Abstract
Open fractures are characterized by disruption of the skin and soft tissue, which allows for microbial contamination and colonization. Preventing infection-related complications of open fractures and other acute wounds remains an evolving challenge due to an incomplete understanding of how microbial colonization and contamination influence healing and outcomes. Culture-independent molecular methods are now widely used to study human-associated microbial communities without introducing culture biases. Using such approaches, the objectives of this study were to 1) define the long-term temporal microbial community dynamics of open fracture wounds and 2) examine microbial community dynamics with respect to clinical and demographic factors. Fifty-two subjects with traumatic open fracture wounds (32 blunt and 20 penetrating injuries) were enrolled prospectively and sampled longitudinally from presentation to the emergency department and at each subsequent inpatient or outpatient encounter. Specimens were collected from both the wound center and adjacent skin. Culture-independent sequencing of the 16S ribosomal RNA gene was employed to identify and characterize microbiota. Upon presentation to the emergency department and time points immediately following, sample collection site (wound or adjacent skin) was the most defining feature discriminating microbial profiles. Microbial composition of adjacent skin and wound center converged over time. Mechanism of injury most strongly defined the microbiota after initial convergence. Further analysis controlling for race, gender, and age revealed that mechanism of injury remained a significant discriminating feature throughout the continuum of care. We conclude that the microbial communities associated with open fracture wounds are dynamic in nature until eventual convergence with the adjacent skin community during healing, with mechanism of injury as an important feature affecting both diversity and composition of the microbiota. A more complete understanding of the factors influencing microbial contamination and/or colonization in open fractures is a critical foundation for identifying markers indicative of outcome and deciphering their respective contributions to healing and/or complication.
Keywords: Traumatic wound, acute wound, microbiome, 16S rRNA gene, skin, wound healing
INTRODUCTION
Open fractures, which occur either from blunt or penetrating trauma, are characterized by disruption and exposure of the skin and soft tissue and an increased risk for wound infection and delayed healing of the skin and bone [1]. The microbial milieu of a wound may impact both local and systemic defense responses, tissue repair, and clinical outcomes [2]. However, surveillance cultures taken at presentation have little predictive value for downstream complication [3]. A major obstacle to identifying contaminating and colonizing microorganisms in cutaneous wounds observed in orthopaedic trauma is the reliance on culture-based approaches that are standard in clinical practice. Culture-based methods are biased toward microorganisms that readily grow in isolation and under artificial laboratory conditions [4,5].
Culture-independent molecular methods, based on DNA sequencing of the gene encoding the prokaryote-specific 16S ribosomal RNA (rRNA), provide greater resolution while eliminating biases associated with cultivation and isolation procedures [6]. Using these methods, we previously demonstrated that cultures severely underestimate microbial burden and diversity in open fractures and other types of cutaneous wounds [7,8]. Sequencing-based methods may be useful for identifying microbial biomarkers of clinical outcomes, including infection- and healing-related outcomes [7,9,10]. Furthermore, understanding how wound- and patient-level clinical factors vary with microbial colonization may reveal useful indicators of problematic bioburden.
The vast majority of studies to date using culture-independent methods for characterizing wound bioburden have been cross-sectional and in subjects with chronic wounds, where factors such as age and diabetes significantly modify the host and its response to microbial threats [11]. For example, the wound environment of a diabetic foot ulcer will be influenced by neuropathy, altered glucose metabolism, and other pathologies related to diabetes mellitus [9,12]. The nature of open fractures and the inherent continuum of care requiring patient follow-up enable longitudinal study designs. In other types of wounds, longitudinal profiling revealed temporally instable microbial communities, suggesting a major limitation of cross-sectional study designs [9,13,14]. Understanding the temporal dynamics of all components of the acute wound environment, including the microbiota, will improve our ability to effectively manage and treat them.
Here, in a cohort of 52 subjects systematically sampled from presentation in the emergency room and at all possible inpatient and outpatient follow-up visits, we tested the hypothesis that microbial community structure in an open fracture would evolve in a manner depending on a blunt or penetrating mechanism of injury. Sequencing and analysis of the 16S rRNA gene was used to: 1) define the long-term temporal microbial community dynamics of open fracture wounds and adjacent skin, and 2) analyze microbial community diversity, composition, and dynamics with respect to injury mechanism.
MATERIALS AND METHODS
Study Design
Fifty-two patients with open fractures presenting to the University of Pennsylvania Orthopaedic Trauma and Fracture Service were enrolled into the study. The study protocol was reviewed and approved by the University of Pennsylvania Institutional Review Board prior to the start of the patient enrollment. A modification of the informed consent process was approved for this investigation to enable sample collection under emergent conditions. Informed consent was obtained from all subjects enrolled in the study. Catch-All Sample Collection Swabs (Epicentre) were used to swab the wound center and the adjacent skin (5 cm away from wound edge) for microbiota. Samples from all 52 patients were collected at all possible clinical encounters, starting in the emergency department (ED) prior to debridement, irrigation, and cleansing of the wound. Management was consistent across all open fractures, with immediate antibiotic administration (48 hours of intravenous antibiotic including a third generation cephalosporin and an aminoglycoside for type II and III fractures) and early operative debridement (within 8 hours for Type I and within 6 hours for Type II and III wounds). Fractures were stabilized with either external or internal fixation depending on fracture pattern and soft tissue envelope. Closure was performed based on type of wound and the need for subsequent debridements. Samples were additionally collected intraoperatively (OR), as well as during all follow-up office (OF) visits or hospital admissions. Clinical, demographic, and behavioral information was collected for each participant and is summarized in Table 1.
Table 1.
Cohort demographics and descriptive summary of sample dataset
| Age | (#) | (% total) |
|---|---|---|
| Mean | 39 | |
| Range | (18–74) | |
|
| ||
| Sex | ||
| Male | 40 | 76.92% |
| Female | 12 | 23.08% |
|
| ||
| Mode of Injury | ||
| Blunt Trauma | 32 | 61.54% |
| Penetrating Trauma | 20 | 38.46% |
|
| ||
| Race | ||
| Asian | 1 | 1.92% |
| Black | 35 | 67.31% |
| Hispanic | 1 | 1.92% |
| White | 15 | 28.85% |
|
| ||
| Total Samples | 477 | |
|
| ||
| Analyzed Wound Samples | 208 | |
| Emergency Department | 32 | 15.38% |
| Operating Room | 36 | 17.31% |
| Office / Follow-up | 140 | 67.31% |
|
| ||
| Analyzed Skin Samples | 269 | |
| Emergency Department | 41 | 15.24% |
| Operating Room | 59 | 21.93% |
| Office / Follow-up | 169 | 62.83% |
|
| ||
| Blunt Patient Samples | 330 | |
| Wound Samples | 145 | 43.94% |
| Skin Samples | 185 | 56.06% |
| Visits (Mean) | 6.21 | |
| Visits (Median) | 6.5 | |
|
| ||
| Penetrating Patient Samples | 147 | |
| Wound Samples | 63 | 42.86% |
| Skin Samples | 84 | 57.14% |
| Visits (Mean) | 4.3 | |
| Visits (Median) | 3.5 | |
|
| ||
| All Visits | ||
| Mean | 5.48 | |
| Range | (1–12) | |
|
| ||
| Enrollment Time (days) | ||
| Mean | 176.13 | |
| Range | (1–560) | |
|
| ||
| Affected Bone | ||
| Tibia/Fibula | 33 | 63.46% |
| Femur/Patella | 11 | 21.15% |
| Humerus | 5 | 9.62% |
| Radius/Ulna | 2 | 3.85% |
| Calcaneus | 1 | 1.92% |
Sequencing and Processing of Microbial Reads
DNA was extracted from swabs as previously described [7,8]. Amplification of the V1-V3 region of the bacterial 16S rRNA gene was performed as described previously [15]. Sequencing was performed at the University of Pennsylvania Next Generation Sequencing Core using V3 chemistry and 300 bp paired-end reads. Controls included: 1) genomic DNA of a mock community of 20 bacterial isolates in even concentration (BEI Resources, Even Low Concentration, v5.1L) and 2) negative controls processed through the DNA extraction, amplification, and sequencing pipeline exactly as swab specimens. Sequences were demultiplexed, quality filtered, and paired ends were assembled using PEAR [16]. Reads between 465 and 535 nucleotides long were retained. A total of 8,627,005 paired-end sequences were considered in the analysis with an average of 18,086 sequences and a median of 15,706 sequences per sample. To normalize sequence counts across samples, a single random subsampling of 1,500 sequences was selected from each sample after singletons had been filtered out to maximize counts while also retaining the most possible samples. To verify adequate community coverage of the filtered data, we calculated Good’s coverage on samples in a similar rarefied dataset with a depth of 1,500 sequences per sample drawing from all reads, including singletons that were filtered out in our main analyses [17]. Good’s coverage estimations yielded an average of 0.932 with a standard deviation of 0.060, indicating that we were capturing most of the community when subsampling at a low enough level to retain the most samples for analysis. Sequence alignment, operational taxonomic unit (OTU) clustering, and taxonomic assignment were performed with the QIIME software package [17]. Raw sequences and metadata are publicly available in the NCBI Short Read Archive (SRA) at BioProject Accession: PRJNA386669; the subsampled dataset of taxonomically assigned reads used in the analysis can be retrieved from FigShare, DOI: 10.6084/m9.figshare.6139841.
Statistical Analysis
The R computing package was used for data processing and statistical analyses. Principal coordinates analyses (PCoA) were performed using the LabDSV R package to reduce the high dimensionality of the data and easily visualize distances based on the most differentiating features of the bacterial communities [18]. The axes (PC1 and PC2) visualized from PCoA analyses were determined by the two eigenvectors explaining the most variance within a multivariate matrix, as described in a 2003 review by Anderson and Willis [19]. ANOSIM and PERMANOVA tests were employed to investigate the significance of associations between factors and community structure. The ANOSIM test was used in conjunction with the PERMANOVA test to control for differences in assumptions and null hypotheses tested by each method [20,21]. ANOSIM and PERMANOVA tests were performed with functions from the vegan R package and used 999 permutations to calculate p-values [22]. Wilcoxon rank-sum tests were utilized for non-parametric comparisons of the relative abundance of taxa between groupings of samples to produce p-values that were then adjusted for multiple testing with the Benjamini Hochberg false discovery rate (FDR) [23]. Bacterial genera were included in these tests if they comprised an average relative abundance of at least 0.1% in the dataset being considered. The genus level of identified bacteria was used for comparing relative abundances of taxa. Plotting of data was performed with the ggplot2 R package [24].
RESULTS
Defining open fracture wound and adjacent skin microbial colonization at presentation
Fifty-two subjects were enrolled into a longitudinal, prospective, cohort study to evaluate the microbiota colonizing traumatic open fracture wounds using culture-independent methods. Table 1 summarizes the demographic and clinical status of the cohort. All 20 penetrating open fracture wounds were caused by gunshots while vehicular crashes, bike accidents, and falls caused 30 of the 32 blunt open fracture wounds; 2 patients with blunt open fracture wounds did not disclose the specifics of their injury. Microbiota samples were collected at presentation to the Emergency Department (ED), intraoperatively, and at each outpatient follow up visit and/or inpatient admission. Swab samples were collected from the center of the wound and from the normal, unobscured skin 5 cm adjacent to the wound edge.
To set a baseline for longitudinal analyses, we first compared the microbiota of the wound center to the adjacent skin upon patient presentation to the ED. The distances between sample communities, as measured by the weighted UniFrac metric, which takes into account the different bacterial lineages present and their abundances, were calculated to broadly compare microbial community structure [25]. Skin and wound center microbiota were significantly different, as determined by a PERMANOVA test (p < 0.005) (Fig. 1A). Diversity, as measured by Faith’s Phylogenetic Diversity (PD) metric [26], was significantly reduced in the wound center compared to the intact adjacent skin (p < 0.005; Fig. 1B). The genus-level taxa Corynebacterium, Staphylococcus, Finegoldia (q < 0.01), and Anaerococcus (q < 0.05) all were found in significantly higher relative abundances in adjacent skin compared to the wound center (Fig. 1C).
Figure 1. Microbial communities of open fractures and adjacent skin at presentation to the emergency department.
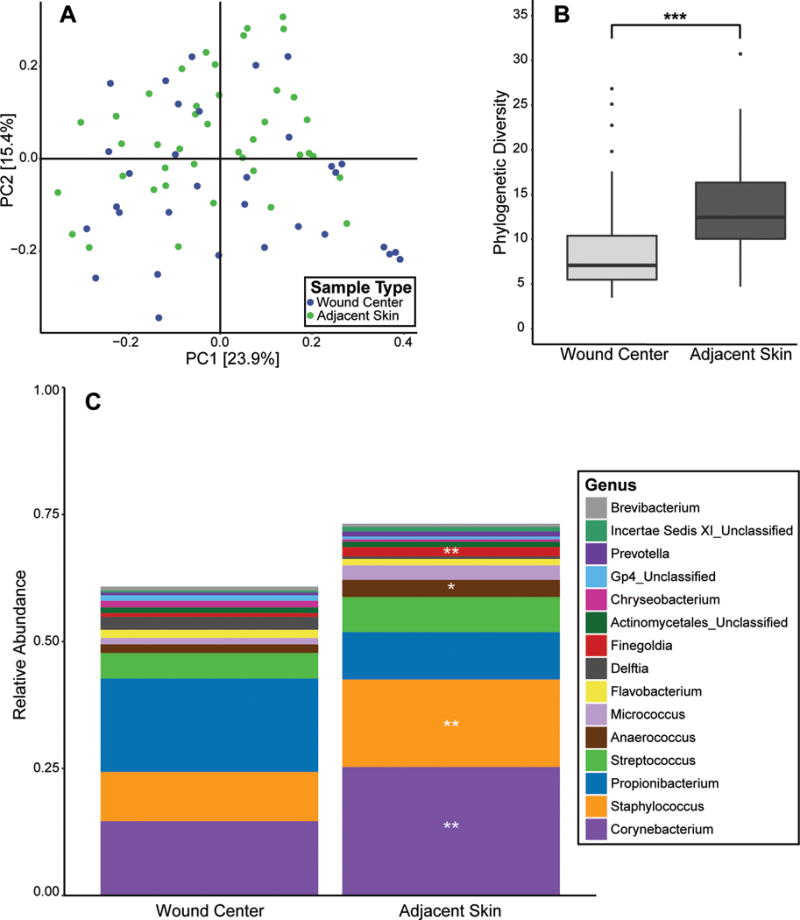
(A) PCoA of the weighted UniFrac inter-sample distances. Each point represents a single patient specimen at the ED time point; one wound sample and one adjacent skin sample from each patient are displayed where data is available. Distance between points (specimens) is indicative of similarity/dissimilarity to other points (specimens). Color indicates sample type (green=skin; blue=wound center). Shown are the first 2 principle coordinates (PC1 and PC2), and percent variance explained by each coordinate is indicated in parentheses by the axis. (B) Comparison of median microbial diversity as measured by Faith’s Phylogenetic diversity metric (y-axis) in wound center and skin specimens (x-axis). A higher metric value indicates higher microbial diversity. Upper and lower box hinges correspond to first and third quartiles, and the distance between these quartiles is defined as the interquartile range (IQR). Lines within the box depict median, and whiskers extend to the highest and lowest values within 1.5 times the IQR. Outliers of the IQR are depicted as dots above or below the whiskers. ***p < 0.005. (C) Average relative abundance/proportion (y-axis) of genus-level taxa in open fracture wounds and adjacent skin (x-axis). Each color represents a different genus-level taxa (identified in the corresponding legend) and its average proportion in the overall sample type indicated. Significant increases are indicated as **q < 0.01; *q < 0.05.
Association of wound microbial community structure with clinical factors
Because the pilot study we previously conducted identified mechanism of injury, severity, and location as significant factors associated with microbial diversity and/or composition [8], we further investigated these associations. Neither injury location nor severity (as measured by the Gustilo-Anderson classification [27]), were significantly associated with microbial community composition, Faith’s Phylogenetic Diversity, or community structure as measured by weighted UniFrac distance of samples collected at the ED time point. There were significant differences in microbial communities when comparing mechanism of injury between blunt and penetrating wounds (p < 0.05; Fig. 2A). The adjacent skin microbiota in blunt force open injuries was more diverse than the wound center microbiota (p < 0.01; Fig. 2B). Relative abundance of Staphylococcus, Anaerococcus, Finegoldia (q < 0.05), and Peptoniphilus (q < 0.01) were significantly increased in the skin community compared to the wound (Fig. 2C). In penetrating injuries, the adjacent skin samples appeared to be qualitatively more diverse than the wound center samples, but the difference was not statistically significant (p = 0.079; Fig. 2B). Only the relative abundance of Corynebacterium (q < 0.05) significantly differentiated adjacent skin from wound microbiota in penetrating injuries (Fig. 2C). Corynebacterium was also significantly elevated in the skin communities of penetrating injuries compared to the skin of blunt injuries (q < 0.01). These findings suggest that even at presentation to the ED, mechanism of injury differentiates the microbiota colonizing open fracture wounds and adjacent skin.
Figure 2. Microbial communities of open fractures differs by mechanism of injury at presentation to the ED.
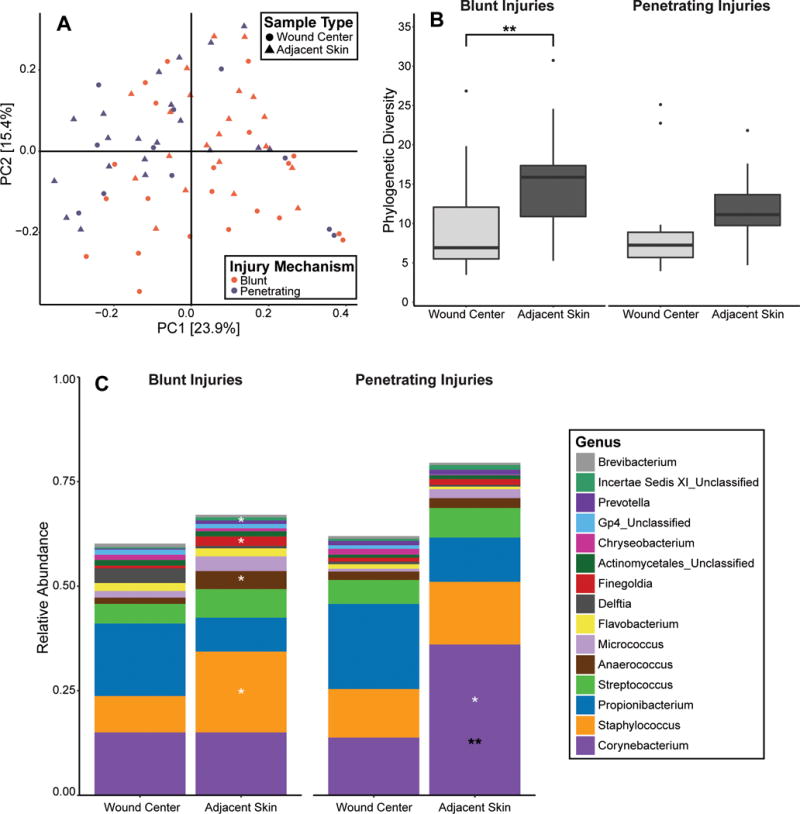
(A) PCoA of the weighted UniFrac inter-sample distances. Each point represents a single patient specimen at the ED time point; one wound sample and one adjacent skin sample from each patient are displayed where data is available. Color indicates mechanism of injury (purple=penetrating; orange=blunt) and shape indicates the sample type (circle=wound center; triangle=adjacent skin). Shown are the first 2 principle coordinates, and percent variance explained by each coordinate is indicated in parentheses by the axis. (B) Alpha diversity, as measured by Faith’s Phylogenetic Diversity index (Y-axis). Blunt and penetrating injuries are depicted on the left and right panels, respectively. Boxplots were generated according to the methods outlined in Figure 1. **p<0.01. (C) Average relative abundance of bacterial genera (y-axis) by sampling site and mechanism of injury. Significant differences in adjacent skin microbiota according to mechanism of injury are indicated by black asterisks. Significant differences between adjacent skin and wound center samples within each injury category are indicated by white asterisks. *q<0.05; **q<0.01
Microbial composition associated with mechanism of injury
During outpatient follow up visits, blunt and penetrating wound microbial communities remained significantly distinct by the weighted UniFrac metric (p = 0.001) as seen in Figure 3. Wound center samples continued to differ from skin samples (p < 0.05), but sample site was less defining than mechanism of injury at these later time points. These findings were apparent in a PCoA of the sample communities (Fig. 3). The first principal coordinate axis (PC1) visually separated the groups by mechanism of injury explaining 23.1% of the variance between all the samples. Comparatively, the second principal coordinate axis (PC2), which visually separates the wound center samples from the adjacent skin samples within each mechanism of injury, explains 11.1% of the variance. In order to rule out potential discrepancies between standards of care between blunt and penetrating wounds, we tested for an association of severity of the open fractures, which could dictate the extent of treatment, with mechanism of injury, and did not find it to be significant (p = 0.68). More specifically, >70% of the wounds caused by each mechanism of injury were assigned a score of IIIA on the Gustilo-Anderson scale [27].
Figure 3. Microbial communities diverge based on mechanism of injury.

A PCoA of the weighted UniFrac distances between all samples collected at office follow up visits. Points are colored by mechanism of injury, and circles represent the wound center while triangles represent the adjacent skin. The centroids represent the average coordinates of samples falling within 4 categories (Blunt Wound, Blunt Adjacent Skin, Penetrating Wound, and Penetrating Adjacent Skin) and can be seen as larger points along with error bars displaying the variance of each grouping. Each centroid contains two error bars, representing the variances along both principal coordinate axes of each group of samples.
All patients with penetrating injuries racially identified as black, whereas subjects with blunt injuries constituted a racially mixed cohort. To address this potentially confounding factor, we performed a separate analysis that included only black subjects and found that the mechanism of injury was still highly significant in separating samples by both ANOSIM and PERMANOVA tests (p = 0.001). When comparing the two most prevalent races in our study, black and white, in the subset of only blunt injuries, race was a less significant factor by the PERMANOVA test (p = 0.005) and not significant by an ANOSIM test (p = 0.068). All subjects with penetrating injuries also identified as male, which we addressed by comparing the communities of both genders in blunt injury patients. Although gender was found to be significant in differentiating samples from blunt injury patients by a PERMANOVA test (p = 0.001), it was not significant by ANOSIM (p = 0.841). The mechanism of injury in only male subjects was found to be highly significant by both ANOSIM and PERMANOVA tests (p = 0.001), further recapitulating our overall finding. Moreover, we further restricted our dataset to only include subjects identifying as black males, yielding a subset of 6 patients with blunt injuries and 20 patients with penetrating injuries. Mechanism of injury was again found to be significant in this much smaller subset of 26 patients, indicating both that our findings were robust and demographics were not biasing our overall conclusions (p = 0.001).
The average age of subjects with penetrating injuries was significantly lower than that of subjects with blunt injuries (p < 0.001). We tested age-related microbial associations by binning office follow-up visits into two groups based on the age of the subject at time of admission to the hospital; 18–26 years old and 27–43 years old. These bins were selected to include the most subjects while still maintaining similar compositions of subjects with different mechanisms of injury in each bin. Independent of mechanism of injury, subjects in the older group had significantly higher relative abundances of Anaerococcus and Finegoldia (q < 0.05) in the adjacent skin samples and no differentially abundant genera in the wound center when compared to subjects in the younger group. In penetrating injuries, only Propionibacterium differed significantly, being present at a lower relative abundance in adjacent skin samples of older subjects compared to younger subjects (q < 0.05). Conversely, when comparing mechanism of injury in the younger group, penetrating injuries had higher relative abundances of Corynebacterium (q < 0.05) and lower relative abundances of Staphylococcus (q < 0.05) in adjacent skin samples. In the older group, penetrating injuries had higher relative abundances of Corynebacterium in both adjacent skin and wound center samples (q < 0.001) as well as lower relative abundances of Clostridium (q < 0.05) in wound center samples.
Time-dependent dynamics of open fracture wound microbiota
Temporal dynamics and convergence between wound and skin microbiota were calculated with weighted UniFrac distances between the wound center and adjacent skin at each sampling date for each patient. Local polynomial regression (LOESS) was performed on paired sample distances for each mechanism of injury, resulting in two distinct regression lines with respective confidence curves representing the 95% confidence interval of the regression (Fig. 4A). The regressions were performed on 382 samples (or 191 paired adjacent skin and wound center samples), with the inclusion criterion requiring at least two visits with paired samples per subject. 44 subjects met this criterion; 29 with blunt injuries contributing 133 paired samples and 15 with penetrating injuries contributing 58 paired samples. The 44 subjects had an average and median of 5.5 visits included in the analysis with a range of 2 to 9 visits.
Figure 4. Microbial community convergence of the wound center and adjacent skin over time.
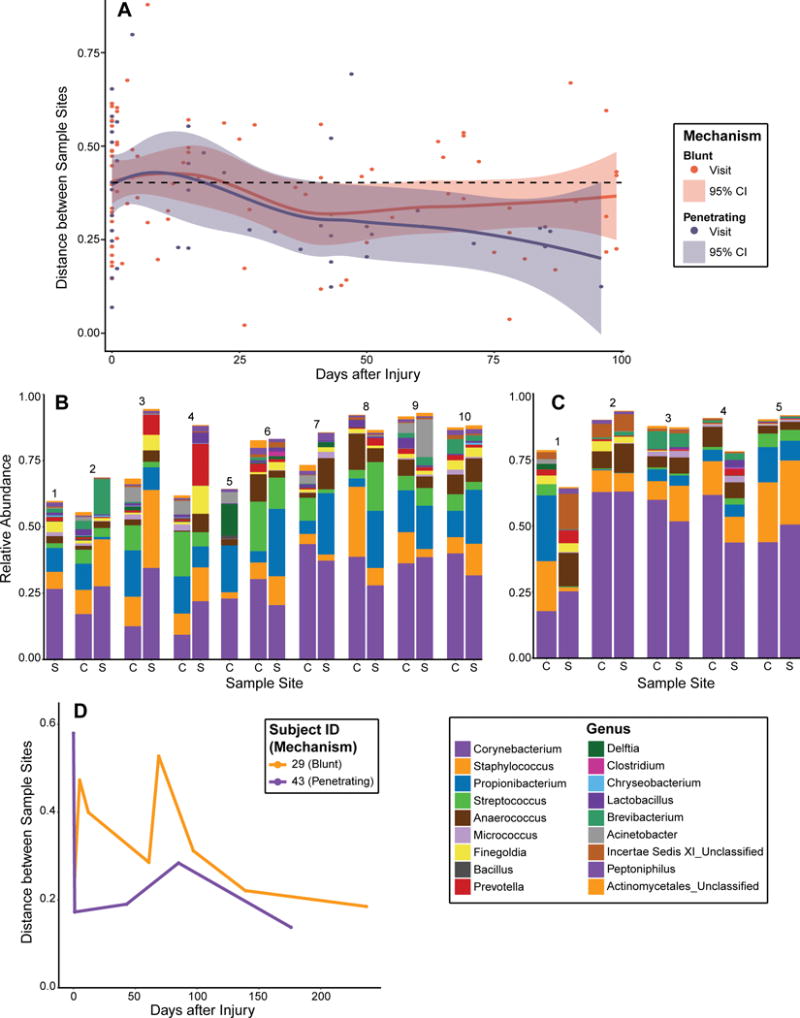
(A) Scatterplot of weighted UniFrac distances between the wound center and adjacent skin microbial communities (Y-axis) over time (X-axis). Purple and orange dots indicate distances between each patient’s skin-wound pair for penetrating and blunt injuries, respectively. Similarly, purple and orange lines represent a Loess curve fit through the points of penetrating and blunt injuries, respectively. Shading represents 95% confidence intervals of the Loess curves. By 100 days following hospital admission for the injury, wound microbiota in penetrating wounds, became more similar in composition to adjacent skin microbiota. The average distance at presentation to the ED was 0.403, indicated as a dashed horizontal line. (B and C) Relative abundance of microbiota colonizing wound center (“C”) and adjacent skin (“S”) in (B) Subject 29 who suffered a blunt injury, and (C) Subject 43 who suffered a penetrating injury. X-axis depicts sequential time points at which microbiota was sampled. (D) Convergence between the adjacent skin and wound microbiota for subjects 29 and 43. Weighted UniFrac distances between skin and wound samples were calculated (y-axis) and plotted over time (x-axis) for each subject. Subject 29 (blunt injury) is depicted in orange; Subject 43 (penetrating injury) is depicted in purple.
In penetrating injuries, the upper bound of the Loess line confidence interval dropped below the baseline distance between all paired samples by day 45, indicating that the wound center and adjacent skin microbiota had converged to more similar microbial communities from the initial sampling. Over the 100 days following initial presentation, the confidence interval of the regression remained below the initial sampling distance.
In blunt injuries, the upper bound of the confidence interval begins to closely trace the baseline distance between paired samples near day 45 as well, but then begins to increase with respect to the confidence interval of the penetrating paired samples, demarcating a qualitative divergence between penetrating and blunt wounds. This divergence demonstrated a delayed convergence between the communities of the adjacent skin and the wound in blunt wounds compared to penetrating wounds. Although the distance appeared to increase at later time points, this trend reflects a decrease in total patients still receiving treatment, which produces a larger confidence interval due to the corresponding decrease in paired sample distances on which to regress, as well as bias from open fractures requiring later follow-ups due to complications or delayed healing.
The convergence between skin and wound microbiota was further illustrated upon examining individual patient profiles of microbial temporal dynamics. Subject 29 suffered a blunt trauma open fracture and exhibited high levels of divergence between skin and wound microbial communities until ~100 days post-injury. Both wound and skin microbiota were unstable at initial time points, with blooms of genera such as Prevotella and Brevibacterium that are rarely found on healthy skin (Fig. 4B). Relative abundance of Corynebacterium gradually increased over time, until the last two visits in which the wound remained fairly stable and more similar in composition to the adjacent skin microbiota. This is in contrast to Subject 43 who sustained a penetrating open fracture. The skin and wound center are markedly different in samples collected at the ED, but then converged quickly during follow up time points (Fig. 4C). This is reflected by the community composition shifting towards a more frequently observed skin-like microbiota consortium, as evident in the increase in relative abundance of Propionibacterium and Streptococcus. These trends are further illustrated by examining longitudinal weighted UniFrac distances of the two subjects (Fig. 4D).
DISCUSSION
Here, we show that acute wounds resulting from open fracture are colonized with microbiota distinct from that of the adjacent skin at presentation to the emergency department (ED), followed by a convergence of wound microbiota with skin microbiota over time. Additionally, we found that mechanism of injury was associated with the microbiota as early as presentation to the ED and continued to distinguish microbiota throughout the course of healing. We further observed a faster rate of convergence of wound and skin microbiota in injuries caused by penetrating trauma compared to blunt trauma.
Our finding of temporally converging wound and adjacent skin microbiota, while to some extent expected, may be indicative of the healing process as the wound center begins to attain a microbial community comprised of skin commensal bacteria. Few studies have measured the dynamics of wound microbiota through longitudinal, serial sampling of the wound. Our previous study of 100 patients with chronic diabetic foot ulcers underscored the importance of longitudinal sampling to measure microbial community dynamics with respect to the tissue environment, and stability was predictive of wound outcomes [9]. In the present study, collection of a corresponding skin specimen adjacent to the open fracture wound allowed us to measure the convergence of these two distinct microenvironments while controlling for interpersonal variation of the microbiota. Although our study intended to further associate convergence of the microbial communities with complications such as nonunions and infections, our cohort only contained two patients with observed and recorded nonunions and three patients with confirmed infections, two of which recovered from their infections within 2 visits or less. We similarly did not find any significant associations between other complications and factors such as demographics or mechanism of injury given the sparse appearances of complications.
We analyzed microbiota with respect to clinical factors that are associated with outcomes of open fractures: injury location, mechanism, and severity. Mechanism of injury robustly associated with microbiota, immediately following injury and temporally. We found a discrepancy in the rate at which convergence occurs between blunt and penetrating wounds. Thus, we conclude that mechanism of injury may dictate a distinct microbial community structure and also alter the time-dependent community dynamics. These findings were robust even after controlling for age, race, and sex in this cohort. We speculate that differences in wound tissue, such as nutrient availability and oxygenation, may drive differences in communities colonizing blunt and penetrating wounds. Alternatively, the surrounding skin microbiota may also contribute in part to these differences. Further research is needed to define the mechanisms whereby microbial community succession occurs in the acute wound environment.
The findings presented here will be instrumental in guiding future work to directly assess the association between the microbiota and outcomes of open fracture wounds. First, we demonstrate the importance of longitudinal study designs in capturing microbial community dynamics. Cross-sectional sampling of the microbiota can only provide a snapshot of an ever-changing microbial landscape. Second, future studies to directly associate outcomes with the microbiome will need to control for mechanism of injury since this variable is associated with the diversity and composition of colonizing microbiota. Moreover, we demonstrate the utility of collecting a sample of the adjacent skin as a baseline by which to measure the convergence of the wound microbiota while controlling for interpersonal variation.
Acknowledgments
We thank Qi Zheng for assistance in processing sequencing reads; the Penn Orthopaedic Trauma and Fracture Service faculty, residents, and staff for assistance in collection of swab samples and gathering patient information.
Source of Funding
Funding was provided by the Orthopaedic Trauma Association, grant #029 (SM). EAG is supported by the National Institutes of Health (NIAMS R00AR060873, NIAMS R01AR066663 and NINR R01NR015639). CBM was supported by a training grant to the University of Pennsylvania Department of Dermatology (NIAMS/NIH T32AR007465) and GDH was supported by the US Department of Defense National Defense Science and Engineering Graduate Fellowship. The content is solely the responsibility of the authors and does not necessarily represent the official view of the funding bodies.
Footnotes
Conflict of interest disclosure: The authors listed in this manuscript have no conflicts of interest to declare.
References
- 1.Harley BJ, Beaupre LA, Jones CA, Dulai SK, Weber DW. The effect of time to definitive treatment on the rate of nonunion and infection in open fractures. J Orthop Trauma. 2002;16(7):484–90. doi: 10.1097/00005131-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Grice EA, Segre JA. Interaction of the microbiome with the innate immune response in chronic wounds. Adv Exp Med Biol. 2012;946:55–68. doi: 10.1007/978-1-4614-0106-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hannigan GD, Pulos N, Grice EA, Mehta S. Current concepts and ongoing research in the prevention and treatment of open fracture infections. Adv Wound Care. 2015;4(1):59–74. doi: 10.1089/wound.2014.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Misic AM, Gardner SE, Grice EA. The wound microbiome: modern approaches to examining the role of microorganisms in impaired chronic wound healing. Adv Wound Care. 2014;3(7):502–10. doi: 10.1089/wound.2012.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajilić‐Stojanović M, Smidt H, De Vos WM. Diversity of the human gastrointestinal tract microbiota revisited. Environ Microbiol. 2007;9(9):2125–36. doi: 10.1111/j.1462-2920.2007.01369.x. [DOI] [PubMed] [Google Scholar]
- 6.Muyzer G, De Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59(3):695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner SE, Hillis SL, Heilmann K, Segre JA, Grice EA. The neuropathic diabetic foot ulcer microbiome is associated with clinical factors. Diabetes. 2013;62(3):923–30. doi: 10.2337/db12-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hannigan GD, Hodkinson BP, McGinnis K, Tyldsley AS, Anari JB, Horan AD, et al. Culture‐independent pilot study of microbiota colonizing open fractures and association with severity, mechanism, location, and complication from presentation to early outpatient follow‐up. J Orthop Res. 2014;32(4):597–605. doi: 10.1002/jor.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loesche M, Gardner SE, Kalan L, Horwinski J, Zheng Q, Hodkinson BP, et al. Temporal stability in chronic wound microbiota is associated with poor healing. J Invest Dermatol. 2017;137(1):237–44. doi: 10.1016/j.jid.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scales BS, Huffnagle GB. The microbiome in wound repair and tissue fibrosis. J Pathol. 2013;229(2):323–31. doi: 10.1002/path.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736–43. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 12.Mayfield JA, Reiber GE, Sanders LJ, Janisse D, Pogach LM. Preventive foot care in people with diabetes. Diabetes care. 2003;26(1 Suppl):S78–9. doi: 10.2337/diacare.26.2007.s78. [DOI] [PubMed] [Google Scholar]
- 13.Tipton CD, Mathew ME, Wolcott RA, Wolcott RD, Kingston T, Phillips CD. Temporal dynamics of relative abundances and bacterial succession in chronic wound communities. Wound Repair Regen. 2017;25(4):673–79. doi: 10.1111/wrr.12555. [DOI] [PubMed] [Google Scholar]
- 14.Sprockett DD, Ammons CG, Tuttle MS. Use of 16S rRNA sequencing and quantitative PCR to correlate venous leg ulcer bacterial bioburden dynamics with wound expansion, antibiotic therapy, and healing. Wound Repair Regen. 2015;23(5):765–71. doi: 10.1111/wrr.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meisel JS, Hannigan GD, Tyldsley AS, SanMiguel AJ, Hodkinson BP, Zheng Q, Grice EA. Skin microbiome surveys are strongly influenced by experimental design. J Invest Dermatol. 2016;136(5):947–56. doi: 10.1016/j.jid.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Kobert K, Flouri T, Stamatakis A. PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics. 2014;30(5):614–20. doi: 10.1093/bioinformatics/btt593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts DW. labdsv: Ordination and multivariate analysis for ecology. Version 1.8-0 [software] 2016 Available from: https://cran.r-project.org/package=labdsv.
- 19.Anderson MJ, Willis TJ. Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology. 2003;84(2):511–25. [Google Scholar]
- 20.Anderson MJ, Walsh DC. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: what null hypothesis are you testing? Ecol Monogr. 2013;83(4):557–74. [Google Scholar]
- 21.Lek E, Fairclough DV, Platell ME, Clarke KR, Tweedley JR, Potter IC. To what extent are the dietary compositions of three abundant, co‐occurring labrid species different and related to latitude, habitat, body size and season? J Fish Biol. 2011;78(7):1913–43. doi: 10.1111/j.1095-8649.2011.02961.x. [DOI] [PubMed] [Google Scholar]
- 22.Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al. vegan: Community Ecology Package. Version 2.4-4[software] 2017 Available from: https://cran.r-project.org/package=vegan.
- 23.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57(1):289–300. [Google Scholar]
- 24.Wickham H. ggplot2: elegant graphics for data analysis. Springer. 2016 [Google Scholar]
- 25.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71(12):8228–35. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faith DP. Conservation evaluation and phylogenetic diversity. Biol Conserv. 1992;61(1):1–0. [Google Scholar]
- 27.Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58(4):453–8. [PubMed] [Google Scholar]


