Abstract
The artificial pancreas combines a hormone infusion pump with a continuous glucose monitoring device, supported by a dosing algorithm currently installed on the pump. It allows for dynamic infusions of insulin (and possibly other hormones such as glucagon) tailored to patient needs. For patients with type 1 diabetes the artificial pancreas has been shown to prevent more effectively hypoglycaemic events and hyperglycaemia than insulin pump therapy and has the potential to simplify care. However, the potential ethical issues associated with the upcoming integration of the artificial pancreas into clinical practice have not yet been discussed. Our objective was to identify and articulate ethical issues associated with artificial pancreas use for patients, healthcare professionals, industry and policymakers. We performed a literature review to identify clinical, psychosocial, and technical issues raised by the artificial pancreas and subsequently analysed them through a common bioethics framework. We identified five sensitive domains of ethical issues. Patient confidentiality and safety can be jeopardized by the artificial pancreas’ vulnerability to security breaches or unauthorized data sharing. Public and private coverage of the artificial pancreas could be cost-effective and warranted. Patient selection criteria need to ensure equitable access and sensitivity to patient-reported outcomes. Patient coaching and support by healthcare professionals or industry representatives could help foster realistic expectations in patients. Finally, the artificial pancreas increases the visibility of diabetes and could generate issues related to personal identity and patient agency. The timely consideration of these issues will optimize the technological development and clinical uptake of the artificial pancreas.
Keywords: artificial pancreas, closed-loop system, type 1 diabetes, ethics, psychosocial
Introduction
People with type 1 diabetes (T1D) need to monitor their blood glucose (glycaemia) frequently and self-inject with appropriate amounts of insulin. To achieve optimal T1D care, patients must carefully plan their schedules around their care activities. They also have to continuously make difficult decisions and calculations regarding their treatment regimen [1], notably by taking into account dietary intakes and lifestyle factors. This behavioural burden of T1D care can elicit distress and frustration among patients [2], especially among those who struggle to attain their target glycaemia levels [1]. Patients also fear both acute (e.g., severe hypoglycaemia) and long-term complications of T1D (e.g., retinopathy) and the fluctuations in mood that accompany frequent glycaemic variations add to the emotional toll of the illness [1].
Both the continuous glucose monitoring device and the insulin pump developed in the last few decades can improve clinical outcomes associated with T1D care compared to punctual glucose readings and self-injections [3, 4]. By their respective abilities to measure interstitial glucose continuously and fine-tune insulin infusions, the continuous glucose monitoring device and the insulin pump alleviate some of the burden associated with T1D care. Yet, learning how to successfully coordinate the use of these complicated and customizable medical devices remains challenging for patients. For example, parameters for basal insulin infusions and prandial boluses of the pump still need to be adjusted according to the patient’s schedule, level of activity, and glycaemic profile, while most continuous glucose monitoring devices still need to be calibrated with traditional capillary blood glucose values a few times per day to provide reliable readings [5].
The advent of the artificial pancreas
The artificial pancreas (AP) has been developed with the intention of simplifying and improving care for patients with T1D [2, 6]. The AP operates in a closed-loop system that consists of three components: a continuous glucose monitoring device, a dosing algorithm and an infusion pump for hormones. Single-hormone versions only infuse insulin, while dual-hormone versions infuse glucagon in addition to insulin. Every ten minutes, interstitial glucose values provided by the continuous glucose monitoring device are transmitted to the algorithm, which calculates hormonal infusions or boluses with the aim of maintaining glucose values within target range. Hence, the resulting infusions of insulin (and possibly glucagon) are tailored to the changes in the patient’s blood glucose. A first commercial version of the AP was approved by the FDA in September 2016 [7]. Most of the currently developed AP versions are hybrid closed-loop systems: they still require the patient to announce exercise and meals, with or without exact carbohydrate counting [8, 9]. This is partly due to the pharmacokinetics of the available insulins and equilibration between interstitial glucose measured by the continuous glucose monitoring device and actual blood glucose values. In the future, faster acting insulins, increased accuracy and reduced lag-time of continuous glucose monitoring device as well as self-learning adapting algorithms will probably improve the AP’s level of automation. These advances could fully close the loop and minimize the need for oversight for AP use [6]. In addition, glycaemia and hormonal infusions data will likely be accessible for users and their health care professionals through a web platform [10].
Clinical benefits of the single-hormone AP for glycaemic control have been established through inpatient and outpatient trials, which indicate that it is more effective at preventing both hypoglycaemic and hyperglycaemic events than conventional insulin pump therapy [11–13]. The magnitude of these benefits may depend on the algorithm developed for the software and the functionalities they offer [6, 11, 14, 15]. A dual-hormone AP, which includes insulin and glucagon infusions, is also being tested to further reduce the occurrence of hypoglycaemic events [16–19]. Yet, the inclusion of glucagon comes with the cost of dealing with a more complex system, which notably has an additional catheter and infusion site. Moreover, as glucagon was initially commercialized for one-time use, a stable glucagon formulation that withstands fibrillation and aggregation in an insulin pump still needs to be developed for its inclusion in the AP [20]. Accordingly, the safety of chronic glucagon use also needs to be established [21].
Psychosocial and ethical issues raised by the artificial pancreas
Preliminary studies have explored the psychosocial benefits and implications of the AP. In a study exploring adolescent and parent perceptions of the AP following a three-week trial at home, participants reported feeling greater assurance in their ability to manage their T1D, as well as a sense of safety and peacefulness [22]. In two other studies, participants reported similar psychosocial benefits, in addition to improved daily functioning, sleep, and overall quality of life, most likely due to a relief from some key components of T1D care [23, 24]. In return, an AP user must be willing to wear at all times all the pieces of the AP and still needs to rotate the sensor and infusion sites [24]. Patients who have tried the AP also expressed concerns about technical difficulties such as calibration or equipment issues, as well as annoyance of alarms, the cumbersomeness of the device and its associated visibility [22, 24, 25]. These concerns of wearability and visibility, which could also be true for patients who currently use an insulin pump with or without a continuous glucose monitoring device, may diminish in the future especially with the integration of various pieces of the AP.
Like for psychosocial issues, anticipating the ethical issues raised by medical devices is useful to inform its optimal technological development and clinical uptake [26]. Ethical issues are those that raise important questions with respect to values and principles and are interwoven with psychosocial as well as other aspects of the AP. Like other medical technologies, the AP is likely accompanied by its own set of ethical issues, however these have been much less investigated than the psychosocial issues raised by the artificial pancreas. For example, given that medical technology is expensive and has limited availability, its allocation involves concerns of fairness and justice. In addition, medical technologies that are used daily generate issues regarding the autonomy of users: while they may improve the user’s control of illness and daily life, they create a sense of dependency. Yet, to our knowledge, the ethical issues associated with the use of the AP have not been extensively studied previously, aside from the tension between its benefits, potential harms, and inconvenience as outlined above. Accordingly, our objective was to identify and articulate the main ethical issues associated with AP use for patients, healthcare professionals, developers, and policymakers. We have identified five domains in which ethically problematic situations involving the AP could arise in the future and generate difficult choices and dilemmas: confidentiality and safety, coverage, patient selection, patient coaching and support, and personal identity and agency. We here report on those categories of issues as encountered in the literature, explain them in terms of ethically problematic situations that patients could face, and discuss possible initial recommendations to tackle them.
Methods
Some general psychosocial issues raised by the AP have already been documented in the literature [22–24, 27–32], but to our knowledge, their ethical ramifications have rarely been addressed. Our strategy therefore consisted in performing a literature review to identify these issues and analyse the latter through an ethical framework (Figure 1). More precisely, we looked for psychosocial and technical issues related to the AP, or issues in neighbouring literature such as the ethical and psychosocial issues of other T1D management strategies. The databases used were PubMed, Scopus, and Philosophers’ Index.
Figure 1.
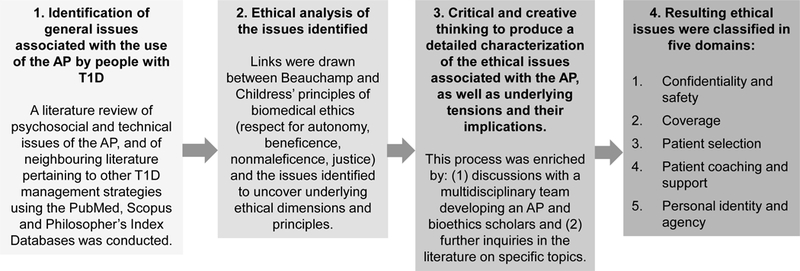
Scheme of the literature review to identify issues and analyse the latter through an ethical framework
We adopted Beauchamp and Childress’ principles of biomedical ethics as an ethical framework to analyse the identified issues. This framework evaluates how four ethical principles, namely respect for autonomy, beneficence, nonmaleficence, and justice, articulate tensions in an ethically problematic situation arising in a clinical or research environment [33]. We chose this framework for its ability to uncover general ethical tensions raised by the AP, while recognizing that more sophisticated frameworks could eventually be mobilized to refine our understanding of some of these issues. The framework was applied to each identified issue identified through the literature review to reveal its ethical ramifications, notably by drawing links between the issue itself and the principles and sub-principles of the framework. The issues raised by the AP and their ethical implications were further articulated through discussions with a multidisciplinary team that develops an AP (clinicians, research personnel, engineer, and healthcare professionals) as well as with bioethics scholars and patients with T1D. Both the discussions and the resulting reflections served as a basis for further inquiry in the literature. We subsequently organized the issues and their associated ethical questions under five domains that pertain to the development, the attribution, or the use of the AP: confidentiality and safety, coverage, patient selection, patient coaching and support, and personal identity and agency.
Domains of ethical issues
Table 1 provides an overview of the issues and potential problematic situations patients could face regarding the five domains of ethical relevance presented below, as well as early recommendations to tackle them.
Table 1.
Summary table of potential ethically problematic situations that could arise through AP use.
| Domains of ethical issues | Issues | Ethically problematic situations | Recommendations |
|---|---|---|---|
| Confidentiality and safety | Information-transmission features | The AP’s information-transmission features makes it vulnerable to cybersecurity breaches, such as unauthorized data access or modification, threatening privacy and confidentiality, as well as physical integrity [36,37,31]. | Regulatory agencies could require more systematic testing for data interception and modification in the research and development stages [36]. |
| Data access | The person with T1D may feel surveilled if he did not personally authorize the healthcare professional and/or his relatives to have access to his data [32,40]. | Data sharing should be established in partnership with the patient and with his consent. | |
| Complexity | Complex systems have an increased number of vulnerabilities, which amplifies security concerns [45]. | Additional features and functionalities should only be added to the AP if their benefits outweigh their risks. | |
| Coverage | Coverage decisions | Without coverage, the AP’s costs will involve additional out-of-pocket expenditures for people with T1D, which already face tangible socio-economic burdens [48]. | AP coverage could be of interest to public and private insurers due to its potential cost-effectiveness (i.e., limiting expenditures by reducing the incidence of T1D complications [51,52]). Further studies are needed to validate cost-effectiveness. |
| Insurability and care | AP coverage could be discontinued through a change in insurability, forcing the patient to resort to a simpler and less effective treatment modality. | When acquiring an AP, patients could be sensitized to the risk of discontinued or terminated coverage. | |
| Patient selection | Clinical needs | Some people with T1D experience poor glycemic control despite rigorous adherence to insulin therapy, which results in a high likelihood of developing long-term complications. | AP allocation could prioritize people with T1D with complex clinical needs, so that maximum clinical benefits are attained. People with T2D could benefit from simpler and less costly interventions than the AP [56]. |
| Patient attitudes and traits | While positive patient attitudes favour clinical benefits, some patient traits (e.g., age, technological ability) are not predictive of success with T1D management technologies [60]. | Patients with positive attitudes and realistic expectations are good AP candidates. The AP should not be allocated simply according to age and technological ability. More studies are needed to establish which traits are predictive of clinical benefits. | |
| Social environment | While a patient with good social support and a flexible schedule may be more likely to benefit from AP use, patients without this socio-economic advantage may equally benefit from the AP. | Social support and flexibility of schedule should not be used as selection criteria, since it could further enhance inequity in T1D care that already exists for people with lower socio-economic status [62–64]. | |
| Adherence to therapy and follow-up | Eligibility to some insulin pump public coverage programs is determined by specific criteria [65]. Some of these criteria could be used for AP attribution, but others may be irrelevant given the AP’s functionalities (e.g., being able to adjust insulin doses to prevent hypo- and hyperglycemic events). | Eligibility criteria for a public coverage program for the AP would favour clinical benefits, safety, and optimal resource use. While these criteria could mirror those of the insulin pump, they should be coherent with the AP’s functionalities and patients’ needs. | |
| Patient coaching and support | Realistic expectations | With unrealistic expectations on the AP’s capabilities, patients could be deceived by the technology despite positive outcomes. | Healthcare professionals could portray the AP as a partner in T1D care by explaining the AP’s capabilities and limits and insisting on the patient’s responsibilities. Providing the patient with a balanced account of the advantages, drawbacks, and capabilities of the AP will also foster realistic expectations [24]. |
| Troubleshooting | Healthcare professionals may not have extensive knowledge and time to assist patients in AP troubleshooting, who may feel overwhelmed when starting to use the technology. | To empower their patients in their T1D management, healthcare professionals could direct patients to written and online troubleshooting resources. AP companies should deliver technical support to patients. | |
| Experiential knowledge | Patients develop experiential knowledge (e.g., personal tricks and habits) to aid them in the management of T1D through trial-and-error [68]. Such manipulations may result in sub-optimal raw glycaemic data. | Healthcare professionals may need to interpret raw glycaemic data by acknowledging contextual factors, such as new AP use to allow patients to develop experiential knowledge. | |
| Lifestyle habits | Healthy nutrition and physical activity are an essential part of T1D management, but may add to the burden of the illness (i.e., through carbohydrate counting and fear of hypoglycemic events during exercise [5]). | An AP that would not require strict carbohydrate counting [19] could help improving diet quality and allow patients to eat and exercise more mindfully. In contrast, patients should not expect the AP to account for high sugar intake through increases in insulin infusions. All upcoming AP versions will require meal notifications. | |
| Personal identity and agency | Agency | Patients who trust the AP are comfortable with surrendering part of their control of T1D to the technology, gaining agency in their daily lives. Yet, trust in the AP could be limited by unfamiliarity, technical limitations, and patient values and preferences. | To ensure that trust toward the AP is built over time, healthcare professionals could be sensitive to values, preferences, and technical abilities in directing patients towards the AP. They could remind patients that the need for sustained oversight will resolve itself over time. |
| Burden of control | Patients often experience frustration when unable to control their T1D despite rigorous self-care [73]. | Shared responsibility with the AP alleviates the burden of successful T1D control. Positive feedback provided by the AP when target glycaemic readings are attained may improve patients’ confidence. | |
| Visibility of T1D management or devices | T1D is visible punctually (i.e., through self-injections or glucose monitoring) or continuously (i.e. by wearing an insulin pump, a continuous glucose monitoring device or an AP). This visibility may limit adherence, elicit stigmatization, or negatively impact relationships (e.g., professional encounters, intimacy)[1,75–77]. | Healthcare professionals should acknowledge patient preferences regarding visibility (i.e., punctual or continuous) in directing the patient towards an appropriate treatment modality. | |
| Dependence and vulnerability | Patients may become dependent on the AP and forget how to resort to conventional management strategies if a technical problem arises. | Healthcare professionals should ensure that patients remain knowledgeable of conventional management strategies (e.g., calculating insulin doses according to blood glucose readings) to avoid jeopardizing their health in the event of a technical failure. The AP could include a feature that suspends insulin infusions in the case of technical issues or hypoglycemic events. |
Domain 1: Confidentiality and safety
The AP’s transmits and shares clinical data (glucose readings and/or insulin infusion doses) and personal information, which involves risks to the patient’s privacy, confidentiality and safety. In addition, the AP’s increased complexity as opposed to conventional T1D management strategies creates additional technological vulnerabilities, which may pose risks to the user’s physical integrity.
Information-transmission features
If hijacked by a malevolent user, the AP’s data transmission features may pose risks to the patient’s privacy and physical integrity. Indeed, clinical data and personal information circulate wirelessly between AP components, a Web platform (e.g. https://carelink.minimed.com or https://diasend.com currently used for insulin pumps or continuous glucose monitoring devices), and to third-party applications installed on a smartphone or a specific interface on the pump, depending on the AP system. While these data transmission features allow patients to readily access patient individual health data and facilitate T1D management decisions [32], they render the device vulnerable to information security breaches [31, 34, 35]. Those breaches could involve unauthorized access, storage, transfer and modification of information [36]. Unauthorized access to the patient’s clinical data or personal information could threaten the patient’s privacy and confidentiality [36, 37]. A malevolent modification of the clinical data could result in physical harms for the patient, notably through an increase in the quantity of insulin to be infused [31].
To limit such risks, regulatory agencies could mandate medical device developers to test systematically various data interception and modification scenarios as part of the research and development process [36], especially given that such risks may also be common to other automated or closed-loop medical technologies (e.g., pacemakers, deep brain stimulators, brain computer interfaces) [38]. AP companies could solicit the expertise of external consultants to test for potential vulnerabilities in the AP’s software.
Data access
Clinical data sharing with healthcare professionals or family members may leave the patient with the impression of being surveilled and monitored, especially if this is done without his or her consent. Access to glycemic and insulin dose data through a web platform by healthcare professionals certainly empowers them to offer better guidance to patients [32, 39]. Yet, patients may not be at ease with unrestricted data access by healthcare professionals. In addition to fears of surveillance, patients could worry about the impact of their experimentation with T1D management would leave on their glycaemic readings, especially when beginning to use an AP. Their raw clinical data could be interpreted as ineffective T1D management by the healthcare professional if taken out of that context.
Similarly, data access by family members may certainly empower them to offer improved care, notably for pediatric patients and elderly patients, but it could also be interpreted as surveillance by some people with T1D [32]. For example, adolescents seeking independence can interpret parental oversight and worry as being intrusive [40]. In such cases, the parents’ benevolence conflicts with the need to recognize the adolescent’s autonomy and claim to privacy [41]. On the contrary, automated T1D management offered by the AP is also an opportunity to empower adolescents in the management of their illness and allow them to be more independent. More generally, data surveillance could also involve tensions between spouses, or between a caregiver and the patient (e.g. adolescent or elderly parent).
How much and what kind of information to share with the healthcare professionals and relatives could be determined in partnership with the patient. Currently, the continuous glucose monitoring device and the insulin pump require that the patient uploads data on a web platform accessible by his or her healthcare professionals on a voluntary basis [42]. If a similar data sharing approach is implemented with the AP, some of the concerns relating to unwarranted surveillance could be mitigated. Data sharing among relatives could be made with the consent of patients (or assent of minor patients). For adolescents specifically, data sharing practices may need to be established according to the patient family’s needs with a progressive recognition of the growing autonomy of the aging adolescent [43, 44].
Complexity
As medical devices like the AP increase in complexity to provide patients with personalized treatment, their number of loopholes and vulnerabilities also increase [23, 45]. For example, the dual-hormone AP includes an additional infusion system for glucagon and promises to further reduce hypoglycaemic events in comparison to single-hormone systems [21, 46], but the addition of glucagon may amplify security concerns. Risks to patient safety and security created by increased AP complexity need to be considered in the backdrop of their related benefits as well as the justification and acceptability of those risks.
Domain 2: Coverage
Coverage intends to improve patient care and quality of life by facilitating access to expensive medical interventions like the AP. Faced with the increase in health expenditures, policymakers and insurers are often encouraged to allocate resources towards cost-effective medical technologies [47], like the AP potentially. Patients forced to change public or private coverage plans could be faced with discontinuity of care and associated challenges.
Coverage decisions
Unavailable public or private coverage for the AP could add to the already tangible socio-economic burden of T1D for those who choose to use the AP. For example, women and older adults with T1D face unemployment and lower quality of life more often than the general population [48]. Moreover, is estimated that the average yearly medical spending for people with T1D of ages 19 to 29 is $3,500 US, but that figure reaches $10,100 US for people of ages 65 to 74 [49]. AP costs will add to these occupational and financial burdens.
Since insulin pumps and less frequently continuous glucose monitoring devices can be covered by private insurers and sometimes by public reimbursement programs [50], it can be expected that AP coverage will be similar, at best. Coverage of the AP could be of interest to policymakers if it is cost-effective, notably by preventing some of the costs associated with the treatment of T1D complications. While the AP represents tangible investments due to the need to wear a continuous glucose monitoring device at all times, medium and long-term AP use could reduce the risk of both acute and chronic complications and their associated costs [51, 52]. The apparent cost-effectiveness of the AP will need to be confirmed through further analyses, such as those performed in health technology assessments, as well as through long-term trials. Moreover, optimal AP uptake will also require allocation of dedicated human resources through patient education and follow-up by healthcare professionals.
Changes in insurability
Patients who opt for an AP due to available coverage could be sensitized to the dependency that they can develop towards the AP, which can be problematic if discontinuity or termination of coverage occurs. Transitioning from the AP’s automated features which require minimal user input, to a less sophisticated treatment modality may elicit anxiety and uncertainty in patients, who would need to revise important diabetes management education knowledge or acquire new knowledge, skills, and trust towards the simpler treatment modality. Moreover, moving towards a treatment modality that does not incorporate a continuous glucose monitoring device may be highly distressing for patients with severe fears of hypoglycaemia.
Domain 3: Patient selection
In the likely scenario of restricted availability and coverage of the AP for financial reasons, fair patient selection criteria that result in optimal clinical benefits and account for patient preferences and values will be needed. While some patients may prefer being closely involved in the control of their DT1 through multiple daily injections, others may feel at ease in surrendering a portion of their T1D management to an AP. Moreover, patients with specific clinical needs, attitudes and traits, and social environments may benefit more from the AP. Such factors will need regular revaluation as patients’ personal situation, experience, insurance coverage, and desires evolve over time.
Clinical needs
People with T1D who experience poor glycaemic control despite rigorous adherence to insulin therapy could be ideal candidates for an AP. Such patients who face recurrent and severe hypoglycaemic events despite intensive insulin therapy and/or persistent high glucose values with progressing chronic complications are already favoured in pancreatic islet transplantation, a costly procedure with limited availability [53–55]. Some of these specific attribution criteria used in pancreatic islet transplantation allocation could be transposed to AP attribution and would therefore ensure maximum clinical benefits. Moreover, such criteria would also ensure that patients would not benefit from a simpler and cheaper intervention such as multiple daily insulin injections, which insurers would rather fund.
Unlike people with T1D with poorly controlled T1D despite good adherence to therapy, people with type 2 diabetes have access to a greater variety of pharmacological, surgical and lifestyle treatment options [56]. Indeed, current use of the AP mainly targets people with T1D and only a few studies with the AP involved patients with type 2 diabetes [57, 58]. Hence, people with type 2 diabetes should not be prioritized in AP allocation at this time.
Patient attitudes and traits
Motivated patients [59, 60] who have a positive outlook on the benefits of the AP with realistic expectations are good candidates for the AP [25, 29], because they will likely persevere when faced with hardships when adapting to the technology. While such patients could be favoured in AP allocation, these attitudes may also be fostered through patient coaching (see below).
Moreover, patient traits which do not translate into optimal clinical benefits should not be used as selection criteria. For instance, age and ability to use technology may be tempting ways to orient AP allocation, because young and tech-savvy smartphone users could be expected to better grasp how to use T1D management technologies than the elderly. Surprisingly, one study showed that some elderly patients tended to be more prudent with insulin pump therapy: they were more attentive in following the instruction manual and less likely to improvise [60]. Some tech-savvy individuals who were assigned insulin pump therapy did not achieve maximum benefits because they would tend to deviate from the instructions they received during their training [60]. Hence, using age and technological ability as a selection criterion would be discriminatory and unfair, knowing that its application may not necessarily translate into optimal clinical benefits. More inquiry will be needed to establish which traits are predictive of optimal clinical outcomes and less harm with the use of the AP.
Social environment
Good social support and flexible schedules may ensure greater clinical benefits, but a strict application of this criterion could disproportionately impact people of lower socio-economic status. Social support and flexible schedules may involve that patients can benefit from greater follow-up by being available for regular follow-up appointments [61]. This is especially true for patients who are less autonomous in the management of their T1D due to their age (e.g., adolescents, elderly). On the other hand, access to T1D care varies according to socio-economic status. In Sweden, a lower socio-economic status doubles or triples the risks for T1D complications such as cardiovascular disease and death [62]. In the United States, non-Hispanic white youth with higher socio-economic status are more susceptible than their counterparts to be attributed insulin pump therapy in the first year following their diagnosis [63]. Similarly, life misfortunes and lower education encountered by families with lower socio-economic status act as barriers for accessing medical care, which translates in reduced access to insulin pump therapy [64]. Moreover, these individuals are often eligible to public health insurance who offers, at best, limited coverage for insulin pump therapy and continuous glucose monitoring devices, as opposed to usually larger coverage by private health insurance. If AP attribution follows similar patterns, equity in access to sophisticated T1D care through the AP could be compromised for people of lower socio-economic status. In addition, individuals faced with social hardships could especially benefit from the AP, given that its automation would alleviate the need for familial support and rigid schedules.
Adherence to therapy and follow-up
Eligibility to some public coverage programs for insulin pump therapy is offered to those demonstrating good knowledge of T1D management, who adhere to therapy and who schedule regular follow-up appointments. In Alberta, patients who want to receive public coverage for an insulin pump must fulfil specific requirements and undergo various assessments in a period of three to six months to qualify for the coverage program. These patients must have two HbA1c readings under 9.0% taken three months apart. They must demonstrate good knowledge of carbohydrate counting and insulin dose adjustment to prevent hypo- and hyperglycaemic events and must also attend regular appointments [65]. At first glance, similar eligibility criteria could be envisioned for a public coverage program for the AP to favour clinical benefits, patient safety, and appropriate use of human and economic resources. Paradoxically, the strict application of such criteria could prevent access to the AP to patients who experience difficulties with their current treatment modality, some of which the AP is well-positioned to solve (e.g., insulin dose adjustments). Hence, AP eligibility criteria could mirror those used with the insulin pump, but they should be adapted to the improved capabilities of the technology and to patients’ needs.
Domain 4: Patient coaching and support
Sufficient support, education, and follow-up for people with T1D are necessary to ensure maximum benefits and safety, as well as to empower patients in autonomously managing their illness [66]. Classic T1D management skills include carbohydrate counting, calculating insulin doses or insulin-to-carbohydrate ratios, and adjusting the treatment regimen to physical exercise. In addition to these skills, AP education includes AP use and maintenance, as well as using data generated to improve glucose control. Additional components of comprehensive patient support include realistic expectations, troubleshooting, experiential knowledge, and lifestyle habits.
Realistic expectations
Without realistic expectations, patients could be disappointed by the AP despite improvements in glycaemic control, and expose themselves to risks by not sufficiently understanding the limits of what the AP can do for them. In itself, the expression “artificial pancreas” is misleading and may cultivate unrealistic expectations: indeed, the AP does not replicate all endocrine functions of a healthy pancreas and it is not integrated seamlessly into the body, despite alleviating important burdens. Patients may only have realistic expectations regarding the AP’s capabilities if they understand that it is not a new organ and not a cure to T1D [61].
Healthcare professionals could help foster realistic expectations among patients by highlighting that the AP constitutes a partner in T1D care. Indeed, the patient performs general maintenance (e.g., replacement of cartridges and catheters) of the AP and oversight (e.g., entering meal carbohydrate content and managing glycaemic variations induced by exercise). In exchange, the AP takes clinical decisions by calculating and administering appropriate insulin doses, and acts as a decision aide, notably by displaying glycaemic curves, which empowers the patient to make reasoned choices regarding activities, meals, and exercise periods. Providing patients with a balanced account of the advantages, drawbacks, and capabilities of the AP may also help foster realistic expectations [24].
Troubleshooting
Long-term AP use will likely impact the use of time during appointments with healthcare professionals. Provided that technical details don’t overrule the discussion, the automation of glycaemic management will allow for greater promotion of healthy lifestyle, more extensive complication screening and treatment, and improved reduction of cardiovascular complications, as opposed to a primary and sometimes exclusive focus on glycaemia fluctuations. Adoption of the AP shortly after a T1D diagnosis could also reduce the risks of acute and long-term complications (e.g., severe hypoglycaemic events and chronic kidney diseases) and their economic, administrative, organizational, and healthcare implications. Yet, when starting to use the technology, AP users may require significant assistance in AP troubleshooting due to its greater complexity. Without sufficient support, AP users could feel disempowered by the technology and not experience the expected clinical outcomes.
Offering sufficient troubleshooting support to patients may be challenging for healthcare professionals, who already face limited availability. Devoting time to AP troubleshooting could involve less time to devote to patients with other treatment modalities or health conditions [32], raising concerns of justice. Moreover, the AP transforms the role of the clinician from a “physiology expert to [a] machine operator” [67] by centring T1D care on the operation of a computerized tool. This shift in the clinician’s responsibilities highlights the need for healthcare professionals to obtain new training on AP troubleshooting that goes beyond the utility of the AP as a decision-making aide in T1D care [67].
Industry representatives should offer troubleshooting help. When acquiring a new insulin pump, in Canada, patients typically benefit from a one-month follow-up by a healthcare professional working for the company and then a help line is made available to them. A similar and ideally improved support system could be envisioned for the AP. The exact division of tasks between the healthcare system and industry representatives would need to be determined and potential conflicts of interests could be made explicit. In both cases, patients could also be directed to written and online resources (e.g., instruction manuals) that could provide guidance regarding troubleshooting. Ultimately, patients should be taught to maintain and operate an AP, as well as to manage acute problems that could arise with the technology (e.g., software malfunction).
Experiential knowledge
A patient’s experiential knowledge is necessary for successful management of T1D [68]. This knowledge, generated through trial-and-error, involves personal tricks, habits, and daily tasks useful to T1D management such as note-taking about wellness, making sense of reactions to insulin, and acquiring subjective understanding about how physical activity, nutrition and stress can affect glycaemia [68]. Transitioning from a conventional T1D management strategy to the AP may not always translate into improved glucose control, especially when patients start to use the device and experiment with it. Thus, healthcare professionals may need to interpret raw data provided by the AP cautiously and in complement to the patient’s evolving experiential knowledge when formulating recommendations related to T1D management.
Lifestyle habits
Physical activity and healthy nutrition are determinants of general health and are relevant to patient education, but respectively involve adjusting insulin doses prior to exercise and carbohydrate counting [5], which both add to the burden of T1D management. By preventing hypoglycaemia more effectively, especially with glucagon, the AP helps people with T1D to practice physical activity more safely and easily. The resulting psychological and physical benefits of exercise could translate into general health improvements for AP users in the context of rapid rising prevalence of cardiometabolic risk factors and complications in patients with T1D [69]. On the other hand, current T1D management is glucocentric, meaning that patients must monitor their carbohydrate intake and correct hypoglycaemic events through sugar intake. Understandably, several patients with T1D view eating as a stressful task, and eating disorders have a higher prevalence especially among women with T1D than in the general population [70]. Current versions of the AP perpetuate glucocentric T1D management by requiring users to enter their carbohydrate intake at every meal [8].
However, experimental versions of the AP which require simpler general meal information (e.g., a small meal that corresponds to a pre-programmed range of carbohydrates) are currently being tested [19]. In addition to alleviating the burden of carbohydrate counting and reducing the risk of hypoglycaemic events, such experimental versions could be more permissive regarding meal intake and allow users to enjoy their meals and eat mindfully. The shift from a conventional glucocentric T1D diet management to a more general healthy eating will allow to shift patients’ attention to other aspects of diet quality (e.g. fiber, protein and fat content of meals). On the other hand, the latter could perhaps be jeopardized by transition to the AP if patients erroneously believe that the AP’s adjustment of glycaemia towards target ranges is sufficient for good health. In this case, patients might be less self-conscious about the foods they consume by expecting the AP to adjust the insulin bolus appropriately [2]. Nevertheless, in the near future, all upcoming AP versions will require meal notification. With current and future AP versions, healthcare professionals should remind their patients of the importance of diet quality and that correcting for glycaemic excursions is not sufficient for good health.
Domain 5: Personal identity and agency
The support offered by the AP in T1D care will likely have benefits for users, who feel more reassured and at peace with the technology with the impression of living a normal life and that their illness is under control [22]. Patients report feeling less stressed, anxious, and worried with an AP [29]. These resulting improvements in quality of life, in addition to the benefits provided by the AP, improve sleep [22] and performance at work [28]. While this support is profitable for AP users, it implies changes to agency, burden of control, and visibility of T1D, and may render patients more vulnerable to malfunctions.
Agency
Agency designates a person’s ability to act autonomously and voluntarily and it is considered a finite resource [71]. People with T1D devote part of their agency to the control of their illness and direct the rest of their agency towards other daily occupations. Patients who opt for an AP agree to surrender part of their T1D control to the technology in exchange of greater agency to devote to their daily activities. This shared control of T1D is conditional to the patient’s trust in the technology, which may be limited by unfamiliarity with the AP, technical limitations, and patient values and preferences. Indeed, patients who start using the AP may initially exert close oversight on the AP’s operations for safety and security concerns. As patients become familiar with the AP’s capabilities and limitations, and as they witness its performance and reliability, they will develop trust in the technology in partly controlling their T1D. Yet, technical problems (e.g., issues with the equipment or software) or limitations of current AP versions (e.g., imprecise glucose sensor readings in hypoglycaemic or hyperglycaemic peaks or due to inappropriate calibration [28, 30, 72]) could diminish users’ trust in the technology.
In addition, healthcare professionals may want to take into account their patients’ values and preferences in directing them towards an appropriate treatment modality. Some patients’ experiential knowledge in using multiple daily injections may allow them to manage rigorously and successfully their T1D. These patients could believe than an AP would not possess the fine knowledge of being able to adjust insulin infusions according to their level of activity and bodily sensations, and would less likely trust it. In all cases of limited trust in the AP, the use of this technology may become self-defeating. Patients will feel the urge to validate that the AP’s operations and decisions are appropriate, forcing them to allocate even greater agency to their T1D management. Given that trust is likely strengthened over time, healthcare professionals could warn patients that initial AP use may warrant closer oversight, but that this situation will resolve over time. Healthcare professionals may also want to encourage AP use in patients who can appropriately deal with the AP’s technical limitations.
Burden of glycaemic control
The sharing of responsibilities and agency with the AP could alleviate some of the psychological toll of T1D control experienced by the patient. Furthermore ongoing glycaemic feedback may foster confidence in T1D control. People with T1D often believe that they are fully responsible for the control of their illness [73]. Thus, the inability to attain target glycaemia can represent a moral failure and a sign of unworthiness to them [73]. The sharing of responsibilities and agency with the AP will likely involve a change in this charged discourse. Patients could feel less guilty and shameful of not attaining adequate glycaemia levels, and shift the blame on the AP, which could alleviate some of the moral and emotional burden they normally feel. In addition, the AP’s ability to provide timely glycaemia readings could mimic the positive feedback provided by clinicians in medical appointments, helping the patient to become more confident in his or her T1D care.
Visibility of T1D management or devices
The visibility of behaviours or technologies involved in T1D management may challenge adherence, especially if their visibility is unwelcomed by the patient. Multiple daily insulin injections and glucose monitoring make T1D a disease that is occasionally visible, while insulin pumps or an AP transform T1D into a readily visible illness. Importantly, the visibility of behaviours or devices used for T1D management may jeopardize adherence to the treatment regimen. For example, social environments do not always offer the privacy or convenience to self-inject [1, 74] and may limit adherence to this treatment modality. As a result, people with T1D, especially younger adults, may avoid self-injections or wearing a pump in public to preserve their self-image, avoid stigmatization, and hide their illness from others [1, 75, 76]. Stigmatization occurs when someone with poor knowledge of the illness can falsely attribute negative characteristics to the person with T1D, such as blaming them for their illness and labelling them as sick [76, 77]. Limits to adherence in public spaces could also be an issue with the AP, namely regarding calibration and replacements of the insulin cartridge, but these steps are easier to dissimulate in public for patients who express the need for privacy. By its design, the AP also facilitates adherence by transforming T1D management from a series of manual operations to essentially entering numerical values and settings, at convenient times, into a programmable and hybrid closed-loop system.
Despite making T1D management more discrete, the AP still contributes to the visibility of the illness by being a cumbersome and an uncomfortable external system for some patients [30, 77]. The insulin pump and the catheter can impose restrictions or adjustments on clothing and are also problematic for intimate relationships [78]. By being visible, the AP could raise questions among the public and act as a conversation starter, to the dismay of patients who would prefer avoiding unsolicited attention and be “treated as ‘normal’” [73]. The AP could also contribute to reducing patients with T1D to their identity as “diabetics”, shedding light mainly on their illness and leaving less space for the rest of their identity [73]. This misconception could be handled by the patient in an informal setting, but in other situations such as the case of a job interview where first impressions might matter more, the patient would prefer avoiding that an interviewer be aware of his or her illness.
To ensure sustained adherence to a treatment modality, healthcare professionals should take into account patient preferences regarding the visibility of T1D when recommending treatment modalities. Healthcare professionals could acknowledge that the potential constant visibility of T1D conferred by the pump or the AP, albeit unwelcomed by some patients, can be informative to the patient’s surroundings in the case of an emergency.
Dependence and vulnerability
By relying on the AP for clinical decisions, patients may become dependent on the AP and vulnerable to malfunctions, especially if they expect the AP to resolve all technical issues without external intervention. Accordingly, some maintenance of basic T1D management skills and refreshers on the principles underlying more conventional forms of T1D management will need to be nurtured by healthcare professionals to increase patients’ resilience in the event of technical failures of the AP. More specifically, to ease potential concerns regarding control and lack of reliability, a feature that temporarily suspends automatic insulin infusions informed by glycaemia and that would allow the patient to switch back to a pre-programmed classic open-loop insulin pump infusions with manual adjustments will be an essential feature of AP devices.
Conclusion
In this paper, we undertook a first review and analysis of ethical issues raised by the artificial pancreas keeping in mind the developers, clinicians, patients, and decisions-makers who are, at this time, key stakeholders in the development of the AP and its clinical adoption. Given the scarcity of relevant literature, we used a combination of reviews and studies on relevant issues raised by the AP analysed through a common bioethics framework. We then identified five initial domains where ethically problematic situations are likely to surface based on further analyses and team discussions. More research will be needed to validate the ethical issues associated with the AP we outlined above, to discard worries which may not become significant as the technology unfolds and improves, and to understand issues that could arise through improvements in the technology. For example, future continuous glucose monitoring devices will be smaller and wearable for a greater length of time, require less calibrations, and offer improved glycaemic reading accuracy during hypoglycaemic and hyperglycaemic events, for instance [79]. Over time, these improvements in technology and their reduced costs could improve patient satisfaction by diminishing some of the concerns pertaining to visibility, reliability, and safety. Inversely, these improvements could give rise to additional ethically problematic situations. For example, the widespread use of extremely effective APs in the future could nurture higher expectations regarding glycaemic control in healthcare professionals, which could be more difficult to attain for those who use multiple insulin injections, by choice or due to a lack of financial resources. For now, understanding patient preferences and attitudes toward different trade-offs offered by the technology, as well as monitoring the translation of the technology in the situations described in this paper and over time, will be key to ensure patient satisfaction and adequate clinical uptake. Hopefully this paper sets the stage for such further work as well as conversations about ethical aspects of this new technology.
Acknowledgements:
Writing of this paper was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases, NIH (RRL; 1 DP3 DK106930–01), a summer internship scholarship from the IRCM, a graduate student award from the Canadian Institutes of Health Research (AQ) and a career award from the Fonds de recherche Québec – Santé (ER). RRL holds the J-A DeSeve chair for diabetes. We would like to thank Jelena Poleksic and other members of the Neuroethics Research Unit, as well as Nadine Taleb and Katherine Desjardins of the Metabolic Diseases Unit for helpful feedback on a previous version of this manuscript.
Disclosure of interest
RRL has received research grants from the Canadian Diabetes Association, Astra-Zeneca, Eli Lilly, Cystic Fibrosis Canada, Merck, Novo-Nordisk, and Sanofi-Aventis. He has been a consultant or member of advisory panels of Abbott, Amgen, Astra-Zeneca, Boehringer, Carlina Technology, Eli Lilly, Janssen, Medtronic, Merck, Neomed, Novo-Nordisk, Roche, Sanofi-Aventis, and Takeda. He has received honoraria for conferences by Abbott, Astra-Zeneca, Eli Lilly, Janssen, Medtronic, Merck, Novo-Nordisk, and Sanofi-Aventis. He has received in kind contributions related to closed-loop technology from Animas, Medtronic, and Roche. He also benefits from unrestricted grants for clinical and educational activities from Eli Lilly, Lifescan, Medtronic, Merck, Novo Nordisk, and Sanofi. He holds intellectual property in the field of type 2 diabetes risk biomarkers, catheter life and the artificial pancreas. RRL and VM received purchase fees from Eli Lilly in relation with closed-loop technology. AQ and ER do not have any competing interests to declare.
References
- [1].Balfe M, Doyle F, Smith D, Sreenan S, Brugha R, Hevey D, et al. What’s distressing about having type 1 diabetes? A qualitative study of young adults’ perspectives. BMC Endocr Disord 2013;13:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gonder-Frederick L Lifestyle modifications in the management of type 1 diabetes: still relevant after all these years? Diabetes Technol Ther 2014;16(11):695–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mauras N, Fox L, Englert K, Beck RW. Continuous glucose monitoring in type 1 diabetes. Endocrine 2013;43(1):41–50. [DOI] [PubMed] [Google Scholar]
- [4].Misso ML, Egberts KJ, Page M, O’Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev 2010(1):CD005103. [DOI] [PubMed] [Google Scholar]
- [5].Walsh J, Roberts R. Pumping insulin: everything you need for success on a smart insulin pump. Torrey Pines Press; 2006. [Google Scholar]
- [6].Peyser T, Dassau E, Breton M, Skyler JS. The artificial pancreas: current status and future prospects in the management of diabetes. Ann N Y Acad Sci 2014;1311:102–23. [DOI] [PubMed] [Google Scholar]
- [7].FDA. FDA’s efforts to advance artificial pancreas device systems, https://www.fda.gov/medicaldevices/productsandmedicalprocedures/homehealthandconsumer/consumerproducts/artificialpancreas/default.htm; 2016 [accessed August 30,.2017].
- [8].Gingras V, Taleb N, Roy-Fleming A, Legault L, Rabasa-Lhoret R. The challenges of achieving postprandial glucose control using closed-loop systems in patients with type 1 diabetes. Diabetes Obes Metab 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Quemerais MA, Doron M, Dutrech F, Melki V, Franc S, Antonakios M, et al. Preliminary evaluation of a new semi-closed-loop insulin therapy system over the prandial period in adult patients with type 1 diabetes: the WP6.0 Diabeloop study. J Diabetes Sci Technol 2014;8(6):1177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lazaro C, Oruklu E, Sevil M, Turksoy K, Cinar A. Implementation of an artificial pancreas system on a mobile device. 2016 IEEE International Conference on Electro Information Technology (EIT). 2016. [Google Scholar]
- [11].Blauw H, van Bon AC, Koops R, DeVries JH. Performance and safety of an integrated bihormonal artificial pancreas for fully automated glucose control at home. Diabetes Obes Metab 2016;18(7):671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Del Favero S, Place J, Kropff J, Messori M, Keith-Hynes P, Visentin R, et al. Multicenter outpatient dinner/overnight reduction of hypoglycemia and increased time of glucose in target with a wearable artificial pancreas using modular model predictive control in adults with type 1 diabetes. Diabetes Obes Metab 2015;17(5):468–76. [DOI] [PubMed] [Google Scholar]
- [13].Anderson SM, Raghinaru D, Pinsker JE, Boscari F, Renard E, Buckingham BA, et al. Multinational home use of closed-loop control is safe and effective. Diabetes Care 2016;39(7):1143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Doyle FJ, Huyett LM, Lee JB, Zisser HC, Dassau E. Closed-loop artificial pancreas systems: engineering the algorithms. Diabetes Care 2014;37(5):1191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].El-Khatib FH, Balliro C, Hillard MA, Magyar KL, Ekhlaspour L, Sinha M, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. Lancet 2017;389(10067):369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Haidar A, Legault L, Messier V, Mitre TM, Leroux C, Rabasa-Lhoret R. Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open-label randomised controlled crossover trial. Lancet Diabetes Endocrinol 2015;3(1):17–26. [DOI] [PubMed] [Google Scholar]
- [17].Haidar A, Rabasa-Lhoret R, Legault L, Lovblom LE, Rakheja R, Messier V, et al. Single- and dual-hormone artificial pancreas for overnight glucose control in type 1 diabetes. J Clin Endocrinol Metab 2016;101(1):214–23. [DOI] [PubMed] [Google Scholar]
- [18].Taleb N, Emami A, Suppere C, Messier V, Legault L, Ladouceur M, et al. Efficacy of single-hormone and dual-hormone artificial pancreas during continuous and interval exercise in adult patients with type 1 diabetes: randomised controlled crossover trial. Diabetologia 2016;59(12):2561–71. [DOI] [PubMed] [Google Scholar]
- [19].Gingras V, Rabasa-Lhoret R, Messier V, Ladouceur M, Legault L, Haidar A. Efficacy of dual-hormone artificial pancreas to alleviate the carbohydrate-counting burden of type 1 diabetes: A randomized crossover trial. Diabetes Metab 2016;42(1):47–54. [DOI] [PubMed] [Google Scholar]
- [20].Taleb N, Coriati A, Khazzaka C, Bayonne J, Messier V, Rabasa-Lhoret R. Stability of commercially available glucagon formulation for dual-hormone artificial pancreas clinical use. Diabetes Technol Ther 2017;19(10):589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Taleb N, Haidar A, Messier V, Gingras V, Legault L, Rabasa-Lhoret R. Glucagon in the artificial pancreas systems; potential benefits and safety profile of future chronic use. Diabetes Obes Metab 2016. [DOI] [PubMed] [Google Scholar]
- [22].Barnard KD, Wysocki T, Allen JM, Elleri D, Thabit H, Leelarathna L, et al. Closing the loop overnight at home setting: psychosocial impact for adolescents with type 1 diabetes and their parents. BMJ Open Diabetes Res Care 2014;2(1):e000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Barnard KD, Aldred C, Hood KK, Weissberg-Benchell J, Oliver N, Laffel L. Psychosocial assessment of artificial pancreas (AP): commentary and review of existing measures and their applicability in AP research. Diabetes Technol Ther 2015;17(4):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Iturralde E, Tanenbaum ML, Hanes SJ, Suttiratana SC, Ambrosino JM, Ly TT, et al. Expectations and attitudes of individuals with type 1 diabetes after using a hybrid closed loop system. Diabetes Educ 2017;43(2):223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Barnard KD, Wysocki T, Thabit H, Evans ML, Amiel S, Heller S, et al. Psychosocial aspects of closed- and open-loop insulin delivery: closing the loop in adults with Type 1 diabetes in the home setting. Diabet Med 2015;32(5):601–8. [DOI] [PubMed] [Google Scholar]
- [26].Citron P Ethics considerations for medical device R&D. Prog Cardiovasc Dis 2012;55(3):307–15. [DOI] [PubMed] [Google Scholar]
- [27].Barnard KD, Venkat MV, Close K, Heinemann L, Weissberg-Benchell J, Hood KK, et al. PsychDT working group: report psychosocial aspects of artificial pancreas systems. J Diabetes Sci Technol 2015;9(4):925–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Young AJ, Thabit H, Heller SR, Evans ML, Amiel SA, Hovorka R, et al. Holistic impact of closed-loop technology on people with type 1 diabetes. J Diabetes Sci Technol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].van Bon AC, Kohinor MJE, Hoekstra JBL, von Basum G, DeVries JH. Patients’ perception and future acceptance of an artificial pancreas. J Diabetes Sci Technol 2010;4(3):596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ang KH, Tamborlane WV, Weinzimer SA. Combining glucose monitoring and insulin delivery into a single device: current progress and ongoing challenges of the artificial pancreas. Expert Opin Drug Deliv 2015;12(10):1579–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].O’Keeffe DT, Maraka S, Basu A, Keith-Hynes P, Kudva YC. Cybersecurity in Artificial Pancreas Experiments. Diabetes Technol Ther 2015;17(9):664–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Weissberg-Benchell J, Hood K, Laffel L, Heinemann L, Ball D, Kowalski A, et al. Toward development of psychosocial measures for automated insulin delivery. J Diabetes Sci Technol 2016;10(3):799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Beauchamp TL. Principles of biomedical ethics. 7th ed New York: Oxford University Press; 2013. [Google Scholar]
- [34].Maisel WH, Kohno T. Improving the security and privacy of implantable medical devices. N Engl J Med 2010;362(13):1164–6. [DOI] [PubMed] [Google Scholar]
- [35].Government Accountability Office of the United States. Medical devices : FDA should expand its consideration of information security for certain types of devices : report to congressional requesters. 2012.
- [36].FDA. The Content of Investigational Device Exemption (IDE) and Premarket Approval (PMA) Applications for Artificial Pancreas Device Systems.
- [37].Camara C, Peris-Lopez P, Tapiador JE. Security and privacy issues in implantable medical devices: A comprehensive survey. J Biomed Inform 2015;55:272–89. [DOI] [PubMed] [Google Scholar]
- [38].Chinthapalli K Experts call for greater regulation of deep brain stimulation. BMJ : British Medical Journal 2013;346. [DOI] [PubMed] [Google Scholar]
- [39].Cengiz E, Sherr JL, Weinzimer SA, Tamborlane WV. Clinical equipoise: an argument for expedited approval of the first small step toward an autonomous artificial pancreas. Expert Rev Med Devices 2012;9(4):315–7. [DOI] [PubMed] [Google Scholar]
- [40].Weinger K, O’Donnell KA, Ritholz MD. Adolescent views of diabetes-related parent conflict and support: a focus group analysis. J Adolesc Health 2001;29(5):330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mellin AE, Neumark-Sztainer D, Patterson JM. Parenting adolescent girls with type 1 diabetes: parents’ perspectives. J Pediatr Psychol 2004;29(3):221–30. [DOI] [PubMed] [Google Scholar]
- [42].Ng SM. Improving patient outcomes with technology and social media in paediatric diabetes. BMJ Qual Improv Rep 2015;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pinzon J, Harvey J. Care of adolescents with chronic conditions. Paediatrics & Child Health 2006;11(1):43–8. [Google Scholar]
- [44].Consent Informed Permission Parental, and Assent in Pediatric Practice. Pediatrics 1995;95(2):314–7. [PubMed] [Google Scholar]
- [45].Kriewall TJ. Ethics of medical device safety. J Long Term Eff Med Implants 2008;18(2):167–74. [DOI] [PubMed] [Google Scholar]
- [46].Haidar A, Legault L, Matteau-Pelletier L, Messier V, Dallaire M, Ladouceur M, et al. Outpatient overnight glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or conventional insulin pump therapy in children and adolescents with type 1 diabetes: an open-label, randomised controlled trial. Lancet Diabetes Endocrinol 2015;3(8):595–604. [DOI] [PubMed] [Google Scholar]
- [47].Ciani O, Wilcher B, van Giessen A, Taylor RS. Linking the regulatory and reimbursement processes for medical devices: the need for integrated assessments. Health Econ 2017;26 Suppl 1:13–29. [DOI] [PubMed] [Google Scholar]
- [48].Nielsen HB, Ovesen LL, Mortensen LH, Lau CJ, Joensen LE. Type 1 diabetes, quality of life, occupational status and education level - A comparative population-based study. Diabetes Res Clin Pract 2016;121:62–8. [DOI] [PubMed] [Google Scholar]
- [49].Tao B, Pietropaolo M, Atkinson M, Schatz D, Taylor D. Estimating the cost of type 1 diabetes in the U.S.: a propensity score matching method. PLoS One 2010;5(7):e11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tanenbaum ML, Adams RN, Hanes SJ, Barley RC, Miller KM, Mulvaney SA, et al. Optimal use of diabetes devices: clinician perspectives on barriers and adherence to device use. J Diabetes Sci Technol 2017;11(3):484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Thabit H, Tauschmann M, Allen JM, Leelarathna L, Hartnell S, Wilinska ME, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med 2015;373(22):2129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kropff J, Del Favero S, Place J, Toffanin C, Visentin R, Monaro M, et al. 2 month evening and night closed-loop glucose control in patients with type 1 diabetes under free-living conditions: a randomised crossover trial. Lancet Diabetes Endocrinol 2015;3(12):939–47. [DOI] [PubMed] [Google Scholar]
- [53].Othonos N, Choudhary P. Who should be considered for islet transplantation alone? Curr Diab Rep 2017;17(4):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, et al. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 2016;39(7):1230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Shapiro AMJ, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006;355(13):1318–30. [DOI] [PubMed] [Google Scholar]
- [56].Wu Y, Ding Y, Tanaka Y, Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int J Med Sci 2014;11(11):1185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Thabit H, Hartnell S, Allen JM, Lake A, Wilinska ME, Ruan Y, et al. Closed-loop insulin delivery in inpatients with type 2 diabetes: a randomised, parallel-group trial. Lancet Diabetes Endocrinol 2017. [DOI] [PubMed] [Google Scholar]
- [58].Ruan Y, Thabit H, Wilinska ME, Hovorka R. Modelling endogenous insulin concentration in type 2 diabetes during closed-loop insulin delivery. Biomed Eng Online 2015;14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Franklin V Influences on technology use and efficacy in type 1 diabetes. J Diabetes Sci Technol 2016;10(3):647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lawton J, Kirkham J, Rankin D, White DA, Elliott J, Jaap A, et al. Who gains clinical benefit from using insulin pump therapy? A qualitative study of the perceptions and views of health professionals involved in the Relative Effectiveness of Pumps over MDI and Structured Education (REPOSE) trial. Diabet Med 2016;33(2):243–51. [DOI] [PubMed] [Google Scholar]
- [61].Bell E, Mathieu G, Racine E. Preparing the ethical future of deep brain stimulation. Surg Neurol 2009;72(6):577–86. [DOI] [PubMed] [Google Scholar]
- [62].Rawshani A, Svensson A-M, Rosengren A, Eliasson B, Gudbjörnsdottir S. Impact of socioeconomic status on cardiovascular disease and mortality in 24,947 individuals with type 1 diabetes. Diabetes Care 2015;38(8):1518–27. [DOI] [PubMed] [Google Scholar]
- [63].Lin MH, Connor CG, Ruedy KJ, Beck RW, Kollman C, Buckingham B, et al. Race, socioeconomic status, and treatment center are associated with insulin pump therapy in youth in the first year following diagnosis of type 1 diabetes. Diabetes Technol Ther 2013;15(11):929–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Powell PW, Chen R, Kumar A, Streisand R, Holmes CS. Sociodemographic effects on biological, disease care, and diabetes knowledge factors in youth with type 1 diabetes. J Child Health Care 2013;17(2):174–85. [DOI] [PubMed] [Google Scholar]
- [65].Alberta Health Services. Process for New Patients Interested in Insulin Pump Therapy, https://www.albertahealthservices.ca/assets/programs/ps-1061556-insulin-pump-process.pdf; 2013. [accessed April 12 2018].
- [66].McGibbon A, Richardson C, Hernandez C, Dornan J. Pharmacotherapy in Type 1 Diabetes. Can J Diabetes;37:S56–S60. [DOI] [PubMed] [Google Scholar]
- [67].Kietzman L, Slotnick S. Diabetes technology: ‘fear not’, http://www.medscape.com/viewarticle/881741; 2017. [accessed August 30.2017]. [Google Scholar]
- [68].Storni C Patients’ lay expertise in chronic selfcare: a case study in type 1 diabetes. Health Expect 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gingras V, Leroux C, Fortin A, Legault L, Rabasa-Lhoret R. Predictors of cardiovascular risk among patients with type 1 diabetes: A critical analysis of the metabolic syndrome and its components. Diabetes Metabol 2017;43(3):217–22. [DOI] [PubMed] [Google Scholar]
- [70].Goebel-Fabbri AE. Disturbed eating behaviors and eating disorders in type 1 diabetes: clinical significance and treatment recommendations. Curr Diab Rep 2009;9(2):133–9. [DOI] [PubMed] [Google Scholar]
- [71].Baumeister RF, Bratslavsky E, Muraven M, Tice DM. Ego depletion: is the active self a limited resource? J Pers Soc Psychol 1998;74(5):1252–65. [DOI] [PubMed] [Google Scholar]
- [72].Barnard KD, Wysocki T, Allen JM, Elleri D, Thabit H, Leelarathna L, et al. Closing the loop overnight at home setting: psychosocial impact for adolescents with type 1 diabetes and their parents. BMJ Open Diabetes Research & Care 2014;2(1):e000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Broom D, Whittaker A. Controlling diabetes, controlling diabetics: moral language in the management of diabetes type 2. Soc Sci Med 2004;58(11):2371–82. [DOI] [PubMed] [Google Scholar]
- [74].Wilson V Students’ experiences of managing type 1 diabetes. Paediatr Nurs 2010;22(10):25–8. [DOI] [PubMed] [Google Scholar]
- [75].Dunning T, Duggan N, Savage S, Martin P. Diabetes and end of life: ethical and methodological issues in gathering evidence to guide care. Scand J Caring Sci 2013;27(1):203–11. [DOI] [PubMed] [Google Scholar]
- [76].Browne JL, Ventura A, Mosely K, Speight J. ‘I’m not a druggie, I’m just a diabetic’: a qualitative study of stigma from the perspective of adults with type 1 diabetes. BMJ Open 2014;4(7):e005625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Farrington C Wearable technologies and stigma in diabetes: the role of medical aesthetics. Lancet Diabetes Endocrinol 2016;4(7):566. [DOI] [PubMed] [Google Scholar]
- [78].Racine E, Quintal A, Sample M. Neuroessentialism in discussions about the impact of closed-loop technologies on agency and identity. AJOB Neurosci 2017;8(2):81–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Frieden J FDA Panel Gives Thumbs Up to 90-Day Glucose Monitor, https://www.medpagetoday.com/primarycare/diabetes/72098; 2018. [accessed April 12 2018].


