Abstract
Although hypertension is identifiable in children and adolescents, there are many knowledge gaps regarding how to best define and manage high blood pressure in the young. The Study of High Blood Pressure In Pediatrics: Adult Hypertension Onset in Youth (SHIP-AHOY) is being conducted to address these knowledge gaps. 500 adolescents will be recruited and will undergo ambulatory blood pressure monitoring, echocardiographic, vascular and cognitive assessments, as well as epigenetic studies to identify mechanisms that underlie the development of hypertensive target organ damage. Details of the design and methods that will be utilized in SHIP-AHOY are presented here, as well as baseline characteristics of the first 264 study participants. The primary aim of the study is to develop a risk-based definition of hypertension in the young that will result in better understanding of the transition from blood pressure in youth to adult cardiovascular disease.
Keywords: hypertension, children, adolescents, blood pressure, left ventricular hypertrophy, vascular stiffness, epigenetics
Introduction
Target organ damage (TOD) related to high blood pressure (BP) levels measured in adulthood is strongly associated with significantly increased risk for cardiovascular (CV) events such as stroke and myocardial infarction [1,2]. Ample data from large-scale clinical trials have demonstrated the CV benefits of BP reduction [3]. Since hypertension affects at least one-third of adults, the potential public health burden and associated expenditures related to hypertension are enormous [4]. Given this, substantial effort and resources have been expended to improve awareness and control of hypertension among adults.
Less attention has been paid to the earliest phases of hypertension, which likely have their origins in childhood. Although it is well known that hypertension and hypertensive TOD are detectable in the young, there are limited data available linking childhood BP levels with future cardiovascular disease (CVD). This point was underscored by an evidence review conducted for the US Preventative Services Task Force, which concluded that evidence linking childhood BP levels to the prediction of development of adult CVD is lacking [5]. A shortcoming of that analysis, however, is that it did not examine the adverse impact that elevated BP might have in a more immediate time frame for children and adolescents and also did not evaluate the level of BP at which this might occur.
In contrast to adult hypertension, childhood hypertension is statistically defined based on the distribution of BP in healthy children [6], and treatment recommendations represent consensus opinion based on evidence from cross-sectional and longitudinal cohort studies [6,7]. Clarifying the relationship between BP levels and development of hypertensive TOD in youth is essential to establish evidence-based treatment guidelines. Additionally, data on whether BP phenotype, as defined by the combination of casual and ambulatory BP measurements, can help predict the risk of hypertensive TOD in youth are scant. Finally, a better understanding of epigenetic pathways involved in the development of hypertensive TOD could help identify new targets for early therapy of hypertension in the young.
These knowledge gaps stimulated development of the Study of High Blood Pressure In Pediatrics: Adult Hypertension Onset in Youth (SHIP-AHOY) Study, one of the American Heart Association’s Strategically Focused Research Networks (SFRN) in Hypertension. SHIP-AHOY consists of three projects (population, clinical, and basic) conducted on a single cohort of adolescents. The goals are to: 1) redefine BP thresholds for the diagnosis of childhood hypertension, based on direct evidence; 2) better define the clinical phenotype of BP associated TOD; and 3) focus on high BP in adolescence as an actual disease-causing, treatable condition in the young, rather than a risk factor.
Methods
At the completion of the study, data can be requested from the SHIP-AHOY Data Coordinating Center (DCC).
Study Population
The SHIP-AHOY study is recruiting a multiethnic cohort of 500 otherwise healthy adolescents across the BP spectrum, divided into 3 BP strata (see below). The BP groups are balanced for BMI category so that similar proportions of subjects in each BP group will be lean (BMI <85th percentile) or overweight/obese (BMI ≥ 85th percentile). Inclusion and exclusion criteria are presented in Table 1.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion: |
|
|
|
| Exclusion: |
|
Abbreviations: ADHD, attention-deficit hyperactivity disorder; BMI, body mass index; BP, blood pressure.
The study protocol has undergone institutional review board (IRB) review and approval, and all study participants and their parents have provided written informed consent and/or assent according to local IRB requirements.
Study Organization
Each of the three projects of the SHIP-AHOY study have individual principal investigators (PI) at 5 clinical sites (Figure 1) who regularly interact with the SFRN center director, who also oversees the DCC. Additional network oversight is provided by the Consultants and Executive Steering Committee. The Cardiovascular Research Center (CVRC) in Cincinnati oversees echocardiography and pulse wave velocity (PWV) studies, the ambulatory BP monitoring (ABPM) Center in Seattle oversees performance and initial analysis of ABPM studies, and cognitive questionnaires and tests are scored at the Psychology Core in Chapel Hill. Blood and urine samples, including genetic samples used in the Basic Science project, are processed at each site, stored at −80°C and shipped in batches quarterly to Cincinnati Children’s Hospital Medical Center (CCHMC) laboratories for analysis. Processing of samples for epigenetic studies is performed at the University of Cincinnati, with analyses of these data performed in conjunction with genetic and bioinformatics experts at CCHMC.
Figure 1.
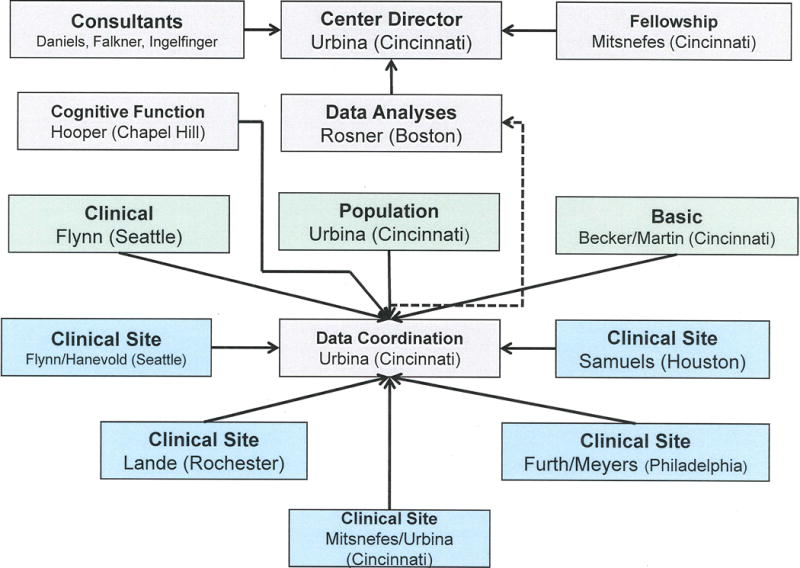
Illustration of SHIP-AHOY study organization.
Study assessments
Casual BP measurement
SHIP-AHOY participants have casual BP measurements obtained in the right arm by auscultation at study entry, then again at the main study visit. All participating sites use the same aneroid sphygmomanometer (Mabis MedicKit 5, Mabis Healthcare, Waukegan, IL). Standardized training and certification in the auscultatory BP measurement protocol were provided to all study personnel responsible for casual BP measurement.
At each study visit, prior to BP determination, arm circumference is measured with a plastic measuring tape at the midpoint of the upper arm between the acromion and olecranon and a cuff is then selected so that the length of the cuff bladder is equal to 80%-100% of the arm circumference. Following cuff selection, the peak inflation pressure is determined by inflating the cuff to 60 mmHg and then gradually continuing to inflate in increments of 10 mmHg until the radial pulse is no longer felt – thereby determining the pulse obliteration pressure. An additional 30 mmHg is added to this value and recorded as the peak inflation pressure. The cuff is then inflated to this value for all BP measurements in each participant at that study visit.
After 5 minutes of rest, BP measurement begins. First, pulse is measured by palpation of the radial artery. Then, four BPs at 30-second intervals are obtained by auscultation of the brachial artery, using the first Korotkoff sound for systolic BP (SBP) and the fifth Korotkoff sound for diastolic BP (DBP). The average of the last 3 BPs is recorded as the participant’s BP for the study visit. According to the average systolic BP, BP percentile rank is determined and the participant is classified into one of 3 BP groups, which are based upon published data demonstrating that hypertensive TOD may occur at BP levels as low as the 80th percentile [9]:
High-Risk BP: Average SBP ≥ 90th percentile
Mid-Risk BP: Average SBP ≥ 80th and < 90th percentiles
Normal BP: Average SBP <75th percentile
Ambulatory BP Monitoring (ABPM)
Ambulatory BP is measured with an oscillometric device (SpaceLabs OnTrak™, Spacelabs Healthcare, Issaquah, WA, USA). Using the arm circumference obtained during the auscultatory BP measurement, a properly sized cuff is selected and the monitor placed on the participant. Three resting BP’s are obtained immediately after monitor placement and recorded for confirmation of correct placement and function of the device. BP monitoring is performed for 26 hours, with measurements obtained every 20 minutes. The monitor is returned to the site and the data are downloaded to a laptop computer. Studies are divided into sleep-wake periods according to diaries provided by the participants. No hours of monitoring are discarded, consistent with current AHA recommendations for pediatric ABPM [9]. Data files are transmitted electronically to the ABPM center in Seattle.
The ABPM center then performs preliminary quality review of the ABPM data prior to transmittal to the DCC. Summary variables are generated, including mean 24-hour, wake and sleep BP, BP load (percentage of readings > 95th percentile), heart rate, pulse pressure and BP variability. The casual BP and ABPM results are then used to derive the categorical assignment of BP phenotype (Table 2) according to current guidelines [8].
Table 2.
BP Phenotypes
| Phenotype* | Casual BP | Ambulatory BP |
|---|---|---|
| Normotensive | Normal | Normal |
| White Coat Hypertension | Elevated | Normal |
| Ambulatory Hypertension | Elevated | Elevated |
| Masked Hypertension | Normal | Elevated |
Categories defined as per 2014 American Heart Association Scientific Statement on pediatric ABPM [8].
BP, blood pressure
Cardiac Measurements
Echocardiography is performed using standard cardiac ultrasound systems at each site. Cardiac sonographers participated in a web-based training in the echocardiographic protocol. Fourteen images are obtained with the participant supine. The images planes include: parasternal long-axis view, parasternal short-axis view, apical 4-chamber view, apical 2-chamber view, high parasternal short axis view, and suprasternal notch view. Loops of at least 3 cardiac cycles are obtained. All images are uploaded to a cloud-based image repository for later analysis by the CVRC. The absence of structural heart disease is confirmed and any abnormalities found in the study are flagged and sent to the clinical site PI.
Left ventricular end-diastolic dimension (LVED), left ventricular end-systolic dimension (LVES), end-diastolic interventricular septal thickness (IVSd), and left ventricular end-diastolic and end-systolic posterior wall thicknesses (LVPWd, LVPWs) measurements are obtained off-line by a trained sonographer using a Cardiology Analysis System (Digisonics, Houston, Texas). Left ventricular mass (LVM) is calculated from 2D-guided M-Mode images of the left ventricle at end diastole [10,11] using the Devereux equation [12]. To adjust for body size without overcompensating for the adverse effect of obesity, LVMI is calculated as LVM/ht2.7 as described by DeSimone [13].
Relative wall thickness (RWT) at end-diastole is also calculated as the ratio of the sum of the interventricular septum and posterior wall divided by the end diastolic dimension [(LVPWd + IVSd)/LVED]. LV geometry is categorized as normal, concentric remodeling, concentric hypertrophy, or eccentric hypertrophy based on whether a participant has normal or elevated LVMI and/or RWT [14].
Systolic function is evaluated by calculation of mid-wall fractional shortening (SFmid), a measure that better reflects myocardial performance in hypertrophied hearts [15]. LV strain and strain-rate imaging is performed using TOMTEC software (TOMTEC Corporation, Chicago, IL) to quantify intraventricular dyssynchrony and evaluate components of myocardial function [16].
For diastolic function, mitral inflow velocities are obtained with pulsed wave Doppler in the apical 4-chamber view. The Doppler cursor is placed parallel to mitral inflow and maximal velocity is measured with the sample volume at the mitral valve leaflet tips. The mitral peak E (early filling) and A (inflow with atrial contraction) waves are measured offline and E/A ratio is calculated. Tissue Doppler imaging myocardial flow velocities are acquired in the apical 4-chamber view. The peak (Ea) and late velocities (Aa) of mitral annular flow were recorded at the septal and lateral annulus; both lateral and septal Ea/Aa ratios and their averages are calculated. 2D measures of left atrium by body surface area are also obtained [17]. Each of the above parameters has been shown to correlate with invasive measures of diastolic function and LV end-diastolic pressure [18].
Vascular Measurements
Pulse wave velocity (PWV) is measured using the SphygmoCor CPV System (AtCor Medical, Sydney, Australia) as an assessment of vascular stiffness [19]. All research coordinators from the clinical sites were trained by the CVRC to perform the PWV measures. The patient is asked to lie supine in a comfortable position with their head flat on the bed. Using calipers, the average of 3 measures of sternal notch to femoral artery distance are obtained and entered into the software as the “distal femoral distance”. The distance from the carotid artery to the suprasternal notch is then obtained and entered as the “proximal” carotid measurement. Electrocardiogram (ECG) leads are then placed on the chest in standard positions. A tonometer is then used to obtain arterial waveforms gated to the R-wave on the ECG tracing from the carotid and femoral artery. PWV is the difference in the carotid-to-femoral path length divided by the difference in R-wave-to-foot of the pressure wave times. The software requires a minimum of 3 data pairs to calculate a result, and includes specific quality control indicators. Data is then downloaded from the device and sent to the CVRC.
Cognitive Measurements
Cognitive testing in SHIP-AHOY focuses on assessment of executive functions as an estimate of hypertensive TOD. Executive functions provide critical support for goal-directed behaviors that include inhibition, working memory, and attention regulation, abilities that are critical for the regulation of learning and behavior. Each of the tasks chosen for this study (Supplemental table S1) were selected because: 1) they represent key cognitive functions that have previously been identified as important in the hypertension literature [20, 21]; 2) they are age-appropriate and have well established age-based normative data; 3) they maintain strong psychometric properties; 4) they are well standardized and relatively straightforward with respect to their administration, assuring that non-psychologists can be trained to administer them; and 5) they have few language barriers in their administration. All components of the test battery have been validated in children [22–25].
The executive function tasks for this study were selected to measure attention regulation and inhibitory control, working memory, and nonverbal problem solving. Ratings of executive functions also will be obtained from the parent and participant. As with other assessments in SHIP-AHOY, standardized training and certification in the cognitive protocol have been provided to all study personnel responsible for conducting these assessments. Sites send the cognitive test results to the Cognitive Reading Center at the University of North Carolina for scoring and quality assurance checks.
Laboratory Analyses
Analyses of blood and urine samples are performed by the CCHMC clinical laboratory. Laboratory measures include fasting lipids, glucose, insulin, hsCRP, and uric acid level. Urine albumin and creatinine, obtained from a properly collected first morning urine sample, are used for calculation of urinary albumin excretion and urine sodium (Na) and potassium (K) for calculation of the urinary Na/K ratio. Girls have a urine pregnancy test performed at the local site since early pregnancy may affect BP. Microalbuminuria is defined as 30-300 mg albumin/g creatinine. Values ≥300 mg/g are considered proteinuria (macroalbuminuria). Glucose and insulin are used to estimate insulin resistance using the Homeostasis Model Assessment (HOMA) equation [26]. This equation correlates significantly (p<0.001) with insulin sensitivity measured directly with the insulin clamp [27]. HOMA will be analyzed as a continuous variable; and we will use HOMA >3.25 as a categorical criterion for insulin resistance. Total cholesterol (TC), total triglycerides (TG), and high-density lipoprotein-cholesterol (HDL-C) are measured and low-density lipoprotein cholesterol (LDL-C) is calculated.
Evaluation of Epigenetic Changes
One of the major challenges in studying hypertension is the marked heterogeneity in outcomes. Potential sources for phenotypic variation are acquired genetic, also known as epigenetic, modifications and alterations in gene regulation. Epigenetic processes alter gene activity without fundamentally changing the DNA [28] and have been shown to play a major role in the phenotypic expression of diseases such as hypertension. Multiple studies have supported a role for epigenetics in BP control and downstream or tissue-level sequela from poor control. Gene methylation alterations have been implicated in blood pressure control [29,30] and carotid intima-media thickness [31]. Micro-RNA (miRNA), as major regulators of gene activity, are associated with cardiac injury, angiogenesis, and cellular changes [32–36].
While genome-wide evaluation of epigenetic changes is possible, this approach would require a prohibitively large cohort. Thus, we opted to leverage existing biologic data to select candidate genes for our epigenetic study. First, from studies performed in hypertensive adults we compiled a list of genes which had DNA hypertension-associated alterations [37–40]. From this list we identified 106 genes that had also been reported to have either altered methylation or to be targets for miRNAs with resulting altered gene expression. We subsequently used ToppFun (http://toppgene.cchmc.org/) [41], a gene list enrichment software to narrow the number of candidate genes for additional testing. Lastly, we used the GRAIL system to identify functional interactions, epigenetic modifications and altered regulatory miRNAs between genes associated with hypertension and LVH. The final list of 14 candidate genes (Supplemental table S2) cluster with the following biologically plausible pathway for the development of hypertension and BP-related TOD:
This targeted approach allows us to evaluate how environmental influences such as obesity, metabolic derangement, and lifestyle, might influence gene expression and regulation leading to BP-related TOD.
DNA Methylation Measurement by Pyrosequencing
Individual bisulfite-treated DNA samples are subject to PCR amplification [42–44]. Pyrosequencing generates continuous measurements of DNA methylation ranging from 0% to 100% [methylation%=peak height methylated/(peak height methylated + peak height unmethylated)]. Methylation% is also logit transformed [log2(methylation%/(1-methylation%))]. After standard normalization procedures, we will use ANOVAs to test for differences between groups and t-tests for pairwise comparisons between groups.
Micro-RNA Profiling
In addition to the targeted approach previously summarized, we also performed unbiased genome-wide miR-sequencing. The Illumina TruSeq small RNA kit (Illumina Inc., San Diego, CA) was used as per the manufacturer’s recommendations, except for the library size selection (18-25 bases), resulting in a minimum of 2M reads per sample. To produce miRNA profiles, we used the miRExpress software package (http://mirexpress.mbc.nctu.edu.tw) [45] to successively group identical reads, trimming the library adaptor sequence off the end of the reads and aligning the reads to the pre-miR reference sequences in the latest release of the mirBase database. Finally, the alignments to each pre-miR are tallied into individual miR counts to obtain a digital readout of how many molecules of each miRNA was sampled in the experiment. That number is proportional to the miR’s expression blood level. After appropriate normalization, we search for miR that are upregulated or down regulated between groups. As we expect many genes to be differentially expressed, we will use pathway analyses to identify the key biologic processes underpinning the differential expression patterns.
Multicenter Study Coordination
Uniform methods of collecting data were developed across projects. Questionnaire (medical, family, diet, exercise) and other phenotypic data (age, race/ethnicity, sex, anthropometrics, casual/office BP) are entered into a REDCap web-based database (www.projectredcap.org) hosted at CCHMC. Lab results processed in Cincinnati are also transmitted to the DCC in electronic format, where abnormal clinical values are flagged and transmitted to the site PIs to communicate to the participants. Echocardiograms and PWV data are downloaded and transmitted electronically to the DCC for reading at the CVRC. Similarly, ABPM data and cognitive testing scores are transmitted electronically to the DCC from the ABPM center and cognitive reading center, respectively.
Data quality is monitored and controlled at many points in the data collection process. The DCC at CCHMC is staffed by a biostatistician, programmer and coordinator. The DCC evaluates all data sources for outliers and biologically implausible results every 6 months and then adjudicates them. Reproducibility of repeat measures of LV structure and PWV are calculated and monitored.
Statistical Analyses
The cross-sectional nature of the study will allow us to test the hypotheses that adolescents with high risk BP will have significantly greater LVMI, and PWV compared to adolescents with normal BP. Sample size calculations (see online supplement) were performed to ensure that we would have sufficient power to test this hypothesis.
Chi-square analyses will be used to relate BP category (low-risk/mid-risk/high-risk) to TOD at baseline. In addition, logistic regression will be used to relate TOD to BP phenotype after adjusting for: (a) age, sex, BMI at baseline; and (b) all variables in (a) plus additional CVD risk factors (e.g., lipids, insulin resistance, glucose). The primary analyses will be linear regression models. Similar analyses will be applied for TOD measures of executive function (individually and then combined executive composite) and UAE.
Primary Outcomes
Outcome variables of TOD include LVMI, PWV, urine albumin excretion (UAE), and cognitive function scores with a focus on executive functions.
Results
To date, 264 adolescents have been recruited into SHIP-AHOY. Participant characteristics and cardiovascular variables of interest are summarized in Table 3. Mean age is 15.7 ±7.7 (range 11.2-18.9), 51% are male, and 61% are Caucasian. BMI and other measures of obesity are generally highest in the high-risk BP group.
Table 3.
SHIP-AHOY Participant Characteristics*
| Variable | Low-risk BP (N=130) |
Mid-risk BP (N=60) |
High-risk BP (N=74) |
|---|---|---|---|
| Demographics/Anthropometrics | |||
| Age (years) | 15.7±1.5 | 16.1±1.7 | 15.4±1.6 |
| Sex (%male) | 50.0 | 65.0 | 58.1 |
| Race (%Caucasian) | 63.9 | 60.0 | 56.8 |
| Hispanic (%) | 12.3 | 15.0 | 16.2 |
| Height (meters) | 1.68±0.09 | 1.72±0.10 | 1.69±0.09 |
| Weight (kilograms) | 74.2±22.5 | 85.7±26.4 | 85.6±26.7 |
| Waist (centimeters) | 83.6±17.7 | 89.7±20.4 | 93.3±21.0 |
| BMI (kg/m2) | 26.3±6.7 | 29.3±9.6 | 29.8±8.3 |
| Waist/Height ratio | 49.7±9.8 | 52.1±11.4 | 55.1±11.7 |
| Hemodynamics | |||
| K1 SBP (mmHg) | 110.8±10.0 | 126.0±5.6 | 133.6±7.4 |
| K5 DBP (mmHg) | 74.5±9.6 | 82.3±7.5 | 86.1±9.3 |
| MAP (mmHg) | 86.8±9.3 | 96.9±5.5 | 101.9±7.3 |
| SBP percentile (%) | 43.6±26.3 | 83.0±9.4 | 95.0±4.3 |
| DBP percentile (%) | 71.0±23.5 | 87.0±11.5 | 91.4±12.7 |
| Heart rate (bpm) | 71.4±11.9 | 68.3±12.4 | 71.4±12.6 |
| ABPM parameters† | |||
| Mean Wake SBP | 116.7±9.2 | 125.5±9.1 | 130.4±9.9 |
| Wake SBP Index | 0.87±0.07 | 0.93±0.07 | 0.97±0.08 |
| Wake SBP Load (%) | 9.6±13.5 | 23.7±20.9 | 35.7±28.0 |
| Mean Wake DBP | 68.6±6.5 | 73.6±6.6 | 75.2±7.8 |
| Wake DBP Index | 0.83±0.08 | 0.89±0.08 | 0.91±0.10 |
| Wake DBP Load (%) | 9.6±11.7 | 21.3±15.9 | 27.0±21.5 |
| Mean Sleep SBP | 103.1±9.3 | 109.8±11.1 | 114.0±10.1 |
| Sleep SBP Index | 0.88±0.07 | 0.93±0.10 | 0.96±0.09 |
| Sleep SBP Load (%) | 11.4±17.0 | 26.6±27.5 | 34.4±29.0 |
| Mean Sleep DBP | 55.8±5.7 | 58.4±6.0 | 60.7±8.5 |
| Sleep DBP Index | 0.84±0.09 | 0.88±0.09 | 0.91±0.13 |
| Sleep DBP Load (%) | 12.7±16.7 | 19.7±18.5 | 27.8±26.8 |
| SBP Dipping (%) | 11.6±6.1 | 12.6±6.0 | 12.5±5.7 |
| DBP Dipping (%) | 18.4±7.6 | 20.6±6.2 | 19.4±7.4 |
Data expressed as Mean and standard deviation, or as frequencies
Index is calculated as the subject’s BP value divided by the 95th percentile BP; Load is defined as the percentage of readings above the 95th percentile for that time period; Dipping percentage is calculated as: ((mean wake S/DBP – mean sleep S/DBP)/mean wake S/DBP) × 100
There has been good separation of casual BP between the low, medium and high-risk BP groups (table 3). Similarly, there has been good separation of mean ambulatory BP among the three BP groups. Nocturnal dipping has not significantly varied between groups thus far. Success in obtaining research-quality ambulatory BP studies has been good, with average recording times of 25.5 hours, and 82% of planned readings obtained.
Discussion
Although the association between primary hypertension and CV death in adults has been well described, the causality and natural history, including genetic, epigenetic, and environmental influences that impact the clinical expression of primary hypertension in youth remains unclear. A better understanding of primary hypertension over time, from childhood forward, will be facilitated by long-term population studies that include a mechanistic backbone. At some point in the continuum of primary hypertension, LVH, increased arterial stiffness, and renal damage can develop. Similarly, measures of TOD such as LVH, increased PWV, decline in renal function, and impaired cognitive function themselves become preclinical diseases and powerful, independent risk factors for ischemic heart disease, congestive heart failure, arrhythmias and sudden cardiac death. Understanding the determinants of these two transition points (from risk factor to TOD to clinical disease) may greatly impact the investigation and examination, management, and health of patients with primary hypertension. Understanding the epigenetic modifications of the existing genetic background may also explain the heterogeneity in the hypertensive phenotype.
The SHIP-AHOY study has several unique design elements that should allow us to answer many of the questions surrounding the development of hypertension starting in the young. First, it is the largest multicenter, multiethnic study of BP and BP-associated TOD in adolescents. Second, it includes youth across the BP distribution to determine the risk for TOD, even in adolescents with BP < 90th percentile. Third, it combines auscultatory casual BP measurements with ABPM data to determine the BP phenotype, which will better characterize BP and what BP parameter signifies greater risk. Finally, the inclusion of advanced echocardiographic measures, such as Tissue Doppler Imaging and strain, arterial stiffness measurements (PWV), and cognitive functioning as secondary measures of TOD, is unique. This will provide significant additional evidence on the intermediate effects of high BP in the young.
The characteristics of the participants enrolled thus far demonstrate the feasibility of recruiting a well-characterized cohort of adolescents that will allow us to address fundamental questions regarding the effects of high BP in youth. The SHIP-AHOY study is likely to change how BP is evaluated and diagnosed in youth, moving from a statistical definition of risk to one that is based on thresholds for intervention based on intermediate measures of TOD. These data may also inform development of more specific treatment guidelines, and by identifying those at highest risk, may be able to modify the CVD risk trajectory into adulthood.
Perspectives
Although much is known about childhood hypertension, uncertainty remains regarding how to best define BP thresholds for diagnosis and treatment. The SHIP-AHOY study will provide new data that should help to close these and other knowledge gaps, thereby advancing our understanding of the earliest phases of hypertension and hypertension-associated CVD.
Supplementary Material
Novelty and Significance.
1) What Is New?
Study includes youth across the BP distribution to determine the risk for TOD, including BP well below the 90th percentile.
Combination of auscultatory casual BP measurements with ABPM data, to better characterize BP and what BP parameter signifies greater risk.
Inclusion of advanced echocardiographic measures, arterial stiffness and cognitive function assessment.
2) What Is Relevant?
Study allows a better understanding of the determinants of how BP in youth may transition from a risk factor to TOD to clinical disease, which may greatly impact the health of patients with primary hypertension.
3) Summary
The SHIP-AHOY study is likely to change how hypertension is defined in youth, moving from a statistical definition to one based on intermediate measures of TOD.
These data should inform development of new treatment guidelines that will identify those at highest risk and modify the CVD risk trajectory into adulthood.
Acknowledgments
Funding
This study was supported by AHA grant 15SFRN23680000 and partially by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1 TR001425. The content is solely the responsibility of the authors and does not necessarily represent the official views of the American Heart Association or the National Institutes of Health.
Footnotes
Disclosures
None
References
- 1.Vasan RS, Larsen M, Leip EP, et al. Impact of high normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 2.Psaty BM, Ferburg CD, Kuller LH, et al. Association between blood pressure level and the risk of myocardial infarction, stroke, and total mortality: the cardiovascular health study. Arch Intern Med. 2001;161:1183–1192. doi: 10.1001/archinte.161.9.1183. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris Gl, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–1572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Mozaffairan D, Roger VL, et al. Heart Disease and Stroke Statistics—2014 Update: A Report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson M, Dana T, Bougatsos C, et al. Screening for hypertension in children and adolescents to prevent cardiovascular disease. Pediatrics. 2013;131:490–525. doi: 10.1542/peds.2012-3523. [DOI] [PubMed] [Google Scholar]
- 6.Flynn JT, Kaelber D, Baker-Smith CM, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140 doi: 10.1542/peds.2017-1904. pii: e20171904. [DOI] [PubMed] [Google Scholar]
- 7.Flynn JT. High blood pressure in the young: why should we care? Acta Paediatrica. 2018;107:14–19. doi: 10.1111/apa.14110. [DOI] [PubMed] [Google Scholar]
- 8.Flynn JT, Daniels S, Hayman L, et al. Update: ambulatory blood pressure monitoring in children and adolescents. a scientific statement from the American Heart Association. Hypertension. 2014;63:1116–1135. doi: 10.1161/HYP.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falkner B, DeLoach S, Keith SW, Gidding SS. High risk blood pressure and obesity increase the risk for left ventricular hypertrophy in African-American adolescents. J Pediatr. 2013;162:94–100. doi: 10.1016/j.jpeds.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottdiener JS, Bednarz J, Devereux R, et al. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17:1086–1119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 13.de Simone G, Devereux RB, Daniels SR, et al. Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–1062. doi: 10.1016/0735-1097(94)00540-7. [DOI] [PubMed] [Google Scholar]
- 14.Ganau A, Devereux RB, Roman MJ, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–1558. doi: 10.1016/0735-1097(92)90617-v. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu G, Hirota Y, Kita Y, et al. Left ventricular midwall mechanics in systemic arterial hypertension. Myocardial function is depressed in pressure-overload hypertrophy. Circulation. 1991;83:1676–1684. doi: 10.1161/01.cir.83.5.1676. [DOI] [PubMed] [Google Scholar]
- 16.Dandel M, Lehmkuhl H, Knosalla C, et al. Strain and strain rate imaging by echocardiography - basic concepts and clinical applicability. Curr Cardiol Rev. 2009;5:133–148. doi: 10.2174/157340309788166642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farias CA, Rodriguez L, Garcia MJ, et al. Assessment of diastolic function by tissue Doppler echocardiography: comparison with standard transmitral and pulmonary venous flow. J Am Soc Echocardiogr. 1999;12:609–617. doi: 10.1053/je.1999.v12.a99249. [DOI] [PubMed] [Google Scholar]
- 18.Kasner M, Westermann D, Steendijk P. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: A comparative Doppler-conductance catheterization study. Circulation. 2007;116:637–647. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- 19.Savant JD, Furth SL, Meyers KE. Arterial stiffness in children: pediatric measurement and considerations. Pulse (Basel) 2014;2:69–80. doi: 10.1159/000374095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lande MB, Kupferman JC, Adams HR. Neurocognitive Alterations in Hypertensive Children and Adolescents. J Clin Hypertens. 2012;14:353–359. doi: 10.1111/j.1751-7176.2012.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lande MB, Adams H, Falkner B, et al. Parental assessment of executive function and internalizing and externalizing behavior in primary hypertension after anti-hypertensive therapy. J Pediatr. 2010;157:114–119. doi: 10.1016/j.jpeds.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conners CK. Conners Continuous Performance Test Third Edition. North Tonawanda, NY: Multi-Health Systems Inc.; 2014. [Google Scholar]
- 23.Shrank F, Mather N, McGrew KS. Woodcock-Johnson IV Tests of Cognitive Abilities. Itasca, IL: Riverside Publishing; 2014. [Google Scholar]
- 24.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Functions. Lutz, FL: Psychological Assessment Resources, Inc; 2015. [Google Scholar]
- 25.Hammill DD, Pearson NA, Wiederholt JL. Test of Nonverbal Intelligence-2. Lutz, FL: Psychological Assessment Resources, Inc; 1990. [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Cheng C, Campbell KL, Kushner H, Falkner BE. Correlation of oral glucose tolerance test-derived estimates of insulin sensitivity with insulin clamp measurements in an African-American cohort. Metabolism. 2004;53:1107–1112. doi: 10.1016/j.metabol.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Weinhold B. Epigenetics: The Science of Change. Environmen Health Perspective. 2006;114:A160–A167. doi: 10.1289/ehp.114-a160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Udali S, Guarini P, Moruzzi S, Choi SW, Friso S. Cardiovascular epigenetics: from DNA methylation to microRNAs. Mol Aspects Med. 2013;34:883–901. doi: 10.1016/j.mam.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Alexeeff SE, Baccarelli AA, Halonen J, et al. Association between blood pressure and DNA methylation of retrotransposons and pro-inflammatory genes. Int J Epidemiol. 2013;42:270–280. doi: 10.1093/ije/dys220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breton CV, Park C, Siegmund K, et al. NOS1 methylation and carotid artery intima-media thickness in children. Circ Cardiovasc Genet. 2014;7:116–22. doi: 10.1161/CIRCGENETICS.113.000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Rosa S, Curcio A, Indolfi C. Emerging role of microRNAs in cardiovascular diseases. Circ J. 2014;78:567–575. doi: 10.1253/circj.cj-14-0086. [DOI] [PubMed] [Google Scholar]
- 33.Li RC, Tao J, Guo YB, et al. In vivo suppression of microRNA-24 prevents the transition toward decompensated hypertrophy in aortic-constricted mice. Circ Res. 2013;112:601–605. doi: 10.1161/CIRCRESAHA.112.300806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan W, Zhong Y, Cheng C, et al. MiR-30-regulated autophagy mediates angiotensin II-induced myocardial hypertrophy. PloS One. 2013;8:e53950. doi: 10.1371/journal.pone.0053950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thum T, Lorenzen JM. Cardiac fibrosis revisited by microRNA therapeutics. Circulation. 2012;126:800–802. doi: 10.1161/CIRCULATIONAHA.112.125013. [DOI] [PubMed] [Google Scholar]
- 36.Villar AV, Merino D, Wenner M, et al. Myocardial gene expression of microRNA-133a and myosin heavy and light chains, in conjunction with clinical parameters, predict regression of left ventricular hypertrophy after valve replacement in patients with aortic stenosis. Heart. 2011;97:1132–1137. doi: 10.1136/hrt.2010.220418. [DOI] [PubMed] [Google Scholar]
- 37.Johnson AD, Newton-Cheh C, Chasman DI, et al. Association of hypertension drug target genes with blood pressure and hypertension in 86,588 individuals. Hypertension. 2011;57:903–910. doi: 10.1161/HYPERTENSIONAHA.110.158667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy D, DeStefano AL, Larson MG, et al. Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the Framingham heart study. Hypertension. 2000;36:477–483. doi: 10.1161/01.hyp.36.4.477. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell GF, Guo CY, Kathiresan S, et al. Vascular stiffness and genetic variation at the endothelial nitric oxide synthase locus: the Framingham Heart study. Hypertension. 2007;49:1285–1290. doi: 10.1161/HYPERTENSIONAHA.106.085266. [DOI] [PubMed] [Google Scholar]
- 40.International Consortium for Blood Pressure Genome-Wide Association S. Ehret GB, Munroe PB, Rice KM, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J, Xu H, Aronow BJ, Jegga AG. Improved human disease candidate gene prioritization using mouse phenotype. BMC Bioinformatics. 2007;8:392. doi: 10.1186/1471-2105-8-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji H, Ehrlich LI, Seita J, et al. Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature. 2010;467:338–342. doi: 10.1038/nature09367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Ulm A, Somineni HK, et al. DNA methylation dynamics during differentiation and maturation of human dendritic cells. Epigenetics Chromatin. 2014;7:21. doi: 10.1186/1756-8935-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji H, Zhang X, Oh S, et al. Dynamic transcriptional and epigenomic reprogramming from pediatric nasal epithelial cells to induced pluripotent stem cells. J Allergy Clin Immunol. 2015;135:236. doi: 10.1016/j.jaci.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang W-C, Lin F-M, Chang W-C, et al. miRExpress: Analyzing high-throughput sequencing data for profiling microRNA expression. BMC Bioinformatics. 2009;10:328. doi: 10.1186/1471-2105-10-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


