Abstract
Objective
Gut microbiota-dependent metabolites, in particular trimethylamine N-oxide (TMAO), have recently been reported to promote atherosclerosis and thrombosis. Here, we examined for the first time the relation of TMAO and the risk of incident cardiovascular events (CVE) in patients with recent first-ever ischemic stroke in two independent prospective cohorts. Moreover, the link between TMAO and proinflammatory monocytes as a potential contributing factor for cardiovascular risk in stroke patients was studied.
Approach and Results
In a first study (n=78), higher TMAO plasma levels were linked with an increased risk of incident CVE including myocardial infarction, recurrent stroke and cardiovascular death (Quartile (Q)4 vs Q1; HR: 2.31; 95% CI: 1.25–4.23; P<0.01). In the second independent validation cohort (n=593), high TMAO levels again heralded marked increased risk of adverse CVE (Q4 vs Q1; HR: 5.0; 95% CI: 1.7–14.8; P<0.01), and also after adjustments for cardiovascular risk factors including hypertension, diabetes, LDL cholesterol and eGFR (HR: 3.3 95% CI: 1.2–10.9; P=0.04).
A significant correlation was also found between TMAO levels and percentage of proinflammatory intermediate CD14++CD16+ monocytes (r=0.70; P<0.01). Moreover, in mice fed a diet enriched with choline to increase TMAO synthesis, levels of proinflammatory murine Ly6Chigh monocytes were higher than in the chow-fed control group (choline: 9.2±0.5 x 103 per ml vs. ctr.: 6.5±0.5 x 103 per ml; P<0.01). This increase was abolished in mice with depleted gut microbiota (choline + ABS: 5.4±0.7 x 103 per ml; P<0.001 vs. choline).
Conclusions
The present study demonstrates for the first time a graded relation between TMAO levels and the risk of subsequent CVE in patients with recent prior ischemic stroke. Our data support the notion that TMAO-related increase of proinflammatory monocytes may add to elevated cardiovascular risk of patients with increased TMAO levels.
Keywords: gut microbiota, trimethylamine N-oxide, stroke, cardiovascular risk, proinflammatory monocyte
Introduction
The risk of secondary cardiovascular events (CVE) varies across the spectrum of patients with ischemic stroke. Despite guideline-based optimal treatment of patients with TIA and stroke including urgent management in specialized units, broad diagnostic work-up, rapid treatment with antithrombotic agents and other stroke-prevention strategies, recent studies suggest a considerable residual risk of 6.2% for CVE and 5.1% for stroke within 1-year follow-up1. In view of these data, there is still great need to identify additional factors impacting the patients’ risk beyond traditional risk factors that may help to more accurately estimate patient vulnerability for subsequent CVE improving preventive concepts.
Recently, an important contribution of gut microbiota in pathobiological processes including metabolic2, immunological3, and cardiovascular diseases4 has been suggested. Notably, metabolomics combined with mechanistic animal model studies have led to a proposed potential link between dietary nutrients, such as choline and carnitine, and their gut microbiota-dependent metabolism to trimethylamine N-oxide (TMAO), and atherosclerosis4, 5. There is considerable evidence linking the metaorganismal TMAO pathway to the development of atherosclerosis and thrombosis in animal models and incident cardiovascular disease risks. In particular, proatherogenic effects of microbe-dependent TMAO formation include enhanced macrophage cholesterol accumulation and subsequent foam cell formation4, proinflammatory changes in the artery wall6, and promotion of both platelet hyperreactivity and enhanced arterial thrombosis potential7.
With regard to recent observations on the relation between TMAO and the risk for CVE in patients with acute coronary syndrome8 we sought to evaluate the prognostic value of TMAO levels in patients with recent first ever ischemic stroke and investigate a potential interaction between TMAO and proinflammatory circuits that promote thrombotic complications of patients at high risk.
In recent years a critical role of monocyte subsets with distinct expressions of the lipopolysaccharide (LPS) receptor CD14 and the FcγIII receptor CD16 has been unveiled for formation, propagation and vulnerability of atherosclerotic lesions9, 10. In particular the intermediate CD14++CD16+ monocytes have been identified to independently predict future cardiovascular events in large patient cohorts of coronary artery disease, chronic kidney disease and peripheral artery disease11–13. The intermediate CD14++CD16+ monocytes are considered as a subset with particular proinflammatory functions14 known to secrete high amounts of inflammatory cytokines, such as TNF-α, upon activation15. In patients with ischemic stroke the increased number of the intermediate monocyte subset has been strongly correlated with the progression and severity of brain infarction16. Based on advanced cluster analysis the intermediate subset has been closely related to proinflammatory murine Ly6Chigh monocytes10, 15, 17 allowing to investigate the biology of these monocytes in experimental settings.
Here, we evaluated for the first time the relationship between circulating TMAO levels and incident CVE in two independent prospective cohorts of patients with ischemic stroke. Moreover, we examined the relation between TMAO and the level of proinflammatory monocyte subsets as a potential contributing factor to trigger cardiovascular events both in patients and in a mouse model of antibiotics-induced depletion of the gut microbiota. The results may provide novel insights into the relationship between the gut microbiota-related metabolite TMAO, proinflammatory monocytes and clinical prognosis in patients with stroke.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. Details of the major resources and detailed methods can be found in the Supplemental Material.
Study populations and study design
Two prospective cohorts of patients with first-ever ischemic stroke were examined. The primary outcome measure of both cohorts was a combined vascular end-point composed of myocardial infarction (MI), recurrent stroke and cardiovascular death during 1-year follow-up on a structured interview with the patient or their relatives and, if indicated, with additional information from the hospital or physician.
The inclusion criteria of both studies were: (1) Age 18 years; (2) first ever acute stroke that occurred with stroke onset in the last seven-days and (3) written informed consent by patient or legal guardian prior to study participation. The exclusion criteria were: (1) Prior stroke; (2) patients presenting brain tumor or brain metastasis and (3) participation in an intervention study. Both studies were approved by the respective local research ethics committee. All participants with adjudicated diagnosis of ischemic stroke provided written informed consent and were prospectively enrolled. For evaluation of acute ischemic stroke, diffusion weighted magnetic resonance imaging (Siemens Verio 3.0 Tesla) was performed.
In the first cohort, 78 patients with complete data on primary outcome were enrolled. The relation of the gut microbe-dependent metabolite TMAO with the risk of subsequent (1-year) cardio- and cerebrovascular events (CVE) including myocardial infarction, recurrent stroke and cardiovascular death was examined.
After finding a significant relation between TMAO with the cardiovascular risk in the first cohort, a second larger independent validation cohort including 608 patients from the ‘Prospective Cohort with Incident Stroke’ study (PROSCIS) with first-ever stroke conducted at the Center for Stroke Research Berlin in Germany (PROSCIS-B: Center for Stroke Research Berlin, Charité University Hospital) was examined with 1-year of follow-up to evaluate if the observed relation persists in an independent non-overlapping patient population. Study protocol and design of the PROSCIS study were described previously18.
Blood tests
Laboratory workup was performed for total cholesterol, low- and high-density lipoproteins, triglyceride, C-reactive protein, and full blood count. Blood samples were immediately processed and frozen at −80°C for further analyses. Levels of choline and TMAO were quantified by stable isotope dilution liquid chromatography with online tandem mass spectrometry as previously described19, using a Shimadzu LCMS-8050 CL Triple Quadrupole Mass Spectrometer interfaced with a Nexera LC-30AD CL Ultra High Performance Liquid Chromatograph (UHPLC) system.
Analysis of monocyte subsets in humans
Flow cytometry was used to determine human monocyte subsets as previously described20. Blood samples were taken by venipuncture and transferred immediately on ice to the lab and prepared directly without time delay for flow cytometry analysis (antibodies are shown in the major resources table in the Supplemental Material). After pre-selection (side scatter and forward scatter) monocytes were identified as HLA-DR positive and classified according to CD14 and CD16 expression: CD14++CD16− as classical monocytes, CD14+CD16++ as non-classical monocytes and CD14++CD16+ as intermediate monocytes. Detailed gating strategy for identification of monocyte subsets is shown in Supplemental Figure I.
Mouse experiments
All animals were bred, raised and housed in the facilities of the “Forschungseinrichtungen für Experimentelle Medizin” (FEM, Charité – University Medicine Berlin, Germany) under specific pathogen-free (SPF) conditions. Adult (10 weeks of age) C57BL/6J mice (Charles River) were placed on normal chow (0.2% choline) or choline supplemented diets (1.3% choline) (Ssniff, Soest, Germany) for 3 weeks prior to monocyte subset analysis. At the end of the 3 weeks period mice were sacrified for collection of blood and bone marrow (BM). Mouse experiments were approved by the research advisory committee and permitted by LAGeSo (Landesamt für Gesundheit und Soziales Berlin).
Generation of secondary abiotic (gnotobiotic) mice
Secondary abiotic mice were generated through quintuple antibiotic treatment (+ABS) for 6 weeks via the drinking water. The intestinal colonization status of the mice was controlled once a week by highly sensitive cultural analysis of fecal samples as described earlier21.
Analysis of blood monocyte subsets in mice
Blood samples were collected by cardiac puncture using EDTA-coated syringes. Whole blood (100 μl) or BM cell suspension was incubated with antibodies for 20 minutes at room temperature in the dark (major resources table in the Supplemental Material). After doublet exclusion monocytes were identified as CD45 positive and CD115 positive and further selected by highly positive expression of CD11b, a marker of dendritic cells, monocytes and granulocytes, shown in the CD11b/Ly6C plot. Monocytes were further classified according to Ly6C expression. Detailed gating strategy for identification of monocyte subsets is shown in Supplemental Figure II.
Analysis of bone marrow hematopoietic stem cells and progenitor cells in mice
BM cells were collected from both femur and tibia of mice by flushing the BM with Dulbecco’s PBS. Harvested cells were filtered through a 70mm cell strainer and washed with PBS. Erythrocytes from all samples were lysed with red blood cell (RBC) lysis buffer. BM cell suspension was incubated with antibodies for 20 minutes at room temperature in the dark (major resources table in the Supplemental Material). After pre-selection (side scatter and forward scatter) the Lineage negative cells were gated in a histogram and hematopoietic stem cells (HSC) were identified as the C-kit positive and Sca1 positive population and further selected by positive expression of CD135 and CD115 as monocyte and dendritic cell progenitors (MDP).
Statistical Analyses
Database management and statistical analyses were performed with PRISM version 5.0a (GraphPad Software Inc., USA) and SPSS version 23 (IBM SPSS, USA).
Mouse studies
Continuous data were subjected to the Kolmogorov–Smirnov test to determine their distribution and were expressed as mean±standard deviation (SD) or median and range. Comparison of means was performed by independent t-test and Wilcoxon rank-sum test of medians was used if data were not normally distributed. For continuous variables, univariate correlation analyses were performed.
Human studies
For comparison of basic patients’ characteristics continuous data were subjected to the Kolmogorov–Smirnov test to determine their distribution and were expressed as mean±standard deviation (SD) or median and range. Comparison of means was performed by independent t-test and Wilcoxon rank-sum test of medians was used if data were not normally distributed. Kaplan–Meier survivor curves were examined to assess the relationship between quartiles of TMAO or choline levels timing of events during follow-up, and the log-rank test for statistical assessment. Cox regression forward selection was used to further characterize the relation of TMAO and choline to outcome. To determine whether TMAO was independently related to outcome, three cox regression models were used: In model 1 the outcome was adjusted for sex and age, in model 2 outcome was adjusted for model 1 and cardiovascular risk factors including hypertension, diabetes, LDL cholesterol and eGFR and in model 3 the outcome was adjusted for model 1 and additionally for stroke severity, stroke etiology (TOAST)22, cardiovascular risk factors including hypertension, diabetes, LDL cholesterol, history of peripheral or coronary artery disease or myocardial infarction. For additional comparison of the prognostic value of TMAO with regard to CVE or stroke at 1-year, receiver operating characteristic (ROC) curves were generated, and the areas under the curves (AUC) were calculated. To assess the prognostic value of TMAO in patient subgroups Chi-Square and Odds ratio analyses were performed. Additionally, combined ROC curve analyses of the cardiovascular risk factors diabetes, hypertension and dyslipidemia with and without TMAO were performed to assess the additive prognostic value of TMAO over classical cardiovascular risk factors.
Parametric correlation analyses were used to evaluate the correlation between TMAO and intermediate CD14++CD16+ monocytes. Multiple linear regression analysis was performed to further assess the correlation between TMAO levels and intermediate monocyte subsets after adjustment for age, sex and the cardiovascular risk factors including diabetes, hypertension, dyslipidemia and smoking. Significance was assumed at a two-sided value of P≤0.05.
Results
Patient Characteristics
Demographic and clinical parameters, as well as laboratory test results of all patients in the first study are shown in Table 1, and for the validation study in Table 2. We also compared these parameters between patients with the lowest plasma levels of TMAO (first quartile in the first study n=20; in the validation study n=148) and those with the highest plasma levels of TMAO (fourth quartile in the first study n=20; in the validation study n=148). In the first study, the cohort was composed of 78 sequential consenting patients with adjudicated diagnosis of ischemic stroke. In this cohort, subjects with higher TMAO levels tended to have higher creatinine levels as compared to subjects with low TMAO levels, while otherwise no differences in baseline clinical or laboratory parameters were observed between patients in the highest quartile versus the lowest quartile of TMAO levels (Table 1). The second larger validation population included 593 patients in the ‘Prospective Cohort with Incident Stroke’ study (PROSCIS) who had suffered with first-ever stroke. In this cohort, subjects in the highest TMAO quartile tended to be older, had higher creatinine levels, and had lower hemoglobin levels as well as higher leucocyte counts (Table 2) as compared with subjects in the lowest TMAO quartile. Outcomes at 1-year included myocardial infarction in 6 patients, stroke in 21 patients, cardiovascular death in 19 patients, and the composite CVE in 46 patients.
Table 1.
Baseline clinical parameters and laboratory test results of the first study
| Parameters | All patients (n=78) | Patients in the 1st quartile (n=20) | Patients in the 4st quartile (n=20) | p-value 1st vs 4th quartile |
|---|---|---|---|---|
| Age (years) (mean±SD) | 59 ± 14 | 59 ± 13 | 59 ± 11 | 0.65 |
| Sex (% male) | 69 | 70 | 75 | 1.00 |
| Systolic BP (mmHg) (mean±SD) | 122 ± 23 | 128 ± 26 | 131 ± 21 | 0.33 |
| Diastolic BP (mmHg) (mean±SD) | 66 ± 12 | 68 ± 13 | 67 ± 9 | 0.11 |
| Total Cholesterol (mg/dl) (mean±SD) | 202 ± 14 | 193 ± 53 | 208 ± 51 | 0.92 |
| LDL (mg/dl) (mean±SD) | 143 ± 39 | 138 ± 42 | 144 ± 36 | 0.53 |
| HDL (mg/dl) (mean±SD) | 49 ± 14 | 46 ± 14 | 49 ± 11 | 0.22 |
| Triglyceride (mg/dl) (median, range) | 120 (35 –664) | 102 (35 –664) | 104 (59 –656) | 0.93 |
| CRP (mg/l) (mean±SD) | 13 ± 29 | 29 ± 53 | 6 ± 7 | 0.09 |
| Creatinine (μmol/l) (median, range) | 84 (36 – 706) | 81 (52 – 120) | 93 (64 – 706) | 0.02 |
| Hb (g/dl) (mean±SD) | 14 ± 1.4 | 14.0 ± 1.5 | 13.9 ± 1.4 | 0.92 |
| Leukocytes (tsd/μl) (mean±SD) | 7.7 ± 2.8 | 8.3± 4.4 | 7.8 ± 2.3 | 0.57 |
| Platelets (tsd/μl) (median, range) | 217 (90–445) | 214 (104–257) | 220 (113–306) | 0.66 |
BMI: body mass index; HR: heart rate; BP: blood pressure; LDL: low-density lipoprotein; HDL: high-density lipoprotein; CRP: C-reactive protein; Hb: hemoglobin;
Table 2.
Baseline clinical parameters and laboratory test results of the second independent validation cohort
| Parameters | All patients (n=593) | Patients in the 1st quartile (n=148) | Patients in the 4st quartile (n=148) | p-value 1st vs 4th quartile |
|---|---|---|---|---|
| Age (years) (mean±SD) | 67±13 | 62±14 | 70±12 | 0.01 |
| Sex (% male) | 61 | 57 | 60 | 0.64 |
| Systolic BP (mmHg) (mean±SD) | 139±22 | 136±22 | 140±22 | 0.10 |
| Diastolic BP (mmHg) (mean±SD) | 78±14 | 76±15 | 76±15 | 0.80 |
| Total Cholesterol (mg/dl) (mean±SD) | 200±48 | 201±42 | 198±49 | 0.50 |
| LDL (mg/dl) (mean±SD) | 123±41 | 123±38 | 119±40 | 0.40 |
| HDL (mg/dl) (mean±SD) | 51±16 | 54±18 | 51±8 | 0.30 |
| Triglyceride (mg/dl) (median, range) | 117 (35–577) | 109 (36–553) | 122 (36–492) | 0.09 |
| CRP (mg/l) (mean±SD) | 3.2±10.8 | 4.7±14.5 | 3.2±11.9 | 0.30 |
| Creatinine (μmol/l) (median, range) | 80 (31 – 460) | 74 (31 – 159) | 91 (42 – 460) | <0.01 |
| Hb (g/dl) (mean±SD) | 14.2±1.7 | 14.3±1.6 | 13.8±1.7 | 0.02 |
| Leukocytes (tsd/μl) (mean±SD) | 8.1±3.5 | 7.5±2.2 | 8.3±3.0 | 0.02 |
| Platelets (tsd/μl) (median, range) | 221 (80–526) | 213 (87–393) | 227 (100–451) | 0.13 |
Plasma TMAO Levels in Relation to Subsequent CVE and in the First Study
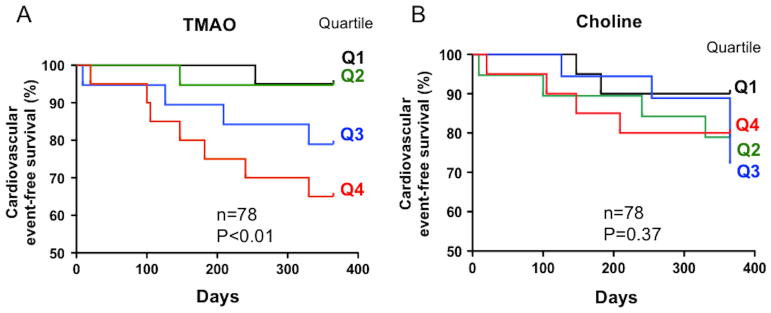
In the first study cohort (n=78), Kaplan-Meier analyses of TMAO levels, but not its precursor, the trimethylamine-containing nutrient choline, showed a dose dependent relationship with the risk of incident CVE including myocardial infarction, recurrent stroke and cardiovascular death (TMAO, P<0.01, Figure 1A; choline, P=0.37, Figure 1B). The association between TMAO and CVE was further adjusted for age, sex, hypertension, diabetes, LDL-cholesterol and smoking resulting in a significant enhanced risk with an HR 3.5 (95% CI: 1.66 – 7.21; P=0.001). Analysis of the linear trend across quartiles also showed a significant enhanced risk with a Chi2 of 7.0 (P<0.01).
Figure 1. Plasma TMAO levels in relation to subsequent cardiovascular events in patients with ischemic stroke from the first study.
In the first cohort, plasma levels of TMAO (A) but not choline (B) dose-dependently trace the risk of incident CVE including myocardial infarction, stroke and death.
TMAO Levels and the Risk of Subsequent CVE or Recurrent Stroke in the Validation Study
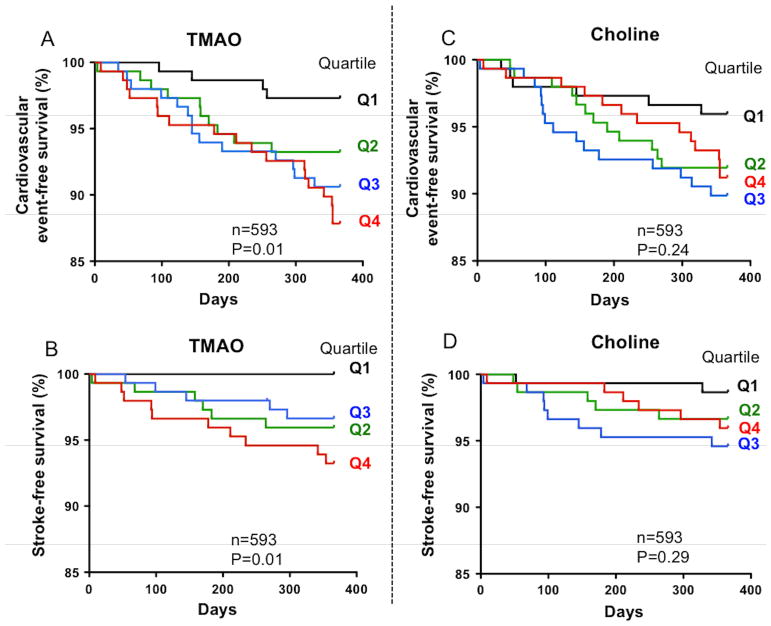
In the validation study cohort (n=593), Kaplan-Meier plots indicated a graded increased risk of combined CVE including myocardial infarction, recurrent stroke and cardiovascular death (Figure 2A, P=0.01) and also recurrent stroke alone (Figure 2B, P=0.01) with increasing plasma TMAO levels, but not with plasma choline levels (Figure 2C and D, P=0.24 and P=0.29, respectively). The linear trend across quartiles results in a significant enhanced risk of combined CVE with a Chi2 of 9.8 (p<0.01).
Figure 2. Circulating TMAO levels and risk of incident cardiovascular events or recurrent stroke in the validation study.
Kaplan-Meier plots of the validation study confirm that patients in the 4th quartile for plasma TMAO (A, B), but not for plasma choline (C, D), had a significantly increased risk for experiencing a recurrent CVE and recurrent stroke (A and B, respectively).
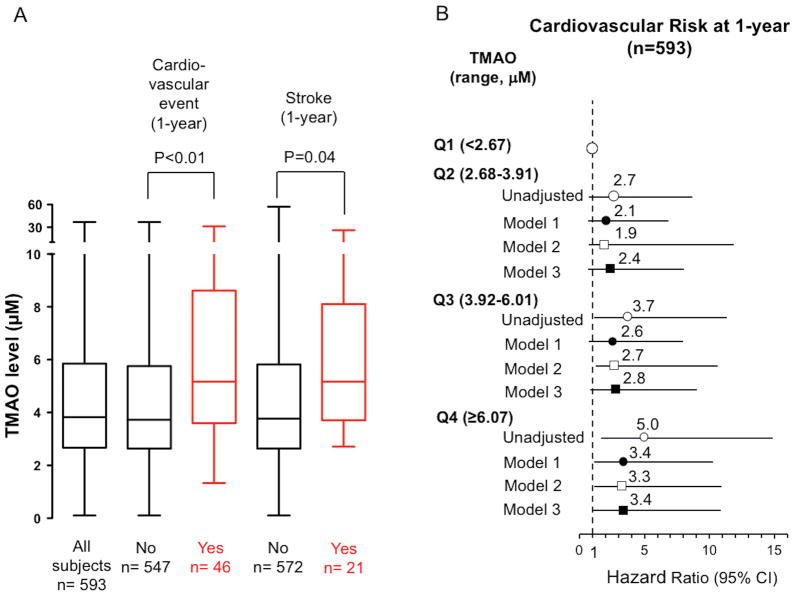
Subjects who experienced incident CVE had higher plasma TMAO levels at presentation than those without an event at 1-year follow-up (no event: 4.91 ± 0.18 μM vs event: 7.28 ± 0.91 μM, P<0.01, Figure 3A). Similarly, subjects who particularly experienced recurrent stroke displayed also higher plasma TMAO levels than those without recurrent stroke at 1-year follow-up (no recurrent stroke: 4.92 ± 0.18 μM vs recurrent stroke: 6.86 ± 1.11 μM, P=0.04, Figure 3A). The dose-dependent relationship between TMAO levels and incident CVE was particularly noticeable in patients in the 4th quartile (6.07–122 μM) of plasma TMAO levels compared to those in the first quartile (<2.7 μM) (HR unadjusted: 5.0; 95% CI: 1.7–14.8; P<0.01; Figure 3B). Moreover, patients in the highest quartile of TMAO levels had an increased risk of CVE over the ensuing 1-year follow-up period after the adjustments for both age and sex (HR model 1: 3.4; 95% CI: 1.2–10.2; P<0.01), and even after further adjustments for cardiovascular risk factors including hypertension, diabetes, LDL cholesterol and eGFR (HR model 2: 3.3 95% CI: 1.2–10.9; P=0.04). In a third model, we adjusted for age and sex and additionally for stroke severity, stroke etiology (TOAST), hypertension, diabetes, LDL cholesterol, Lp(a) and history of peripheral (PAD) or coronary artery disease (CAD) or myocardial infarction (MI) (HR model 2: HR 3.4; 95% CI: 1.1–10.1; P=0.02). Moreover, patients in the 4th vs. 1st quartile of plasma TMAO levels were at higher risk of recurrent stroke over 1-year follow-up interval (HR 2.7; 95% CI: 1.1–6.1; P<0.01).
Figure 3. Relationship between plasma TMAO levels and risk of subsequent cardiovascular events or recurrent stroke.
(A) Data from the second lager validation cohort demonstrate higher TMAO levels (Box-Whisker plots) in patients with subsequent CVE or recurrent stroke as compared with patients without events or recurrent stroke, respectively. (B) Illustrates Forest plots of the hazard ratio of 1-year cardiovascular risk according to the quartiles of blood TMAO levels. Bars represent 95% CI. Model 1: adjusted for sex and age. Model 2: adjusted for model 1 plus hypertension, diabetes, LDL cholesterol and eGFR. Model 3: adjusted for model 1 plus stroke severity, stroke etiology (TOAST), hypertension, diabetes, LDL cholesterol, Lp(a), peripheral artery disease, and history of coronary artery disease or myocardial infarction.
ROC Curve Analyses of TMAO and Prognostic Utility in Patient Subgroups
Receiver operating characteristic curve analyses of TMAO further illustrated that TMAO is a considerable indicator of cardiovascular risk with an AUC of 0.66. (Supplemental Figure IIIA). Moreover, combined ROC curve analyses of the cardiovascular risk factors diabetes, hypertension and dyslipidemia with and without TMAO were performed to assess the additive prognostic value of TMAO over classical cardiovascular risk factors. Adding TMAO to classical risk factors including diabetes, hypertension and LDL increased AUC (AUC with TMAO: 0.63; p<0.01vs. AUC without TMAO: 0.57; p=0.09) (Supplemental Figure IIIB). A TMAO value of 4.86 μM yielded the highest sensitivity (63%) and specificity (66%) for the prediction of 1-year cardiovascular risk.
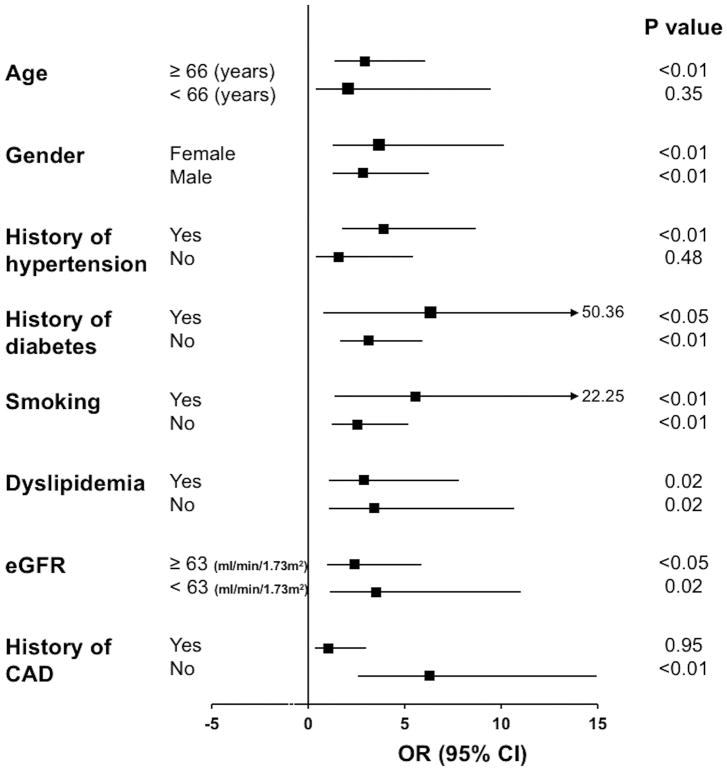
Moreover, the prognostic value of TMAO was assessed in several patient subgroups (Figure 4). A TMAO level > 4.86 μM was associated with an increased cardiovascular risk in patients above 66 years of age, in both males and females, and in patients with hypertension. Moreover, TMAO showed clinical prognostic utility independent of traditional cardiovascular risk factors including within both diabetic and non-diabetic patients, in smokers and non-smokers, in patients with and without dyslipidemia, in patients with normal or impaired renal function, and in patients without history of CAD (Figure 4).
Figure 4. TMAO and cardiovascular risk in patient subgroups.
Cardiovascular risk during 1-year follow-up associated with a TMAO level above 4.86 μM in subgroups of patients from the second validation study. Odds ratio (OD) with 95% CIs and P values are shown.
Plasma TMAO Levels in Relation to Monocyte Subsets
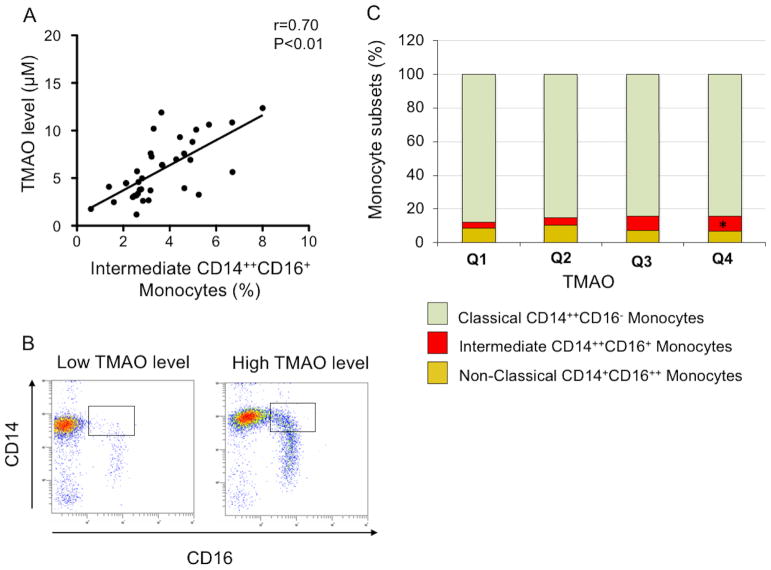
Assessment of the proportion of intermediate CD14++CD16+ monocytes revealed significant correlation with plasma levels of TMAO (r=0.70, P<0.01) (Figure 5A, B). Additional multiple linear regression analysis was performed to assess the correlation between TMAO levels and intermediate monocyte subsets after adjustment with age, sex and the cardiovascular risk factors diabetes, hypertension, dyslipidemia and smoking. After adjustment, the results still demonstrated significant correlation between intermediate monocyte levels with plasma TMAO concentrations (r=0.38, P<0.01). Further analyses determined the distribution of monocyte subsets in these patients according to TMAO levels as assessed by quartiles (Figure 5C). Classical CD14++CD16− monocytes were not significantly altered between patients with the highest and lowest TMAO levels (Q4: 84.3% vs Q1: 87.8%; P=0.20). Non-Classical CD14+CD16++ monocytes tended to be lower in patients with the highest TMAO levels, although this was not statistically significant (Q4: 6.7% vs Q1: 8.6%; P=0.17). Whereas, the proportion of intermediate CD14++CD16+ monocytes showed a dose-dependent increase according to TMAO levels with the highest levels being observed in patients with the highest TMAO concentrations (Q4: 9.0% vs Q1: 3.6%; P<0.01).
Figure 5. Relation of TMAO concentrations with the level of intermediate CD14++CD16+ monocytes.
(A) Graph depicts correlation between TMAO concentrations and the level of intermediate CD14++CD16+ monocytes. (B) shows representative FACS plots of blood monocyte subsets in a patient with low TMAO levels (left plot) and in a patient with high TMAO levels (right plot). (C) Comparison of the monocyte subset distribution between patients with increasing quartiles of TMAO levels (* indicate P<0.05 as compared to CD14++CD16+ monocytes in patients from the first quartiles).
Impact of the trimethylamine-containing nutrient choline on blood and bone marrow monocyte subset distribution in mice
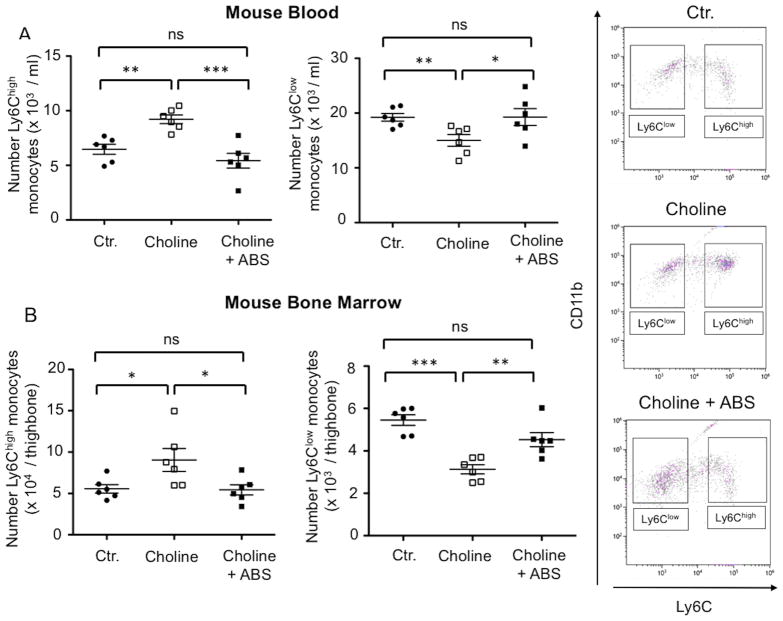
The levels of Ly6Chigh monocytes were increased in mice on choline (1.3%) supplemented diet as compared to normal chow (ctr.) (choline: n=6, 9.2±0.5 x 103 per ml vs. ctr.: n=6, 6.5±0.5 x 103 per ml; P=0.001). This increase was abolished in mice with depleted gut microbiota (choline + ABS: n=6, 5.4±0.7 x 103 per ml; P<0.001 vs. choline) (Figure 6A). Opposingly, the levels of blood Ly6Clow monocytes decreased in high choline-fed mice (choline: n=6, 15.0±1.1 x 103 per ml vs. ctr.: n=6, 19.2±0.7 x 103 per ml, P<0.01). This effect was abolished in gut microbiota-depleted mice (choline +ABS: n=6, 19.3±1.5 x 103 per ml; P=0.03 vs. choline) (Figure 6A). To evaluate whether the shift in monocyte subsets occurs at the level of BM exit the levels of Ly6Chigh and Ly6Clow monocytes in the BM were analyzed. Similar to the results in the circulation, the levels of Ly6Chigh monocytes were increased in the BM in mice on choline-supplemented diet as compared to normal chow (choline: n=6, 9.0.8±1.3 x 104; vs ctrl.: n=6, 5.6±0.5 x 104 per thighbone; P=0.03) (Figure 6B) which was prevented in mice with depleted gut microbiota (choline + ABS: n=6, 5.4±0.6 x 104 per thighbone; P=0.03 vs. choline). Consistently, the levels of Ly6Clow monocytes decreased in the BM in choline fed mice (choline: n=6, 3.1±0.2 x 103 per thighbone vs ctr.: n=6: 5.5±0.2 x 103 per thighbone; P<0.001) but not in gut microbiota depleted mice (choline + ABS: n=6, 4.5±0.3 x 103 per thighbone; P<0.01 vs choline.) (Figure 6B).
Figure 6. Impact of choline supplemented diet on levels of mouse monocyte subsets in the blood and bone marrow.
(A) graphs depict proportions of Ly6Chigh (left graph) and ly6Clow (right graph) blood monocytes in normal chow diet (ctr.) and changes in monocyte subsets in mice on choline supplemented diet with intact gut microbiome (choline) or with depleted gut microbiome (choline + ABS). Corresponding changes in bone marrow monocyte subsets are shown in (B). (C) shows representative FACS plots of blood monocyte subsets in a mouse on normal chow diet (ctr., upper plot), upon choline supplementation with intact gut microbiota (choline, middle plot) or with depleted gut microbiota (choline + ABS, lower plot).
Impact of the trimethylamine-containing nutrient choline on bone marrow hematopoietic stem and progenitor cells in mice
Analysis of hematopoietic stem and progenitor cells in BM showed no alteration of HSC (choline: n=8, 1.7±0.2% of lineage negative (neg) cells vs ctr.: n=7, 1.1±0.2% of lineage neg cells; P=0.06) (Figure S4A) nor MDP levels (choline: n=8, 1.6±0.1% of lineage neg cells vs ctr.: n=7, 1.2±0.2% of lineage neg cells; P=0.08) (Figure S4B) in choline supplemented mice as compared to normal chow. Accordingly, depletion of the gut microbiota had no significant effects on HSC or on MDP levels in the BM (Supplemental Figure IV).
Discussion
Our study examines for the first time the relationship between the gut microbe-derived metabolite TMAO and recurrent stroke and CVE risk among patients with ischemic stroke in two independent cohorts and the relationship between baseline TMAO with the level of intermediate CD14++CD16+ monocytes in patients with stroke. The results indicate the following: (i) elevated TMAO levels are dose-dependently associated with an increased risk of recurrent stroke and subsequent CVE in patients after first-ever stroke; (ii) this relationship holds true even after adjusting for traditional cardiovascular and cerebrovascular risk factors and initial stroke severity; (iii) blood concentrations of TMAO are closely related to the levels of proinflammatory intermediate CD14++CD16+ monocytes.
Importantly, the results of the second independent validation cohort demonstrate that the observed clinical association remains robust, indicating the prognostic clinical utility of TMAO for predicting incident adverse event or thrombosis risk replicates in a totally distinct yet clinically relevant cohort.
ROC curve analyses further revealed that TMAO provides added prognostic value to traditional risk factors as a marker of 1-year cardiovascular mortality risk in these patients. At the best cutoff value with the highest sensitivity and specificity, TMAO provided prognostic information in clinically relevant patient subgroups defined according to age, sex, underlying cardiovascular risk factors, and renal dysfunction.
Notably, we found reduced renal function in patients with the highest TMAO levels. This observation may be partly due to impaired renal elimination of TMAO in these patients. However, it was also previously shown that TMAO likely contributes to the progression of renal disease by increasing tubulointerstitial fibrosis and collagen deposition23. Moreover, it was shown that elevated TMAO levels increased the risk of mortality in patients with chronic kidney disease23. Subgroup analyses in the present study showed that clinical prognostic utility of elevated TMAO was observed in both subgroups possessing normal versus impaired renal function.
Recently, intestinal microbes and their metabolic products have been identified as potential promoters for the development of cardio-metabolic diseases and atherosclerosis24. Microbial composition studies combining clinical phenotypic data with microbial sequencing analysis have identified that an altered gut metagenome is associated, for example, with the development of metabolic disorders such as type 2 diabetes mellitus, obesity, and symptomatic atherosclerotic heart disease24. Early metabolomics studies, coupled with mechanistic investigations, have unveiled a specific pathway by which gut microbes contribute to atherosclerosis and cardiovascular disease. This pathway is based on particular dietary nutrients containing a TMA group such as phosphatidylcholine, choline, and carnitine. In the intestine, the TMA containing nutrient is subsequently degraded by a TMA lyase from specific bacterial strains, and, after absorption into the portal circulation, it is further metabolized into TMAO by hepatic flavin monooxygenases (FMOs), in particular FMO34, 5, 25–27.
A potential mechanistic link of TMAO to the promotion of atherosclerosis was first demonstrated in animal models, in which TMAO led to increased cholesterol accumulation in macrophages and the formation of foam cells in atherosclerotic lesions by increasing the expression of the scavenger receptors (CD36 and SR-A1) on macrophages4. Interestingly, a recent clinical study examining coronary artery anatomy with the SYNTAX score showed a dose-dependent relationship between TMAO levels and enhanced atherosclerotic burden in subjects28. Moreover, recent studies suppressing TMAO generation with a small molecule inhibitor of microbial TMA production led to inhibition of atherosclerosis27, and suppression of FMO3 has been demonstrated to both lead to TMAO reduction and inhibition in atherosclerosis in animal models29. Notably, TMAO has been shown to promote vascular inflammation by activating mitogen-activated protein kinase, extracellular signal–related kinase, and nuclear factor-κB signaling cascade in endothelial cells6. Furthermore, direct injection of TMAO in rodent models promotes aortic endothelial cell activation and up-regulation of adhesion proteins6. Recent studies with cultured endothelial cells indicate that TMAO activates the NLRP3 inflammasome, a multi-enzyme proinflammatory complex thought to be involved in the development of atherosclerosis30.
While in experimental studies a role of TMAO for inflammatory processes has been shown, our study links increased TMAO values to proinflammatory CD14++CD16+ monocytes in humans. The intermediate CD14++CD16+ monocytes are considered to promote inflammatory responses14, e.g. by secreting high amounts of inflammatory cytokines, such as TNF-α15. Moreover, high expression of adhesion molecules, such as CD162/PSGL-1, as well as myeloperoxidase, a major source of reactive oxidant species in innate immune responses13 has been identified in intermediate monocytes contributing to their pro-thrombotic and pro-atherogenic properties.
Our mouse experiments further support this proposed link and suggest a shift of monocyte subset distribution towards proinflammatory Ly6Chigh monocytes in the bone marrow without affecting hematopoietic stem cells or monocyte progenitor cells.
Recent lineage-tracing and adoptive transfer studies have supported the concept that Ly6Chigh monocytes are the precursors to Ly6Clow monocytes31. Thus, alteration of subset levels reflects a difference in subset conversion. Among molecular pathways to control differentiation of Ly6Clow monocytes orphan nuclear receptor mediated signaling, such as the Nr4a1 signaling, has been identified32. Further studies are required to dissect the underlying molecular mechanisms by which TMAO regulates monocyte subset conversion and promotes the shift towards increased Ly6Chigh monocytes.
Despite the observed link between TMAO and its precursor choline and proinflammatory monocytes, future studies will need to clarify whether chronic exposure to TMA-containing nutrients and sustained elevation in TMAO are indeed a causal trigger of (low-grade) inflammatory processes leading to enhanced cardiovascular risk, and whether targeting gut microbiota-dependent TMAO generation in humans can similarly to animal models reduce incident event risks in patients.
The relationship between altered gut microbiota composition/function and different levels of the microbiota-dependent metabolite TMAO has been recently demonstrated. For example, plasma TMAO concentrations were found to be significantly associated with Prevotella and Peptococcaceae5. Additionally, a significant positive correlation between plasma TMAO concentrations with Clostridiales and negative correlation with F. prausnitzii was identified33. Interestingly, microbial transplantation studies have also shown that TMAO production potential and both atherosclerosis26 and thrombosis7 and thrombosis susceptibility are transmissible traits, further supporting a role for gut microbiota-generated TMAO in atherogenesis and thrombosis risk. These findings align with our observation that the increase in Ly6Chigh monocytes could be prevented upon depletion of the gut microbiota suggesting an instrumental role for the gut microbiota for processing inflammation promoting metabolites.
It is tempting to speculate that metagenomics analyses of feces could reveal alterations in the abundance of specific microbes, or more importantly, microbial enzyme pathways, known to be involved in TMA and TMAO formation. Measurement of the downstream functional output of such microbial alterations (i.e. TMAO levels) serves as an integrated sensor of the global changes in microbial community structure and function, with respect to the meta-organismal TMAO pathway. In future investigations, it will be of interest to explore whether demonstration of an altered fecal microbiota serves as an additive predictor beyond TMAO levels of enhanced cardiovascular risk in patients at risk for ischemic stroke. A better understanding of the role of microbiota-related metabolism in patients with cerebrovascular disease may contribute to the development of novel strategies targeting the gut microbiota (e.g. its enzyme systems) for both primary and secondary prevention in these patients.
Our analysis of incident CVE data in patients with stroke suggests that elevated TMAO levels are linked with an increased risk of future cardiovascular and cerebrovascular thrombotic events. This association is consistent with the recently observed role of TMAO in enhancing platelet hyper-responsiveness and thrombosis potential in vivo7. In addition to platelet hyper-responsiveness, the link between microbiota-dependent TMA/TMAO formation and increased levels of proinflammatory monocytes may add to potential pathophysiological mechanisms ultimately cumulating in increased incidence of cardiovascular or cerebrovascular events.
In conclusion, our study suggests a novel link between TMAO and proinflammatory monocytes, as well as with an adverse cardiovascular prognosis in patients after stroke. Our findings contribute to the understanding of a potential role of gut microbiota related mechanisms in patients with cerebrovascular disease progression and encourages further investigation of therapeutic strategies aimed at fostering a reduction in systemic levels of the gut microbial metabolite TMAO.
Supplementary Material
Highlights.
Elevated TMAO levels are dose-dependently related with an increased risk of recurrent stroke and subsequent cardiovascular events in patients after first-ever stroke, also after adjustment for traditional cardiovascular and cerebrovascular risk factors and initial stroke severity.
TMAO adds significant prognostic information in patient subgroups defined by age, sex, cardiovascular risk factors and glomerular filtration rate
Blood concentrations of TMAO are closely related with the levels of proinflammatory intermediate CD14++CD16+ monocytes in patients after ischemic stroke.
Supplementation of choline, the TMA-containing precursor of TMAO, in mice increases the levels of proinflammatory Ly6Chigh monocytes in a gut microbiota dependent manner
Acknowledgments
We thank Ulrike Escher for her assistance in this study.
Sources of funding
This study was supported by grants from the Deutsche Stiftung für Herzforschung, the Else Kröner-Fresenius-Stiftung and Deutsches Zentrum für Herz Kreislaufforschung (Rotation Grant) to Dr. Arash Haghikia and the Foundation Leducq network “Gut microbiome as a target for the treatment of cardiometabolic diseases” as well as the Fondation Leducq network “Evoked Neuronal Activity: A New Therapy for Acute Ischemic Stroke“. The PROSCIS-B study was funded by the German Federal Ministry of Education and Research (BMBF funding for The Center for Stroke Research Berlin). Mass spectrometry studies were supported in part by grants from the National Institutes of Health and the Office of Dietary Supplements (R01HL103866, R01DL106000 and R01HL130819). Mass spectrometry studies were performed on instruments housed in a facility supported in part by a Center of Excellence Award from Shimadzu Corporation.
Nonstandard Abbreviations and Acronyms
- AUC
Area under the curve
- BM
Bone marrow
- CAD
Coronary artery disease
- CD
Cluster of Differentiation
- CVE
Cardiovascular events
- EGFR
estimated glomerular filtration rate
- HR
Hazard Ratio
- HSC
hematopoietic stem cells
- LDL
Low-density lipoprotein
- Lp (a)
Lipoprotein a
- Ly6C
Lymphocyte antigen 6C
- MDP
Monocyte and dendritic cell progenitor
- MI
Myocardial infarction
- ROC
Receiver operating characteristic
- PAD
Peripheral artery disease
- Q
Quartile
- TMA
Trimethylamine
- TMAO
Trimethylamine N-oxide
Footnotes
Disclosure
Drs. Wang and Hazen are named as co-inventor on patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Drs. Wang and Hazen report that they have the right to receive royalty payment for inventions or discoveries related to cardiovascular diagnostics and therapeutics from Cleveland Heart Lab and P&G. Dr. Hazen reports having been paid as a consultant for the following companies: Esperion and P&G. Dr. Hazen reports receiving research funds from Astra Zeneca, P&G, Pfizer Inc. and Takeda. All other authors have no relationships to disclose.
References
- 1.Amarenco P, Lavallee PC, Labreuche J, Albers GW, Bornstein NM, Canhao P, Caplan LR, Donnan GA, Ferro JM, Hennerici MG, Molina C, Rothwell PM, Sissani L, Skoloudik D, Steg PG, Touboul PJ, Uchiyama S, Vicaut E, Wong LK Investigators TIo. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533–1542. doi: 10.1056/NEJMoa1412981. [DOI] [PubMed] [Google Scholar]
- 2.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haghikia A, Jorg S, Duscha A, Berg J, Manzel A, Waschbisch A, Hammer A, Lee DH, May C, Wilck N, Balogh A, Ostermann AI, Schebb NH, Akkad DA, Grohme DA, Kleinewietfeld M, Kempa S, Thone J, Demir S, Muller DN, Gold R, Linker RA. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity. 2015;43:817–829. doi: 10.1016/j.immuni.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, Lusis AJ, Shih DM. Trimethylamine n-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-kappab. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut microbial metabolite tmao enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Raber L, Windecker S, Rodondi N, Nanchen D, Muller O, Miranda MX, Matter CM, Wu Y, Li L, Wang Z, Alamri HS, Gogonea V, Chung YM, Tang WH, Hazen SL, Luscher TF. Gut microbiota-dependent trimethylamine n-oxide in acute coronary syndromes: A prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J. 2017;38:814–824. doi: 10.1093/eurheartj/ehw582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilgendorf I, Swirski FK. Making a difference: Monocyte heterogeneity in cardiovascular disease. Curr Atheroscler Rep. 2012;14:450–459. doi: 10.1007/s11883-012-0274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libby P, Nahrendorf M, Swirski FK. Monocyte heterogeneity in cardiovascular disease. Semin Immunopathol. 2013;35:553–562. doi: 10.1007/s00281-013-0387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, Grosse-Dunker G, Heisel I, Hornof F, Jeken J, Rebling NM, Ulrich C, Scheller B, Bohm M, Fliser D, Heine GH. Cd14++cd16+ monocytes independently predict cardiovascular events: A cohort study of 951 patients referred for elective coronary angiography. J Am Coll Cardiol. 2012;60:1512–1520. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Rogacev KS, Seiler S, Zawada AM, Reichart B, Herath E, Roth D, Ulrich C, Fliser D, Heine GH. Cd14++cd16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J. 2011;32:84–92. doi: 10.1093/eurheartj/ehq371. [DOI] [PubMed] [Google Scholar]
- 13.Wildgruber M, Aschenbrenner T, Wendorff H, Czubba M, Glinzer A, Haller B, Schiemann M, Zimmermann A, Berger H, Eckstein HH, Meier R, Wohlgemuth WA, Libby P, Zernecke A. The “intermediate” cd14(++)cd16(+) monocyte subset increases in severe peripheral artery disease in humans. Sci Rep. 2016;6:39483. doi: 10.1038/srep39483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shantsila E, Tapp LD, Wrigley BJ, Pamukcu B, Apostolakis S, Montoro-Garcia S, Lip GY. Monocyte subsets in coronary artery disease and their associations with markers of inflammation and fibrinolysis. Atherosclerosis. 2014;234:4–10. doi: 10.1016/j.atherosclerosis.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Schlitt A, Heine GH, Blankenberg S, Espinola-Klein C, Dopheide JF, Bickel C, Lackner KJ, Iz M, Meyer J, Darius H, Rupprecht HJ. Cd14+cd16+ monocytes in coronary artery disease and their relationship to serum tnf-alpha levels. Thromb Haemost. 2004;92:419–424. doi: 10.1160/TH04-02-0095. [DOI] [PubMed] [Google Scholar]
- 16.Kaito M, Araya S, Gondo Y, Fujita M, Minato N, Nakanishi M, Matsui M. Relevance of distinct monocyte subsets to clinical course of ischemic stroke patients. PLoS One. 2013;8:e69409. doi: 10.1371/journal.pone.0069409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D’Cruz D, Casanova JL, Trouillet C, Geissmann F. Human cd14dim monocytes patrol and sense nucleic acids and viruses via tlr7 and tlr8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liman TG, Zietemann V, Wiedmann S, Jungehuelsing GJ, Endres M, Wollenweber FA, Wellwood I, Dichgans M, Heuschmann PU. Prediction of vascular risk after stroke - protocol and pilot data of the prospective cohort with incident stroke (proscis) Int J Stroke. 2013;8:484–490. doi: 10.1111/j.1747-4949.2012.00871.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL. Measurement of trimethylamine-n-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40. doi: 10.1016/j.ab.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuser J, Galuppo P, Fraccarollo D, Willig J, Kempf T, Berliner D, Bauersachs J, Widder JD. Intermediate cd14++cd16+ monocytes decline after transcatheter aortic valve replacement and correlate with functional capacity and left ventricular systolic function. PLoS One. 2017;12:e0183670. doi: 10.1371/journal.pone.0183670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, Jahn HK, Dunay IR, Moter A, Gescher DM, Schumann RR, Gobel UB, Liesenfeld O. Gram-negative bacteria aggravate murine small intestinal th1-type immunopathology following oral infection with toxoplasma gondii. J Immunol. 2006;177:8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 22.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 23.Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota-dependent trimethylamine n-oxide (tmao) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–455. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown JM, Hazen SL. The gut microbial endocrine organ: Bacterially derived signals driving cardiometabolic diseases. Annu Rev Med. 2015;66:343–359. doi: 10.1146/annurev-med-060513-093205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregory JC, Buffa JA, Org E, Wang Z, Levison BS, Zhu W, Wagner MA, Bennett BJ, Li L, DiDonato JA, Lusis AJ, Hazen SL. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2015;290:5647–5660. doi: 10.1074/jbc.M114.618249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senthong V, Li XS, Hudec T, Coughlin J, Wu Y, Levison B, Wang Z, Hazen SL, Tang WH. Plasma trimethylamine n-oxide, a gut microbe-generated phosphatidylcholine metabolite, is associated with atherosclerotic burden. J Am Coll Cardiol. 2016;67:2620–2628. doi: 10.1016/j.jacc.2016.03.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shih DM, Wang Z, Lee R, Meng Y, Che N, Charugundla S, Qi H, Wu J, Pan C, Brown JM, Vallim T, Bennett BJ, Graham M, Hazen SL, Lusis AJ. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56:22–37. doi: 10.1194/jlr.M051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen ML, Zhu XH, Ran L, Lang HD, Yi L, Mi MT. Trimethylamine-n-oxide induces vascular inflammation by activating the nlrp3 inflammasome through the sirt3-sod2-mtros signaling pathway. J Am Heart Assoc. 2017:6. doi: 10.1161/JAHA.117.006347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kratofil RM, Kubes P, Deniset JF. Monocyte conversion during inflammation and injury. Arterioscler Thromb Vasc Biol. 2017;37:35–42. doi: 10.1161/ATVBAHA.116.308198. [DOI] [PubMed] [Google Scholar]
- 32.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC. The transcription factor nr4a1 (nur77) controls bone marrow differentiation and the survival of ly6c- monocytes. Nat Immunol. 2011;12:778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Org E, Blum Y, Kasela S, Mehrabian M, Kuusisto J, Kangas AJ, Soininen P, Wang Z, Ala-Korpela M, Hazen SL, Laakso M, Lusis AJ. Relationships between gut microbiota, plasma metabolites, and metabolic syndrome traits in the metsim cohort. Genome Biol. 2017;18:70. doi: 10.1186/s13059-017-1194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.