Abstract
Background and purpose
MRI-visible perivascular spaces in the centrum semiovale (CSO-PVS) have been associated with cerebral amyloid angiopathy (CAA).We aimed to further confirm this link by evaluating CSO-PVS volume in pathologically-demonstrated sporadic and genetically-demonstrated hereditary forms of the disease.
Methods
We studied a retrospective hospital-based cohort consisting of 63 individuals aged>55 having brain MRI and pathological assessment of CAA (mean age 73.6±8.5, 46% female), and a separate cohort consisting of 26 carriers and 28 non-carriers of the hereditary cerebral hemorrhage with amyloidosis–Dutch type (HCHWA-D)(mean age 46.7±12.8, 61.1% female). CSO-PVS volume was quantified on a single MRI slice using a computer-assisted segmentation method and expressed as the relative volume of the intracranial volume in that particular slice (CSO-PVS relative volume). We compared CSO-PVS relative volume: 1) between subjects with and without the disease in both cohorts; 2) between non-CAA, CAA without hemorrhage and CAA with hemorrhage cases in the sporadic CAA cohort. All variables reaching p<0.1 in bivariate analyses were entered in logistic regression models.
Results
In both sporadic and Dutch cohorts, cases with CAA had significantly higher CSO-PVS relative volume than cases without (median[IQR] 3.7%[2.5–5.3] vs 1.8%[1.2–2.4], p<0.0001; 3.8%[0.6–6.2] vs 0.7%[0.4–1.6], p=0.007, respectively). In linear regression models, sporadic CAA was associated with higher CSO-PVS relative volume (p=0.008). In the sporadic CAA cohort, compared to non-CAA cases, CSO-PVS relative volume was higher in both CAA with hemorrhage and without hemorrhage (4.4%[2.6–6.1] and 3%[2.4–3.6] vs 1.8%[1.2–2.4], p<0.001 and p=0.005 respectively). Higher CSO-PVS relative volume was associated with CAA in regression models, both when hemorrhage was present (OR[95% CI]=2.63[1.33–5.18], p=0.005) and absent (OR[95% CI]=4.55[0.98–21.04], p=0.05).
Conclusions
Increased CSO-PVS volume is a consistent MRI marker of cerebrovascular amyloid deposition, and a promising diagnostic tool for sporadic CAA without hemorrhagic manifestations.
MeSH key words: sporadic cerebral amyloid angiopathy, HCHWAD, Hereditary Cerebral Amyloid Angiopathy, Dutch Type Angiopathy, cerebral hemorrhage, neuroimaging
Subject terms: sporadic cerebral amyloid angiopathy, hereditary cerebral amyloid angiopathy, Dutch type, perivascular spaces, dilated perivascular spaces, lobar hemorrhage, lobar microbleed
INTRODUCTION
Perivascular spaces (PVS) surround arteries, arterioles and venules as they run through the brain parenchyma1. PVS are filled with interstitial fluid (ISF), which contains plasma solutes and waste products from brain metabolism (including β-amyloid). Theoretical and animal models have suggested that ISF flow within PVS is largely driven by the blood flow in the associated vessel2–4 and that these two flows follow opposite directions3, 5. The periarterial pathway is a main clearance mechanism for soluble β-amyloid (Aβ), and it was shown to be defective in ageing6 and in the context of the two main disorders associated with amyloid accumulation in the brain: Alzheimer’s disease (AD)2 and cerebral amyloid angiopathy (CAA)6. CAA, in turn, may further impair ISF drainage by physically obstructing PVS at a cortical level, setting in motion a forward feedback loop.7,8 Thus accumulating evidence supports the view that PVS dysfunction may be a strong determinant of Aβ build-up in AD/CAA.
PVS are usually microscopic and hence not visible on conventional MRI; presumably when PVS are dilated they become increasingly visible on neuroimaging. Increased MRI-visible PVS have been reported as a frequent feature in ageing9 and in relation to several pathologic conditions10–13. Extensive PVS may ultimately reflect a state of ISF stagnation, and be an indication of poor drainage of certain solutes toxic to the brain, such as Aβ species. In line with this hypothesis, two landmark radiological studies using different patient populations found a consistent association between increased PVS in the whole-brain white matter (WM-PVS), and the centrum semiovale (CSO-PVS) in particular, and presence and number of strictly lobar hemorrhages, a putative marker of CAA14, 15. Also, high grade of CSO-PVS has been associated with higher total cortical retention of Pittsburgh B compound (PiB) in a PET study of non-demented elderly individuals, including cases with “probable CAA”16. Although these findings are potentially of great clinical interest, the association between increased CSO-PVS and neuropathologically-confirmed CAA has been explored only in one small study17. Therefore, it is still uncertain whether increased CSO-PVS are linked to the presence of CAA and, importantly, whether CSO-PVS may have a diagnostic role for CAA independent of strictly lobar hemorrhages, the most salient putative biomarker of the disease18, 19.
One further point to consider when addressing the role of PVS dilation in CAA comes from a recent memory cohort study, which suggests that ageing and hypertension, which had been linked to PVS dilation in the basal ganglia regions14, 15, could also contribute to dilation of CSO-PVS20. One way to overcome this potential overlap is to target younger individuals carrying hereditary forms of CAA, as the confounding effect of advanced age and long exposure to vascular risk factors is virtually avoided. To our knowledge, no neuroimaging studies on PVS have been conducted in hereditary CAA populations to date.
In this study, we aimed to confirm whether increased CSO-PVS may have an independent diagnostic role for CAA. For this purpose, we evaluated MRI-visible CSO-PVS in: 1) a hospital-based cohort of elderly subjects having neuropathological assessment of CAA (sporadic CAA); and 2) a young cohort of carriers and non-carriers of the hereditary cerebral hemorrhage with amyloidosis–Dutch type (HCHWA-D) mutation, a ‘pure’ early-onset cerebrovascular amyloidosis. In a second step, we assessed in the sporadic CAA cohort whether CSO-PVS could independently predict the presence of underlying CAA pathology even in the absence of any hemorrhagic lesions, including cerebral microbleeds.
SUBJECTS AND METHODS
(The data that support the findings of this study are available from the corresponding author upon reasonable request).
Study cohorts
“Sporadic cohort”
We searched across datasets for patients seen at Massachusetts General Hospital (MGH) between 1997 and 2012 who were older than 55 years and had records of both brain MRI and neuropathological examination of the brain. We applied an exclusion algorithm to obtain a cohort of individuals having high-quality, complete MRI brain studies (including hemosiderin-specific sequences) and explicit CAA assessment. Details on inclusion/exclusion workflow are described in Figure 1. A total of 63 individuals met criteria for inclusion.
Figure 1.
Inclusion/exclusion workflow for the hospital-based cohort.
“Hereditary cohort”
Subjects were identified and selected via the HCHWA-D patient association in Katwijk (the Netherlands) and the outpatient clinic of the Department of Neurology of the Leiden University Medical Center based on DNA analysis for confirmation of the codon 693 mutation in the amyloid-β precursor protein (AβPP) gene. Twenty-six DNA-proven HCHWA-D mutation carriers were included in the present study. These subjects had a mean age of 45.9 years (25th –75th percentile 35 to 55 years). We included both symptomatic (n=15) and pre-symptomatic (n=11) mutation carriers. Control subjects were recruited from relatives at risk for HCHWA-D but who tested genetically negative. Additional control subjects were recruited from subject spouses, relatives or friends, who also underwent genetic testing for inclusion. Twenty-eight controls were included in the present study. These subjects had a mean age of 47.5 years (25th –5th percentile 37 to 57 years).
Definition of sporadic and hereditary CAA diagnosis
Sporadic CAA (hospital-based cohort)
Brain pathology reports were reviewed by a neurologist blinded to radiological and clinical data (SMR). Specific studies for CAA detection included Congo red staining and/or β-amyloid immunostaining (DAKO) of paraffin-embedded sections of neocortex and leptomeninges21. CAA diagnosis was made if vascular amyloid deposition (≥ 1 and ≥ 2 in Vonsattel’s severity scale for CAA on brain biopsy and full autopsy respectively) was documented.
HCHWA-D mutation (Dutch cohort)
DNA isolation and analysis for identification of presence/absence of E22Q point mutation within AβPP gene was performed in all participants. Methodology has been described in detail elsewhere22, 23.
Imaging acquisition and analysis
MRI studies were obtained using 1.5T and 3T scanners. Sequences used in this study were T2-weighted fast spin echo (FSE), FLAIR and either T2*-gradient echo (GRE) or susceptibility-weighted imaging (SWI). Imaging protocols and technical parameters of MRI sequences for both study cohorts have been described elsewhere19, 24.
Microbleeds (MBs) were defined as focal, small (<10 mm), round or ovoid areas of marked signal-loss on either GRE or SWI sequences, different from vascular flow voids, calcifications, cavernous malformations and basal ganglia mineralization. On the same MRI sequences, intracerebral hemorrhages (ICHs) were defined as parenchymal defects, generally >10 mm, with evidence of hemosiderin deposition. Rating of microbleeds and ICHs was done following previously published guidelines25.
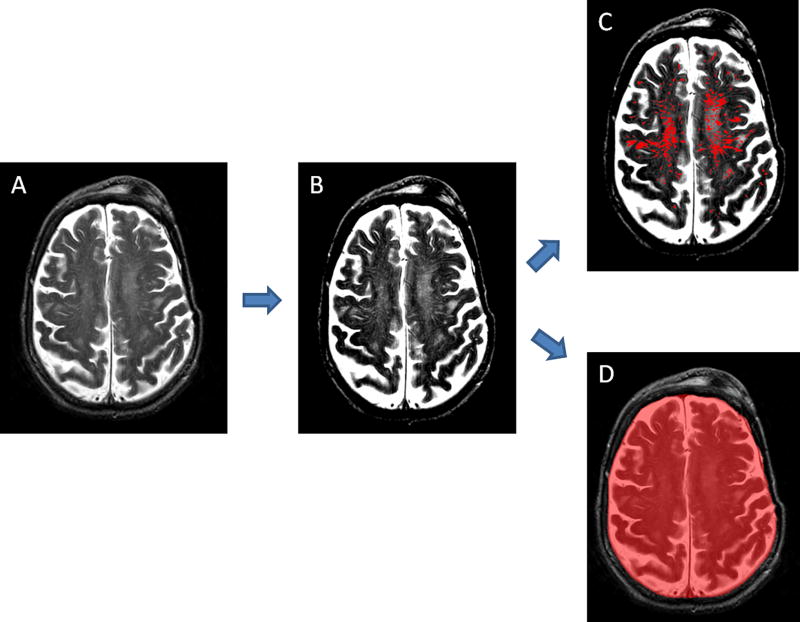
WMH volumes were quantified on FLAIR sequences. We used a previously described method consisting of the substraction of a first layer of regions of interest (ROIs) corresponding to WMH created semiautomatically based on intensity thresholding, and a second layer of ROIs manually outlined by gross contouring of all WMHs26. A slightly modified version of this method was used to calculate CSO-PVS relative volume on T2-FSE images. The PVS segmentation technique comprised the following steps: 1) selection of the first brain slice immediately above the lateral ventricles; 2) adjustment of brightness and contrast to highlight PVS against the white matter in the background; 3) thresholding and creation of an intensity-based mask that includes as many visible PVS as possible; 4) drawing manually a second mask over areas of the white matter containing dilated PVS; 5) subtraction of both masks; 6) manual correction of the resulting mask to ensure that it contains only PVS. As the plane of MRI acquisition and other factors could substantially modify the volume of brain where PVS were assessed from subject to subject, we adjusted the absolute PVS volume by the intracranial (IC) volume in the measured slice. Final CSO-PVS relative volume for each individual was expressed as a percentage of the IC volume in that slice that corresponded to CSO-PVS. The method for IC volume calculation was also based on thresholding and mask subtraction. Figure 2 provides a visual example of the process. Although CSO-PVS and IC volumes were generally determined in both hemispheres, cases with ICH distorting CSO required measurements to be done in the unaffected side only, and then multiplied ×2 assuming symmetric PVS burden.
Figure 2.
Segmentation of CSO-PVS and intracranial (IC) structures. A) Raw T2 scan; B) Adjustment of brightness and contrast to optimize PVS visualization; C) Final PVS mask; D) Final IC area mask. CSO-PVS relative volume was calculated as (volume of mask in image C/volume of mask in image D) × 100.
Lastly, PVS in the basal ganglia were rated on axial T2-FSE images, using a previously described validated 4-point visual rating scale (0=no PVS, 1=<10 PVS, 2=11–20 PVS, 3=21–40 PVS and 4=>40 PVS)27 and following STRIVE definitions28. We dichotomized basal ganglia PVS burden in “low” (degrees 0, 1 and 2) and “high” (degrees 3 and 4) as in previous analyses14.
Rating of MB and quantitative analyses of WMH, PVS and IC structures were assisted by the image-processing software MRIcron (www.cabiatl.com/mricro/mricron/). All neuroimaging ratings at MGH and Leiden Medical Center were performed by expert readers blind to the subjects’ demographics, clinical characteristics and neuropathological/genetic findings. We have previously reported a very high inter-rater reliability for MB detection29 and WMH volume quantification26. Intraclass correlation coefficient for CSO-PVS relative volume measurement between MGH and Leiden readers (SMR and SvR, respectively) over 14 scans was excellent (0.93).
Clinical variables and sub-classification of study subjects
For each individual in both cohorts we collected age at the time of MRI study, gender and presence of hypertension (defined as a diagnosis of hypertension and/or use of anti-hypertensive drugs based on medical records review).
Patients from the hospital-based cohort were initially classified by neuropathology into non-CAA (CAA−) and CAA (CAA+) cases. Individuals from the Dutch cohort were classified into HCHWA-D mutation carriers(HCHWA-D+) and non-carriers (HCHWA-D−). In a further step, sporadic CAA cases were subclassified in 3 diagnostic categories: non-CAA(CAA−), CAA without hemorrhage (CAA+/H−) and CAA with hemorrhage (CAA+/H+). The term “hemorrhage” referred to the presence of lobar MB and/or lobar ICH.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved at Massachusetts General Hospital and Leiden University Medical Center by the local Institutional Review Board and the local ethics committee, respectively.
Written informed consent was obtained from all subjects enrolled in the Leiden prospective cohort. MGH subjects (or their surrogates) had consented in the past to brain biopsy or autopsy for clinical and/or research purposes but were not requested additional consent for the present study given the retrospective nature of it and the anonymized treatment of data.
Statistics
CSO-PVS relative volume was compared between CAA and non-CAA subjects and between HCHWA-D carriers and non-carriers using the median test (non-normal continuous variable). Other variables were compared with the Wilcoxon test, Student’s t test (normal continuous variables), and Fisher’s exact test (proportions). To compare CSO-PVSrv between the 3 diagnostic categories (CAA−, CAA+/H− and CAA+/H+) in the hospital cohort we used 3-way ANOVA test to look for global differences, and Wilcoxon’s test to explore differences by pairs. Besides CSO-PVS relative volume, all variables reaching a global p value <0.1 in bivariate analyses were selected to enter logistic regression models.
Nominal logistic regression models were used to study whether increased CSO-PVS relative volume is independently associated with the presence(yes/no) of sporadic CAA and HCHWA-D carrier status (dependent variables). In order to study whether CSO-PVS relative volume may be predictive of sporadic CAA in the absence of hemorrhage, the multiple diagnostic classification (CAA− vs CAA+/H− vs CAA+/H+) was chosen as the dependent variable and an alternative regression strategy was applied: multiple nominal logistic regressions, excluding one of the diagnostic categories at a time.
Significance level was set at 0.05 and all tests of significance were two-tailed. JMP Pro 10 (SAS institute, Cary, NC) and Stata 11.2 (StataCorp, College Station, TX) software were used for data analysis.
RESULTS
The sporadic CAA cohort consisted of sixty-three individuals (mean age 73.6±8.5 years, 46% female) and the Dutch cohort consisted of 54 individuals (mean age 46.7±12.8 years, 61.1% female). Table 1 shows the basic demographics for each study cohort.
Table 1.
Description of hospital-based and Dutch cohorts.
| Sporadic CAA cohort (n=63) | Dutch cohort (n=54) | |
|---|---|---|
| Age by MRI, mean ± SD | 73.6 ± 8.5 | 46.7 ± 12.8 |
| Female sex, n(%) | 29(46) | 33(61.1) |
| Hypertension, n(%) | 41(65) | 12(22.2) |
| High burden basal ganglia-PVS, n(%) | 15(23.8) | 4(7.4) |
| WMH volume in cc, median[IQR] | 26.5[11.3 – 45.8] | 2.2[0.4 – 38.9] |
| Presence of any lobar hemorrhage, (%) | 45(71.4) | 19(35.2) |
| CSO-PVS relative volume, median% [IQR] | 3[1.8 – 4.9] | 1.5[0.5 – 3.6] |
CSO-PVS relative volume was significantly higher in patients with sporadic CAA compared to those without. Similarly, HCHWA-D mutation carriers had significantly higher CSO-PVS relative volume than non-carriers (Table 2). Logistic regression models revealed that increasing CSO-PVS relative volume was predictive of sporadic CAA diagnosis (OR 95%CI 2.74[1.3–5.75]). The only other independent predictor of sporadic CAA was the presence of any lobar hemorrhage (OR 95%CI 5.93[1.11–31.75]). By contrast, a high burden of basal ganglia PVS (OR 95%CI 0.05[0.007–0.39]) independently predicted the absence of CAA on pathology.
Table 2.
Comparison of characteristics between CAA and non-CAA cases (hospital-based pathology cohort) and between HCHWA-D mutation carriers and non-carriers (Dutch cohort).
| Sporadic CAA cohort (n=63) | CAA+ cases | CAA− cases | p value |
|
| |||
| Age by MRI, mean ± SD | 73.1 ± 8.2 | 74.9 ± 9.3 | 0.46 |
| Female sex, n(%) | 22(47.9) | 7(41.2) | 0.78 |
| Hypertension, n(%) | 30(65.2) | 11(64.7) | 0.99 |
| High burden basal ganglia PVS, n(%) | 5(10.9) | 10(58.8) | 0.0002 |
| WMH volume in cc, median[IQR] | 26.3[11.6 – 44.8] | 26.5[5.6 – 58.9] | 0.84 |
| Presence of any lobar hemorrhage, n(%) | 38(82.6) | 7(41.2) | 0.003 |
| CSO-PVS relative volume, median % [IQR] | 3.7[2.5 – 5.3] | 1.8[1.2 – 2.4] | <0.0001 |
|
| |||
| Dutch cohort (n=54) | Mutation carriers | Non-carriers | p value |
|
| |||
| Age by MRI, mean ± SD | 45.8 ± 13.9 | 47.5 ± 11.8 | 0.55 |
| Female sex, n(%) | 16(61.5) | 17(60.7) | 0.99 |
| Hypertension, n(%) | 6(23) | 6(21.4) | 0.99 |
| High burden basal ganglia PVS, n(%) | 2(7.7) | 2(7.1) | 0.99 |
| WMH volume in cc, median[IQR] | 38.8[1.5 – 117.9] | 0.6[0.2 – 2.3] | 0.0007 |
| Presence of any lobar hemorrhage, n(%) | 17(65.4) | 2(7.1) | <0.0001 |
| CSO-PVS relative volume, median% [IQR] | 3.8[0.6 – 6.2] | 0.7[0.4 – 1.6] | 0.007 |
A further diagnostic subgroup analysis was conducted in the hospital-based cohort (Table 3). While CSO-PVS relative volume was not different between CAA+/H− and CAA+/H+ cases, it was significantly higher in these 2 diagnostic categories compared to CAA−. Supplemental Figure I shows graphically the unadjusted comparison of CSO-PVS relative volume between the 3 subgroups. As a complimentary subanalysis, we compared CSO-PVS relative volume between MB-only vs. ICH cases within the CAA+/H+ subgroup, but no significant differences were observed (data not shown). In adjusted nominal regression models, higher CSO-PVS relative volume was an independent predictor of the CAA+/H+ diagnostic category, and borderline predictive of CAA+/H− (Table 4).
Table 3.
Bivariate comparison of clinical and radiological variables between diagnostic subgroups: non-CAA (CAA−), CAA without hemorrhage (CAA+/H−) and CAA with hemorrhage (CAA+/H+).
| Sporadic CAA cohort (n=63) | CAA− | CAA+/H− | CAA+/H+ | Global p value |
|---|---|---|---|---|
| Age by MRI, mean ± SD | 74.9 ± 9.3 | 71.6 ± 8.1 | 73.4 ± 8.3 | 0.67 |
| Female sex, n(%) | 7(41.2) | 1(12.5) | 21(55.3) | 0.08 |
| Hypertension, n(%) | 11(64.7) | 6(75) | 24(63.2) | 0.92 |
| High burden basal ganglia PVS, n(%) | 10(58.8) | 1(12.5) | 4(10.5) | 0.0006 |
| WMH volume in cc, median[IQR] | 26.5[5.6–58.9] | 10.9[8.9–15.8] | 30[15.5–47.9] | 0.06 |
| CSO-PVS relative volume, median% [IQR] | 1.8[1.2–2.4]a | 3[2.4–3.6]b | 4.4[2.6–6.1]c | 0.0001* |
Based on p values obtained from Wilcoxon each pair tests: c > a, b > a, b ≈ c.
Table 4.
Multivariable nominal logistic regression to study differences in CSO-PVS volume between diagnostic categories (non-CAA [CAA−], CAA without hemorrhage [CAA+/H−] and CAA with hemorrhage [CAA+/H+]) adjusted by covariates. Hospital-based cohort.
| Dependent variable : CAA+/H− vs CAA−* | Odds Ratio [95% CI] | P value |
|
| ||
| Female gender | 0.10[0.003–2.70] | 0.17 |
| WMH volume | 0.96[0.85–1.08] | 0.53 |
| High burden basal ganglia PVS | 0.02[0.0001–3.07] | 0.13 |
| CSO-PVS relative volume | 4.55[0.98–21.04] | 0.05 |
|
| ||
| Dependent variable: CAA+/H+ vs CAA−† | Odds Ratio [95% CI] | P value |
|
| ||
| Female gender | 2.84[0.44–18.10] | 0.27 |
| WMH volume | 1.02[0.99–1.04] | 0.15 |
| High burden basal ganglia PVS | 0.02[0.002–0.30] | 0.004 |
| CSO-PVS relative volume | 2.63[1.33–5.18] | 0.005 |
excluding CAA+/H+ cases
excluding CAA+/H− cases
DISCUSSION
In this study, we demostrated increased PVS (specifically in the CSO region) in CAA patients with pathologically-proven sporadic CAA as well as genetic Dutch-type CAA. The consistent results in the younger hereditary CAA cohort in addition to the older sporadic CAA, suggest that increased MRI-visible CSO-PVS are a characteristic marker of the disease independent of additional factors, including advanced age. Additionally, we showed that high CSO-PVS volume is an independent predictor of neuropathologically-defined sporadic CAA even in the absence of lobar hemorrhages (MB or ICH), which supports its potential utility in the diagnosis of non-hemorrhagic or even early forms of the disease.
Sporadic CAA is the form of amyloid angiopathy that accounts for the vast majority of CAA cases in the elderly.30 The Boston Criteria rely on the presence of strictly lobar hemorrhages for the clinical diagnosis of CAA18, 31. Little additional diagnostic value is gained when superficial siderosis is also incoroporated in the Criteria32. Validation studies of the Boston Criteria have overall shown the need for new markers to improve their sensitivity, which is estimated to be only around 42% – 63%21,33. The present study provides preliminary evidence for CSO-PVS volume as an independent marker for the diagnosis of sporadic CAA and may be a useful marker to be further explored in larger studies.
PVS in basal ganglia regions have been typically linked to hypertension14, 34. Although increased PVS in white matter areas have been repeatedly associated to CAA markers across different populations14, 15, recent work has also reported associations with hypertension and markers of hypertensive vasculopathy20, 35. This suggests the potential for overlapping etiologies and risk factors contributing to CSO-PVS, for which it may be difficult to dissect their independent role. The importance of the finding of increased CSO-PVS in HCHWA-D mutation carriers lies in the fact that the younger age of these patients (mean age 45.8 years) virtually excludes the impact of prolonged exposure to vascular risk factors on PVS dilation. Thus, in this cohort, vascular Aβ emerges as the main single suspect associated with PVS dilation in CSO.
Another valuable aspect of finding increased CSO-PVS volumes in both hereditary and sporadic CAA relates to our understanding of the mechanisms leading to PVS dilation. The pathophysiology of the Dutch-type amyloidosis is mainly driven by the production of toxic amyloid species, with enhanced fibrillogenic properties and resistance to enzymatic degradation36–38. This allows us to speculate that in these young HCHWA-D mutation carriers, the increased PVS dilation seen in CSO may be simply a direct consequence of extensive perivascular amyloid deposition in the cortex, with secondary retrograde dilation of PVS into the white matter. Interestingly, this hypothesis of cortical PVS blockage by amyloid had been previously postulated for sporadic CAA in AD patients7. It is possible that multiple mechanisms contribute to PVS dilation in the CSO in the elderly. It is conceivable that increased PVS dilation in the white matter may be a surrogate of chronic poor perivascular drainage caused by ageing and exposure to vascular risk factors, which predisposes individuals to amyloid stagnation and CAA development. This hypothesis would align with the current concept of sporadic CAA as a result of cumulative age-related defects in amyloid clearance pathways6.
The use of both pathologically and genetically confirmed cases from 2 different types of CAA respectively, and the application of a novel quantitative method to estimate CSO-PVS burden are the main strengths of this study. This method allowed for a continuous quantification of PVS burden, not subjected to the arbitrary thresholds of visual scales used in earlier studies27, 39. In populations like the ones studied here, quantitative measurements may be more useful than visual scales since they avoid potential ceiling effects. Our study has some limitations. The hospital cohort was retrospective and thus some radiological studies were obtained at 1.5T MRI, which depicts tiny structures like PVS with less resolution and may have resulted in systematically lower volumes in a subset of patients. However, the fact that the same associations were present in the Dutch cohort, which included an homogenous 3T MRI protocol, is reassuring. Our method to quantitatively assess CSO-PVS has excellent interrater reliability. However, we note that this tool might have somewhat limited applicability in a clinical setting as it is time-consuming and requires good quality imaging sequences. Also, it is uncertain whether CSO-PVS are a consistent good estimator of the whole PVS load in the brain, or at least in the white matter. Standardized, fully automated and reliable whole-brain assessment techniques for PVS volume quantification are desirable and should be developed in the near future to further improve generalizability of results in the PVS field40.
SUMMARY AND CONCLUSIONS
Increased CSO-PVS volume is identified in both hereditary and sporadic forms of amyloid angiopathy, and appears as a promising marker of sporadic CAA, even in the absence of any lobar hemorrhages. Whether PVS volume increases early in the disease course, or even before any vascular Aβ accumulates, is currently unknown. Prospective, population-based neuroimaging studies are needed to clarify these important questions.
Supplementary Material
Acknowledgments
SOURCE OF FUNDING:
This work was supported by the following NIH grants: R01 NS070834, R01AG047975, R01AG026484, P50AG005134 and K23AG02872605.
M.J.H.W. was supported by a personal grant from the Netherlands Heart Foundation.
Footnotes
DISCLOSURES:
None
References
- 1.Zhang ET, Inman CB, Weller RO. Interrelationships of the pia mater and the perivascular (virchow-robin) spaces in the human cerebrum. J Anat. 1990;170:111–123. [PMC free article] [PubMed] [Google Scholar]
- 2.Arbel-Ornath M, Hudry E, Eikermann-Haerter K, Hou S, Gregory JL, Zhao L, et al. Interstitial fluid drainage is impaired in ischemic stroke and alzheimer's disease mouse models. Acta neuropathologica. 2013;126:353–364. doi: 10.1007/s00401-013-1145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schley D, Carare-Nnadi R, Please CP, Perry VH, Weller RO. Mechanisms to explain the reverse perivascular transport of solutes out of the brain. Journal of theoretical biology. 2006;238:962–974. doi: 10.1016/j.jtbi.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: Significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathology and applied neurobiology. 2008;34:131–144. doi: 10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 5.Weller RO, Djuanda E, Yow HY, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009;117:1–14. doi: 10.1007/s00401-008-0457-0. [DOI] [PubMed] [Google Scholar]
- 6.Hawkes CA, Hartig W, Kacza J, Schliebs R, Weller RO, Nicoll JA, et al. Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta neuropathologica. 2011;121:431–443. doi: 10.1007/s00401-011-0801-7. [DOI] [PubMed] [Google Scholar]
- 7.Roher AE, Kuo YM, Esh C, Knebel C, Weiss N, Kalback W, et al. Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in alzheimer's disease. Mol Med. 2003;9:112–122. [PMC free article] [PubMed] [Google Scholar]
- 8.van Swieten JC, van den Hout JH, van Ketel BA, Hijdra A, Wokke JH, van Gijn J. Periventricular lesions in the white matter on magnetic resonance imaging in the elderly. A morphometric correlation with arteriolosclerosis and dilated perivascular spaces. Brain. 1991;114(Pt 2):761–774. doi: 10.1093/brain/114.2.761. [DOI] [PubMed] [Google Scholar]
- 9.Heier LA, Bauer CJ, Schwartz L, Zimmerman RD, Morgello S, Deck MD. Large virchow-robin spaces: Mr-clinical correlation. AJNR Am J Neuroradiol. 1989;10:929–936. [PMC free article] [PubMed] [Google Scholar]
- 10.Maclullich AM, Wardlaw JM, Ferguson KJ, Starr JM, Seckl JR, Deary IJ. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry. 2004;75:1519–1523. doi: 10.1136/jnnp.2003.030858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patankar TF, Baldwin R, Mitra D, Jeffries S, Sutcliffe C, Burns A, et al. Virchow-robin space dilatation may predict resistance to antidepressant monotherapy in elderly patients with depression. J Affect Disord. 2007;97:265–270. doi: 10.1016/j.jad.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson SC, Blane A, Perros P, McCrimmon RJ, Best JJ, Wardlaw J, et al. Cognitive ability and brain structure in type 1 diabetes: Relation to microangiopathy and preceding severe hypoglycemia. Diabetes. 2003;52:149–156. doi: 10.2337/diabetes.52.1.149. [DOI] [PubMed] [Google Scholar]
- 13.Wuerfel J, Haertle M, Waiczies H, Tysiak E, Bechmann I, Wernecke KD, et al. Perivascular spaces--mri marker of inflammatory activity in the brain? Brain. 2008;131:2332–2340. doi: 10.1093/brain/awn171. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Ramirez S, Pontes-Neto OM, Dumas AP, Auriel E, Halpin A, Quimby M, et al. Topography of dilated perivascular spaces in subjects from a memory clinic cohort. Neurology. 2013;80:1551–1556. doi: 10.1212/WNL.0b013e31828f1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charidimou A, Meegahage R, Fox Z, Peeters A, Vandermeeren Y, Laloux P, et al. Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: A multicentre mri cohort study. J Neurol Neurosurg Psychiatry. 2013;84:624–629. doi: 10.1136/jnnp-2012-304434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charidimou A, Hong YT, Jager HR, Fox Z, Aigbirhio FI, Fryer TD, et al. White matter perivascular spaces on magnetic resonance imaging: Marker of cerebrovascular amyloid burden? Stroke; a journal of cerebral circulation. 2015 doi: 10.1161/STROKEAHA.115.009090. [DOI] [PubMed] [Google Scholar]
- 17.Charidimou A, Jaunmuktane Z, Baron JC, Burnell M, Varlet P, Peeters A, et al. White matter perivascular spaces: An mri marker in pathology-proven cerebral amyloid angiopathy? Neurology. 2014;82:57–62. doi: 10.1212/01.wnl.0000438225.02729.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: Validation of the boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Ramirez S, Romero JR, Shoamanesh A, McKee AC, Van Etten E, Pontes-Neto O, et al. Diagnostic value of lobar microbleeds in individuals without intracerebral hemorrhage. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2015 doi: 10.1016/j.jalz.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shams S, Martola J, Charidimou A, Larvie M, Granberg T, Shams M, et al. Topography and determinants of magnetic resonance imaging (mri)-visible perivascular spaces in a large memory clinic cohort. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg SM, Vonsattel JP. Diagnosis of cerebral amyloid angiopathy. Sensitivity and specificity of cortical biopsy. Stroke. 1997;28:1418–1422. doi: 10.1161/01.str.28.7.1418. [DOI] [PubMed] [Google Scholar]
- 22.Luyendijk W, Bots GT, Vegter-van der Vlis M, Went LN, Frangione B. Hereditary cerebral haemorrhage caused by cortical amyloid angiopathy. J Neurol Sci. 1988;85:267–280. doi: 10.1016/0022-510x(88)90186-4. [DOI] [PubMed] [Google Scholar]
- 23.Bakker E, van Broeckhoven C, Haan J, Voorhoeve E, van Hul W, Levy E, et al. DNA diagnosis for hereditary cerebral hemorrhage with amyloidosis (dutch type) American journal of human genetics. 1991;49:518–521. [PMC free article] [PubMed] [Google Scholar]
- 24.van Opstal AM, van Rooden S, van Harten T, Ghariq E, Labadie G, Fotiadis P, et al. Cerebrovascular function in presymptomatic and symptomatic individuals with hereditary cerebral amyloid angiopathy: A case-control study. Lancet Neurol. 2017;16:115–122. doi: 10.1016/S1474-4422(16)30346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: A guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurol ME, Irizarry MC, Smith EE, Raju S, Diaz-Arrastia R, Bottiglieri T, et al. Plasma beta-amyloid and white matter lesions in ad, mci, and cerebral amyloid angiopathy. Neurology. 2006;66:23–29. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- 27.Potter GM, Chappell FM, Morris Z, Wardlaw JM. Cerebral perivascular spaces visible on magnetic resonance imaging: Development of a qualitative rating scale and its observer reliability. Cerebrovasc Dis. 2015;39:224–231. doi: 10.1159/000375153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viswanathan A, Guichard JP, Gschwendtner A, Buffon F, Cumurcuic R, Boutron C, et al. Blood pressure and haemoglobin a1c are associated with microhaemorrhage in cadasil: A two-centre cohort study. Brain. 2006;129:2375–2383. doi: 10.1093/brain/awl177. [DOI] [PubMed] [Google Scholar]
- 30.Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: Recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry. 2012;83:124–137. doi: 10.1136/jnnp-2011-301308. [DOI] [PubMed] [Google Scholar]
- 31.Greenberg SM, Briggs ME, Hyman BT, Kokoris GJ, Takis C, Kanter DS, et al. Apolipoprotein e epsilon 4 is associated with the presence and earlier onset of hemorrhage in cerebral amyloid angiopathy. Stroke; a journal of cerebral circulation. 1996;27:1333–1337. doi: 10.1161/01.str.27.8.1333. [DOI] [PubMed] [Google Scholar]
- 32.Linn J, Halpin A, Demaerel P, Ruhland J, Giese AD, Dichgans M, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74:1346–1350. doi: 10.1212/WNL.0b013e3181dad605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Rooden S, van der Grond J, van den Boom R, Haan J, Linn J, Greenberg SM, et al. Descriptive analysis of the boston criteria applied to a dutch-type cerebral amyloid angiopathy population. Stroke. 2009;40:3022–3027. doi: 10.1161/STROKEAHA.109.554378. [DOI] [PubMed] [Google Scholar]
- 34.Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on mri are a feature of cerebral small vessel disease. Stroke; a journal of cerebral circulation. 2010;41:450–454. doi: 10.1161/STROKEAHA.109.564914. [DOI] [PubMed] [Google Scholar]
- 35.Yakushiji Y, Charidimou A, Hara M, Noguchi T, Nishihara M, Eriguchi M, et al. Topography and associations of perivascular spaces in healthy adults: The kashima scan study. Neurology. 2014;83:2116–2123. doi: 10.1212/WNL.0000000000001054. [DOI] [PubMed] [Google Scholar]
- 36.Tsubuki S, Takaki Y, Saido TC. Dutch, flemish, italian, and arctic mutations of app and resistance of abeta to physiologically relevant proteolytic degradation. Lancet. 2003;361:1957–1958. doi: 10.1016/s0140-6736(03)13555-6. [DOI] [PubMed] [Google Scholar]
- 37.Van Nostrand WE, Wagner SL, Haan J, Bakker E, Roos RA. Alzheimer's disease and hereditary cerebral hemorrhage with amyloidosis-dutch type share a decrease in cerebrospinal fluid levels of amyloid beta-protein precursor. Ann Neurol. 1992;32:215–218. doi: 10.1002/ana.410320214. [DOI] [PubMed] [Google Scholar]
- 38.Lin YS, Bowman GR, Beauchamp KA, Pande VS. Investigating how peptide length and a pathogenic mutation modify the structural ensemble of amyloid beta monomer. Biophys J. 2012;102:315–324. doi: 10.1016/j.bpj.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu YC, Tzourio C, Soumare A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated virchow-robin spaces is associated with age, blood pressure, and mri markers of small vessel disease: A population-based study. Stroke. 2010;41:2483–2490. doi: 10.1161/STROKEAHA.110.591586. [DOI] [PubMed] [Google Scholar]
- 40.Hernandez Mdel C, Piper RJ, Wang X, Deary IJ, Wardlaw JM. Towards the automatic computational assessment of enlarged perivascular spaces on brain magnetic resonance images: A systematic review. J Magn Reson Imaging. 2013;38:774–785. doi: 10.1002/jmri.24047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.