Downregulation of miR-362-5p inhibits proliferation, migration and invasion of human breast cancer MCF7 cells. Oncol Lett. 2016;11:1155–60. doi: 10.3892/ol.2015.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu K, Yang L, Chen J, Zhao H, Wang J, Xu S, Huang Z. miR-362-5p inhibits proliferation and migration of neuroblastoma cells by targeting phosphatidylinositol 3-kinase-C2beta. FEBS Lett. 2015;589:1911–9. doi: 10.1016/j.febslet.2015.05.056. [DOI] [PubMed] [Google Scholar]
- 68.Yang P, Ni F, Deng RQ, Qiang G, Zhao H, Yang MZ, Wang XY, Xu YZ, Chen L, Chen DL, Chen ZJ, Kan LX, Wang SY. MiR-362-5p promotes the malignancy of chronic myelocytic leukaemia via down-regulation of GADD45alpha. Mol Cancer. 2015;14:190. doi: 10.1186/s12943-015-0465-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Ni F, Guo C, Sun R, Fu B, Yang Y, Wu L, Ren S, Tian Z, Wei H. MicroRNA transcriptomes of distinct human NK cell populations identify miR-362-5p as an essential regulator of NK cell function. Sci Rep. 2015;5:9993. doi: 10.1038/srep09993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shifrin N, Raulet DH, Ardolino M. NK cell self tolerance, responsiveness and missing self recognition. Semin Immunol. 2014;26:138–44. doi: 10.1016/j.smim.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ye SB, Li ZL, Luo DH, Huang BJ, Chen YS, Zhang XS, Cui J, Zeng YX, Li J. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget. 2014;5:5439–52. doi: 10.18632/oncotarget.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walsh PT, Buckler JL, Zhang J, Gelman AE, Dalton NM, Taylor DK, Bensinger SJ, Hancock WW, Turka LA. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J Clin Invest. 2006;116:2521–31. doi: 10.1172/JCI28057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma MD, Shinde R, McGaha TL, Huang L, Holmgaard RB, Wolchok JD, Mautino MR, Celis E, Sharpe AH, Francisco LM, Powell JD, Yagita H, Mellor AL, Blazar BR, Munn DH. The PTEN pathway in Tregs is a critical driver of the suppressive tumor microenvironment. Sci Adv. 2015;1:e1500845. doi: 10.1126/sciadv.1500845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol. 2015;16:178–87. doi: 10.1038/ni.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ding G, Zhou L, Qian Y, Fu M, Chen J, Chen J, Xiang J, Wu Z, Jiang G, Cao L. Pancreatic cancer-derived exosomes transfer miRNAs to dendritic cells and inhibit RFXAP expression via miR-212-3p. Oncotarget. 2015;6:29877–88. doi: 10.18632/oncotarget.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou M, Chen J, Zhou L, Chen W, Ding G, Cao L. Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203. Cell Immunol. 2014;292:65–9. doi: 10.1016/j.cellimm.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 77.Lin EY, Pollard JW. Macrophages: modulators of breast cancer progression. Novartis Found Symp. 2004;256:158–68. discussion 68–72, 259–69. [PubMed] [Google Scholar]
- 78.Szebeni GJ, Vizler C, Kitajka K, Puskas LG. Inflammation and Cancer: Extra- and Intracellular Determinants of Tumor-Associated Macrophages as Tumor Promoters. Mediators Inflamm. 2017;2017:9294018. doi: 10.1155/2017/9294018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, Zanesi N, Crawford M, Ozer GH, Wernicke D, Alder H, Caligiuri MA, Nana-Sinkam P, Perrotti D, Croce CM. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109:E2110–6. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cervantes JL, Weinerman B, Basole C, Salazar JC. TLR8: the forgotten relative revindicated. Cell Mol Immunol. 2012;9:434–8. doi: 10.1038/cmi.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Challagundla KB, Wise PM, Neviani P, Chava H, Murtadha M, Xu T, Kennedy R, Ivan C, Zhang X, Vannini I, Fanini F, Amadori D, Calin GA, Hadjidaniel M, Shimada H, Jong A, Seeger RC, Asgharzadeh S, Goldkorn A, Fabbri M. Exosome-mediated transfer of microRNAs within the tumor microenvironment and neuroblastoma resistance to chemotherapy. J Natl Cancer Inst. 2015:107. doi: 10.1093/jnci/djv135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guo XL, Ma NN, Zhou FG, Zhang L, Bu XX, Sun K, Song JR, Li R, Zhang BH, Wu MC, Wei LX. Up-regulation of hTERT expression by low-dose cisplatin contributes to chemotherapy resistance in human hepatocellular cancer cells. Oncol Rep. 2009;22:549–56. doi: 10.3892/or_00000470. [DOI] [PubMed] [Google Scholar]
- 83.Jang JY, Lee JK, Jeon YK, Kim CW. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC Cancer. 2013;13:421. doi: 10.1186/1471-2407-13-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hagemann T, Biswas SK, Lawrence T, Sica A, Lewis CE. Regulation of macrophage function in tumors: the multifaceted role of NF-kappaB. Blood. 2009;113:3139–46. doi: 10.1182/blood-2008-12-172825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fanini F, Fabbri M. Cancer-derived exosomic microRNAs shape the immune system within the tumor microenvironment: State of the art. Semin Cell Dev Biol. 2017;67:23–8. doi: 10.1016/j.semcdb.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eichmuller SB, Osen W, Mandelboim O, Seliger B. Immune Modulatory microRNAs Involved in Tumor Attack and Tumor Immune Escape. J Natl Cancer Inst. 2017:109. doi: 10.1093/jnci/djx034. [DOI] [PubMed] [Google Scholar]
- 88.Alfonsi R, Grassi L, Signore M, Bonci D. The Double Face of Exosome-Carried MicroRNAs in Cancer Immunomodulation. Int J Mol Sci. 2018:19. doi: 10.3390/ijms19041183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Viaud S, Terme M, Flament C, Taieb J, Andre F, Novault S, Escudier B, Robert C, Caillat-Zucman S, Tursz T, Zitvogel L, Chaput N. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PLoS One. 2009;4:e4942. doi: 10.1371/journal.pone.0004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Madhavan B, Yue S, Galli U, Rana S, Gross W, Muller M, Giese NA, Kalthoff H, Becker T, Buchler MW, Zoller M. Combined evaluation of a panel of protein and miRNA serum-exosome biomarkers for pancreatic cancer diagnosis increases sensitivity and specificity. Int J Cancer. 2015;136:2616–27. doi: 10.1002/ijc.29324. [DOI] [PubMed] [Google Scholar]
- 91.Sugimachi K, Matsumura T, Hirata H, Uchi R, Ueda M, Ueo H, Shinden Y, Iguchi T, Eguchi H, Shirabe K, Ochiya T, Maehara Y, Mimori K. Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Br J Cancer. 2015;112:532–8. doi: 10.1038/bjc.2014.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J, Chow A, O’Connor ST, Li S, Chin AR, Somlo G, Palomares M, Li Z, Tremblay JR, Tsuyada A, Sun G, Reid MA, Wu X, Swiderski P, Ren X, Shi Y, Kong M, Zhong W, Chen Y, Wang SE. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17:183–94. doi: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Deng Z, Rong Y, Teng Y, Zhuang X, Samykutty A, Mu J, Zhang L, Cao P, Yan J, Miller D, Zhang HG. Exosomes miR-126a released from MDSC induced by DOX treatment promotes lung metastasis. Oncogene. 2017;36:639–51. doi: 10.1038/onc.2016.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Redzic JS, Ung TH, Graner MW. Glioblastoma extracellular vesicles: reservoirs of potential biomarkers. Pharmgenomics Pers Med. 2014;7:65–77. doi: 10.2147/PGPM.S39768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kai K, Dittmar RL, Sen S. Secretory microRNAs as biomarkers of cancer. Semin Cell Dev Biol. 2017 doi: 10.1016/j.semcdb.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 96.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–5. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 97.Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, Kuroda M. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21:185–91. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P. Delivery of Functional Anti-miR-9 by Mesenchymal Stem Cell-derived Exosomes to Glioblastoma Multiforme Cells Conferred Chemosensitivity. Mol Ther Nucleic Acids. 2013;2:e126. doi: 10.1038/mtna.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kosaka N, Iguchi H, Yoshioka Y, Hagiwara K, Takeshita F, Ochiya T. Competitive interactions of cancer cells and normal cells via secretory microRNAs. J Biol Chem. 2012;287:1397–405. doi: 10.1074/jbc.M111.288662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nguyen J, Szoka FC. Nucleic acid delivery: the missing pieces of the puzzle? Acc Chem Res. 2012;45:1153–62. doi: 10.1021/ar3000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 102.Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest. 2016;126:1216–23. doi: 10.1172/JCI81136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Puzar Dominkus P, Stenovec M, Sitar S, Lasic E, Zorec R, Plemenitas A, Zagar E, Kreft M, Lenassi M. PKH26 labeling of extracellular vesicles: Characterization and cellular internalization of contaminating PKH26 nanoparticles. Biochim Biophys Acta. 2018;1860:1350–61. doi: 10.1016/j.bbamem.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 104.Wiklander OP, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mager I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L, Smith CI, Le Blanc K, Macchiarini P, Jungebluth P, Wood MJ, Andaloussi SE. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bahrami A, Aledavood A, Anvari K, Hassanian SM, Maftouh M, Yaghobzade A, Salarzaee O, ShahidSales S, Avan A. The prognostic and therapeutic application of microRNAs in breast cancer: Tissue and circulating microRNAs. J Cell Physiol. 2018;233:774–86. doi: 10.1002/jcp.25813. [DOI] [PubMed] [Google Scholar]
- 106.Fabbri M. MicroRNAs and miRceptors: a new mechanism of action for intercellular communication. Philos Trans R Soc Lond B Biol Sci. 2018:373. doi: 10.1098/rstb.2016.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mirzaei H, Sahebkar A, Jaafari MR, Goodarzi M, Mirzaei HR. Diagnostic and Therapeutic Potential of Exosomes in Cancer: The Beginning of a New Tale? J Cell Physiol. 2017;232:3251–60. doi: 10.1002/jcp.25739. [DOI] [PubMed] [Google Scholar]