Abstract
Oxytocin (OT),a neuropeptide that acts in the brain as a neuromodulator,has been long known to shape maternal physiology and behavior in mammals, however its role in regulating social cognition and behavior in primates has come to the forefront only in the recent decade. Many of the current perspectives on the role of OT in modulating social behavior emerged first from studies in rodents, where invasive techniques with a high degree of precision have permitted the mechanistic dissection of OT-related behaviors, as well as their underlying neural circuits in exquisite detail. In parallel, behavioral and imaging studies in humans have suggested that brain OT may similarly influence human social behavior and neural activity. These studies in rodents and humans have spurred interest in the therapeutic potential of targeting the OT system to remedy deficits in social cognition and behavior that are present across numerous psychiatric disorders. Yet there remains a tremendous gap in our mechanistic understanding of the influence of brain OT on social neural circuitry between rodents and man. In fact, very little is known regarding the neural mechanisms by which exogenous or endogenous OT influences human social cognition, limiting its therapeutic potential. Here we discuss how non-human primates (NHPs) are uniquely positioned to now bridge the gaps in knowledge provided by the precise circuit-level approaches widely used in rodent models and the behavioral, imaging, and clinical studies in humans. This review provides a perspective on what has been achieved, and what can be expected from exploring the role of OT in shaping social behaviors in NHPs in the coming years.
Keywords: non-human primates, oxytocin, social behavior
1 |. INTRODUCTION
The goal of this review is to provide a brief summary of the studies that have examined the role of oxytocin (OT) in modulating social cognition and behavior in mammals, with an emphasis on the potential of nonhuman primate (NHP) social behavior for enhancing our understanding of OT social neurobiology. Historically, much of our understanding of the role of OT, beyond reproductive and offspring-rearing behaviors, started with significant discoveries in rodents (reviewed below), which in turn motivated a search for similar effects in NHPs as well as in humans. Current work in NHPs is uniquely positioned to bridge the divide between levels of analyses available in human and rodent research; specifically, the behavioral and functional imaging data in humans and the large body of mechanistic research now routinely performed in rodents. We propose that knowledge gained from OT research on NHPs may be more translatable by virtue of the behavioral and anatomical similarities between NHPs and humans, relative to rodents. This advantage combined with the accessibility of the brain in NHPs (that allows invasive manipulations not possible in humans) makes NHPs a critical intermediary animal model for exploring the social-behavioral effects of OT.
The role of OT as a neuromodulator with specific social-behavioral functions emerged from pharmacological or genetic manipulations of central OT levels in rodents, beginning with studies investigating its role in regulating the onset of maternal behaviors in rats and mother-infant bonding in sheep (Rilling & Young, 2014). For example, virgin female rats normally ignore or attack pups,yet when they receive an intra-ventricular infusion of OT into the brain they display maternal behaviors (Pedersen & Prange, 1979). The socially monogamous prairie vole (Microtus ochrogaster) later emerged as an excellent model for examining the interactions between OT and other complex social behaviors, including pair bonding, alloparental care, and empathy, each of which may have evolutionary roots in OT's functions in maternal behavior. Unlike other species of voles, such as the meadow vole (Microtus pennsylvanicus), monogamous prairie voles are highly social and form long-term, socially monogamous relationships with their mates. Prairie voles express higher densities of OT receptors (OXTR) in regions of the brain involved in reward and reinforcement than other species of voles that do not form long-term, socially monogamous relationships (McGraw & Young, 2010; Young & Wang, 2004). Pair-bonding in these monogamous voles is critically dependent on the action of OT in specific brain circuits, including the nucleus accumbens and prefrontal cortex (Donaldson & Young, 2008; Johnson & Young, 2015, 2017; Johnson et al., 2016; Young & Wang, 2004). The social functions of OT are not restricted to reproductive behaviors such as pair bonding and parental care. For example, when OT function is disrupted in the medial amygdala of mice they fail to recognize familiar conspecifics despite repeated previous exposures (Ferguson, Aldag, Insel, & Young, 2001). The explanatory mechanisms for these observations started to emerge as a result of recent breakthroughs in gene-specific manipulations, for example, optogenetics. For instance, virgin female mice normally do not respond to the ultrasonic distress calls of pups as quickly as mothers. However, when these distress calls were paired with optogenetically evoked endogenous OT release into the auditory cortex, virgin females quickly responded to the calls, and retrieved the pups. The optogenetically stimulated release of OT balanced the magnitude and timing of inhibitory and excitatory responses in the auditory cortex, resulting in a pattern of activity that resembles responses found in experienced mothers (Marlin, Mitre, D'amour, Chao, & Froemke, 2015). This, and similar experiments in rodents examining olfactory processing (Oettl et al., 2016)have ledtothe hypothesis that OT tunes inhibitory circuits to increase the salience (signal/noise ratio) of important social stimuli (Marlin & Froemke, 2017; Mitre, Minder, Morina, Chao, & Froemke, 2017).
Natural variation in OXTR expression contributes to individual variation in social behavioral phenotypes as well. For example, OXTR density is highly variable among prairie voles and is tightly regulated by genetic polymorphisms in the OXTR gene (King, Walum, Inoue, Eyrich, & Young, 2016). This variation in density has been linked to individual differences in alloparental behavior (Olazábal & Young, 2006a, 2006b), pair-bonding (King et al., 2016; Ross, Cole, et al., 2009; Ross, Freeman, et al., 2009), and resilience to early-life social neglect (Barrett, Arambula, & Young, 2015). Striatal OT receptors also mediate the onset of depressive-like “grieving” behavior in prairie voles following loss of a partner (Bosch et al., 2016), a phenomenon that has been proposed to maintain social bonds (Bosch & Young, 2017; Pohl, Young, & Bosch, 2018). OXTR signaling coordinates brain activity across a social salience network (involving the nucleus accumbens, amygdala, prefrontal cortex, and other brain regions) during mating, facilitating the flow of social information across the network in prairie voles, and nucleus accumbens OXTRs appear to serve as a hub for facilitating coordinated activity across the social salience network (Johnson et al., 2016; Johnson, Walum, Xiao, Riefkohl, & Young, 2017). Finally, OXTRs in the anterior cingulate cortex, a region implicated in human empathy, mediate empathy-based consoling behavior in male and female prairie voles, a behavior once thought to exist only in primates (Burkett et al., 2016). Continuing research in rodents, which enable the use of precise circuit-level and genetic manipulations, is essential to developing a mechanistic understanding of the role of OT in the brain. However, these studies explore innate behaviors (e.g., pair-bonding) that are often species-specific. Furthermore, many neuroanatomical differences exist between rodents and primates (Murray, Wise, & Graham, 2017; Phillips et al., 2014; Wise, 2008), providing additional obstacles in translating results obtained in rodents to primates.
Parallel work in humans has demonstrated several important roles of OT in social behavior. Genetic polymorphisms in the human OT receptor gene have suggested a role for OT in face recognition abilities, reminiscent to the olfactory-based social recognition deficits in OT mutant mice (Skuse et al., 2014). Peripheral administration of OT increased the time healthy adults spent viewing the eyes of others (Auyeung et al., 2015; Guastella, Mitchell, & Dadds, 2008), enhanced the ability of subjects to discriminate facial expressions in others (Domes, Heinrichs, Michel, Berger, & Herpertz, 2007; Lischke et al., 2012), promoted trust (Kosfeld, Heinrichs, Zak, Fischbacher, & Fehr, 2005), and improved socially reinforced learning (Hurlemann et al., 2010), although the robustness of these pharmacological studies in healthy subjects has recently been called into question (Walum, Waldman, & Young, 2015). Both functional magnetic resonance imaging (fMRI) in healthy humans and behavioral studies of patients with selective calcifications of the amygdala (Urbach-Wiethe disease) have implicated the amygdala as an anatomical substrate of OTmediated behavioral changes (Domes, Steiner, Porges, & Heinrichs, 2013; Gamer, 2010; Hurlemann et al., 2010; Kirsch et al., 2005; Tully, Gabay, Brown, Murphy, & Blackwood, 2018). Compared to rodent studies, however, these studies only localize the site of increased neural activation, but cannot reveal the mechanism by which OT alters social behavior, for example, the location of OT acting on OXTR to modulate neural activity. These studies in both animal models and humans mentioned above, as well as many others, have fueled enthusiasm for targeting the OT system to improve various aspects of social cognition in psychiatric disorders such as autism (ShamayTsoory & Young, 2016; Tully et al., 2018; Watanabe et al., 2015; Yatawara, Einfeld, Hickie, Davenport, & Guastella, 2016; Young, 2015).
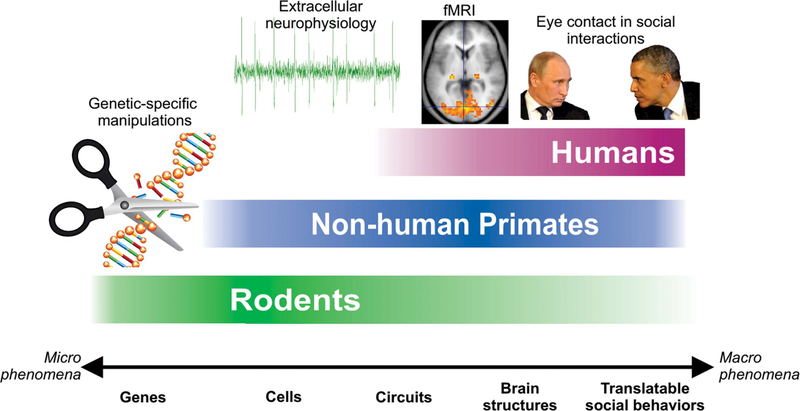
But there is a tremendous opportunity to begin to close the gap in precision and circuit-level knowledge of OT action between rodents and humans through the effective use of NHPs in combination with highly precise existing and emerging neuroscience techniques, including electrophysiology, optogenetics, and even gene editing techniques such as CRISPR. Compared to rodents, NHPs can perform tasks with cognitive demands comparable to humans, and can be tested on speciesspecific social behaviors that have human equivalents (e.g., communication with facial expressions or eye contact) (Figure 1). Moreover, NHPs are the species of choice for certain invasive procedures, not feasible in healthy human subjects, such as recording both extracellular single unit activity and local field potentials in the context of primate-specific social behaviors (Chang, 2017). The advantages of NHP models for studying OT have been previously outlined by Chang and Platt (2014). While techniques allowing gene-specific manipulations, such as inducible geneknockouts or optogenetic stimulation of genetically-specific neuronal populations are not yet widely available in NHPs, ongoing efforts already show promise (Eldridge et al., 2016; Stauffer et al., 2016). In the following paragraphs we review the outcome of experimental work with NHPs, and highlight possible future directions that either fill gaps in, or expand, our current understanding of OT.
FIGURE 1.
A diagram illustrating the experimental approaches and levels of analysis available in rodents, NHPs, and humans for studying the effects and mechanisms of OT
2 |. STUDIES OF OXYTOCIN IN NON-HUMAN PRIMATES
2.1 |. Peripheral oxytocin administration elevates OT levels in the primate brain
Given that the OT molecule is too large to efficiently and passively move through the blood-brain barrier, it is critical to establish whether peripheral administration of OT influences the concentrations of OT in the brain. Furthermore, the method of administration may also influence central concentrations of OT. The two most common methods of administration in primates are intranasal sprays (IN), and aerosolized inhalation via nebulization (AE). To determine whether peripheral OT administration penetrates the brain, and by proxy the cerebrospinal fluid (CSF) in NHPs, Chang and Platt (2014) measured the levels of OT in the CSF of rhesus monkeys (Macaca mulatta) that inhaled 24IU of OT by AE administration. They found a 2.5-fold (compared to vehicle) increase in the levels of OT in the CSF, 30 min after OT administration (Chang, Barter, Ebitz, Watson, & Platt, 2012). A subsequent study by Modi, Connor-Stroud, Landgraf, Young, and Parr (2014) compared delivery of OT via both AE and IN on levels of OT in the CSF, finding that AE treatments resulted in the greatest increase of OT in the CSF, however this was only significant compared to baseline measurements and not to the vehicle controls. A more comprehensive approach was taken by Dal Monte, Noble, Costa, and Averbeck (2014) who set up a balanced design to compare IN and AE administration of OT in a large number of animals. They found that both methods robustly increased OT levels in the brain at 40 min post-treatment delays (Dal Monte, Noble, Turchi, Cummins, & Averbeck, 2014). The first study examining female rhesus monkeys also found that IN treatments (as well as intravenous administration) of OT significantly raised CSF OT levels (Freeman et al., 2016). This work also contains a detailed table and discussion clearly highlighting the methodological differences between the studies carried out to that point. The strongest evidence for peripherally administered OT crossing into the brain was brought by a recent study that demonstrated that deuterated-OT (isotopically labeled) crossed from the periphery into the CSF (Lee et al., 2018). Importantly, this novel assay allowed Lee and colleagues to differentiate between the levels of endogenous and exogenous OT in the CSF. They concluded that IN treatment of OT does not lead to central release of endogenous OT, instead the administered deuterated-OT penetrates into the CSF (Lee et al., 2018) crossing the blood-brain barrier via a yet unknown mechanism. Taken together with similar results from studies performed in humans (Gossen et al., 2012; Striepens et al., 2013), these results clearly suggest that OT enters the CSF of primates and elevates OT levels in the brain when peripherally administered at appropriate doses.
2.2 |. Oxytocin receptors are expressed sparsely and in selective areas of the primate brain
Once in the brain, the neuroanatomical site of action for OT remains unclear. The distribution of OXTRs has been well characterized across numerous roden species, where generally OXTRs are widely distributed across the brain, particularly in areas that process olfactory information (Freeman & Young, 2016). This mapping enabled subsequent rodent studies to perform site-specific pharmacological manipulations in regions where OXTRs were expressed to delineate circuits where OT affects behavior (Bosch et al., 2016; Burkett et al., 2016). Early attempts to localize OXTRs in NHPs were inconclusive (Toloczko, Young, & Insel, 1997) due to the cross-reactivity of the OT radioligand used for OXTR autoradiography and structurally similar arginine vasopressin receptor (AVPR1A) (Manning et al., 2012). In situ hybridization has permitted a more selective localization of both OXTR and AVPR expressing neurons (i.e., mRNA localization) in the NHP brain, however where receptor proteins are localized on these neurons (somatodendrically or on axonal projections) remains unclear. In other words, localization of OXTR mRNA with in situ hybridization identifies the location of the cell bodies synthesizing OXTR, but the OXTR protein could be on axon terminals of those cells in distant brain regions as has been previously shown in mice (Dölen, Darvishzadeh, Huang, & Malenka, 2013). Just as rodents express OXTRs in the brain regions dedicated to their primary sensory modality (olfaction), NHPs express OXTR, and AVPR in regions devoted to attention and visual and/or multisensory processing. Thus an intriguing characteristic of the OXTR system is the phylogenetic plasticity of the expression pattern and distribution in the brain, which may provide a mechanism of increased diversity in socio-sexual behaviors across and within species in rodents and in primates (Johnson & Young, 2017).
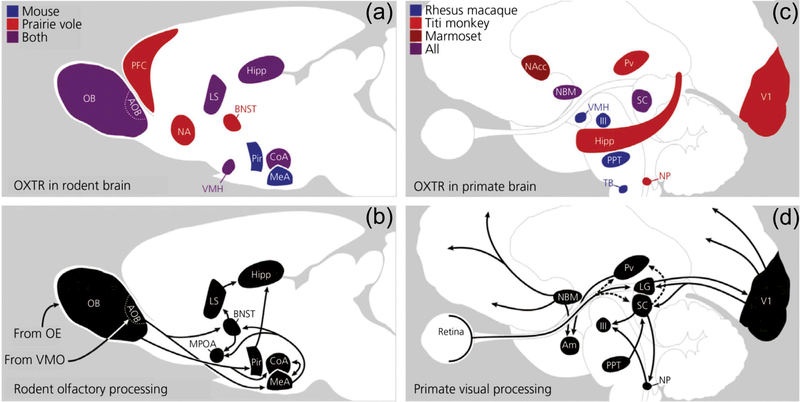
In rhesus monkeys OXTR expression is most evident in the nucleus basalis of Meynert (NBM), pedunculopontine tegmental nucleus (PPT), superficial gray layer of the superior colliculus, the trapezoid body, and the ventromedial hypothalamus (Freeman, Inoue, Smith, Goodman, & Young, 2014). Particularly vigilance in general (Bentley, Vuilleumier, Thiel, Driver, & Dolan, 2003; Gill, Sarter, & Givens, 2000; Robbins, 1997; Sarter & Bruno, 1997). Indeed OXTRs have been found in the NBM of multiple primate species, including Titi monkeys (Callicebus cupreus) (Freeman, Walum, et al., 2014), marmosets (Callithrix jacchus) (Schorscher-Petcu, Dupré, & Tribollet, 2009), and humans (Loup, Tribollet, Dubois-Dauphin, & Dreifuss, 1991; Loup, Tribollet, Dubois-Dauphin, Pizzolato, & Dreifuss, 1989). The differences and similarities in OXTR expression between these species are summarized in Figure 2, which has been adapted from the excellent review by Freeman and Young (2016). Interestingly there seems to be notable variation of OXTR expression across various primate species, which may be linked to species-specific behavioral differences (e.g., arboreal primates rely more on auditory social signals, whereas terrestrial primates rely more on visual signals). The expression patterns that are similar in Titi monkeys, marmosets, and monogamous voles (Freeman, Walum et al., 2014) might be related to a similar mating system in these species. In this light, Titi monkeys and marmosets may serve as primate counterparts to monogamous voles (Bales et al., 2017) for the examination of partner preference formation.
FIGURE 2.
Comparative OXTR expression in brain regions that modulate attention to relevant social stimuli. (a) OXTR expression across two rodent species; (b) oxytocinergic areas in the rodent olfactory processing pathway; (c) OXTR expression across three primate species; (d) oxytocinergic areas in the primate visual pathways. III, oculomotor nucleus; Am, amygdala; AOB, accessory olfactory bulb; BNST, bed nucleus of stria terminalis; CoA, cortical nucleus of the amygdala; Hipp, hippocampus; LS, lateral septum; MeA, medial amygdala; MPOA, medial preoptic area; NA, nucleus accumbens; NAcc, nucleus accumbens; NBM, nucleus basalis of Meynert; NP, nucleus prepositus; OB, olfactory bulb; OE, olfactory epithelium; Pir, piriform cortex; PPT, pedunculopontine tegmental nucleus; Pv, pulvinar; PFC, prefrontal cortex; SC, superior colliculus; TB, trapezoid body; V1, primary visual cortex; VMH, ventromedial hypothalamus; VMO, vomeronasal organ. Reprinted with permission from Freeman and Young (2016)
Few studies have successfully examined the differential expressions of OXTRs and AVPRs in humans. Early studies employing autoradiography with human brain tissue detected OXTRs in areas which overlapped with NHPs, most intensely in the NBM (Loup et al., 1991, 1989), however the pharmacological crosstalk between OXTR and AVPR receptors prevented selective localization of OXTRs in these studies. More recently in human brainstem tissue, the selective expression of OXTRs was found in the spinal trigeminal nucleus, and inferior olivary nucleus. This distribution was identified by highly selective competitors for OXTRs and AVPRs (Freeman, Smith, Goodman, & Bales, 2017). While the mapping of these receptors in human tissue is incomplete, it is already suggestive of where and how OT might modulate neural activity in the human brain. More recent transcriptomic analysis of OXTR mRNA distribution in the human brain has revealed more distributed OXTR expression, perhaps due to the increased sensitivity of this technology, with the nucleus accumbens showing some of the highest levels of expression, resembling that of prairie voles and marmosets (Bethlehem et al., 2017). This may have important implications for our ability to form social bonds, including pair bonds.
A fundamental difficulty in determining the localization of OT action in the brain is that OT may not bind to OXTRs alone, but can also bind to the structurally similar AVPRs (Young & Flanagan-Cato, 2012). It appears that in primates AVPRs are expressed more widely, or at least more easily detectable, and in areas where OXTR is not abundantly detectable, notably the amygdala, and throughout the cortex (Freeman et al., 2017; Schorscher-Petcu et al., 2009; Young, Toloczko, & Insel, 1999). The complexities of examining both OXTR and AVPR expression in primates is thoroughly discussed in a recent review (Freeman & Young, 2016). While the conclusions which can be drawn from these studies are limited, two distinct features stand out: (i) unlike in rodents, the distribution of OXTR synthesis in the primate brain is relatively scarce and (ii) there is variation across primate species, perhaps suggesting that flexible but sparse expression of OXTRs receptors could account for some of the behavioral variation between these species. However, it should be noted that receptor with permission from Freeman and Young (2016)
autoradiography used to detect OXTR in the NHP studies is not sufficiently sensitive to detect OXTRs on axon terminals, so while the combination of in situ hybridization to detect mRNA and receptor autoradiography to detect abundant protein most likely on cell bodies provide strong evidence of where the cells that make OXTR are located, they do not provide information on where the functional OXTR protein on terminal fields are located. One can surmise, based on the known projection sites of these OXTR synthesizing areas, the major sites of action of OT signaling, but visualizing the receptors that may be modulating social behavior in primates is currently not possible. In other words, the distribution of OXTR described in NHPs from Freeman and colleagues provides some insights into circuits modulated by OT (e.g., NBM), but OT may be acting on the axon terminals of those regions on OXTRs that are not detectable by receptor autoradiography.
2.3 |. Oxytocin plays a role in socio-cognitive development in primates
Humans and many species of NHPs share two features of their sociocognitive development: (i) the presence of a critical period when certain circuits are laid down that will govern social cognition throughout adult life and (ii) the protracted development and refinement of social behaviors often spanning several years. During this period, rhesus infants form bonds not only with their mothers but also with other adult females in the matriline and develop social skills that will support social bonding with peers later in life (Corcoran et al., 2012; Dettmer et al., 2016; Simpson, Sclafani, et al., 2017; Vanderwert et al., 2015). In this stage of development, face recognition, the discrimination of individuals, and most importantly, the discrimination of social signals such as facial expression, gestures, and postures is perfected (Dettmer et al., 2016; Harlow, 1962; Ruppenthal, Harlow, Eisele, Harlow, & Suomi, 1974; Sclafani et al., 2016; Simpson, Miller, Ferrari, Suomi, & Paukner, 2016). In the second year of their life, juveniles start to learn and understand the social hierarchy of the troupe, and their place within this hierarchy. These qualities enable NHPs to serve as an excellent model for studying socio-cognitive processes in place of humans, and unlike humans, NHPs can be subjected to pharmacological manipulations such as chronic administration of OT or other agents that are expected to influence development.
Not surprisingly, numerous research groups are actively exploring the role of OT in socio-cognitive development. For example, Weinstein, Bales, Maninger, Hostetler, and Capitanio (2014) demonstrated that in female rhesus monkeys, the number of reciprocal friendships at age 1 year significantly predicted later blood plasma OT levels. The relationship between the number of reciprocal friendships and plasma OT was best described by a U-shaped quadratic function (middle- and high-ranking juveniles with either low or high numbers of reciprocal friendships had higher OT levels) (Weinstein et al., 2014). Recently Madrid et al. (2017) showed that the preference for novel faces of infant rhesus monkeys predicted the concentration of OT in the CSF. The ability to recognize others is an essential social behavior in primates (Gothard & Hoffman, 2009; Leonard, Blumenthal, Gothard, & Hoffman, 2012), and deficits in facial processing are early predictors of social deficits later in life (Sclafani et al., 2016). It appears that early features of social behavior are also predictive of OT levels later in life, although the mechanism underlying these effects remains unknown. Maternal care, or lack thereof, also seems to impact the expression of OXTR in rhesus monkeys. Indeed, peer-reared infants, compared to their mother-reared counterparts, showed altered epigenetic regulation of the OXTR gene (resulting in decreased OXTR mRNA in the hippocampus), and higher levels of separation anxiety and arousal (Baker et al., 2017). Subsequently it was shown that a naturally occurring gain-of-function nonsynonymous OXTR polymorphism partially rescued these behavioral differences. While the precise mechanisms by which OT influences socio-cognitive development are yet to be discovered, the available data strongly suggests the involvement of OT in this developmental process. These studies are consistent with studies in voles that demonstrate that early striatal OT signaling can profoundly influence later social attachment behaviors (Barrett et al., 2015).
2.4 |. Multiple task-specific social behaviors are altered by OT in monkeys
A key advantage of working with NHPs is their propensity to spontaneously engage in natural social behaviors which are oftentimes highly similar to those of humans. For example, both species prefer looking more at the eyes than at any other facial feature (Gothard, Brooks, & Peterson, 2009; Gothard, Erickson, & Amaral, 2004; Keating & Keating, 1982; Mackworth & Morandi, 1967; Mosher, Zimmerman, & Gothard, 2011; Walker-Smith, Gale, & Findlay, 1977). The innate preference for the eyes is further enhanced by IN OT in both species (Auyeung et al., 2015; Dal Monte, Noble, Costa, et al., 2014; Guastella et al., 2008). While highly quantifiable, eye looking is only one facet of primate social communication. To fully explore the role of OT in altering social perception and social behavior it would be useful to measure the more subtle, yet profoundly meaningful, aspects of social communication mediated by gaze. For example, a viewer may fixate differentially on averted eyes compared to eyes which are directed at the viewer (eye contact). These dynamics may be different during faceto-face interactions with social partners and even when watching videos, where the social stimulus behaves more naturally (Gothard et al., 2018). The effect of OT on blinking should also be quantified, as blinking is an integral component of a gaze-mediated social interactions (Ballesta & Duhamel, 2015; Ballesta, Mosher, Szep, Fischl, & Gothard, 2016; Cummins, 2012). The more socially engaging the partners in a dyadic interaction, the more likely it is that they coordinate their blinks—that is, the more likely they blink within 500 ms after the partner blinks (Ballesta et al., 2016; Cummins, 2012).
Furthermore, gaze interactions occur within the context of other communicative signals, such as facial expressions and head/gaze direction. The literature is replete with studies using images of stereotypical facial expressions in their maximal and unambiguous version as stimuli. Naturally occurring facial expressions are rarely pure, rather they appear as partial expressions or mixtures that, despite their “imperfection,” are highly interpretable and meaningful. Consider for example, a raised eyebrow combined with direct gaze from a dominant animal and compare its significance for the receiver to a maximal open-mouth-threat displayed by an unfamiliar juvenile. Likewise, consider gaze avoidance with both head and eyes averted or gaze avoidance by a single sideways glance, with a furtive saccade returning to the face of the partner to check whether the perceived challenge is still on display. We have often observed adult male recipients of OT unwilling to look at certain individuals depicted in videos. It is unclear why the viewer was avoiding a video that did not contain overt threats, but looked at videos of threats displayed by other individuals. One likely explanation is that exposing monkeys to videos or real-life interactions with unfamiliar individuals, triggers a spontaneous negotiation of status. It is unclear whether some of the effects of OT may address the default preoccupation with status in rhesus monkeys. These questions might be important to further explore given the effects of OT on lowering social anxiety or awareness of potential threats carried by various dimensions of the stimulus. The most obvious dimensions to explore would include: age, size, sex, reproductive status (visible in females), confident or hesitant demeanor, and the amount of time the animal in the video is looking at the viewer.
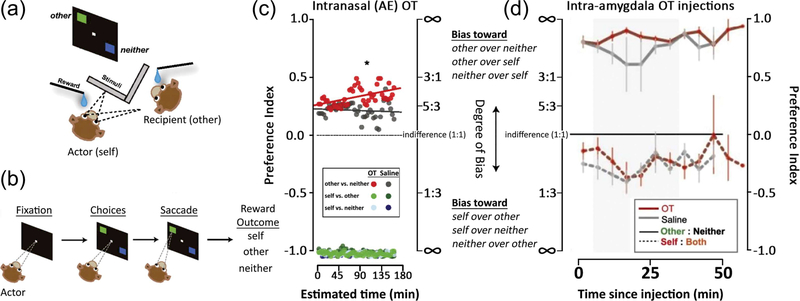
A major step that moved the field forward was implementing real life interactions between NHP social partners created in laboratory settings. A form of the dictator game was set up in the laboratory between two monkeys where one was the actor and the other the passive recipient (Figure 3). The actor monkey's choices determined where a juice reward was delivered. The juice could be delivered to the actor alone (selfish choice, to the recipient alone (pro-social choice), to an empty container (i.e., no one), or combinations of these options (such as to both the actor and recipient). Of particular interest were the trials where the actor choose between delivering juice to the passive recipient or to no-one, where it was shown that AE OT inhalation increased the frequency of pro-social choices (reward to the social partner) (Chang et al., 2012). In this behavioral context, OT increased pro-social choices. The delayed emergence of pro-social choices displayed by the OT recipient(s) (Figure 3) may suggest that the prosocial choices become available only after the monkey satisfied his own needs. It is also possible, however, that the effects of OT manifest later because of some form of plasticity that takes tens of minutes to develop. If this were the case, a rodent model would be better suited to address possible mechanisms of plasticity, but it is unlikely that the dictator game, or an equivalent task could be implemented in rodents. Equivalent tasks in humans would allow us to better understand which component of the social behavior is specifically enhanced or altered by OT. Although the dictator game or an equivalent task has not been tested in both NHPs and humans in parallel, the common feature that emerges from the task that are at least comparable is that OT enhances pro-social behaviors relative to allies or to in-group individuals (De Dreu, Greer, Van Kleef, Shalvi, & Handgraaf, 2011).
FIGURE 3.
Oxytocin, both inhaled and injected intracranially, increases pro-social donations (adapted with permission from Chang et al., 2012, 2015). (a) Two rhesus monkeys, the actor and recipient, were seated adjacent to each other. (b) The actor monkey was able to choose, by making a saccade, between the following pairwise options of juice delivery: (i) to the recipient or to neither monkey;(ii) to the actor (self) or to the recipient (other); and (iii) or to the actor (self) or to neither. (c) Intranasal (AE) OT increased pro-social donations of juice (other vs. neither trials) to the recipient, particularly in the latter half of a session (Chang et al., 2012). (d) Intra-amygdala injections of OT (red lines) similarly increased pro-social donations of juice to the recipient. This was observed during a 30-min time window beginning 5 min after the completion of each injection (shaded gray area) (Chang et al., 2015). Preference index between options A or B was calculated by (A−B)/ (A + B), and ranged between −1 and 1 (where 1 is the value of the index if the monkey always chooses the “pro-social” option, and −1 is the value of the index if the monkey always chooses the “anti-social” option)
A second important effect of OT is the selective reduction of attention to negative or threatening stimuli. This was first demonstrated by Ebitz and colleagues who trained rhesus monkeys to saccade to a target while a distractor image of a conspecific was presented. They found that AE OT treatment diminished the normal interference of threatening or fearful distractor images on performance (Ebitz, Watson, & Platt, 2013). This study also reported an increase in the time spent viewing the eye regions of face stimuli. Like wise when rhesus monkeys performed a dot-probe task, where images of conspecifics displaying facial expressions were presented next to images of conspecifics displaying neutral facial expressions or clip art, AE OT administration reduced attention to images with negative facial expressions (Parr, Modi, Siebert, & Young, 2013).
Marmosets will typically form and maintain monogamous sociosexual relationships (Evans, 1983; French, Cavanaugh, Mustoe, Carp, & Womack, 2018), however exhibit flexibility in their willingness to interact with opposite-sex strangers in the absence of their pair-mate much like humans (Buss & Schmitt, 1993). Treatment of OT via IN inhalation increased the time spent huddling with a partner, while OTreceptor antagonists decreased partner proximity, huddling, and food sharing (Smith, Ågmo, Birnie, & French, 2010). Subsequent studies found that administration of the marmoset-specific variant of the OT ligand (Pro(8)-OT) reduced time that marmosets spent in close proximity to opposite-sex strangers (Cavanaugh, Mustoe, Taylor, & French, 2014). It appears that in this case OT does not increase all social behaviors, but selectively reinforces fidelity to the bonded partner. Further studies showed that the social signals emitted by the recipient animals were sufficiently altered by OT to cause an increase in the amount of grooming received by OT-treated females from their bonded mate. When males were treated with OT the untreated female partners approached them more frequently (Cavanaugh, Huffman, Harnisch, & French, 2015). In both cases OT may alter in subtle ways the signals emitted by recipient that modified the partner's social behavior. Interestingly IN treatment of Pro(8)-OT also decreased latencies to respond to infant stimuli in males (Taylor & French, 2015).
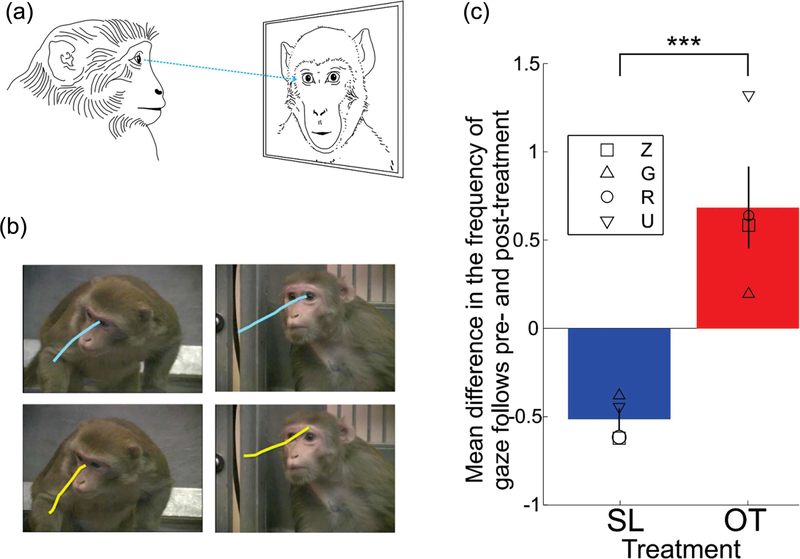
We have tested the role of OT in enhancing the active engagement of the male monkeys with perceived social partners, such as monkeys presented in videos. Videos are known to engage monkeys in the reciprocation of facial expressions and in reciprocating or soliciting eye contact (Mosher et al., 2011). Rhesus monkeys that received AE OT before they freely viewed videos of conspecifics, showed a robust increase in the frequency of gaze following saccades, a behavior that indicates an explicit interest in where others are looking (Putnam, Roman, Zimmerman, & Gothard, 2016) (Figure 4). A gazefollowing saccade is defined as a saccade that is preceded by a fixation on the eye and is parallel with the line of sight of the social partner's eyes. Typically, repeated viewing of the same videos causes habituation, a reduction in the viewer's interest, and implicitly of the time spent looking at the monkeys shown in the videos. The less the monkeys look at the videos the fewer gaze-following saccades are expected. We compared the frequency of gaze-following saccades after saline and OT inhalation, and found that under OT treatment the subjects showed a smaller decrease in the frequency of gaze-following saccades over time. Remarkably, we did not find an increase in attention to the eye region while our subjects viewed these videos after OT administration. When, however, the same subject viewed static images extracted from the same videos, the OT treatment showed the expected effect: increase in the time looking at the eyes. This finding suggests that: (i) OT increases interactive social behaviors; (ii) gaze-following, an interactive behavior elicited by videos, is unrelated to the time spent looking at the eyes; and (iii) looking at the eyes of static images may not be a good measure for the effect of OT on natural, interactive social behaviors. A similar finding was reported in infant rhesus monkeys but the increase in the frequency of gaze-following behaviors was observed only in males and not females (Simpson, Paukner, et al., 2017). It is possible that many of these socio-behavioral effects of OT are sex-specific. Most of the available data has been acquired from male monkeys therefore requiring careful interpretation and further investigation.
FIGURE 4.
Gaze-following saccades are increased by AE OT in male rhesus monkeys. (a) Rhesus watched videos of conspecifics displaying natural social behaviors, while their eye movements were tracked. (b) Examples of gaze-following saccades from two monkeys (each row) are overlaid on the frames from two videos (each column). (c) Mean difference in gaze-following saccades between pre- and post-treatment blocks. Means for data from four monkeys are shown scattered as different symbols for each treatment
2.5 |. From behavioral observations to neural mechanisms
Given the similarity of behaviors altered by OT in humans and NHPs, one can start to address the neural mechanism underlying these effects. A first step in this direction is to localize the brain regions where OT may modify neural activity. Multiple studies in humans have shown that intranasal OT reliably modulates hemodynamic activity in the amygdala (Domes et al., 2013; Gamer, 2010; Kirsch et al., 2005; Tully et al., 2018). Likewise in rhesus monkeys, IN OT reduced the functional coupling between the amygdala and areas in the occipital and inferior temporal cortices but only when the subjects viewed aggressive or fearful faces (Liu et al., 2015). Together these studies suggested that the amygdala is a critical site for the action of OT, however given that peripherally administered OT presumably could act globally in the brain, the possibility that these hemodynamic changes in the amygdala are downstream effects from an unknown source cannot be excluded. The amygdala, as the most interconnected structure of the primate brain, orchestrates social and affective behavior through enlisting multiple cortical and subcortical areas involved in the perception and interpretation of social stimuli as well as in the coordination of behavioral responses (Figure 5). Intra-amygdala injections of OT might be more likely to replicate the effect of endogenous OT, as focal increases of OT may better represent the natural mechanisms of OT release in the brain (Chini, Verhage, & Grinevich, 2017). Indeed, intra-amygdala injection of OT increased pro-social behavior in rhesus monkeys performing the previously described dictator game (Chang et al., 2015). It appears, therefore, that the amygdala may be a critical site where OT is interacting with neural circuits that govern social behavior (Figure 5). Paradoxically, this conclusion would not have emerged from the examination of the distribution of OXTRs in the monkey brain using in situ hybridization or receptor autoradiography as the amygdala does not show signals above background. These two observations can be reconciled if we consider the possibility that OT binds to the AVPR in the amygdala, or that the effect of OT on the amygdala is mediated through binding sites in NBM that projects strongly to the amygdala, OXTR protein on terminals from the NMB in the amygdala that cannot be detected by receptor autoradiography.
FIGURE 5.
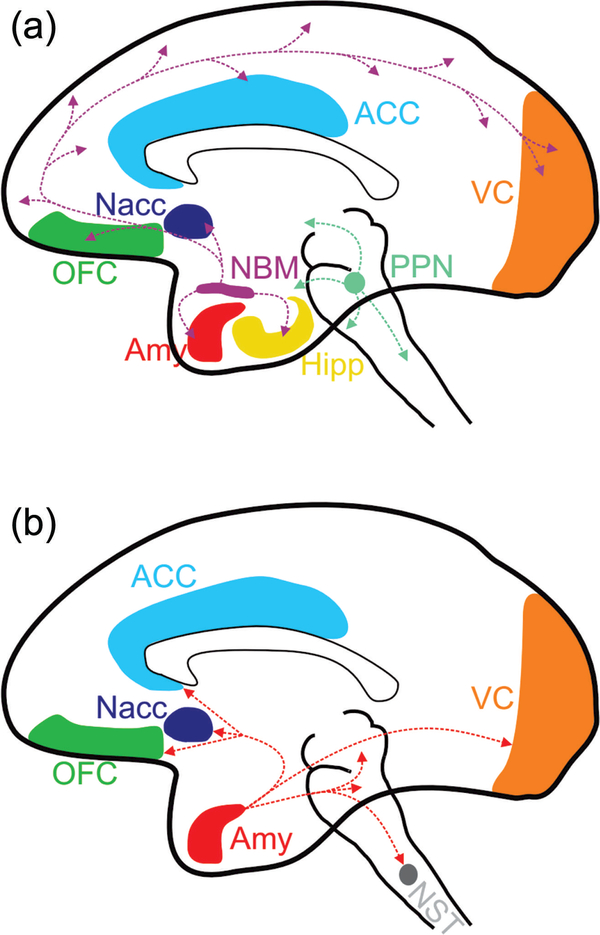
(a) Regions of the NHP brain with high density of OXTR, the nucleus basalis of Meynert (NBM) and pedunculopontine nucleus (PPN), drawn with their interconnectivity to social neural networks. Several areas highlighted in color are components of the social brain. These include the orbitofrontal cortex (OFC), the anterior cingulate cortex (ACC), and the amygdala (Amy). Other areas are highlighted such as the nucleus accumbens (Nacc), known to modulate reward and regulate vigilance/arousal, the solitary tract (NST), an autonomic center in the brainstem, and the visual cortices (VC). Note the rich connectivity of the NBM, which provides cholinergic innervation to the entire cortical mantle. (b) Selected projections of the Amy to other components of the social brain. While the primate Amy does not contain detectable OXTRs it receives strong cholinergic innervation from the NBM. Note that OT could be acting on any of the terminal fields projecting from the NBM or PPN, despite these receptors not being detectable by autoradiography
2.6 |. The neuroanatomical targets of oxytocin
A basic and yet unresolved question is the cellular site of action of OT. It is unclear what types of cells are the targets of OT action and where the OXTRs are located that produce OT signaling. Neurons are not spherical, but can project long distances through the brain. While we know where the cells that synthesize OXTR are, we cannot be sure of where the OXTR protein that signals to influence behavior is located. For example, OT could be modulating the activity of cells in the amygdala or cortex by activating OXTR on terminals originating from the NBM. If those NBM terminals are cholinergic, then a major mechanism of action of OT could be widespread modulation of the cholinergic system. Clues to these questions may lie in the mechanisms by which endogenous OT is distributed in the brain. One theory is that following dendritic release in the hypothalamus, OT passively diffuses in the extracellular space and acts upon OXTRs located throughout the brain, that is, through diffusion of volume transmission. New insights suggest that there may be an additional process by which OT reaches target areas. In rodents the discovery of long-range axonal projections from the hypothalamus to various regions, including the amygdala, have suggested that axonal release of OT can elevate local concentrations in punctate areas and effectively activate OXTRs (Chini et al., 2017; Ross & Young, 2009; Ross, Cole, et al., 2009; Ross, Freeman, et al., 2009). Indeed, when these projections have been optogenetically stimulated, rats showed decreased freezing responses in a fear-conditioning paradigm (Knobloch et al., 2012). It is likely that axonal release is present also in NHPs, and the anatomical targets of these projections could reveal the site of action of OT.
As previously described, rodents and NHPs show considerable differences in the expression and pharmacological properties of OXTRs, which limits our ability to extrapolate findings from rodents to humans and NHPs. A potential alternative for future studies is to make use of recently available selective antagonists which penetrate into the CSF (Smith et al., 2017). When combined with precise microinjection techniques it would be possible to selectively block OT receptors in various brain regions, and assess both behavioral and neurophysiological changes induced by IN, AE, or peripheral administration of OT. While current evidence points to the amygdala as a principal site of OT action (Gamer, 2010), it is likely that OT coordinates neural activity across several brain structures, not all of which necessarily have OXTR (Johnson et al., 2016) (See Figure 5). In other words, an fMRI study could reveal that OT massively activates or inhibits a brain region, but that could be due to OT acting in a separate brain region that sends excitatory or inhibitory inputs to the affected brain region. So, while OT may modulate amygdala activity, it could be through its actions on OXTR in a different region. The only way to resolve this question is through site-specific infusions of OT or OT antagonist combined with in vivo electrophysiology, as is commonly performed in rodents.
3 |. PROMISING FUTURE DIRECTIONS
If OT is similar to other neurohormones and neuromodulators, it is unlikely to influence the activity of a single brain region or to exert its action by itself, without significant interactions with other neurotransmitter systems. For example, mating, a complex behavior known to involve OT, also involves: dopaminergic signaling as part of the reward system, the opioid-endorphin pleasure system, cholinergic signaling as part of attention and vigilance, and patterned sympathetic and parasympathetic activation (Young & Alexander, 2014). Exploring the interaction between OT and other neurotransmitter systems is a promising avenue for future research in NHPs. The first steps in this direction have been highly productive in rodents. For example, the development of partner preference in voles requires not only OT, but also dopaminergic signaling in the nucleus accumbens and opioid signaling in the dorsal striatum (Burkett, Spiegel, Inoue, Murphy, &Young, 2011; Numan & Young, 2016). It was recently demonstrated that medial prefrontal cortex modulation of nucleus accumbens activity is involved in the formation of the partner preference, and that activation of this circuit in a social context can bias preference toward a partner without mating (Amadei et al., 2017). Furthermore, in mice, the optogenetically induced release of OT in the ventral tegmental area not only increased pro-social behaviors, but also increased excitatory drive to dopamine neurons as part of reward specific responses (Hung et al., 2017). Similar links between the dopaminergic system and OT in NHPs have been evidenced by IV administration of a non-selective dopamine agonist that resulted in peripheral OT secretion (Amico, Layden, Pomerantz, & Cameron, 1993). Recently, Hostetler et al. (2017) demonstrated that the formation of pair bonds increases dopamine D1 receptor binding (as detected by PET scans) in the lateral septum of Titi monkeys, but not the nucleus accumbens, caudate, putamen, or ventral pallidum. This is notable because it suggests that while dopaminergic mechanisms underlie pair bond formation in both rodents and primates, the specific brain regions involved differ. Future research in NHPs could employ techniques such as fast-scan cyclic voltammetry to monitor real-time dopamine concentrations in vivo in response to OT manipulations (Schluter, Mitz, Cheer, & Averbeck, 2014).
Likewise, both OT and opioid mechanism are involved in partner preference formation in monogamous voles (M. ochrogaster) (Resendez et al., 2013). Recent studies in NHPs have also suggested a regulatory relationship between the opioid and OT systems. When AE OT was paired with a ¼-opioid receptor antagonist, rhesus monkeys increased the number of fixations on a partner monkey, beyond the effect of either OT or the antagonist alone (Dal Monte et al., 2017). The mechanism of this supralinear effect is not yet understood, but this observation strongly suggests that OT may potentiate the effects of neurotransmitter system with broader (and better understood) control over the circuits that govern socio-emotional behavior.
An interaction between OT and the serotonergic system strongly suggested by evidence that serotonin is involved in social behaviors. In rodents, OT exerts anxiolytic effects through activation of OXTRexpressing serotonergic neurons in the raphe nuclei (Yoshida et al., 2009). Similar interactions regulate resident-intruder aggression in male mice (Pagani et al., 2015) and are crucial for social interactions to be rewarding (as demonstrated by a conditioned place preference) (Dölen et al., 2013). Interestingly, in this social reward study, OT acted on OXTRs on serotonergic terminals arising from the dorsal raphe. This is notable since receptor autoradiography in mice fails to detect OXTR in the nucleus accumbens, demonstrating the principle that OXTR on terminals are not detectable by autoradiography and therefore one cannot deduce all possible sites of OT action based on autoradiography. In rhesus monkeys intramuscular administration of the immediate serotonin precursor L-5-hydroxytryptophan (5-HTP) not only increased levels of serotonin in the CSF, but also interacted with individual variations in baseline attention and baseline levels of CSF serotonin to differentially modulate attentional changes (WeinbergWolf et al., 2018). Interestingly 5-HTP decreased the duration of looking at static images of conspecifics in animals with high baseline attention, but increased looking duration in low baseline attention animals (Weinberg-Wolf et al., 2018). Subcutaneous injections of a selective 5-HTP1A agonist in male Titi monkeys decreased the social behaviors directed at a female pair-mate (Larke, Maninger, Ragen, Mendoza, & Bales, 2016), further implicating serotonergic activity in the regulation of primate social behavior. A recent study using PET neuroimaging provided evidence for interactions between OT and serotonin in NHPs. Direct injection of OT into the lateral ventricle of rhesus monkeys, combined with radiotracers marking the serotonin transporter and the serotonin 1A receptor, revealed a release of serotonin in the amygdala, insula, and hippocampus, and an increase in the availability of 5-HT1AR receptors in the same regions (Lefevre et al., 2017). Future research will be required to understand the nature of these OT-serotonin interactions in NHPs.
The interaction of OT with the cholinergic system is perhaps the most unexplored and promising area for future study as both major sources of acetylcholine (ACh) in the primate brain, the NBM and the pedunculopontine nucleus (PPN) in rhesus monkeys, contain a high density of OXTRs. Indeed, the expression of OXTRs across primate species seems to be most conserved in these cholinergic regions, and their terminal fields are likely candidate sites of OT action. Vigilance and attention to visual signals is under cholinergic control in primates (Monosov, Leopold, & Hikosaka, 2015) and this may be potentiated for social stimuli by OT (Freeman & Young, 2016). The NBM sends dense cholinergic projections to the basal and accessory basal nucleus of the amygdala in primates (Jones, Burton, Saper, & Swanson, 1976; Mesulam et al., 1983) and these projections may be ideally positioned to influence the process of stimulus evaluation that takes place in the these nuclei. The basal and accessory basal nuclei also receive highly processed sensory inputs from the lateral nucleus (that does not contain cholinergic fibers) and for the ventromedial prefrontal cortex. This pattern of inputs may account for the value-related response properties of neuron in these nuclei (Paton, Belova, Morrison, & Salzman, 2006). These nuclei are also the source of glutaminergic excitatory projections to numerous cortical areas, including primary sensory areas such as V1 (Freese & Amaral, 2005, 2006), where OT receptors are also abundant. The basal and accessory basal nuclei are also in position to activate the ventral striatum thereby transforming social perception into social reward and actions
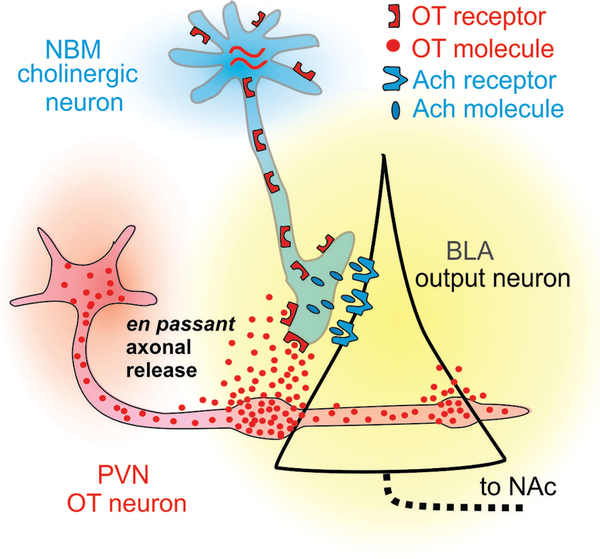
In primates OT may modulate amygdala activity by binding to OXTR-containing neurons in the NBM that project to the amygdala (the activated OXTR may be located either on the soma of cholinergic neurons in the NBM, or on the axons of the amygdala-projecting NMB cholinergic neurons) (Figure 6). It is highly likely that OT released in the NBM or in the amygdala will modulate cholinergic activity. Investigating the interactions between the OT and the cholinergic system holds important translational implications as there exist many reliable and proven methods for manipulation of cholinergic transmission. The cholinergic inputs into the amygdala may enhance the salience of social stimuli processed therein. The precise cellular mechanism by which neurons in the NBM might enhance the salience of social stimuli in the monkey amygdala are unknown. It is known, however, that the impact of Ach on cells in the basolateral amygdala depends on how strongly these cells are activated (Unal, Pare, & Zaborszky, 2015). Weakly activated cells are inhibited, while strongly activated cells are excited. Indeed, neurons in the BLA can be strongly activated by external stimuli, such as neurons that respond to faces or eye contact (Gothard, Battaglia, Erickson, Spitler, & Amaral, 2007; Mosher, Zimmerman, & Gothard, 2014). Thus, Ach effectively increases the signal/noise ratio in the basolateral amygdala (Hasselmo & Sarter, 2011), an effect that could be enhanced by OT.
FIGURE 6.
Putative circuit of interaction between cholinergic inputs from NBM and OT releasing neurons in the BLA. OT is released from the axons of neurons located in the paraventricular nucleus (PVN) of the hypothalamus. OT binds receptors on the terminals of cholinergic neurons from the NBM that contact projection cells in the BLA. These cells project to the NAc but also to many other targets included in a circuit labeled as the “social brain”
Beyond enhancing the representation of rewarding stimuli in the amygdala itself, it is possible that OT also enhances activity in the ventral striatum via projections from the amygdala. In rodents, inputs from the amygdala to the ventral striatum are required for the dopaminergic enhancement of cue-evoked neural responses (Ambroggi, Ishikawa, Fields, & Nicola, 2008). Furthermore, optogenetically stimulating fibers projecting from the amygdala to the NAc increases behavioral responding to reward (Stuber et al., 2011). The downstream effects of the amygdala in the ventral striatum is to link positive value to external stimuli and trigger overt social behaviors aimed at obtaining the predicted rewards (Roesch, Singh, Brown, Mullins, & Schoenbaum, 2009). These hypothetical mechanisms could account for many of the behavioral effects of OT, for example, increased attention to the eyes is a result of increased salience for eye stimuli which are represented in the amygdala (Mosher et al., 2014).
Future studies are expected to confirm or disprove these hypotheses by further exploring the interactions of OT with other neurotransmitters and neuromodulator systems in the primate brain. Even though optogenetics and DREADD-based (Designer Receptors Exclusively Activated by Designer Drugs) chemogenetic tools have limited efficacy in NHPs, a judicious combination of tried-and-true techniques readily available in NHPs holds the promise of important answers. Many outstanding questions will likely be answered by neurophysiological recordings in multiple brain regions combined with targeted microinjections of OT, OXTR antagonists, or other pharmacological manipulations in the context of ethologically meaningful behavior. Viral vector mediated gene editing approaches involving CRISPR may also be useful for determining the role of OXTR in specific brain regions in the future.
Even before these techniques become available, there are important questions to be addressed with conventional methods. Many mental processes involved in social behavior emerge from the coordinated activity of large population of neurons that are often distributed across multiple brain areas. The mental processes implemented in these circuits must have electrophysiological signatures that can be captured either at the level of single neurons or at the level of neural ensembles. There is a lot to be learned from simultaneous recordings from brain areas clearly involved in socialcognitive processing. When combined with pharmacological manipulations of OT or ACh, these approaches hold the promise to understand the changes induced by OT at the systems level. Given the conserved systems-level architecture of the social brain, the knowledge gained from NHPs would become comparable to the deeper understanding achieved in rodents but will be so much richer in translational value. If indeed OT has much of its effects through modulating ACh activity, the therapeutic potential of OT or drugs that evoke OT release (Modi et al., 2015) will go well beyond autism and may include disorders associated with cholinergic deficits such as cognitive decline.
4 |. CONCLUDING REMARKS
Despite several decades of research, demonstrating numerous behavioral changes elicited by OT administration, it remains unclear where and how in the primate brain OT is acting, and with which other neurotransmitter systems it interacts. The role of NHPs in answering this question fits firmly between rodents, where highly precise genetic manipulations are available, and humans, who can perform behaviors relevant for the translation value of OT. Research in NHPs allows the examination of natural social behaviors combined with the feasibility of invasive techniques for the mechanistic dissection of systems and circuits involved. To date, the studies in NHPs have already yielded key findings including evidence that peripheral administration of OT penetrates into the CSF, that OXTR mRNA in primates is markedly sparser than in rodents, but that OXTR is expressed in cholinergic regions that have powerful modulatory actions on social neural networks, and that OT modulates social and cognitive behaviors shared by humans and NHPs. Further research is required to understand both the location of and mechanisms through which OT acts in the primate brain, however current data implicates the amygdala and interactions with other neurotransmitter and neuromodulator systems.
It is critical to establish both the mechanism and the site of action for OT in the brain, with an emphasis on expanding investigation to multiple, interacting brain regions. Once we identify the circuit-level changes, even those downstream from the site of OT action, we can start to design systems-level interventions that may mimic the effects of OT thereby maximizing the translational potential of OT research in NHPs.
ACKNOWLEDGMENTS
Preparation of this manuscript was supported by NIH grants P50MH100023 to LJY and KMG, R01MH096983 to LJY, and OD P51OD011132 to YNPRC. All experiments referenced in this review adhered to the principles for the ethical treatment of NHPs, as formulated by the American Society of Primatologists Research and Development Committee.
Funding information
National Institute of Mental Health, Grant numbers: OD P51OD011132, P50MH100023, R01MH096983
Footnotes
How to cite this article: Putnam PT, Young LJ, Gothard KM. Bridging the gap between rodents and humans: The role of non-human primates in oxytocin research. Am J Primatol. 2018;e22756. https://doi.org/10.1002/ajp.22756
REFERENCES
- Amadei EA, Johnson ZV, Kwon YJ, Shpiner AC, Saravanan V, Mays WD,... Liu RC (2017). Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature, 546(7657), 297–301. 10.1038/nature22381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambroggi F, Ishikawa A, Fields HL, & Nicola SM (2008). Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron, 59(4), 648–661. 10.1016/j.neuron.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico JA, Layden LM, Pomerantz SM, & Cameron JL (1993). Oxytocin and vasopressin secretion in monkeys administered apomorphine and a D2 receptor agonist. Life Sciences, 52(15), 1301–1309. [PubMed] [Google Scholar]
- Auyeung B, Lombardo MV, Heinrichs M, Chakrabarti B, Sule A, Deakin JB, ... Baron-Cohen S (2015). Oxytocin increases eye contact during a real-time, naturalistic social interaction in males with and without autism. Translational Psychiatry, 5, e507 10.1038/tp.2014.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Lindell SG, Driscoll CA, Zhou Z, Yuan Q, Schwandt ML, ... Barr CS (2017). Early rearing history influences oxytocin receptor epigenetic regulation in rhesus macaques. Proceedings of the National Academy of Sciences of the United States of America, 114(44), 11769–11774. 10.1073/pnas.1706206114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Arias del Razo R, Conklin QA, Hartman S, Mayer HS, Rogers FD, ... Wright EC (2017). Titi monkeys as a novel nonhuman primate model for the neurobiology of pair bonding. The Yale Journal of Biology and Medicine, 90(3), 373–387. [PMC free article] [PubMed] [Google Scholar]
- Ballesta S, & Duhamel J-R (2015). Rudimentary empathy in macaques’ social decision-making. Proceedings of the National Academy of Sciences of the United States of America, 112(50), 15516–15521. 10.1073/pnas.1504454112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesta S, Mosher CP, Szep J, Fischl KD, & Gothard KM (2016). Social determinants of eyeblinks in adult male macaques. Scientific Reports, 6, 38686 10.1038/srep38686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE, Arambula SE, & Young LJ (2015). The oxytocin system promotes resilience to the effects of neonatal isolation on adult social attachment in female prairie voles. Translational Psychiatry, 5(7), e606 10.1038/tp.2015.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P, Vuilleumier P, Thiel CM, Driver J, & Dolan RJ (2003). Cholinergic enhancement modulates neural correlates of selective attention and emotional processing. NeuroImage, 20(1), 58–70. 10.1016/S1053-8119(03)00302-1 [DOI] [PubMed] [Google Scholar]
- Bethlehem RAI, Lombardo MV, Lai M-C, Auyeung B, Crockford SK, Deakin J, ... Baron-Cohen S (2017). Intranasal oxytocin enhances intrinsic corticostriatal functional connectivity in women. Translational Psychiatry, 7(4), e1099 10.1038/tp.2017.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Dabrowska J, Modi ME, Johnson ZV, Keebaugh AC, Barrett CE, ... Young LJ (2016). Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology, 64, 66–78. 10.1016/j.psyneuen.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, & Young LJ (2017). Oxytocin and social relationships: From attachment to bond disruption (pp. 1–21). Berlin, Heidelberg: Springer Berlin Heidelberg; 10.1007/7854_2017_10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FBM, & Young LJ (2016). Oxytocin-dependent consolation behavior in rodents. Science (New York, N.Y.), 351(6271), 375–378. 10.1126/science.aac4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Spiegel LL, Inoue K, Murphy AZ, & Young LJ (2011). Activation of ¼-opioid receptors in the dorsal striatum is necessary for adult social attachment in monogamous prairie voles. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 36(11), 2200–2210. 10.1038/npp.2011.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss DM, & Schmitt DP (1993). Sexual strategies theory: An evolutionary perspective on human mating. Psychological Review, 100(2), 204–232. [DOI] [PubMed] [Google Scholar]
- Cavanaugh J, Huffman MC, Harnisch AM, & French JA (2015). Marmosets treated with oxytocin are more socially attractive to their long-term mate. Frontiers in Behavioral Neuroscience, 9, 251 10.3389/fnbeh.2015.00251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, Mustoe AC, Taylor JH, & French JA (2014). Oxytocin facilitates fidelity in well-established marmoset pairs by reducing sociosexual behavior toward opposite-sex strangers. Psychoneuroendocrinology, 49, 1–10. 10.1016/j.psyneuen.2014.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SWC (2017). An emerging field of primate social neurophysiology: Current developments. ENeuro, 4(5), [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SWC, Barter JW, Ebitz RB, Watson KK, & Platt ML (2012). Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta). Proceedings of the National Academy of Sciences of the United States of America, 109(3), 959–964. 10.1073/pnas.1114621109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SWC, Fagan NA, Toda K, Utevsky AV, Pearson JM, & Platt ML (2015). Neural mechanisms of social decision-making in the primate amygdala. Proceedings of the National Academy of Sciences, 112(52), 16012–16017. 10.1073/pnas.1514761112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SWC, & Platt ML (2014). Oxytocin and social cognition in rhesus macaques: Implications for understanding and treating human psychopathology. Brain Research, 1580, 57–68. 10.1016/j.brainres.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini B, Verhage M, & Grinevich V (2017). The action radius of oxytocin release in the mammalian CNS: From single vesicles to behavior. Trends in Pharmacological Sciences, 38(11), 982–991. 10.1016/j.tips.2017.08.005 [DOI] [PubMed] [Google Scholar]
- Corcoran CA, Pierre PJ, Haddad T, Bice C, Suomi SJ, Grant KA, ... Bennett AJ (2012). Long-term effects of differential early rearing in rhesus macaques: Behavioral reactivity in adulthood. Developmental Psychobiology, 54(5), 546–555. 10.1002/dev.20613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins F (2012). Gaze and blinking in dyadic conversation: A study in coordinated behaviour among individuals. Language and Cognitive Processes, 27(10),1525–1549. 10.1080/01690965.2011.615220 [DOI] [Google Scholar]
- Dal Monte O, Noble PL, Costa VD, & Averbeck BB (2014). Oxytocin enhances attention to the eye region in rhesus monkeys. Frontiers in Neuroscience, 8, 41 10.3389/fnins.2014.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O, Noble PL, Turchi J, Cummins A, & Averbeck BB (2014). CSF and blood oxytocin concentration changes following intranasal delivery in macaque. PLoS ONE, 9(8), e103677 10.1371/journal.pone.0103677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O, Piva M, Anderson KM, Tringides M, Holmes AJ, & Chang SWC (2017). Oxytocin under opioid antagonism leads to supralinear enhancement of social attention. Proceedings of the National Academy of Sciences of the United States of America, 114(20), 5247–5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu CKW, Greer LL, Van Kleef GA, Shalvi S, & Handgraaf MJJ (2011). Oxytocin promotes human ethnocentrism. Proceedings of the National Academy of Sciences of the United States of America, 108(4), 1262–1266. 10.1073/pnas.1015316108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, Kaburu SSK, Simpson EA, Paukner A, Sclafani V, Byers KL, ... Ferrari PF (2016). Neonatal face-to-face interactions promote later social behaviour in infant rhesus monkeys. Nature Communications, 7, 11940 10.1038/ncomms11940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, & Malenka RC (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature, 501(7466), 179 10.1038/nature12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, & Herpertz SC (2007). Oxytocin improves “Mind-Reading” in humans. Biological Psychiatry, 61(6), 731–733. 10.1016/j.biopsych.2006.07.015 [DOI] [PubMed] [Google Scholar]
- Domes G, Steiner A, Porges SW, & Heinrichs M (2013). Oxytocin differentially modulates eye gaze to naturalistic social signals of happiness and anger. Psychoneuroendocrinology, 38(7), 1198–1202. 10.1016/j.psyneuen.2012.10.002 [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, & Young LJ (2008). Oxytocin, vasopressin, and the neurogenetics of sociality. Science, 322(5903), 900–904. 10.1126/science.1158668 [DOI] [PubMed] [Google Scholar]
- Ebitz RB, Watson KK, & Platt ML (2013). Oxytocin blunts social vigilance in the rhesus macaque. Proceedings of the National Academy of Sciences of the United States of America, 110(28), 11630–11635. 10.1073/pnas.1305230110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge MAG, Lerchner W, Saunders RC, Kaneko H, Krausz KW, Gonzalez FJ, ... Richmond BJ (2016). Chemogenetic disconnection of monkey orbitofrontal and rhinal cortex reversibly disrupts reward value. Nature Neuroscience, 19(1), 37 10.1038/nn.4192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S (1983). The pair-bond of the common marmoset, Callithrix jacchus jacchus: An experimental investigation. Animal Behaviour, 31(3), 651–658. 10.1016/S0003-3472(83)80220-6 [DOI] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, & Young LJ (2001). Oxytocin in the medial amygdala is essential for social recognition in the mouse. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 21(20), 8278–8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Inoue K, Smith AL, Goodman MM, & Young LJ (2014). The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta). Psychoneuroendocrinology, 45, 128–141. 10.1016/j.psyneuen.2014.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Samineni S, Allen PC, Stockinger D, Bales KL, Hwa GGC, & Roberts JA (2016). Plasma and CSF oxytocin levels after intranasal and intravenous oxytocin in awake macaques. Psychoneuroendocrinology, 66, 185–194. 10.1016/j.psyneuen.2016.01.014 [DOI] [PubMed] [Google Scholar]
- Freeman SM, Smith AL, Goodman MM, & Bales KL (2017). Selective localization of oxytocin receptors and vasopressin 1a receptors in the human brainstem. Social Neuroscience, 12(2), 113–123. 10.1080/17470919.2016.1156570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Walum H, Inoue K, Smith AL, Goodman MM, Bales KL, & Young LJ (2014). Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus). Neuroscience, 273, 12–23. 10.1016/j.neuroscience.2014.04.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, & Young LJ (2016). Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: Translational implications. Journal of Neuroendocrinology, 28(4), 1–12. 10.1111/jne.12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese JL, & Amaral DG (2005). The organization of projections from the amygdala to visual cortical areas TE and V1 in the macaque monkey. The Journal of Comparative Neurology, 486(4), 295–317. 10.1002/cne.20520 [DOI] [PubMed] [Google Scholar]
- Freese JL, & Amaral DG (2006). The synaptic organization of projections from the amygdala to visual cortical areas TE and V1 in the macaque monkey. The Journal of Comparative Neurology, 496(5), 655–667. 10.1002/cne.20945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Cavanaugh J, Mustoe AC, Carp SB, & Womack SL (2018). Social monogamy in nonhuman primates: Phylogeny, phenotype, and physiology. Journal of Sex Research, 55(4–5), 410–434. 10.1080/00224499.2017.1339774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer M (2010). Does the amygdala mediate oxytocin effects on socially reinforced learning? The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30(28), 9347–9348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill TM, Sarter M, & Givens B (2000). Sustained visual attention performance-associated prefrontal neuronal activity: Evidence for cholinergic modulation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 20(12), 4745–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen A, Hahn A, Westphal L, Prinz S, Schultz RT, Gründer G, & Spreckelmeyer KN (2012). Oxytocin plasma concentrations after single intranasal oxytocin administration—A study in healthy men. Neuropeptides, 46(5), 211–215. 10.1016/j.npep.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Gothard KM, Battaglia FP, Erickson CA, Spitler KM, & Amaral DG (2007). Neural responses to facial expression and face identity in the monkey amygdala. Journal of Neurophysiology, 97(2), 1671–1683. 10.1152/jn.00714.2006 [DOI] [PubMed] [Google Scholar]
- Gothard KM, Brooks KN, & Peterson MA (2009). Multiple perceptual strategies used by macaque monkeys for face recognition. Animal Cognition, 12(1), 155–167. 10.1007/s10071-0080179-7 [DOI] [PubMed] [Google Scholar]
- Gothard KM, Erickson CA, & Amaral DG (2004). How do rhesus monkeys (Macaca mulatta) scan faces in a visual paired comparison task? Animal Cognition, 7(1), 25–36. 10.1007/s10071003-0179-6 [DOI] [PubMed] [Google Scholar]
- Gothard KM, & Hoffman KL (2009). Circuits of emotion in the primate brain Primate neuroethology (pp. 292–315). London: Oxford University Press. [Google Scholar]
- Gothard KM, Mosher CP, Zimmerman PE, Putnam PT, Morrow JK, & Fuglevand AJ (2018). New perspectives on the neurophysiology of primate amygdala emerging from the study of naturalistic social behaviors. Wiley Interdisciplinary Reviews. Cognitive Science, 9(1), 10.1002/wcs.1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, & Dadds MR (2008). Oxytocin increases gaze to the eye region of human faces. Biological Psychiatry, 63(1), 3–5. 10.1016/j.biopsych.2007.06.026 [DOI] [PubMed] [Google Scholar]
- Harlow HF (1962). The development of learning in the rhesus monkey. Science Progress, 12, 239–269. [PubMed] [Google Scholar]
- Hasselmo ME, & Sarter M (2011). Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 36(1), 52–73. 10.1038/npp.2010.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler CM, Hinde K, Maninger N, Mendoza SP, Mason WA, Rowland DJ, ... Bales KL (2017). Effects of pair bonding on dopamine D1 receptors in monogamous male titi monkeys (Callicebus cupreus). American Journal of Primatology, 79(3), 1–9. 10.1002/ajp.22612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, ... Malenka RC (2017). Gating of social reward by oxytocin in the ventral tegmental area. Science (New York, N.Y.), 357(6358), 1406–1411. 10.1126/science.aan4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, ... Kendrick KM (2010). Oxytocin enhances amygdaladependent, socially reinforced learning and emotional empathy in humans. The Journal of Neuroscience, 30(14), 4999–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Walum H, Jamal YA, Xiao Y, Keebaugh AC, Inoue K, & Young LJ (2016). Central oxytocin receptors mediate mating-induced partner preferences and enhance correlated activation across forebrain nuclei in male prairie voles. Hormones and Behavior, 79, 8–17. 10.1016/j.yhbeh.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Walum H, Xiao Y, Riefkohl PC, & Young LJ (2017). Oxytocin receptors modulate a social salience neural network in male prairie voles. Hormones and Behavior, 87, 16–24. 10.1016/j.yhbeh.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, & Young LJ (2015). Neurobiological mechanisms of social attachment and pair bonding. Current Opinion in Behavioral Sciences, 3, 38–44. 10.1016/j.cobeha.2015.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, & Young LJ (2017). Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neuroscience and Biobehavioral Reviews, 76(Pt A), 87–98. 10.1016/j.neubiorev.2017.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Burton H, Saper CB, & Swanson LW (1976). Midbrain, diencephalic and cortical relationships of the basal nucleus of Meynert and associated structures in primates. The Journal of Comparative Neurology, 167(4), 385–419. 10.1002/cne.901670402 [DOI] [PubMed] [Google Scholar]
- Keating CF, & Keating EG (1982). Visual scan patterns of rhesus monkeys viewing faces. Perception, 11(2), 211–219. 10.1068/p110211 [DOI] [PubMed] [Google Scholar]
- King LB, Walum H, Inoue K, Eyrich NW, & Young LJ (2016). Variation in the oxytocin receptor gene predicts brain region-specific expression and social attachment. Biological Psychiatry, 80(2), 160–169. 10.1016/j.biopsych.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, ... MeyerLindenberg A (2005). Oxytocin modulates neural circuitry for social cognition and fear in humans. Journal of Neuroscience, 25(49), 11489–11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitt CA, Mitchell SJ, DeLong MR, Wainer BH, & Price DL (1987). Fiber pathways of basal forebrain cholinergic neurons in monkeys. Brain Research, 406(1–2), 192–206. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, ... Grinevich V (2012). Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron, 73(3), 553–566. 10.1016/j.neuron.2011.11.030 [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, & Fehr E (2005). Oxytocin increases trust in humans. Nature, 435(7042), 673–676. 10.1038/nature03701 [DOI] [PubMed] [Google Scholar]
- Larke RH, Maninger N, Ragen BJ, Mendoza SP, & Bales KL (2016). Serotonin 1A agonism decreases affiliative behavior in pair-bonded titi monkeys. Hormones and Behavior, 86, 71–77. 10.1016/j.yhbeh.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Scheidweiler KB, Diao XX, Akhlaghi F, Cummins A, Huestis MA, ... Averbeck BB (2018). Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: Determination using a novel oxytocin assay. Molecular Psychiatry, 23(1), 115–122. 10.1038/mp.2017.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre A, Richard N, Jazayeri M, Beuriat P-A, Fieux S, Zimmer L, ... Sirigu A (2017). Oxytocin and serotonin brain mechanisms in the nonhuman primate. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 37(28), 6741–6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard TK, Blumenthal G, Gothard KM, & Hoffman KL (2012). How macaques view familiarity and gaze in conspecific faces. Behavioral Neuroscience, 126(6), 781–791. 10.1037/a0030348 [DOI] [PubMed] [Google Scholar]
- Lischke A, Berger C, Prehn K, Heinrichs M, Herpertz SC, & Domes G (2012). Intranasal oxytocin enhances emotion recognition from dynamic facial expressions and leaves eye-gaze unaffected. Psychoneuroendocrinology, 37(4), 475–481. 10.1016/j.psyneuen.2011.07.015 [DOI] [PubMed] [Google Scholar]
- Liu N, Hadj-Bouziane F, Jones KB, Turchi JN, Averbeck BB, & Ungerleider LG (2015). Oxytocin modulates fMRI responses to facial expression in macaques. Proceedings of the National Academy of Sciences of the United States of America, 112(24), E3123–E3130. 10.1073/pnas.1508097112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, & Dreifuss JJ (1991). Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Research, 555(2), 220–232. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Pizzolato G, & Dreifuss JJ (1989). Localization of oxytocin binding sites in the human brainstem and upper spinal cord: An autoradiographic study. Brain Research, 500(1–2), 223–230. [DOI] [PubMed] [Google Scholar]
- Mackworth NH, & Morandi AJ (1967). The gaze selects informative details within pictures. Perception & Psychophysics, 2(11), 547–552. 10.3758/BF03210264 [DOI] [Google Scholar]
- Madrid JE, Oztan O, Sclafani V, Rosso LA, Calonder LA, Chun K, ... Parker KJ (2017). Preference for novel faces in male infant monkeys predicts cerebrospinal fluid oxytocin concentrations later in life. Scientific Reports, 7(1), 12935 10.1038/s41598-01713109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, ... Guillon G (2012). Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. Journal of Neuroendocrinology, 24(4), 609–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin BJ, & Froemke RC (2017). Oxytocin modulation of neural circuits for social behavior. Developmental Neurobiology, 77(2), 169–189. 10.1002/dneu.22452 [DOI] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D’amour JA, Chao MV, & Froemke RC (2015). Oxytocin enables maternal behavior by balancing cortical inhibition. Nature, 520(7548), 499–504. 10.1038/nature14402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw LA, & Young LJ (2010). The prairie vole: An emerging model organism for understanding the social brain. Trends in Neurosciences, 33(2), 103 10.1016/j.tins.2009.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, & Wainer BH (1983). Cholinergic innervation of cortex by the basal forebrain: Cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. The Journal of Comparative Neurology, 214(2), 170–197. 10.1002/cne.902140206 [DOI] [PubMed] [Google Scholar]
- Mitre M, Minder J, Morina EX, Chao MV, & Froemke RC (2017). Oxytocin modulation of neural circuits SpringerLink (pp. 1–23). Berlin, Heidelberg: Springer; 10.1007/7854_2017_7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi ME, Connor-Stroud F, Landgraf R, Young LJ, & Parr LA (2014). Aerosolized oxytocin increases cerebrospinal fluid oxytocin in rhesus macaques. Psychoneuroendocrinology, 45, 49–57. 10.1016/j.psyneuen.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi ME, Inoue K, Barrett CE, Kittelberger KA, Smith DG, Landgraf R, & Young LJ (2015). Melanocortin receptor agonists facilitate oxytocin-dependent partner preference formation in the prairie vole. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 40(8), 1856–1865. 10.1038/npp.2015.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monosov IE, Leopold DA, & Hikosaka O (2015). Neurons in the primate medial basal forebrain signal combined information about reward uncertainty, value, and punishment anticipation. Journal of Neuroscience, 35(19), 7443–7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher CP, Zimmerman PE, & Gothard KM (2011). Videos of conspecifics elicit interactive looking patterns and facial expressions in monkeys. Behavioral Neuroscience, 125(4), 639–652. 10.1037/a0024264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher CP, Zimmerman PE, & Gothard KM (2014). Neurons in the monkey amygdala detect eye contact during naturalistic social interactions. Current Biology: CB, 24(20), 2459–2464. 10.1016/j.cub.2014.08.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Wise SP, & Graham KS (2017). Representational specializations of the hippocampus in phylogenetic perspective. Neuroscience Letters, 10.1016/j.neulet.2017.04.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M, & Young LJ (2016). Neural mechanisms of mother-infant bonding and pair bonding: Similarities, differences, and broader implications. Hormones and Behavior, 77, 98–112. 10.1016/j.yhbeh.2015.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettl L-L, Ravi N, Schneider M, Scheller MF, Schneider P, Mitre M, ... Kelsch W (2016). Oxytocin enhances social recognition by modulating cortical control of early olfactory processing. Neuron, 90(3), 609–621. 10.1016/j.neuron.2016.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olazábal DE, & Young LJ (2006a). Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience, 141(2), 559–568. 10.1016/j.neuroscience.2006.04.017 [DOI] [PubMed] [Google Scholar]
- Olazábal DE, & Young LJ (2006b). Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Hormones and Behavior, 49(5), 681–687. 10.1016/j.yhbeh.2005.12.010 [DOI] [PubMed] [Google Scholar]
- Pagani JH, Williams Avram SK, Cui Z, Song J, Mezey,, Senerth JM, ... Young WS (2015). Raphe serotonin neuron-specific oxytocin receptor knockout reduces aggression without affecting anxiety-like behavior in male mice only. Genes, Brain, and Behavior, 14(2), 167–176. 10.1111/gbb.12202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, Modi M, Siebert E, & Young LJ (2013). Intranasal oxytocin selectively attenuates rhesus monkeys’ attention to negative facial expressions. Psychoneuroendocrinology, 38(9), 1748–1756. 10.1016/j.psyneuen.2013.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, & Salzman CD (2006). The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature, 439(7078), 865–870. 10.1038/nature04490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, & Prange AJ (1979). Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proceedings of the National Academy of Sciences of the United States of America, 76(12), 6661–6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, ‘t Hart BA, ... Voytko ML (2014). Why primate models matter. American Journal of Primatology, 76(9), 801–827. 10.1002/ajp.22281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl TT, Young LJ, & Bosch OJ (2018). Lost connections: Oxytocin and the neural, physiological, and behavioral consequences of disrupted relationships. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, Epub ahead of print. 10.1016/j.ijpsycho.2017.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam PT, Roman JM, Zimmerman PE, & Gothard KM (2016). Oxytocin enhances gaze-following responses to videos of natural social behavior in adult male rhesus monkeys. Psychoneuroendocrinology, 72, 47–53. 10.1016/j.psyneuen.2016.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Dome M, Gormley G, Franco D, Nevárez N, Hamid AA, & Aragona BJ (2013). ¼-Opioid receptors within subregions of the striatum mediate pair bond formation through parallel yet distinct reward mechanisms. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 33(21), 9140–9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, & Young LJ (2014). The biology of mammalian parenting and its effect on offspring social development. Science (New York, N.Y.), 345(6198), 771–776. 10.1126/science.1252723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW (1997). Arousal systems and attentional processes. Biological Psychology, 45(1–3), 57–71. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Singh T, Brown PL, Mullins SE, & Schoenbaum G (2009). Ventral striatal neurons encode the value of the chosen action in rats deciding between differently delayed or sized rewards. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29(42), 13365–13376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, & Young LJ (2009). Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience, 162(4), 892–903. 10.1016/j.neuroscience.2009.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, & Young LJ (2009). Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29(5), 1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, & Young LJ (2009). Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Frontiers in Neuroendocrinology, 30(4), 534–547. 10.1016/j.yfrne.2009.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppenthal GC, Harlow MK, Eisele CD, Harlow HF, & Suomi SJ (1974). Development of peer interactions of monkeys reared in a nuclear-family environment. Child Development, 45(3), 670–682. [PubMed] [Google Scholar]
- Sarter M, & Bruno JP (1997). Cognitive functions of cortical acetylcholine: Toward a unifying hypothesis. Brain Research. Brain Research Reviews, 23(1–2), 28–46. [DOI] [PubMed] [Google Scholar]
- Schluter EW, Mitz AR, Cheer JF, & Averbeck BB (2014). Real-time dopamine measurement in awake monkeys. PLoS ONE, 9(6), e98692 10.1371/journal.pone.0098692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Dupré A, & Tribollet E (2009). Distribution of vasopressin and oxytocin binding sites in the brain and upper spinal cord of the common marmoset. Neuroscience Letters, 461(3), 217–222. 10.1016/j.neulet.2009.06.016 [DOI] [PubMed] [Google Scholar]
- Sclafani V, Rosso LAD, Seil SK, Calonder LA, Madrid JE, Bone KJ, ... Parker KJ (2016). Early predictors of impaired social functioning in male rhesus macaques (Macaca mulatta). PLoS ONE, 11(10), e0165401 10.1371/journal.pone.0165401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory S, & Young LJ (2016). Understanding the oxytocin system and its relevance to psychiatry. Biological Psychiatry, 79(3), 150–152. 10.1016/j.biopsych.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EA, Miller GM, Ferrari PF, Suomi SJ, & Paukner A (2016). Neonatal imitation and early social experience predict gaze following abilities in infant monkeys. Scientific Reports, 6, 20233 10.1038/srep20233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EA, Paukner A, Sclafani V, Kaburu SSK, Suomi SJ, & Ferrari PF (2017). Acute oxytocin improves memory and gaze following in male but not female nursery-reared infant macaques. Psychopharmacology, 234(3), 497–506. 10.1007/s00213-016-4480-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EA, Sclafani V, Paukner A, Kaburu SSK, Suomi SJ, & Ferrari PF (2017). Handling newborn monkeys alters later exploratory, cognitive, and social behaviors. Developmental Cognitive Neuroscience, 10.1016/j.dcn.2017.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse DH, Lori A, Cubells JF, Lee I, Conneely KN, Puura K, ... Young LJ (2014). Common polymorphism in the oxytocin receptor gene (OXTR) is associated with human social recognition skills. Proceedings of the National Academy of Sciences of the United States of America, 111(5),1987–1992 10.1073/pnas.1302985111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AL, Walum H, Connor-Stroud F, Freeman SM, Inoue K, Parr LA, ... Young LJ (2017). An evaluation of central penetration from a peripherally administered oxytocin receptor selective antagonist in nonhuman primates. Bioorganic & Medicinal Chemistry, 25(1), 305–315. 10.1016/j.bmc.2016.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Ågmo A, Birnie AK, & French JA (2010). Manipulation of the oxytocin system alters social behavior and attraction in pairbonding primates, Callithrix penicillata. Hormones and Behavior, 57(2), 255–262. 10.1016/j.yhbeh.2009.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer WR, Lak A, Yang A, Borel M, Paulsen O, Boyden ES, & Schultz W (2016). Dopamine neuron-specific optogenetic stimulation in rhesus macaques. Cell, 166(6), 1564–1571e6. 10.1016/j.cell.2016.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Hanking V, Landgraf R, Wüllner U, Maier W, & Hurlemann R (2013). Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Scientific Reports, 3, 3440 10.1038/srep03440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, ... Bonci A (2011). Amygdala to nucleus accumbens excitatory transmission facilitates reward seeking. Nature, 475(7356), 377–380. 10.1038/nature10194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JH, & French JA (2015). Oxytocin and vasopressin enhance responsiveness to infant stimuli in adult marmosets. Hormones and Behavior, 75, 154–159. 10.1016/j.yhbeh.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toloczko DM, Young LJ, & Insel TR (1997). Are there oxytocin receptors in the primate brain? Annals of the New York Academy of Sciences, 807, 506–509. [DOI] [PubMed] [Google Scholar]
- Tully J, Gabay AS, Brown D, Murphy DGM, & Blackwood N (2018). The effect of intranasal oxytocin on neural response to facial emotions in healthy adults as measured by functional MRI: A systematic review. Psychiatry Research: Neuroimaging, 272, 17–29. 10.1016/j.pscychresns.2017.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal CT, Pare D, & Zaborszky L (2015). Impact of basal forebrain cholinergic inputs on basolateral amygdala neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 35(2), 853–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwert RE, Simpson EA, Paukner A, Suomi SJ, Fox NA, & Ferrari PF (2015). Early social experience affects neural activity to affiliative facial gestures in newborn nonhuman primates. Developmental Neuroscience, 37(3), 243–252. 10.1159/000381538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Smith GJ, Gale AG, & Findlay JM (1977). Eye movement strategies involved in face perception. Perception, 6(3), 313–326. 10.1068/p060313 [DOI] [PubMed] [Google Scholar]
- Walum H, Waldman ID, & Young LJ (2015). Statistical and methodological considerations for the interpretation of intranasal oxytocin studies. Biological Psychiatry, 10.1016/j.biopsych.2015.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Kuroda M, Kuwabara H, Aoki Y, Iwashiro N, Tatsunobu N, ... Yamasue H (2015). Clinical and neural effects of six-week administration of oxytocin on core symptoms of autism. Brain: A Journal of Neurology, 138(Pt 11), 3400–3412. 10.1093/brain/awv249 [DOI] [PubMed] [Google Scholar]
- Weinberg-Wolf H, Fagan NA, Anderson GM, Tringides M, Dal Monte O, & Chang SWC (2018). The effects of 5-hydroxytryptophan on attention and central serotonin neurochemistry in the rhesus macaque. Neuropsychopharmacology, 1 10.1038/s41386-0170003-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein TAR, Bales KL, Maninger N, Hostetler CM, & Capitanio JP (2014). Early involvement in friendships predicts later plasma concentrations of oxytocin and vasopressin in juvenile rhesus macaques (Macaca mulatta). Frontiers in Behavioral Neuroscience, 8, 295 10.3389/fnbeh.2014.00295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SP (2008). Forward frontal fields: Phylogeny and fundamental function. Trends in Neurosciences, 31(12), 599–608. 10.1016/j.tins.2008.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, & Guastella AJ (2016). The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: A randomized clinical crossover trial. Molecular Psychiatry, 21(9), 1225–1231. 10.1038/mp.2015.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, & Nishimori K (2009). Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29(7), 2259–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ (2015). Oxytocin, social cognition and psychiatry. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 40(1), 243–244. 10.1038/npp.2014.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, & Alexander B (2014). The chemistry between us: Love, sex, and the science of attraction Reprint edition Penguin. [Google Scholar]
- Young LJ, & Flanagan-Cato LM (2012). Editorial comment: Oxytocin, vasopressin and social behavior. Hormones and Behavior, 61(3), 227–229. 10.1016/j.yhbeh.2012.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Toloczko D, & Insel TR (1999). Localization of vasopressin (V1a) receptor binding and mRNA in the rhesus monkey brain. Journal of Neuroendocrinology, 11(4), 291–297. 10.1046/j.13652826.1999.00332.x [DOI] [PubMed] [Google Scholar]
- Young LJ, & Wang Z (2004). The neurobiology of pair bonding. Nature Neuroscience, 7(10), 1048–1054. 10.1038/nn1327 [DOI] [PubMed] [Google Scholar]