Abstract
Objective
The cell-cholesterol efflux capacity (CEC) of high-density lipoprotein (HDL) is inversely associated with coronary heart disease (CHD) risk. ATP-binding cassette transporter-A1 (ABCA1) plays a crucial role in cholesterol efflux from macrophages to preβ-1-HDL. We tested the hypothesis that CHD patients have functionally abnormal preβ-1-HDL.
Approach and Results
HDL CEC via the ABCA1 and the scavenger receptor class B type-I (SR-BI) pathways, HDL anti-oxidative capacity, apolipoprotein A-I-containing HDL particles, and inflammatory- and oxidative-stress markers were measured in a case-control study of 100 CHD cases and 100 gender-matched controls. There were significant positive correlations between ABCA1-dependent cholesterol efflux and the levels of small lipid-poor preβ-1-particles (R2=0.535) and between SR-BI-dependent cholesterol efflux and the levels of large lipid-rich (α-1+α-2) HDL particles (R2=0.712). Cases had significantly higher (87%) preβ-1 concentrations than controls but the functionality of their preβ-1 particles (preβ-1-concentration-normalized ABCA1-dependent efflux capacity) was significantly lower (−31%). Cases had significantly lower (−12%) mean concentration of large HDL particles, but the functionality of their particles (α-1+α-2-concentration-normalized SR-BI-dependent efflux capacity) was significantly higher (22%) compared to controls. HDL anti-oxidative capacity was significantly lower (−16%) in cases than in controls. There were no significant correlations between either preβ-1 functionality or large-HDL-particle functionality with HDL anti-oxidative capacity or the concentrations of inflammatory- and oxidative-stress markers.
Conclusions
HDL CEC is significantly influenced by both the concentration and the functionality of specific HDL particles participating in cell-cholesterol efflux. CHD patients have higher than normal preβ-1 concentrations with decreased functionality, and lower than normal large-HDL particle concentrations with enhanced functionality.
Keywords: cell-cholesterol efflux, HDL particles, preβ-1, CHD risk, ABCA1, SR-BI
Subject codes: Cardiovascular Disease, Metabolism, Biomarkers, Lipids and Cholesterol, Inflammation
INTRODUCTION
In the last several decades, cross-sectional and interventional trials have indicated that high-density lipoprotein cholesterol (HDL-C) concentration is inversely associated with coronary heart disease (CHD) risk(1–3). Although a large spectrum of HDL functions has been described, the underlying mechanisms of how HDL protects against CHD are not fully understood. Moreover, randomized drug-intervention trials have failed to prove that increasing HDL-C level decreases CHD risk(4–6). It has been shown that apolipoprotein (apo) A–I concentration in individual HDL subpopulations is superior to HDL-C concentration in predicting risk for cardiovascular disease (CVD)(7–10). Recently several HDL functionality markers, most importantly the cell-cholesterol efflux capacity (CEC) of HDL via the ATP-binding cassette transporter A1 (ABCA1) pathway, have emerged as better CVD-risk markers than HDL-C and/or apoA-I concentration. First, Khera et al. reported strong inverse associations between CEC and both carotid intimae-media thickness and the likelihood of angiographic coronary artery disease, independent of HDL-C level(11). Then the Dallas Heart Study investigators reported an inverse association between CEC and incidence of cardiovascular events(12). The EPIC-Norfolk investigators also reported, in a nested case-control study, a significant inverse association between CEC and incidence of CHD, independent of HDL-C concentration(13). However, Li et al. reported higher CEC in patients with myocardial infarction compared to those without such events(14). Unfortunately, CEC studies are difficult to compare because different methods have been used to determine HDL CEC: J774 mouse macrophages with 3H-labeled cholesterol were used in Khera’s studies and in the Epic-Norfolk Study(11,13), J774 cells and BODYPI-labeled cholesterol was used in the Dallas Heart Study(12), and RAW 264.7 mouse macrophages were used by Li et al.(14).
HDL removes cholesterol from cells by multiple mechanisms. Scavenger receptor class B type I (SR-BI)-dependent CEC occurs primarily with the large lipid-rich α-1 and α-2 HDL particles (15,16), which are deficient in CHD subjects compared to controls(8,9,17). ABCA1-dependent CEC is mediated by the small lipid-poor preβ-1-HDL particles(15,16,18), whose concentration is known to be higher in CHD patients compared to controls(17,19–21).
We hypothesized that CHD patients have functionally abnormal preβ-1 particles. We tested this hypothesis by measuring the preβ-1-concentration-normalized efflux capacities of CHD and gender-matched control subjects. In an attempt to elucidate the mechanism of this dysfunction, we also assessed the influence of HDL anti-oxidative capacity and lipid and inflammatory markers on HDL functionality.
Materials and Methods
Study design and population
The data that support the findings of this study are available from the corresponding author upon reasonable request. The study protocol was approved by the Institutional Review Board of Tufts University. The study utilized a case-control design of 200 total subjects with a 1:1 allocation of CHD cases to gender-matched controls. Cases (70 males and 30 females) were randomly selected from the baseline samples of a randomized clinical trial(22). Participants in this trial had stable, established coronary artery disease (including previous myocardial infarction [≥6 months prior], coronary artery bypass surgery [>12months prior] or angioplasty, stable angina, or evidence of CHD on prior imaging), an abnormal exercise tolerance test result, or ischemia by nuclear imaging, deemed by the patients’ clinical cardiologist not to require current intervention. Additional inclusion criteria were age 21–75 years, body mass index (BMI) >25 and <35 in women or <40 in men, a stable regimen of statin dosing, and an estimated creatinine clearance of at least 60 mL/min/1.73m2. Participants had at least 1 evaluable segment with plaque on baseline multi-detector computed tomography angiography. Controls (70 males and 30 females) were selected from our blood bank, comprised of a large out-patient sample pool, with no history of cardiovascular, kidney, thyroid, or liver disease or diabetes, no use of lipid-lowering medication, low-density lipoprotein cholesterol (LDL-C) level < 5.18 mmol/L, and triglyceride (TG) level < 3.39 mmol/L.
Laboratory measurements
Plasma and serum samples were collected after an overnight fast. Blood chemistries were determined by standard methods on a COBAS 8000 analyzer at Boston Heart Diagnostics (Framingham, MA): total cholesterol, TG, LDL-C, HDL-C, apoA-I, apoB, and high-sensitivity C-reactive Protein using kits from Roche Diagnostics (Indianapolis, IN), small-dense LDL-C using kits from Denka-Seiken (Tokyo, Japan), and Fibrinogen using kits from Medtest (Cortland Manor, NY). Myeloperoxidase (MPO) was measured on a Dimension clinical chemistry system using kits from Siemens (Newark, DE). ApoA-I concentration in HDL subpopulations were measured in plasma by 2-dimensional native agarose-polyacrylamide gel electrophoresis, immunoblot, and image analysis as previously described(17) and shown in Supplemental Figure I. Serum amyloid A (SAA) was measured by ELISA (Invitrogen, Waltham, MA) at Tufts University. HDL CEC was measured in all subjects at Vascular Strategies (Plymouth Meeting, PA) using methods previously described(11,15). Briefly, ABCA1-dependent CEC was measured in J774 cells radiolabeled with 2μCi of 3H-cholesterol/mL. ABCA1 was upregulated by 6-hour incubation with 0.3 mM of 8-(4-chlorophenylthio)-cyclic- adenosine monophosphate (cAMP). Medium, containing 2.8% apoB-depleted serum, was then added for 4 hours to cAMP-stimulated and unstimulated cells. Liquid scintillation counting was used to quantify the effluxed radio-labeled cholesterol in the medium. Total (global) efflux was defined as the efflux measured from cAMP-stimulated J774 cells. Non-ABCA1-specific (basal) efflux was measured from unstimulated J774 cells. ABCA1-specific efflux was calculated as the difference between cAMP-stimulated (total) and unstimulated (non-ABCA1) cells. SR-BI-mediated CEC was measured as the fraction of radio-labeled cholesterol released from radio-labeled Fu5AH cells to the media containing 2.8% apoB-free serum. Efflux data were expressed as percent cholesterol efflux/4 hour. In the efflux assays, cells were incubated with serum. When only plasma samples were available, plasma was converted to serum by adding 25 mM CaCl2 to plasma for clotting, and then the clot was removed by low-speed centrifugation. HDL anti-oxidative (anti-ox) capacity was measured in 64 cases and 64 gender-matched controls also at Vascular Strategies. This test assessed the ability of apoB-depleted serum to inhibit or enhance the oxidation of LDL in the presence of a fluorescent organic substrate dichlorofluorescein (DCF) (2′,7′-Dichlorodihydrofluorescein diacetate, Sigma-Aldrich). DCF was dissolved in methanol at 2.0 mg/mL and was incubated at room temperature in dark for 1 hour with isolated LDL in the presence or absence of apoB-depleted plasma as surrogate for HDL(23). Fluorescence intensity was determined with a spectrofluorometer set at an excitation wavelength of 485 nm and an emission wavelength of 530 nm. HDL anti-ox capacity was expressed in arbitrary units. Values > 1.0 indicated that HDL had anti-ox capacity while values < 1.0 indicated that HDL was pro-oxidative. To correct for inter-assay variability, a pool of human serum was tested in parallel with the test samples in every efflux- and anti-ox capacity assay. For inter-day comparability, measurements were normalized to a standard pool.
Statistical analysis
The study size was based on power calculations with at least 90% power to detect significant differences in CEC, HDL particle concentrations, and HDL-particle-concentration-normalized CEC between cases and controls. Differences in outcomes were assessed using multivariate linear regression. All correlations reported were coefficients of determination (R2). Cases and controls were gender matched, and all analyses were adjusted for age. Information about any other potential confounders such as anthropometrics or smoking status was not available and thus not included in the analyses. All analyses were performed using the R Language for Statistical Computing version 3.2.2.
RESULTS
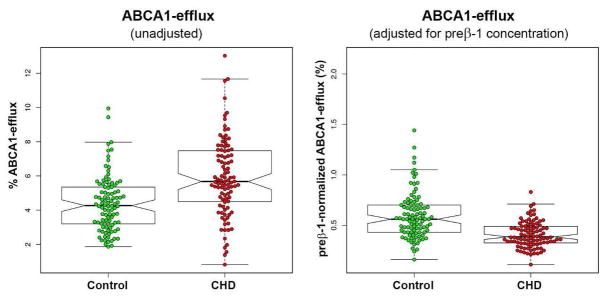
Major characteristics of the CHD and the control groups are presented in Table 1. Compared to controls, cases had significantly lower concentrations of total cholesterol (−7%), LDL-C (−11%), and HDL-C (−20%) and significantly higher concentrations of small, dense LDL-C (61%) and TG (62%). There was no difference in apoA-I concentration between cases and controls but the apoA-I-containing HDL subpopulation profiles were different. Cases had significantly higher concentrations of the small preβ-1 (87%) and α-3 (22%) particles and significantly lower concentration of the large α-1 particles (−28%). There was no correlation between ABCA1-dependent CEC and HDL-C (R2=0.001) and a weak correlation between ABCA1-dependent CEC and apoA-I concentrations (R2=0.110) (Figure 1). ABCA1-dependent CEC was strongly positively correlated with preβ-1 concentration (R2=0.535) but not with the concentration of any other HDL particle (Figure 1 and Supplemental Figure II). Even though the mean preβ-1 concentration was 87% higher, ABCA1-dependent CEC was only 30% higher in the CHD group compared to the control group. Preβ-1-normalized CEC (a metric of preβ-1 functionality) was calculated for each subject by dividing ABCA1-dependent CEC values with preβ-1 concentrations. Cases had significantly (31%) lower preβ-1 functionality than controls. Figure 2 demonstrates that there was large biological variability in ABCA1-dependent CEC in both cases and controls and a large overlap prior to normalization for preβ-1 concentration (left panel). However, the inter-individual variability in preβ-1 functionality was much less in cases than in controls (right panel). There were positive correlations between TG level and ABCA1-dependent CEC (R2=0.317) and preβ-1 level (R2=0.345), but not preβ-1 functionality (R2=0.01) (Figure 3). TG concentration was associated with the concentrations of small HDL particles, preβ-1 (R2=0.345) and α-3 (R2=0.254) (Supplemental Figure III). Moreover, there was a positive correlation between the concentrations of preβ-1 and α-3 particles (R2=0.329). In Supplemental Table I, we compared the measured parameters by gender. Mean preβ-1 concentration and ABCA1-dependent CEC were significantly higher in cases compared to controls in both genders (p<0.01). Preβ-1 functionality was lower in cases compared to controls (males 0.38 vs. 0.61, p<0.01; females 0.53 vs. 0.57, ns).
Table 1.
Characteristics of the study groups
| Control | CHD | P | |
|---|---|---|---|
| Male; Female | 70; 30 | 70; 30 | |
| Age (year) | 55 ± 16 | 61 ± 7 | |
| BMI (kg/m2) | NA | 31.1 ± 3.2 | |
| TC (mmol/L) | 4.3 ± 0.8 | 4.0 ± 0.9 | 0.012 |
| LDL-C (mmol/L) | 2.5 ± 0.6 | 2.2 ± 0.7 | 1.1×10−8 |
| sdLDL-C (mmol/L) | 0.5 ± 0.3 | 0.8 ± 0.4 | 0 |
| HDL-C (mmol/L) | 1.5 ± 0.3 | 1.2 ± 0.4 | 1.6×10−7 |
| TG (mmol/L) | 1.0 ± 0.4 | 1.6 ± 0.8 | 5.0×10−9 |
| apoB (mg/dL) | 81 ± 17 | 79 ± 22 | 0.388 |
| apoA-I (mg/dL) | 156 ± 23 | 156 ± 33 | 0.978 |
| Preβ-1 (mg/dL) | 8.0 ± 3.2 | 15.1 ± 6.9 | 2.4×10−14 |
| α-1 (mg/dL) | 34.0 ± 11.9 | 24.8 ± 13.7 | 1.3×10−6 |
| α-2 (mg/dL) | 63.7 ± 11.6 | 61.6 ± 15.1 | 0.292 |
| α-1+α-2 (mg/dL) | 96.4 ± 19.7 | 86.4 ± 26.7 | 0.001 |
| α-3 (mg/dL) | 21.5 ± 3.9 | 26.0 ± 4.7 | 1.3×10−11 |
| α-4 (mg/dL) | 16.0 ± 4.2 | 16.8 ± 3.8 | 0.025 |
| Global efflux (%) | 10.3 ± 2.2 | 11.4 ± 2.9 | 0.029 |
| ABCA1-efflux (%) | 4.4 ± 1.6 | 5.9 ± 2.3 | 1.3×10−5 |
| Preβ-1-normalized ABCA1 efflux* | 0.59 ± 0.23 | 0.43 ± 0.20 | 7.9×10−7 |
| SR-BI-efflux (%) | 3.4 ± 0.7 | 3.6 ± 0.8 | 0.270 |
| Large-HDL-normalized SR-BI efflux† | 0.035 ± 0.004 | 0.043 ± 0.007 | 4.7×10−15 |
| HDL anti-oxidative capacity‡ | 3.4 ± 0.6 | 2.9 ± 0.5 | 2.5×10−7 |
| hsCRP (mg/L) | 1.9 ± 2.5 | 2.5 ± 4.2 | 0.284 |
| SAA (mg/L) | 29.3 ± 19.5 | 30 ± 20 | 0.178 |
| Fibrinogen (mg/dL) | 329 ± 68 | 453 ± 133 | 1.7×10−13 |
| MPO (pMol/L) | 303 ± 199 | 535 ± 437 | 4.0×10−6 |
Data are expressed as mean ± SD.
Differences between cases and controls were calculated after adjusting data for age.
Preβ-1-normalized ABCA1-efflux (preβ-1-particle functionality) was calculated by dividing ABCA1-dependent cholesterol efflux (%) with preβ-1 concentration (mg/dL).
Large-HDL-normalized SR-BI-efflux (large-HDL-particle functionality) was calculated by dividing SR-BI-dependent cholesterol efflux (%) with the combined concentration of α-1+α-2 (mg/dL).
Anti-oxidative capacity was measured in 64 cases and 64 gender-matched controls and is expressed in arbitrary units.
Abbreviations: BMI body mass index, NA not available, TC total cholesterol, LDL-C low-density lipoprotein cholesterol, sd small dense, HDL-C high-density lipoprotein cholesterol, TG triglycerides, apo apolipoprotein, ABCA1 ATP-binding cassette transporter A1, SR-BI Scavenger receptor class B type I, hsCRP high-sensitivity C-reactive protein, SAA serum amyloid A, MPO myeloperoxidase
Figure 1.
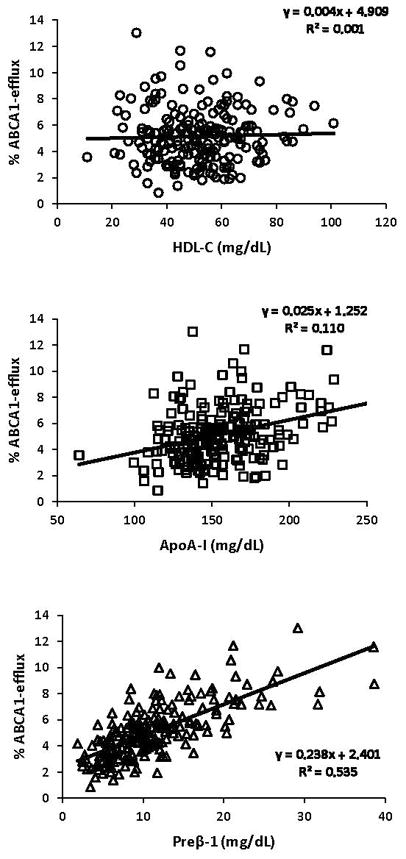
Linear regression analysis on ABCA1-dependent cell-cholesterol efflux capacity with HDL-C, apoA-I, and preβ-1-HDL particle levels (n=200).
Figure 2.
Box-plot analysis of unadjusted and adjusted (preβ-1-concentration-normalized) data on cell-cholesterol efflux capacity via the ABCA1 pathway.
Figure 3.
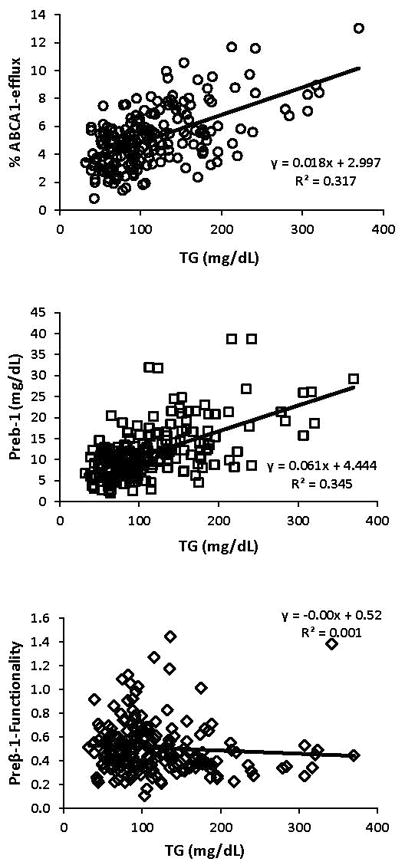
Linear regression analyses on ABCA1-dependent cell-cholesterol efflux capacity, preβ-1, and preβ-1 functionality with TG (n=200).
Compared to controls, cases had significantly lower (−12%) concentrations of large (α-1+α-2) HDL particles but no difference in CEC via the SR-BI pathway. Cases had significantly higher (22%) large-HDL-concentration-normalized SR-BI efflux (a metric of large-HDL-particle functionality). Strong positive correlations were observed between SR-BI-dependent CEC and the levels of HDL-C (R2=0.564), apoA-I (R2=0.685), and large (α-1+α-2) HDL particles (R2=0.712) (Figure 4). There were strong correlations between SR-BI-dependent efflux and the large lipid-rich HDL particles (α-1 R2=0.542, α-2 R2=0.618) but not the smaller lipid-poor HDL particles (Supplemental Figure IV). There was large biological variability in SR-BI-dependent efflux in both the normalized and non-normalized models (Figure 5). Compared to control males, control females had significantly higher SR-BI-dependent CEC due to higher levels of large HDL particles, but there was no gender difference in large-HDL-particle functionality (Supplemental Table I).
Figure 4.
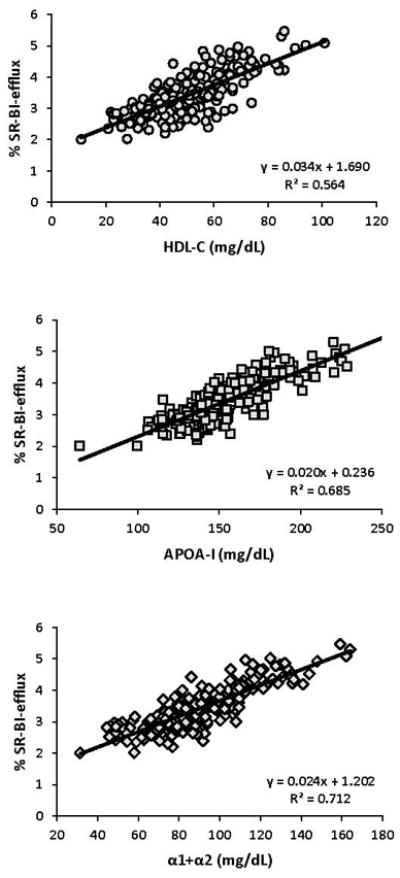
Linear regression analysis on SR-BI-dependent cell-cholesterol efflux capacity with HDL-C, apoA-I, and large-HDL particle concentrations (n=200).
Figure 5.
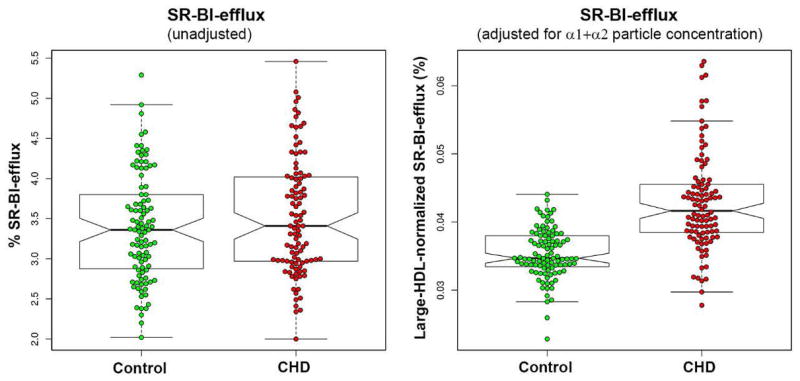
Box-plot analysis of unadjusted and adjusted (large-HDL-particle-normalized) data on cell-cholesterol efflux via the SR-BI pathway
Data on BMI were available only in cases; therefore, we investigated the relationship between BMI and ABCA1- and SR-BI-dependent CEC only in this group (Supplemental Figure V). There were no significant correlations between BMI and either ABCA1-dependent CEC (R2=0.002) or preβ-1 functionality (R2=0.0003). There was a weak inverse correlation between BMI and SR-BI-dependent CEC (R2=−0.043) and a weak positive correlation between BMI and the functionality of large (α1+α2) HDL particles (R2=0.034).
The mean level of HDL anti-ox capacity was significantly lower (−16%) in CHD cases compared to controls. Cases had higher levels of inflammatory- (C-reactive Protein, SAA, and fibrinogen) and oxidative-stress (MPO) markers than controls, but only fibrinogen and MPO were significantly different. Linear regression analysis indicated weak inverse correlations between preβ-1 functionality and the concentrations of SAA (R2=0.021), fibrinogen (R2=0.046), and MPO (R2=0.031), and weak positive correlations between large-HDL-particle functionality and the concentrations of fibrinogen (R2=0.179) and MPO (R2=0.030) (Supplemental Figure VI). The correlations between HDL anti-ox capacity and the concentrations of SAA (R2=0.002), fibrinogen (R2=0.056), and MPO (R2=−0.013) were weak and inconsistent (Supplemental Figure VII). There was a positive correlation between HDL anti-ox capacity and preβ-1 functionality (R2=0.135) and an inverse correlation between HDL anti-ox capacity and large-HDL-particle functionality (R2=0.117) (Supplemental Figure VIII).
DISCUSSION
ABCA1 plays a crucial role in cholesterol efflux from macrophages, and small lipid-poor preβ-1 particles are the primary acceptors of cholesterol via this pathway(15,16,18). We have demonstrated that although CHD patients have higher preβ-1 concentration, the functionality of their preβ-1 particles is reduced compared to controls. ABCA1-dependent CEC was strongly correlated with the concentrations of preβ-1 particles, but very weakly with the levels of HDL-C, apoA-I, and other HDL particles (Figure 1 and Supplemental Figure II). We have found that the 87% higher preβ-1 level in CHD patients was associated with only a 30% higher ABCA1-dependent CEC as compared to controls (Table 1). Most importantly, we have shown that preβ-1 functionality was significantly lower (−31%) and less variable in cases as compared to controls indicating that preβ-1 particles were altered in a similar way in cases (Figure 2). We corroborated previous findings(15,16) that SR-BI-dependent CEC was strongly correlated with the concentrations of HDL-C, apoA-I, and the highly-lipidated large HDL particles (α-1+α-2) (Figure 4). In contrast to preβ-1 particles, the concentration of large-HDL particles was significantly lower in CHD cases compared to controls, but their functionality was significantly higher (Table 1). These findings clearly demonstrate that ABCA1-dependent and SR-BI-dependent CEC are influenced by both the concentration and the functionality of the HDL particles participating in these efflux pathways.
The mechanisms that alter ABCA1-dependent efflux in CHD cases are not well understood. There are a number of possibilities, some of which were addressed by this study and many more which are yet to be investigated. Since the cells used in the efflux analyses are constant, the variability in ABCA1-dependent CEC must relate to variability in the concentration, composition, and physicochemical properties of preβ-1 particles. As the influence of particle concentration on CEC was eliminated by the normalization, the variability in CEC can only be explained by differences in the composition and/or physicochemical properties of preβ-1 particles. Linear regression analysis showed better correlation between preβ-1 concentration and ABCA1-dependent CEC in CHD patients (R2=0.481) as compared to controls (R2=0.312) suggesting that specific alteration(s) generated morphologically more similar, although less functional, preβ-1 particles in cases. Precisely how these particles are altered and why those alterations reduced their functionality requires further exploration.
One initial proposed mechanism was that low preβ-1 functionality in CHD patients was the result of high levels of recycled preβ-1 particles due to elevated TG levels and increased cholesteryl ester transfer protein (CETP) and hepatic lipase activities. Preβ-1 particles are formed in two different ways: 1) de novo preβ-1 particles form from newly synthesized apoA-I; 2) recycled preβ-1 particles are generated during the remodeling of mature large HDL particles. An elevated TG level, which is common in CHD patients, increases CETP and hepatic lipase activities. CETP exchanges cholesterol for TG between TG-rich lipoproteins and large HDL particles(24,25). Hepatic lipase digests TG in large HDL particles and, as their core shrinks, some surface lipids and apoA-I are shed and are recycled as preβ-1 particles(26). At that time, we assumed that recycled preβ-1 particles had altered composition with low functionality. However, there was no association between preβ-1 functionality and TG levels in this study (Figure 3). Moreover, in previous studies, positive associations have been shown between TG levels and preβ-1 functionality in subjects with high TG levels without CHD as well as between TG levels and CEC(16,27,28). Therefore, we concluded that an increased level of recycled preβ-1 particles, as a consequence of elevated TG level, was not the cause of low preβ-1 functionality in CHD patients.
Another possibility was that inflammatory and oxidative stress damage HDL and impair reverse cholesterol transport(29–31). It is well documented that MPO oxidizes apoA-I(32). In this study, CHD patients had significantly increased MPO levels compared to controls but the influence of MPO level on preβ-1 functionality was weak (R2=0.031) (Supplemental Figure VI). Moreover, neither in this study nor in an earlier study, have we found significant associations between increased levels of MPO, SAA and fibrinogen and preβ-1 functionality(16), therefore, we excluded high levels of circulating inflammatory- and oxidative-stress markers as significant players in the decreased preβ-1 functionality in CHD patients.
We have also investigated how HDL anti-ox capacity, a key athero-protective function of HDL(33), was associated with HDL CEC. HDL anti-ox capacity was significantly lower (−16%) in cases compared to controls; however, it is worth noting that none of the participants had pro-oxidative HDL (anti-ox capacity <1.0). The relationships between HDL anti-ox capacity and HDL-particle functionality were equivocal. HDL anti-ox capacity was associated positively with preβ-1 functionality (R2=0.135) and inversely with large-HDL-particle functionality (R2=0.117) (Supplemental Figure VIII). Moreover, there were no significant correlations between HDL anti-ox capacity and the concentrations of inflammatory- and oxidative-stress markers (Supplemental Figure VII).
We have investigated the influence of BMI on ABCA1- and the SR-BI-dependent CEC in the CHD group where BMI data were available. We found no correlation between BMI and either ABCA1-dependent CEC or preβ-1 functionality (Supplemental Figure IV). There is no consensus on the influence of BMI on CEC. Attia et al. reported increased ABCA1-dependent CEC in 12 overweight to obese women compared to 9 normal-weight women and a significant positive correlation between BMI and ABCA1-dependent CEC(42). In contrast, Talbot et al. reported 9% lower ABCA1-dependent CEC in 52 obese men compared to 25 normal-weight men and a significant inverse correlation between BMI and ABCA1-dependent CEC(43). We believe that BMI has no direct effect on ABCA1-dependent CEC, but may have an indirect influence on it by modifying the concentrations of TG and/or HDL particles.
We assume that the physicochemical properties of apoA-I in preβ-1 particles are altered in CHD patients compared to controls and are responsible for the reduced ABCA1-dependent CEC. Preliminary data generated in our laboratory indicate that the lipid composition of preβ-1 is more complex than previously thought and may be different between cases and controls. We also think that the higher functionality of large-HDL particles in cases compared to controls may partially be caused by the elevated TG levels. Generally, increased plasma TG level is associated with increased CETP activity, which in turn decreases cholesteryl ester concentration and increases the phospholipid/cholesterol ratio in large HDL particles. These alterations make large-HDL particles better acceptors of cell cholesterol. However, hepatic lipase remodels the TG-enriched large-HDL particles into smaller HDL particles(16). In our view, high TG level induces the formation of more functional large HDL particles, but simultaneously enhances the degradation of these particles.
Our findings are in line with previous ones showing that drug-induced changes in preβ-1 concentration correlate significantly and positively with changes in ABCA1-dependent CEC(34–36). As CHD patients have significantly higher preβ-1 levels than controls(8,9,19–21) and there is a strong positive correlation between preβ-1 level and CEC, one would expect to see elevated CEC in cases compared to controls. Our findings are in line with this assumption. However, others reported lower CEC in CHD cases compared to controls(11–13). One reason for this discrepancy is that the nearly 2-fold higher preβ-1 level in cases may have almost perfectly offset the decreased preβ-1 functionality as seen in this study. Other reasons may be related to differences in the study populations or in lipid-modifying interventions. It has been documented that both statins and niacin decrease preβ-1 concentration in a drug- and dose-specific way(37,38). However, there is no consensus on the effects of lipid-modifying interventions on CEC. Studies have reported either an increase, a decrease, or no change in ABCA1-dependent CEC after statin or niacin treatment(27,28,39–41). Moreover, it is possible that lipid-modifying interventions influence the concentration and the functionality of HDL particles independently.
There are several limitations to this study. Since all cases were on statins but none of the controls, we could not dissect the drug effects from the effects of CHD prevalence on HDL CEC. Further limitations include the relatively low number of subjects and the inability to adjust for all potential confounders (e.g. BMI, smoking, and diabetes). Due to these limitations, our results might not be comparable to other populations having different pathological conditions and receiving different medications. A significantly larger and better-characterized population is needed to dissect the effects of CHD from other parameters on HDL CEC.
The strength of this study is that we were able to determine not only the efflux capacity of HDL, but also the functionality of key HDL particles that mediate cell-cholesterol efflux. However, further studies are needed to determine the physicochemical and compositional properties of preβ-1 HDL particles to explain why they are dysfunctional in CHD patients.
Conclusions
CHD patients had higher than normal preβ-1 particle concentrations but those particles had lower than normal CEC reducing their maturation into larger HDL particles. We excluded high plasma levels of TG, MPO, and fibrinogen as significant contributors to the decreased preβ-1 functionality in CHD patients. Cases had lower than normal concentrations of α-1+α-2 particles but those particles had higher CEC compared to controls indicating that the functionality of large-HDL particles was not compromised in CHD patients. Our data clearly support the concept that reverse cholesterol transport (the sum of HDL’s cell-cholesterol efflux and transport capacities from peripheral cells to the bile) is influenced not only by the concentrations of individual HDL particles but also by their functional properties. These properties are altered in CHD cases compared to controls. We strongly believe that measurement of both the concentration and the functionality of HDL particles, involved in cell-cholesterol efflux, is critical for better understanding HDL’s role in CHD and in designing interventions that increase not only the concentration, but also the functionality of HDL.
Supplementary Material
Highlights.
Preβ-1 HDL is responsible for about 54% of ABCA1-dependent cell-cholesterol efflux.
Large HDL particles (α-1+α-2) are responsible for about 72% of SR-BI-dependent cell cholesterol efflux.
CHD patients have elevated preβ-1 levels (87%) but lower preβ-1 functionality (−31%) compared to controls.
CHD patients have lower concentrations of large-HDL particles (−12%) but higher large-HDL particle functionality (22%) compared to controls.
HDL cell-cholesterol efflux capacity is influenced by both the concentrations and the functionality of HDL particles; therefore, to better understand the roles of HDL in health and disease, both HDL properties should be measured.
Acknowledgments
The authors thank Dr. Brett Milburn for his help with the statistical analyses and Drs. Allison Goldfine and Francine Welty for providing plasma samples for the study.
Sources of funding: This work was supported by grants to Dr. Asztalos from the NIH/NHLBI (HL117933) and from Boston Heart Diagnostics, Framingham, MA.
Abbreviations
- anti-ox
Anti-oxidative
- apo
Apolipoprotein
- ABCA1
ATP-binding cassette transporter A1
- BMI
body mass index
- CEC
Cell cholesterol efflux capacity
- CETP
Cholesteryl ester transfer protein
- CHD
Coronary heart disease
- HDL
High-density lipoprotein
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- MPO
Myeloperoxidase
- SR-BI
Scavenger receptor class B type I
- SAA
Serum Amyloid A
- TG
Triglyceride
Footnotes
Disclosures: EJ Schaefer is an employee of Boston Heart Diagnostics.
References
- 1.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR, Jr, Bangdiwala S, Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Ballantyne CM, Herd JA, Ferlic LL, Dunn JK, Farmer JA, Jones PH, Schein JR, Gotto AM., Jr Influence of low HDL on progression of coronary artery disease and response to fluvastatin therapy. Circulation. 1999;99:736–743. doi: 10.1161/01.cir.99.6.736. [DOI] [PubMed] [Google Scholar]
- 3.Nicholls SJ, Tuzcu EM, Sipahi I, Grasso AW, Schoenhagen P, Hu T, Wolski K, Crowe T, Desai MY, Hazen SL, Kapadia SR, Nissen SE. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA. 2007;297:499–508. doi: 10.1001/jama.297.5.499. [DOI] [PubMed] [Google Scholar]
- 4.Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesäniemi YA, Sullivan D, Hunt D, Colman P, d’Emden M, Whiting M, Ehnholm C, Laakso M FIELD study investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 5.The AIM-HIGH Investigators. Niacin in patients with low HDL-cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS dal-OUTCOMES Investigators. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 7.Ballantyne FC, Clark RS, Simpson HS, Ballantyne D. High-density and low-density lipoprotein subfractions in survivors of myocardial infarction and in control subjects. Metabolism. 1982;31:433–437. doi: 10.1016/0026-0495(82)90230-x. [DOI] [PubMed] [Google Scholar]
- 8.Asztalos BF, Cupples LA, Demissie S, Horvath KV, Cox CE, Batista MC, Schaefer EJ. High-density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants of the Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 2004;24:2181–2187. doi: 10.1161/01.ATV.0000146325.93749.a8. [DOI] [PubMed] [Google Scholar]
- 9.Asztalos BF, Collins D, Cupples LA, Demissie S, Horvath KV, Bloomfield HE, Robins SJ, Schaefer EJ. Value of high-density lipoprotein (HDL) subpopulations in predicting recurrent cardiovascular events in the Veterans Affairs HDL-Intervention Trial. Arterioscler Thromb Vasc Biol. 2005;25:2185–2191. doi: 10.1161/01.ATV.0000183727.90611.4f. [DOI] [PubMed] [Google Scholar]
- 10.Williams PT. Fifty-three year follow-up of coronary heart disease versus HDL2 and other lipoproteins in Gofman’s Livermore Cohort. J Lipid Res. 2012;53:266–272. doi: 10.1194/jlr.M019356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DJ, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, Lukmanova D, Mucksavage ML, Luben R, Billheimer J, Kastelein JJ, Boekholdt SM, Khaw KT, Wareham N, Rader DJ. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3:507–513. doi: 10.1016/S2213-8587(15)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li XM, Tang WH, Mosior MK, Huang Y, Wu Y, Matter W, Gao V, Schmitt D, Didonato JA, Fisher EA, Smith JD, Hazen SL. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol. 2013;33:1696–1705. doi: 10.1161/ATVBAHA.113.301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asztalos BF, de la Llera-Moya M, Dallal GE, Horvath KV, Schaefer EJ, Rothblat GH. Differential effects of HDL subpopulations on cellular ABCA1- and SR-BI-mediated cholesterol efflux. J Lipid Res. 2005;46:2246–2253. doi: 10.1194/jlr.M500187-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Asztalos BF, Horvath KV, Mehan M, Yokota Y, Schaefer EJ. Influence of HDL particles on cell-cholesterol efflux under various pathological conditions. J Lipid Res. 2017;58:1238–1246. doi: 10.1194/jlr.M075648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asztalos BF, Roheim PS, Milani RL, Lefevre M, McNamara JR, Horvath KV, Schaefer EJ. Distribution of apoA-I-containing HDL subpopulations in patients with coronary heart disease. Arterioscler Thromb Vasc Biol. 2000;20:2670–2676. doi: 10.1161/01.atv.20.12.2670. [DOI] [PubMed] [Google Scholar]
- 18.Favari E, Lee M, Calabresi L, Franceschini G, Zimetti F, Bernini F, Kovanen PT. Depletion of pre-beta-high density lipoprotein by human chymase impairs ATP-binding cassette transporter A1- but not scavenger receptor class B type I-mediated lipid efflux to high density lipoprotein. J Biol Chem. 2004;279:9930–9936. doi: 10.1074/jbc.M312476200. [DOI] [PubMed] [Google Scholar]
- 19.Guey LT, Pullinger CR, Ishida BY, O’Connor PM, Zellner C, Francone OL, Laramie JM, Naya-Vigne JM, Siradze KA, Deedwania P, Redberg RF, Frost PH, Seymour AB, Kane JP, Malloy MJ. Relation of increased prebeta-1 high-density lipoprotein levels to risk of coronary heart disease. Am J Cardiol. 2011;108:360–366. doi: 10.1016/j.amjcard.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 20.de Vries R, Perton FG, van Tol A, Dullaart RP. Carotid intima media thickness is related positively to plasma preß-high-density lipoproteins in non-diabetic subjects. Clin Chim Acta. 2012;413:473–477. doi: 10.1016/j.cca.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Miida T, Nakamura Y, Inano K, Matsuto T, Yamaguchi T, Tsuda T, Okada M. Prebeta-1 high-density lipoprotein increases in coronary artery disease. Clin Chem. 1996;42:1992–1995. [PubMed] [Google Scholar]
- 22.Hauser TH, Salastekar N, Schaefer EJ, Desai T, Goldfine HL, Fowler KM, Weber GM, Welty F, Clouse M, Shoelson SE, Goldfine AB Targeting Inflammation Using Salsalate in Cardiovascular Disease (TINSAL-CVD) Study Team. Effect of targeting inflammation with salsalate: The TINSAL-CVD randomized clinical trial on progression of coronary plaque in overweight and obese patients using statins. JAMA Cardiol. 2016;1:413–423. doi: 10.1001/jamacardio.2016.0605. [DOI] [PubMed] [Google Scholar]
- 23.Navab M, Hama SY, Hough GP, Subbanagounder G, Reddy ST, Fogelman AM. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J Lipid Res. 2001;42:1308–1317. [PubMed] [Google Scholar]
- 24.Asztalos BF, Schaefer EJ, Horvath KV, Yamashita S, Miller M, Franceschini G, Calabresi L. Role of LCAT in HDL remodeling: investigation of LCAT-deficiency states. J Lipid Res. 2007;48:592–599. doi: 10.1194/jlr.M600403-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Tani M, Horvath KV, Lamarche B, Couture P, Burnett JR, Schaefer EJ, Asztalos BF. High-density lipoprotein subpopulation profiles in lipoprotein lipase and hepatic lipase deficiency. Atherosclerosis. 2016;253:7–14. doi: 10.1016/j.atherosclerosis.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rye K, Barter PJ. Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler Tromb Vasc Biol. 2004;24:421–428. doi: 10.1161/01.ATV.0000104029.74961.f5. [DOI] [PubMed] [Google Scholar]
- 27.Khera AV, Demler O, Adelman SJ, Collins HL, Glynn RJ, Ridker PM, Rader DJ, Mora S. Cholesterol efflux capacity, HDL particle number, and incident cardiovascular events. An analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin) Circulation. 2017;135:2494–2504. doi: 10.1161/CIRCULATIONAHA.116.025678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khera AV, Patel PJ, Reilly MP, Rader DJ. The addition of niacin to statin therapy improves high-density lipoprotein cholesterol levels but not metrics of functionality. J Am Coll Cardiol. 2013;62:1909–1910. doi: 10.1016/j.jacc.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 29.Banka CL, Yuan T, de Beer MC, Kindy M, Curtiss LK, de Beer FC. Serum amyloid A (SAA): influence on HDL-mediated cellular cholesterol efflux. J Lipid Res. 1995;36:1058–1065. [PubMed] [Google Scholar]
- 30.Vaisar T, Tang C, Babenko I, Hutchins P, Wimberger J, Suffredini AF, Heinecke JW. Inflammatory remodeling of the HDL proteome impairs cholesterol efflux capacity. J Lipid Res. 2015;56:1519–1530. doi: 10.1194/jlr.M059089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenson RS, Brewer HB, Jr, Ansell BJ, Barter P, Chapman MJ, Heinecke JW, Kontush A, Tall AR, Webb NR. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol. 2016;13:48–60. doi: 10.1038/nrcardio.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao B, Pennathur S, Heinecke JW. Myeloperoxidase targets apolipoprotein A-I, the major high-density lipoprotein protein, for site-specific oxidation in human atherosclerotic lesions. J Biol Chem. 2012;287:6375–6386. doi: 10.1074/jbc.M111.337345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabet F, Rye KA. High-density lipoproteins, inflammation and oxidative stress. Clinical Science. 2009;116:87–98. doi: 10.1042/CS20080106. [DOI] [PubMed] [Google Scholar]
- 34.Diditchenko S, Gille A, Pragst I, Stadler D, Waelchli M, Hamilton R, Leis A, Wright SD. Novel formulation of a reconstituted high-density lipoprotein (CSL112) dramatically enhances ABCA1-dependent cholesterol efflux. Arterioscler Thromb Vasc Biol. 2013;33:2202–2211. doi: 10.1161/ATVBAHA.113.301981. [DOI] [PubMed] [Google Scholar]
- 35.Nicholls SJ, Ruotolo G, Brewer HB, Kane JP, Wang MD, Krueger KA, Adelman SJ, Nissen SE, Rader DJ. Cholesterol efflux capacity and pre-beta-1 HDL concentrations are increased in dyslipidemic patients treated with evacetrapib. J Am Coll Cardiol. 2015;66:2201–2210. doi: 10.1016/j.jacc.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 36.Kempen HJ, Asztalos BF, Moerland M, Jeyarajah E, Otvos J, Kallend DG, Bellibas SE, Wijngaard PL. High-density lipoprotein subfractions and cholesterol efflux capacities after infusion of MDCO-216 (Apolipoprotein A-IMilano/Palmitoyl-Oleoyl-Phosphatidylcholine) in healthy volunteers and stable coronary artery disease patients. Arterioscler Thromb Vasc Biol. 2016;36:736–742. doi: 10.1161/ATVBAHA.115.307052. [DOI] [PubMed] [Google Scholar]
- 37.Asztalos BF, Horvath KV, McNamara JR, Roheim PS, Rubinstein JJ, Schaefer EJ. Comparing the effects of five different statins on the HDL subpopulation profiles of coronary heart disease patients. Atherosclerosis. 2002;164:361–369. doi: 10.1016/s0021-9150(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 38.Asztalos BF, Batista M, Horvath KV, Cox CE, Dallal GE, Morse JS, Brown GB, Schaefer EJ. Change in alpha-1 HDL concentration predicts progression in coronary artery stenosis. Arterioscler Thromb Vasc Biol. 2003;23:847–852. doi: 10.1161/01.ATV.0000066133.32063.BB. [DOI] [PubMed] [Google Scholar]
- 39.El Khoury P, Waldmann E, Huby T, Gall J, Couvert P, Lacorte JM, Chapman J, Frisdal E, Lesnik P, Parhofer KG, Le Goff W, Guerin M. Extended-release Niacin/Laropiprant improves overall efficacy of postprandial reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2016;36:285–294. doi: 10.1161/ATVBAHA.115.306834. [DOI] [PubMed] [Google Scholar]
- 40.Ronsein GE, Hutchins PM, Isquith D, Vaisar T, Zhao XQ, Heinecke JW. Niacin therapy increases high-density lipoprotein particles and total cholesterol efflux capacity but not ABCA1-specific cholesterol efflux in statin-treated subjects. Arterioscler Thromb Vasc Biol. 2016;36:404–411. doi: 10.1161/ATVBAHA.115.306268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franceschini G, Calabresi L, Colombo C, Favari E, Bernini F, Sirtori CR. Effects of fenofibrate and simvastatin on HDL-related biomarkers in low-HDL patients. Atherosclerosis. 2007;195:385–391. doi: 10.1016/j.atherosclerosis.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 42.Attia N, Fournier N, Vedie B, Cambillau M, Beaune P, Ziegler O, Grynberg A, Paul JL, Guerci B. Impact of android overweight or obesity and insulin resistance on basal and postprandial SR-BI- and ABCA1-mediated serum cholesterol efflux capacities. Atherosclerosis. 2010;209:422–9. doi: 10.1016/j.atherosclerosis.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 43.Talbot CPJ, Plat J, Joris PJ, Konings M, Kusters YHAM, Schalkwijk CG, Ritsch A, Mensink RP. HDL cholesterol efflux capacity and cholesteryl ester transfer are associated with body mass, but are not changed by diet-induced weight loss: A randomized trial in abdominally obese men. Atherosclerosis. 2018;274:23–28. doi: 10.1016/j.atherosclerosis.2018.04.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.