Abstract
Although preterm infants are at risk for social deficits, interventions to improve mother-infant interaction in the NICU are not part of standard care (SC). Study participants were a subset from a randomized controlled trial of a new intervention for premature infants, Family Nurture Intervention (FNI), designed to help mothers and infants establish an emotional connection. At 4 months corrected age, mother-infant face-to-face interaction was filmed and coded on a 1-second time base for mother touch, infant vocal affect, mother gaze and infant gaze. Time-series models assessed self- and interactive contingency. Comparing FNI to SC dyads, FNI mothers showed more touch and calmer touch patterns, and FNI infants showed more angry-protest but less cry. In maternal touch self-contingency, FNI mothers were more likely to sustain positive touch, and to repair moments of negative touch by transitioning to positive touch. In maternal touch interactive contingency, when infants looked at mothers, FNI mothers were likely to respond with more positive touch. In infant vocal affect self-contingency, FNI infants were more likely to sustain positive vocal affect, and to transition from negative to positive vocal affect. In maternal gaze interactive contingency, following infant looking at mother, FNI mothers of male infants were more likely to look at their sons. In maternal gaze self-contingency, following mother looking away, FNI mothers of male infants were more likely to look at their sons. Documentation of positive effects of FNI for 4-month mother-infant face-to-face communication is useful clinically and has important implications for an improved developmental trajectory of these infants.
Keywords: prematurity, NICU Family Nurture Intervention, mother-infant communication, self- and interactive contingency
Preterm infants are at increased risk for adverse neurodevelopmental outcomes in infancy, childhood, and adolescence (Feldman, Rosenthal, & Eidelman, 2014; Johnson et al., 2012). Early maternal deprivation is associated with multiple deficits later in life in animals and humans (Haller, Harold, Sandi, & Neumann, 2014). Family Nurture Intervention (FNI) was designed to overcome negative effects of maternal deprivation in the NICU by fostering mother-infant emotional connection (Welch, 2016b; Welch et al., 2012, 2013). This study tests the hypothesis that by four months (corrected age [CA]), FNI improves multiple aspects of mother-infant social engagement associated with emotional connection.
Maternal postpartum nurturing is critical for mother-infant social development but is compromised following preterm birth due to prolonged maternal separation that occurs in the NICU, disrupting mother-infant emotional connection (Flacking et al., 2012; Welch & Myers, 2016). Maternal nurturing provides the context for the infant’s repertoire of social contingencies. Mother-infant interaction is characterized by second-by-second shifts of gaze, affect, vocalization, and touch that require contingent coordination by both partners. Mothers and infants reciprocally coordinate communication behaviors from birth (Lavelli & Fogel, 2005). Early patterns of mother-infant coordination establish the foundation for infant development in socio-emotional, cognitive, and regulatory domains (Beebe et al., 2010; Feldman, 2007a, b, c; Jaffe, Beebe, Feldstein, Crown, & Jasnow, 2001; Tronick, 1989).
Physical separation of mother and infant in the NICU impairs the early mutual physiological/emotional connection necessary for optimal co-regulated social contingencies (Welch, 2016b; Feldman, 2002). With underdeveloped neurobehavioral systems, premature (vs. full term) infants can be difficult-to-read and less socially responsive (Feldman & Eidelman, 2007; Malatesta, Grigoryev, Lamb, Albin, & Culver, 1986). Mothers of premature (vs. full term) infants look at, talk to, and touch their infants less frequently (Davis & Thoman, 1988); and are less able to co-regulate cycles of attention and affect with their infants (Lester, Hoffman, & Brazelton, 1985). Recent studies also show deficits in preterm infant-mother dyads during face-to-face and Still-Face paradigms (Feldman & Eidelman, 2007; Jean & Stack, 2012; Montirosso, Borgatti, Trojan, Zanini, & Tronick, 2010). These difficulties predict sub-optimal infant biosocial outcomes (Feldman & Eidelman, 2006; van Baar, van Waaenaer, Briet, Dekker & Kok, 2005). Specifically, these dyads are at risk for dysregulated self- and interactive processes during social interaction, the focus of our study.
Despite a number of promising NICU interventions, there is no consensus on which interventions are of greatest benefit (Symington & Pinelli, 2006). NICU intervention studies often lack randomization and blind assessmemts (Hussey-Gardner & Famuyide, 2009), and many interventions commence after the period of isolette confinement, depriving the infant of critical maternal involvement during the first weeks of life.
Family Nurture Intervention (FNI)
Here we assess whether Family Nurture Intervention (FNI) in the NICU improves mother-infant face-to-face communication at 4 months CA. FNI facilitates emotional connection and autonomic co-regulation between mother and infant, starting with isolette confinement and continuing throughout the NICU stay (Welch, 2012). FNI is not didactic but rather involves direct participation in nurturing activities facilitated by a nurture-specialist, with several new constructs (Welch, 2016a):
-
(1)
FNI aims to create emotional connection and autonomic co-regulation between mother and infant through calming sessions, which include scent cloth exchange, skin-to-skin care, holding, comfort touch, eye contact, vocal soothing and listening
-
(2)
FNI aims to utilize autonomic conditioning via repeated calming sessions to counter adverse NICU experiences and strengthen attraction between mother and infant
-
(3)
mother and infant are equal agents of FNI.
Previous analyses showed that FNI (vs. Standard Care [SC]) mothers exhibited increased maternal sensitivity during caregiving behavior in the NICU (Hane et al. 2015), and fewer anxiety and depressive symptoms when infants were 4 months CA; by 18 months FNI (vs. SC) infants had improved cognitive and language scores (Bayley III), fewer attention problems (Child Behavioral Check List), and decreased risk for autism spectrum disorders (Welch et al., 2015). Because these improved outcomes were independent of specific components of amount of holding and skin-to-skin care, emotional connection may be an aggregate construct that is greater than the sum of the component parts.
The foundation of FNI was first developed to overcome negative consequences of separations such as those that occur with preterm birth or maternal depression (Welch, 1988). While the subcomponents of FNI, olfaction, touch, calming, and vocal expression are analogous to Feldman’s regulatory framework and Hofer’s ‘hidden regulator’ sub-processes (Hofer, 1994), Welch argues that Pavlovian autonomic co-conditioning governs these hidden regulators (Welch, 2016a). Hidden regulators are co-conditioned in the viscera/autonomic nervous systems of fetus and mother during gestation. With normal birth, the co-conditioning triggers attraction behaviors and physiological calming, facilitating emotional connection and visceral/autonomic co-regulation. With preterm birth (or other adverse events), co-conditioning can be interrupted. Repeated calming sessions restore emotional connection and physiological co-regulation. We hypothesize FNI paved the way for the dyad to achieve more optimal patterns of self- and interactive contingency at the 4-month CA follow-up.
Mother-Infant Communication
A dyadic systems view of face-to-face communication, in which both partners contribute to the face-to-face exchange through a bi-directional co-regulation, informed the study. Because each person regulates ongoing behavior and at the same time coordinates with the partner, all dyadic interactions simultaneously reflect self- and interactive processes (Beebe, Messinger, Bahrick, Margolis, Buck & Chen, 2016; Gianino & Tronick, 1988). Fogel (1993) described all behavior as unfolding in the individual, while at the same time modifying and being modified by the changing behavior of the partner. Both self- and interactive processes are essential to face-to-face communication. Both intrapersonal and interpersonal behavioral rhythms provide ongoing temporal information necessary to coordinate with one’s partner, so that each can anticipate how the other will proceed (Beebe et al., 2010; Feldman, 2016).
In this and past studies we quantify self- and interactive processes by coding behavior second-by-second and generating measures of contingency, a term we use interchangeably with predictability and coordination. Contingencies are quantified using time-series methods. Interactive contingency assesses predictable moment-to-moment adjustments that each individual makes in response to the partner’s prior behavior. Self-contingency measures the degree to which prior behavior predicts current behavior: the degree of stability/variability within an individual’s own rhythms of behavior (in the presence of a particular partner) (Beebe et al., 2016; Messinger, Ekas, Ruvolo, & Fogel, 2012). In prior research on full term samples, both heightened and lowered degrees of mother and infant contingency may be associated with maternal distress and infant insecure attachment (Beebe et al., 2007, 2008, 2010; Jaffe et al., 2001; Malatesta et al., 1989).
Approach
Mothers and premature infants participated in a randomized controlled trial of FNI. We aimed to specify the self- and interactive contingency patterns of these infants and mothers, during videotaped mother-infant face-to-face communication at 4 months CA.
We chose to study 4 months because it is the age at which infant social capacities flower (Beebe et al., 2016; Tronick, 1989). We chose to assess mother-infant face-to-face communication because it provides critical inputs for maturation of the social brain and sensitizes infants to temporal and emotional resonances that underlies human relationships (Feldman, 2007, 2015; Jaffe et al., 2001). By 4 months, face-to-face communication taps the infant’s most advanced social capacities (Tronick, 1989).
Videotaped interactions were coded for mother and infant gaze, infant vocal affect, and maternal touch. Mother and infant gaze patterns generate greater/lesser likelihood of mutual gaze, the foundation of face-to-face engagement (Stern, 1985). Attention to, and contingent coordination with, the partner’s direction of gaze-at and -away from one’s own face provide a foundation for coordination in other modalities, and are compromised with infant prematurity (Feldman, Eidelman, Sirota & Weller, 2002). Lower maternal gaze coordination with infant gaze is associated with higher maternal self-criticism and maternal depression (Beebe et al., 2007, 2008). Less predictable maternal gaze patterns predict infant insecure (vs. secure) attachment (Beebe et al., 2010).
Maternal touch may be the most basic mammalian maternal behavior (Feldman, 2012). Less affectionate and more intrusive maternal touch is associated with infant prematurity (Feldman & Eidelman, 2003, 2007) and maternal depression (Beebe et al., 2008). Lower maternal coordination of touch with infant touch patterns is associated with maternal depression (Beebe et al, 2008) and disorganized attachment (Beebe et al., 2010). Mothers of premature infants who participate in Kangaroo Care provide more affectionate touch (Feldman, Eidelman et al., 2002; Feldman, Weller, Sirota & Eidelman (2002).
Infant vocal affect measures positive to distressed affect. Infant vocal distress predicts social/cognitive risk (NICHD Network, 2004) and disorganized infant attachment (Beebe et al., 2010). Positive infant vocalization is associated with greater maternal attunement (Markova & Legerstee, 2006).
The specificity of the behavioral coding approach and data-analytic strategy allowed evaluation of the following dimensions of the 4-month interaction which may be influenced by FNI: (1) partner (mother/infant), (2) type of measure (behavioral frequency/contingency), (3) type of contingency (self/interactive), and (4) modality of behavior (attention, affect, touch).
We hypothesized that FNI would optimize mother-infant social development assessed at 4 months (CA). Because prior studies have shown lower social coordination in premature infant-mother dyads, we predicted that FNI would increase maternal and infant coordination with the partner’s behavior as evidenced by increased interactive contingency. Lacking sufficient prior literature, we made no specific hypotheses regarding self-contingency.
Method
FNI Trial Design and Intervention
Participants were enrolled in a single-center, parallel group, randomized controlled trial. The study design is published (Welch, et al., 2012, 2013) and was registered (ClinicalTrials.gov: NCT01439269). We excluded: mothers who did not speak English, had a history of drug addiction or mental illness; infants with birth weight below the third percentile for gestational age or significant congenital defect. A total of 115 mothers and 150 infants (35 sets of twins, 80 singletons, delivered 26–34 weeks gestation) were enrolled.
As soon after delivery as possible, mothers provided consent, completed baseline assessments, and were randomly assigned to Standard Care (SC) or FNI. A research assistant drew a sealed envelope containing a group indicator from a box holding a numbered sequence of such envelopes, prepared using block randomization. Mothers assigned to FNI met with Nurture Specialists, former NICU nurses trained in implementing the intervention, who guided mothers and families throughout the study regarding all aspects of the intervention. Mothers and infants assigned to the SC condition received standard NICU care: (a) parent education by the bedside nurse in infant touch, handling, skin-to-skin care, feeding, bathing and diapering; skin-to-skin care and breast feeding were determined by the mother’s preferences; (b) availability of a social worker, infant mental health psychologist, and parent groups led by a social worker.
Initial FNI activities took place when infants were in incubators. As soon as possible after birth, two small cotton cloths were given to the mother, one worn in her bra and the other placed under her infant’s head. Each day, the cloths were exchanged. Mothers were encouraged to sniff the cloth suffused with their infant’s smell when going home at night; the cloth suffused with the mother’s smell was placed by the infant’s head. As infants became more stable, Nurture Specialists facilitated FNI mothers in making contact with their infants through the ports of the incubator, using firm and sustained touch, speaking and singing emotionally to their infants in their native languages, and making eye contact as often as possible. Later, mothers were encouraged to engage in holding (skin-to-skin or non-skin-to-skin). FNI mothers engaged in these activities ~6 hours/week until discharge (Welch, Hofer, Brunelli, Stark, Andrews, Austin, Myers, et al., 2012; Welch et al., 2013).
Procedures at Infant Age 4 Months Corrected Age
At 4 months CA, 80 (N = 37 SC, 43 FNI) of the original 115 mothers returned with their infants for face-to-face play with split-screen filming. New York State Psychiatric Institute Institutional Review Board approved study procedures for “Data Analysis 4 Months: NICU Follow Up” #6718 (expiration 1.21.19). Of N = 115 dyads, 30 were lost at term due to: transfer to other facilities (SC 4, FNI 4), infant death (SC 1, FNI 1), withdrawal (SC 0, FNI 4), or loss to follow up (SC 10, FNI 6). An additional 5 were lost to follow up by 4 months CA. Parents from dyads with 4-month video data were better educated (mother χ2 = 8.10, p = .017; father χ2 = 6.78, p = .034), and more likely to be married (χ2 = 5.48, p = .019), but did not differ with respect to either parent’s age and race/ethnicity, or household income category.
Singletons and first-born twins were filmed. Mothers (seated opposite infants seated in an infant seat on a table) were instructed to play with their infants as they would at home, but without toys, for approximately 10 minutes. A special-effects generator created a split-screen view from input of 2 synchronized cameras (mounted on opposite walls) focused on head and upper torso of mother and infant. Of N = 80, 9 recordings were lost due to poor film quality, camera angle inadequate for gaze coding, static audio, or failure to record audio. Thus, analyses on 71 dyads compared 39 FNI and 32 SC dyads.
By virtue of the intervention, FNI (vs. SC) mothers engaged in more hours of skin-to-skin contact per week (FNI = 3.6, SC = 1.5, p <.001). Infant gestational age at birth, birth weight, number of NICU visits per week, and hours of clothed holding per week, did not differ for 39 FNI vs. 32 SC dyads.
Behavioral coding
The first 2.5 minutes1 of uninterrupted mother-infant interaction were coded on a one-second time base by coders blind to FNI/SC status. If more than one behavior occurred in the same second, the behavior occurring in the second half of the second was privileged (Beebe et al., 2010; Tronick & Weinberg, 1990). Behaviors were coded with ordinal scales from high to low except gaze, coded on/off the partner’s face. Infant vocal affect (vocal contour) was coded as high positive, neutral/positive, none, fuss/whimper, angry-protest, cry.
Mother touch was coded from affectionate to intrusive: affectionate (stroke, kiss), static (hold, provide finger for infant to hold), playful (tap, tickle), none, caregive, jiggle/ bounce, infant-directed oral-touch (e.g. put finger in infant’s mouth), object-mediated, centripetal (body center: face, body, head), rough (scratch, push, pinch), high intensity/intrusive (both rough touch and high intensity touch are considered intrusive). This coding considered type of touch, location, and intensity (mild/moderate vs. intense/intrusive); touch to the body-periphery was considered less stimulating than touch to the body-center (Stepakoff, 1999; Stepakoff, Beebe & Jaffe, 2000; Beebe et al., 2010). This maternal touch scale has yielded informative results (Beebe et al., 2007, 2008, 2010, 2016). For all coding details, see Web Appendix A (or Beebe et al., 2010). Inter-coder reliability estimates were conducted on 20% of the dyads and generated mean Cohen’s Kappa per modality as follows: infants: gaze .95; vocal affect .98; mothers: gaze 0.92; touch .90.
From these assessments, we generated 4 mother-infant modality pairings for analyses of self- and interactive contingency:
-
(1)
infant gaze - mother gaze
-
(2)
infant gaze - mother touch
-
(3)
infant vocal affect - mother touch
-
(4)
infant vocal affect - mother gaze.
Data Analysis
Analyses compared 39 FNI dyads and 32 SC dyads at 4 months CA, using all 150 sec coded from video for each individual. First we tested whether FNI vs. SC dyads differed in means and frequencies of behavior. Then we created indices of self- and interactive contingency. Traditional time-series approaches model each dyad individually and enter model coefficients into analyses of variance. In contrast, multi-level time-series approaches model the group as a whole,2 creating estimates of both fixed effects3 in the sample (group-level), and random effects (individual variation in those effects). Advantages of this approach include more appropriate statistical assumptions, more accurate estimates of parameters, and increased power. These models are designed to quantify patterns over time, here the course of behavior second-by-second, within the individual (self-contingency), and between two individuals (interactive contingency).
SAS statistical software was used to estimate random and fixed effects on patterns of self- and self-with-other behaviors over 150 seconds. SAS PROC MIXED was used to examine ordinally-coded mother touch and infant vocal affect behaviors (McArdle & Bell, 2000; Singer, 1998). SAS PROC GLIMMIX was used to evaluate mother and infant gaze behaviors (dichotomously-coded) (Cohen, Chen, Hamigami, Gordon, & McArdle, 2000; Goldstein, Healy, & Rasbash, 1994; Littell, Miliken, Stoup, & Wolfinger, 1996). Repeated second-by-second observations on individuals formed the basic random data, just as in cross-sectional data single individual variables are the basic units of analyses. For details of statistical models see Chen and Cohen (2006). Self- and interactive contingency were first calculated for all mothers and infants for all modality pairings. A second set of analyses tested conditional effects of FNI vs. SC group on self- and interactive contingency.
Two types of multi-level time-series models: Weighted lag and Individual lags Weighted lag time-series analysis.
Consistent with previous studies, we used a weighted lag approach (Beebe et al., 2007; 2010; 2016). Using a 4-second moving window,4 the prior 3 seconds (lags 1, 2 and 3: L1, L2, L3) of behavior were used to predict t0, the behavior at the current moment. All 3 prior seconds were condensed to one assessment (“weighted lag”) by weighting each prior second by its relative association with t0. For each dependent variable, standardized (x̅ = 0; SD = 1) measures of prior self or partner behavior, “lagged variables,” were computed as a weighted average of the recent prior seconds, based on these analyses. Estimated coefficients for effects of these standardized lagged variables on current behavior (t0) over the duration of the interaction (150 seconds) indicate the level of self- or interactive contingency: larger coefficients reflect stronger contingencies. Each analysis included both self- and interactive contingency; thus estimated coefficients of one form of contingency control for the other.
Individual seconds (lags) time-series analysis.
This approach is supplemental to the weighted lag approach. Behaviors at each of the 3 prior lags were evaluated individually with a separate model for each second’s association with behavior at the current moment: (L1 → t0; L2 → t0, L3 → t0). A key difference between the weighted lag and individual seconds analyses is that, in the latter, the values used in the analyses are simply those obtained at each of 3 lags; in the former, the values at the 3 lags are weighted by their respective correlations with t0 and then are combined into a single value. Otherwise the models are identical. The individual seconds approach applies a more precise lens to the identification of differences in FNI vs. SC groups. For simplicity of interpretation, the individual seconds approach does not accommodate the interaction terms of control variables with individual lags and group. The weighted lag approach has more power in detecting differences between groups when each individual second of the 3 prior seconds is not sufficiently strong, but collectively the 3 prior seconds are sufficiently strong to detect differences. Reciprocally, the individual seconds approach has more power when differences are primarily located in particular seconds of the 3 prior seconds. Nevertheless, where findings from the individual seconds analyses are not consistent with those of the weighted lag analyses, we present with caution.
Tests of hypotheses used fixed effects (FNI vs. SC groups). In addition to the intercept, fixed effects included: (1) lagged effects of self- and partner behavior (self- and interactive contingency); (2) differences in behavioral frequencies (e.g. infant vocal affect) associated with group; (3) differences in self- and interactive contingency associated with group. After removing non-significant terms, the final model was the simplest consistent with the data. Significance level was set at p < .05. All tests were 2-tailed. With 71 dyads (39 FNI, 32 SC) and 150 seconds of behavior per individual, the resulting 10,650 seconds for mother (or infant) per communication modality generated ample power to detect effects. In the weighted lag models we included maternal age, education, and ethnicity as covariates but these were dropped because they did not contribute to the model; however, gender was significant and was retained as a covariate.
Analysis of Predicted Values: Illustrations of Behavioral Details of Time-Series Models
Multi-level time-series analyses identify overall group differences in the level of self- and interactive contingency between FNI and SC groups but cannot tell us where differences in specific behaviors lie. Further post-hoc descriptive analyses are required to explicate specific patterns of behavioral predictors across L1, L2, and L3 that contribute to any significant group differences at t0 identified by multi-level models. We used an approach termed analysis of predicted values to identify specific behavioral patterns that underlie significant group differences (see Searle & Gruber, 2016). Because the analysis of predicted values comes directly from the individual seconds time-series models, it is more accurate (than, for example, percent time transition-matrices) and represents the temporal dynamics.
Our analysis of predicted values derived predicted values at t0 for FNI vs. SC groups. For ordinal scales, the resulting value was the predicted level of the behavioral code at t0. For gaze (binary variable), the resulting value was the predicted probability of being gaze-on at t0. To locate sources of difference between FNI and SC contrasts identified by significant time-series models, we generated every possible combination of behavioral codes for mother at L1, L2, L3, and infant at L1, L2, L3 (within a particular modality pairing) in relation to a behavior predicted at t0. We then computed estimated values (level of behavior or probability) at t0 for FNI vs. SC groups for the significant finding in question, using the equations generated by the individual seconds time-series analyses. We identified absolute values of differences in predicted values at t0 for the two groups, ranking the absolute differences from largest to smallest. To ascertain where FNI and SC groups differed the most, we examined the behavior combinations with the 10 highest differences in predicted value at t0. For each combination of behaviors, the significant difference in predicted value of t0 indicates that, although the FNI and SC dyads behaved in the same way over the prior 3s, they behaved differently at t0.
In the individual lags time-series approach, we can interpret relevant findings at each lag of L1, L2, and L3, for mother and infant. But in the weighted lag time-series approach, where the information of L1, L2, and L3 has been aggregated into one value, we used L1 to interpret effects in the analysis of predicted values approach, as we observed that L1 always had the largest association with t0, as we expect.
Results
Descriptive Statistics
The first goal was a descriptive evaluation of differences between FNI vs. SC groups. Comparing FNI and SC dyads in mean levels of behaviors using independent t-tests, we found no differences (see Web Appendix B1). Testing percent time spent in each behavior produced no group differences in mother gaze (χ2 = 0.007, p = 0.931), or infant gaze (χ2 = 2.323, p = 0.128); but did produce significant differences in mother touch patterns (χ2 = 197.272, p < 0.001) and infant vocal affect levels (χ2 = 81.166, p < 0.001). FNI (vs. SC) mothers touched their infants a greater percentage of time (less time coded as no-touch: 16.5% vs. SC 20.7%); used more static touch, a more positive, calming pattern (43% vs. SC 36.5%); used less caregiving touch (which interrupts the ongoing communication) (0.7% vs. SC 1.9%); used more object-mediated touch (2.5% vs. SC 0.5%); and used more intrusive touch (1.2% vs. SC 0.6%). The latter two types of touch are rare. FNI (vs. SC) infants used more angry-protest (1.8% vs. SC 0.8%) but less cry (0.4% vs. SC 1.8%).
Influenced by Feldman and colleagues (Feldman, 2007b; Feldman & Eidelman, 2003, 2006), we pursued the possibility of other differences in gaze behavior in FNI vs. SC dyads. However, we found no differences after testing the following variables: number and average length of mutual gaze episodes; proportion of time in mutual gaze; latency to, and duration of, first mutual gaze; which partner breaks the first mutual gaze; percentage of all mutual gaze episodes broken by mother or by infant; latency to first infant gaze aversion; likelihood of extensive infant gaze aversion (80% time+); or co-occurrence within the same second of mutual gaze and mother positive touch (affectionate/static/playful patterns) (see Web Appendix B2).
Self- and Interactive Contingencies in FNI vs. SC Dyads
The second goal was to evaluate differences between the FNI vs. SC groups in levels of self- and interactive contingency for 4 modality pairings: (1) infant gaze - mother touch; (2) infant vocal affect - mother gaze; (3) infant gaze -mother gaze; (4) infant vocal affect - mother touch. For these analyses behavior in the current second is represented as t0; behavior one second prior to the current second is represented as L1 (t-1); behavior two seconds prior as L2 (t-2); behavior three seconds prior as L3 (t-3).
(1). Infant Gaze – Mother Touch
(1a). Infant gaze self-contingency (controlling for mother touch).
Testing across the prior 3 seconds with a weighted lag, Table 1 shows no difference between FNI and SC infants in gaze self-contingency (controlling for prior mother touch). Testing for the predictability of each individual second, Table 2 shows that gaze self-contingency of FNI (vs. SC) infants was marginally significantly lower (more variable) from L2 (IG L2 → IG β = −.298, p = .051). The β is an index of degree of contingency.
Table 1.
Infant Gaze - Mother Touch: Weighted Lag Analysis for Standard Care (SC) and Family Nurture Intervention (FNI) and their Differences (Δ)
| Infant Gaze | β | SEβ | p | |
|---|---|---|---|---|
| IG → IG1 | 1.338 | .055 | <.001 | |
| MT → IG | .029 | .093 | .755 | |
| SC | I Sex2 | −.091 | .179 | .613 |
| IG * Sex→ IG3 | .062 | .061 | .310 | |
| MT * Sex→ IG | .196 | .084 | .021 | |
| FNI | IG → IG | 1.412 | .051 | <.001 |
| MT → IG | .061 | .033 | .064 | |
| Group | −.012 | .180 | .945 | |
| Δ4 | IG * Group → IG5 | .074 | .062 | .232 |
| MT* Group →IG | .032 | .097 | .738 | |
| Mother Touch | β | SEβ | p | |
| MT → MT | 5.783 | .154 | <.001 | |
| IG → MT | .261 | .120 | .029 | |
| SC | I Sex | −.185 | .207 | .376 |
| MT * Sex→ MT | −.278 | .139 | .045 | |
| IG * Sex→ MT | −.403 | .131 | .002 | |
| FNI | MT → MT | 4.709 | .086 | <.001 |
| IG → MT | .601 | .111 | <.001 | |
| Group | .236 | .208 | .260 | |
| Δ | MT * Group → MT | −1.074 | .170 | <.001 |
| IG * Group → MT | .340 | .132 | .010 | |
IG → IG = infant gaze predicting infant gaze: infant gaze self-contingency; MT → IG = mother touch predicting infant gaze: infant gaze interactive contingency with mother touch; these contingency terms represent baseline effects for male infants. Arrow = direction of prediction; predicted variable is to the right of the arrow; weighted lag term is to the left of arrow. In these weighted lag models, the weighted lag term is calculated in relation to the outcome variable, whereas lags in the Individual Seconds models (see Table 2) are not.
Sex = difference in level of infant gaze for female (vs. male) infants.
IG * Sex → IG: additional effect of being female on contingency (female = 1, male = 0).
Δ = difference between FNI (Family Nurture Intervention) vs. SC (Standard Care) groups.
IG * Group → IG: additional effect of being in FNI intervention group (FNI = 1, SC = 0).
Note. Models included time and intercept. Beta values are represented as standardized effect sizes. We evaluated whether contingencies of FNI vs. SC dyads differed, and determined the significance of baseline contingencies for SC dyads and the additional effect of being in FNI group. To determine significance of contingencies of FNI group, we reversed the 0/1 coding of FNI vs. SC and re-ran the models. We include main effects for these models and show significance of baseline contingencies for FNI group (without other terms in the model). We include terms for sex, or 3-way interaction terms (*sex*group), only where significant.
Table 2.
Infant Gaze – Mother Touch: Individual Seconds Time Series Analysis for Standard Care (SC) and Family Nurture Intervention (FNI) and their Differences (Δ)
| Infant Gaze | β | SEβ | p | Mother Touch | β | SEβ | p | |
|---|---|---|---|---|---|---|---|---|
| IG L1 → IG1 | 2.24 | .098 | <.001 | MT L1 → MT | .725 | .034 | <.001 | |
| IG L2 → IG | .573 | .112 | <.001 | MT L2 → MT | .090 | .042 | .031 | |
| SC | IG L3 → IG | .336 | .107 | .002 | MT L3 → MT | −.030 | .034 | .367 |
| MT L1 → IG2 | −.017 | .015 | .257 | IG L1 → MT | .120 | .256 | .640 | |
| MT L2 → IG | .016 | .020 | .429 | IG L2 → MT | −.010 | .281 | .973 | |
| MT L3 → IG | −.002 | .019 | .918 | IG L3 → MT | −.043 | .253 | .865 | |
| IG L1 → IG | 2.47 | .090 | <.001 | MT L1 → MT | .408 | .011 | <.001 | |
| IG L2 → IG | .276 | .103 | .008 | MT L2 → MT | .115 | .012 | <.001 | |
| FNI | IG L3 → IG | .562 | .093 | <.001 | MT L3 → MT | .147 | .011 | <.001 |
| MT L1 → IG | .003 | .005 | .487 | IG L1 → MT | 1.246 | .227 | <.001 | |
| MT L2 → IG | .018 | .005 | <.001 | IG L2 → MT | −.454 | .256 | .076 | |
| MT L3 → IG | −.015 | .005 | .002 | IG L3 → MT | −.506 | .224 | .024 | |
| Group (GP) | −.037 | .183 | .840 | Group (GP) | .263 | .204 | .202 | |
| IGL1*GP→IG | .231 | .133 | .083 | MTL1*GP→MT | −.317 | .035 | <.001 | |
| IG L2* GP→IG | −.298 | .152 | .051 | MTL2*GP→MT | .025 | .043 | .557 | |
| Δ | IG L3* GP→IG | .226 | .142 | .112 | MTL3*GP→MT | .178 | .035 | <.001 |
| MTL1*GP→IG | .020 | .016 | .200 | IG L1*GP→ MT | 1.127 | .342 | .001 | |
| MTL2*GP→IG | .002 | .021 | .927 | IG L2*GP→ MT | −.445 | .380 | .242 | |
| MTL3*GP→IG | −.013 | .019 | .513 | IG L3*GP→ MT | −.463 | .338 | .171 | |
L1 = 1 sec lag (1 second prior); L2 = 2 sec lag; L3 = 3 sec lag.
Model testing for MT L1, L2, L3 also includes IG L1, L2, L3; that is, of the six terms, all control for the other five.
Analysis of predicted values was used to clarify the details of these results (see Web Appendix C, Table C1). Given infant gaze-on at L2, both FNI and SC infants are likely to be gaze-off at t0 (both probabilities of gaze-on are less than 50%), but this is significantly more likely for FNI than SC infants (mean of the top 10 probability values at t0 = .184 for FNI; .282 for SC).
In summary, given infants were gaze-on 2 seconds prior, both FNI and SC infants were likely to be gaze-off at t0, but this was significantly more likely for FNI infants. FNI infants have a more variable gaze process when controlling for mother touch.
(1b). Infant gaze interactive contingency (mother touch predicting infant gaze).
Testing with weighted lag and individual seconds approaches, Tables 1 and 2 show no differences between FNI and SC groups in degree of infant gaze coordination with mother touch (controlling for prior infant gaze).
(1c). Mother touch self-contingency (controlling for infant gaze).
Testing with a weighted lag, Table 1 shows lowered (more variable) touch self-contingency in FNI (vs. SC) mothers (MT* Group → MT β = −1.074, p < .001) (controlling for prior infant gaze). Testing the predictability of each individual second, Table 2 shows that FNI (vs. SC) mothers had more variable touch self-contingency from L1 (MT L1 → MT β = −.317, p < .001), and heightened touch self-contingency from L3 (MT L3 → MT β = .178, p < .001).
Analysis of predicted values (see Web Appendix C, Table C2) showed that, given mother touch tending toward the most negative values at L1, or the most positive values at L3, FNI (vs. SC) mothers showed more positive touch (about four levels higher) at t0 (mean of the top 10 probability values at t0 = 6.804 for FNI; = 2.749 for SC).
In summary, FNI (vs. SC) mothers are more likely to sustain positive touch and to repair moments of negative touch into positive touch.
(1d). Mother touch interactive contingency (infant gaze predicting mother touch).
Testing with a weighted lag, Table 1 shows heightened maternal touch coordination with prior infant gaze in FNI (vs. SC) mothers (IG *Group → MT β = .340, p = .010) (controlling for prior maternal touch). Testing with an individual seconds approach, Table 2 also shows heightened maternal touch coordination with infant gaze in FNI (vs. SC) mothers, from L1 (IG L1 → MT β = 1.127, p = .001). Because the β is standardized, it is a measure of effect size. We note that the effect size is over 3× greater using the individual seconds approach, from L1. Thus, mother’s touch coordination with infant gaze primarily occurs in the next second (from mother touch t−1 → infant gaze t0).
Analysis of predicted values (see Web Appendix C, Table C2) showed that, given infant gaze-on at L1, mother touch was more positive (4 levels higher) at t0 in FNI (vs. SC) mothers (mean of the top 10 probability values at t0 = 6.804 for FNI; 2.749 for SC).
In summary, FNI (vs. SC) mothers showed a heightened positive touch response to infant gaze-on (vs. -off) mother’s face. When infants look, FNI mothers are likely to greet infants with much more positive forms of touch, in the next second.
(2). Infant Vocal Affect - Mother Gaze
(2a). Infant vocal affect self-contingency (controlling for mother gaze).
Testing with a weighted lag, Table 3 shows lowered (more variable) infant vocal affect self-contingency (IVA → IVA β = −2.424, p < .001) (controlling for prior mother gaze). Testing for the predictability of each individual second, Table 4 shows lowered (more variable) FNI (vs. SC) infant vocal affect self-contingency from L1 (IVA L1 → IVA β = −.903, p < .001), and increased infant vocal affect self-contingency from L2 (IVA L2 → IVA β = .166, p < .001).
Table 3.
Infant Vocal Affect - Mother Gaze: Weighted Lag Analysis for Standard Care (SC) and Family Nurture Intervention (FNI) and their Differences (Δ)
| Infant Vocal Affect | β | SE β | p | |
|---|---|---|---|---|
| IV → IV | 2.987 | .228 | <.001 | |
| MG → IV | .033 | .150 | .824 | |
| SC | Sex | .777 | .492 | .119 |
| IV * Sex→ IV | 4.335 | .240 | <.001 | |
| MG * Sex → IV | .048 | .162 | .769 | |
| FNI | IV → IV | .553 | .224 | .014 |
| MG → IV | .123 | .138 | .375 | |
| Group | .110 | .494 | .824 | |
| Δ | IV * Group → IV | −2.424 | .257 | <.001 |
| MG * Group → IV | .089 | .162 | .582 | |
| Mother Gaze | β | SEβ | p | |
| MG → MG | .647 | .055 | <.001 | |
| IV → MG | .140 | .122 | .251 | |
| SC | I Sex | .029 | .206 | .890 |
| MG * Sex → MG | −.091 | .075 | .225 | |
| IV * Sex → MG | −.042 | .096 | .662 | |
| FNI | MG → MG | .428 | .053 | <.001 |
| IV → MG | −.051 | .082 | .534 | |
| Group | −.086 | .207 | .680 | |
| MG * Group → MG | −.219 | .076 | .004 | |
| Δ | IV * Group → MG | −.190 | .137 | .165 |
| MG * Group* Sex → MG1 | .303 | .103 | .003 | |
| MG*Group*Sex →MG | SC | FNI | SC vs. FNI | |||
|---|---|---|---|---|---|---|
| β | p | β | p | β | p | |
| Male infant | .647 | < .001 | .428 | <.001 | .220 | .004 |
| Female infant | .556 | <.001 | .640 | <.001 | −.084 | .226 |
| Male vs. Female | .091 | .225 | −.212 | .003 | ||
Note. See Table 1 footnotes for explanation of terms. IV = infant vocal affect.
The table below shows the betas, and significance tests of their differences, relevant to the 3-way interaction effect.
Table 4.
Infant Vocal Affect – Mother Gaze: Individual Seconds Time Series Analysis for Standard Care (SC) and Family Nurture Intervention (FNI) and their Differences (Δ)
| Infant Vocal Affect | β | SEβ | p | Mother Gaze | β | SEβ | p | |
|---|---|---|---|---|---|---|---|---|
| IV L1 → IV | .647 | .030 | <.001 | MG L1 → MG | 1.578 | .109 | <.001 | |
| IV L2 → IV | .046 | .037 | .213 | MG L2 → MG | .604 | .120 | <.001 | |
| IV L3 → IV | .173 | .033 | <.001 | MG L3 → MG | .435 | .119 | <.001 | |
| SC | MG L1 → IV | −.396 | .361 | .273 | IV L1 → MG | −.008 | .013 | .515 |
| MG L2 → IV | .014 | .374 | .970 | IV L2 → MG | .049 | .055 | .366 | |
| MG L3 → IV | −.244 | .360 | .498 | IV L3 → MG | .010 | .029 | .739 | |
| IV L1 → IV | −.256 | .012 | <.001 | MG L1 → MG | 1.549 | .099 | <.001 | |
| IV L2 → IV | .212 | .012 | <.001 | MG L2 → MG | .555 | .108 | <.001 | |
| FNI | IV L3 → IV | .205 | .012 | <.001 | MG L3 → MG | .309 | .109 | .005 |
| MG L1 → IV | .592 | .322 | .066 | IV L1 → MG | −.005 | .004 | .282 | |
| MG L2 → IV | −.533 | .332 | .109 | IV L2 → MG | −.008 | .004 | .068 | |
| MG L3 → IV | .319 | .319 | .318 | IV L3 → MG | −.003 | .005 | .580 | |
| Group (GP) | .110 | .494 | .824 | Group (GP) | −.086 | .207 | .680 | |
| IVL1*GP → IV | −.903 | .032 | <.001 | MGL1*GP→ MG | −.029 | .148 | .843 | |
| IVL2*GP → IV | .166 | .039 | <.001 | MGL2*GP→ MG | −.049 | .162 | .763 | |
| Δ | IVL3*GP → IV | .032 | .036 | .375 | MGL3*GP→ MG | −.127 | .162 | .434 |
| MGL1*GP → IV | .988 | .484 | .041 | IVL1*GP → MG | .003 | .013 | .795 | |
| MGL2*GP → IV | −.546 | .500 | .275 | IVL2*GP → MG | −.057 | .055 | .295 | |
| MGL3*GP → IV | .563 | .482 | .242 | IVL3*GP → MG | −.012 | .030 | .677 | |
Analysis of predicted values (see Appendix C, Table C3) showed that, as infant vocal affect tended toward the most negative level at L1, or toward the most positive at L2, FNI (vs. SC) infant vocal affect was more positive at t0, by over 4 vocal affect levels (mean of the top 10 probability values at t0 = 6.677 for FNI; 1.850 for SC).
In summary, FNI (vs. SC) infants are more likely to sustain positive vocal affect and to transition from negative to more positive vocal affect (controlling for mother gaze).
(2b). Infant vocal affect interactive contingency (mother gaze predicting infant vocal affect).
Testing with a weighted lag, Table 5 shows no difference between FNI and SC infants (β = .089, p = .528). Testing with an individual seconds approach, Table 4 shows that vocal affect of FNI (vs. SC) infants is more coordinated with prior mother gaze (controlling for prior infant vocal affect), from L1 (MG L1 → IVA β = .988, p = .041).
Table 5.
Infant Gaze - Mother Gaze: Weighted Lag Analyses for Standard Care (SC) and Family Nurture Intervention (FNI) and their Differences (Δ)
| Infant Gaze | β | SEβ | p | |
|---|---|---|---|---|
| SC | IG → IG | 1.365 | .047 | <.001 |
| MG → IG | .067 | .046 | .148 | |
| FNI | IG → IG | 1.431 | .041 | <.001 |
| MG → IG | .024 | .042 | .561 | |
| Group | .005 | .179 | .979 | |
| Δ | IG * Group → IG | .062 | .062 | .322 |
| MG * Group → IG | −.043 | .062 | .491 | |
| Mother Gaze | β | SE6 | p | |
| MG → MG1 | .642 | .056 | <.001 | |
| IG → MG1 | .100 | .082 | .224 | |
| I Sex | .013 | .206 | .951 | |
| SC | MG * Sex → MG2 | −.104 | .077 | .176 |
| IG * Sex → MG2 | .161 | .114 | .159 | |
| FNI | MG → MG | .378 | .055 | <.001 |
| IG → MG | .358 | .073 | <.001 | |
| Group | −.066 | .206 | .750 | |
| MG * Group → MG3 | −.264 | .078 | <.001 | |
| Δ | IG * Group → MG3 | .258 | .110 | .019 |
| MG * Group * Sex → MG4 | .371 | .105 | <.001 | |
| IG * Group * Sex → MG4 | −.443 | .152 | .004 | |
| MG*Group*Sex →MG | SC | FNI | SC vs. FNI | |||
|---|---|---|---|---|---|---|
| β | p | β | p | β | p | |
| Male infant | .642 | < .001 | .378 | <.001 | .264 | <.001 |
| Female infant | .538 | <.001 | .645 | <.001 | −.107 | .128 |
| Male vs. Female | .104 | .176 | −.267 | <.001 | ||
| MG*Group*Sex →IG | SC | FNI | SC vs. FNI | |||
| β | p | β | p | β | p | |
| Male infant | .099 | .224 | .358 | <.001 | −.258 | .019 |
| Female infant | .260 | .001 | .075 | .268 | .185 | .076 |
| Male vs. Female | −.161 | .159 | .283 | .005 | ||
Note. See Table 1 footnotes for explanation of terms.
MG → MG; IG → MG: contingency term alone represents the baseline effect for males.
MG * Sex → MG; IG * Sex → MG: effect of being female on contingency (female 1, male 0).
MG * Group → MG; IG * Group → MG: effect of being in FNI group (FNI = 1, SC = 0).
MG * Group * Sex → MG; IG * Group*Sex → MG: 3-way interaction among prior mother gaze, group, and sex, for mother self-contingency (β = .371, p < .001), and mother interactive contingency (β = −.443, p < .001). Tables below show betas and significance tests of differences relevant to the 3-way interaction effects.
Analysis of predicted values (see Web Appendix C, Table C3) showed that, given mother gaze-on at L1, FNI (vs. SC) infants are likely to show more positive vocal affect in the current moment, by over 4 vocal affect levels (mean of the top 10 probability values at t0 = 6.677 for FNI; = 1.851 for SC).
In summary, given mother gaze-on in the prior second, FNI (vs. SC) infants show more positive vocal affect in the current second. However, the weighted lag approach generated no corresponding finding. Because this was the only significant infant interactive contingency finding of 12 possible equations using the individual seconds approach, it was not pursued.
(2c). Mother gaze self-contingency (controlling for infant vocal affect).
Testing with a weighted lag approach, FNI vs. SC differences in maternal gaze self-contingency (controlling for prior infant vocal affect) were a function of infant sex. The 3-way interaction effect (MG *Group*Sex → MG β = .303, p = .003) in Table 3 represents the interaction of being in the FNI (vs. SC) group, and being mothers of female (vs. male) infants, above and beyond either alone, on maternal self-contingency. As shown in Table 3, footnote 1, the self-contingency of FNI mothers of males (β = .428) was lower than that of SC mothers of males (β = .647), and significantly different (β = .220; p = .004); but the self-contingency of FNI vs. SC mothers of females did not differ (β = −.084; p = .226). Thus, FNI vs. SC differences in mother gaze self-contingency were seen only in mothers of male infants. Within the FNI group, the self-contingency of mothers of male infants was lower than that of females (β = −.212, p = .003); within the SC group, mothers of male vs. female infants did not differ (β = .091, p =.225). Thus, differences in the effect of gender on mother gaze self-contingency were seen only in the FNI group. Testing for the predictability of each individual second, Table 4 shows no findings.
Analysis of predicted values (see Web Appendix C, Table C4) showed that, given mother gaze-off at L1, FNI (vs. SC) mothers of males are more likely to be gaze-on in the current moment. The mean probability of the top 10 values at t0 is .762 for FNI vs. .436 for SC.
In summary, given mothers gazing away in the just prior second, FNI (vs. SC) mothers of males are more likely to look at their sons in the current second.
(2d). Mother gaze interactive contingency (infant vocal affect predicting mother gaze).
Testing with weighted lag and individual seconds approaches, Tables 3 and 4 show no FNI vs. SC differences in mother gaze coordination with prior infant vocal affect.
(3). Infant Gaze – Mother Gaze
(3a). Infant gaze self-contingency (controlling for mother gaze).
Testing with a weighted lag approach, Table 5 shows no difference between FNI and SC groups (β = .062, p = .322). Testing with the individual seconds approach, Table 6 shows that FNI (vs. SC) infants are less predictable in gaze on-and-off mother’s face, from L2 (β = −.298, p = .05) (controlling for prior mother gaze).
Table 6.
Infant Gaze – Mother Gaze: Individual Seconds Time Series Analysis for Standard Care (SC) and Family Nurture Intervention (FNI) and their Differences (Δ)
| Infant Gaze | β | SEβ | p | Mother Gaze | β | Seβ | p | |
|---|---|---|---|---|---|---|---|---|
| IG L1 → IG | 2.278 | .099 | <.001 | MG L1 → MG | 1.548 | .111 | <.001 | |
| IG L2 → IG | .546 | .113 | <.001 | MG L2 → MG | .565 | .121 | <.001 | |
| SC | IG L3 → IG | .335 | .108 | .002 | MG L3→ MG | .461 | .121 | <.001 |
| MG L1 → IG | .187 | .138 | .175 | IG L1 → MG | .286 | .136 | .036 | |
| MG L2 → IG | −.062 | .142 | .662 | IG L2 → MG | .026 | .148 | .858 | |
| MG L3 → IG | .128 | .137 | .350 | IG L3 → MG | .061 | .136 | .654 | |
| IG L1 L IG | 2.449 | .089 | <.001 | MG L1 L MG | 1.506 | .101 | <.001 | |
| IG L2 L IG | .248 | .012 | .015 | MG L2 L MG | .564 | .110 | <.001 | |
| FNI | IG L3 L IG | .604 | .093 | <.001 | MG L3 L MG | .269 | .112 | .016 |
| MG L1 → IG | .139 | .122 | .252 | IG L1 → MG | .439 | .120 | <.001 | |
| MG L2 → IG | −.099 | .123 | .422 | IG L2 → MG | −.299 | .132 | .024 | |
| MG L3 L→ IG | −.027 | .120 | .823 | IG L3 → MG | .363 | .119 | .002 | |
| Group (GP) | −.037 | .183 | .841 | Group (GP) | −.072 | .211 | .735 | |
| IGL1*GP → IG | .171 | .133 | .199 | MGL1*GP → MG | −.042 | .150 | .780 | |
| IGL2*GP → IG | −.298 | .152 | .050 | MGL2*GP → MG | −.001 | .164 | .995 | |
| Δ | IGL3*GP → IG | .269 | .142 | .059 | MGL3*GP → MG | −.192 | .165 | .244 |
| MGL1*GP → IG | −.048 | .184 | .795 | IGL1* GP → MG | .154 | .182 | .397 | |
| MGL2*GP → IG | −.037 | .188 | .845 | IGL2* GP → MG | −.323 | .198 | .101 | |
| MGL3*GP → IG | −.155 | .182 | .396 | IGL3* GP → MG | .301 | .181 | .096 | |
Analysis of predicted values (see Web Appendix C, Table C5) showed that, given infant gaze-off at L2, the probability of infant gaze-on in the current moment is higher in FNI (vs. SC) infants. The mean probability of the top 10 values at t0 = .643 for FNI infants and = .556 for SC infants.
In summary, FNI (vs. SC) infants are more likely to seek visual re-engagement with their mothers. We note that there was no corresponding finding from the weighted lag approach.
(3b). Infant gaze interactive contingency (mother gaze predicting infant gaze).
Testing with weighted lag and individual seconds approaches, Tables 5 and 6 show no FNI (vs. SC) differences. Note that infant contingent gaze coordination with mother gaze is not significant in FNI or SC dyads.
(3c). Mother gaze self-contingency (controlling for prior infant gaze).
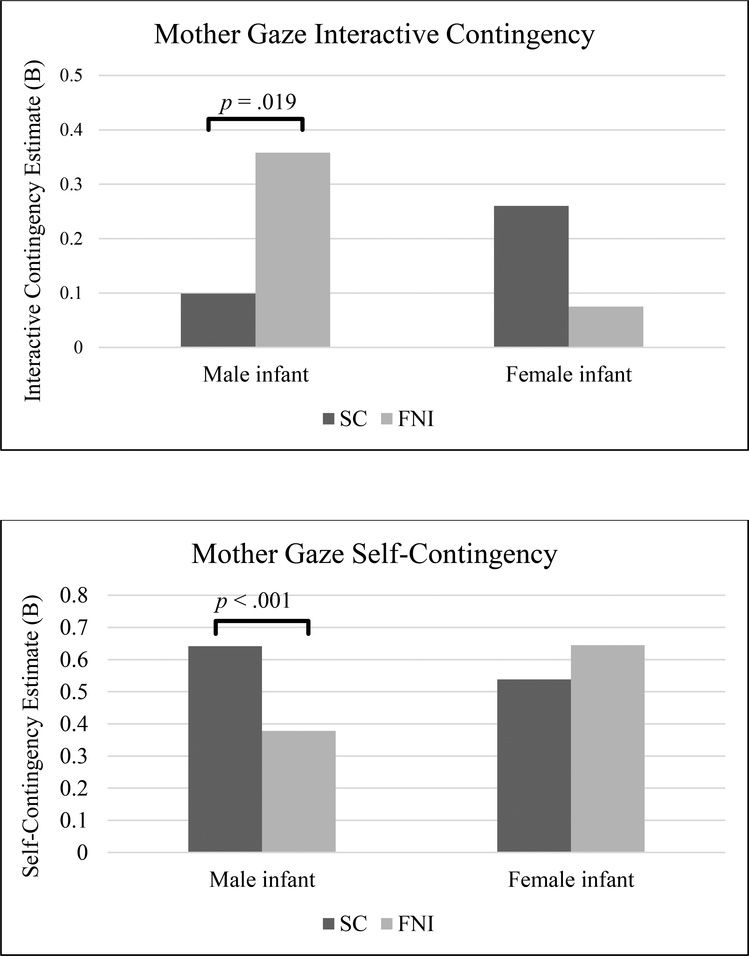
Testing with a weighted lag approach, FNI (vs. SC) differences in maternal gaze self-contingency (controlling for prior infant gaze) were a function of infant sex. The 3-way interaction effect (MG *Group*Sex → MG β = .371, p < .001) in Table 5 represents the interaction of being in the FNI (vs. SC) group, and being mothers of female (vs. male) infants, above and beyond either alone, on maternal self-contingency. As shown in Table 5, footnote 4, the self-contingency of SC mothers of male infants (β = .642) was higher than that of FNI mothers of males (β = .378), and significantly different (β =.264; p < .001); but the self-contingency of FNI vs. SC mothers of females did not differ (β = −.107; p = .128) (see Figure 1). Thus, differences in the effect of FNI (vs. SC) on mother gaze self-contingency were present only in mothers of male infants. Within the FNI group, the self-contingency of mothers of males was lower (more variable) than that of mothers of females (β= −.267; p < .001); within the SC group, mothers of male vs. female infants did not differ (β= .104; p = .176). Testing for the predictability of each individual second, Table 2 shows no findings.
Figure 1.
Differences in Mother Gaze Interactive Contingency and Self-Contingency between Family Nurture Intervention (FNI) and Standard Care (SC) groups for mothers of male infants and for mothers of female infants.
Note For mothers of male infants: (1) mother gaze interactive contingency of FNI mothers was higher than that of SC mothers; (2) mother gaze self-contingency of FNI mothers was lower than that of SC mothers. For mothers of female infants, there were no significant group differences in mother gaze self- or interactive contingencies.
Analysis of predicted values (Web Appendix C, Table C6) showed that, given mother gaze-off at L1, FNI (vs. SC) mothers of male (vs. female) infants were more likely to be gaze-on in the current second. The mean probability of the top 10 values at t0 = .851 for FNI mothers of males, compared to .613 for SC mothers of males.
In summary, the findings for mother gaze self-contingency (controlling for infant gaze) are similar to those in pairing (1) (controlling for infant vocal affect). Given mother gaze-off in the prior second, FNI mothers of males are more likely to be gaze-on in the current second.
(3d). Mother gaze interactive contingency (infant gaze predicting mother gaze).
Testing with a weighted lag, Table 5 shows that differences in FNI vs. SC maternal interactive contingency (controlling for prior mother gaze) were a function of infant sex. Testing for the predictability of each individual second, Table 6 shows no findings. The 3-way interaction effect (IG*Group*Sex → MG β = −.443, p < .004) in Table 5 represents the interaction of being in the FNI (vs. SC) group, and being mothers of female (vs. male) infants, on maternal interactive contingency. As shown in Table 5, footnote 4, the interactive contingency of SC mothers of male infants (β =.099) was lower than that of FNI mothers of males (β =.358), and significantly different (β = −.258; p = .019); but the interactive contingency of FNI vs. SC mothers of female infants did not differ (β =.185; p = .076) (see Figure 1). Thus, the effect of FNI (vs. SC) on mother gaze interactive contingency was seen only in mothers of male infants. Within the FNI group, the interactive contingency of mothers of male infants was higher than that of females (β = .283, p = .005); within the SC group, mothers of male vs. female infants did not differ (β = −.161, p =.159).
Analysis of predicted values showed that FNI (vs. SC) mothers of male infants coordinated to a greater degree with their infants (see Web Appendix C, Table C6). Given male infants were gaze-on at L1, FNI (vs. SC) mothers of males were more likely to gaze at their sons in the current moment. The mean probability of mother gaze-on (for the top 10 values) in the current moment = .851 for FNI mothers of males, compared to .613 for SC mothers of males.
In summary, given infant gaze-on in the prior second, FNI (vs. SC) mothers of males are more likely to join their sons in gaze-on in the current second.
(4). Infant Vocal Affect – Mother Touch
The results of these analyses are presented in Tables 7 and 8, and the analysis of predicted values can be found in Web Appendix C, Tables C7 and C8. Because the results of this modality pairing were redundant with those presented above, we describe them in Web Appendix D.
Table 7.
Infant Vocal Affect - Mother Touch: Weighted Lag Analysis for Standard Care (SC) and Family Nurture Intervention (FNI) and their Differences (Δ)
| Infant Vocal Affect | β | SEβ | p | |
|---|---|---|---|---|
| IV → IV | 2.638 | .209 | <.001 | |
| MT → IV | .180 | .236 | 0.445 | |
| SC | I Sex | .783 | .552 | .160 |
| IV * Sex → IV | 4.124 | .233 | <.001 | |
| MT * Sex→ IV | −.050 | .174 | .775 | |
| FNI | IV → IV | .312 | .219 | .155 |
| MT → IV | .024 | .102 | .814 | |
| Group | .149 | .553 | .792 | |
| Δ | IV * Group → IV | −2.327 | .247 | <.001 |
| MT * Group→ IV | −.156 | .249 | .532 | |
| Mother Touch | β | SEβ | p | |
| SC | MT → MT | 5.593 | .159 | <.001 |
| IV → MT | .039 | .128 | .760 | |
| FNI | MT → MT | 4.286 | .070 | <.001 |
| IV → MT | .069 | .084 | .412 | |
| Group | .187 | .226 | .411 | |
| Δ | MT * Group→ MT | −1.307 | .174 | <.001 |
| IV * Group → MT | .030 | .153 | .844 | |
Note. See Table 1 footnotes for explanation of terms.
Table 8.
Infant Vocal Affect – Mother Touch: Individual Seconds Time Series Analysis for Standard Care (SC) and Family Nurture Intervention (FNI) and their Differences (Δ)
| Infant Vocal Affect | β | SE β | p | Mother Touch | β | Seβ | p | |
|---|---|---|---|---|---|---|---|---|
| IV L1 → IV | .330 | .022 | <.001 | MT L1 → MT | .714 | .033 | <.001 | |
| IV L2 → IV | .134 | .033 | <.001 | MT L2 → MT | .093 | .041 | .022 | |
| SC | IV L3 → IV | .236 | .032 | <.001 | MT L3 → MT | −.026 | .033 | .430 |
| MT L1 → IV | −.049 | .042 | .241 | IV L1 → MT | .013 | .017 | .461 | |
| MT L2 → IV | .082 | .051 | .107 | IV L2 → MT | −.012 | .026 | .644 | |
| MT L3 → IV | −.028 | .042 | .509 | IV L3 → MT | .001 | .025 | .967 | |
| IV L1 → IV | −.226 | .011 | <.001 | MT L1 → MT | .437 | .010 | <.001 | |
| IV L2 → IV | .207 | .012 | <.001 | MT L2 → MT | .060 | .011 | <.001 | |
| FNI | IV L3 → IV | .187 | .012 | <.001 | MT L3 → MT | .145 | .010 | <.001 |
| MT L1 → IV | .005 | .013 | .669 | IV L1 → MT | .011 | .008 | .207 | |
| MT L2 → IV | .011 | .014 | .444 | IV L2 → MT | .002 | .008 | .807 | |
| MT L3 → IV | −.025 | .013 | .056 | IV L3 → MT | −.016 | .009 | .070 | |
| Group (GP) | .221 | .553 | .691 | Group (GP) | .213 | .222 | .342 | |
| IVL1*GP → IV | −.556 | .025 | <.001 | MTL1*GP → MT | −.277 | .035 | <.001 | |
| IVL2*GP → IV | .073 | .035 | .038 | MTL2*GP → MT | −.033 | .042 | .435 | |
| Δ | IVL3*GP → IV | −.049 | .034 | .150 | MTL3*GP → MT | .171 | .035 | <.001 |
| MTL1* GP → IV | .054 | .044 | .213 | IVL1*GP → MT | −.002 | .019 | .903 | |
| MTL2* GP → IV | −.072 | .053 | .176 | IVL2*GP → MT | .014 | .028 | .607 | |
| MTL3* GP → IV | .003 | .044 | .944 | IVL3*GP ↔ MT | −.017 | .027 | .525 | |
Across all equations, FNI (vs. SC) differences were documented in 50% of weighted lag time-series equations, and 25% of individual seconds time-series equations. Differences were more evident in self-contingency processes than interactive contingency. Across weighted lag models, self-contingency differences in FNI (vs. SC) were found in 50% of infant, and 100% of mother, equations; interactive contingency differences were found in no infant equations and in 50% of mother equations. Across individual seconds models, self-contingency differences were found in 50% of infant, and 33% of mother equations; interactive contingency differences were found in 8.3% of infant equations and 8.3% of mother equations.
Discussion
The Family Nurture Intervention (FNI) in the NICU (compared to standard care) facilitated more optimal mother-infant face-to-face interaction at 4 months CA. Dyads who received FNI demonstrated: (a) greater frequency of maternal touch and more optimal maternal touch patterns; (b) greater frequency of more optimal (less extreme) infant expression of vocal distress; (c) more optimal maternal coordination of touch patterns with infant gaze patterns; (d) greater likelihood of sustaining positive patterns, specifically, maternal positive touch patterns and infant positive vocal affect patterns; (e) greater likelihood of repair patterns, whereby moments of negative maternal touch, or of negative infant vocal affect, transitioned into positive behavioral patterns; (f) greater likelihood of infant visual re-engagement after a moment of infant looking away; and (g) for mothers of male infants, greater likelihood of maternal visual re-engagement after a moment of mother looking away, and of maternal joining infants in looking. Together these findings document an improved social engagement reflective of greater emotional connection in the FNI preterm infants and their mothers at 4 months (CA).
Maternal Touch
Maternal touch is compromised in mothers of preterm infants (Davis & Thoman, 1988; Feldman et al., 2003), and interventions utilizing touch improve infant outcomes (Álvarez et al., 2017; Moore, Bergman, Anderson & Medley, 2016). FNI had an extensive impact on maternal touch patterns. Our findings extend the literature by specifying further dimensions of maternal touch at 4 months that changed with the FNI intervention. FNI (vs. SC) mothers not only showed a greater amount of touch, and more positive touch patterns (particularly static, calming touch); but also the capacity to sustain positive touch, and to repair negative touch patterns. Moreover, we documented increased maternal capacity to reciprocate infant gaze through heightened contingent touch responsivity, and with much more positive touch, in the very next second.
Infant Distress
The FNI (vs. SC) infants used less extreme forms of distress (angry-protest rather than cry). Feldman, Weller et al. (2002) similarly found that infant distress at 3 and 6 months decreased with a NICU Kangaroo Care intervention, using a global coding scheme (CIB). Using a more detailed coding of infant vocal distress, our findings refine our understanding of how the FNI improved the premature infant’s ability to engage in the face-to-face exchange at 4 months. The infant is not only less negative, but is also more likely to repair negative affect and to sustain positive affect. This is an important contribution of the infant to the improved emotional connection with the mother.
Infant Gaze
Testing FNI vs. SC dyads with frequency and duration measures of mother and infant gaze behavior yielded no differences. In future studies, longer observations may yield differences. Instead, testing with time-series models, we documented differences in the process of relating through gazing and gazing away.
There were two infant gaze findings, interpreted with caution. Analyzing infant gaze controlling for maternal gaze, when infants gazed away, FNI (vs. SC) infants were more likely to look back, seeking visual re-engagement. Analyzing infant gaze controlling for mother touch, when infants gazed at their mothers, both FNI and SC infants were then likely to gaze away, but this was more likely for FNI infants, indicating a more variable gaze process.
Mother Gaze
FNI (vs. SC) mothers of male infants were more visually engaged. When mothers looked away, FNI (vs. SC) mothers of male infants were more likely to look back, seeking visual re-engagement. When infants looked at their mothers, FNI (vs. SC) mothers of males were more likely to join their sons in looking, thus more contingently responsive. Intervening in the NICU with kangaroo care, Feldman, Weller et al. (2002) similarly found improved mother–infant shared attention at infant age 6 months.
Sex effects have been extensively documented in preterm infants. Preterm male (vs. female) infants are at greater risk for multiple deficits (Spinillo et al., 2009), perform less optimally on neonatal neurobehavioral tests (Alvarez-Garcia, Fornieles-Deu, Costas-Moragas, & Botet-Mussons, 2015), and are less alert and have more diffuse (immature) sleep states (Foreman, Thomas, & Blackburn, 2008). FNI mothers may have higher levels of gaze vigilance with their male (vs. female) infants due to the initial greater vulnerability of male infants.
Self-Contingency
Our hypothesis that FNI would increase the capacity of infants and mothers to contingently coordinate with each other was upheld, in maternal touch coordination with infant gaze, and in maternal gaze coordination with infant gaze for mothers of males. However, the bulk of the findings concerned self-contingency.
Whereas interactive contingency measures adjustments an individual makes in response to a partner’s prior behavior, self-contingency measures the individual’s likelihood of maintaining (or changing) behavior from moment-to-moment. Self-contingency generates procedural expectancies of how predictable (stable/variable) one’s behaviors are, and where one’s behavior is tending in the next moment, contributing to a sense of temporal coherence. It is this aspect of relatedness that the FNI intervention substantially altered.
In touch self-contingency, FNI mothers were more likely to sustain positive touch, and to transition from negative to positive forms of touch. In vocal affect self-contingency, FNI infants were more likely to sustain positive vocal affect, and to transition from negative to more positive vocal affect. In gaze self-contingency, FNI mothers of male infants were more likely to visually re-engage. We note that the effect sizes were twice as large for infant vocal affect self-contingency as for mother touch self-contingency. Thus, the intervention had a particularly large effect on infant vocal affect, pointing to the sensitivity of the infant to the intervention, and to the importance of the infant’s contribution to the co-regulation of the interactive system.
In prior work, self-contingency has frequently been a more sensitive variable than interactive contingency (Beebe et al., 2007, 2008, 2010). Beebe et al. (2016) documented that the effects of self-contingency are substantially greater than those of interactive contingency, and that self- and interactive contingency are co-constituted, with each process affecting the other. This co-constitution is consistent with Calming Cycle Theory (Welch, 2016a), which describes mother and infant as an open biobehavioral system of feedback loop co-regulation. We speculate that the self-contingency processes that we documented at 4 months stemmed from this co-regulation which began in utero, continued after birth, and was shaped by FNI conditioning.
Clinical Implications
The specificity of our findings can inform NICU interventions and, more generally, clinical work with premature infants and their mothers. For example, our findings of maternal static touch, sustained positive forms of touch, the rapid repair of intrusive touch, and immediate positive maternal touch response to infant looking, can generate specific intervention targets.
Limitations/Future Directions
Lacking sufficient prior literature, we made no specific hypotheses regarding self-contingency. We did not code maternal vocalization because it will be coded by an automated method, reserved for a future report. A comparison of these preterm infants with a term sample is under way.
Conclusion
Our randomized control trial of Family Nurture Intervention in the NICU generated more positive forms of mother and infant engagement at 4 months CA. Our micro-level behavioral coding and time-series approach revealed dimensions of maternal touch, infant vocal affect, and mother and infant gaze hitherto undetected by global coding methods. These results, suggesting greater positive emotional connection, add to our published findings showing immediate and long-term improvements for the FNI group. Because mother-infant coordination during face-to-face communication in the early months of life is a critical foundation for development, this documentation of positive effects of FNI for 4-month mother-infant face-to-face communication has important implications for an improved developmental trajectory of these infants.
Supplementary Material
Acknowledgments
This work was funded through grants from the Bernard and Esther Besner Infant Research Fund (Beebe), the Einhorn Family Nurture Intervention Facilities Mother-Infant Engagement
Family Charitable Trust, Fleur Fairman Family, Mary Stephenson Fund, and the Irving Institute for Clinical and Translational research (Myers, Hane, Austin, Ludwig, Welch), and NIEHS awarded to Margolis (K23ES026239).
Footnotes
A 2.5 minute sample of behavior is standard in the literature (Beebe et al., 2010; Cohn & Tronick, 1988). Mother-infant face-to-face interaction has a relatively stable structure with robust session-to-session reliability (Cohn & Tronick, 1989; Moore, Cohn, & Campbell, 1997; Weinberg & Tronick, 1991; Zelner, Beebe, & Jaffe, 1982).
Compared to traditional time-series techniques, multilevel models (Singer & Willett, 2003) have more power, take into account error structures, and estimate individual effects with empirical Bayesian (maximum likelihood) techniques (rather than Ordinary Least Squares), which take into account prior distributions. Because the prior probability of error is greatest for the extreme parameters, this method tends to pull in such extremes. Advantages of this approach include: (a) multiple time-series (in our case, self- and interactive contingency) can be modeled simultaneously, (b) an average effect of key parameters (e.g., infant behavior contingent on mother behavior) is estimated for the group and allows the investigator to ask how that group mean changes in the context of other factors (such as infant gender), (c) SC variables and their conditional effects can be included as necessary, (d) potential nonlinear relations can be examined in the same analyses, (e) more appropriate statistical model assumptions are made.
A “random effect” is the term used for identifying the differences in a variable (function, or association) among the study participants. These always include variation in the mean of the dependent variable across observations, and variation in the variance of the dependent variable across observations; they usually include variation in the linear change in the dependent variable over time, and in our case it includes between-dyad variation in the auto-regressive effect. A “fixed effect” is the average association across study units (in our case, dyads), just as it would be in an ordinary regression analysis. These average effects will account for some fraction of the random effects, just as in an ordinary regression analysis the predictors account for some fraction of the variance in the dependent variable.
To determine optimum window size for calculating contingency estimates, in prior work (Beebe et al., 2007; 2010; 2016) we estimated the number of seconds over which lagged effects were significant and their magnitude for the pairs as a whole (fixed model estimates). For each dependent variable, measures of prior self- or partner-behavior, “lagged variables,” were computed as a weighted average of recent prior seconds, based on these analyses. The beta weight of each lag is divided by the sum of the significant beta weights (up to 3). Typically, the prior 3 seconds sufficed to account for these lagged effects on subsequent behavior (t0). Across the modality pairings studied, mother was significant at 2–3 lags (2–3 seconds) for both self- and interactive contingency; evaluation of longer lags yielded non-significant results. Significant infant lags varied: for self-contingency, 4 lags (vocal affect), 3 (gaze); infant interactive contingency varied from 6 to 3 lags, but the amount of variance accounted for was very small for lags longer than 3 seconds. Note that in the weighted lag analyses, no more than 3 lags, and no fewer than 2, were used in any weighted mean lag, to maintain consistent sample size. By using a standard 3-second unit for both self- and interactive contingency, it is possible that there were subtle differences in the duration of the relevant prior window that we would not be able to determine in this model.
References
- Alvarez-Garcia A, Fornieles-Deu A, Costas-Moragas A, & Botet-Mussons F (2015). Neurobehavioral conditions and effects of gender, weight and severity in preterm infants according to the Neonatal Behavioral Assessment Scale Annals of Psychology, 31(3), 818–824. [Google Scholar]
- Beebe B, Jaffe J, Buck K, Chen H, Cohen P, Blatt S, . . . Andrews H (2007). Six-week postpartum maternal self-criticism and dependency and 4-month mother-infant self- and interactive contingencies. Dev Psychol, 43(6), 1360–1376. doi: 10.1037/0012-1649.43.6.1360 [DOI] [PubMed] [Google Scholar]
- Beebe B, Jaffe J, Buck K, Chen H, Cohen P, Feldstein S, & Andrews H (2008). Six-week postpartum maternal depressive symptoms and 4-month mother-infant self- and interactive contingency Infant Mental Health Journal, 29(5), 442–471. doi: 10.1002/imhj.20191 [DOI] [PubMed] [Google Scholar]
- Beebe B, Jaffe J, Markese S, Buck K, Chen H, Cohen P, . . . Feldstein S (2010). The origins of 12-month attachment: a microanalysis of 4-month mother-infant interaction. Attach Hum Dev, 12(1–2), 3–141 doi: 10.1080/14616730903338985. Supplemental Materials are adapted from Appendices A., B. and Web Appendix 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe B, Messinger D, Bahrick LE, Margolis A, Buck KA, & Chen H (2016). A systems view of mother-infant face-to-face communication. Developmental Psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, & Cohen P (2006). Using individual growth model to analyze the change in quality of life from adolescence to adulthood. Health and Quality of Life Outcomes, 4(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Chen H, Hamigami F, Gordon K, & McArdle J (2000). Multilevel analyses for predicting sequence effects of financial and employment problems on the probability of arrest. Journal of Quantitative Criminology, 16(2), 223–235. doi: 10.1023/A:1007568606759 [DOI] [Google Scholar]
- Cohn J, & Tronick E (1989). Specificity of infants’ response to mothers’ affective behavior. Journal of the American Academy of Child & Adolescent Psychiatry, 28(2), 242–248. doi: 10.1097/00004583-198903000-00016 [DOI] [PubMed] [Google Scholar]
- Cohn J & Tronick E (1988). Mother-infant face-to-face interaction: Influence is bidirectional and unrelated to periodic cycles in either partner’s behavior. Dev Psychol, 24, 386–392. [Google Scholar]
- Davis D, & Thoman E (1988). The early social environment of premature and fullterm infants. Early Hum Dev, 17(2–3), 221–232. [PubMed] [Google Scholar]
- Demetri-Friedman D, Beebe B, Jaffe J Ross D & Triggs S (2010). Microanalysis of 4-month infant vocal affect qualities and maternal postpartum depression. Clinical Social Work Journal, 38, 8–16. [Google Scholar]
- Feldman R (2007a). Parent-infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. J Child Psychol Psychiatry, 48(3–4), 329–354. doi: 10.1111/j.1469-7610.2006.01701.x [DOI] [PubMed] [Google Scholar]
- Feldman R (2007b). Parent-Infant Synchrony: Biological Foundations and Developmental Outcomes. Current Directions in Psychological Science, 16, 340–345. [Google Scholar]
- Feldman R (2007c). Mother-infant synchrony and the development of moral orientation in childhood and adolescence: direct and indirect mechanisms of developmental continuity. American Journal of Orthopsychiatry, 77(4), 582–97. [DOI] [PubMed] [Google Scholar]
- Feldman R (2012). Parent-infant synchrony: A bio-behavioral model of mutual influences in the formation of affiliative bonds. Monographs of the Society for Research in Child Development, 77(2), 42–51. [Google Scholar]
- Feldman R (2015). The adaptive human parental brain: implications for children’s social development. Trends Neurosci, 38(6), 387–399. doi: 10.1016/j.tins.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Feldman R (2016). The neurobiology of mammalian parenting and the biosocial context of human caregiving. Horm Behav, 77, 3–17. doi: 10.1016/j.yhbeh.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Feldman R, & Eidelman A (2003). Skin-to-skin contact (Kangaroo Care) accelerates autonomic and neurobehavioural maturation in preterm infants. Dev Med Child Neurol, 45(4), 274–281. [DOI] [PubMed] [Google Scholar]
- Feldman R, & Eidelman A (2006). Neonatal state organization, neuromaturation, mother-infant interaction, and cognitive development in small-for-gestational-age premature infants. Pediatrics, 118(3), e869–878. doi: 10.1542/peds.2005-2040 [DOI] [PubMed] [Google Scholar]
- Feldman R, & Eidelman A (2007). Maternal postpartum behavior and the emergence of infant-mother and infant-father synchrony in preterm and full-term infants: the role of neonatal vagal tone. Dev Psychobiol, 49(3), 290–302. doi: 10.1002/dev.20220 [DOI] [PubMed] [Google Scholar]
- Feldman R, Eidelman AI, Sirota L, & Weller A (2002). Comparison of skin-to-skin (kangaroo) and traditional care: parenting outcomes and preterm infant development. Pediatrics, 110(1 Pt 1), 16–26. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Sirota L, & Eidelman A (2002). Skin-to-Skin contact (Kangaroo care) promotes self-regulation in premature infants: sleep-wake cyclicity, arousal modulation, and sustained exploration. Dev Psychol, 38(2), 194–207. [DOI] [PubMed] [Google Scholar]
- Flacking R, Lehtonen L, Thomson G, Axelin A, Ahlqvist S, Moran V, Ewald U & Dykes F (2012). Closeness and separation in neonatal intensive care. Acta Paediatr. 101(10):1032–1037. doi: 10.1111/j.1651-2227.2012.02787.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel A (1993). Developing through relationships: Origins of communication, self, and culture. Chicago: University of Chicago Press. [Google Scholar]
- Foreman S, Thomas K, & Blackburn S (2008). Individual and gender differences matter in preterm infant state development. J Obstet Gynecol Neonatal Nurs, 37(6), 657–665. doi: 10.1111/j.1552-6909.2008.00292.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianino A, & Tronick E (1988). The mutual regulation model: The infant’s self and interactive regulation, coping and defense In Field T, McCabe P, & Schneiderman N (Eds.), Stress and coping (pp. 47–68). Hillsdale, NJ: Lawrence Erlbaum Assoc. [Google Scholar]
- Goldstein H, Healy M, & Rasbash J (1994). Multi-level time-series models with applications to repeated measures data. Statistics in Medicine, 13, 1643–1655. [DOI] [PubMed] [Google Scholar]
- Haller J, Harold G, Sandi C, & Neumann I (2014). Effects of adverse early-life events on aggression and anti-social behaviours in animals and humans. Journal of Neuroendocrinology, 26(10), 724–738. [DOI] [PubMed] [Google Scholar]
- Hane A, Myers M, Hofer M, Ludwig R, Halperin M, Austin J, Glickstein S & Welch M (2015). Family Nurture Intervention Improves the Quality of Maternal Caregiving in the Neonatal Intensive Care Unit: Evidence from a Randomized Controlled Trial. J. Dev Behav Pediatr 36:188–196. [DOI] [PubMed] [Google Scholar]
- Hofer M (1994). Early relationships as regulators of infant physiology and behavior. Acta Paediatr Suppl, 397, 9–18. [DOI] [PubMed] [Google Scholar]
- Hussey-Gardner B, & Famuyide M . (2009). Developmental Interventions in the NICU. NeoReviews, 10(3). doi: 10.1542/neo.10-3-e113 [DOI] [Google Scholar]
- Jaffe J, Beebe B, Feldstein S, Crown CL, & Jasnow MD (2001). Rhythms of dialogue in infancy: coordinated timing in development. Monogr Soc Res Child Dev, 66(2), 1–132. [PubMed] [Google Scholar]
- Jean A, & Stack D (2012). Full-term and very-low-birth-weight preterm infants’ self-regulating behaviors during a Still-Face interaction: influences of maternal touch. Infant Behav Dev, 35(4), 779–791. [DOI] [PubMed] [Google Scholar]
- Johnson M, Schmeid V, Lupton S, Austin M, Matthey S, Kemp L, & Yeo A (2012). Measuring perinatal mental health risk. Archives of Women’s Mental Health, 15, 375–386. doi: 10.1007/s00737-012-0297-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelli M, & Fogel A (2005). Developmental changes in the relationship between the infant’s attention and emotion during early face-to-face communication: the 2-month transition. Dev Psychol, 41(1), 265–280. doi: 10.1037/0012-1649.41.1.265 [DOI] [PubMed] [Google Scholar]
- Lester BM, Hoffman J, & Brazelton TB (1985). The rhythmic structure of mother-infant interaction in term and preterm infants. Child Dev, 56(1), 15–27. [PubMed] [Google Scholar]
- Littell R, Miliken G, Stoup W, & Wolfinger R (1996). SAS system for mixed models. Cary, NC: SAS Institute. [Google Scholar]
- Malatesta C, Grigoryev P, Lamb C, Albin M, & Culver C (1986). Emotion socialization and expressive development in preterm and full-term infants. Child Dev, 57(2), 316–330. [DOI] [PubMed] [Google Scholar]
- Markova G & Legerstee M, (2006). Contingency, imitation, and affect sharing: Foundations of infants’ social awareness. Developmental Psychology 42, 132–141 [DOI] [PubMed] [Google Scholar]
- McArdle J, & Bell R (2000). An introduction to latent growth models for developmental data analysis. Mahwah, NJ: Lawrence Erlbaum. [Google Scholar]
- Messinger D, Ekas N, Ruvolo P, & Fogel A (2012). “Are you interested, baby?” Young infants exhibit stable patterns of attention during interaction. Infancy, 17(2), 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montirosso R, Borgatti R, Trojan S, Zanini R, & Tronick E (2010). A comparison of dyadic interactions and coping with still-face in healthy pre-term and full-term infants. British Journal of Developmental Psychology, 28(Pt 2), 347–368. [DOI] [PubMed] [Google Scholar]
- Moore G, Cohn J, & Campbell S (1997). Mothers’ affective behavior with infant siblings: stability and change. Dev Psychol, 33(5), 856–860. [DOI] [PubMed] [Google Scholar]
- Moore E, Bergman N, Anderson G, Medley N (2016). Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database of Systematic Reviews, 11, 1–120. CD003519. DOI: 10.1002/14651858.CD003519.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHD Network NECCR (2004). Affect dysregulation in the mother-child relationship in the toddler years: Antecedents and consequences. Development and Psychopathology, 16(1), 43–68. doi: 10.1017/S0954579404044402 [DOI] [PubMed] [Google Scholar]
- Searle S & Gruber M (2016). Linear models. John Wiley & Sons. [Google Scholar]
- Singer JD, & Willett JB (2003). Applied longitudinal data analysis: Modeling change and event occurrence. New York, NY: Oxford University Press. [Google Scholar]
- Spinillo A, Montanari L, Gardella B, Roccio M, Stronati M, & Fazzi E (2009). Infant sex, obstetric risk factors, and 2-year neurodevelopmental outcome among preterm infants. Dev Med Child Neurol, 51(7), 518–525. doi: 10.1111/j.1469-8749.2009.03273.x [DOI] [PubMed] [Google Scholar]
- Stepakoff S (1999). Mother-infant tactile communication at four months: Effects of infant gender, maternal ethnicity, and maternal depression Doctoral Dissertation, Ferkauf Graduate School of Psychology, Yeshiva University. [Google Scholar]
- Stepakoff S, Beebe B, & Jaffe J (2000, July). Infant gender, maternal touch, and ethnicity. International Conference of Infant Studies, Brighton, England. [Google Scholar]
- Stern D (1985). The interpersonal world of the infant. New York: Basic Books. [Google Scholar]
- Symington A, & Pinelli J (2006). Developmental care for promoting development and preventing morbidity in preterm infants. Cochrane Database Syst Rev(2), CD001814. doi: 10.1002/14651858.CD001814.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick E, & Weinberg M (1990). The infant regulatory scoring system. Unpublished Manuscript. Children’s Hospital. Harvard Medical School; Boston, MA. [Google Scholar]
- Tronick E (1989). Emotions and emotional communication in infants. American Psychologist, 44(2), 112–119. [DOI] [PubMed] [Google Scholar]
- van Baar A, van Waaenaer A, Briet J, Dekker F, & Kok J (2005). Very preterm birth is associated with disabilities in multiple developmental domains. J Pediatr Psychol, 30(3), 247–255. doi: 10.1093/jpepsy/jsi035 [DOI] [PubMed] [Google Scholar]
- Weinberg K, & Tronick E (1991). Stability of infant social and coping behaviors and affective displays between 6 and 15 months: Age-appropriate tasks and stress bring out stability. Paper presented at the Society for Research in Child Development, Seattle, Washington. [Google Scholar]
- Welch MG (1988). Holding time: how to eliminate conflict, temper tantrums, and sibling rivalry and raise happy, loving, successful children. New York, NY: Simon and Schuster. [Google Scholar]
- Welch MG (2016a). Calming cycle theory: The role of visceral/autonomic learning in early mother and infant/child behaviour and development. Acta Pediatr, 105(11), 1266–1274. doi: 10.1111/apa.13547 [DOI] [PubMed] [Google Scholar]
- Welch MG (2016b). Nurture in the neonatal intensive care unit. Acta Pediatr, 105(7), 730–731. doi: 10.1111/apa.13294 [DOI] [PubMed] [Google Scholar]
- Welch MG, Firestein M, Austin J, Hane AA, Stark R., Hofer M, . . . Myers MM (2015). Family Nurture Intervention in the Neonatal Intensive Care Unit improves social-relatedness, attention, and neurodevelopment of preterm infants at 18 months in a randomized controlled trial. J Child Psychol Psychiatry, 56(11), 1202–1211. doi: 10.1111/jcpp.12405 [DOI] [PubMed] [Google Scholar]
- Welch MG, Hofer M, Brunelli S, Stark R, Andrews H, Austin J, & Myers MM (2012). Family nurture intervention (FNI): Methods and treatment protocol of a randomized controlled trial in the NICU. BMC Pediatr, 12, 14. doi: 10.1186/1471-2431-12-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch MG, Hofer M, Brunelli S, Stark R, Andrews H, Austin J, . . . Family Nurture Intervention Trial, G. (2012). Family nurture intervention (FNI): methods and treatment protocol of a randomized controlled trial in the NICU. BMC Pediatr, 12, 14. doi: 10.1186/1471-2431-12-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch MG, Hofer M, Stark R, Andrews H, Austin J, Glickstein S, . . . Myers MM (2013). Randomized controlled trial of Family Nurture Intervention in the NICU: assessments of length of stay, feasibility and safety. BMC Pediatr, 13, 148. doi: 10.1186/1471-2431-13-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch MG, & Myers MM (2016). Advances in family-based interventions in the neonatal ICU. Curr Opin Pediatr, 28(2), 163–169. [DOI] [PubMed] [Google Scholar]
- Zelner S, Beebe B, & Jaffe J (1982). The organization of vocalization and gaze in early mother-infant interactive regulation. Paper presented at the International Conference Infant Studies, New York City, NY. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.