Abstract
Unilateral primary aldosteronism is the most common surgically curable form of hypertension that must be accurately differentiated from bilateral primary aldosteronism for therapeutic management (surgical versus medical). Adrenalectomy results in biochemical cure (complete biochemical success) in almost all patients diagnosed with unilateral primary aldosteronism; the remaining patients with partial or absent biochemical success comprise those with persisting aldosteronism who were misdiagnosed as unilateral primary aldosteronism pre-operatively. To identify determinants of post-surgical biochemical outcomes, we compared the adrenal histopathology and the peripheral venous steroid profiles of patients with partial and absent or complete biochemical success after adrenalectomy for unilateral primary aldosteronism. A large multicentre cohort of adrenals from patients with absent and partial biochemical success (N=43) displayed a higher prevalence of hyperplasia (49% versus 21%, P=0.004) and a lower prevalence of solitary functional adenoma (44% versus 79%, P<0.001) compared with adrenals from age- and sex-matched patients with primary aldosteronism with complete biochemical success (N=52). We measured the peripheral plasma steroid concentrations in a subgroup of these patients (N=43) and in a group of patients with bilateral primary aldosteronism (N=27). Steroid profiling was associated with histopathological phenotypes (solitary functional adenoma, hyperplasia and aldosterone-producing cell clusters) and classified patients according to biochemical outcome or diagnosis of bilateral primary aldosteronism. If validated, peripheral venous steroid profiling may be a useful tool to guide the decision to perform surgery based on expectations of biochemical outcome after the procedure.
Keywords: Primary aldosteronism, aldosterone producing adenoma, bilateral adrenal hyperplasia, adrenalectomy, endocrine hypertension, Hyperaldosteronism, aldosterone, postsurgical outcomes, adrenal gland, immunohistochemistry, steroid profiling
Summary:
Immunohistopathology may help determine which patients are likely to need ongoing follow-up for persistent PA and steroid profiling may be useful to guide the decision to perform surgery
Introduction
Primary aldosteronism (PA) is a form of endocrine hypertension caused by the overproduction of aldosterone from one or both adrenal glands mainly due to a unilateral aldosterone-producing adenoma (APA) or bilateral adrenal hyperplasia.1 Specific treatment by unilateral laparoscopic adrenalectomy (unilateral PA) or medical therapy with mineralocorticoid antagonists (bilateral PA) reverses the increased risk of stroke and heart disease in patients with PA compared with patients with essential hypertension.2–4 Adrenal venous sampling (AVS) or adrenal computed tomography (CT) scanning is used to differentiate unilateral from bilateral PA although alternative approaches are currently being investigated including functional imaging using positron emission tomography/CT scanning with a radiolabelled tracer and peripheral venous steroid profiling. 5–10
Immunohistochemistry using polyclonal11 or monoclonal antibodies12–14 to key enzymes involved in adrenal steroidogenesis (CYP11B2, CYP11B1 and CYP17A1) has aided identification of pathological features contributing to aldosterone overproduction.15-17 The histopathological abnormalities present in unilateral PA are highly heterogeneous and include solitary functional unilateral adenoma, adenoma with adjacent hyperplasia of the zona glomerulosa or unilateral diffuse hyperplasia with functional micronodules or hyperplasia with macronodules.17–21 Nests of CYP11B2-positive cells (APCCs, aldosterone-producing cell clusters) have been identified beneath the adrenal capsule and are present in normal adrenals and in the adrenal cortex adjacent to an APA. 11,16,19,22,23 The occurrence of APCCs increases with age and they frequently have somatic CACNA1D, ATP1A1 and ATP2B3 mutations that drive dysregulated aldosterone production in APAs and have been proposed as a likely source of constitutive aldosterone production and possible precursors to APAs.6,22,24
Unlike unilateral PA, the pathophysiology of bilateral PA remains poorly understood, hampered in part by the scarce availability of resected adrenal specimens due to the medical, rather than surgical, management of bilateral PA. The Primary Aldosteronism Surgical Outcome (PASO) study established criteria to assess outcomes (complete, partial or absent clinical and biochemical success) of patients after adrenalectomy for unilateral PA.25 Clinical outcomes were defined by blood pressure measurements and antihypertensive medication dosage, biochemical outcomes by plasma potassium, aldosterone and renin measurements.25 Biochemical outcomes provide a quality measure of patient diagnosis with complete biochemical success defining the correct diagnosis and appropriate treatment whereas absent and partial biochemical success indicate persistent aldosteronism after surgery. Absent and partial biochemical success combined comprises around 1 in every 20 patients with aldosterone lateralisation performed by AVS which presumably results from bilateral asymmetrical aldosterone overproduction.25
We hypothesised that patients with an absent or partial biochemical outcome comprise mainly cases of bilateral PA misdiagnosed as unilateral pre-operatively. In a large multicentre study with outcomes assessed in accordance with an international consensus, we analysed the histopathology of 95 adrenals from patients operated for unilateral PA (43 from patients with absent and partial biochemical success matched with 52 cases of complete biochemical success) and determined peripheral venous steroid profiles in a subgroup of these patients compared with patients diagnosed with bilateral PA.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Patient selection
The study included surgically resected adrenals of 95 patients collected from 9 international centres that were diagnosed with unilateral PA and classified with absent, partial or complete biochemical success at 6–12 months after unilateral adrenalectomy in accordance with the PASO consensus (Table S1).25 Patients with absent and partial biochemical success were matched for age (± 5 years) and sex with patients with complete biochemical success from the same centre. Peripheral venous plasma samples from patients of the Munich cohort (N=70) were analysed by steroid profiling (absent or partial biochemical success, N=15, age- and sex-matched with complete success, N=28 and bilateral PA, N=27). All patients were diagnosed according to the Endocrine Society Guideline or the Japan Endocrine Society Guideline.1,26
Baseline and follow-up parameters of patients providing resected adrenals are shown in Table S2. Baseline parameters at study entry of patients providing peripheral plasma samples are shown in Table S3. Blood pressure measurements were recorded as described previously.25 Study approval was obtained from the appropriate institutional review committees and all patients gave informed consent in accordance with local ethical guidelines.
Histopathology
Successive paraffin-embedded adrenal tissue sections (4 μm thick) were immunostained for CYP11B2 (clone 41–17B), CYP11B1 (clone 80–7-5) and CYP17A1 (clone 10–19-G6) developed by C.E.G-S.12,13 All haematoxylin and eosin (H&E) stained sections and immunostained sections were independently assessed by a specialist in adrenal histopathology (C.E.G-S.) and an experienced pathologist (I.C.). Both investigators were blinded for the surgical outcome of enrolled patients and agreement was reached in cases of discordant scoring. The samples were scored for solitary functional adenoma (a single well defined adenoma with positive CYP11B2 staining), hyperplasia (multiple CYP11B2-positive macronodules, focal thickening of the zona glomerulosa with CYP11B2-positive immunostaining or CYP11B2-positive diffuse hyperplasia with or without CYP11B2-positive micronodules), APCCs (clusters of zona glomerulosa cells, CYP11B2-positive and CYP11B1- and CYP17A1-negative, localised in the subcapsular region extending into the zona fasciculata).17,23 There were no significant differences in sex distribution or the average age of patients with a solitary functional adenoma, hyperplasia or APCCs (Table S4).
Steroid profiling using liquid chromatography-tandem mass spectrometry (LC_MS/MS)
Blood was drawn by venipuncture at time of diagnosis of PA between 8:00 and 10:00 am in a fasting state and processed according to standard operational procedures. The measurement of 15 adrenal steroids using LC-MS/MS was performed in plasma of 15 patients with absent or partial biochemical success, 28 patients with complete biochemical success and 27 patients with a diagnosis of bilateral PA as described.9,10
Statistical analysis
Statistical analyses were performed using SPSS Version 24, Data are shown as mean ± SD, as medians and interquartiles or as absolute numbers and percentages. Quantitative normally distributed variables were analysed using one-way ANOVA with a post hoc Bonferroni or a t test, group differences by Kruskal-Wallis or Mann-Whitney U tests for quantitative non-normally distributed variables, and χ² or Fisher’s exact tests for categorical variables. A P-value of less than 0.05 was considered significant. Linear discriminant analyses were performed in R and decision tree analyses used MATLAB R2017b.
Results
Patient characteristics
Patients with post-surgical complete biochemical success (N=52) had lower serum potassium concentrations at baseline relative to patients with an absent + partial biochemical outcome (P=0.035) (Table S2). No significant differences were detected in nodule size (at pathology or imaging) and in the appearance of the contralateral adrenal at imaging with respect to biochemical outcome. However, patients with absent + partial success displayed a lower lateralisation index and a higher contralateral ratio compared with patients with a complete biochemical outcome (Figure 1, Table S5). Genotype data were available for 46 of the 95 specimens; the proportion of adrenals with a KCNJ5 mutation was not significantly higher in the complete biochemical outcome group (18 adrenals carrying a KCNJ5 mutation of 30 [58%] genotyped samples compared with 7 of 16 [37%] in the absent group, P=0.292) (Table S5).
Figure 1. Adrenal venous sampling results stratified for biochemical outcomes.
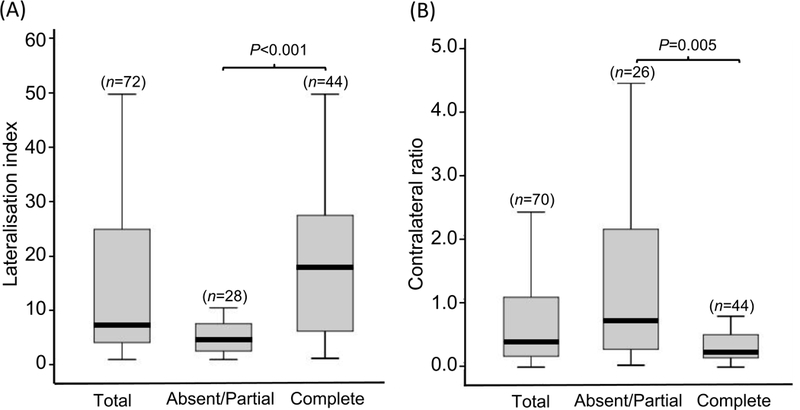
Box and whisker plots showing AVS results stratified for biochemical outcomes. Patients with absent or partial biochemical success compared with complete success after adrenalectomy have lower lateralisation indices (Panel A) and higher contralateral ratios (Panel B). Horizontal lines within boxes indicate the median, and box and whiskers represent the 25th to 75th and 5th to 95th percentiles, respectively. n indicates the number of patients in each group and a Mann-Whitney test was used to calculate P values.
Adrenal histopathology of resected sample specimens according to biochemical outcome
The distribution of solitary functional adenoma, hyperplasia or APCCs in the complete, partial and absent biochemical success groups is shown in Figure 2A. In the total sample set the majority of adrenals showed a solitary functional adenoma (60 of 95 samples, 63%) with 50% (30 of 60) displaying concurrent APCC in the adjacent cortex, 15% (9 of 60) associated with cortical hyperplasia and 48% (29 of 60) without hyperplasia or APCC (normal appearing adjacent cortex). The complete biochemical outcome group displayed a significantly higher prevalence of solitary functional adenomas compared with the absent + partial group (79% versus 44%, P < 0.001) (Table 1). The immunohistopathology of the adjacent cortex surrounding a functional solitary adenoma was not perceivably different in patients with complete biochemical success compared with an absent + partial biochemical outcome (Table 1).
Figure 2. Heterogeneous histopathology of resected adrenals from patients with PA.
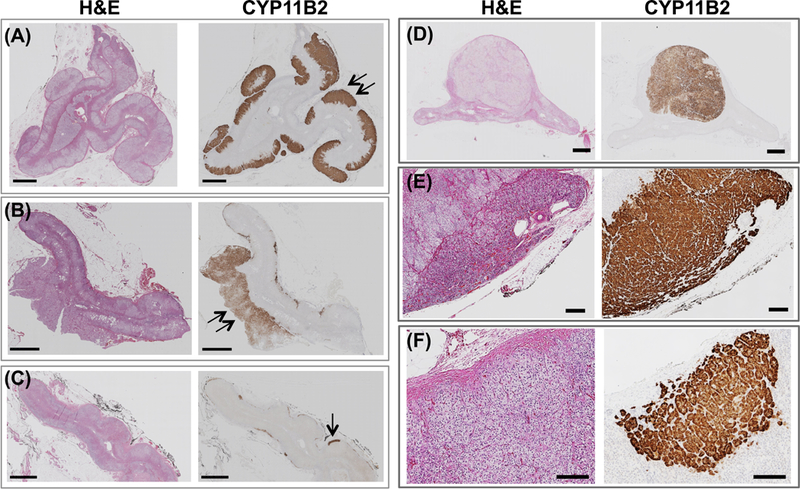
The diverse histopathology of resected adrenals in this cohort is shown with H&E staining and CYP11B2 immunostaining as indicated. Panels A-C: adrenals without a functional adenoma showing the various histopathology of this subgroup. APCC indicated with a single arrow, hyperplasia with a double arrow. Scale bar represents 2 mm. These 3 adrenals were from patients with post-surgical absent or partial biochemical success; Panels D-E: examples of histopathological features classified in this study: solitary functional adenoma (Panel D), hyperplasia of the zona glomerulosa (Panel E) and an APCC (Panel F). Scale bars represent 200 μm.
Table 1.
Histopathology of adrenals from patients stratified by biochemical outcome after adrenalectomy
| VARIABLE | Total cohort N (%) |
BIOCHEMICAL OUTCOME |
P-value |
|
|---|---|---|---|---|
| A + P | C | |||
| Total | 95 (100 %) | 43 (45 %) | 52 (55 %) | |
| Solitary functional adenoma | 60 (63 %) | 19 (44 %) | 41 (79 %) | < 0.001 |
| Normal appearing adjacent cortex | 29 (48 %) | 9 (47 %) | 20 (49 %) | 0.919 |
| Hyperplasia | 9 (15 %) | 5 (26 %) | 4 (10 %) | 0.200 |
| APCC | 30 (50 %) | 10 (53 %) | 20 (49 %) | 0.781 |
| No functional adenoma | 35 (37 %) | 24 (56 %) | 11 (21 %) | < 0.001 |
| CYP11B2 negative adenoma | 9 (26 %) | 7 (29 %) | 2 (18 %) | 0.403 |
| Hyperplasia | 23 (66 %) | 16 (67 %) | 7 (64 %) | 0.576 |
| APCC | 27 (77 %) | 18 (75 %) | 9 (81 %) | 0.508 |
| Hyperplasia | 32 (34 %) | 21 (49 %) | 11 (21 %) | 0.004 |
| APCC | 57 (60 %) | 28 (65 %) | 29 (56 %) | 0.355 |
| APCC number (sample section) | 3.2 ± 2.9 | 3.2 ± 3.1 | 3.3 ± 2.8 | 0.641 |
APA, aldosterone-producing adenoma; A, P and C refer to absent, partial and complete biochemical success after surgery; APCC, aldosterone-producing cell cluster; CYP11B2, aldosterone synthase. Values indicate absolute numbers with proportions in parenthesis (%) or average numbers ± SD. P values were calculated using a χ² or Fisher’s exact tests or Mann-Whitney tests as appropriate.
Adrenals without a functional adenoma (without CYP11B2-positive immunostaining) comprised 37% of the total sample set (35 of 95 samples) with a higher prevalence noted in the absent + partial compared with the complete biochemical success group (56%, 24 of 43 versus 21%, 11 of 52, P < 0.001). These adrenals showed a combination of mainly APCC and cortical hyperplasia but non-functional adenomas (CYP11B2-negative), without a concurrent functional adenoma, were present in 9 adrenals with 7 in the absent + partial group and 2 in the complete biochemical success group (Table 1).
Adrenals from the absent + partial group had a higher prevalence of cortical hyperplasia (49% versus 21%, P = 0.004) but no differences were observed in the proportion of adrenals with APCC or the average number of APCC per tissue section compared with the complete biochemical success group (Table 1).
LC-MS/MS peripheral venous steroid profiling
There were no significant differences in concentrations of peripheral venous adrenal steroids according to histopathological feature (Table S6). Linear discriminant analyses of adrenal steroids correctly classified the presence or absence of solitary functional adenoma, hyperplasia or APCC in 84% - 88% of samples (misclassification rate, 0.12–0.16) (Figure 3B) and decision tree analysis using steroids selected from estimate prediction certainties improved the accuracy of prediction to 91% - 93% (misclassification rate, 0.07–0.09) (Figure 3C-F).
Figure 3. Classification of adrenal histopathology in PA according to peripheral venous steroid concentrations.
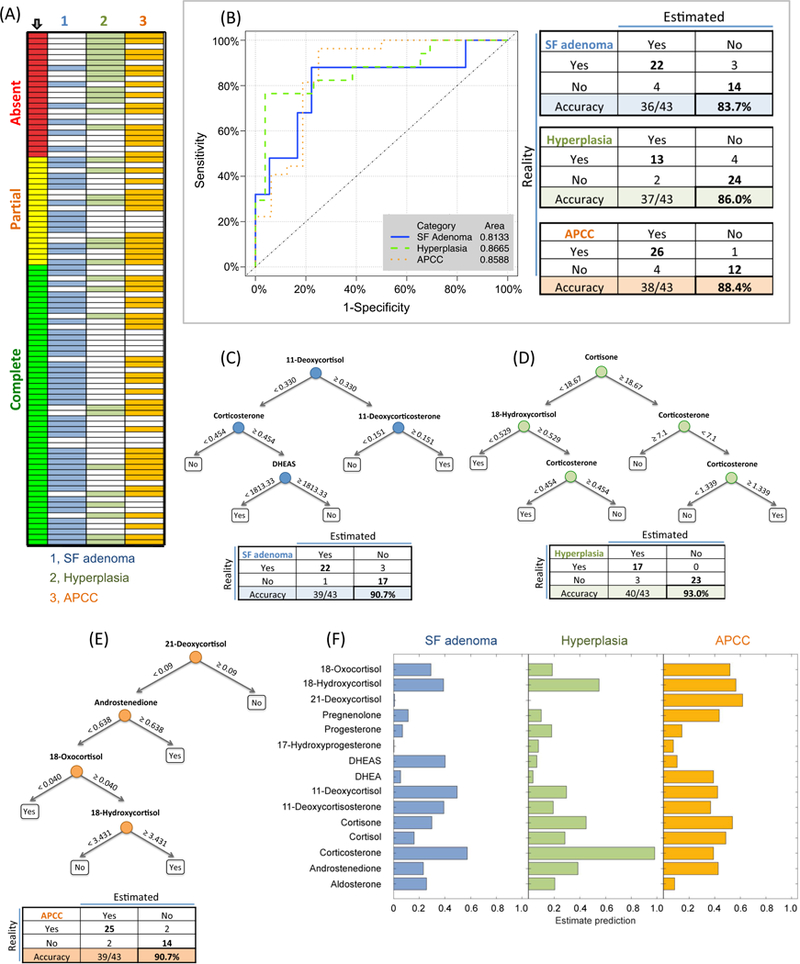
The distribution of histopathological features (1, solitary functional adenoma; 2, hyperplasia; 3, APCC, aldosterone-producing cell clusters) stratified for biochemical outcome (indicated by an arrow) is represented in Panel A. Linear discriminant analyses using peripheral venous steroid concentrations was used to generate receiver-operating characteristic (ROC) curves with areas under the curves (inset) and tables showing the real and estimated presence (yes) or absence (no) of solitary functional adenomas (SF adenomas), hyperplasia and APCCs (Panel B). The steroids used in each model are shown in Table S9 with linear discriminant coefficients and cut-offs for prediction of the presence of SF adenoma, hyperplasia or APCC. Decision tree analyses improved the prediction of histopathology by steroid measurements: decision trees with numbers indicating steroid concentrations in ng/mL predicting the presence (yes) or absence (no) of a solitary functional (SF) adenoma (Panel C); hyperplasia (Panel D) and APCCs (panel E) are shown with an accompanying table with the real and estimated presence (yes) and absence (no) of each histopathological feature. Steroids used for decision tree analysis were selected from their estimated predictive performance (Panel F).
For the absent + partial group, concentrations of aldosterone were higher in peripheral venous plasma compared with the bilateral PA group (P <0.001) and cortisone and 11-deoxycortisol concentrations were significantly higher than in the complete group (P = 0.021 and P = 0.017, respectively) (Table S7). Discriminant analysis correctly predicted biochemical outcome after adrenalectomy and diagnosis of bilateral PA in 53 of 70 patients (76%) (Figure 4A). Decision tree analysis improved the correct classification to 60 of 70 cases (86%, misclassification rate, 0.14): all 15 patients with an absent + partial biochemical outcome after surgery were correctly predicted albeit 5 patients with complete biochemical success were incorrectly classified with an absent or partial biochemical outcome (Figure 4C). Linear discriminant analysis and decision trees of steroid measurements resulted in a higher accuracy for the classification of biochemical outcomes compared with predictive models using AVS parameters (lateralisation index and contralateral ratio) (Table S8).
Figure 4. Classification of biochemical outcomes after adrenalectomy and diagnosis of bilateral adrenal hyperplasia according to peripheral venous steroid concentrations.
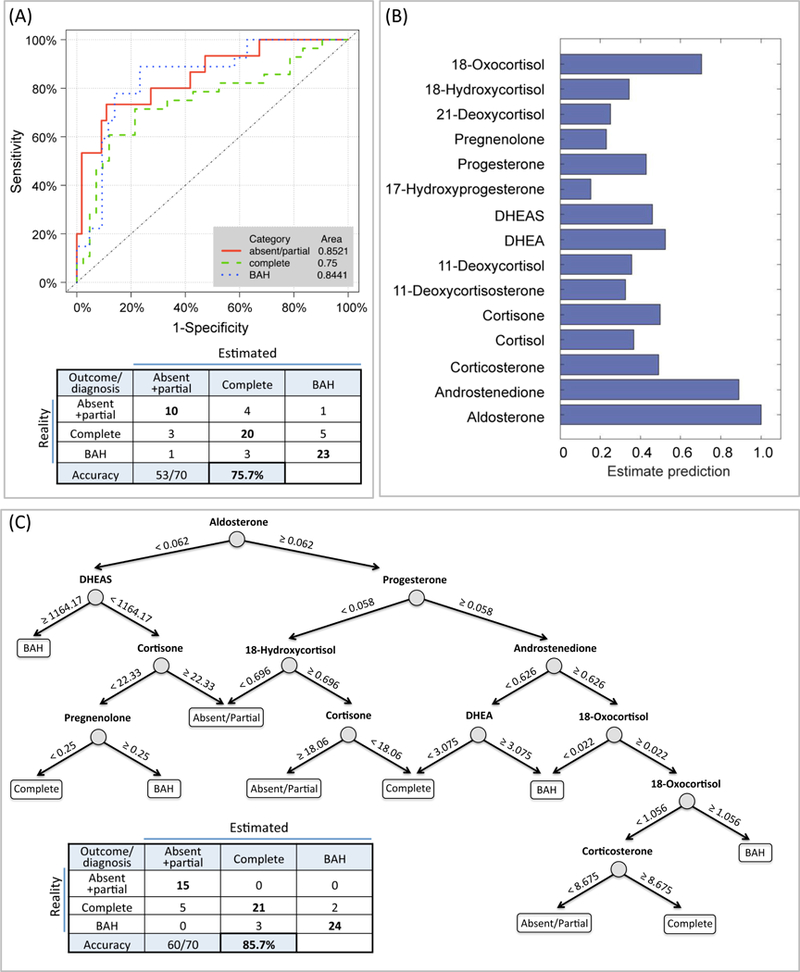
Discriminant analysis with 9 steroids (androstenedione, cortisol, cortisone, 11-deoxycortisol, DHEA, DHEA sulphate, pregnenolone, 18-hydroxycortisol and 18-oxocortisol) generated receiver-operating characteristic (ROC) curves with areas under the curves (inset) and a table showing real and estimated biochemical outcomes (absent + partial and complete biochemical success) and diagnosis of bilateral PA (BAH) (Panel A). The steroids used in each model are shown in Table S9 with linear discriminant coefficients and cut-offs for prediction of absent + partial biochemical success. Decision tree analysis using steroids based on estimated predictive performance (Panel B) improved the correct classification of biochemical outcomes and diagnosis of bilateral PA. Numbers in the decision tree indicate steroid concentrations in ng/mL (Panel C).
Discussion
We report the histopathology and peripheral venous steroid profiles associated with biochemical outcome after adrenalectomy for unilateral PA. In a multicentre international study with differentiation of unilateral from bilateral PA by AVS, adrenalectomy for unilateral PA resulted in biochemical cure (complete biochemical success) in 94% of patients thereby indicating the correct diagnosis and appropriate treatment.25,27,28 Partial or absent biochemical success classifies patients with bilateral aldosterone excess who were presumably misdiagnosed as unilateral (instead of bilateral) pre-operatively.25 In the present study, the lower lateralisation index and the higher contralateral ratio of patients with absent + partial biochemical outcomes would be consistent with the higher aldosterone production from the contralateral adrenal compared with the complete biochemical success group despite a similar incidence of abnormalities detected by adrenal imaging.
The development of specific antibodies to CYP11B2 and CYP11B1 has revealed the complex heterogeneity of adrenal histopathology in PA.11,12,17 Immunostaining of CYP11B2 identifies cells comprising the likely origin of constitutive aldosterone production and classifies diverse histopathological subtypes of PA.15–17 Unilateral aldosterone excess is usually produced from an APA29 frequently accompanied by APCCs in the hyperplastic adjacent cortical tissue.15,19
In a multicentre study of patients diagnosed with unilateral PA, Åkerström et al.21 reported adenomas without associated hyperplasia in 287 of 348 (82%), adenomas with associated hyperplasia in 52 of 348 (15%) and hyperplasia with macro- or micronodules in 9 of 348 (3%) of sample specimens. A higher prevalence of cortical hyperplasia has been reported by others30,31 with multinodular hyperplasia or diffuse hyperplasia present in 54 and 12 resected adrenals, respectively, from 122 patients with post-surgical biochemical cure.31 No association of histopathology with persistent PA was found in 6 patients with persistent PA and recurrent PA reported in 3 of 79 patients with long-term follow-up data who were previously biochemically cured.31 Few studies have addressed the histopathology of bilateral PA. A study on 25 resected adrenals from patients with undetectable abnormalities by CT scanning included 13 adrenals from patients with bilateral PA that displayed an increased incidence of diffuse functional hyperplasia compared with adrenals from unilateral PA.16 In a large sample set of 43 resected adrenals from patients with absent + partial biochemical success after adrenalectomy for unilateral PA, we show an increased prevalence of cortical hyperplasia in adrenals in agreement with the proposal that nodular hyperplasia may comprise a risk factor for persistent aldosteronism after surgery.32 We also show the increased incidence of solitary functional adenomas (APAs) in the complete biochemical success group. Functional adenomas were often associated with APCCs in the adjacent cortex, more frequently than with hyperplasia. There were no perceivable differences in the prevalence or numbers of APCC per sample section between biochemical outcomes although the potential association of somatic mutations in APCCs with biochemical outcomes cannot be excluded.
LC-MS/MS measurements of plasma adrenal steroids predicted the presence or absence of a solitary functional adenoma, hyperplasia or APCCs. The association of histopathology in PA with adrenal steroid concentrations ostensibly underlies or contributes to the classification of post-surgical biochemical outcomes by steroid profiling which herein identified all patients with absent + partial biochemical success from patients with biochemical cure or from non-operated patients.
Strengths and limitations of the study
The strengths of the study are the large multicentre sample cohort comprising the largest reported sample set of resected adrenals from patients with post-surgical absent + partial biochemical success that were matched with a control group (complete biochemical success) and the strict standardised PASO criteria used to assess biochemical outcomes. A limitation is the small size of the study population used for steroid profiling in particular the number of patients with absent + partial biochemical success. Nonetheless, in the Munich cohort, adrenal steroid concentrations in peripheral plasma correctly predicted post-surgical absent + partial biochemical success in all 15 patients, an association possibly driven by the underlying adrenal histopathological features. The 5 of 28 patients with biochemical cure, predicted by steroid profiling to have absent + partial biochemical success at 6–12 months post-adrenalectomy, potentially comprise patients who develop long-term recurrent PA.30,31 A prospective validation study with long-term follow-up should address this possibility.
Perspectives
The histopathology of adrenals from patients who are biochemically cured after adrenalectomy for unilateral PA is quantitatively different from the adrenals from patients with absent + partial biochemical success. The absence of a functional adenoma at pathology or the presence of cortical hyperplasia may indicate patients in whom follow-up, including assessment of biochemical parameters, should be considered mandatory. Measurements of adrenal steroids in peripheral venous plasma are associated with adrenal histopathology and biochemical outcomes after surgery. This highlights the potential application of steroid profiling to guide the decision to perform surgery in patients who are being considered for adrenalectomy on the basis of a pre-operative diagnosis of unilateral PA.
Supplementary Material
Novelty and Significance:
- 1) What is New?
- The absence of a functional solitary adenoma or the presence of cortical hyperplasia is associated with partial + absent biochemical success after surgery for unilateral PA
- Steroid profiling was associated with the presence or abence of solitary functional adenomas, cortical hyperplasia and APCCs
- Steroid profiling classifies the majority of patients according to complete or partial + absent biochemical success after unilateral adrenalectomy
- 2) What is Relevant?
- Peripheral venous steroid profiling may be useful to select patients with a pre-operative diagnosis of unilateral PA for surgery based on expectations of biochemical outcome
Acknowledgements
We gratefully acknowledge Petra Rank for help with immunohistochemistry.
Sources of Funding
This work was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No [694913] to M. Reincke) and by the Deutsche Forschungsgemeinschaft (DFG) (within the CRC/Transregio 205/1 “The Adrenal: Central Relay in Health and Disease” to F. Beuschlein, G. Eisenhofer, S. Hahner, J.W.M. Lenders, M. Peitzsch, M. Reincke and T.A. Williams; and grants RE 752/20–1 to M. Reincke and grants BE 2177/13–1 and BE 2177/18–1 to F. Beuschlein) and the Else Kröner-Fresenius Stiftung in support of the German Conns Registry-Else-Kröner Hyperaldosteronism Registry (2013_A182 and 2015_A171 to M. Reincke). C. E. Gomez-Sanchez is supported by the National Heart, Lung and Blood Institute grant R01 HL27255 and the National Institute of General Medical Sciences grant U54 GM115428. This study was also supported by the Ministry of Health of Slovenia (Tertiary Care Scientific grant number 20170018 of the University Medical Centre Ljubljana to T. Kocjan), a grant from MIUR (ex-60% 2016–2017 to P. Mulatero), the Japan Agency for Medical Research and Development (AMED) for the Practical Research Project for Rare/Intractable Disease (grants JP17ek0109122 and JP18ek0109352 to M. Naruse) and a Grant for Research on Intractable Diseases provided by the Japanese Ministry of Health, Labour and Welfare (to T. Nishikawa).
Footnotes
Conflicts of Interest/Disclosures
None
References
- 1).Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF Jr. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101:1889–1916. [DOI] [PubMed] [Google Scholar]
- 2).Sechi LA, Novello M, Lapenna R, Baroselli S, Nadalini E, Colussi GL, Catena C. Long-term renal outcomes in patients with primary aldosteronism. JAMA. 2006;295:2638–2645. [DOI] [PubMed] [Google Scholar]
- 3).Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A, Crudo V, Burrello J, Milan A, Rabbia F, Veglio F. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98:4826–4833. [DOI] [PubMed] [Google Scholar]
- 4).Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, Mulatero P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50. [DOI] [PubMed] [Google Scholar]
- 5).Burton TJ, Mackenzie IS, Balan K, Koo B, Bird N, Soloviev DV, Azizan EA, Aigbirhio F, Gurnell M, Brown MJ. Evaluation of the sensitivity and specificity of (11)C-metomidate positron emission tomography (PET)-CT for lateralizing aldosterone secretion by Conn’s adenomas. J Clin Endocrinol Metab. 2012;97:100–109. [DOI] [PubMed] [Google Scholar]
- 6).Abe T, Naruse M, Young WF Jr, Kobashi N, Doi Y, Izawa A, Akama K, Okumura Y, Ikenaga M, Kimura H, Saji H, Mukai K, Matsumoto H. A Novel CYP11B2-Specific Imaging Agent for Detection of Unilateral Subtypes of Primary Aldosteronism.J Clin Endocrinol Metab. 2016;101:1008–1015. [DOI] [PubMed] [Google Scholar]
- 7).Heinze B, Fuss CT, Mulatero P, et al. , Targeting CXCR4 (CXC Chemokine Receptor Type 4) for Molecular Imaging of Aldosterone-Producing Adenoma. Hypertension. 2018;71:317–325. [DOI] [PubMed] [Google Scholar]
- 8).Satoh F, Morimoto R, Ono Y, et al. , Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension. 2015;65:1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Williams TA, Peitzsch M, Dietz AS, Dekkers T, Bidlingmaier M, Riester A, Treitl M, Rhayem Y, Beuschlein F, Lenders JW, Deinum J, Eisenhofer G, Reincke M.Genotype-Specific Steroid Profiles Associated With Aldosterone-Producing Adenomas. Hypertension. 2016;67:139–145. [DOI] [PubMed] [Google Scholar]
- 10).Eisenhofer G, Dekkers T, Peitzsch M, Dietz AS, Bidlingmaier M, Treitl M, Williams TA, Bornstein SR, Haase M, Rump LC, Willenberg HS, Beuschlein F, Deinum J, Lenders JW, Reincke M. Mass Spectrometry-Based Adrenal and Peripheral Venous Steroid Profiling for Subtyping Primary Aldosteronism. Clin Chem. 2016;62:514–524. [DOI] [PubMed] [Google Scholar]
- 11).Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, Ogishima T, Suematsu M, Mukai K. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. 2010;95:2296–305. [DOI] [PubMed] [Google Scholar]
- 12).Gomez-Sanchez CE, Qi X, Velarde-Miranda C, Plonczynski MW, Parker CR, Rainey W, Satoh F, Maekawa T, Nakamura Y, Sasano H, Gomez-Sanchez EP. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol. 2014;383:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Uchida T, Nishimoto K, Fukumura Y, et al. , Disorganized Steroidogenesis in Adrenocortical Carcinoma, a Case Study. Endocr Pathol. 2017;28:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Gomez-Sanchez CE, Gomez-Sanchez EP. Immunohistochemistry of the adrenal in primary aldosteronism. Curr Opin Endocrinol Diabetes Obes. 2016;23:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Nishimoto K, Koga M, Seki T, et al. , Immunohistochemistry of aldosterone synthase leads the way to the pathogenesis of primary aldosteronism. Mol Cell Endocrinol. 2017;441:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Yamazaki Y, Nakamura Y, Omata K, Ise K, Tezuka Y, Ono Y, Morimoto R, Nozawa Y, Gomez-Sanchez CE, Tomlins SA, Rainey WE, Ito S, Satoh F, Sasano H. Histopathological Classification of Cross-Sectional Image-Negative Hyperaldosteronism. J Clin Endocrinol Metab. 2017;102:1182–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Gomez-Sanchez CE, Kuppusamy M, Reincke M, Williams TA. Disordered CYP11B2 Expression in Primary Aldosteronism. Horm Metab Res. 2017;49:957–962. [DOI] [PubMed] [Google Scholar]
- 18).Gomez-Sanchez CE, Rossi GP, Fallo F, Mannelli M. Progress in primary aldosteronism: present challenges and perspectives. Horm Metab Res. 2010;42:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Boulkroun S, Samson-Couterie B, Dzib JF, Lefebvre H, Louiset E, Amar L, Plouin PF, Lalli E, Jeunemaitre X, Benecke A, Meatchi T, Zennaro MC. Adrenal cortex remodeling and functional zona glomerulosa hyperplasia in primary aldosteronism. Hypertension. 2010;56:885–892. [DOI] [PubMed] [Google Scholar]
- 20).Dekkers T, ter Meer M, Lenders JW, Hermus AR, Schultze Kool L, Langenhuijsen JF, Nishimoto K, Ogishima T, Mukai K, Azizan EA, Tops B, Deinum J, Küsters B. Adrenal nodularity and somatic mutations in primary aldosteronism: one node is the culprit? J Clin Endocrinol Metab. 2014;99:E1341–E1351. [DOI] [PubMed] [Google Scholar]
- 21).Åkerström T, Crona J, Delgado Verdugo A, et al. , Comprehensive re-sequencing of adrenal aldosterone producing lesions reveal three somatic mutations near the KCNJ5 potassium channel selectivity filter. PLoS One 2012;7:e41926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Nishimoto K, Tomlins SA, Kuick R, Cani AK, Giordano TJ, Hovelson DH, Liu CJ, Sanjanwala AR, Edwards MA, Gomez-Sanchez CE, Nanba K, Rainey WE. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci U S A. 2015;112:E4591–E4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Omata K, Tomlins SA, Rainey WE. Aldosterone-Producing Cell Clusters in Normal and Pathological States. Horm Metab Res. 2017;49:951–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Nishimoto K, Seki T, Kurihara I, Yokota K, Omura M, Nishikawa T, Shibata H, Kosaka T, Oya M, Suematsu M, Mukai K. Case Report: Nodule Development From Subcapsular Aldosterone-Producing Cell Clusters Causes Hyperaldosteronism. J Clin Endocrinol Metab. 2016;101:6–9. [DOI] [PubMed] [Google Scholar]
- 25).Williams TA, Lenders JWM, Mulatero P, et al. , Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5:689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Nishikawa T, Omura M, Satoh F, Shibata H, Takahashi K, Tamura N, Tanabe A; Task Force Committee on Primary Aldosteronism, The Japan Endocrine Society. Guidelines for the diagnosis and treatment of primary aldosteronism--the Japan Endocrine Society 2009. Endocr J. 2011;58:711–721. [DOI] [PubMed] [Google Scholar]
- 27).Muth A, Ragnarsson O, Johannsson G, Wängberg B. Systematic review of surgery and outcomes in patients with primary aldosteronism. Br J Surg. 2015;102:307–317. [DOI] [PubMed] [Google Scholar]
- 28).Rutherford JC, Taylor WL, Stowasser M, Gordon RD. Success of surgery for primary aldosteronism judged by residual autonomous aldosterone production. World J Surg. 1998;22:1243–1245. [DOI] [PubMed] [Google Scholar]
- 29).Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery 2004;136:1227–1235. [DOI] [PubMed] [Google Scholar]
- 30).Iacobone M, Citton M, Viel G, Boetto R, Bonadio I, Tropea S, Mantero F, Rossi GP, Fassina A, Nitti D, Favia G. Unilateral adrenal hyperplasia: a novel cause of surgically correctable primary hyperaldosteronism. Surgery 2012;152:1248–1255. [DOI] [PubMed] [Google Scholar]
- 31).Citton M, Viel G, Rossi GP, Mantero F, Nitti D, Iacobone M. Outcome of surgical treatment of primary aldosteronism. Langenbecks Arch Surg. 2015;400:325–331. [DOI] [PubMed] [Google Scholar]
- 32).Lee J, Oltmann SC, Woodruff SL, Nwariaku FE, Holt SA, Rabaglia JL. Contralateral adrenal abnormalities in Conn’s syndrome. J Surg Res. 2016;200:183–188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


