Abstract
Objective
Macrophages play important roles in the pathogenesis of atherosclerosis, but their dynamics within plaques remain obscure. We aimed to quantify macrophage positional dynamics within progressing and regressing atherosclerotic plaques.
Approach and Results
In a stable intravital preparation, large asymmetrical “foamy” macrophages in the intima of carotid artery plaques were sessile, but smaller rounded cells nearer plaque margins, possibly newly recruited monocytes, mobilized laterally along plaque boarders. Thus, to test macrophage dynamics in plaques over a longer period of time in progressing and regressing disease, we quantified displacement of nondegradable phagocytic particles within macrophages for up to 6-weeks. In progressing plaques, macrophage-associated particles appeared to mobilize to deeper layers in plaque, whereas in regressing plaques, the label was persistently located near the lumen. By measuring the distance of the particles from the floor of the plaque, we discovered that particles remained at the same distance from the floor regardless of plaque progression or regression. The apparent deeper penetration of labeled cells in progressing conditions could be attributed to monocyte recruitment that generated new superficial layers of macrophages over the labeled phagocytes.
Conclusions
While there may be individual exceptions, as a population, newly differentiated macrophages fail to penetrate significantly deeper than the limited depth they reside upon initial entry, regardless of plaque progression or regression. These limited dynamics may prevent macrophages from escaping areas with unfavorable conditions (such as hypoxia) and pose a challenge for newly recruited macrophages to clear debris through efferocytosis deep within plaque.
Keywords: Arteriosclerosis, inflammation, monocyte, migration, cell adhesion, intravital microscopy
Introduction
Atherosclerotic disease remains one of the leading causes of morbidity and mortality in the Western world. Hallmarks of this disease include accumulation of lipids in the vessel wall and a chronic inflammatory state that is orchestrated by several immune cells such as macrophages, dendritic cells and T lymphocytes. In particular, recruited monocyte-derived macrophages and proliferating macrophages within the arterial wall take up cholesterol-rich lipoproteins and play a critical role in the pathogenesis of atherosclerosis by developing into foam cells 1, 2. For example, activated macrophages within early atherosclerotic plaques secrete proinflammatory cytokines and chemokines 3, which results in the recruitment of immune cells such as monocytes and migration of smooth muscle cells from the media into the intima. Plaque progression can eventually result in its rupture and catastrophic vessel occlusion.
Morphologically, atherosclerotic plaques bear some resemblance to granulomas, developing marked clusters of macrophages that can progress to incorporate a necrotic core. In atherosclerosis, necrotic core debris is a major driver of thrombotic risk and rupture 4. An unanswered question, however, is whether the limited macrophage dynamics previously described for mycobacterial granulomas 5 applies to atherosclerotic plaques. If so, then one can envision that necrotic core formation and expansion could partly be a consequence of macrophages having limited ability to emigrate away from any microenvironment that might preferentially predispose to their death or to mobilize toward dying cells in order to clear them. Indeed, to employ mathematical modeling in the future to predict plaque dynamics that lead to stable or unstable lesions requires that we understand how well macrophages are able to move around within plaques in different conditions.
To address the issue of defining macrophage dynamics in plaque, we undertook a two-pronged approach modeled after that used by Egen et al. 5, first employing intravital imaging to examine macrophage behavior in the living mouse and then adding a second approach to track cells over a much longer period of time than is possible with intravital imaging. Intravital two-photon microscopy has advanced our understanding of immune responses during steady state and in a wide variety of disease models, and some recent advances have been made in imaging atherosclerotic disease using this technique 6–11. Here, we have applied a stable and high resolution imaging approach to visualize native atherosclerotic plaques in the carotid artery to allow for short-term observation of macrophage motility within the developing plaque. In addition, for long-term assessment of macrophage positioning, we quantified the location of stable phagocytic cargo carried by macrophages within plaques during progressive or regressive atherosclerotic disease. Use of phagocytic cargo with differing fluorescent colors at different time points supported the conclusion that macrophages within the atherosclerotic plaques are largely sessile cells, regardless of plaque progression or regression. Macrophages appear especially unlikely to deeply penetrate plaques, such that successive waves of recruited monocytes create layers somewhat reminiscent of growth rings in trees.
Methods
Mice
LysMcre/+ (B6.129P2-Lyz2tm1(cre)lfo/J, Jackson Laboratories, #004781), RosaLsl-Tomato (B6; 129S6-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J, Jackson Laboratories, #007905), Ldlr−/− (B6.129S7-Ldlrtm1Her/J, Jackson Laboratories, #002207), and B6 Apoe−/− (B6.129P2-Apoetm1Unc/J, Jackson Laboratories, #002052) mice were purchased from The Jackson Laboratories and then bred at Washington University or Mount Sinai. CCR2gfp/+ (B6(C)-Ccr2tm1.1Cln/J, Jackson Laboratories #027619)12 mice were kindly provided by Dr. Marco Colonna (Washington University in St. Louis). Strains were crossed for intravital imaging to generate LysMcre/+ RosaLSL-Tomato Ldlr−/− and CCR2gfp/+ Ldlr−/− mice, whereas Apoe−/− mice were used for progression and regression studies. For atherosclerosis studies, mice were placed on a high fat diet (21% milk fat, 0.15% cholesterol; Envigo Teklad TD.88137) at the age of 6 weeks for 12–16 weeks. For all progression studies (including intravital imaging), both male and female mice were used and combined for analysis. For microarray analysis, male mice were used. Regression experiments using the AAV8 ApoE vector (Vector Biolabs) showed a low infection efficacy in female animals; therefore, male mice were used for studies involving reversal of hypercholesterolemia. However, regression studies involving use of adenovirus encoding hApoE3 (gift from Dr. Dan Rader) were carried out in female mice. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Washington University School of Medicine, St. Louis, Missouri or Mount Sinai School of Medicine, New York, NY.
Two-photon microscopy and data analysis
For intravital carotid imaging, following 12–16 weeks HFD feeding, hair was removed using a depilatory cream and a small neck incision was opened to expose the carotid artery. Plaque regions in close proximity to the common carotid bifurcation were gently elevated out of the cavity and placed under an adjustable coverslip for stability and to facilitate access of the microscope’s objective. Imaging was performed on a modified Leica SP8 2-photon imaging system with a custom temperature controlled hood. GFP and tomato-labeled cells were visualized using a Mai Tai Deepsee laser (Specta Physics, Santa Clara Ca, USA) optimally tuned to 920nm and 950nm consecutively. Fluorescence emission passed through 458nm, 495nm, and 560nm long-pass mirrors, with the 458 and 560 placed below and above the 495. Emission was detected by ultrasensitive hybrid detectors with tomato >560 nm, GFP 495 to 560 nm, autofluorescence 458–495, and second harmonic <458 nm. Time-lapse recordings were acquired using Leica Application Suite, LAS X (Leica Microsystems). Twenty-five to thirty frames (2.5 μm stack size, 62.5–75 μm total) were acquired every 15 seconds. Multidimensional rendering and manual cell tracking were analyzed with Imaris (Bitplane, Zurich, Switzerland) to generate speed of migration and displacement data. Data were transferred and plotted in GraphPad Prism 6.0 (GraphPad Software Inc., La Jolla, CA, USA)
Cellular bead labeling
Classical Ly-6Chi monocytes were labeled with beads 3 days after intravenous injection of 250 μl clodronate-loaded liposomes (purchased at clodronateliposomes.org). 1-μm Fluoresbrite yellow-green or red fluorescent plain microspheres (Polysciences Inc., Warrington, PA) diluted 1:4 in sterile PBS were administered i.v. (200 μl). Nonclassical Ly-6Clo monocytes were labeled with beads similarly, but without the use of clodronate-loaded liposomes 13. Labeling efficiency of blood monocytes was verified by flow cytometry 1 day after labeling.
Atherosclerosis development and plaque regression after viral vector-induced expression of ApoE
At the age of 5–6 weeks, ApoE−/− mice were transitioned to a high fat, cholesterol-enriched diet (Envigo Teklad TD.88137) and maintained on this diet for an additional 12–16 weeks, as indicated. In some experiments, matched cohorts of mice were divided into 3 groups. In all groups, monocytes were labeled with fluorescent beads as described above. Then, 5–8 days later, one group was sacrificed as a baseline cohort. The two other groups remained on a high fat diet for an additional 4–6 weeks and were infected either with ApoE-encoding adenoviral vector (ad-hApoE3) to rapidly reduce total plasma cholesterol and induce regression of plaque macrophages 13–15 or with its control empty adenoviral vector (ad-Empty) intravenously. Experiments were repeated using adeno-associated viral (AAV-hApoE3) (Vector Biolabs). Infection with the ApoE vector was routinely verified in each animal by measuring reduction of total cholesterol levels after blood collection at the submandibular vein. Following sacrifice, mice were perfused with 4% paraformaldehyde (PFA)-saline, hearts were isolated, and tissue was fixed in a solution of 4% PFA and 30% sucrose. Tissue was embedded in OCT compound (Tissue-Tek), and 10 μm cryosections were made spanning the depth of the entire aortic sinus. Images of plaque sections prepared at the aortic sinus were collected using a Nikon E800 bright field microscope, and the fluorescent bead number in plaque was counted using a Leica SPE confocal microscope. Image J software was used to assess the distance that each bead in plaque sections was positioned relative to the luminal endothelial surface and, separately, to the floor of the plaque demarcated by elastic lamina and a smooth muscle cell layer.
Gene Expression Analysis
As described above, C57Bl/6 mice were injected i.v. with latex beads (diluted 1:4 in PBS) three days following clodronate-loaded liposome mediate monocyte depletion. Then four days later, classical and nonclassical blood monocytes were FACS sorted (FACSAria, BD Biosciences) for presence or absence of latex beads. RNA was extracted from sorted cells using TRIzol and RNeasy mRNA isolation kit (Qiagen). RNA amplification, direct labeling, hybridization, and chip scanning were performed as previously described 16. Microarray was performed on an Affymetrix GeneChip 430 2.0. R packages “Oligo” and “Limma” were used to read the microarray expression data, normalize, access QC and perform DE. Gene set enrichment analysis with “fgsea” package was used to probe for pathway enrichment in hallmark and canonical pathways. Benjamini-Hochberg correction was used to correct p-vlaue for multiple comparisons. Gene expression data were submitted to the Gene Expression Omnibus (GEO) data bank (accession number pending).
Statistics
Values are presented as the mean ± standard error of the mean (SEM). Data were assessed for homogeneity of variance by Bartlett’s test. Samples not conforming to equal variance were assayed by Kruskal-Wallis rank test where adjusted p-values less than 0.05 was considered significant (p-value, *≤0.05, **≤0.01, ***≤0.001, ****≤0.001). For samples conforming to equal variance, data were analyzed further by two-tailed ANOVA with Tukey correction for multiple comparison, where a p-value less than 0.0332 was considered significant (p-value, *≤0.0332, **≤0.0021, ***≤0.0002, ****≤0.00001).
Results
Visualization of monocytes and monocyte-derived macrophages in atherosclerotic plaques
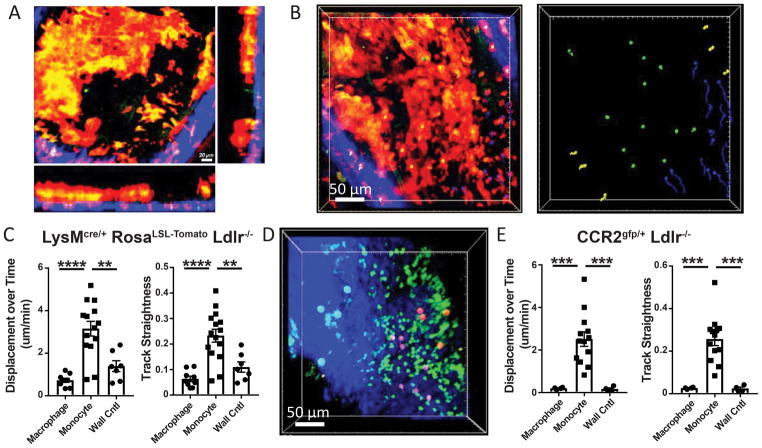
Stable intravital imaging of the carotid artery of plaque-bearing LysMcre/+ RosaLSL-Tomato Ldlr−/− mice was performed to examine intra-plaque macrophage motility. As illustrated by 3-dimensional image reconstruction, the preparation allowed for examination through the vessel wall to visualize plaque and the lumen of the artery (Figure 1A). Acellular necrotic regions within plaques, commonly associated with late stage atherosclerosis, were not observed in the carotid artery following 12–16 week HFD feeding. Tomato-expressing asymmetric cells, resembling foamy macrophages based on size, location and morphology, were the primary cell type present in the carotid plaques (Figure 1A, Supplemental Movie 1). Asymmetric morphology is a consistent feature of cells that have undergone transendothelial migration and therefore are within the plaque intima region 17. Similarly, cells with a more rounded symmetrical morphology are potentially within the vasculature and interacting with the endothelial surface.
Figure 1. 3D-myeloid cell dynamics within established atherosclerotic plaque using intravital imaging.
A) Image from intravital imaging from the carotid artery of plaque bearing LysMcre/+ RosaLSL-Tomato Ldlr−/− mouse showing established plaque myeloid cells (red), arterial wall (blue, second harmonic signaling), and lumen (black). Green is autofluorescent background signal. B) Foamy macrophages within the plaque (identified by large Tomato+ asymmetric cells found within the lumen of the plaque, identified with green balls), monocytes (small Tomato+ symmetric cells found on plaque boarders, identified with blue balls), and macrophages within the arterial wall (Tomato+ cells found in the wall of the artery, identified with yellow balls) were charted for their motility over ten minutes of continuous imaging. Cell tracks were assayed for (C) displacement length over time and track straightness (track displacement/track length). D) CCR2gfp/+ Ldlr−/− mice were used to track intraplaque dynamics following 12 weeks HFD feeding. Foamy macrophages (identified by orange balls), monocytes (purple balls), and wall macrophages (blue balls) were used to track cell dynamics over 1 hour of continuous imaging, and plotted for (F) displacement/time and track straightness. Data are representative from individual videos that are representative of at least four independent experiments for each model. Statistical significance was assayed by Kruskal-Wallis rank test where adjusted p-values less than 0.05 was considered significant (p-value, *≤0.05, **≤0.01, ***≤0.001, ****≤0.001).
Plaque macrophages, identified as indicated by asymmetric morphology and expression of the fluorescent Td-Tomato molecule, were observed to be particularly sessile during intravital imaging. However, a particular pattern of motility was evident during intravital two-photon microscopy of progressive plaques (Supplemental Movie 1). This motility appeared to be restricted to the smaller-sized and symmetric Tomato+ cells, resembling monocytes, and motility appeared to be distinct to regions on plaque borders. Migration on the plaque border is consistent with the proposed primary location of monocyte recruitment to plaque 18–20. Tomato-labeled asymmetric (marked by green dots) and symmetric, rounded cells (marked by blue dots) were charted for trajectories of individual cells over a ten-minute video segment (Figure 1B, 1C). To control for movement associated with the live animal preparation, motility was compared against Tomato+ asymmetric cells observed in the outer arterial wall and not in the plaque as a control for vibrations associated with heart beat and blood flow, labeled by yellow dots. Dots used to label cells in videos are overlaid with a 3D image, therefore bead color (particularly the green beads) merge to appear yellow in the overlay, left panel of Figure 1B. Correct bead colors can be seen on the right panel of Figure 1B and in Supplemental Movie 1). Asymmetric cells found within plaque displayed little net displacement over the imaging period, and no significant migratory potential over control cells identified in the wall (Figure 1C, 1D). This is consistent with fully differentiated macrophages within many other tissues where they are thought to be non-motile cells. Furthermore, the motion detected in asymmetric cells was not directed movement and showed little straightness (net displacement/track length) (Figure 1C). By contrast, the small symmetrical Tomato+ cells outside of the main plaque area, but near the plaque border, showed marked displacement and a high degree of directed motility over the imaging period. Thus, the Tomato+ cells in plaques could be divided into two populations based on behavior, with central macrophages, resembling foamy macrophages, in the high cellular density areas of plaque exhibiting minimal motility and a second population, resembling monocytes, on the plaque periphery showing high motility. A few small Tomato+ cells were also non-motile, suggestive of the possibility that these cells were recently recruited across the endothelial layer and were still undergoing differentiation into mature macrophages in tissue. Unfortunately, the limited continuous imaging time did not allow for observation of these cells transitioning to mature foam cells. Regardless of this caveat, these observations support a concept that differentiation of monocytes to macrophages in plaque yields macrophages with limited displacement potential.
The LysMcre reporter system is an efficient approach for labeling macrophages within atherosclerotic plaque. However, it introduces difficulty in separating neutrophils from monocytes. By flow cytometric analysis, both neutrophil and monocytes express the fluorescent Tomato protein in LysMcre/+ RosaLSL-Tomato Ldlr−/− mice (Supplemental Figure IA–B). This raises the possibility that small migratory cells observed in and around foamy macrophages in Figure 1A–C could potentially be neutrophils, and not monocytes as anticipated. To confirm whether monocytes were the motile cells observed in our intravital preparations, we employed the use of the CCR2gfp/+ Ldlr−/− reporter mouse12, where blood monocytes, but no other blood cell, expressed GFP (Supplemental Figure IB). This mouse was suitable for intravital imaging and the GFP label also identified a small subset, but not all macrophages within the atherosclerotic plaque (Figure 1D, Supplemental Figure IC). Performing intravital imaging on CCR2gfp/+ Ldlr−/− mice fed HFD for 12 weeks showed similar kinetics as seen in our previous model (Supplemental Movie 2). Asymmetric macrophages displayed sessile behavior, but small rounded symmetric cells (in this scenario, monocytes) were observed to be motile on the plaque boarders. Importantly, the monocytes displayed the same relative displacement/time and straightness measurements as found in our previous LysMcre/+ Tomato-expressing model (Figure 1E). These features of plaque were observed in both male and female mice. Together, these data suggest that motile monocytes migrate into and along plaque borders, but that macrophages that have undergone transendothelial migration into plaque regions display limited motility, at least during acute time periods of intravital imaging.
Recruited monocytes deposit on the surface and do not penetrate deep within progressing plaques
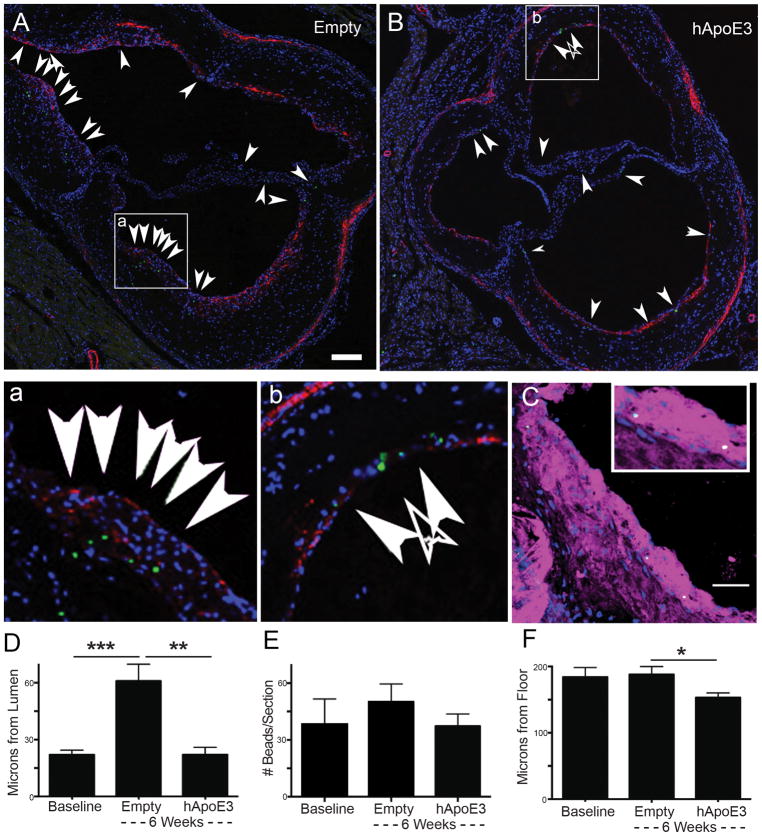
Intravital imaging presented a limited time interval for the examination of monocyte and macrophage motility within the plaque. Therefore, approaches allowing for longer tracking needed to be implemented. We had previously established that macrophage loss from plaques can be triggered by virally induced apoE expression in plaque-baring ApoE−/− mice. AAV8-ApoE virial transduction was efficient at reversing plasma cholesterol levels rapidly and stably 15 in male mice (Supplemental Figure IIA, 2B), with the labeling and infection protocol outlined in Supplemental Figure IIC. Particles carried by macrophages are not removed from plaques during the period of macrophage loss in this model 13, leading us to conclude that migratory egress of macrophages was not necessary for their removal and such migration was not occurring in this model. However, upon subsequent closer inspection of plaque sections in these experiments there was a striking pattern in which particles appeared further from the lumen during disease progression (ad-Empty) (Figure 2A, with inset in Figure 2a) and near the lumen in the regression treatment (ad-hApoE3) (Figure 2B, with inset in Figure 2b). Importantly, beads were observed to be largely present in the cytoplasm of macrophages within the plaque for either treatment condition (Figure 2C). Indeed, quantification of particle distance from the lumen confirmed the deeper positioning of particles in the ad-Empty group compared with the ad-hApoE3 group (Figure 2D). We further confirmed that total bead counts in the various groups were not statistically different, as expected 13 (Figure 2E).
Figure 2. Positional tracking using phagocytic cargo labeling suggests little displacement of central plaque macrophages over several weeks during plaque progression or regression.
A–B) Image shows aortic sinus plaque section from ApoE−/− mice treated with adenovirus, following model II in Supplemental Figure IIC, that was empty (control, panel A) or that encoded hApoE3 to promote regression (panel B). Tissues were recovered 6 weeks after monocyte labeling with green beads and vector treatment. Arrows point to green beads within plaques (marked by green dots), including beads within the valve leaflets, although beads in leaflets were not counted as being part of the plaque. To help highlight the floor of the plaque, counterstaining for smooth muscle actin was included (red). Nuclei are stained with DAPI (blue). Insets (2a and 2b) show higher magnification of images to visualize green bead deposition in the plaque. Scale bar, 50 μm. C) Green beads within plaques were present within macrophage rich regions, identified by CD115 and MOMA2 co-staining (magenta). Sample from an Empty-vector treated animal. Nuclei stained by DAPI in blue, scale bar 10 μm. D) Bead distance from the plaque lumen was assessed 5 days after bead labeling of blood monocytes, just prior to adenovirus treatment (baseline) or 6 weeks after Empty or ApoE3 vectors were administered. E) Total beads in plaque sections were enumerated. F) Distance of beads from the floor of the plaque, defined by elastic lamina and muscle layer, was quantified. Data is from a single experiment in which 7–10 mice were included in each group. Experiments are representative of a total of 6 independent experiments performed, each with slightly different timing or design, but all providing support for the conclusions drawn here. Statistics were performed by two-way ANOVA with Tukey correction for multiple comparison test (p-value, *≤0.0332, **≤0.0021, ***≤0.0002).
We initially wondered if macrophages containing beads as cargo had wandered closer to the lumen during regression even if they had not egressed with such cargo. However, we negated this possibility when we quantified whether the particles were deeper within the plaques when their position was determined relative to the internal elastic laminal border just above the internal elastic lamina and smooth muscle actin staining medial layer (Figure 2A, 2B, 2F). In this assessment, beads in the regressing group were slightly closer to the plaque floor, perhaps due to the modest reduction in plaque area during regression (Figure 2F). Most significantly, the distance of the cargo from the floor of the plaque was the same at day 5 after labeling (baseline) and did not change for the following 6 weeks of HFD feeding in the Empty vector treated animals (Figure 2F). Thus, the apparent penetration of bead-bearing phagocytes to progressing plaques and the apparent retention (or return) of phagocytic cargo to the vessel lumen in regressive conditions was an optical aberration related to luminal growth of the plaque, without bead-bearing cells moving deeper into plaque. The difference in bead positioning relative to the endothelial lumen during plaque regression versus progression, then, could be explained by the cessation of monocyte recruitment that is induced by ad-hApoE3 13. That is, overall, the data suggested that particles brought in by monocyte-derived macrophages have no net displacement in the depth of the plaque (we have no landmarks to assess lateral movement) within plaques for weeks. Instead, under conditions when monocyte recruitment is robust, the particle-bearing cells are layered upon by the newly recruited cells. However, if monocyte recruitment abates, such as in our regression model, the phagocytic cargo remains relatively nearer the vessel lumen.
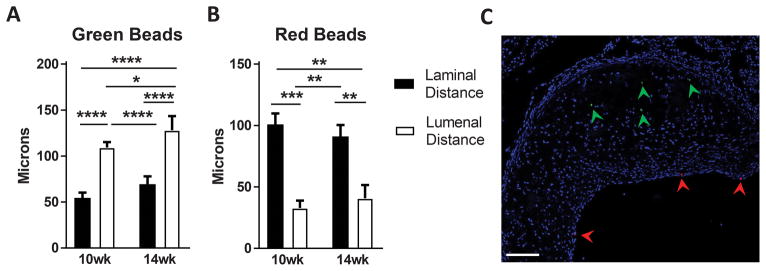
To further test the concept that macrophage positioning in plaques is primarily the product of a layering phenomenon that does not allow for significant penetration of newly recruited cells into the deeper layers of the plaque, we carried out experiments with ApoE−/− mice on high fat diet for different periods in which particle labeling of monocytes occurred in two kinetic waves using green or red particles in succession (modeled in Supplemental Figure III). Just as above, this approach allows for measurements in the Z-axis to quantify depth within the plaques, but does not allow for measurements of side-to-side motility. Labeling of monocytes early in plaque progression (green beads) resulted in labeled cells positioned nearer the plaque floor than the luminal border (Figure 3A). Conversely, later labeling (red beads) showed positioning of the second wave of particles nearer the endothelial surface (Figure 3B, 3C). In addition to the bead labeling of monocytes at different time periods resulting in bead positioning within plaque being restricted by relative location, we observed no scenario in which a cell had taken up both bead colors, arguing against significant bead transfer, or cell death and bead efferocytosis by a phagocyte containing a bead of another color in the time points studied. Furthermore, the dramatic separation in localization of the red and green beads supports limited access of circulating monocytes to established regions of the plaque. Taken together, these data support that macrophage motility in the plaque z-axis is very low. If it occurs, macrophage motility leads to no net displacement in macrophage positioning within plaques.
Figure 3. Positional tracking of phagocytic cargo shows minimal displacement in progressing plaques in relation to the plaque laminal boarder.
A–B) Green beads were used to label blood monocytes given two weeks after ApoE−/− mice were transitioned to a high fat diet following weaning. Then, in the same mice, red beads were used to label monocytes 7 days before the end of the 10- or 14-week period of high fat diet feeding (modeled in Supplemental Figure III). The position of each bead type was recorded with respect to plaque lumen or plaque floor, with representative image (C) showing localization of green and red beads in plaque (marked by arrows, scale bar 100 μm). Displayed data combine two independent experiments using two time points (10 or 14 weeks of high fat diet), with experimental group sizes of 4–6 mice. For each mouse, 5 sections were charted with approximately 20–40 beads of each color per section and distance measurements averaged for each mouse. Statistics were performed by two-way ANOVA with Tukey correction for multiple comparison test (p-value, *≤0.0332, **≤0.0021, ***≤0.0002, ****≤0.00001).
Phagocytic cargo shows minimal gene expression changes on circulating monocytes
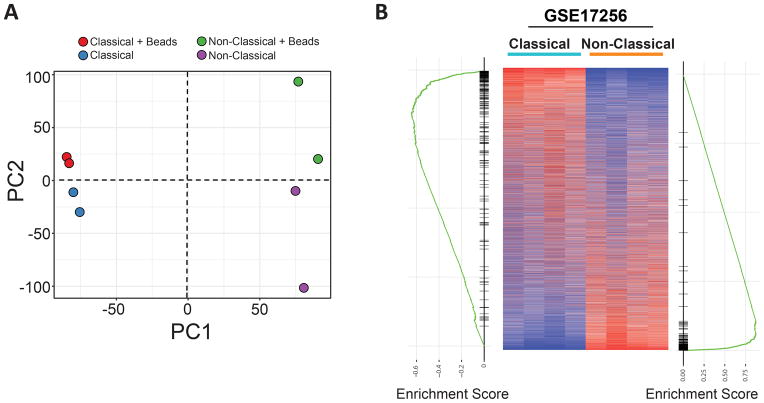
Finally, we carried out quality control experiments to assess whether the phagocytic labeling of macrophages with bead particles might strongly alter their phenotype. Previous studies using plain polystyrene particle-labeled monocytes to assess recruitment into tissues have demonstrated that bead labeling did not affect the ability of monocytes to enter tissues 21, and particle uptake did not activate p38/MAPK or NFκB pathways 22. Placement of particles in the airway does not induce inflammation, as indicated by lack of neutrophil recruitment 23. However, the possibility remains that bead labeling could have effects on global gene expression profiles. To address this prospect, we performed gene expression profiling of classical and non-classical monocytes that had taken up latex particle in vivo against cells from the same mouse that had not taken up particles. Unbiased clustering analysis of gene expression data revealed the primary driver of population differences (principal component 1, PC1) was the cell type being assessed (classical vs. non-classical) and the presence of latex beads was a secondary function (PC2) of diversity in the array (Figure 4A). In classical monocytes, only 5 individual mRNA transcripts were significantly upregulated by bead uptake, including Spic, Fabp5, and three complement component transcripts C1qa, C1qb, C1qc. There were no significant transcripts downregulated. With respect to nonclassical monocytes, bead labeling induced significant upregulation of CD209a, Klrd1, Slc40a1, F13a1, Vcan. Again, there were no significantly downregulated transcripts in bead-labeled nonclassical monocytes compared to their unlabeled counterparts. With these few changes in gene expression, pathway analysis yielded no pathways that were significantly altered using an adjusted p value < 0.01.
Figure 4. Monocytes show minimal gene expression changes in response to bead loading in vivo.
Classical and nonclassical monocytes which were loaded with beads or not (cntl) were assayed for gene expression changes associated with the presence of beads. A) Principle component analysis (PCA) analysis shows unbiased clustering of samples. B) Monocyte gene expression data from WT untreated mice (heatmap, GSE17256) was compared with the top 150 genes associated with classical or nonclassical monocytes from animals that were treated with bead labeling approach. Comparison showed significant enrichment of genes between sample groups, with the left GSEA plot representing classical monocyte genes and right GSEA plot representing nonclassical monocyte genes.
Given that the data collected on the impact of bead labeling was performed on monocytes with or without bead uptake in mice that had been treated so as to label monocytes, we carried out further analysis to determine whether there were gene expression changes in association with monocytes whose gene expression was analyzed from previously unmanipulated mice 24. Using the top 150 genes enriched in classical or nonclassical monocytes from mice subjected to bead labeling, we assessed the distribution of these genes against previously published dataset of monocytes from untreated mice (Figure 4B). Heat maps of rank-listed genes in either classical or nonclassical monocytes revealed a high degree of enrichment between experimental groups comparing monocytes from unmanipulated and bead-manipulated mice. All together, these data suggest that neither the labeling of monocytes with latex beads nor the procedure associated with it leads to dramatic changes in overall gene expression compared to circulating monocytes from naïve mice. Thus, the conclusions drawn regarding the relative immobility of monocyte-derived cells in plaques is not likely an artifact of changes associated with bead labeling.
Discussion
Using an established model of plaque regression, this study brings technical and conceptual advances to our understanding of experimental atherosclerosis. The underlying question and dilemma driving the study was to better understand the migratory capacity and behavior of macrophages in plaques. Intravital imaging of atherosclerotic plaques is challenging, not least because of the need to confront motion associated with breathing and heart rhythm in the arterial tree. However, using different approaches, these hurdles are being overcome in different groups, largely either with the use of software that collects images in rhythm with the heart beat 7 or with physical stabilization of arterial tissue to reduce motion 6, 11. We developed a preparation capable of direct imaging of established plaque in the carotid artery. To our knowledge, our preparation is the first to image high fat-diet induced established plaques native to the animal being evaluated, as compared with injury-induced plaques 6, transplanted vessels 11, or monocyte interactions with the vessel wall prior to plaque progression 8. Having developed a stable and high resolution imaging approach, we found that macrophages piled in clusters within the plaque were highly sessile, displaying minimal net displacement. Smaller, monocyte-like cells were motile in regions adjacent to the established plaque. At least a few of these cells may have been undergoing recruitment and integration into plaques. However, even these monocytes, while motile, did not appear to penetrate deep into the plaque.
These results motivated us to test the migratory potential of plaque macrophages using long term progressive or regressive conditions. We charted the position of phagocytic bead cargo transported into plaque by recruited monocytes and asked whether there were z-axis shifts (shifts in depth) in cargo localization within the plaque following progressive or regressive conditions. If random motility occurred in plaque macrophages throughout the plaque (including plaque depth) 25, or if directed migration promoted movement to a particular part of plaque, we would expect to be able to quantify this motility as a net displacement of phagocytic cargo over time. With the data showing no depth displacement in bead localization within the plaque over up to a six-week period, we conclude that macrophage motility in plaques is quite limited. Previous studies have noted that macrophage phenotype is segregated between regions within plaque, with classically activated macrophage found near the necrotic core/unstable plaque regions and alternatively activated macrophages near the border/stable regions 26. Our data suggest that these observations may be explained by stable, positional differences in phenotype: the microenvironment of the plaque controls the macrophage phenotype and this phenotype is not diluted by the ability of macrophages to readily relocate to other areas that would simultaneously allow macrophages with different phenotypes to mix. That macrophages may take on particular functional phenotypes as a result of their positioning within plaques underscores the concept that although we conclude that macrophages are largely immobile, they are not functionally inert, but indeed may carry out pivotal roles in the spatial confines in which they reside.
It remains unclear whether the motile monocytes observed near the surface of the plaque shoulders frequently or infrequently integrate into the plaque itself. However, some smaller cells were observed having lost mobility at the edges of the plaque, suggesting they may have begun differentiation programs to develop into foamy macrophages within the arterial wall. Clear evidence of monocyte recruitment into and integration within plaques was not observed in the videos we obtained. This is an important issue as a picture starts to emerge about the dynamics of changes that occur within plaques. In particular, the data presented here, when considered with the literature at large, most strongly support a model in which monocyte differentiation to macrophages results in cells with a very limited range of mobility regardless of whether a plaque is regressing, creating not a swarm as seen in lymph nodes for lymphocytes 25, but rather a zone of collected cells with fixed spatial arrangements, much as reported in liver granulomas 5. The data presented here fit with our previous observation that congenically marked monocytes layer over older macrophages in plaques 27. Furthermore, it is unlikely that presence of the bead within phagocytes causes sessility, because we have reported numerous instances in which beads were carried by mononuclear phagocytes for long distances 21, 28–30. Additionally, we performed gene expression profile analysis between monocytes loaded with beads and those that had not and found minimal changes in overall gene expression, which was consistent with previous reports suggesting that bead loading had not dramatic effects on activation or survival pathways 21, 22. Since our bead tracking studies included analysis of regressing conditions and these data support relative immobility of macrophages during disease reversal, it is unlikely that plaque regression is associated with a marked increase in migratory capacity for most plaque macrophages.
With respect to the use of beads to track the position of macrophages in plaques, one immediately may consider whether the technique is limited by the possibility that beads are transferred from one macrophage to another under conditions in which the bead-carrying macrophage may undergo death. However, engulfment of beads or engulfment of a bead-bearing dying macrophage would necessarily require that the efferocytic macrophage localize close to a dying macrophage. Once engulfed, the efferocytic macrophage would in turn have the potential to migrate or remain relatively immobile. When this consideration is taken into account, it seems unlikely that even death of the original macrophage would greatly alter the interpretation of the experiment, as long as the beads remained within macrophages as a population, which we show is the case. To guard against the possibility that the phagocytic labeling technique might yield incorrect conclusions, we developed a live imaging approach. The live imaging supported the conclusion that macrophages in plaques are scarcely mobile.
The issue of relative mobility is important because once macrophages have differentiated and thereby become spatially confined, they may become vulnerable to adverse changes within their micro-environment that they cannot escape, including changes in which incoming macrophages pile over them. Indeed, the inability to escape may be a force underlying the development of a necrotic core and its reticence for clearance. Indeed, the two instances where macrophage motility has been defined as limited, granulomas 5 and the present work on atherosclerotic plaque, are two instances that are prone to emergence of necrotic core. Future incorporation of our findings into mathematical modeling of plaque progression may allow us to better understand conditions that control plaque vulnerability to rupture.
Supplementary Material
Following 12 weeks of HFD feeding, LysMcre/+ RosaLSL-Tomato Ldlr−/− mice were assessed for myeloid cell motility within atherosclerotic plaques on the right common carotid artery by intravital 2-photon microscopy. Video shows Tomato expressing cells in red, secondary harmonic in blue, and autofluorescence in green. Blue balls represent monocyte, yellow balls represent vessel wall macrophages, and green balls represent lesion foamy macrophages. Lines track migration patterns of individual cells over the length of the video. Video is representative of 5 independent experiments.
Following 12 weeks of HFD feeding, CCR2gfp/+ Ldlr−/− mice were assessed for monocyte and macrophage motility within atherosclerotic plaques on the right common carotid artery by intravital 2-photon microscopy. In the video green identifies CCR2-expressing cells and blue represents second harmonic signal. Magenta balls represent monocyte, cyan balls represent vessel wall macrophages, and red balls represent lesion foamy macrophages. Lines track migration patterns over the video. Video is representative of 3 independent experiments.
Highlights.
Intravital imaging of carotid atherosclerotic plaques displays motile monocytes and sessile foamy macrophages.
Monocytes, tracked with a long-term label, are recruited to the superficial surface of atherosclerotic plaques and as a population have limited penetration into the plaque, resulting in a tree ring-like growth pattern for atherosclerotic plaque as incoming monocytes overlay earlier recruited ones.
The spatial confinement of recruited monocytes is operative in both regressing and progressing plaques, indicating that limited motility is not linked to hypercholesterolemia or plaque status.
Limited spatial dynamics may impact macrophage survival and phagocytic cleanup of other macrophages, and thus necrotic core formation, because macrophages have limited capacity to remove themselves from environments that may impinge upon viability.
Acknowledgments
We would like to thank Daniel J. Rader (University of Pennsylvania) for kindly providing Ad-hApoE3 vector.
Sources of funding
Major support for this work was supplied by National Institutes of Health (NIH) R01/R37 AI049653 and NIH RO1 HL118206 to GJR. Additional support to GJR includes NIH DP1DK109668. JWW was supported by NIH training grant 2T32DK007120-41, American Heart Association (AHA) grant 17POST33410473, and NIH K99HL138163. AE was supported by NIH training grant T32-HL07081-38 and BHZ was supported by AHA grant 16SDGG30480008.
Abbreviations
- HFD
High Fat Diet
- Apoe
Apolipoprotein E
- Ldlr
Low Density Lipoprotein
References
- 1.Randolph GJ. Mechanisms that regulate macrophage burden in atherosclerosis. Circ Res. 2014;114:1757–71. doi: 10.1161/CIRCRESAHA.114.301174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hilgendorf I, Swirski FK, Robbins CS. Monocyte fate in atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:272–9. doi: 10.1161/ATVBAHA.114.303565. [DOI] [PubMed] [Google Scholar]
- 3.Ai D, Jiang H, Westerterp M, Murphy AJ, Wang M, Ganda A, Abramowicz S, Welch C, Almazan F, Zhu Y, Miller YI, Tall AR. Disruption of mammalian target of rapamycin complex 1 in macrophages decreases chemokine gene expression and atherosclerosis. Circ Res. 2014;114:1576–84. doi: 10.1161/CIRCRESAHA.114.302313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–8. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 5.Egen JG, Rothfuchs AG, Feng CG, Winter N, Sher A, Germain RN. Macrophage and T cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity. 2008;28:271–84. doi: 10.1016/j.immuni.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevre R, Gonzalez-Granado JM, Megens RT, Sreeramkumar V, Silvestre-Roig C, Molina-Sanchez P, Weber C, Soehnlein O, Hidalgo A, Andres V. High-resolution imaging of intravascular atherogenic inflammation in live mice. Circ Res. 2014;114:770–9. doi: 10.1161/CIRCRESAHA.114.302590. [DOI] [PubMed] [Google Scholar]
- 7.McArdle S, Chodaczek G, Ray N, Ley K. Intravital live cell triggered imaging system reveals monocyte patrolling and macrophage migration in atherosclerotic arteries. J Biomed Opt. 2015;20:26005. doi: 10.1117/1.JBO.20.2.026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quintar A, McArdle S, Wolf D, Marki A, Ehinger E, Vassallo M, Miller J, Mikulski Z, Ley K, Buscher K. Endothelial Protective Monocyte Patrolling in Large Arteries Intensified by Western Diet and Atherosclerosis. Circ Res. 2017;120:1789–1799. doi: 10.1161/CIRCRESAHA.117.310739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcovecchio PM, Thomas GD, Mikulski Z, Ehinger E, Mueller KAL, Blatchley A, Wu R, Miller YI, Nguyen AT, Taylor AM, McNamara CA, Ley K, Hedrick CC. Scavenger Receptor CD36 Directs Nonclassical Monocyte Patrolling Along the Endothelium During Early Atherogenesis. Arterioscler Thromb Vasc Biol. 2017;37:2043–2052. doi: 10.1161/ATVBAHA.117.309123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams JW, Randolph GJ, Zinselmeyer BH. A Polecat’s View of Patrolling Monocytes. Circ Res. 2017;120:1699–1701. doi: 10.1161/CIRCRESAHA.117.311021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Luehmann H, Hsiao H, Tanaka S, Ryuiji H, Gauthier J, Sultan DLK, Brody S, Gelman A, Gropler R, Liu Y, Kreisel D. Visualization of Monocytic Cells in Regressing Atherosclerotic Plaques by Intravital 2-Photon and Positron Emission Tomography-Based Imaging. Arterioscler Thromb Vasc Biol. 2018 doi: 10.1161/ATVBAHA.117.310517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satpathy AT, Briseno CG, Lee JS, Ng D, Manieri NA, Kc W, Wu X, Thomas SR, Lee WL, Turkoz M, McDonald KG, Meredith MM, Song C, Guidos CJ, Newberry RD, Ouyang W, Murphy TL, Stappenbeck TS, Gommerman JL, Nussenzweig MC, Colonna M, Kopan R, Murphy KM. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nat Immunol. 2013;14:937–48. doi: 10.1038/ni.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J Clin Invest. 2011;121:2025–36. doi: 10.1172/JCI43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsukamoto K, Smith P, Glick JM, Rader DJ. Liver-directed gene transfer and prolonged expression of three major human ApoE isoforms in ApoE-deficient mice. J Clin Invest. 1997;100:107–14. doi: 10.1172/JCI119501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim IH, Jozkowicz A, Piedra PA, Oka K, Chan L. Lifetime correction of genetic deficiency in mice with a single injection of helper-dependent adenoviral vector. Proc Natl Acad Sci U S A. 2001;98:13282–7. doi: 10.1073/pnas.241506298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grabner R, Lotzer K, Dopping S, Hildner M, Radke D, Beer M, Spanbroek R, Lippert B, Reardon CA, Getz GS, Fu YX, Hehlgans T, Mebius RE, van der Wall M, Kruspe D, Englert C, Lovas A, Hu D, Randolph GJ, Weih F, Habenicht AJ. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J Exp Med. 2009;206:233–48. doi: 10.1084/jem.20080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berman ME, Muller WA. Ligation of platelet/endothelial cell adhesion molecule 1 (PECAM-1/CD31) on monocytes and neutrophils increases binding capacity of leukocyte CR3 (CD11b/CD18) J Immunol. 1995;154:299–307. [PubMed] [Google Scholar]
- 18.Haka AS, Potteaux S, Fraser H, Randolph GJ, Maxfield FR. Quantitative analysis of monocyte subpopulations in murine atherosclerotic plaques by multiphoton microscopy. PLoS One. 2012;7:e44823. doi: 10.1371/journal.pone.0044823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang CK, Pang H, Wang L, Niu Y, Luo J, Chang E, Sparks JD, Lee SO, Chang C. New therapy via targeting androgen receptor in monocytes/macrophages to battle atherosclerosis. Hypertension. 2014;63:1345–53. doi: 10.1161/HYPERTENSIONAHA.113.02804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131–8. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- 21.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–94. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yates RM, Russell DG. Phagosome maturation proceeds independently of stimulation of toll-like receptors 2 and 4. Immunity. 2005;23:409–17. doi: 10.1016/j.immuni.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Jakubzick C, Helft J, Kaplan TJ, Randolph GJ. Optimization of methods to study pulmonary dendritic cell migration reveals distinct capacities of DC subsets to acquire soluble versus particulate antigen. J Immunol Methods. 2008;337:121–31. doi: 10.1016/j.jim.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, Habenicht AJ, Ziegler-Heitbrock L, Randolph GJ. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:e10–9. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cahalan MD, Parker I, Wei SH, Miller MJ. Real-time imaging of lymphocytes in vivo. Curr Opin Immunol. 2003;15:372–7. doi: 10.1016/s0952-7915(03)00079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol. 2015;12:10–7. doi: 10.1038/nrcardio.2014.173. [DOI] [PubMed] [Google Scholar]
- 27.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–84. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van Rooijen N, Grainger JR, Belkaid Y, Ma’ayan A, Riches DW, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jakubzick C, Tacke F, Llodra J, van Rooijen N, Randolph GJ. Modulation of dendritic cell trafficking to and from the airways. J Immunol. 2006;176:3578–84. doi: 10.4049/jimmunol.176.6.3578. [DOI] [PubMed] [Google Scholar]
- 30.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–61. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Following 12 weeks of HFD feeding, LysMcre/+ RosaLSL-Tomato Ldlr−/− mice were assessed for myeloid cell motility within atherosclerotic plaques on the right common carotid artery by intravital 2-photon microscopy. Video shows Tomato expressing cells in red, secondary harmonic in blue, and autofluorescence in green. Blue balls represent monocyte, yellow balls represent vessel wall macrophages, and green balls represent lesion foamy macrophages. Lines track migration patterns of individual cells over the length of the video. Video is representative of 5 independent experiments.
Following 12 weeks of HFD feeding, CCR2gfp/+ Ldlr−/− mice were assessed for monocyte and macrophage motility within atherosclerotic plaques on the right common carotid artery by intravital 2-photon microscopy. In the video green identifies CCR2-expressing cells and blue represents second harmonic signal. Magenta balls represent monocyte, cyan balls represent vessel wall macrophages, and red balls represent lesion foamy macrophages. Lines track migration patterns over the video. Video is representative of 3 independent experiments.